Abstract
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, are a group of fatal neurodegenerative diseases detected in a wide range of mammalian species. The “protein-only” hypothesis of TSE suggests that prions are transmissible particles devoid of nucleic acid and the primary pathogenic event is thought to be the conversion of cellular prion protein (PrPC) into the disease-associated isoform (PrPSc). According to susceptibility to TSEs, animals can be classified into susceptible species and low susceptibility species. In this review we focus on several species with low susceptibility to TSEs: dogs, rabbits, horses and buffaloes. We summarize recent studies into the characteristics of low susceptibility regarding protein structure, and biochemical and genetic properties.
Keywords: Transmissible spongiform encephalopathy, Low susceptibility, Dog, Rabbit, Horse, Buffalo, PRNP, SPRN
Transmissible spongiform encephalopathy (TSE), or prion disease, is an invariably fatal neurodegenerative disease detected in a wide range of mammalian species, including Scrapie in goats (Capra hircus) and sheep (Ovis aries); bovine spongiform encephalopathy (BSE) in cattle (Bos taurus); chronic wasting disease (CWD) in elaphure (Elaphurus davidianus) and moose (Alces americanus); feline spongiform encephalopathy (FSE) in cats (Felis catus); transmissible mink encephalopathy (TME) in minks (Mustla vison); and Creutzfeldt-Jakob disease (CJD), variant Creutzfeldt-Jakob disease (vCJD), fatal familial insomnia (FFI), Gerstmann-Straussler-Scheinker syndrome (GSS) and Kuru in humans (Homo sapiens) (Collins et al, 2004; Prusiner, 1982). Humans and other animals infected with TSE are clinically and pathologically characterized with neuronal progressive vacuolation, stellate cell gliosis, spongiform lesions in gray matter, amyloid deposition and eventually disastrous degeneration and death (Prusiner, 1998). No effective treatments have been found and the World Health Organization has named TSE and AIDS as two major health problems of the 21st century.
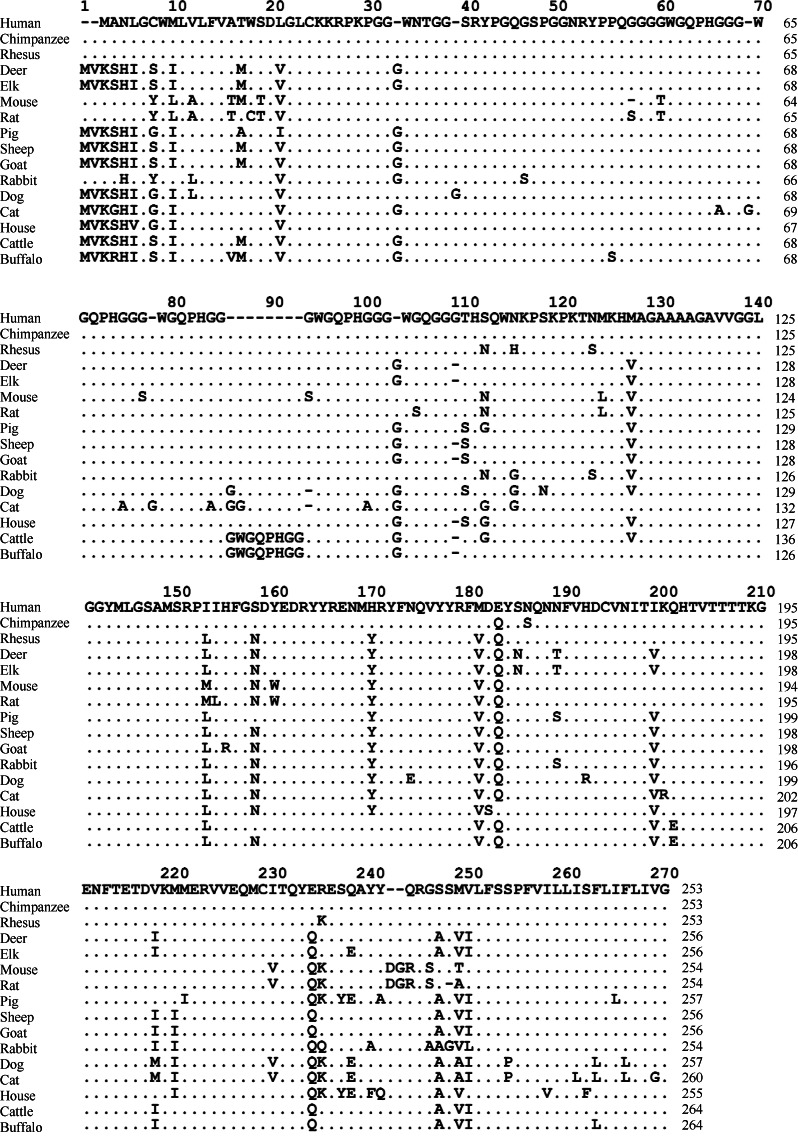
The “protein-only” hypothesis of TSE suggests that the pathogenic factors of TSE are not bacteria or a virus, but a protein devoid of nucleic acid, which has been named prion protein (PrP). PrP is encoded by the prion protein gene (PRNP) (Prusiner, 1982). Normal cellular PrP (PrPC) expresses in the cells of mammalian species and the number of amino acid residues varies from 253 to 264 across species (Wopfner et al, 1999) and are all highly conserved (Figure 1). PrP has two signal peptide sequences, a N-terminal and a C-terminal. Mature PrP has an intra-molecular disulfide bond and two glycosylation sites, and is anchored on the cell membrane surface via glycosylphosphatidylinositol (GPI) at the C-terminal (Aguzzi et al, 2008). According to the protein-only hypothesis TSE is a conformational disease and under certain circumstances cellular PrPC mistakenly converts into the disease-associated isoform (PrPSc). Although the primary structures of PrPC and PrPSc are the same, their secondary structures are quite different. PrPC is enriched with α-helix (42% are α-helix, 3% are β-fold), whereas PrPSc is enriched with β-fold (43% are β-fold, 30% are α-helix) and is protease resistant (McKinley et al, 1983; Pan et al, 1993; Prusiner, 1982). The massive intracellular accumulation of PrPSc induces formations of oligomer and amyloid fibrils, and eventually neuronal degeneration (Barron et al, 2007; Caughey et al, 2009). PrP plays vital roles in the pathological process of TSE. Knockout and low expression of PrP effectively abolishes or reduces susceptibility to TSEs, respectively (Brandner et al, 1996; Büeler et al, 1993), whereas high expression is associated with susceptibility and a shortened incubation time for disease development (Manson et al, 1994).
Figure 1.
Amino acid sequences of PrP in 16 mammals (data from GenBank)
Human (NM_000311.3), chimpanzee (NM_001009093.3), Rhesus (NM_001047152.1), deer (AY330343.1), elk (EU082291.1), mouse (NM_011170.3), rat (NM_012631.2), pig (NM_001008687.1), sheep (NM_001009481.1), goat(JF729302.1), rabbit (NM_001082021.1), dog (NM_001013423.1), cat (EU341499.1), horse(NM_001143798.1), cattle (NM_181015.2), buffalo ( KC.1). 137634.
TSE susceptibility is species-specific. Previous studies show high susceptibility in hamsters (Mesocricetus auratus) to TSE, as they can be infected by various PrPSc virus strains isolated from human, cattle, goats, mice (Mus musculus) and minks (Bessen & Marsh, 1992; Gibbs & Gajdusek, 1973; Kimberlin & Walker, 1977; Thomzig et al, 2006). Similar high susceptibility is also found in mice (Chandler, 1961; Gibbs & Gajdusek, 1973; Hill et al, 2000; Lasmézas et al, 1997; Thomzig et al, 2006). However, rabbits could not be infected by PrPSc strains isolated from human, goats and mice (Barlow & Rennie, 1976; Gibbs & Gajdusek, 1973). During the outbreak of BSE in the UK, infections in humans and several species of feline were reported, but no infection was found in dogs (Canis familiaris) or horses (Equus caballus) (Aldhous, 1990; Kirkwood & Cunningham, 1994). Collectively, species with confirmed susceptibility to TSE include humans, rhesus monkeys (Macaca mulatta), hamsters, mice, minks, elaphures, moose, goats, sheep, cattle and raccoons (Procyon lotor) (Imran & Mahmood, 2011). Only a few species, such as dogs (Canis familiaris), rabbits (Oryctolagus cuniculus) and horses (Equus caballus) have been recognized as TSE resistant (Fernandez-Funez et al, 2011; Yuan et al, 2013; Zhang, 2011a). Interestingly, although more than 190,000 cattle were infected by BSE, and buffaloes (Bubalus bubalis) and cattle are closely related, no buffalo has been reported with BSE infection (http://www.oie.int) and are of low susceptibility to BSE (Zhao et al, 2012). In this review, based on TSE susceptibility, animals have been classified into TSE susceptible animals and TSE low susceptible animals.
The pathological mechanisms of TSE are yet to be clarified. Although highly susceptible animals are important to understanding this disease, studies on animals with low susceptibility provide a new angle from which to examine TSE. Here, we review recent research developments on protein structures, biochemical characteristics and genetic features of four animals (dogs, rabbits, horses and buffaloes) with low susceptibly to TSE.
Dogs
During the outbreak of BSE in the UK, several species of feline were reportedly infected, including cheetahs (Acinonyx jubatus), pumas (Pumaconcolor) and cats (Kirkwood & Cunningham, 1994). Since 1990, about 100 cats and 29 captive felines, including 15 cheetahs, four lions (Panthera leo), three leopard cats (Prionailurus bengalensis), three pumas, three tigers (Panthera tigris) and one Asian golden cat (Catopuma temminckii), have been diagnosed with FSE (Imran & Mahmood, 2011). The presumed infection source was PrPSc-contaminated food; however, dogs and cats are provided similar food and no dogs were reported with TSE (Imran & Mahmood, 2011; Kirkwood & Cunningham, 1994; Wopfner et al, 1999). With further laboratory cell experiments, dogs have been recognized as a species with low TSE susceptibility. For example, when Madin-Daby canine kidney cells (MDCK) were infected with brain tissue homogenates from CJD patients or RML prion strain isolated from scrapie animals, although the biosynthesis and processing of PrPC in MDCK are similar with those in N2aPK1 cells of murine neuroblastoma, which are highly susceptible to TSE, no PrPSc was found in MDCK. When infected MDCK were used to infect N2aPK1 cells, no PrPSc was found in N2aPK1 cells either (Ploymenidou et al, 2008; Zhang & Liu, 2011).
The gene polymorphism of PRNP is correlated with TSE susceptibility (Westaway et al, 1994). In humans, at least 30 mutations of PRNP are intertwined with TSE susceptibility (Lloyd et al, 2011). In dogs, the amino acid residue 187 and 229 of the PrP sequence are histidine and glycine, respectively, whereas, they both are arginine in cats (Wopfner et al, 1999). No FSE-related polymorphic site was found by screening encoding sequences of PRNP in 609 animals (including 15 FSE infected cases) and 29 species from 22 genera of the Order Carnivora, but Stewart et al (2012) did notice that amino acid residue 163 in all canines is either aspartate or glutamic acid, indicating this locus may have some connection to TSE susceptibility.
The three-dimensional structure of PrPC may be another tool in resolving the puzzle of TSE susceptibility (Lin & Wen, 2011). To understand the structural differences of PrPC in animals with low and high susceptibility to TSE, Lysek et al (2005) carried out a study on nuclear magnetic resonance (NMR) structures of PrPC in dogs (canine PrP,cPrP), cats (feline PrP, fPrP), pigs (sus scrofa PrP, scPrP) and goats (ovine PrP, ovPrP). Their overall three-dimensional structures are quite close, consisting of a N-terminal (constituted of about 100 amino acid residues in random coil) and a globular domain in the C-terminal (including three α-helixes and a pair of short, reverse paralleled β-folds constituting about 100 amino acid residues). The globular domain in the C-terminal is species-specific, e.g., four amino acids (Asp159Asn, Arg177His, Lys185 Arg and Gly229Arg) are different between cPrPC and fPrPC; Asp-159 and Arg-177 in cPrPC make it unique in potential distribution; in fPrPC, scPrPC and ovPrPC same positive potential distribution patterns are observed in their C-terminals.
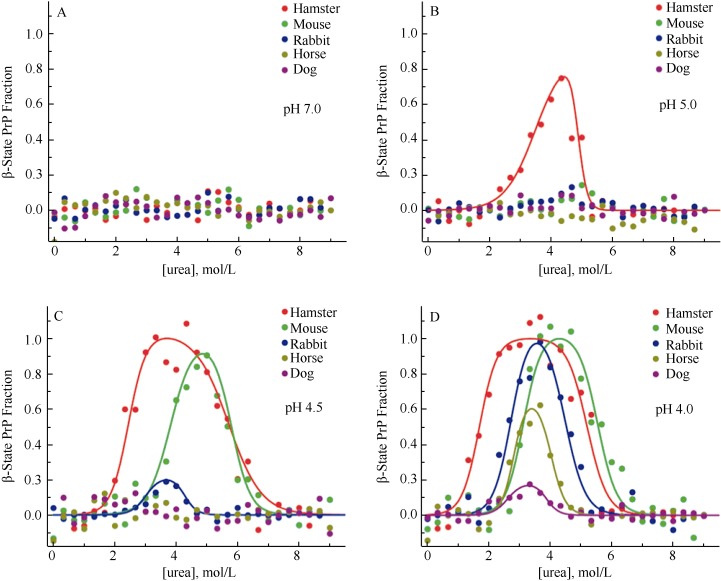
The conversion of PrPC into PrPSc is critical in TSE pathogenesis. Besides PrPC and PrPSc, the existence of the third state-β intermediate state, consisting mainly of β-fold in circular dichroism (CD) (Hornemann & Glockshuber, 1998), has raised attention due to its capability in introducing TSE (Collinge & Clarke, 2007). Khan et al (2010) claimed that in different species, the propensities in forming the β intermediate state are one of the vital factors influencing TSE susceptibility. Using dual wavelength CD, Khan et al (2010) compared the structures of the globular domain in high-susceptible (hamsters and mice) and low-susceptible species to TSE (rabbits, horses and dogs) and found that under inducing conditions with different pH values and urea concentrations, the propensities of forming the β intermediate state vary in different species. At pH 7.0, urea concentrations have no effect on PrPC and no β intermediate state is observed in all five species; at pH 5.0, the structure of hamster PrPC (hPrPC) is most unstable and easily forms the β intermediate state; at pH 4.0, the PrPC in all five species is unstable and easily forms the β intermediate state, the lowest concentration of β intermediate state occurs in dogs (Figure 2). The propensities in forming the β intermediate state (hamsters > mice > rabbits > horses > dogs) can also be adopted in evaluating species’ susceptibilities to TSE (Fernandez-Funez et al, 2011). Using molecular kinetic methods, Zhang & Liu (2011) found stable molecular structures of wild cPrPC under both neutral and acidic pH conditions, and in the neutral condition the salt bridge between D177 and R163 improves structural stability. These studies help to explain the mechanism of low TSE susceptibility in dogs and general TSE pathogenesis.
Figure 2.
Propensities of conversions of PrPC into the β intermediate state in different species at pH 7.0 (A), 5.0 (B), 4.5 (C) and 4.0 (D) and different concentrations of urea (modified from Khan et al, 2010)
Rabbits
No cases of spontaneous TSE infection in rabbits have been reported to date. In 1973, Gibbs & Gajdusek failed to infect rabbits with either brain tissues from human CJD or Kuru patients or brain tissues from scrapie animals and minks with TME. In 1976, Barlow & Rennie failed to infect rabbits with ME7 strains isolated from animals with scrapie. By constructing rabbit PrPC (RaPrPC) over-expressed murine neuroblastoma tumor cell lines, Vorberg et al (2003) confirmed that RaPrPC can neither be infected by RML strains nor convert into the PrPSc. The in vivo experiments conducted by Fernandez-Funez et al (2010) support the view that rabbits are TSE resistant species. Fernandez-Funez et al (2010) expressed full length PrP of hamsters, mice and rabbits in drosophilae voided of endogenous PrP, and found that cavernous transformation and isomers similar with PrPSc can only be found in the brains of transgenic drosophilae expressing shPrPC and mouse PrPC (moPrPC), but not in drosophilae expressing RaPrPC. Moreover, Bellotti & Chiti (2008) reported that TSE correlates with the deposition of amyloid fibrils. Zhou et al (2011) found that Ficoll 70 and dextran 70 significantly accelerate the fibration of hPrPC and bPrPC, but prevent fibration of RaPrPC; however, different from hPrPC and bPrPC, RaPrPC does not have fragments resisting protease K digestion.
Initially it was presumed that one of the possible reasons rabbits are resistant to TSE infection is that certain transforming factors are lacking in their cellular environment or some inhibitory factors are expressed and the conversion of PrPC to PrPSc is prevented. This presumption was later proved wrong. When PK13 cells, expressing shPrPC, moPrPC and vole (Microtus spp.) PrPC, respectively, were infected with shPrPSc, moPrPSc and vole PrPSc, massive replications of PrPSc were observed, indicating that factors necessary for PrP conversion exist in rabbit cells (Courageot et al, 2008; Vilette et al, 2001). Rabbits and mice share 87% similarity in amino acid sequences (33 amino acids are different, including 22 in mature peptides). When amino acid residues 99, 108, 173 and 214 in moPrPC were mutated into the corresponding amino acid residues in RaPrP (Asn99Gly, Leu108Met, Asn173Ser and Val214Ile, respectively) and were overexpressed in mouse neuroblastoma (MNB), and then RML prion strains were used to infect MNB, the mutants of moPrPC could not convert into PrPSc, indicating that several amino acid residues in RaPrPC can prevent the replication of isomers of PrPSc (Vorberg et al, 2003). About 33% of the different amino acids in moPrPC and RaPrPC locate around the attachment site of the GPI-anchor. Nisbet et al (2010) stably transfected RK13 cells voided of endogenous PrPC with a constructed double-mutant (Ser230Gly and Ser231Val) model of moPrPC, MoPrP-RbGPI, and then infected RK13 cells expressing MoPrP-RbGPI using human prion strains M1000 or MU-02 isolated from mice brain homogenates, The results showed that MoPrP-RbGPI could change neither the attachment of the GPI-anchor nor the location of PrPC on cells, but no PrPSc produce either, indicating that rabbit-specific amino acids may interfere with PrPSc and PrPC contact and eventually prevent conversion of PrPC to PrPSc (Nisbet et al, 2010). These findings indicate that the resistance of rabbits to TSE may be attributable to their unique RaPrPC structure (Lin & Wen, 2011). Wen et al (2010a) adopted NMR techniques to study the solution structure of RaPrPC and found that compared to hPrPC, mPrPC and bPrPC, RaPrPC features a unique charge distribution pattern. A large consecutive positive potential area exists on the protein surface of RaPrPC which may interfere to interact with molecules such as chaperon protein X, prevents the proliferation of PrPSc (Wen et al, 2010a). Khan et al (2010) found a critical helix-capping motif interacting with the third α-helix and regulating the β-intermediate state by exploring the crystal structure of RaPrPC. As we mentioned earlier, the complexities of PrPC forming the β-intermediate state in different species are correlated with their susceptibility to TSE. Compared with ShPrPC and moPrPC, RaPrPC is difficult to transform into the β-intermediate state (Figure 2) (Khan et al, 2010). The irregular curling fragment, called an α2-β2 loop, locates between the second β-fold and the second α-helix (165-172). The epitope consisting of the α2-β2 loop and the C-terminal of the third α-helix is considered capable of recognizing protein X and regulating progression of TSE (Kaneko et al, 1997). Protein dynamics analysis shows that RaPrPC has a constructively highly ordered β2-α2 loop (Wen et al, 2010a) which may function as a species barrier for TSE dissemination (Lin & Wen, 2011). However, compared with wild RaPrPC, S173N and I124V mutations affect the interactions of the β2-α2 loop with the third α-helix, and thereafter decrease the stability of the entire construct (Wen et al, 2010a, b). In addition, when the salt bridges between D202-R156 and D178-R164 were removed in hPrPC and moPrPC, although secondary protein structures remained intact, the helix structures of RaPrPC were destroyed, indicating that salt bridges are important to the stability of RaPrPC (Zhang, 2009, 2010, 2011a).
Recently, Joaquin Castilla’s research group has raised questions about the view that rabbits are resistant to TSE. They amplified rabbit brain homogenates using serial automated protein misfolding cyclic amplification (saPMCA) and then inoculated this in vitro novel PrP into the brains of three other rabbits. One rabbit was found with TSE symptoms 766 days after inoculation even although no exogenous PrPSc was involved. Then the brain homogenates from this infected rabbit could 100% infect RaPrPC over-expressed transgenic mice. Therefore, Chianini et al (2012) claims that rabbits are not TSE resistant. Furthermore, using saPMCA, when the amplified proteins from mixtures of rabbit brain homogenates and BSE prion strains were inoculated into the brains of RaPrPC over-expressed transgenic mice, the resultant strains similar to BSE prion strains were discovered (Vidal et al, 2013). Fernández-Borges et al (2012) claims that in vivo infective experiments are imperfect when forming the conclusion that rabbits are resistant to TSE, especially when supported only by the observation that rabbits can not be infected with TSE naturally.
Horses
As there is no reports of horses being naturally infected with TSE, horses are recognized as low susceptibility species (Zhang, 2011a). Relative to dogs and rabbits, fewer studies have looked at low susceptibility in horses. Studies on the conversion of PrPC into the β intermediate state show that under unstable conditions at pH 4, the PrPC of hamsters, mice, rabbits, dogs and horses can convert into the β intermediate state, but the lowest level of β intermediate state is found in horses (Figure 2), indicating that equus caballus PrPC (ecPrPC) is relatively stable (Khan et al, 2010). Structural NMR on ecPrPC found two horse-specific amino acid alterations in its β2-α2 loop (Ser-167 and Lys-173, respectively), among which, S167 affects the highly ordered solution structure of the β2-α2 loop and may influence the low susceptibility of horses to TSE (Pérez et al, 2010). However, when amino acid residue 167 in moPrPC was mutated from asparagine into ecPrPC-specific serine (MoPrPD167S), although MoPrPD167S and ecPrPC share similar NMR structures and their β2-α2 loops in solutions are both relatively highly ordered, spongiform lesions were found in mice expressing MoPrPD167S and neural diseases can be induced with the accumulation of PrPSc in the brain (Sigurdson et al, 2011). Therefore, the ordered state of the β2-α2 loop in solution alone does not fully explain different susceptibilities to TSE (Lin & Wen, 2011). Moreover, as the salt bridge in RaPrPC stabilizes protein structures, similar salt bridges consisting of GLU196-ARG156-HIS187, ARG156-ASP202 and GLU211-HIS177 are also found in ecPrPC (Zhang, 2011b). The structures of ecPrPC and cPrPC are stable under both neutral and acidic conditions (Zhang, 2011a). A common phenomenon found among RaPrPC, ecPrPC and cPrPC, is the salt bridge ASP177-ARG163 connects with the β2-α2 loop of PrP and is probably correlated with TSE susceptibility (Zhang, 2011a). Nevertheless, the low susceptibility of horses to TSE requires further work.
Buffalo
BSE was initially found in the UK in 1986, rapidly spread to over 25 countries, and caused major economic losses (Harman & Silva, 2009; Wells et al, 1987). BSE can also infect humans via the food chain and cause human vCJD (Collinge et al, 1996; Hill et al, 1997). The multiple pathogenic pathways of TSE, including spontaneous mutant, inheritance and infection (Nicholson et al, 2008), may explain why even after meat and bone meal was strictly forbidden, more than 15 000 BSE infected cattle were found in the UK (http://www.oie.int). Worldwide, there were over 190 000 taurus cattle, 1 Bos indicus, and 1 Bos indicus × Bos taurus cross reported with BSE infections (data of OIE, Novakofski et al, 2005; Seuberlich et al, 2006). Although buffaloes and cattle are quite close phylogenetically, no case of BSE infected buffalo was ever reported, suggesting that genetic factors are crucial to BSE susceptibility (Zhao et al, 2012). The expression level of PrP is closely correlated with BSE susceptibility. Studies show that the PRNP gene of cattle has two indel (insertion and deletion) polymorphisms (a 23-bp indel in putative promoter, and a 12-bp indel in intron 1). These Indel polymorphisms affect gene expression (Msalya et al, 2011; Sander et al, 2005) and eventually BSE susceptibility (Haase et al, 2007; Juling et al, 2006; Sander et al, 2004). Studies on polymorphisms in buffalo in Anatolia (Oztabak et al, 2009), Pakistan (Imran et al, 2012), Indonesia and Thailand (Uchida et al, 2014) show signifycant differences in frequency distributions between buffaloes and cattle. Recently, genotyping analysis on Chinese buffalo showed that the distribution frequencies of BSE susceptibility related to genotypes and alleles, including the 23-bp deletion allele (D23) and 12-bp deletion allele (D12), were significantly lower than those of healthy cattle and BSE infected cattle, indicating that the low PrP expressed in buffalo may influence BSE susceptibility. Our later experiments proved that in tissue of the cerebellum, brain stem, mesenteric lymph nodes and bronchial lymph nodes, the expression of PrP is lower in buffalo than in cattle (submitted data).
Although PRNP play a vital role in the pathogenesis of TSE, the underlying pathological mechanisms of TSE remain unclear. Some propose that other than prions, there may be other factors or proteins regulating the pathogenesis and pathological process of TSE (Daude & Westaway, 2011; Watts et al, 2007). The SPRN (shadow of prion protein) gene and its encoded protein Shadoo (Sho) have drawn lots of attention due to their roles in the pathogenesis of TSE. Comparative genomics analysis indicates that Sho is a newly discovered member of the prion protein family. Sho has been found in mammals such as mice and humans and is highly conserved from fish to mammals (Premzl et al, 2003). Sho and PrPC have a lot in common regarding structure and expression (Wang et al, 2014). In PrPSc infected animal brains or nervous cell, with increasing PrPSc expression, the level of Sho decreases dramatically (Watts et al, 2007, 2011; Westaway et al, 2011). Beck et al (2008) reported that the insertion of a base (heterozygous) within the encoding area of SPRN induces a frame-shift mutation which is correlated with vCJD. So, it is highly possible that Sho regulates the process of TSE by functioning as an inhibitory factor (Daude & Westaway, 2011). Our analysis of differences in the genetics and expression of SPRN between buffalo and cattle show that in the hydrophobic domain (HD) within the encoding area, cattle have a 12-bp indel polymorphism which induces insertion/deletion of four amino acids. However, this phenomenon was not observed in buffalo (Zhao et al, 2012). The HD of Sho not only protects against physiological stressors in nervous cell, but also helps Sho to interconnect with PrPC (Wang et al, 2010). This interconnection is a prerequisite of Sho regulating the pathogenesis of disease (Wang et al, 2010). The exploration of the indel polymorphism within the Sho HD structural area is critical in fully understanding underlying mechanisms of TSE. Our luciferase reporter and immuno-blotting experiments confirm that compared to cattle, buffaloes have higher promoter activity and higher Sho expression, consistent with our prediction that buffalos have more transcription factor binding sites than cattle (Zhao et al, 2012). These findings suggest that the low susceptibility of buffalos to BSE is probably attributable to significant genetic differences in SPRN.
Further Research
Joaquin Castilla’s research group denies there are TSE resistant mammals, and believes that with improvements in detection any species can be found to be at risk of TSE infection (Fernandez-Borges et al, 2012). However, from available data, TSE susceptibility does vary between species and we can classify animals as high susceptibility species and low susceptibility species. Scientists worldwide have applied various techniques to the study of TSE pathogenesis and have mainly focused on the genetic polymorphism of PRNP, expression levels of PRNP/PrP, three-dimensional structure and stability of PrPC and dynamics of PRNP. However, the pathogenesis of TSE remains unclear. Lin & Wen (2011) claim that the three-dimensional structure of PrPSc and the physiological function of PrPC are keys to resolving this puzzle but these two research directions have proved extremely difficult, even after two decades of attention. With breakthroughs in novel technologies and methods however, progress is likely. Studies on TSE low susceptibility molecules and newly discovered SPRN (Wang et al, 2014) provide important clues about the formation of PrPSc and our understanding of TSE pathogenesis. Due to similarities in Sho and PrPC regarding structure and function, especially their important roles in the pathogenesis and development of TSE, exploration of the biological functions of Sho and its regulatory effect on TSE will be vital.
Funding Statement
This item was supported by the National Natural Science Foundation of China (31060302 and 31260032), the Transgene Special Project of the Ministry of Agriculture of China (2011ZX08009-003-006) and the Natural Science Foundation of Yunnan Province (2010CD010)
Footnotes
The authors have declared that no competing interests exist.
References
- [1].Aguzzi A, Sigurdson C, Heikenwaelder M. 2008. Molecular mechanisms of prion pathogenesis. Annual Review of Pathology: Mechanisms Of Disease, 3(1): 11-40. [DOI] [PubMed] [Google Scholar]
- [2].Aldhous P. 1990. BSE: Spongiform encephalopathy found in cat. Nature, 345(6272): 194. [DOI] [PubMed] [Google Scholar]
- [3].Barlow RM, Rennie JC. 1976. .The fate of ME7 scrapie infection in rats, guinea-pigs and rabbits. Research in Veterinary Science, 21(1): 110-1. [PubMed] [Google Scholar]
- [4].Barron RM, Campbell SL, King D, Bellon A, Chapman KE, Williamson RA, Manson JC. 2007. High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. Journal of Biological Chemistry, 282(49): 35878-86. [DOI] [PubMed] [Google Scholar]
- [5].Beck JA, Campbell TA, Adamson G, Poulter M, Uphill JB, Molou E, Mead S. 2008. Association of a null allele of SPRN with variant Creutzfeldt-Jakob disease. Journal of Medical Genetics, 45(12): 813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bellotti V, Chiti F. 2008. Amyloidogenesis in its biological environment: challenging a fundamental issue in protein misfolding diseases. Current Opinion in Structural Biology, 18(6):771-779. [DOI] [PubMed] [Google Scholar]
- [7].Bessen RA, Marsh RF. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. Journal of General Virology, 73(2): 329-34. [DOI] [PubMed] [Google Scholar]
- [8].Brandner S, Raeber A, Sailer A, Blättler T, Fischer M, Weissmann C, Aguzzi A. 1996. Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 93(23): 13148-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Büeler H, Aguzzi A, Sailer A, Greiner, RA, Autenried P, Aguet M, Weissmann C. 1993. Mice devoid of PrP are resistant to scrapie. Cell, 73(7): 1339-47. [DOI] [PubMed] [Google Scholar]
- [10].Caughey B, Baron GS, Chesebro B, Jeffrey M. 2009. Getting a grip on prions: oligomers, amyloids and pathological membrane interactions. Annual Review of Biochemistry, 78(1): 177-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chandler RL. 1961. Encephalopathy in mice produced by inoculation with scrapie brain material. The Lancet, 277(7191): 1378-9. [DOI] [PubMed] [Google Scholar]
- [12].Chianini F, Fernández-Borges N, Vidal E, Gibbard L, Pintado B, Castro JD, Priola SA, Hamilton S, Eaton SL, Finlayson J, Pang Y, Steele P, Reid HW, Dagleish MP, Castilla J. 2012. Rabbits are not resistant to prion infection. Proceedings of the National Academy of Sciences of the United States of America, 109(13): 5080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Collinge J, Clarke AR. 2007. A general model of prion strains and their pathogenicity. Science, 318(5852): 930-6. [DOI] [PubMed] [Google Scholar]
- [14].Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF. 1996. Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature, 383(6602): 685-90. [DOI] [PubMed] [Google Scholar]
- [15].Collins SJ, Lawson VA, Masters CL. 2004. Transmissible spongiform encephalopathies. The Lancet, 363(9402): 51-62. [DOI] [PubMed] [Google Scholar]
- [16].Courageot MP, Daude N, Nonno R, Paquet S, Di Bari MA, Le Dur A, Chapuis J, Hill AF, Agrimi U, Laude H, Vilette D. 2008. A cell line infectible by prion strains from different species. Journal of General Virology, 89(1): 341-7. [DOI] [PubMed] [Google Scholar]
- [17].Daude N, Westaway D. 2011. Biological properties of the PrP-like Shadoo protein. Frontiers in Bioscience, 16: 1505-16. [DOI] [PubMed] [Google Scholar]
- [18].Fernández-Borges N, Chianini F, Eraña H, Vidal E, Eaton SL, Pintado B, Finlayson J, Dagleish MP, Castilla J. 2012. Naturally prion resistant mammals: A utopia? Prion, 6(5): 425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernandez-Funez P, Zhang Y, Casas-Tinto S, Xiao X, Zou WQ, Rincon-Limas DE. 2010. Sequence-dependent prion protein misfolding and neurotoxicity. Journal of Biological Chemistry, 285(47): 36897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fernandez-Funez P, Zhang Y, Sanchez-Garcia J, Jensen K, Zou WQ, Rincon-Limas DE. 2011. Pulling rabbits to reveal the secrets of the prion protein. Communicative & Integrative Biology, 4(3): 262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gibbs CJ, Gajdusek DC. 1973. Experimental subacute spongiform virus encephalopathies in primates and other laboratory animals. Science, 182(4107): 67-8. [DOI] [PubMed] [Google Scholar]
- [22].Haase B, Doherr MG, Seuberlich T, Drögemüller C, Dolf G, Nicken P, Schiebel K, Ziegler U, Groschup MH, Zurbriggen A, Leeb T. 2007. PRNP promoter polymorphisms are associated with BSE susceptibility in Swiss and German cattle. BMC Genet, 8(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harman JL, Silva CJ. 2009. Bovine spongiform encephalopathy. Journal of the American Veterinary Medical Association, 234(1): 59-72. [DOI] [PubMed] [Google Scholar]
- [24].Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P. 1997. The same prion strain causes vCJD and BSE. Nature, 389(6650): 448-50. [DOI] [PubMed] [Google Scholar]
- [25].Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J. 2000. Species-barrier-independent prion replication in apparently resistant species. Proceedings of the National Academy of Sciences of the United States of America, 97(18): 10248-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hornemann S, Glockshuber R. 1998. A scrapie-like unfolding intermediate of the prion protein domain PrP (121-231) induced by acidic pH. Proceedings of the National Academy of Sciences of the United States of America, 95(11): 6010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imran M, Mahmood S, Babar ME, Hussain R, Yousaf MZ, Abid NB, Lone KP. 2012. PRNP gene variation in Pakistani cattle and buffaloes. Gene, 505(1): 180-5. [DOI] [PubMed] [Google Scholar]
- [28].Imran M, Mahmood S. 2011. An overview of animal prion diseases. Virology Journal, 8(1): 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Juling K, Schwarzenbacher H, Williams JL, Fries R. 2006. A major genetic component of BSE susceptibility. BMC Biology, 4(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaneko K, Zulianello L, Scott M, Cooper CM, Wallace AC, James TL, Cohen FE, Prusiner SB. 1997. Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proceedings of the National Academy of Sciences of the United States of America, 94(19): 10069-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Khan MQ, Sweeting B, Mulligan VK, Arslan PE, Cashman NR, Pai EF, Chakrabartty A. 2010. Prion disease susceptibility is affected by β-structure folding propensity and local side-chain interactions in PrP. Proceedings of the National Academy of Sciences of the United States of America, 107(46): 19808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kimberlin RH, Walker CA. 1977. Characteristics of a short incubation model of scrapie in the golden hamster. Journal of General Virology, 34(2): 295-304. [DOI] [PubMed] [Google Scholar]
- [33].Kirkwood JK, Cunningham AA. 1994. Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Veterinary Record, 135(13): 296-303. [DOI] [PubMed] [Google Scholar]
- [34].Lasmézas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science, 275(5298): 402-4. [DOI] [PubMed] [Google Scholar]
- [35].Lin DH, Wen W. 2011. Progresses on prion proteins. Scientia China: Chimica, 41(4): 683-98. [Google Scholar]
- [36].Lloyd S, Mead S, Collinge J. 2011. Genetics of prion disease. Topics in Current Chemistr, 305: 1-22. [DOI] [PubMed] [Google Scholar]
- [37].Lysek DA, Schorn C, Nivon LG, Esteve-Moya V, Christen B, Calzolai L, Schroetter CV, Fiorito F, Herrmann T, Guntert P, Wüthrich K. 2005. Prion protein NMR structures of cats, dogs, pigs, and sheep. Proceedings of the National Academy of Sciences of the United States of America, 102(3): 640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Manson JC, Clarke AR, McBride PA, McConnell I, Hope J. 1994. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration, 3(4): 331-40. [PubMed] [Google Scholar]
- [39].McKinley MP, Bolton DC, Prusiner SB. 1983. A protease-resistant protein is a structural component of the scrapie prion. Cell, 35(1): 57-62. [DOI] [PubMed] [Google Scholar]
- [40].Msalya G, Shimogiri T, Ohno S, Okamoto S, Kawabe K, Minezawa M, Maeda Y. 2011. Evaluation of PRNP expression based on genotypes and alleles of two indel loci in the medulla oblongata of Japanese Black and Japanese Brown cattle. PLoS One, 6(5): e18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nicholson EM, Brunelle BW, Richt JA, Kehrli Jr ME, Greenlee, JJ. 2008. Identification of a heritable polymorphism in bovine PRNP associated with genetic transmissible spongiform encephalopathy: evidence of heritable BSE. PLoS One, 3(8): e2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nisbet RM, Harrison CF, Lawson VA, Masters CL, Cappai R, Hill AF. 2010. Residues surrounding the glycosylphosphatidylinositol anchor attachment site of PrP modulate prion infection: insight from the resistance of rabbits to prion disease. Journal of Virology, 84(13): 6678-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Novakofski J, Brewer MS, Mateus-Pinilla N, Killefer J, McCusker RH. 2005. Prion biology relevant to bovine spongiform encephalopathy. Journal of Animal Science, 83(6):1455-76. [DOI] [PubMed] [Google Scholar]
- [44].Oztabak K, Ozkan E, Soysal I, Paya I, Ün C. 2009. Detection of prion gene promoter and intron1 indel polymorphisms in Anatolian water buffalo (Bubalus bubalis). Journal of Animal Breeding and Genetics, 126(6): 463-7. [DOI] [PubMed] [Google Scholar]
- [45].Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorm I, Huang Z, Fletterick RJ, Cohen FE. 1993. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proceedings of the National Academy of Sciences of the United States of America, 90(23): 10962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pérez DR, Damberger FF, Wüthrich K. 2010. Horse prion protein NMR structure and comparisons with related variants of the mouse prion protein. Journal of Molecular Biology, 400(2): 121-8. [DOI] [PubMed] [Google Scholar]
- [47].Polymenidou M, Trusheim H, Stallmach L, Moos R, Julius C, Miele G, Lenz-Bauer C, Aguzzi A. 2008. Canine MDCK cell lines are refractory to infection with human and mouse prions. Vaccine, 26(21): 2601-14. [DOI] [PubMed] [Google Scholar]
- [48].Premzl M, Sangiorgio L, Strumbo B, Marshall Graves JA, Simonic T, Gready JE. 2003. Shadoo, a new protein highly conserved from fish to mammals and with similarity to prion protein. Gene, 314: 89-102. [DOI] [PubMed] [Google Scholar]
- [49].Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science, 216(4542): 136-44. [DOI] [PubMed] [Google Scholar]
- [50].Prusiner SB. 1998. Nobel lecture: Prions. Proceedings of the National Academy of Sciences of the United States of America, 95(23): 13363-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sander P, Hamann H, Drögemüller C, Kashkevich K, Schiebel K, Leeb T. 2005. Bovine Prion protein gene (PRNP) promoter polymorphisms modulate PRNP expression and may be responsible for differences in bovine spongiform encephalopathy susceptibility. Journal of Biology Chemistry, 280(45): 37408-14. [DOI] [PubMed] [Google Scholar]
- [52].Sander P, Hamann H, Pfeiffer I, Wemheuer W, Brenig B, Groschup MH, Ziegler U, Distl O, Leeb T. 2004. Analysis of sequence variability of the bovine prion protein gene (PRNP) in German cattle breeds. Neurogenetics, 5(1): 19-25. [DOI] [PubMed] [Google Scholar]
- [53].Seuberlich T, Botteron C, Wenker C, Café-Marçal V, Oevermann A, Haase B, Leeb T, Heim D, Zurbriggen A. 2006. Spongiform encephalopathy in a miniature zebu. Emerging Infectious Diseases, 12(12): 1950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sigurdson CJ, Joshi-Barr S, Bett C, Winson O, Manco G, Schwarz P, Rulicke T, Nilsson KPR, Margalith I, Raeber A, Peretz D, Hornemann S, Wuthrich K, Aguzzi A. 2011. Spongiform encephalopathy in transgenic mice expressing a point mutation in the β2-α2 loop of the prion protein. Journal of Neuroscience, 31(39): 13840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stewart P, Campbell L, Skogtvedt S, Griffin KA, Arnemo JM, Tryland M, Girling S, Miller MW, Tranulis MA, Goldmann W. 2012. Genetic predictions of prion disease susceptibility in carnivore species based on variability of the prion gene coding region. PloS One, 7(12): e50623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Thomzig A, Cardone F, Krüger D, Pocchiari M, Brown P, Beekes M. 2006. Pathological prion protein in muscles of hamsters and mice infected with rodent-adapted BSE or vCJD. Journal of Neuroscience, 87(1): 251-4. [DOI] [PubMed] [Google Scholar]
- [57].Uchida L, Heriyanto A, Thongchai C, Hanh TT, Horiuchi M, Ishihara K, Tamura Y, Muramatsu Y. 2014. Genetic diversity in the prion protein gene (PRNP) of domestic cattle and water buffaloes in Vietnam, Indonesia and Thailand. Journal of Veterinary Medical Science, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vidal E, Fernández-Borges N, Pintado B, Ordóñez M. , Márquez M, Fondevila D, Torres JM, Pumarola M, Castilla J. 2013. Bovine spongiform encephalopathy induces misfolding of alleged prion-resistant species cellular prion protein without altering its pathobiological features. Journal of Neuroscience, 33(18): 7778-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vilette D, Andreoletti O, Archer F, Madelaine MF, Vilotte JL, Lehmann S, Laude H. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proceedings of the National Academy of Sciences of the United States of America, 98(7): 4055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vorberg I, Groschup MH, Pfaff E, Priola SA. 2003. Multiple amino acid residues within the rabbit prion protein inhibit formation of its abnormal isoform. Journal of Virology, 77(3): 2003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang JY, Hao Z, Xu M, Wang X, Wu SB, Song BC, Liu WS, Li JP, Meng KY, Li ZY, Gao HW. 2010. Mapping the interaction site of prion protein and Sho. Molecular Biology Reports, 37(5): 2295-300. [DOI] [PubMed] [Google Scholar]
- [62].Wang SQ, Zhao H, Zhang YP. 2014. Advances in research on Shadoo, Shadow of prion protein. Chinese Science Bulletin, 59(9): 821-7. [Google Scholar]
- [63].Watts JC, Drisaldi B, Ng V, Yang J, Strome B, Horne P, Sy M, Young R, Mastrangelo P, Bergeron C, Fraser PE, Carlson GA, Mount HTJ, Schmitt-Ulms G, Westaway D. 2007. The CNS glycoprotein Shadoo has PrPC-like protective properties and displays reduced levels in prion infections. The EMBO Journal, 26(17): 4038-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Watts JC, Stöhr J, Bhardwaj S, Wille H, Oehler A, DeArmond SJ, Giles K, Prusiner SB. 2011. Protease-resistant prions selectively decrease Shadoo protein. PLoS Pathogens, 7(11): e1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. 1987. A novel progressive spongiform encephalopathy in cattle. Veterinary Record, 121(18): 419-20. [DOI] [PubMed] [Google Scholar]
- [66].Wen Y, Li J, Yao WM, Xiong MQ, Hong J, Peng Y, Xiao GF, Lin DH. 2010. a. Unique structural characteristics of the rabbit prion protein. Journal of Biological Chemistry, 285(41): 31682-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wen Y, Li J, Xiong M, Peng Y, Yao WM, Hong J, Lin DH. 2010. b. Solution structure and dynamics of the I214V mutant of the rabbit prion protein. PLoS One, 5(10): e13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Westaway D, Genovesi S, Daude N, Brown R, Lau A, Lee I, Mays CE, Coomaraswamy J, Canine B, Pitstick R, Herbst A, Yang J, Ko KWS, Schmitt-Ulms G, Dearmond SJ, Mckenzie D, Hood L, Carlson GA. 2011. Down-regulation of Shadoo in prion infections traces a pre-clinical event inversely related to PrPSc accumulation. PLoS Pathogens, 7(11): e1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Westaway D, Mirenda C A, Foster D, Zebarjadian Y, Scott M, Torchia M, Yang S, Serban H, Dearmond SJ, Ebeling C, Prusiner SB, Carlson GA. 1991. Paradoxical shortening of scrapie incubation times by expression of prion protein transgenes derived from long incubation period mice. Neuron, 7(1): 59-68. [DOI] [PubMed] [Google Scholar]
- [70].Westaway D, Zuliani V, Cooper C M, Costa MD, Neuman S, Jenny AL, Detwiler L, Prusiner SB. 1994. Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes and Development, 8(8): 959-69. [DOI] [PubMed] [Google Scholar]
- [71].Wopfner F, Weidenhöfer G, Schneider R, Brunn AV, Gilch S, Schwarz TF, Werner T, Schätzl HM. 1999. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. Journal of Molecular Biology, 289(5): 1163-78. [DOI] [PubMed] [Google Scholar]
- [72].Yuan Z, Zhao D, Yang L. 2013. Decipher the mechanisms of rabbit's low susceptibility to prion infection. Acta Biochimica et Biophysica Sinica, 45(11): 899-903. [DOI] [PubMed] [Google Scholar]
- [73].Zhang JP. 2009. Studies on the structural stability of rabbit prion probed by molecular dynamics simulations. Journal of Biomolecular Structure and Dynamics, 27(2): 159-62. [DOI] [PubMed] [Google Scholar]
- [74].Zhang JP. 2010. Studies on the structural stability of rabbit prion probed by molecular dynamics simulations of its wild-type and mutants. Journal of Theoretical Biology, 264(1): 119-22. [DOI] [PubMed] [Google Scholar]
- [75].Zhang JP. 2011. a. The nature of the infectious agents: PrP models of resistant species to prion diseases (dog, rabbit and horses). In: Verdier JM. Prions and prion diseases: New developments. Chapter 2. New York: NOVA Science Publishers, 41-8. [Google Scholar]
- [76].Zhang JP, Liu DDW. 2011. Molecular dynamics studies on the structural stability of wild-type dog prion protein. Journal of Biomolecular Structure and Dynamics, 28(6): 861-9. [DOI] [PubMed] [Google Scholar]
- [77].Zhang JP. 2011. b. The structural stability of wild-type horse prion protein. Journal of Biomolecular Structure and Dynamics, 29(2): 369-77. [DOI] [PubMed] [Google Scholar]
- [78].Zhao H, Liu LL, Du SH, Wang SQ, Zhang YP, Forloni G. 2012. Comparative analysis of the Shadoo gene between cattle and buffalo reveals significant differences. PloS One, 7(10): e46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhou Z, Yan X, Pan K, Chen J, Xie ZS, Xiao GF, Yang FQ, Liang Y. 2011. Fibril formation of the rabbit/human/bovine prion proteins. Biophysical Journal, 101(6): 1483-92. [DOI] [PMC free article] [PubMed] [Google Scholar]