Abstract
The findings about the progressive brain changes in schizophrenia are controversial, and the potential confounding effect of antipsychotics on brain structure is still under debate. The goal of the current article was to review the existing longitudinal neuroimaging studies addressing the impact of antipsychotic drug treatment on brain changes in schizophrenia. A comprehensive search of PubMed was performed using combinations of key terms distributed into four blocks: “MRI”, “longitudinal”, “schizophrenia” and “antipsychotic”. Studies were considered to be eligible for the review if they were original articles. Studies that examined only changes in brain density were excluded. A total of 41 MRI studies were identified and reviewed. Longitudinal MRI studies did not provide a consistent notion of the effects of antipsychotic treatment on the pattern of brain changes over time in schizophrenia. Overall, most of the included articles did not find a linear relationship between the degree of exposure and progressive brain changes. Further short- and longterm studies are warranted to a better understanding of the influence of antipsychotics in brain structural changes in schizophrenia and also to verify whether first and second generation antipsychotics may differentially affect brain morphometry.
Keywords: Antipsychotic, longitudinal studies, schizophrenia, structural magnetic resonance imaging.
INTRODUCTION
Schizophrenia is a common chronic and disabling brain disorder. The nature of the disease process remains obscure. “Imaging evidence indicates the consistent association between brain structural abnormalities and schizophrenia” [1]. These brain alterations are already present prior to the development of the disease [2, 3]. Although the pattern of brain changes over time is still under debate, a recent meta-analytic study suggests that these brain alterations may progress over time [4]. “Progressive brain changes could be associated with neurodegenerative or neurotoxic processes” [5], “attributed to a plastic adaptation of the brain to the environment” [6], possible neurotoxic effects of hyper-dopaminergia as well as interactions with the glutamatergic system [7], oxidative stress [8], and related to exposure to pharmacological treatment, as antipsychotic drugs [9].
“The potential effects of antipsychotic medication on brain structure might represent a key factor in understanding brain changes in psychosis” [10]. Therefore, the investigation of the “effect of antipsychotic drugs on progressive brain tissue loss has attracted much interest in the field. The therapeutics and deleterious actions of antipsychotic drugs could be mediated, in part, by their cellular effects and consequently also linked to morphometric changes” [11]. Moreover, “brain volume abnormalities appear associated to the outcome of the illness” [12]. First and second generation antipsychotics (FGAs and SGAs) “may exert neurotrophic, neurogenetic and neuroprotective effects differentially” [13]. Animal and cell biology studies have demonstrated the differential effects of FGAs and SGAs on cellular morphology and brain growth factors. And SGAs seem to have differential long-term neuroprotective actions compared with haloperidol [14, 15]. The goal of the current article was to review longitudinal neuroimaging studies exploring the impact of antipsychotic drug treatment on progressive brain changes in schizophrenia.
METHODS
We systematically reviewed the literature to identify journal articles reporting antipsychotic effects on brain morphometry detected with neuroimaging techniques. Studies were searched in PubMed from January 1994 to July 2014, using combination of key terms distributed in four blocks: “Magnetic Resonance Imaging (MRI)”, “schizophrenia”, “longitudinal” and “antipsychotic”. The identified publications were hand-searched in order to select longitudinal neuroimaging studies considering the effects of antipsychotic medication. Studies were considered to be legible for the current review if they met the following inclusion criteria: 1) they were original publications written in English in a peer-reviewed journal; 2) they were longitudinal structural MRI studies; 3) they included brain volumes or cortical thickness or surface area variables; 4) they examined the effects of antipsychotic medication on brain structure; 5) patients were diagnosed with schizophrenia spectrum disorders. Studies were excluded for any of the following reasons: 1) other designs, such as case-reports, series of cases, etc; 2) non-original studies, including editorials, prefaces, brief communications and letters to the editor, literature reviews or meta-analyses; 3) Results no described the effect of antipsychotics in brain changes; and 4) studies examined only changes in brain density. “Interpreting data across Voxel-Based Morphometry (VBM) studies is a problem because there are a large number of factors with studies using different degrees of smoothing and different registration and segmentation algorithms that can vary and influence the results” [16].
Each article was read in its entirely, and data elements were then extracted and entered in a customized database. Data were extracted from the source documents independently by two investigators (RRS, PSP). The information collected from each article included the following: 1) authors; 2) publication year; 3) ethical approval; 4) sample size; 5) type of patients (chronic or first episode of psychosis (FEP)); 6) form of analysis completion/intention to treat; 7) period of follow-up; 8) antipsychotic drugs and doses; 9) MRI analyses type and software; 10) brain structures involved; and 11) main findings.
The literature investigating the effects of antipsychotic treatment on progressive brain changes includes different study designs: 1) longitudinal studies evaluating the effect of different antipsychotic treatments; 2) longitudinal studies evaluating the impact on brain morphology of a switch from FGAs to SGAs; 3) longitudinal studies conducted in healthy controls and medicated patients evaluating the association between progressive brain changes and antipsychotic exposure in patients. Therefore, to provide a better analysis, the studies were grouped into three categories: i) effect of different antipsychotic treatments; ii) effect of medication switch; and iii) association with the amount of antipsychotic exposure.
RESULTS
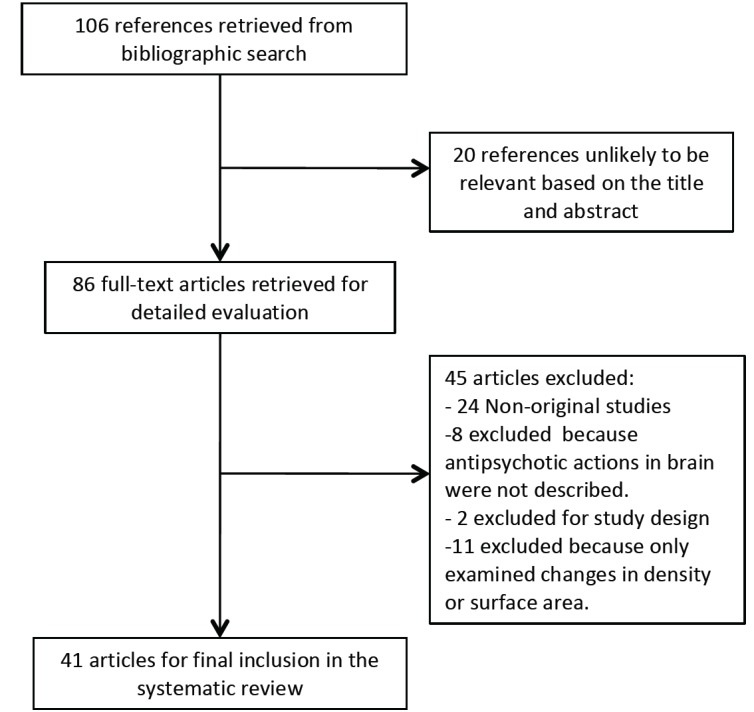
Electronic database search yielded 106 references. Fig. 1 illustrates the flow diagram of the study selection process. After the selection process, we identified 41 longitudinal studies that satisfied the inclusion criteria (Table 1). Twelve studies compared the effect of different treatments on brain morphometry, 31 were focused on the association between progressive brain change and antipsychotic medication exposure, and two analysed the effect on brain morphometry of an antipsychotic medication switch. Four studies were included within more than one group.
Fig. (1).
Flow diagram process of study selection.
Table 1.
Overview of studies included in the review.
| Healthy Controls | Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Study | N | Age Years, mean (SD) | N | Age Years, mean (SD) | Status | Medication | CPZ E mean (SD) | MRI Software and Analysis Type | Outcome Variables | Follow-up Duration (weeks) mean (SD) |
| Association | Arango C et al. 2012 | QUE, OLA, RIS, ZIP, ARI, others | Cumulative: 165658 (90011) mg | In house software Automated ROI | WBV, GM, WM, CSF | 104 | |||||
| Association | Boonstra G et al. 2011 | 20 | 28.0 (5.6) | 16 | 28.8 (6.9) | FEP | OLA, RIS, QUE | Cumulative: 42827.5 (43192.5) mg | Automated and manually traced ROI | ACCU, PUT | 52 |
| Association | Cahn W et al. 2002 | 36 | 24.5 (5.8) | 34 | 26.2 (5.31) | FEP | FGA, SGA | Cumulative: 103675 (48135) mg | In house software Semi-automated ROI | BV, GM, LV | 50.8 (4.4) |
| Association | Chakos MH et al. 1994 | 10 | 30.5 (4.9) | 29 | 25.2 (6.3) | FEP | FGA, SGA | n.s | Manually traced ROI | CAU, LV | 72 |
| Association | Cobia DJ et al. 2012 | 20 | 30.4 (12.8) | 20 | 31.9 (11.1) | Chronic | FGA, SGA | FGA: 0.9 (2.9) dose-year SGA: 3.5 (3.4) dose-year | FreeSurfer Automated ROI | FRO, TEM CT | 104 |
| Different treatments | Corson PW et al. 1999 | --- | --- | 23 | 22.6 (6.2) | Chronic | FGA | FGA: 5.6 (6.%) dose year SGA: 5.3 (7.8%) dose year | BRAINS Manually traced ROI | CAU, PUT, ACU | 112.8 (21.8) |
| Different treatments | Crespo-Facorro B et al. 2008 | 38 | Matched | 18 16 18 | 29.8 (7.9) 28.0 (5.1) 25.0 (6.0) | FEP | HAL RIS OLA | 244.1 mg/d 183.6 mg/d 289.0 mg/d | BRAINS2 Semiautomated ROI | WBV, GM, WM, LV, CAU | 54.6 (4.6) |
| Different treatments | DeLisi LE et al. 2004 | 20 | 25.5 | 26 | 26.8 (7.1) | FEP | FGA, SGA | n.s | ANALYZE Semi-automated | LV | 520 |
| Association | Edbrup BH et al. 2011 | 28 | 28.4 (6.0) | 13: low dose | 26.2 (5.7) | FEP | QUE | < 430.4 mg/d | SPM5 ROI mask | ACCU, CAU, PUT, HP | 36 |
| 9: high dose | 27.8 (5.1) | ≥ 430.4 mg/d | |||||||||
| Different treatments | Glenthoj A et al. 2007 | 19 | 27.5 (5.3) | 16 | 25.9 (5.1) | FEP | ZUCLO, RIS | Cumulative: ZUCLO: 168 mg RIS: 100-240 mg | Manually traced ROI | CAU, PUT, ACU | 12 |
| Association | Frazier JA et al. 1996 | 8 | 15.1 (2.3) | 8 | 15.4 (3.1) | Chronic | CLOZ | 400 (128.9) mg/d | Manually traced ROI | ACCU, CAU, PUT, HP | 104 |
| Different treatments | Garver DL et al. 2005 | 7 | 29.0 (9.0) | 19 | 33.0 (12.0) | Mixed | RIS, ZIP, HAL | RIS: 400 mg/d ZIP: 300 mg/d HAL: 350 mg/d | In house software Automated ROI | GM | 4 |
| Association | Goghari VM et al. 2013 | 26 | 20.9 (2.1) | 19 | 18.9 (3.6) | FEP | RIS (16), QUE (3) | 144 mg/d | FreeSurfer Automated ROI | PreFRO CT | 8 |
| Association | Gur RE et al.1998 | 17 | 31.9 (8.9) | 20 drug-naive | 27.8 (8.2) | Mixed | RIS, CLOZ, CPZ, HAL, FLUP, LOX, MESO, MOLI, THIO, TRIFLU | 259.9 (165.6) mg/kg | Manually traced ROI | CSF, BV, FRO and TEM lobes | 122.5 (51.7) |
| 20 medicated | 30.6 (7.7) | ||||||||||
| 513.3 (224.0) mg/kg | |||||||||||
| Association | Heitmiller DR et al. 2004 | 14 | 26.7 (11.3) | 14 | 26.3 (6.8) | FEP | SGA | 7.4 (5.5) dose-year | BRAINS2 Semiautomated ROI | CAU | Patients: 120.8 (53.2) Controls: 129.6 (48.4) |
| Association | Ho BC et al. 2003 | 23 | 26.9 (5.3) | 72 | 24.5 (4.67) | FEP | FGA, CLOZ, SGA | n.s | BRAINS2 Semiautomated ROI | BV, LV, CSF, cerebellum, FRO, TEM, PAR | Patients: 171.1(77) Controls: 176.3 (83.2) |
| Association; different treatments | Ho BC et al. 2011 | --- | --- | 70 high dose 70 intermediate dose 71 low dose | 26.3 (7.6) | FEP | FGA, CLOZ, SGA | 924.4 mg/d 391.7 mg/d 111.5 mg/d | BRAINS2 Semi-automated ROI | WBV, GM, WM, TEM, FRO, PAR , LV, CSF, cerebellum, PUT, CAU, THA | 374.4 (202.8) |
| Association | Keshavan MS et al.1994 | --- | --- | 11 | n.s | FEP | HAL, FLUP, PERH | 112 (60) mg/d | NIH Image Manually Traced ROI | CAU, pre FRO CT, GM, WM, WBV. | 43.6 (31.1) |
| Switch treatments | Lang DJ et al. 2004 | 23 | 23.2 (7.4) | 10 | 35.3 (8.8) | Chronic | FGA, RIS | 170 (64) mg/d | NIH Image Manually Traced ROI | CAU, PUT, PALL | 56 (17.1) |
| Different treatments | Lieberman JA et al. 2005 | 62 | 25.5 (4.1) | 79 | 24.1 (4.6) | FEP | HAL, OLA | HAL: 135-500 mg/d OLA: 100-1000 mg/d | Automated and manually traced ROI | WBV, GM, LV | 52 |
| Association | Massana G et al. 2005 | --- | --- | 11 drug-naive | 23.0 (4.0) | FEP | RIS | 605 mg/d | Automated ROI | PUT, CAU, PALL, AMIG | 12 |
| Association | McClure RK et al. 2008 | --- | --- | 10 medicated | 36.7 (7.7) | Chronic | SGA | 400 mg/d | ITK-SNAP Semi-automated ROI | CAU, FRO and TEM GM, WM, CSF, LV | 12 |
| Different treatments | McCormick L et al. 2005 | 18 | 30.5 (6.9) | 31 | 24.8 (5.9) | FEP | SGA, FGA, both | n.s | BRAINS2 Manually traced ROI | Cingulate volume | 104-156 |
| Different treatments; association | Molina V et al. 2005 | 11 | 28.4 (6.2) | 17 drug naive | 25.6 (4.0) | FEP | RIS | 500 (200) mg/d | In house software Automated ROI | GM, WM | 104 |
| 12 medicated | 31.0 (5.9) | Chronic resistant | CLOZ | 410 (339) mg/d | |||||||
| Association | Nakamura M et al. 2007 | 26 | 22.9 (3.8) | 17 | 24.3 (5.8) | FEP | SGA* | 266.3 mg/d | In house software Manually traced ROI | NCGM, CSF, GM, WM, LV | 78 |
| Association | Okugawa G et al. 2007 | 10 | 31.9 (5,1) | 10 | 31.6 (6.3) | Chronic | OLA | 357.5 mg/d | BRAINS2 Semi-automated ROI | CAU | 26.6 (8.6) |
| Association | Puri BK et al. 2001 | 12 | 27.9 (6.1) | 24 | 28.5 (8.4) | FEP | 3 drug-naive, 4 RIS, 5 FGA | Cumulative: 68365.9 (53879.5) mg | semi-automated computerised technique | LV | 30.9 (6.2) |
| Association | Reig S et al. 2009 | 34 | 15.2 (1.4) | 21 | 15.7 (1.7) | FEP | QUE, OLA, RIS, CLOZ, others | n.s | In house software Automated ROI | GM, WBM, CSF | 104 |
| Different treatments | Roiz-Santiañez R et al. 2012 | 52 | 28.8 (7.4) 27.3 (5.9) 29.6 (6.1) | 45 | Matched | FEP | HAL (18) OLA (18) RIS (16) | 244.1 mg/d 282.0 mg/d 183.6 mg/d | BRAINS2 Semi-automated ROI | CT | 52 |
| Association | Roiz-Santiañez R et al. 2012 | 70 | Matched | 93 | 28.3 (7.8) | FEP | HAL (11), OLA (19), RIS (22), QUE (16), ZIP (12), ARI (11), no antipsychotic (2) | 224.9 mg/d | BRAINS2 Semi-automated ROI | SG | 54.9 (5.3) |
| Association | Roiz-Santiañez R et al. 2014 | 76 | Matched | 109 | 28.4 (7.8) | FEP | HAL(19), OLA (19), RIS (20), QUE (17), ZIP (18), ARI (16) | Cumulative: 99149 (86605) mg | BRAINS2 Semi-automated ROI | WBV, GM, WM, CAU, CSF, LV, THA | 156 |
| Association | Saijo T et al. 2001 | 12 | 37.1 (4.2) | 18 | 37.5 (8.9) | Chronic | HAL | 2,075 (1080) mg/kg/d | NIH Image Manually traced ROI | LV | 520 |
| Switch treatments | Scheepers FE et al. 2001 | --- | --- | 29 | 35.2 (10.3) | FEP | Swiching from FGA to CLOZ | 345.6 (63.4) | ANALYZE Manually traced ROI | WBV, CAU | 24 |
| Association, different treatments | Sporn AR et al. 2003 | 43 | 14.8 (2.2) | 39 | 15.0 (2.3) | FEP | CLOZ, SGA, CLOZ + FGA, CLOZ +SGA | n.s | Fully automated technique | WM, WBV, LV | 176.8 (72.8) |
| Association | Takahashi T et al. 2009 | 26 | 25.6 (9.1) | 23 | 21.6 (3.5) | FEP | SGA, FGA | n.s | Dr View Manually traced ROI | WBV | 105 (39.2) |
| Association | Tauscher-Wisniewski S et al. 2002 | 10 | 29.4 (8.6) | 15 | 23.0 (6.2) | FEP | FGA, SGA | Cumulative: 15093 (387) mg | BrainImage Manually traced ROI | CAU | 260 |
| Association | Tauscher-Wisniewski S et al. 2005 | 37 | 25.8 (6.2) | 14 | 33.6 (3.7) | FEP | QUE | 395.2 (103.2) mg/d | BrainImage Manually traced ROI | CAU | 12 |
| Association | Taylor S et al. 2005 | 11 | 26.8 (6.6) | 11 | 34.7 (12.4) | FEP | FGA, SGA | n.s | Amira FreeSurfer Automated ROI | CAU, PUT | 4 |
| Association | Van Haren N et al. 2008 | 113 | 35.3 (12.3) | 96 | 32.2 (11.1) | Chronic | FGA, CLOZ, SGA | Cumulative: FGA: 1828 (1238) mg SGA: 1719 (1949) mg CLOZ: 126615 (42247) mg Swiched and used: typical:366 (451) mg Atypical: 819 (726) mg CLOZ: 80932 (48811) mg | In house software Automated ROI | WBV, GM, WM, LV | 260 |
| Association, different treatments | Van Haren N et al. 2011 | 113 | 35.3 (12.3) | 96 | 32.2 (11.1) | Chronic | FGA, CLOZ, SGA | Cumulative: FGA: 1828 (1238) mg SGA: 1719 (1949) mg CLOZ: 126615 (42247) mg Swiched and used: FGA:366 (451) mg SGA: 819 (726) mg CLOZ: 80932 (48811) mg | FreeSurfer Automated ROI | CT | 260 |
| Association | Whitworth AB et al. 2005 | 20 | 31.5 (4.9) | 21 | 25.0 (4.7) | FEP | n.s. | n.s. | Manually traced ROI | WBV | 104-208 |
| 17 | 28.4 (4.0) | Chronic | |||||||||
Association with Antipsychotic Exposure
There were thirty MRI longitudinal studies that performed correlations or regression analyses to investigate if there is any association between the degree of exposure to antipsychotic drugs and progressive brain changes. Most of the studies (N=20) enrolled patients with an FEP, seven included patients with chronic schizophrenia, and four articles covered a mixed sample of drug naïve FEP and chronic patients. Fifteen articles enrolled patients taking FGAs and SGAs, eleven studies included patients with SGAs, one study investigated patients treated only with clozapine, in two articles patients were taking only FGAs, and in one study the type of medication was not specified.
With regard to gray matter (GM) and white matter (WM) volume, results are disparate. Certain studies failed to demonstrate any significant correlation between progressive GM changes and antipsychotic exposure. No significant correlation between SGA treatment in children and adolescent with early onset of schizophrenia and brain changes over two years was found [17, 18]. In line with these results, after one year and a half, 17 patients with FEP did not show differences in neocortical GM changes when they were divided in two groups: compliance antipsychotic medication and non-compliance treatment groups [19]. In these three studies, patients were only treated with SGAs (measured in chlorpromazine equivalents). Similarly, McClure et al. (2008) described an absence of regional GM volume changes after a short period (four months) of SGA exposure in a sample of chronic schizophrenia patients (N=10; mean age: 36.7 years) [20]. In contrast, some other studies have observed significant associations between progressive GM changes and antipsychotic medication treatment. Thus, a loss of GM was observed in 34 FEP patients during the first year of the illness. GM loss was significantly correlated with higher cumulative dosage of antipsychotic medication used during the follow-up period. No interaction with type of antipsychotic (FGA and SGA) was found, but it is of note that only a reduced number of subjects was taking FGAs (N=5) [21]. Gur and colleagues (1995) exploring a sample of 20 FEP patients showed that higher doses of antipsychotics, both FGAs and SGAs, were associated with greater reduction in frontal (r=-0.75) and temporal (r=-0.66) GM volume across time. However, these results were not repeated when chronic schizophrenia patietns were analyzed [22]. Concerning only SGAs, a lengthy intake of olanzapine during five years was correlated with less reduction in GM volume, and even it was related with a subtle GM increment [23]. On the other hand, risperidone treatment increased GM volume in the right and left caudate nuclei and the left accumbens in 11 FEP patients after three months of treatment. In this latter study, it is worth noting that the dose of risperidone (605 mg/day, in CPZ equivalents) was more than twice higher than the usual dose used in the most of neuroimagen studies [24]. In 2005, Molina et al. obtained similar results in a longitudinal study during two years. There was an increase in GM in 17 patients using risperidone. Frontal GM was also increased in 12 chronic patients taking clozapine. In this study, clozapine and risperdone also produced a reduction in WM volumen [25]. In 2001, Ho and colleagues performed the largest sample size study included in the present review (N=211) exploring FEP patients over a period of seven years. Patients were taking FGAs, SGAs or clozapine. WM but not GM volumes showed a significant time by treatment interaction. Therefore, patients of the highest dose group had longitudinal WM volume reductions [26].
Among the research conducted on the whole brain volume (WBV), results did not show a direct relationship between changes and drug exposure. Three studies based in children and adolescents with childhood onset of schizophrenia did not find any association between any treatment features with SGA, such as specific drug or dosage, and WBV. Patients were followed during two [17, 18] and three years [27]. In 2003, Ho and colleagues conducted a longitudinal study of 171 weeks in 72 patients with a recent onset of schizophrenia, taking FGAs and SGAs. They reported no significant effects between any of the studied brain regions, including WBV, and cumulative antipsychotic doses, treatment duration, or percentage of time treated with FGAs, SGAs or both types of drugs together [28]. One study based on drug-naïve FEP patients found independence between antipsychotic treatment (FGA, SGA) and changes in straight gyrus [29]. Moreover, there was no association between annual change insular cortex and cumulative days of inpatient treatment per year during two and four years of follow-up [30].
The studies investigating the relation between antipsychotics and cortical thickness changes have also shown mixed results. In 20 adult patients (mean age = 31.9 years), frontal and temporal cortical thickness was not associated with interval antipsychotic medication use, both FGAs and SGAs in CPZ equivalents, after two years [31]. However, in 2013, Goghari and colleagues (2013) demonstrated that short-term atypical treatment (eight weeks) with risperidone or quetiapine increased prefrontal cortical thickness in 19 FEP drug-naïve patients (mean age = 18.9 years) [32]. In 2011, Van Haren observed that higher cumulative intake of FGAs “during a five-year scan interval was associated with more pronounced cortical thinning, whereas higher cumulative intake of SGA medication was associated with less pronounced cortical thinning” [33] in long-term schizophrenia patients (mean age = 32.2 years).
Several studies have addressed the issue of antipsychotic medication effects on basal ganglia morphometry. Tauscher-Wisniewski and colleagues found a decline in caudate volume over time in a sample of FEP patients and healthy controls but it was independent of medication [34, 35]. However, other two studies showed a caudate enlargement during the course of treatment with FGAs and SGAs in young drug-naïve schizophrenia patients [36, 37]. This increment was associated with greater amounts of antipsychotic medication received by patients before the first scan [36]. In chronic schizophrenia patients (mean age=31.6 years), treatment with olanzapine seemed to increase the caudate volume after six months [38]. Similarly, Frazier and colleagues (1996) showed a caudate enlargement in patients with childhood onset of schizophrenia who were taking FGAs during two years. In this sample, caudate volume decreased in the subgroup of patients who were changed to clozapine [39]. McClure and colleagues (2008) found no longitudinal changes in caudate volume after brief periods of SGA exposure [20]. On the other hand, in 2005, Taylor and colleagues reported an “increase in left striatum that was not associated with drug treatment, but with a reduction of positive symptoms” [40] over the first month. The sample included patients with an FEP (mean age= 34.7 years) treated with FGAs and SGAs and followed during four weeks [40]. Ebdrup et al. (2011) reported that high doses of quetiapine may attenuate the striatal volume loss over time in FEP patients. When patients were compared to healthy control subjects, this volume loss was more prominent in those patients treated with low dose of quetiapine and less apparent in those treated with higher doses of quetiapine. “Post-hoc analyses revealed that the volume loss was pronounced in caudate and putamen nucleus, but not in accumbens nucleus” [41]. They also described that hippocampal volume loss appeared more pronounced in the group of patients treated with higher quetiapine doses [41]. When treatment discontinuation effects were investigated, it seemed to reverse effects of SGAs. A decrease in the volume of accumbens and putamen nucleous occurred in eight FEP patients who discontinued the treatment with SGAs. Conversely, an increment in those volumes was found in patients who did not discontinue the treatment, during one-year follow-up [42]. Gender effect of SGAs on progressive caudate changes has been also considered. SGAs treatment had a negative correlation (r= -0.74) with caudate volume in females, and a positive correlation (r= 0.63) in males [43]. A reduction in caudate volume after three years of the illness onset was also observed in a sample of 109 early onset schizophrenia patients (mean age= 28.4 years) treated with antipsychotics, both typical and atypical, comparing with 76 healthy volunteers [44].
Lateral ventricle (LV) changes were investigated in the lengthiest study included in the review. It covered a period of ten-year follow-up in chronic patients with schizophrenia (mean age= 37.5 years) taking haloperidol. Authors concluded that changes in LV were not associated with daily haloperidol exposure [45]. Puri and colleagues obtained similar results in a sample of FEP patients (mean age=28.5 years) treated with different types of FGAs and SGAs. Changes in LV size were not associated with total duration of treatment or with total cumulative dose over 30 weeks [46].
Effect of Different Antipsychotic Treatments
The differential effects of FGAs and SGAs, which have different profiles of affinity for dopamine D2 receptors on brain structure have attracted much interest since medications are taking chronically.
In 1999, Corson et al. published a two-year longitudinal MRI study with 23 male patients (mean age 25.57 years) with diagnoses in the schizophrenia spectrum. During the two-year period, “mean basal ganglia volume of patients receiving predominantly FGAs increased, while the opposite was observed for patients receiving mostly SGAs” [47]. Basal ganglia volumes have been also examined in a longitudinal, open-label study conducted on “drug-naïve first-episode schizophrenia before and after short-term treatment with either” [48] a FGA and SGA drug. Nineteen FEP patients (mean age 26 years) and 19 matched healthy controls were included. From the patient group, 16 were antipsychotic drug-naïve, and three minimally medicated first-episode schizophrenia patients. Overall, typical (zuclopenthixol) and atypical (risperidone) “medication groups did not differ significantly with respect to volume changes after three months of low-dose treatment” [48] in basal ganglia volumes. “Nevertheless, when medication groups were examined separately, a significant volume increase in the putamen was detected in the risperidone group. Furthermore, a time by gender effect was found in the accumbens nucleus, which was explained by an increase in accumbens volume in the male patients and a decrease in female patients. There were no significant main or interaction effects in the FGA group” [48] treated with zuclopenthixol.
Garver and colleagues (2005) examined cerebral GM in 19 patients (mean age 33 years) “with schizophrenia before and after 28 days of treatment with FGAs (haloperidol; N=6) or SGAs (risperidone and ziprasidone; N=13)” [49] medication. During treatment with the atypical medication, “cerebral cortical GM of 13 patients with schizophrenia expanded” [49]. However, the group of six patients that received “haloperidol, as well as the seven control subjects, showed no change in cortical GM volumes [49]” at the time of reassessment.
In a “longitudinal, randomized, controlled, multisite, double-blind study” [50] , Liberman and colleagues (2005) studied the antipsychotic drug effects on brain morphology in FEP. “Patients were randomized to double-blind treatment with olanzapine, 5 to 20 mg/day, or haloperidol, 2 to 20 mg/day for up 104 weeks. MRI assessments were performed at weeks 0 (baseline), 12, 24, 52, and 104” [50]. One hundred sixty-one patients (mean age 23.85 years) received a baseline and at least one follow-up MRI measure. Authors found that haloperidol was associated with significant reductions in GM volume, whereas olanzapine failed to show this association. “A matched sample of healthy volunteers (N=58) examined contemporaneously showed no change in GM volume” [50]. Authors then hypothesized that these “differential treatment effects on brain morphology could be due to haloperidol-associated toxicity or greater therapeutic effects of olanzapine” [50].
McCormick et al., (2005) investigated whether SGAs differed from FGAs “in their effect on anterior cingulate volume change over time” [51]. They studied 31 antipsychotic-naïve subjects (mean age 24.8 years) diagnosed with schizophrenia. The average follow-up period was 3 years (range 2 to 5 years). They found that increased FGA “exposure over time was correlated to increased anterior cingulate volume over time” [51], whereas increased SGA exposure was correlated to the opposite effect and the cingulated volume decreased [51].
In 2008, Crespo-Facorro and colleagues studied the “effects of risperidone (N=16), olanzapine (N=18) and low doses of haloperidol (N=18) in brain volume changes during one-year follow-up period in a large and heterogeneous sample of first episode schizophrenia spectrum patients” [52] (mean age 29.76 years). They found “a significant increase in lateral ventricles in patients treated with risperidone. Patients exposed to atypical drugs (olanzapine and risperidone) exhibited a decrease in caudate nucleus volume“ [52].
In 2011, Ho and colleagues explored whether FGAs, non-clozapine SGAs, and clozapine might “have differential effects on brain volumes in schizophrenia” [26]. They studied 211 patients with schizophrenia (mean age 26.3 years) “who underwent repeated neuroimaging beginning soon after illness onset” [26]. The sample included 674 MRI scans “covering a mean follow-up period of 7.2 years (range, 1.9–14.0 years), and inter-scan intervals were approximately three years” [26]. Higher FGA doses “were associated with smaller total cerebral GM and frontal GM volumes. Higher doses of non-clozapine SGAs were associated with lower frontal and parietal GM volumes, and higher clozapine doses were associated with smaller total cerebral and lobar GM volumes. For WM volumes, higher non-clozapine SGA doses were significantly associated with larger parietal WM volumes. Higher clozapine doses were associated with larger sulcal cerebrospinal fluid (CSF) volumes and smaller subcortical (caudate, putamen, and thalamic) volumes” [26].
Variations in cortical thickness in relation with different drugs have been also investigated. In 2012, Roiz-Santiañez and colleagues, with the same sample used by Crespo-Facorro et al., (2008), examined cortical thickness changes but they did not find significant effects of time, treatment group or group-by-time interaction for any of the cortical thickness variables evaluated [53]. Recently, van Haren et al. (2011) examined cortical thickness and change in cortical thickness on a vertex-by-vertex basis across the cortical mantle. They performed a five-year follow-up MRI study that included 96 schizophrenia patients (mean age: 32.22 years) and 113 healthy controls (mean age: 35.28 years). “Significant correlations between medication variables and cortical thickness change” [33] showed that, for FGAs, correlations were negative (i.e. higher intake was associated with more pronounced decreases in cortical thickness), whereas correlations with SGAs were positive (i.e., higher intake was related to less decreases in cortical thickness) [33].
Effect of Antipsychotic Medication Switch
Two studies have examined changes in brain structure after a change in medication. A first study performed by Scheepers et al., in 2001 examined “the effect of clozapine on caudate nucleus volume in schizophrenia patients previously treated with FGAs” [54]. Twenty-six patients (mean age: 35.23 years) participated in this open design study. Treatment with clozapine resulted in a decrease in caudate volume. However, no differences in caudate volume changes were found between responders and non-responders to clozapine [54]. In other study, Lang and colleagues (2004) examined 37 patients with schizophrenia. From them, those who were receiving FGAs (N=10) or those taking risperidone with “limited response (N=13), were switched to treatment with olanzapine (N=23). Patients receiving risperidone and exhibiting a good response (N=14) continued treatment with the same treatment” [55]. Authors observed that “olanzapine reversed putamen and globus pallidus enlargement induced by FGAs but did not alter volumes in patients previously treated with risperidone” [55].
DISCUSSION
The present review article investigated the effect of antipsychotic medication on brain structural changes found in patients with schizophrenia. Forty-one longitudinal MRI studies with antipsychotic medication administration were included with a final database of 1391 schizophrenia patients and 1135 controls. The influence of antipsychotic medication in relation to longitudinal brain changes remains inconclusive. There was a large inconsistency among the results from the different studies and also a great heterogeneity with regard to sample size, follow-up duration, antipsychotic medication type and dose, MRI processing analyses or illness duration. This makes not possible to achieve clear conclusions regarding the effects of antipsychotic medication on brain structure.
The analyses of the relationship between the degree of exposure to antipsychotic medication and brain morphometry change have also led to mixed results. Overall, most of the included studies did not find a linear relationship between the degree of exposure and progressive brain changes. Although some studies found different associations, these were inconsistent. Whereas some studies suggested associations between antipsychotic medication exposure and a decrease in global grey matter [21], others found regional volume loss to be dose-dependent. Massana and colleagues (2005) reported that treatment with risperidone was associated, in contrast to the findings for other SGAs [39, 56], with increases in the caudate volume. Keshaban and colleagues (1994) showed a caudate increment across time but using FGAs [37]. Ebdrup and colleagues (2011) directly correlated quetiapine doses with hippocampal volume loss but they found an inverse correlation between quetiapine doses and striatal volume loss [41].
The different effects of FGAs and SGAs on brain morphometry gave also contradictory results. It is been suggested that SGAs could delay, prevent or even reverse cortical loss [57, 58]. Interestingly, Lieberman and colleagues (2005) observed that the differential effects on brain structure of FGAs and SGAs lost significance across time [50]. The effects of FGAs and SGAs in subcortical structures remain also ambiguous. Corson et al. (1999) reported an increment in basal ganglia volume in patients receiving predominantly FGAs, “while the opposite was observed for patients receiving mostly SGAs” [47]. However, Glenthoj et al., (2007) reported no differences with respect to basal ganglia volume changes after three months of low-dose treatment with FGAs and SGAs [48]. A recent literature review has conclude that the assumed “increase of basal ganglia volumes in patients with schizophrenia appears to be related to the D2 blockage due to antipsychotic administration, which is particularly relevant for the FGAs and chronic administration” [59]. Some authors have also suggested “that gross striatal morphological changes may represent candidate biomarker for the risk and outcome of the illness” [60].
A volume reduction has been associated with a medication switch in both studies focused in medication switch to SGAs in patients who had been previously treated with FGAs [54, 55]. It is been suggested that this reduction might represent normalization rather than atrophy.
The mechanisms of antipsychotics action on brain structure can be better understood from animal and in vitro studies. Some authors described similar effects for typical and atypical agents. Thus, in a recent in vivo study, Vernon and colleagues (2014) “developed a rodent model that uses clinically relevant doses and serial magnetic resonance imaging (MRI), followed by postmortem histopathological analysis to study the effects of antipsychotics on brain structures” [61]. This longitudinal study found that chronic (8 weeks) antipsychotic treatment results in altered brain morphology in rodents. “The exposure to both haloperidol and olanzapine resulted in significant decreases in WBV (6% to 8%)” [61] and in the cerebral cortex volume (8% to 12%) compared with the control group. “Hippocampal, corpus striatum, lateral ventricles, and corpus callosum volumes were not significantly different from control subjects, suggesting a differential effect of antipsychotics on the cortex” [61]. Similarly, Dorph-Petersen et al., in 2005, found “that macaque monkeys chronically treated (2.5 years) with haloperidol or olanzapine showed an approximate 10% reduction in brain weight relative to monkeys receiving placebo” [9]. However, Andersson et al., (2002) reported “different effects of FGAs and SGAs in brain morphometry. In this case, haloperidol exposure was associated with a significant increase in caudate–putamen volume and olanzapine exposure with a reduction” [62] in these structures. Evidence from animal and cell biology studies have suggested that dopamine “receptor blockade might influence cell proliferation by modulating brain growth factors” [15] and cellular morphology [14]. Nevertheless, some “caution needs to be exerted when extrapolating results from animals studies to humans” [61].
Previous studies have suggested that “dynamic alterations in gray matter structure can occur very rapidly within a time range of a single week” [63]. A recent “ in vivo multimodal pharmaconeuroimaging study in seven neuroleptic-naive healthy male volunteers undergoing a haloperidol challenge (5 mg per 70 kg of body weight, intravenous application) found reversible striatal volume changes and structural-functional decoupling in motor circuits within hours” [64]. Therefore, changes in brain volumes could occur at the very beginning of antipsychotic treatment and seem to be nonlinear [65]. Hence, the follow-up periods of the articles included in this review (range from 4 to 500 weeks) could be considered too long to detect potential effects of antipsychotic medication on brain structure.
There is not, to the best of our knowledge, any longitudinal MRI study conducted on untreated schizophrenia patients. All available studies were conducted on chronic medicated patients, or FEP drug-naive patients that were treated since the illness onset, and thereby they were taking medication during the follow-up. Therefore, it remains unclear whether potential structural changes are due to the ongoing illness process or they may be mediated by antipsychotic medication and other confounder factors, such as illness duration, changes in clinical severity, cognitive deficits or substance abuse. In this context, larger ventricles and decreased prefrontal volumes have been correlated with worse outcome [66]. Therefore, clinical heterogeneity, small sample sizes, and the effect of other confounding factors such as poor nutrition [67], diminished social and environmental stimuli [68], alcohol, tobacco or cannabis consumption [69-71], and lifestyle, like physical exercise [72, 73] may account for discrepancies between studies.
“Differences in the methodology may also account for inconsistencies between investigations. Reliability in MRI-derived automated morphometric measures can be influenced by several sources of variance” [74]. Therefore, reliability can be affected by subject-related factors, such as hydration status [75], instrument-related factors, such as field strength, scanner manufacturer, imaging magnetic gradients and pulse sequence [76], and data processing-related factors, including the software package and version and the parameters used in the analysis [77-79].
Although we choose to group the studies in three categories: effect of different antipsychotic treatment, effect of switch, association with antipsychotic medication exposure, we acknowledge that other choices could have been made (comparing studies with FGAs vs SGAs, or according to the stage of illness (first episode vs chronic patients) or according to brain areas or tissue).
In conclusion, current longitudinal MRI studies do not provide a consistent description of the effects of antipsychotic treatment on the pattern of brain changes over time in schizophrenia patients. Further short- and long-term studies longitudinal studies are warranted to verify whether antipsychotics may produce brain structural changes or whether FGAs and SGAs may differentially affect brain morphometry.
ACKNOWLEDGEMENTS
The present study was conducted at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: Instituto de Salud Carlos III PI020499, PI050427, PI060507, Plan Nacional de Drugs Research Grant 2005- Orden sco/3246/2004, SENY Fundació Research Grant CI 2005-0308007 and Fundación Marqués de Valdecilla API07/011. We thank IDIVAL Neuroimaging Unit for its help in the execution of this work.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Haijma S.V., Van Haren N., Cahn W., Koolschijn P.C., Hulshoff Pol H.E., Kahn R.S. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steen R. G., Mull C., McClure R., Hamer R. M., Lieberman J. A. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–8. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 3.Vita A., De Peri L., Silenzi C., Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr. Res. 2006;82(1):75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Olabi B., Ellison-Wright I., McIntosh A.M., Wood S.J., Bullmore E., Lawrie S.M. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70(1):88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Molina V., Reig S., Sanz J., Palomo T., Benito C., Sánchez J., Sarramea F., Pascau J., Desco M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr. Res. 2005;80(1):61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman J.A. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol. Psychiatry. 1999;46(6):729–739. doi: 10.1016/S0006-3223(99)00147-X. [DOI] [PubMed] [Google Scholar]
- 7.Ho B.C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger D.R., McClure R.K. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch. Gen. Psychiatry. 2002;59(6):553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- 9.Sporn A. L., Greenstein D. K., Gogtay N., Jeffries N. O., Lenane M., Gochman P., Clasen L. S., Blumenthal J., Giedd J. N., Rapoport J. L. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am. J. Psychiatry. 2003;160(12):2181–9. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- 10.Hastings T.G., Lewis D.A., Zigmond M.J. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. U. S. A. 1996;93(5):1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho B.C., Andreasen N.C., Nopoulos P., Arndt S., Magnotta V., Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 12.Prabakaran S., Swatton J. E., Ryan M. M., Huffaker S. J., Huang J. T., Griffin J. L., Wayland M., Freeman T., Dudbridge F., Lilley K. S., Karp N. A., Hester S., Tkachev D., Mimmack M. L., Yolken R. H., Webster M. J., Torrey E. F., Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry. 2004;9(7):684–97. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 13.Roiz-Santiáñez R., Pérez-Iglesias R., Ortíz-García de la Foz V., Tordesillas-Gutiérrez D., Mata I., González-Mandly A., Pazos A., Tabarés-Seisdedos R., Vázquez-Barquero J.L., Crespo-Facorro B. One year longitudinal study of the straight gyrus morphometry in first-episode schizophrenia-spectrum patients. Psychiatry Res. 2012;202(1):80–83. doi: 10.1016/j.pscychresns.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Dorph-Petersen K.A., Pierri J.N., Perel J.M., Sun Z., Sampson A.R., Lewis D.A. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T., Wood S.J., Soulsby B., McGorry P.D., Tanino R., Suzuki M., Velakoulis D., Pantelis C. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr. Res. 2009;108(1-3):49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Navari S., Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009;39(11):1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 17.Cobia D. J., Smith M. J., Wang L., Csernansky J. G. Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr. Res. 2012;139(1-3):1–6. doi: 10.1016/j.schres.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton S.S., Duman R.S. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21(9):715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]
- 19.Goghari V. M., Smith G. N., Honer W. G., Kopala L. C., Thornton A. E., Su W., Macewan G. W., Lang D. J. Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug-naive first-episode psychosis patients. Schizophr. Res. 2013;149(1-3):149–55. doi: 10.1016/j.schres.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 20.van Haren N.E., Cahn W., Hulshoff Pol H.E., Kahn R.S. The course of brain abnormalities in schizophrenia: can we slow the progression? J. Psychopharmacol. (Oxford) 2012;26(5) Suppl.:8–14. doi: 10.1177/0269881111408964. [DOI] [PubMed] [Google Scholar]
- 21.van Haren N.E., Schnack H.G., Cahn W., van den Heuvel M.P., Lepage C., Collins L., Evans A.C., Hulshoff Pol H.E., Kahn R.S. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry. 2011;68(9):871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- 22.Konradi C., Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol. Psychiatry. 2001;50(10):729–742. doi: 10.1016/S0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauscher-Wisniewski S., Tauscher J., Christensen B.K., Mikulis D.J., Zipursky R.B. Volumetric MRI measurement of caudate nuclei in antipsychotic-naive patients suffering from a first episode of psychosis. J. Psychiatr. Res. 2005;39(4):365–370. doi: 10.1016/j.jpsychires.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Pillai A., Parikh V., Terry A.V., Jr, Mahadik S.P. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007;41(5):372–386. doi: 10.1016/j.jpsychires.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Tauscher-Wisniewski S., Tauscher J., Logan J., Christensen B.K., Mikulis D.J., Zipursky R.B. Caudate volume changes in first episode psychosis parallel the effects of normal aging: a 5-year follow-up study. Schizophr. Res. 2002;58(2-3):185–188. doi: 10.1016/S0920-9964(01)00406-6. [DOI] [PubMed] [Google Scholar]
- 26.Parikh V., Khan M.M., Terry A., Mahadik S.P. Differential effects of typical and atypical antipsychotics on nerve growth factor and choline acetyltransferase expression in the cortex and nucleus basalis of rats. J. Psychiatr. Res. 2004;38(5):521–529. doi: 10.1016/j.jpsychires.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Chakos M.H., Lieberman J.A., Bilder R.M., Borenstein M., Lerner G., Bogerts B., Wu H., Kinon B., Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151(10):1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 28.Whitwell J.L., Josephs K.A., Rossor M.N., Stevens J.M., Revesz T., Holton J.L., Al-Sarraj S., Godbolt A.K., Fox N.C., Warren J.D. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch. Neurol. 2005;62(9):1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- 29.Keshavan M.S., Bagwell W.W., Haas G.L., Sweeney J.A., Schooler N.R., Pettegrew J.W. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344(8934):1434. doi: 10.1016/S0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 30.Arango C., Rapado-Castro M., Reig S., Castro-Fornieles J., González-Pinto A., Otero S., Baeza I., Moreno C., Graell M., Janssen J., Parellada M., Moreno D., Bargalló N., Desco M. Progressive brain changes in children and adolescents with first-episode psychosis. Arch. Gen. Psychiatry. 2012;69(1):16–26. doi: 10.1001/archgenpsychiatry.2011.150. [DOI] [PubMed] [Google Scholar]
- 31.Okugawa G., Nobuhara K., Takase K., Saito Y., Yoshimura M., Kinoshita T. Olanzapine increases grey and white matter volumes in the caudate nucleus of patients with schizophrenia. Neuropsychobiology. 2007;55(1):43–46. doi: 10.1159/000103575. [DOI] [PubMed] [Google Scholar]
- 32.Reig S., Moreno C., Moreno D., Burdalo M., Janssen J., Parellada M., Zabala A., Desco M., Arango C. Progression of brain volume changes in adolescent-onset psychosis. Schizophr. Bull. 2009;35(1):233–243. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frazier J.A., Giedd J.N., Kaysen D., Albus K., Hamburger S., Alaghband-Rad J., Lenane M.C., McKenna K., Breier A., Rapoport J.L. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am. J. Psychiatry. 1996;153(4):564–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M., Salisbury D.F., Hirayasu Y., Bouix S., Pohl K.M., Yoshida T., Koo M.S., Shenton M.E., McCarley R.W. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol. Psychiatry. 2007;62(7):773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor S., Christensen J. D., Holcomb J. M., Garver D. L. Volume increases in striatum associated with positive symptom reduction in schizophrenia: a preliminary observation. Psychiatry Res. 2005;140(1):85–9. doi: 10.1016/j.pscychresns.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.McClure R.K., Carew K., Greeter S., Maushauer E., Steen G., Weinberger D.R. Absence of regional brain volume change in schizophrenia associated with short-term atypical antipsychotic treatment. Schizophr. Res. 2008;98(1-3):29–39. doi: 10.1016/j.schres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Cahn W., Hulshoff Pol H. E., Lems E. B., van Haren N. E., Schnack H. G., van der Linden J. A., Schothorst P. F., van Engeland H., Kahn R. S. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch. Gen. Psychiatry. 2002;59(11):1002–10. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 38.Ebdrup B.H., Skimminge A., Rasmussen H., Aggernaes B., Oranje B., Lublin H., Baaré W., Glenthøj B. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. Int. J. Neuropsychopharmacol. 2011;14(1):69–82. doi: 10.1017/S1461145710000817. [DOI] [PubMed] [Google Scholar]
- 39.Gur R.E., Cowell P., Turetsky B.I., Gallacher F., Cannon T., Bilker W., Gur R.C. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch. Gen. Psychiatry. 1998;55(2):145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 40.Boonstra G., van Haren N.E., Schnack H.G., Cahn W., Burger H., Boersma M., de Kroon B., Grobbee D.E., Hulshoff Pol H.E., Kahn R.S. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. J. Clin. Psychopharmacol. 2011;31(2):146–153. doi: 10.1097/JCP.0b013e31820e3f58. [DOI] [PubMed] [Google Scholar]
- 41.van Haren N. E., Hulshoff Pol H. E., Schnack H. G., Cahn W., Brans R., Carati I., Rais M., Kahn R. S. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol. Psychiatry. 2008;63(1):106–13. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Heitmiller D.R., Nopoulos P.C., Andreasen N.C. Changes in caudate volume after exposure to atypical neuroleptics in patients with schizophrenia may be sex-dependent. Schizophr. Res. 2004;66(2-3):137–142. doi: 10.1016/j.schres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Massana G., Salgado-Pineda P., Junqué C., Pérez M., Baeza I., Pons A., Massana J., Navarro V., Blanch J., Morer A., Mercader J.M., Bernardo M. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J. Clin. Psychopharmacol. 2005;25(2):111–117. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- 44.Roiz-Santiáñez R., Ayesa-Arriola R., Tordesillas-Gutiérrez D., Ortiz-García de la Foz V., Pérez-Iglesias R., Pazos A., Sánchez E., Crespo-Facorro B. Three-year longitudinal population-based volumetric MRI study in first-episode schizophrenia spectrum patients. Psychol. Med. 2014;44(8):1591–1604. doi: 10.1017/S0033291713002365. [DOI] [PubMed] [Google Scholar]
- 45.Saijo T., Abe T., Someya Y., Sassa T., Sudo Y., Suhara T., Shuno T., Asai K., Okubo Y. Ten year progressive ventricular enlargement in schizophrenia: an MRI morphometrical study. Neurosci. 2001;55(1):41–7. doi: 10.1046/j.1440-1819.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- 46.Andersson C., Hamer R.M., Lawler C.P., Mailman R.B., Lieberman J.A. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2002;27(2):143–151. doi: 10.1016/S0893-133X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- 47.Driemeyer J., Boyke J., Gaser C., Büchel C., May A. Changes in gray matter induced by learning--revisited. PLoS One. 2008;3(7):e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puri B.K., Hutton S.B., Saeed N., Oatridge A., Hajnal J.V., Duncan L., Chapman M.J., Barnes T.R., Bydder G.M., Joyce E.M. A serial longitudinal quantitative MRI study of cerebral changes in first-episode schizophrenia using image segmentation and subvoxel registration. Psychiatry Res. 2001;106(2):141–150. doi: 10.1016/S0925-4927(01)00072-5. [DOI] [PubMed] [Google Scholar]
- 49.Tost H., Braus D.F., Hakimi S., Ruf M., Vollmert C., Hohn F., Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat. Neurosci. 2010;13(8):920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- 50.Corson P.W., Nopoulos P., Miller D.D., Arndt S., Andreasen N.C. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am. J. Psychiatry. 1999;156(8):1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- 51.Andreasen N.C., Nopoulos P., Magnotta V., Pierson R., Ziebell S., Ho B.C. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry. 2011;70(7):672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glenthoj A., Glenthoj B.Y., Mackeprang T., Pagsberg A.K., Hemmingsen R.P., Jernigan T.L., Baaré W.F. Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res. 2007;154(3):199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Bellani M., Dusi N., Brambilla P. Longitudinal imaging studies in schizophrenia: the relationship between brain morphology and outcome measures. Epidemiol. Psichiatr. Soc. 2010;19(3):207–210. [PubMed] [Google Scholar]
- 54.Garver D. L., Holcomb J. A., Christensen J. D. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol. Psychiatry. 2005;58(1):62–6. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Hulshoff Pol H.E., Hoek H.W., Susser E., Brown A.S., Dingemans A., Schnack H.G., van Haren N.E., Pereira Ramos L.M., Gispen-de Wied C.C., Kahn R.S. Prenatal exposure to famine and brain morphology in schizophrenia. Am. J. Psychiatry. 2000;157(7):1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 56.Lieberman J.A., Tollefson G.D., Charles C., Zipursky R., Sharma T., Kahn R.S., Keefe R.S., Green A.I., Gur R.E., McEvoy J., Perkins D., Hamer R.M., Gu H., Tohen M., HGDH Study Group Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 57.Diamond G., Siqueland L. Current status of family intervention science. Child Adolesc. Psychiatr. Clin. N. Am. 2001;10(3):641–661. [PubMed] [Google Scholar]
- 58.McCormick L., Decker L., Nopoulos P., Ho B.C., Andreasen N. Effects of atypical and typical neuroleptics on anterior cingulate volume in schizophrenia. Schizophr. Res. 2005;80(1):73–84. doi: 10.1016/j.schres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Rais M., Cahn W., Van Haren N., Schnack H., Caspers E., Hulshoff Pol H., Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am. J. Psychiatry. 2008;80(1):73–84. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- 60.Van Haren N. E., Koolschijn P. C., Cahn W., Schnack H. G., Hulshoff Pol H. E., Kahn R. S. Cigarette smoking and progressive brain volume loss in schizophrenia. 2010. [DOI] [PubMed]
- 61.Crespo-Facorro B., Roiz-Santianez R., Perez-Iglesias R., Pelayo-Teran J. M., Rodriguez-Sanchez J. M., Tordesillas-Gutierrez D., Ramirez M., Martinez O., Gutierrez A., de Lucas E. M., Vazquez-Barquero J. L. 2008. [DOI] [PubMed]
- 62.Smith M.J., Wang L., Cronenwett W., Goldman M.B., Mamah D., Barch D.M., Csernansky J.G. Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophr. Res. 2011;131(1-3):174–183. doi: 10.1016/j.schres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roiz-Santiáñez R., Tordesillas-Gutiérrez D., Ortíz-García de la Foz V., Ayesa-Arriola R., Gutiérrez A., Tabarés-Seisdedos R., Vázquez-Barquero J.L., Crespo-Facorro B. Effect of antipsychotic drugs on cortical thickness. A randomized controlled one-year follow-up study of haloperidol, risperidone and olanzapine. Schizophr. Res. 2012;141(1):22–28. doi: 10.1016/j.schres.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Pajonk F.G., Wobrock T., Gruber O., Scherk H., Berner D., Kaizl I., Kierer A., Muller S., Oest M., Meyer T., Backens M., Schneider-Axmann T., Thornton A.E., Honer W.G., Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. 2010. [DOI] [PubMed]
- 65.Scheepers F. E., de Wied C. C., Hulshoff Pol H. E., van de Flier W., van der Linden J. A., Kahn R. S. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. 2001. [DOI] [PubMed]
- 66.Falkai P., Malchow B., Wobrock T., Gruber O., Schmitt A., Honer W.G., Pajonk F.G., Sun F., Cannon T.D. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(6):469–473. doi: 10.1007/s00406-012-0383-y. [DOI] [PubMed] [Google Scholar]
- 67.Lang D.J., Kopala L.C., Vandorpe R.A., Rui Q., Smith G.N., Goghari V.M., Lapointe J.S., Honer W.G. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. Am. J. Psychiatry. 2004;161(10):1829–1836. doi: 10.1176/appi.ajp.161.10.1829. [DOI] [PubMed] [Google Scholar]
- 68.Chakos M.H., Lieberman J.A., Alvir J., Bilder R., Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345(8947):456–457. doi: 10.1016/S0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 69.Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B., Pacheco J., Albert M., Killiany R., Blacker D., Maguire P., Rosas D., Makris N., Gollub R., Dale A., Dickerson B.C., Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters R.J., Fox N.C., Crum W.R., Taube D., Thomas D.J. Haemodialysis and cerebral oedema. Nephron. 2001;87(2):143–147. doi: 10.1159/000045903. [DOI] [PubMed] [Google Scholar]
- 71.van Haren N.E., Hulshoff Pol H.E., Schnack H.G., Cahn W., Mandl R.C., Collins D.L., Evans A.C., Kahn R.S. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32(10):2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 72.Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R., Kennedy D., Schmitt F., Brown G., Macfall J., Fischl B., Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. 2006. [DOI] [PubMed]
- 73.Tomelleri L., Jogia J., Perlini C., Bellani M., Ferro A., Rambaldelli G., Tansella M., Frangou S., Brambilla P., Neuroimaging Network of the ECNP networks initiative Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. Eur. Neuropsychopharmacol. 2009;19(12):835–840. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Zampieri E., Bellani M., Crespo-Facorro B., Brambilla P. Basal ganglia anatomy and schizophrenia: the role of antipsychotic treatment. Epidemiol. Psychiatr. Sci. 2014;23(4):333–336. doi: 10.1017/S204579601400064X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senjem M. L., Gunter J. L., Shiung M. M., Petersen R. C., Jack C. R., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. 2005. [DOI] [PMC free article] [PubMed]
- 76.Hutcheson N.L., Clark D.G., Bolding M.S., White D.M., Lahti A.C. Basal ganglia volume in unmedicated patients with schizophrenia is associated with treatment response to antipsychotic medication. Psychiatry Res. 2014;221(1):6–12. doi: 10.1016/j.pscychresns.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gronenschild E. H., Habets P., Jacobs H. I., Mengelers R., Rozendaal N., van Os J., Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. 2012. [DOI] [PMC free article] [PubMed]
- 78.Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 79.Vernon A.C., Crum W.R., Lerch J.P., Chege W., Natesan S., Modo M., Cooper J.D., Williams S.C., Kapur S. Reduced cortical volume and elevated astrocyte density in rats chronically treated with antipsychotic drugs-linking magnetic resonance imaging findings to cellular pathology. Biol. Psychiatry. 2014;75(12):982–990. doi: 10.1016/j.biopsych.2013.09.012. [DOI] [PubMed] [Google Scholar]