Abstract
Depression is a serious psychiatric illness that affects millions of people worldwide. Weeks of antidepressant therapy are required to relieve depressive symptoms, and new drugs are still being extensively researched. The latest studies have shown that in depression, there is an imbalance between the main excitatory (glutamatergic) and inhibitory (GABAergic) systems. Administration of antagonists of the glutamatergic system, including zinc, has shown an antidepressant effect in preclinical as well as clinical studies. Zinc inhibits the NMDA receptor via its binding site located on one of its subunits. This is thought to be the main mechanism explaining the antidepressant properties of zinc. In the present review, a link between zinc and the glutamatergic system is discussed in the context of depressive disorder.
Keywords: Depression, GABA, glutamate, GPR39, NMDA, zinc.
INTRODUCTION
Depression is a serious psychiatric illness that is associated with a high risk of morbidity and mortality. Understanding the neurobiological mechanisms that underlie the development of major depression is a challenge of the 21st century. Recently available antidepressants such as tricyclic antidepressants and selective serotonin/noradrenaline reuptake inhibitors are based on the monoaminergic theory of depression, which views inappropriate serotonin, noradrenaline and/or dopamine levels in the brain as being responsible for the condition [1]. However, more than 30% of patients do not respond to this treatment [2]. Due to the unsatisfactory clinical efficacy and numerous side effects of commonly used drugs, as well as the fact that weeks of therapy are required to relieve symptoms, new antidepressant strategies are being extensively researched. Over the past decades, a body of evidence has emerged linking the pathophysiology of depressive disorder to glutamatergic hyperactivity and identifying the N-methyl D-aspartate (NMDA) receptor and glutamatergic synapse as a potential target for pharmacologic intervention. Preclinical studies have been conducted to evaluate glutamate-based antidepressants, which modulate not only ionotropic but also metabotropic glutamate (mGlu) receptors and specialized transporters regulating synaptic glutamate concentrations, such as glial glutamate transporter 1 [3,4]. Yet there are also other putative pathomechanisms of depression (Fig. 1) which conceptualize depression as an immuno-inflammatory and neuroprogressive disorder [5-9]. Phenomena such as cell-mediated immune (CMI) activation, induction of indoleamine 2,3-dioxygenase (IDO), oxidative and nitrosative stress (O&NS), mitochondrial dysfunctions, hypothalamic-pituitary-adrenal (HPA) axis dysregulations and neurotrophic disturbances have been proved to induce apoptosis and inhibit neuronal growth and plasticity [5,6,10]. Consequently, many depressed patients display cognitive and functional decline, as well as structural brain abnormalities, as indicated, for example, by reduced hippocampal volume [7,11]. In such patients, longer and more frequent depressive episodes increase their susceptibility to future relapses.
Fig. (1).
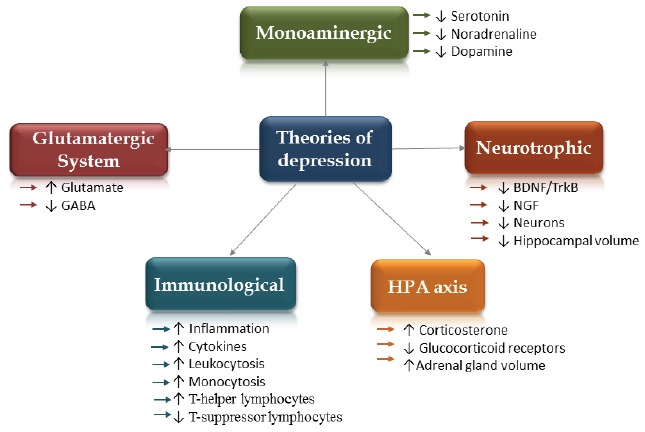
Theories of depression: Glutamatergic Theory of Depression (imbalances between glutamatergic and GABAergic systems in the brain [38]); Monoaminergic Theory of Depression (insufficient concentrations of monoamines in the brain [103,104]); Neurotophic Theory of Depression (reduction in brain derived neurotrophic factor, BDNF [102] and nerve growth factor, NGF as well as decreased amount of neurons and reduced hippocampal volume); HPA Theory of Depression (hyperactivation of the hypothalamic-pituitary-adrenal axis, an increased corticosterone concentrations and reduced glucocorticoid receptors, enlarged adrenal gland); Immunological Theory of Depression (inflammation, an increased cytokines levels [5]).
GLUTAMATERGIC SYSTEM IN THE BRAIN
Glutamate is the main excitatory neurotransmitter in the central nervous system (CNS) and binds to a variety of ionotropic as well as metabotropic receptors (Fig. 2). Some of them are located at pre- or postsynaptic membranes, and some are on glial cells. The ionotropic receptors (ion channels) include N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) and kainate receptors; the metabotropic receptors include three groups of G protein-coupled receptors (mGluRs): (I) mGluR1 and mGluR5; (II) mGluR2 and mGluR3; and (III) mGluR4, mGluR6 and mGluR7 [12,13].
Fig. (2).
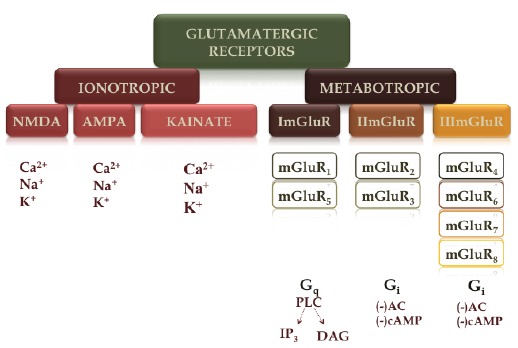
Glutamatergic receptors: ionotropic (ion channels) – (i) N-methyl-D-aspartate (NMDA), (ii) α-amino-3-hydroxy-5-methylisoxazole- 4-propionic acid (AMPA) and (iii) kainate receptors; metabotropic (mGluRs) – (i) mGluR1 and mGluR5; (ii) mGluR2 and mGluR3; and (iii) mGluR4, mGluR6 and mGluR7.
Glutamate is released to the synaptic cleft from depolarized presynaptic neurons and then taken up to astrocytes via excitatory amino acid transporters (EAATs), where the so-called glutamine cycle begins [14]. In the astrocytes, glutamate is converted by glutamine synthetase into glutamine, which is passed from the astrocytes to the neurons via specific glutamine transporters. In the neurons, glutamine is reconverted to glutamate and to GABA via glutamic acid decarboxylase [12]. Another process leading to glutamate production from the beginning (de novo) involves glucose and amino acids derived from energy metabolism [14]. To maintain homeostasis in the brain, the release of glutamate is required. This is possible via presynaptic mGluR2/3 that regulates glutamate release or via an appropriate inhibitory potential triggered by GABA.
Dysregulation between main excitatory glutamatergic neurotransmission and main inhibitory GABA-ergic neuro-transmission results in cellular damage called “excitotoxicity”. This phenomenon is thought to be a cause of depressive disorder and as such is considered to be a potential pharmacological target for the treatment of depression.
GLUTAMATE AND DEPRESSION – PRECLINICAL EVIDENCE (EXAMPLES)
Studies over the past few years have shown that the glutamatergic system plays an important role in both the pathophysiology and the treatment of depression. Suppressing glutamatergic neurotransmission as well as inhibiting the NMDA receptor seem to be important strategies in the pharmacological treatment of depression.
NMDA receptors, as described above, are ion channels that flux the cations Ca2+ and Na+, and they are tetrameric complexes that consists of two obligatory NR1 subunits and two NR2 subunits that have been identified as NR2A-D or NR3A-B [14,15]. Multiple allosteric regulatory sites that modulate glutamatergic neurotransmission are located at the NMDA receptors. Preclinical behavioral tests such as the forced swim test (FST, known as the ‘Porsolt Test’) or the tail suspension test (TST) have shown a reduction in immobility scores/antidepressant activity of AP-7, CGP 39551 and CGP 37849 (competitive NMDA antagonist), dizocilpine - MK-801 and memantine (uncompetitive antagonist), eliprodil (polyamine NR2B antagonist), 1-aminocyclopropanecarboxylic acid - ACPC (glycine partial agonist), and L-701,324 (highly selective glycine B receptor antagonist) [16,17]. The forced swim test and the tail suspension test were designed as screening tests for potential antidepressant drugs [18,19], although there are also other preclinical models that reflect the depressive symptoms of this illness [20]. Aversive stimulus/stressors, when repeated for several weeks, were found to lead to a significant reduction in sucrose consumption by rodents and to the development of anhedonia. It was found that administration of glutamatergic antagonists such as CGP 37849 or MK-801 reversed this reduced sucrose consumption [21,22], and ketamine (channel blocker) or Ro 25-6981 (selective NMDA-2B antagonist) also restored sucrose preference within 24 hours, even after a single dose [23].
Like NMDA receptors, AMPA receptors seem to be pharmacological targets for the treatment of depression, because of their function of creating homeostasis between glutamate and GABA. It is thought that positive allosteric modulators of AMPA receptors could act as a novel antidepressant [24]. Selective positive allosteric modulators of AMPA receptors, such as LY 404187 and LY 392098, were found to be active in both the tests and the models of depression [25-27].
The last decade has also produced strong evidence indicating the involvement of metabotropic mGluRs, which modulate glutamatergic transmission, in the treatment of depression [13,28,29]. Antagonists of group I mGluR, such as MTEP (3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine) and MPEP (2-methyl-6-(phenylethynyl)pyridine), or of group II – namely, mGluR 2 and mGluR3 (e.g. LY 341495, MGS0039) – reduced immobility time in the FST and TST [13,30].
NMDA receptors are also modulated by trace elements such as magnesium or zinc (which will be discussed in the next sections) [31,32]. Magnesium is present at the NMDA receptor as a channel blocker. Its removal is required to facilitate ion flow and NMDA activation. A diet low in magnesium has been shown to result in depressive-like [33] and anxiety-like behaviors [33,34], as well as in a significant reduction in amygdala-hypothalamic protein, but not in mRNA levels of GluN1 subunits [35]. Chronic magnesium treatment caused a reduction in the olfactory bulbectomy (OB)-induced hyperactivity. Moreover, magnesium supplementation significantly increased the protein levels of BDNF, GluN2B (an NMDA-receptor subunit), P-S831 (phospho-Ser_831_GluA1) and P-S845 (phosphor-Ser845-GluA1) in the hippocampus as well as the prefrontal cortex of rats following an olfactory bulbectomy [36]. According to the authors, the results suggest the possible involvement of the AMPA/BDNF pathway in the antidepressant response of magnesium. Pretreatment with of magnesium with D-serine (an agonist for the glycineB site of the NMDA receptors) abolished the antidepressant response of magnesium, as measured by the FST [37].
It has only been possible to present a few examples because of the breadth of the topic width of the topic, but for more details regarding glutamate and depression, see the following reviews [13,16,38].
GLUTAMATE AND DEPRESSION – CLINICAL EVIDENCE (EXAMPLES)
Major depressive disorder is linked to a high risk of suicide. Postmortem studies have confirmed the glutamatergic hypothesis of depression. Hashimoto et al. found increased glutamate concentrations in the frontal cortex of suicide victims who had suffered from major depression and bipolar disorder [39]. Also in the frontal cortex of suicide victims who had suffered from depression, Nowak et al. [40] observed reduced glycine displaceable [3H]CGP-39653 binding to the glutamate receptors, which, according to the authors, means that dysfunction of the glutamatergic system is involved in the pathophysiology of depression and suicidal behavior [40].
Until now only one drug – namely, ketamine – had been found to produce a rapid (i.e. after a single dose) antidepressant response, even in treatment-resistant depression [41,42]. Ketamine is a high-affinity NMDA antagonist. In a randomized, placebo-controlled, double-blind study, in contrast to untreated controls, patients suffering from treatment-resistant depression showed a significant improvement within 110 minutes of receiving a single intravenous dose of ketamine [41]. Ketamine was also found to be effective after a single dose in bipolar depression. In a randomized controlled trial, ketamine improved depressive symptoms and suicidal ideation within 40 minutes, compared with patients receiving a placebo, who showed no improvement [43]. This improvement was observed through the next 3 days. In a study by Berman et al. [44], patients receiving ketamine showed significant improvement, as measured by the Hamilton Depression Rating Scale (HDRS). There are also other NMDA antagonists that produce antidepressant effects, but not after a single dose. Patients suffering from treatment-resistant major depression showed significant improvement after 3 weeks of treatment with riluzole (a neuroprotective agent with glutamatergic modulating and anticonvulsant properties) [45]. In a study by Preskorn et al. [46], in contrast to untreated controls, nonresponders to antidepressant therapy showed mood improvement as measured by the Montgomery-Asberg Depression Rating Scale and the HDRS after a single infusion (in addition to 6-week paroxetine treatment) with CP-101,606, an NR2B subunit-selective NMDA receptor antagonist.
ZINC AT THE GLUTAMATERGIC SYNAPSES
Zinc is a multifunctional element that is important for DNA replication and transcription as well as protein synthesis, and that therefore influences cell division and differentiation [47]. Zinc plays an important role in the mammalian brain. It exists in a histochemically reactive form, and also as zinc metaloprotein and free zinc [48]. It has been found that zinc is mostly present in the regions of the brain involved in emotions, such as the hippocampus, the frontal cortex, the amygdala and the olfactory bulb [47,49,50]. These structures are rich in so-called zincergic neurons that contain glutamate and Zn2+, which may be released upon excitation [51]. The term ‘gluzinergic neurons’ has been proposed for neurons that release zinc and glutamate [48]. Because of its location, zinc can modulate excitability in the brain via its influence on the glutamatergic and GABAergic receptors [52]. Although it is the potent action of zinc ions on the NMDA receptor that is most often described, zinc also influences the AMPA and kainate receptors, and its activity is dependent on its concentration. It has been found that at high concentrations, zinc increases the response of the AMPA and kainate receptors, while at much higher concentrations it inhibits their response [52]. Inhibition of the NMDA receptor by zinc depends on the combination of NMDA subunits. The NR2A subunit has a much higher affinity than the NR2B subunit [8]. Receptors that contain NR1/NR2A subunits can be inhibited by a high-affinity, voltage-independent mechanism as well as by a low affinity, while receptors containing NR1/NR2B subunits are inhibited only by moderate-affinity, voltage-independent blocking [52].
ZINC IN PRECLINICAL STUDIES
Zinc Deficiency
It is believed that zinc deficiency can have an influence on mood, leading to the development of depressive-like symptoms; however it was still not known whether hypozincemia leads to the development of depressive behavior or whether zinc deficiency is a result of depression. In preclinical studies a specially prepared zinc-deficient diet was used to investigate behavioral as well as biochemical and molecular changes. Following administration of the zinc-deficient diet (containing 40% of the daily requirement of zinc) there was an increase in immobility time in both the FST and the TST [53]. Additionally, mice receiving a diet low in zinc exhibited enhanced immediate-to-early gene Zif268 expression in the amygdala. It should be mentioned that antagonist binding to the NMDA receptor attenuates activation of Zif268 in the basolateral amygdala [54]. All of these changes observed under zinc-deficient/stress conditions were normalized/reversed by desipramine (selective serotonic reuptake inhibitor, SSRI) treatment [53]. Other studies have also shown that administration of a diet low in zinc leads to the development of depressive-like behavior in mice [55-58] and rats [59,60]. Moreover zinc-deficient rats showed anhedonia, as measured by the sucrose preference test [59]. In those animals a reduction in saccharin intake was observed in comparison with controls receiving a zinc-adequate diet. Depressive-like behavior observed under zinc-deficient conditions is possibly explained by enhanced excitation. Interestingly, young rats receiving a zinc-deficient diet for 4 weeks showed enhanced exocytosis and, probably as a result, abnormal glutamate release, which was attenuated in the presence of zinc [61]. The higher glutamate concentration that is found in zinc deficiency is thought to be due to enhanced activation of the hypothalamic-pituitary-adrenal (HPA) axis, the main mechanism involved in the stress reaction. Rodents receiving a zinc-deficient diet had significantly elevated corticosterone concentrations [58,60]. It is thought that cortisol/corticosterone may mediate the blocking of glutamate transporter activity under zinc-deficient/stress conditions, leading to the accumulation of excess glutamate [20]. It is also thought that the secretion of excess corticosterone observed in zinc deficiency may be linked to the enhanced excitation of glutamatergic neurons due to intracellular Ca2+ dyshomeostasis [61]. Another explanation for high glutamate release in zinc deficiency is enhanced activity of NMDA receptor. Zinc down-regulates the glutamate response by inhibiting NMDA receptors [62]. This inhibition activity is abolished under zinc-deficient conditions, which results in prolonged intracellular Ca2+ release, as well as excitotoxic damage [63].
Zinc Supplementation
Preclinical studies have shown that zinc supplementation may enhance antidepressant therapy. Moreover, zinc administration has been shown to produce antidepressant action by itself. Zinc has been found to be active in commonly used animal tests and models of depression. Administration of diverse types of zinc salt, such as zinc sulphate (30 mg/kg body weight of rats or mice) [64,65], zinc hydroaspartate (65 mg/kg body weight of rats) [66] and zinc chloride (30 mg/kg body weight of mice) [67], caused a reduction in immobility time in the forced swim test. In the tail suspension test immobility scores were also reduced following zinc chloride (10-30 mg/kg) treatment in mice. Other studies have shown that joint administration of an antidepressant (such as imipramine, citalopram, fluoxetine, reboxetine, desipramine or bupropion) and zinc salt (sulphate, hydrospartate or chloride), both in ineffective doses, reduced immobility time in the FST and TST in mice or rats [65,68-70]. These independent studies have shown that zinc may effectively supplement antidepressant therapy, thereby reducing persistent side effects of commonly used antidepressants. Zinc was also found to be active in animal models of depression. Exposure to chronic mild stress (CMS) or chronic unpredictable stress (CUS) leads to the development of several symptoms of depressive disorder, including anhedonia, as measured by the sucrose preference test [20]. Zinc hydroaspartate reversed those changes in both CMS [71] and CUS [72] conditions. Moreover, joint administration of zinc hydroaspartate and imipramine, both in ineffective doses, also reversed negative symptoms developed through the CUS procedure [72]. Olfactory bulbectomy is another model of depression which causes abnormalities in behavior as well as in neurotransmitter (serotonergic, noradrenergic, glutamatergic, GABAergic and cholinergic) release that are similar to those observed in mood disorders [20]. A study by Nowak et al. [73] showed that both acute and chronic administration of zinc hydroaspartate reduced negative symptoms induced by the removal of the olfactory bulb in rats.
Other authors have examined the effect of zinc hydroaspartate on postpartum depression in mice [74]. Postpartum depression is a major depressive disorder that can happen in women at any time up to 1 year after they have given birth [75]. They found that acute combined administration of zinc, magnesium and vitamin D on postpartum day 3 significantly improves depressive symptoms [74].
Inhibition of the NMDA receptor is thought to be one of the main mechanisms explaining the antidepressant properties of zinc. A study by Rosa et al. [76] has shown that inhibition of the NMDA receptor and interaction with the L-arginine-nirtic oxide (NO) pathway may be the mechanisms by which the antidepressant properties of zinc take effect. Pre-treatment with L-arginine (a substrate for NO synthase) abolished the anti-immobility effect of zinc chloride. In another study, as with magnesium, administration of D-serine abolished the antidepressant effect of zinc in the forced swim test (FST), indicating the importance of the glycineB site in the antidepressant response of zinc [37].
ZINC IN CLINICAL STUDIES
About 50% of the world’s population may suffer from zinc deficiency due to a low supply [77]. It has been reported that patients suffering from depression showed lower serum zinc than healthy controls [78,79]. Significant differences between patients who respond to antidepressant therapy and non-responders were also found [80]. Clinical studies focusing on zinc concentration in depressed subjects have been reviewed by [81], who have summarized clinical data comparing zinc levels in depressed and non-depressed patients. Based on the literature, which included 1643 depressed and 804 non-depressed subjects, they found generally lower peripheral blood zinc concentrations in those suffering from depressive disorder. Lower serum zinc levels were also found in women suffering from postpartum depression [82]. Patients were assessed for postpartum depressive symptoms using the Edinburgh Postnatal Depression Rating Scale (EPDRS). On the 3rd day after childbirth, the EPDRS score increased by 45%, and a 24% reduction in serum zinc levels was observed when compared with the 30th postpartum day. In the study by Nowak et al. [83], patients suffering from unipolar depression had significantly decreased zinc concentrations in the blood, although it normalized after successful antidepressant therapy.
Zinc may be a therapeutic agent or supplement that could help to reverse the symptoms of unipolar depression. In a placebo-controlled, double-blind pilot study by Nowak et al. [84], zinc supplementation of triycyclic reuptake inhibitors as well as of selective serotonin reuptake inhibitors reduced the scores in scales commonly used to assess depressive symptoms – namely, the Hamilton Depression Rating Scale (HDRS) and the Beck Depression Inventory (BDI) – after 6 and 12 weeks, in comparison with placebo-supplemented subjects. Likewise, Siwek et al. [80] showed that the supplementation of imipramine treatment with zinc significantly reduced depression scores in comparison with placebo-supplemented groups.
A postmortem study found a decrease in zinc’s potency to inhibit 3HMK-801 binding to the NMDA receptor in the hippocampus, but not in the frontal cortex of suicide victims [85, 86].
Based on glutamatergic theory of depression, zinc can potentially be beneficial to help uncover novel synaptic avenues for the development of fast-acting antidepressants. Antidepressant action of zinc observed in preclinical as well as clinical studies may results from inhibition of the NMDA receptor. Zinc influences also other types of receptors, such as the AMPA, metabotropic (mGluR) and GABA. It is speculated that zinc may maintain homeostasis between excitatory and inhibitory systems via zinc receptor - GPR39, which seems to be a promising target in depression. Although further studies with agonists and antagonists are required to better understand the role of GPR39 in the antidepressant action.
THE GPR39 ZINC RECEPTOR AS A NEW TARGET FOR ANTIDEPRESSANT TREATMENT
Recent studies indicate that zinc may act as a neurotransmitter in the central nervous system via the GPR39 receptor [87]. The authors found that the GPR39 receptor is activated by zinc ions. GPR39 is a metabotropic receptor widely expressed in regions of the brain that are involved in emotional processes – the frontal cortex, the amygdala and the hippocampus [88], the last specifically in its CA3 neurons [89]. In our previous studies we found that mice or rats receiving a zinc-deficient diet had a lower expression of the GPR39 receptor in the hippocampus as well as in the frontal cortex [55,90]. Moreover, we observed GPR39 down-regulation in both the hippocampus and the frontal cortex in suicide victims [90]. Because these reports were the first indicating the possible role of the zinc receptor in depressive disorder, it could only be speculated that the changes observed may have resulted from decreased CREB and BDNF levels in the hippocampus. The role of the GPR39 is widely reviewed by Młyniec et al. [91]. The GPR39 receptor activates diverse pathways in the brain, including Gαs and Gαq, which lead to CREB expression via cAMP responsive element (CRE)-mediated transcription [87,89,92-94]. CREB increases BDNF expression, and a significant decrease in CREB and BDNF levels in the hippocampus of mice receiving a zinc-deficient diet for 6 weeks has been observed [90]. The CREB/BDNF neuronal pathway seems to play a pivotal role in the pathophysiology of depression and the antidepressant response [95]. In our next study we found that GPR39 knockout mice (GPR39 KO) showed a depressive-like phenotype, as measured by the FST and TST [96]. They also exhibited significantly decreased CREB and BDNF levels in the hippocampus. This indicates that GPR39 may modulate the CREB/BDNF pathway, but further studies are needed to confirm this finding. The GPR39 receptor seems to be involved in the antidepressant response. This zinc receptor was found to be up-regulated in the frontal cortex of mice receiving selective antidepressants such as escitalopram, reboxetine and bupropion, but not imipramine in chronic doses [97].
There is evidence that the GPR39 receptor may modulate glutamatergic neurotransmission (Fig. 3). This could also possibly be the mechanism underlying the depressive-like phenotype found in zinc-deficient or GPR39 KO mice. Chorin et al. [98] reported that activation of the GPR39 receptor in the CA3 region of the hippocampus leads to increased activity of the K+/Cl- cotransporter 2 (KCC2). KCC2 plays a pivotal role in maintaining hyperpolarizing GABAA reversal potentials, which are based on Cl- currents mediated by glycine-gated receptor channels or GABA [99]. This leads to the regulation of excitatory glutamatergic synapses, which is crucial, according to the glutamatergic theory of depression. A study by Matrisciano et al. [100] showed an increased behavioral response to diazepam in a genetic rat model of unipolar depression is associated with an increased expression of KCC2 in the central nervous system, suggesting a potential role of KCC2 in depressive disorders. It appears that KCC2 acts through the GPR39 receptor to maintain. The GPR39 receptor, via KCC2 activation, seems to be involved in the maintenance of homeostasis between inhibitory and excitatory signaling in the brain, but this needs to be confirmed by additional studies. Enhanced activity of KCC2 was found to be up-regulated by synaptic Zn2+ [98]. Synaptic zinc also inhibits glutamate release by enhancing endocanabionoid synthesis. According to Perez-Rosello et al., synaptic zinc, as well as the GPR39 receptor, is required for triggering the synthesis of endocannabinoid 2-arachinoglycerol (2AG) [101]. This seems to be necessary for glutamate inhibition triggered by synaptic Zn2+. The authors found that there is no initiation of 2-AG synthesis mediated by zinc in mice lacking a metabotropic zinc receptor.
Fig. (3).
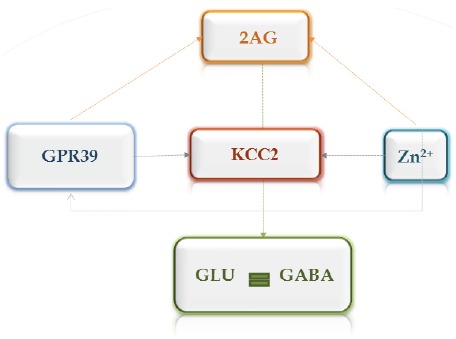
Possible mechanisms regulating homeostasis between glutamatergic and GABAergic systems in the brain via zinc and GPR39 receptor. Activation of the GPR39 zinc receptor leads to increased activity of the K+/Cl- cotransporter 2 (KCC2), which plays an important role in the inhibition. Synaptic zinc and GPR39 receptor are required for triggering the synthesis of endocannabinoid 2-arachinoglycerol (2AG), which inhibits presynaptic glutamate release.
CONCLUSIONS
According to the glutamatergic theory of depression, an imbalance between the main excitatory and inhibitory systems leads to the development of depressive symptoms. Drugs that attenuate glutamatergic neurotransmission showed antidepressant properties in preclinical and clinical studies. Because of strong evidence of decreased zinc concentrations in depressive disorder, it is believed that zinc may be a possible state marker of that illness. Zinc is an antagonist of the glutamatergic NMDA receptor and seems to play a significant role in the treatment of depression by influencing neurotransmission via the recently discovered GPR39 zinc receptor. This may be an important target for new antidepressants, but further studies are required.
ACKNOWLEDGEMENTS
This article was supported by a grant from the National Science Centre K/PBO/000106 (contract DEC-2011/03/B/NZ7/01999).
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
References
- 1.Hollander E. Advances in the treatment of depression. CNS Spectr. 1999;4(7):13. doi: 10.1017/s1092852900011962. [DOI] [PubMed] [Google Scholar]
- 2.Fava M., Davidson K.G. Definition and epidemiology of treatment-resistant depression. Psychiatr. Clin. North Am. 1996;19(2):179–200. doi: 10.1016/S0193-953X(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 3.Di Benedetto B., Rupprecht R. Targeting glia cells: novel perspectives for the treatment of neuropsychiatric diseases. Curr. Neuropharmacol. 2013;11(2):171–85. doi: 10.2174/1570159X11311020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilc A., Wierońska J.M., Skolnick P. Glutamate-based antidepressants: preclinical psychopharmacology. Biol. Psychiatry. 2013;73(12):1125–1132. doi: 10.1016/j.biopsych.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20(3):127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- 6.Maes M., Kubera M., Obuchowiczwa E., Goehler L., Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol. Lett. 2011;32(1):7–24. [PubMed] [Google Scholar]
- 7.Moylan S., Maes M., Wray N.R., Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol. Psychiatry. 2013;18(5):595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 8.Swardfager W., Herrmann N., McIntyre R.S., Mazereeuw G., Goldberger K., Cha D.S., Schwartz Y., Lanctôt K.L. Potential roles of zinc in the pathophysiology and treatment of major depressive disorder. Neurosci. Biobehav. Rev. 2013;37(5):911–929. doi: 10.1016/j.neubiorev.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Szewczyk B., Kubera M., Nowak G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):693–701. doi: 10.1016/j.pnpbp.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Leonard B., Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Brown E.S., Hughes C.W., McColl R., Peshock R., King K.S., Rush A.J. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014;39(3):770–779. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kugaya A., Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10(10):808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 13.Pilc A., Chaki S., Nowak G., Witkin J.M. Mood disorders: regulation by metabotropic glutamate receptors. Biochem. Pharmacol. 2008;75(5):997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Niciu M.J., Kelmendi B., Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012;100(4):656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skolnick P. Modulation of glutamate receptors: strategies for the development of novel antidepressants. Amino Acids. 2002;23(1-3):153–159. doi: 10.1007/s00726-001-0121-7. [DOI] [PubMed] [Google Scholar]
- 17.Trullas R., Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185(1):1–10. doi: 10.1016/0014-2999(90)90204-J. [DOI] [PubMed] [Google Scholar]
- 18.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 19.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 20.Młyniec K., Davies C.L., de Agüero Sánchez I.G., Pytka K., Budziszewska B., Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol. Rep. 2014;66(4):534–544. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Papp M., Moryl E. New evidence for the antidepressant activity of MK-801, a non-competitive antagonist of NMDA receptors. Pol. J. Pharmacol. 1993;45(5-6):549–553. [PubMed] [Google Scholar]
- 22.Papp M., Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur. J. Pharmacol. 1994;263(1-2):1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 23.Li N., Liu R-J., Dwyer J.M., Banasr M., Lee B., Son H., Li X.Y., Aghajanian G., Duman R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alt A., Nisenbaum E.S., Bleakman D., Witkin J.M. A role for AMPA receptors in mood disorders. Biochem. Pharmacol. 2006;71(9):1273–1288. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Quirk J.C., Nisenbaum E.S. LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Rev. 2002;8(3):255–282. doi: 10.1111/j.1527-3458.2002.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farley S., Apazoglou K., Witkin J.M., Giros B., Tzavara E.T. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int. J. Neuropsychopharmacol. 2010;13(9):1207–1218. doi: 10.1017/S1461145709991076. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Tizzano J.P., Griffey K., Clay M., Lindstrom T., Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology. 2001;40(8):1028–1033. doi: 10.1016/S0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 28.Pałucha-Poniewiera A., Wierońska J.M., Brański P., Burnat G., Chruścicka B., Pilc A. Is the mGlu5 receptor a possible target for new antidepressant drugs? Pharmacol. Rep. 2013;65(6):1506–1511. doi: 10.1016/S1734-1140(13)71511-1. [DOI] [PubMed] [Google Scholar]
- 29.Chaki S., Ago Y., Palucha-Paniewiera A., Matrisciano F., Pilc A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology. 2013;66:40–52. doi: 10.1016/j.neuropharm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Belozertseva I. V, Kos T., Popik P., Danysz W., Bespalov A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 2007;17(3):172–9. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Młyniec K., Davies C.L., de Agüero Sánchez I.G., Pytka K., Budziszewska B., Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol. Rep. 2014;66(4):534–544. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Serefko A., Szopa A., Wlaź P., Nowak G., Radziwoń-Zaleska M., Skalski M., Poleszak E. Magnesium in depression. Pharmacol. Rep. 2013;65(3):547–554. doi: 10.1016/S1734-1140(13)71032-6. [DOI] [PubMed] [Google Scholar]
- 33.Singewald N., Sinner C., Hetzenauer A., Sartori S.B., Murck H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice--influence of desipramine and Hypericum perforatum extract. Neuropharmacology. 2004;47(8):1189–1197. doi: 10.1016/j.neuropharm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Sartori S.B., Whittle N., Hetzenauer A., Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2012;62(1):304–312. doi: 10.1016/j.neuropharm.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghafari M., Whittle N., Miklósi A.G., Kotlowsky C., Schmuckermair C., Berger J., Bennett K.L., Singewald N., Lubec G. Dietary magnesium restriction reduces amygdala-hypothalamic GluN1 receptor complex levels in mice. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0779-8. [DOI] [PubMed] [Google Scholar]
- 36.Pochwat B., Pałucha-Poniewiera A., Szewczyk B., Pilc A., Nowak G. NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin. Investig. Drugs. 2014;23(9):1181–1192. doi: 10.1517/13543784.2014.918951. [DOI] [PubMed] [Google Scholar]
- 37.Poleszak E., Szewczyk B., Wlaź A., Fidecka S., Wlaź P., Pilc A., Nowak G. D-serine, a selective glycine/N-methyl-D-aspartate receptor agonist, antagonizes the antidepressant-like effects of magnesium and zinc in mice. Pharmacol. Rep. 2008;60(6):996–1000. [PubMed] [Google Scholar]
- 38.Skolnick P. Antidepressants for the new millennium. Eur. J. Pharmacol. 1999;375(1-3):31–40. doi: 10.1016/S0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto K., Sawa A., Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry. 2007;62(11):1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Nowak G., Ordway G.A., Paul I.A. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675(1-2):157–164. doi: 10.1016/0006-8993(95)00057-W. [DOI] [PubMed] [Google Scholar]
- 41.Zarate C.A., Jr, Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 42.Hasselmann H.W. Ketamine as antidepressant? Current state and future perspectives. Curr. Neuropharmacol. 2014;12(1):57–70. doi: 10.2174/1570159X113119990043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarate C.A., Jr, Brutsche N.E., Ibrahim L., Franco-Chaves J., Diazgranados N., Cravchik A., Selter J., Marquardt C.A., Liberty V., Luckenbaugh D.A. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 45.Zarate C.A., Jr, Payne J.L., Quiroz J., Sporn J., Denicoff K.K., Luckenbaugh D., Charney D.S., Manji H.K. An open-label trial of riluzole in patients with treatment-resistant major depression. Am. J. Psychiatry. 2004;161(1):171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 46.Preskorn S.H., Baker B., Kolluri S., Menniti F.S., Krams M., Landen J.W. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 47.Frederickson C.J. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 1989;31:145–238. doi: 10.1016/S0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 48.Frederickson C.J., Koh J-Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 49.Frederickson C.J., Suh S.W., Silva D., Frederickson C.J., Thompson R.B. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 2000;130(5S Suppl):1471S–83S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 50.Paoletti P., Vergnano A.M., Barbour B., Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158(1):126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 51.Colvin R.A., Fontaine C.P., Laskowski M., Thomas D. Zn2+ transporters and Zn2+ homeostasis in neurons. Eur. J. Pharmacol. 2003;479(1-3):171–185. doi: 10.1016/j.ejphar.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima A.S., Dyck R.H. Zinc and cortical plasticity. Brain Res. Brain Res. Rev. 2009;59(2):347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Whittle N., Lubec G., Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36(1):147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- 54.Milton A.L., Lee J.L., Butler V.J., Gardner R., Everitt B.J. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J. Neurosci. 2008;28(33):8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Młyniec K., Budziszewska B., Reczyński W., Sowa-Kućma M., Nowak G. The role of the GPR39 receptor in zinc deficient-animal model of depression. Behav. Brain Res. 2013;238:30–35. doi: 10.1016/j.bbr.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Młyniec K., Nowak G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 2012;64(2):249–255. doi: 10.1016/S1734-1140(12)70762-4. [DOI] [PubMed] [Google Scholar]
- 57.Młyniec K., Budziszewska B., Reczyński W., Doboszewska U., Pilc A., Nowak G. Zinc deficiency alters responsiveness to antidepressant drugs in mice. Pharmacol. Rep. 2013;65(3):579–592. doi: 10.1016/S1734-1140(13)71035-1. [DOI] [PubMed] [Google Scholar]
- 58.Młyniec K., Davies C.L., Budziszewska B., Opoka W., Reczyński W., Sowa-Kućma M., Doboszewska U., Pilc A., Nowak G. Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol. Rep. 2012;64(3):567–575. doi: 10.1016/S1734-1140(12)70852-6. [DOI] [PubMed] [Google Scholar]
- 59.Tassabehji N.M., Corniola R.S., Alshingiti A., Levenson C.W. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008;95(3):365–9. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M., Tamano H., Kikuchi T., Takeda A. Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem. Int. 2010;56(3):410–416. doi: 10.1016/j.neuint.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Takeda A., Tamano H., Itoh H., Oku N. Attenuation of abnormal glutamate release in zinc deficiency by zinc and Yokukansan. Neurochem. Int. 2008;53(6-8):230–5. doi: 10.1016/j.neuint.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Grønli O., Kvamme J.M., Friborg O., Wynn R. Zinc deficiency is common in several psychiatric disorders. PLoS One. 2013;8(12):e82793. doi: 10.1371/journal.pone.0082793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jurowski K., Szewczyk B., Nowak G., Piekoszewski W. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. J. Biol. Inorg. Chem. 2014;19(7):1069–1079. doi: 10.1007/s00775-014-1139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kroczka B., Zieba A., Dudek D., Pilc A., Nowak G. Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol. J. Pharmacol. 2000;52(5):403–406. [PubMed] [Google Scholar]
- 65.Kroczka B., Branski P., Palucha A., Pilc A., Nowak G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 2001;55(2):297–300. doi: 10.1016/S0361-9230(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 66.Nowak G., Szewczyk B., Wieronska J.M., Brański P., Palucha A., Pilc A., Sadlik K., Piekoszewski W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 2003;61(2):159–164. doi: 10.1016/S0361-9230(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 67.Rosa A.O., Lin J., Calixto J.B., Santos A.R., Rodrigues A.L. Involvement of NMDA receptors and l-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav. Brain Res. 2003;144(1-2):87–93. doi: 10.1016/S0166-4328(03)00069-X. [DOI] [PubMed] [Google Scholar]
- 68.Szewczyk B., Brański P., Wierońska J.M., Pałucha A., Pilc A., Nowak G. Interaction of zinc with antidepressants in the forced swimming test in mice. Pol. J. Pharmacol. 2002;54(6):681–685. [PubMed] [Google Scholar]
- 69.Cunha M.P., Machado D.G., Bettio L.E., Capra J.C., Rodrigues A.L. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(8):1913–1920. doi: 10.1016/j.pnpbp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Szewczyk B., Poleszak E., Wlaź P., Wróbel A., Blicharska E., Cichy A., Dybała M., Siwek A., Pomierny-Chamioło L., Piotrowska A., Brański P., Pilc A., Nowak G. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(2):323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Sowa-Kućma M., Legutko B., Szewczyk B., Novak K., Znojek P., Poleszak E., Papp M., Pilc A., Nowak G. Antidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm (Vienna) 2008;115(12):1621–1628. doi: 10.1007/s00702-008-0115-7. [DOI] [PubMed] [Google Scholar]
- 72.Cieślik K., Klenk-Majewska B., Danilczuk Z., Wróbel A., Łupina T., Ossowska G. Influence of zinc supplementation on imipramine effect in a chronic unpredictable stress (CUS) model in rats. Pharmacol. Rep. 2007;59(1):46–52. [PubMed] [Google Scholar]
- 73.Nowak G., Szewczyk B., Wieronska J.M., Brański P., Pałucha A., Pilc A., Sadlik K., Piekoszewski W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 2003;61(2):159–164. doi: 10.1016/S0361-9230(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 74.Nikseresht S., Etebary S., Karimian M., Nabavizadeh F., Zarrindast M.R., Sadeghipour H.R. Acuteadministration of zn, mg, and thiamine improves postpartumdepression conditions in mice. Arch. Iran Med. 2012;15(5):306–11. doi: 10.012155/AIM.0012.. [DOI] [PubMed] [Google Scholar]
- 75.Etebary S., Nikseresht S., Sadeghipour H.R., Zarrindast M.R. Postpartum depression and role of serum trace elements. Iran. J. Psychiatry. 2010;5(2):40–46. [PMC free article] [PubMed] [Google Scholar]
- 76.Rosa A.O., Lin J., Calixto J.B., Santos A.R., Rodrigues A.L. Involvement of NMDA receptors and l-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav. Brain Res. 2003;144(1-2):87–93. doi: 10.1016/S0166-4328(03)00069-X. [DOI] [PubMed] [Google Scholar]
- 77.Hotz C., Brown K.H. Identifying populations at risk of zinc deficiency: the use of supplementation trials. Nutr. Rev. 2001;59(3 Pt 1):80–84. doi: 10.1111/j.1753-4887.2001.tb06992.x. [DOI] [PubMed] [Google Scholar]
- 78.McLoughlin I.J., Hodge J.S. Zinc in depressive disorder. Acta Psychiatr. Scand. 1990;82(6):451–453. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 79.Maes M., De Vos N., Demedts P., Wauters A., Neels H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J. Affect. Disord. 1999;56(2-3):189–194. doi: 10.1016/S0165-0327(99)00011-7. [DOI] [PubMed] [Google Scholar]
- 80.Siwek M., Dudek D., Paul I.A., Sowa-Kućma M., Zieba A., Popik P., Pilc A., Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J. Affect. Disord. 2009;118(1-3):187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Swardfager W., Herrmann N., Mazereeuw G., Goldberger K., Harimoto T., Lanctôt K.L. Zinc in depression: a meta-analysis. Biol. Psychiatry. 2013;74(12):872–878. doi: 10.1016/j.biopsych.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Wójcik J., Dudek D., Schlegel-Zawadzka M., Grabowska M., Marcinek A., Florek E., Piekoszewski W., Nowak R.J., Opoka W., Nowak G. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 2006;58(4):571–576. [PubMed] [Google Scholar]
- 83.Nowak G., Zięba A., Dudek D., Krośniak M., Szymaczek M., Schlegel-Zawadzka M. Serum Trace Elements in Animal Models and Human Depression. Part I. Zinc. 1999;86:83–86. [Google Scholar]
- 84.Nowak G., Siwek M., Dudek D., Zięba A., Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol. J. Pharmacol. 2003;55(6):1143–1147. [PubMed] [Google Scholar]
- 85.Sowa-Kućma M., Szewczyk B., Sadlik K., Piekoszewski W., Trela F., Opoka W., Poleszak E., Pilc A., Nowak G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013;151(3):924–31. doi: 10.1016/j.jad.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Nowak G., Szewczyk B., Sadlik K., Piekoszewski W., Trela F., Florek E., Pilc A. Reduced potency of zinc to interact with NMDA receptors in hippocampal tissue of suicide victims. Pol. J. Pharmacol. 2003;55(3):455–459. [PubMed] [Google Scholar]
- 87.Holst B., Egerod K.L., Schild E., Vickers S.P., Cheetham S., Gerlach L.O., Storjohann L., Stidsen C.E., Jones R., Beck-Sickinger A.G., Schwartz T.W. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148(1):13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 88.Jackson V.R., Nothacker H-P., Civelli O. GPR39 receptor expression in the mouse brain. Neuroreport. 2006;17(8):813–816. doi: 10.1097/01.wnr.0000215779.76602.93. [DOI] [PubMed] [Google Scholar]
- 89.Besser L., Chorin E., Sekler I., Silverman W.F., Atkin S., Russell J.T., Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009;29(9):2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Młyniec K., Doboszewska U., Szewczyk B., Sowa-Kućma M., Misztak P., Piekoszewski W., Trela F., Ostachowicz B., Nowak G. The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology, 2014;79:290–7. doi: 10.1016/j.neuropharm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Młyniec K., Singewald N., Holst B., Nowak G. GPR39 Zn(2+)-sensing receptor: a new target in antidepressant development? J. Affect. Disord. 2015;174:89–100. doi: 10.1016/j.jad.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 92.Hershfinkel M., Moran A., Grossman N., Sekler I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA. 2001;98(20):11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holst B., Holliday N.D., Bach A., Elling C.E., Cox H.M., Schwartz T.W. Common structural basis for constitutive activity of the ghrelin receptor family. J. Biol. Chem. 2004;279(51):53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- 94.Popovics P., Stewart A.J. GPR39: a Zn(2+)-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell. Mol. Life Sci. 2011;68(1):85–95. doi: 10.1007/s00018-010-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nibuya M., Nestler E.J., Duman R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Młyniec K., Budziszewska B., Holst B., Ostachowicz B., Nowak G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 2015;18(3):pyu002. doi: 10.1093/ijnp/pyu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Młyniec K., Nowak G. GPR39 up-regulation after selective antidepressants. Neurochem. Int. 2013;62(7):936–939. doi: 10.1016/j.neuint.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 98.Chorin E., Vinograd O., Fleidervish I., Gilad D., Herrmann S., Sekler I., Aizenman E., Hershfinkel M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011;31(36):12916–12926. doi: 10.1523/JNEUROSCI.2205-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blaesse P., Schmidt T. K-Cl cotransporter KCC2--a moonlighting protein in excitatory and inhibitory synapse development and function. Pflugers Arch. 2015;467(4):615–624. doi: 10.1007/s00424-014-1547-6. [DOI] [PubMed] [Google Scholar]
- 100.Matrisciano F., Nasca C., Molinaro G., Riozzi B., Scaccianoce S., Raggi M.A., Mercolini L., Biagioni F., Mathè A.A., Sanna E., Maciocco E., Pignatelli M., Biggio G., Nicoletti F. Enhanced expression of the neuronal K+/Cl- cotransporter, KCC2, in spontaneously depressed Flinders Sensitive Line rats. Brain Res. 2010;1325:112–120. doi: 10.1016/j.brainres.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 101.Perez-Rosello T., Anderson C.T., Schopfer F.J., Zhao Y., Gilad D., Salvatore S.R., Freeman B.A., Hershfinkel M., Aizenman E., Tzounopoulos T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. 2013;33(22):9259–9272. doi: 10.1523/JNEUROSCI.0237-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li B., Suemaru K., Kitamura Y., Cui R., Gomita Y., Araki H. [Strategy to develop a new drug for treatment-resistant depression--role of electroconvulsive stimuli and BDNF]. Yakugaku Zasshi. 2007;127(4):735–742. doi: 10.1248/yakushi.127.735. [DOI] [PubMed] [Google Scholar]
- 103.Li B., Zhao J., Lv J., Tang F., Liu L., Sun Z., Wang L., Siwela S.P., Wang Y., Song Y., Manchishi S.M., Cui R. Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:199–206. doi: 10.1016/j.pnpbp.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 104.Marks D.M., Pae C.U., Patkar A.A. Triple reuptake inhibitors: the next generation of antidepressants. Curr. Neuropharmacol. 2008;6(4):338–343. doi: 10.2174/157015908787386078. [DOI] [PMC free article] [PubMed] [Google Scholar]


