Abstract
Depression is one of the prevalent and persistent psychiatric illnesses. It brings heavy socioeconomic burden such as healthcare expenditures and even higher suicide rates. Despite many hypotheses about its mechanism have been put forward, so far it is still unclear, not to mention the precise and effective diagnostic or therapeutic methods. In this paper, the current conditions of pathological and pharmacological mechanism of depression were reviewed systematically. Firstly, the most recent hypotheses and metabolomics based research including hereditary, neurotransmitter systems, brain derived neurotrophic factor (BDNF), hyperactivity of the hypothalamic pituitary adrenal (HPA) axis and inflammatory as well as metabolomics were summarized. Secondly, the present situation and development on antidepressant drugs at home and abroad were reviewed. Finally, a conclusion and prospect on the pathological and pharmacological mechanism of depression were provided primarily.
Keywords: Antidepressant drugs, BDNF, depression, HPA axis, inflammatory, metabolomics, neurotransmitter systems.
1. INTRODUCTION
Depression is a persistent and serious psychiatric illness, affecting over 120 million people worldwide. 13%~20% people have the experience of depressive disorder and the lifetime prevalence rate is 6.1%~9.5%. Moreover, 35.5%~50.3% of seriously depressed patients in developed countries had no appropriate treatment and this rate in less-developed countries has rocketed to 76.3%~85.4% [1]. It has been reported that more than 19 million adults suffer from a depressive disease and the direct or indirect costs are more than $30 billion each year in the United States [2], while the incidence of depression is about 3%~5% and now more than 26 million people develop depression in China [3].
Nowadays, depression has been one of the most important risk factors for lifetime suicide attempts and the population attributable risk proportion has been approximately 28%. According to the World Health Organization (WHO) statistics, depression would be the largest leading cause ended up to death worldwide by 2030 [4, 5]. Both the suffering from depressed patients and the premature deaths by suicide resulted from depression lead to a significant social burden. The primary ways to reduce these burdens are the promotion of health systems efficiency and cost-effective treatment strategies [4].
Considerable experimental and clinical evidences have demonstrated several alterations happened to neuronal serotonergic and noradrenergic function in the central nervous system of depressed patients [6]. Another leading hypothesis on the neurobiological basis of depression suggests the role played by brain-derived neurotrophic factor (BDNF) in the brain [7]. Hyperactivity of the hypothalamic pituitary adrenal (HPA) axis is one of the greatest findings in psychoneuroendocrinology in major depression [8]. Furthermore, the inflammatory cytokines or endogenous metabolites were involved with the mechanism of depression.
The antidepressant drugs were prescribed frequently to treat depressive disorders in clinical practice; however, only one-third of patients performed a beneficial treatment response after drug-treatment. The discovery of tricyclic antidepressants (TCAs) as well as monoamine oxidase inhibitors (MAOIs) in the 1950s gave impetus on research into developing new antidepressant medications with a better safety profile and less side effects. Then, the selective 5-HT reuptake inhibitors (SSRIs) were considered as more effective antidepressants. More recently, 5-HT-norepinephrine reuptake inhibitors (SNRIs) have been widely used in clinic. Though the treatment of depression is efficacious, these antidepressant drugs frequently produce side effects and are generally expensive [9]. For this reason, natural medicine, with a significant treatment effect but relative less side effect and low price, has played an important role in keeping people healthy since ancient times [10, 11] turned to be the hotspot in recent years.
Nowadays, there are many antidepressants in the market, but they are not very ideal because of the side effects. In the present paper, the mechanism of depression and current situation of antidepressant are summarized completely. It is promising to provide a reference for the study on depression as well as research and development of ideal novel antidepressants.
2. PATHOLOGICAL MECHANISM OF DEPRESSION
2.1. Hereditary
There is a low possibility that an unusual neuropsychiatric disorder inherited in autosomal dominant fashion could occur in three successive generations of a family. Study showed that symptoms commenced late in their fifth decade in six affected patients and could lead to death in four to six years. The earliest and most prominent essential symptom was mental depression without any response to either electroconvulsive therapy or antidepressant drugs. The symptoms of these patients were accompanied by frequent exhaustion, sleep disturbances, and marked distinguished weight loss [12]. The probability of heritability in major depression is likely to be in the range of around 31%~ 42%, which is probably and optimistically the lower bound, in fact, the level of heritability is likely to be much substantially higher for reliably diagnosed major depression or for the subtypes such as recurrent major depression. As a complex disorder, major depression is an integrated result of both genetic heredity and environmental disturbance influences. It has been long hypothesized that gene-environment interactions have been hypothesized to be of great importance in the etiology of major depression [13].
2.2. Neurotransmitter Systems
Considerable experimental and clinical evidences suggest that the neurotransmitters are the fundamental roles of neurotransmitters in the etiology of depression [6]. As a biochemical messenger and regulator, serotonin is wide spread in the nervous system of vertebrates and invertebrates. Serotonin is synthesized from the L-tryptophan and acts as a local transmitter at synapses, allowing various "state-dependent" behavioral responses to different stimulations. In vertebrates, deficiency of the serotonergic system can lead to disorders such as depression, phobias, obsessive-compulsive disorder, generalized anxiety disorder as well as posttraumatic stress disorder [14]. For decades, the serotonin hypothesis of depression carried research forward to the etiology of depression; depressed patients may exhibit decreased level of brain serotonin and alterations in 5-HT receptors, such as an up-regulation of the 5-HT2 receptors as well as a down regulation in the 5-HT1A receptors [15]. Brain serotonin systems are involved in adaptive responses to disgusting aversive events. Three involved mechanisms of impaired 5-HT1A function in depression are discussed: 5-HT₂ receptors inhibit 5-HT₁ neurotransmission, hypercortisolaemia inhibits 5-HT₁ neurotransmission, and social isolation reduces 5-HT₁ neurotransmission [16]. It has been shown that BDNF and NT-3, among these endogenous proteins, had a great contribution to the growth as well as function of 5-HT including neurons in the adult brain [17].
As a dominant transmitter in the extra pyramidal system of brain, dopamine is also a precursor to epinephrine and norepinephrine and plays an important role in regulating behavior. More and more human or animal studies proved the close connection between depression and dopamine transmission in the central nervous system. Some researchers also found that there was an up-regulation of dopamine transporter in depressed patients, which makes a re-uptake of dopamine into the presynaptic neurons more effectively.
2.3. Brain-Derived Neurotrophic Factor
BDNF, abundant in periphery and brain, is a significant member of the neurotrophin family in nerve growth factor related proteins. BDNF have been regarded to be of critical contributions to a cellular mechanism on learning and memory in long-term potentiating. It is indicated that the neurotrophic factor is associated with the plasticity [7]. It is known to exert different effects on the nervous system such as synaptic connectivity, differentiation, neuronal outgrowth and neuronal repair. This neurotrophin hypothesis on depression is based mainly on that decreasing in levels of hippocampal BDNF are related to stress-induced depressive behaviors and antidepressant therapy promotes the expression of BDNF [18]. This neurotrophin has been involved in the phenotypic expression of various neurotransmitter systems including Alzheimer’s disease and amyotrophic lateral sclerosis [19].
Recently, several studies have demonstrated neuro-modulatory effects of BDNF on monoamines, neuropeptides and behavior. BDNF as well as other members such as nerve growth factor (NGF) and neurotrophin-3 (NT-3) in neurotrophic factor family, affect cellular function by activation of the respective tyrosine kinase receptors [20]. Studies suggest that infusion of BDNF either directly or intracerebroventricularly into the rat midbrain, near the periaqueductal gray (PAG), median raphe nuclei and dorsal, increased activity within dopamine, serotonin, and/or norepinephrine pathways in different forebrain areas including the hippocampus, cortex, nucleus accumbens and striatum. So, it has been shown that central BDNF administration can regulate the activity of the neurochemical and anatomical systems, which is thought to be involved with depression [21].
Acute and chronic stress, which can make contributions to development of depression in humans, makes decreasing levels of BDNF in the rodent hippocampus, one brain region implicated in the pathophysiology and treatment of depression. While antidepressant treatment makes increasing levels of BDNF in the rodent hippocampus chronically, but not acutely. This finding does a consistency with the delayed onset of efficacy of drugs in clinic. Interestingly, chronic antidepressant treatment can also prevent the decrease of BNDF in the hippocampus induced by stress. What’s more, it produces an antidepressant-like effect when administration of BDNF into either hippocampus or midbrain in rodent models of depression [22].
2.4. Hypothalamic-pituitary-adrenal (HPA) Axis
Hyperactivity of the HPA axis (Fig. 1) stands for the major neuroendocrine stress response system which contributes to maintain stability and health via adapting the organism to change in demand and thereby. Stress as well as acute challenge has long been considered as a potential risk factor for depression, which is often involved in the onset of depressive episodes. Thus, the changes of HPA axis in depression may reflect influences of stress and regulate the manifestation of depressive symptoms. [8]. HPA axis activity is dominated by vasopressin (AVP) and the secretion of adrenocorticotrophic hormone releasing factor (CRF) from the hypothalamus, which in turn activates the secretion of adrenocorticotrophic hormone (ACTH) from the pituitary, finally stimulates the secretion of the glucocorticoids from the adrenal cortex. And then the glucocorticoids make interaction with their receptors from multiple target tissues such as HPA axis, where they serve as feedback inhibition both directly on secretion of ACTH in pituitary corticotrope and on CRF as well as AVP from the hypothalamus [23].
Fig. (1).
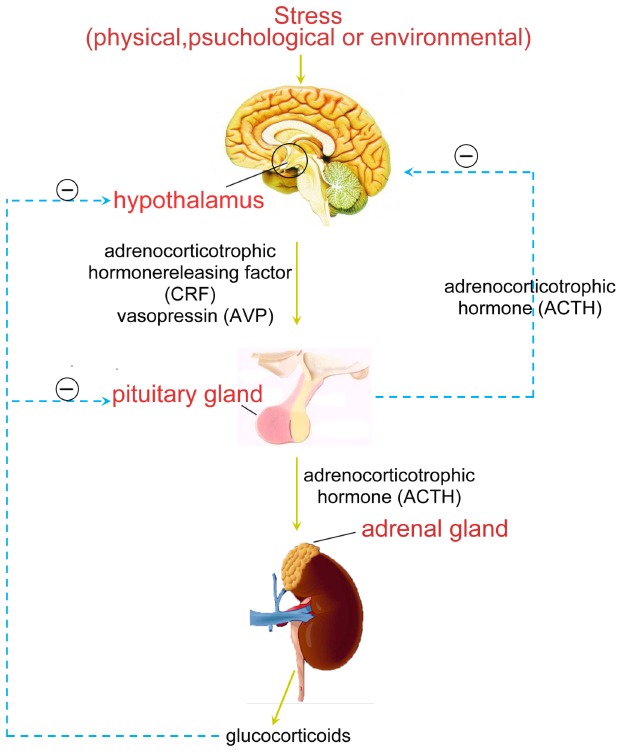
Stress and HPA axis.
The over activity of HPA axis occurs at any level of this axis during stress in depressed patients and normal. This can induce enlargement of the pituitary and adrenals. The integrity of the hippocampus is essential for memory function and, via the high density of its cortisol receptors; cortisol induced inhibitory feedback to the HPA axis [24]. The symptom profile of depressive episodes occuring antenatally and postnatally may be different. Melancholic depression is related to raise cortisol levels, while a typical depression with low cortisol concentration. Additionally, women who have different genetic predispositions are probably at higher risk of depressive episodes at various times in the perinatal period [25]. In accordance with this “stress reactivity” formulation, researchers have discovered that cortisol had a hereditary component and was elevated in 40%~60% of depressed adults, which could be considered as a credible indicator for functioning and stress reactivity of HPA axis. In fact, hypercortisolemia has been assumed to cause the loss of hippocampus neuronal which has also been postulated to be participated in the pathogenesis of depression in turn [26].
2.5. Inflammatory
Over the past In the last t two decades, some new studies on psychiatric have hypothesized that inflammatory processes participated in the pathogenesis of major depression. There is a relationship between T cell activation,lowered levels of serum zinc, and increased acute phase proteins as well as cytokines in patients with depression [27-30]. During immune responses, cytokines produced by monocytes and increasing body temperature and stimulating hepatocytes so that C-reactive protein, haptoglobin and macroglobulin are produced. These proteins activate the complement system and opsonize bacteria [31]. Fostering maladaptive health practices, triggering dysregulation of hormonal systems and increasing susceptibility to infections related with atherosclerosis promotes a mild inflammatory response in depression [32].
It was shown that depression is associated with increased expression of inflammatory molecules [32-34]. A number of the molecules such as C-reactive protein (CRP) and interleukin-6 (IL-6) have been shown to predict cardiac morbidity and mortality [35]. Moreover, the increased production of pro-inflammatory cytokines including IL-6, IL-1β and TNF-α may induce sickness behavior syndromes.
2.6. Metabolomics
Metabolomics, an embranchment of systems biology, has been widely applied in many areas. With the development of effective analytical technologies and methods, it makes a great contribution to understanding the basis of diseases via analyzing fluids as well as tissues from an organism and the discovery of specific biomarkers [36]. Metabolomics information can be obtained by maximum data catching from samples via proton nuclear magnetic resonance (1H NMR), gas chromatography-mass spectrometer (GC-MS), liquid chromatography-mass spectrometry (LC-MS) coupled with multivariate data analysis. Considerable experiment in animal model or clinical practice indicated that metabolomics was a useful tool for researching the pathophysiology and identified potential biomarkers of depression. The general procedures where biomarkers can be discovered via metabolomics are shown in Fig. 2.
Fig. (2).
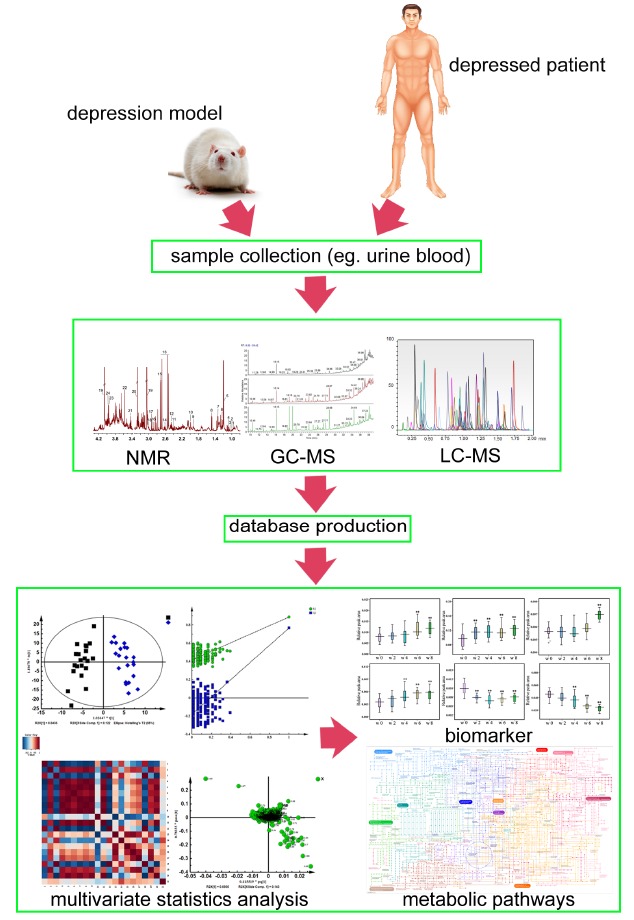
The general procedures where biomarkers can be discovered via metabolomics.
A 1H NMR plasma metabolomics study of chronic and acute stress rat’s models of depression was performed for the identification of crucially endogenous metabolites which can discriminate between chronic and acute stresses. The concentration of proline, valine decreased, while trimethylamine, lactate, aspartate, glutamate, acetoacetate, alanine, leucine/isoleucine, lipids increased in the chronic unpredictable mild stress (CUMS) model, trimethylamine decreased in the FST-1d model, α -glucose, β -glucose, valine and lipids increased in the FST-14d model. It is indicated that metabolomics is a useful tool for selecting suitable animal model on the study of depression [37]. With the use of a proton nuclear magnetic resonance (1H NMR) based urinary metabolomics method, 23 differentially expressed metabolites were initially identified that distinguished major depressive disorder subjects from healthy control subjects, and five metabolite as potential biomarkers including malonate, formate, N-methylnicotinamide, m-hydroxyphenylacetate and alanine could be used to accurately distinguish MDD subjects from healthy control subjects. These metabolites were found to primarily participate in energy metabolism, gut microbial metabolism, and tryptophan-nicotinic acid metabolism [38]. While 1H NMR-based plasma metabolomics were used to distinguish depressed patients and healthy controls, the key metabolites include amino acids, lipid/protein complexes and some molecules related to lipid metabolism and energy metabolism contributing to discrimination between depressed patients and healthy controls were identified [39]. We use urinary 1H NMR-based metabolomics to investigate the effective TCM formula Xiaoyaosan (XYS) in depressed patients, and the results show that the concentrations of creatinine, taurine, 2-oxoglutarate and xanthurenic acid increase significantly after XYS treatment, while the levels of citrate, lactate, alanine and dimethylamine decrease significantly compared with pre-treatment samples. These statistically significant perturbations are involved in energy metabolism, gut microbes, tryptophan metabolism and taurine metabolism. It is a suggestion that urine metabolomics could serve as a perfect way for the investigation of depression and antidepressant.
In order to investigate the biomarkers of depression, GC-MS was applied to the metabolomics analysis of plasma of CUMS rats. We identified 13 metabolites among the detected compounds and the concentrations of 12 metabolites in the CUMS group were significantly changed compared with the control. Via the KEGG pathway database, amino acid metabolism, energy metabolism and glycometabolism were found to be affected by CUMS treatment. Interestingly, we found glycometabolism related to the depression in a plasma metabolomics study on CUMS depression model [40]. Employing a GC-MS-based metabolomics approach, CUMS rats were characterized by lower levels of isoleucine as well as glycerol and higher levels of N-acetylaspartate as well as β-alanine compared to control rats [41]. Therefore, plasma metabolic dynamic variations in a CUMS induced rat model of depression showed that the metabolic responses were systematic changes in glycometabolism, amino acid metabolism and energy metabolism. Six potential biomarkers (glycine, glutamate, fructose, citric acid, glucose, hexadecanoic acid) were closely related to depression [42].
Depression is common across the lifespan, despite the abundance of research into this disease and various hypotheses that have been put forward; the etiology of depressive disorder is still not completely understood. However, since different researchers have performed many tasks on modality from different perspectives, they are necessarily linked, and complementary, it is helpful for us to comprehend depression furthermore. Fig. 3 shows the different pathological mechanism of depression.
Fig. (3).
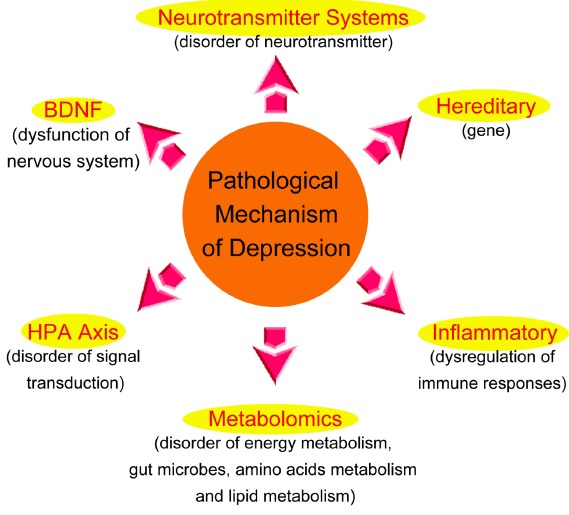
The different pathological mechanism of depression.
3. PHARMACOLOGICAL MECHANISM
3.1. Classic Antidepressant Drugs
Most antidepressant drugs produce their primary pharmacological effects on the brain monoamine neurotransmitters, norepinephrine (NE) and serotonin (5-HT), but differ regarding their selectivity. Tricyclic antidepressants could inhibit the neuronal uptake of NA [43] and some of them also make a contribution to inhibit the uptake of 5-HT [44], which indicate that the treatment response of antidepressants could cause an increased availability of 5-HT or NA at postsynaptic receptor sites. Recently, it has been found that amitriptyline and nortriptyline, two of tricyclic antidepressants used most commonly, have a higher affinity for 3H-d-LSD binding sites of brain and also brought a reduced activity of central 5-HT in a behavioral test [45].
New antidepressant drugs have fewer side effects than non-selective MAO inhibitors (MAOIs) and tricyclics owing to lack of affinity for receptors for acetylcholine and amines. So, the selective serotonin (5-HT) reuptake inhibitors (SSRIs) have become the most commonly used drugs in clinic in several countries. The introduction of the SSRIs, which can be given at a therapeutic dose from the beginning of the treatment, did not shorten the considerable delay for the occurrence of the antidepressant response, even if some of them reach a relatively steady-state level within a few days.
Nearly all antidepressants could be categorized as inhibitors of monoamine reuptake, monoamine oxidase inhibitors, and typical agents. Antidepressants that inhibit monoamine reuptake are available, they selectively block the reuptake of NE, selectively block the reuptake of 5-HT, or are non-selective reuptake inhibitors (NSRIs) with high affinity for both NE and 5-HT transporters. Some antidepressants inhibit NE and dopamine (DA) transporters but not 5-HT. Other atypical drugs also produce changes in neurotransmission of noradrenergic or serotonergic neurons, but their precise mechanism of action may be indirect. Even within these broad categories, there may be important relative differences in selectivity and potency between monoamines, or differences in selectivity for monoamine and non-monoamine targets which may result in a different side-effect profile. The identification of different behavioral components to the antidepressant response may provide a strategy for developing more effective antidepressants [46]. Lots of meta-analyses have indicated that treatment of depressed patients with the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine brings greater response or remission rates than the SSRIs. At present, it remains unclear whether there are differences in terms of efficacy between the SSRIs and a broad and heterogeneous grouping of antidepressant drugs which can enhance noradrenergic as well as serotonergic neurotransmission [47].
3.2. Medicinal Plants
The antidepressants such as TCAs, MAOIs and SSRIs frequently produce side effects in clinical practice. Although some new synthesized antidepressants produce less side-effects, they are extremely expensive, which leads to an urgent need for further research on the findings of novel effective and safe antidepressants. Thus, one of effective approaches was natural medicine [10, 11].
The most widely used medicinal plants for the treatment of depressive disorders in traditional and conventional medicines around world are Hypericum perforatum (Hypericaeae), Uncaria tome (Rubiaceae), Valeriana officinalis (Valerianaceae), Centella asiatica (Apiaceae), Pfaffia paniculata (Amaranthaceae),Rhodiola rosea (Crassulaceae), Schizandra chin (Schizandraceae), Rhododendron molle (Ericaceae), Rauwolfia serpentina (Apocynaceae), Thea sinensis (Theaceae) and Withania somnifera (Solanaceae) [48]. In oriental countries, many medicinal plants from natural resources, especially traditional Chinese medicine, such as Scrophularia ningpoensis, Plantago asiatica and Hypericum perforatum were successfully applied to prevent or treat depression-like disorders [49].
With more attention to be paid to the antidepressant-like effects, some flavonoids in natural plants have been identified to be the main bioactive constituents as they have demonstrated efficacy and safety not only in animal models but also in clinical trials [50, 51]. Isoliquiritin and liquiritinare isolated from G. uralensis appeared to produce antidepressant-like effect in tail suspension test (TST) and forced swimming test (FST) in mice [52]. Phototherapy is widely employed for the treatment of depression in different countries around the world. The flowering tops of Hypericum perforatum (St. John’s wort; SJW) have been used throughout millennia for a range of nervous system conditions, several meta-analysis of randomized controlled trials (RCTs) involving SJW for depression shows that there is non-significant difference on the treatment of depression compared with SSRIs [53,54]. SJW contains a range of constituents, including the naphthodianthrones pseudohypericin and hypericin; the phloroglucinol hyperforin; and a range of flavonoids, volatile oils, and tannins [55]. In vivo and in vitro research has revealed nonselective inhibition of the neuronal re-uptake of serotonin, dopamine, and noradrenalin, and weak monoamine oxidase A and B inhibition. Other biologic activities include a decreased degradation of neurochemicals and a sensitization of and/or increased binding of ligands to various receptors (eg. γ-gaminobutyric acid, glutamate, and adenosine), increased dopaminergic activity in the prefrontal cortex, and neuroendocrine modulation [56,57]. Furthermore, it has also been shown that treatments with such extracts seem to be the tendency to decrease serious side effects and to reduce the cost [50]. Given the lower cost of phytomedicines, they may make a great contribution to the management of depressed patients with a low economic status or who cannot tolerate the side effects produced by them [58]. Genipin, the glycone of geniposide, one of the crucial bioactive constituents, was extracted from fruit of Gardenia jasminoides E. A metabolomics method based on 1H NMR suggest that genipin have antidepressive effect, and lipids, N-acetyl glycoproteins, lactate, alanine and trimetlylamine N-oxide are identified as potential antidepressant biomarkers in plasma. Citrate, dimethylamine, trimetlylamine, creatinine and betaine are identified in urine. Genipin may play an antidepressant role by regulating energy metabolism, gut microbes, and glycometabolism [37].
Currently, the effective ingredients in many plants showed strong antidepressant effects in animal experiments. But there still is a long road to go before being used as a novel antidepressant in clinic. And we still need to do a lot of researches to ensure it is safe, stable and effective.
3.3. Chinese Herbal Formula
Traditional Chinese medicines (TCM) are attracting more and more attention worldwide, owing to their specific theory as well as long historical clinical practice. There are several TCM formulas for the treatment of depression including Ban-xia-hou-pu-tang, Gan-mai-da-zao-tang, Bai-he-di-huang-tang, Chai-hu-jia-long-gu-mu-li-tang, Chai-hu-shu-gan-san [59], and Xiao-yao-san [60-64]. Some of those studies are reviewed in Table 1.
Table 1.
Studies on the antidepressant effect of TCM formulas Chai-hu-shu-gan-san and Xiao-yao-san.
| TCM Formulas | Researcher | Subject | Methods | Results |
|---|---|---|---|---|
| Chai-hu-shu-gan-san | Kim, S.H. et al. (2005) [65] | Rat model | Forced swimming test (FST) and the chronic mild stress (CMS) model |
The therapeutic mechanism of CHSGS was involved with the modification of the disturbed HPA axis and γ-Aminobutyric acid (GABA) system |
| Chai-hu-shu-gan-san | Su, Z. H. et al. (2011) [59] | Rat model | A urine metabolomics based on UPLC–QTOF-MS |
The therapeutic mechanism of Chai-hu-shu-gan-san was involved in regulating the dysfunctions of energy metabolism, tryptophan metabolism, bone loss and liver detoxification |
| Xiao-yao-san | Dai, Y.T. et al. (2010) [61] |
Rat model | A urine metabolomics based on GC-MS | The anti-depression effect of Xiao-yao-san at the dose of 6.75 g/kg was the most remarkable and the effect was time dependent. |
| Xiao-yao-san | Gao, X.X. et al. (2011) [62] |
Rat model | A plasma metabolomics method based on GC-MS |
The therapeutic mechanism of Xiao-yao-san was involved in regulating in amino acid metabolism, energy metabolism and glycometabolism. |
| Xiao-yao-san | Liu, X.J. et al. (2012) [63] |
Rat model | A plasma metabolomics based on NMR spectroscopy |
The therapeutic mechanism of XYS was related to the metabolic pathways of gut microbiota, ketone body formation. |
| Xiao-yao-san | Tian, J.S. et al. (2014) [64] |
Depressed patient | A urine metabonomics based on NMR spectroscopy |
The therapeutic mechanism of xiao-yao-san was related to the energy metabolism, gut microbes, tryptophan metabolism and taurine metabolism. |
Chai-hu-shu-gan-san, which is composed of seven Chinese herbs, is one of the most widely used TCM formulas for the treatment of depression in China. Chai-hu-shu-gan-san produce significant antidepressant-like effect in FST and the CUMS animal models and the possible mechanism is involved with the modification of the disturbed HPA axis and γ-Aminobutyric acid (GABA) system [65]. A urine metabolomics study of anti-depressive effect on a rat model of depression induced by CUMS suggest that the therapeutic effect of Chai-hu-shu-gan-san may involve in regulating the dysfunctions of energy metabolism, tryptophan metabolism, bone loss and liver detoxification [59].
Xiao-yao-san, another popular TCM formula, contains eight herbs. The analysis of behavior and metabolomics indicated that Xiao-yao-san had an exact protective effect on depression [63]. Metabolomics study on the anti-depression effect of Xiao-yao-san in CUMS induced rat depression model demonstrates a relation to the energy metabolism, amino acid metabolism, and glycometabolism [60, 61]. With a study on plasma metabolomics in CUMS model and intervention effects of Xiao-yao-san by using GC-MS, most potential biomarkers such as hexadecanoic acid, glycine and glucose have been identified. These changes of metabolites in plasma indicated that the influence of depression to normal animal may involve in amino acid metabolism, glycometabolism and energy metabolism. And the antidepressant effect of Xiao-yao-san may play via regulating these three metabolic pathways [62]. Recently, Tian J.S. et al. [64] study the endogenous metabolites in depressed patients treated with Xiao-yao-san using urinary 1H NMR-based metabolomics, creatinine, taurine, 2-oxoglutarate and xanthurenic acid increased significantly after Xiao-yao-san treatment, while the levels of citrate, lactate, alanine and dimethylamine decreased significantly compared with pre-treatment urine samples. These statistically significant perturbations are involved in energy metabolism, gutmicrobes, tryptophan metabolism and taurine metabolism.
Classic antidepressant drugs, medicinal plants and traditional Chinese medicines have their own characteristics and advantages in the treatment of depression. These antidepressants can relieve the suffering of the depressed patients on a certain extent. The TCM always carries out dialectical treatment based on the traditional Chinese medicine theory and bring good therapeutic effect owing to their combination of different ingredients.
CONCLUSION AND PROSPECT
Depression is a complex mental disorder with important genetic as well as nongenetic contributory factors. Many approaches and strategies will be applied; some of them are not yet feasible or even imagined. Despite extensive research, the neurobiology of depression remains poorly understood due to the complexity, relatively low rates of heritability and heterogeneity of precipitating factors as well as lack of biomarkers. The focus of existing research was relatively one fold, lack of integration and system. Metabolomics is a comprehensive and simultaneous profiling of metabolic changes occurring in living systems in response to genetic or environmental as well as lifestyle factors. This approach attempts to obtain global changes and overall physiological status in biochemical networks and pathways aiming to elucidate sites of perturbations and has shown a great promise as a means to identify biomarkers. Metabolomics is a powerful tool for discovering novel biomarkers as well as biochemical pathways to improve diagnostic, prognostication, and therapy. It has many potential applications and advantages for research into complex systems, such as the mechanisms of depression and antidepressant medicine.
Nowadays, with the requirement of greater therapeutic efficacy and less adverse effects on depression, metabolomics has attracted great interest in alternative medicine, particularly the use of herbal products. Western herbal medicines are usually standardized extracts from single herbs, such as valerian and St. John's wort. Herbal products in terms of single-chemical entities have been successfully used for the treatment and the action of drug is predictable and fast, bring a favorable resolution of a critical situation in a short period of time. The treatment can also result in serious side effects, particularly in the long term. TCM are decoctions of mixtures of herbs that are customized for each individual patient. Dialectical treatment is energetically emphasized in the theory of TCM. That’s to say, during the treatment of disease, every single herb in traditional herbal formulas plays an important role and interact each other. So we can conjecture the attribution of disease in the TCM theory according to nature of herbs in traditional herbal formulas. When those herbs combined, two biologically active constituents can be found to have following effects: mutual accentuation, mutual enhancement, mutual counteraction, mutual suppression, mutual antagonism, and mutual incompatibility. TCM stands for one aspect of Chinese medical philosophy, which has been characterized by its emphasis on maintaining and restoring balance. This medical method is much more appropriate to disease prevention as well as the treatment of chronic diseases with a less collateral damage unaccepted.
In general, further studies are required to find biomarkers of depression in order to get a much better idea of pathological mechanism and to guide new antidepressant drugs and strategy developed. With the development of relative databases and analytical techniques, the application of integration for TCM theory and metabolomics can bring a better speculation for attributions to diseases including the guidance to the diagnosis, prevention or treatment to disease, and the research or development of novel antidepressant drugs.
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China (No. 81441096 and No. 81173366).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Lépine J.P., Briley M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011;7(Suppl. 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baglioni C., Battagliese G., Feige B., Spiegelhalder K., Nissen C., Voderholzer U., Lombardo C., Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011;135(1-3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 3.He Y.C., Zhang B. Clinic research progress on depression. Intl. J. Lab. Med. 2013;07:832–834. [Google Scholar]
- 4.Miret M., Ayuso-Mateos J.L., Sanchez-Moreno J., Vieta E. Depressive disorders and suicide: Epidemiology, risk factors, and burden. Neurosci. Biobehav. Rev. 2013;37(10 Pt 1):2372–2374. doi: 10.1016/j.neubiorev.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Tian J.S., Shi B.Y., Xiang H., Gao S, Qin XM, Du GH. 1HNMR- Based Metabonomic Studies on the Anti-Depressant Effect of Genipin in the Chronic Unpredictable Mild Stress Rat Model. PLoS One. 2013;8(9):e75721. doi: 10.1371/journal.pone.0075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeroff C.B. Recent advances in the neurobiology of depression. Psychopharmacol. Bull. 2002;36(Suppl. 2):6–23. [PubMed] [Google Scholar]
- 7.Gonul A.S., Akdeniz F., Taneli F., Donat O., Eker C., Vahip S. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255(6):381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 8.Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology, 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Thanacoody H.K., Thomas S.H. Tricyclic antidepressant poisoning : cardiovascular toxicity. Toxicol. Rev. 2005;24(3):205–214. doi: 10.2165/00139709-200524030-00013. [DOI] [PubMed] [Google Scholar]
- 10.Arnold H.J., Gulumian M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984;12(1):35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 11.Zolla C. Traditional medicine in Latin America, with particular reference to Mexico. J. Ethnopharmacol. 1980;2(1):37–51. doi: 10.1016/0378-8741(80)90028-8. [DOI] [PubMed] [Google Scholar]
- 12.Perry T.L., Bratty P.J., Hansen S., Kennedy J., Urquhart N., Dolman C.L. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch. Neurol. 1975;32(2):108–113. doi: 10.1001/archneur.1975.00490440058009. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan P.F., Neale M.C., Kendler K.S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157(10):1552-–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 14.De-Miguel F.F., Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell. Mol. Neurobiol. 2005;25(2):297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 16.Deakin J.F., Graeff F.G. 5-HT and mechanisms of defence. J. Psychopharmacol. (Oxford) 1991;5(4):305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 17.Altar C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999;20(2):59–61. doi: 10.1016/S0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 18.Martinowich K., Manji H., Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 19.Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 20.Shirayama Y., Chen A.C., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siuciak J.A., Lewis D.R., Wiegand S.J., Lindsay R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 22.Monteggia L.M., Luikart B., Barrot M., Theobold D., Malkovska I., Nef S., Parada L.F., Nestler E.J. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Stokes P.E. The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur. Neuropsychopharmacol. 1995;5(Supplement 10):77–82. doi: 10.1016/0924-977X(95)00039-R. [DOI] [PubMed] [Google Scholar]
- 25.Kammerer M., Taylor A., Glover V. The HPA axis and perinatal depression: a hypothesis. Arch. Women Ment. Health. 2006;9(4):187–196. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- 26.Gotlib I.H., Joormann J., Minor K.L., Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 28.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-M. [DOI] [PubMed] [Google Scholar]
- 29.Maes M., Bosmans E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 30.Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 31.Rothermundt M., Arolt V., Peters M., Gutbrodt H., Fenker J., Kersting A., Kirchner H. Inflammatory markers in major depression and melancholia. J. Affect. Disord. 2001;63(1-3):93–102. doi: 10.1016/S0165-0327(00)00157-9. [DOI] [PubMed] [Google Scholar]
- 32.Miller G.E., Stetler C.A., Carney R.M., Freedland K.E., Banks W.A. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol. 2002;90(12):1279–1283. doi: 10.1016/S0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 33.Kop W.J., Gottdiener J.S., Tangen C.M., Fried L.P., McBurnie M.A., Walston J., Newman A., Hirsch C., Tracy R.P. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am. J. Cardiol. 2002;89(4):419–424. doi: 10.1016/S0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 34.Suarez E.C., Krishnan R.R., Lewis J.G. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom. Med. 2003;65(3):362–368. doi: 10.1097/01.PSY.0000035719.79068.2B. [DOI] [PubMed] [Google Scholar]
- 35.Miller G.E., Freedland K.E., Carney R.M., Stetler C.A., Banks W.A. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun. 2003;17(4):276–285. doi: 10.1016/S0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F., Jia Z., Gao P., Kong H., Li X., Lu X., Wu Y., Xu G. Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol. Biosyst. 2010;6(5):852–861. doi: 10.1039/b914751a. [DOI] [PubMed] [Google Scholar]
- 37.Shi B., Tian J., Xiang H., Guo X., Zhang L., Du G., Qin X. A ¹H-NMR plasma metabonomic study of acute and chronic stress models of depression in rats. Behav. Brain Res. 2013;241(0):86–91. doi: 10.1016/j.bbr.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Zheng P., Wang Y., Chen L., Yang D., Meng H., Zhou D., Zhong J., Lei Y., Melgiri N.D., Xie P. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol. Cell. Proteomics. 2013;12(1):207–214. doi: 10.1074/mcp.M112.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng P., Gao H.C., Li Q., Shao W.H., Zhang M.L., Cheng K., Yang Y., Fan S.H., Chen L., Fang L., Xie P. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J. Proteome Res. 2012;11(3):1741–1748. doi: 10.1021/pr2010082. [DOI] [PubMed] [Google Scholar]
- 40.Li Z.Y., Zheng X.Y., Gao X.X., Zhou Y.Z., Sun H.F., Zhang L.Z., Guo X.Q., Du G.H., Qin X.M. Study of plasma metabolic profiling and biomarkers of chronic unpredictable mild stress rats based on gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24(24):3539–3546. doi: 10.1002/rcm.4809. [DOI] [PubMed] [Google Scholar]
- 41.Chen G., Yang D., Yang Y., Li J., Cheng K., Tang G., Zhang R., Zhou J., Li W., Liu Z., Fan S., Xie P. Amino acid metabolic dysfunction revealed in the prefrontal cortex of a rat model of depression. Behav. Brain Res. 2015;278(278C):286–292. doi: 10.1016/j.bbr.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Gao X., Guo B., Yang L., Liu J., Zhang X., Qin X., Du G. Selection and dynamic metabolic response of rat biomarkers by metabonomics and multivariate statistical analysis combined with GC-MS. Pharmacol. Biochem. Behav. 2014;117:85–91. doi: 10.1016/j.pbb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Glowinski J., Axelrod J. Inhibition of uptake of tritiated-noradrenaline in the intact rat brain by imipramine and structurally related compounds. Nature. 1964;204(4965):1318–1319. doi: 10.1038/2041318a0. [DOI] [PubMed] [Google Scholar]
- 44.Carlsson A., Corrodi H., Fuxe K., Hökfelt T. Effect of antidepressant drugs on the depletion of intraneuronal brain 5-hydroxytryptamine stores caused by 4-methyl-α-ethyl-meta-tyramine. Eur. J. Pharmacol. 1969;5(4):357–366. doi: 10.1016/0014-2999(69)90113-7. [DOI] [PubMed] [Google Scholar]
- 45.Ögren S.O., Fuxe K., Agnati L.F., Gustafsson J.A., Jonsson G., Holm A.C. Reevaluation of the indoleamine hypothesis of depression. Evidence for a reduction of functional activity of central 5-HT systems by antidepressant drugs. J. Neural Transm. 1979;46(2):85–103. doi: 10.1007/BF01250331. [DOI] [PubMed] [Google Scholar]
- 46.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8(6-7):523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Papakostas G.I., Thase M.E., Fava M., Nelson J.C., Shelton R.C. 2007.
- 48.Mamedov N. Adaptogenic, geriatric, stimulant and antidepressant plants of Russian Far East. J. Cell Mol. Biol. 2005;4:71–75. [Google Scholar]
- 49.Wang Y., Han T., Zhu Y., Zheng C.J., Ming Q.L., Rahman K., Qin L.P. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J. Nat. Med. 2010;64(1):24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 50.Chatterjee S.S., Bhattacharya S.K., Wonnemann M., Singer A., Müller W.E. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998;63(6):499–510. doi: 10.1016/S0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 51.Mora S., Millán R., Lungenstrass H., Díaz-Véliz G., Morán J.A., Herrera-Ruiz M., Tortoriello J. 2006. [DOI] [PubMed]
- 52.Zhao Z., Wang W., Guo H., Zhou D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res. 2008;194(1):108–113. doi: 10.1016/j.bbr.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 53.Linde K., Berner M.M., Kriston L. St John’s wort for major depression. Cochrane Database Syst. Rev. 2008;4(4):CD000448. doi: 10.1002/14651858.cd000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahimi R., Nikfar S., Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(1):118–127. doi: 10.1016/j.pnpbp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Butterweck V., Schmidt M. St. John’s wort: role of active compounds for its mechanism of action and efficacy. Wien. Med. Wochenschr. 2007;157(13-14):356–361. doi: 10.1007/s10354-007-0440-8. [DOI] [PubMed] [Google Scholar]
- 56.Butterweck V. Mechanism of action of St John’s wort in depression : what is known? CNS Drugs. 2003;17(8):539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- 57.Sarris J., Kavanagh D.J. Kava and St. John’s Wort: current evidence for use in mood and anxiety disorders. J. Altern. Complement. Med. 2009;15(8):827–836. doi: 10.1089/acm.2009.0066. [DOI] [PubMed] [Google Scholar]
- 58.Gaster B., Holroyd J. St John’s wort for depression: a systematic review. Arch. Intern. Med. 2000;160(2):152–156. doi: 10.1001/archinte.160.2.152. [DOI] [PubMed] [Google Scholar]
- 59.Su Z.H., Li S.Q., Zou G.A., Yu C.Y., Sun Y.G., Zhang H.W., Gu Y., Zou Z.M. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats. 2011. [DOI] [PubMed]
- 60.Chen J.X., Li W., Zhao X., Yang J.X. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell. Mol. Neurobiol. 2008;28(5):745–755. doi: 10.1007/s10571-007-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai Y., Li Z., Xue L., Dou C., Zhou Y., Zhang L., Qin X. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 2010;128(2):482–489. doi: 10.1016/j.jep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Gao X.X., Zheng X.Y., Li Z.Y., Sun H., Zhang L., Guo X., Du G., Qin X. Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. J. Ethnopharmacol. 2011;137(1):690–699. doi: 10.1016/j.jep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 63.Liu X.J., Zhou Y.Z., Li Z.F., Cui J., Li Z.Y., Gao X.X., Sun H.F., Zhang L.Z., Du G.H., Qin X.M. Anti-depressant effects of Xiaoyaosan on rat model of chronic unpredictable mild stress: a plasma metabonomics study based on NMR spectroscopy. J. Pharm. Pharmacol. 2012;64(4):578–588. doi: 10.1111/j.2042-7158.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 64.Tian J.S., Peng G.J., Gao X.X., Zhou Y.Z., Xing J., Qin X.M., Du G.H. Dynamic analysis of the endogenous metabolites in depressed patients treated with TCM formula Xiaoyaosan using urinary (1)H NMR-based metabolomics. J. Ethnopharmacol. 2014;158(Pt A):1–10. doi: 10.1016/j.jep.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Kim S.H., Han J., Seog D.H., Chung J.Y., Kim N., Hong Park Y., Lee S.K. Antidepressant effect of Chaihu-Shugan-San extract and its constituents in rat models of depression. Life Sci. 2005;76(11):1297–1306. doi: 10.1016/j.lfs.2004.10.022. [DOI] [PubMed] [Google Scholar]


