Abstract
I am honored and humbled to be one of the awardees of the 2014 A. Champalimaud Vision Award. I offer my heartfelt thanks to the Champalimaud Foundation President, Leonor Beleza, and to the Award Committee Members for this wonderful recognition.
I feel especially fortunate to have had the opportunity to witness my scientific discoveries move from the bench to the clinic. Scientific discovery is hugely exciting, but the ability to translate that work into potentially helping someone lead a better life is even more fulfilling. This Award is dedicated to the patients.
Keywords: VEGF, angiogenesis, tyrosine kinase, age-related macular degeneration, antibody
Introduction
The existence of factors capable of inducing growth of cells and tissues was hypothesized already at the beginning of the last century. In 1913, Carrel1 described the ability of tissues extracts to stimulate cell proliferation in cultured tissue explants. Early investigators also speculated that biochemical mediators are responsible for the growth of blood vessels associated with tumorigenesis and other pathological conditions (reviewed previously2). In 1939, the observation that tumors transplanted in transparent chambers inserted in the rabbit ear induce rapid and extensive neovascular growth, led Ide et al.3 to postulate the existence of a tumor-derived “blood vessel growth stimulating factor.” In 1945, Algire et al.4 announced the seminal hypothesis that “the rapid growth of tumor transplants is dependent upon the development of a rich vascular supply.” In the late 1940s and in the 1950s, other investigators postulated the existence of a diffusible angiogenic factor (“Factor X”), produced in the ischemic retina.5–7 This hypothetical molecule was thought to be responsible for neovascularization associated with diabetic retinopathy and other retinal disorders.5–7 Notwithstanding these seminal studies, very little progress was possible at that time, given the daunting challenge of isolating growth factors, which typically are active at very low concentrations. Beginning in the 1970s, the availability of powerful protein purification techniques, combined with the development of cDNA cloning methodologies, enabled major advances. The greatest challenge at that time was purifying the proteins to homogeneity to obtain a partial amino acid sequence, which could be used to design probes suitable for cDNA cloning, thus, dramatically expanding the possibilities of investigating the molecules of interest.2
In 1971, Folkman8 published an elegant synthesis of the aforementioned early studies and hypotheses, and also proposed that antiangiogenesis could be a novel strategy to inhibit tumor growth. This key hypothesis stimulated the search for regulators of angiogenesis. By the mid 1980s, several proangiogenic molecules had been identified and characterized, including epidermal growth factor (EGF), tumor growth factor (TGF)–α, TGF-β, a-fibroblast growth factor (aFGF), bFGF, and angiogenin (reviewed previously2,9). However, while these factors were able to promote angiogenesis in various bioassays, their role as endogenous mediators of angiogenesis remained uncertain, suggesting that in all likelihood some key molecules remained to be discovered (reviewed previously2,10).
The Discovery of Vascular Endothelial Growth Factor (VEGF)
Independent efforts contributed to the discovery of VEGF. In 1983, Senger et al.11 at Beth Israel Hospital (Boston, MA) reported an initial biochemical characterization of vascular permeability factor (VPF), a permeability-enhancing protein identified in the conditioned media of a guinea pig tumor cell line. However, the lack of amino acid sequence data precluded molecular cloning and establishing whether VPF was distinct from the known mediators of vascular permeability or from other proteins. Therefore, it is not surprising that limited progress in elucidating the significance and function of VPF took place during the next several years.12 Senger et al. reported the full purification of guinea pig VPF in 1990.13
In 1989, we reported the isolation and cloning of a heparin-binding endothelial cell mitogen.14,15 This project began while I was a postdoctoral fellow at the University of California, San Francisco (UCSF) in the mid 1980s. At that time, I was able to isolate and culture a population of nonhormone-secreting cells from bovine pituitary, termed “follicular” or “folliculo-stellate” cells.16 Earlier investigators noted that they establish intimate contacts with the pituitary perivascular spaces, suggesting a role in the development or maintenance of the pituitary vasculature.17 In the course of these studies, I discovered that follicular cells release in their culture supernatants an endothelial cell mitogen. In 1988, I joined Genentech, where I had the opportunity to pursue the isolation of this mitogen. By early 1989, we were able to determine the amino terminal amino acid sequence of the purified protein. We found that this sequence was unique, since it had no match with known sequences in available databases.14,15 Because this molecule appeared to have growth-promoting activity selectively for vascular endothelial cells, we proposed the name “vascular endothelial growth factor” (VEGF). We then isolated bovine and human clones encoding multiple molecular species (isoforms) of VEGF, due to alternative mRNA splicing.15 In this early study, we identified three VEGF isoforms: VEGF121, VEGF165, and VEGF189. Subsequent studies revealed the existence of additional VEGF isoforms (reviewed previously18).
After our cloning paper was accepted for publication,15 we learned that a group at the Monsanto Company had submitted at approximately the same time a manuscript reporting on the cloning of VPF.19 These investigators described a human clone that encoded a protein identical to VEGF189.19 This group followed up on the earlier work by Senger et al.11 and was able to isolate and sequence VPF. Therefore, it appeared that the same molecule possesses mitogenic and permeability-enhancing activities.
VEGF as a Key Regulator of Normal and Tumor Angiogenesis
The cloning of VEGF (today also known as VEGF-A following the discovery of several related molecules, VEGF-B, VEGF-C, VEGF-D, and PlGF) generated significant interest in the angiogenesis field,2 but it took several years before we could establish that VEGF was truly a pathophysiologically relevant mediator. It became clear that the VEGF isoforms are well suited to generate biochemical gradients, a requirement for angiogenesis in vivo, due to their differential diffusibility, which depends on their affinity for heparan-sulfate proteoglycans.20,21 A key question was whether VEGF has a role as an angiogenic factor in vivo. The earliest evidence that VEGF expression is temporally and spatially correlated with neovascularization was from a study published in 1990, where we examined the expression of VEGF mRNA in the rat ovary by in situ hybridization.22 We reported that the expression was low in the avascular granulosa cells, but was strongly upregulated in the highly vascularized corpus luteum.22 Furthermore, in 1992 we reported that the high affinity binding sites for VEGF are selectively expressed in endothelial cells in vivo.23
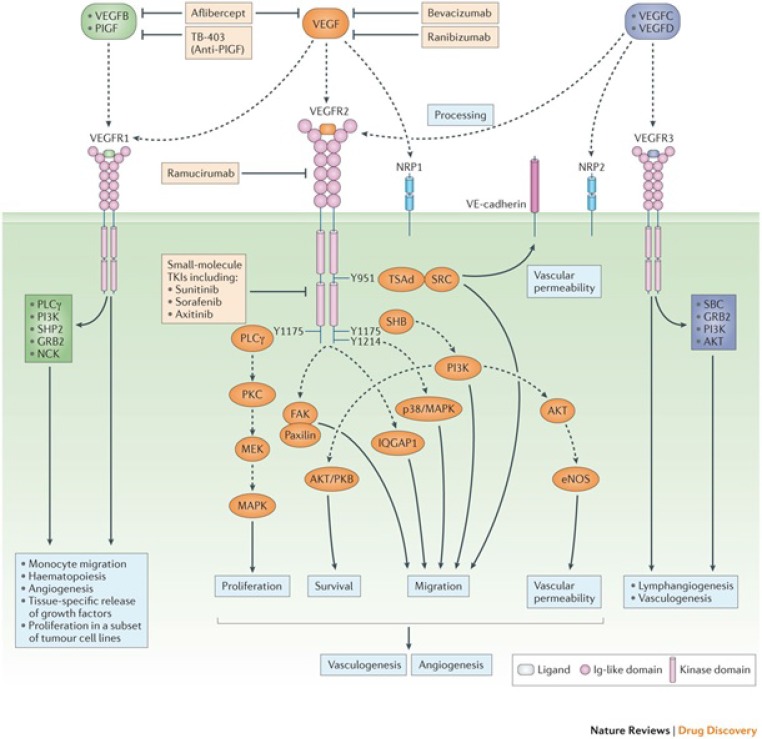
The identification of the VEGF tyrosine kinase receptors represented a major milestone in the quest to understand VEGF action. In 1992, in collaboration with Lewis (Rusty) Williams at UCSF, we identified the fms-like tyrosine kinase24 (presently known as VEGFR-1) as a high-affinity VEGF receptor.25 In the same year, Terman et al.26 identified a highly homologous tyrosine kinase receptor, known as KDR or VEGFR-2. It now is well established that VEGFR-2 is the main signaling VEGF receptor.27 Figure 1 illustrates the current view of the roles of the VEGF receptors and signaling pathways.
Figure 1.
The VEGF signaling pathways and inhibitors. The two VEGF tyrosine kinase receptors are expressed primarily in endothelial cells. The VEGF-related molecules PlGF and VEGFB bind selectively to VEGFR-1, while VEGF binds VEGFR-1 and VEGFR-2. VEGFC and VEGFD bind to VEGF3, a key regulator of lymphangiogenesis, but following proteolytic processing also have the ability to bind and activate VEGFR-2.56 Heparin binding VEGF isoforms and PlGF also bind the coreceptor neuropilin (NRP)1.67 NRP1 increase the binding affinity to VEGFR-2.68 Direct effects of VEGF or PlGF on NRP1, independent of VEGF receptor activation, also have been reported.68 In some circumstances, VEGFR-1 may function as a “decoy” receptor, preventing VEGF from binding to VEGFR-2. However, VEGFR-1 is able to regulate the expression of a variety of genes in the endothelium, including MMP9 and certain growth factors, such as hepatocyte growth factor and connective tissue growth factor, which are known to have an important role in tissue homeostasis and regeneration.69 Also, VEGFR-1 is expressed in monocytes and macrophages and, in some cases, also in tumor cells where in it can mediate tumor proliferation in response to VEGF or PlGF.70 VEGFR-2 is the main signaling receptor that mediates endothelial cell mitogenesis and vascular permeability.56 Multiple inhibitors can block VEGF-induced signaling. Bevacizumab and ranibizumab bind VEGF. The soluble chimeric receptor aflibercept binds VEGF, PlGF, and VEGFB. The anti-VEGFR-2 monoclonal antibody ramucirumab prevents VEGFR-2 dependent signaling. Also, numerous small molecule kinase inhibitors (e.g., sunitinib, sorafenib, and axitinib) inhibit VEGFR signaling (reproduced from the study of Ferrara and Adamis56).
To elucidate the role of VEGF in vivo, we employed multiple strategies to inhibit its function. In 1993, we reported that administration of an anti-VEGF monoclonal antibody substantially reduced the growth of several human tumor cell lines implanted in immunodeficient mice.28 These findings were unexpected at that time, as it was widely believed that tumor angiogenesis is multifactorial and, therefore, reflects the contribution of numerous mediators. They paved the way for subsequent clinical development of VEGF inhibitors as cancer therapeutics, including a humanized variant of this anti-VEGF antibody29 (bevacizumab), which has been approved for therapy of multiple tumor types.30 The impact of these findings, however, went beyond the tumor angiogenesis field. They rapidly stimulated studies aimed at directly probing the role of VEGF in other biological contexts, including intraocular neovascularization.
Inactivation of the vegf gene in mice provided evidence for the crucial role of this molecule in the early development of the vasculature. These findings, reported in 1996 by our group31 and by Carmeliet et al.,32 were nothing short of striking. Loss of even a single vegf allele resulted in defective vascular development and early embryonic lethality. This phenotype was even more dramatic than that of VEGFR2 knockout, which requires inactivation of both alleles to elicit a lethal phenotype.33 A few years later, we developed VEGF loxP mice.34 Crossing these mice with various Cre-transgenic lines enables conditional VEGF inactivation in specific cell types or tissues.34 These studies reinforced the notion that VEGF is required for angiogenesis in many tissues and organs (reviewed previously27). In parallel with the genetic reagents, we developed soluble chimeric VEGF receptors (or “VEGF-traps”), which, unlike many monoclonal antibodies, can block VEGF across species.35 Also, structure–function studies of VEGFR-1 led to the discovery that of the seven extracellular Ig-like domains, domain two is the critical element for high-affinity VEGF binding,36 enabling the design of smaller and more stable soluble receptors. The availability of these tools allowed us to establish the role of VEGF in neovascularization associated not only with such essential physiological processes as organ and skeletal growth34,37 or cyclical growth of the ovarian corpus luteum,38 but also with pathological retinal neovascularization.39
Looking back at that period, it is almost impossible not to recall a sense of excitement permeating through the angiogenesis field. After decades of largely descriptive work, it finally was possible to unravel some of the secrets of this process and provide a molecular explanation for a variety of fundamental pathophysiological processes. A commentary by Klagsbrun and Soker,40 published in 1993, reflects this excitement. According to the authors, “…VEGF/VPF may be the best candidate for the principle regulator of normal and tumor angiogenesis.”40 I feel extremely fortunate that my lab was at the forefront of this revolution.
VEGF as a Mediator of Intraocular Neovascularization
As pointed out above, by the early 1990s it was apparent that VEGF was implicated in normal as well as in pathologic angiogenesis. Vascular endothelial growth factor also had several features consistent with “Factor X,”5 being diffusible and selective for vascular endothelial cells. Also, in 1992 two studies reported that VEGF mRNA expression is induced by hypoxia.41,42 Therefore, it is not surprising that VEGF became the top candidate as a mediator of retinal ischemia-related neovascularization. In 1994, in a collaborative study with Lloyd Aiello and George King at the Joslin Diabetes Center in Boston, we tested this hypothesis. Taking advantage of sensitive assays newly developed in our group, we measured the VEGF levels in the eye fluids from 164 patients.43 We found a striking correlation between VEGF concentrations and active proliferative retinopathy associated with diabetes, occlusion of central retinal vein, or prematurity.43 Adamis et al.44 at the Massachusetts Eye & Ear Infirmary in Boston also reported elevated VEGF levels in the vitreous of patients with diabetic retinopathy.44 At approximately the same time, a French group also reported similar findings.45
Subsequent studies revealed that VEGF upregulation in the eye is not limited to ischemic retinal disorders. In 1996, two groups reported the immunohistochemical localization of VEGF in choroidal neovascular membranes from patients with wet age-related macular degeneration (AMD), the leading cause of irreversible severe vision loss in the adult population.46,47
Proof-of-concept studies supported the hypothesis that VEGF is, indeed, a major mediator of intraocular neovascularization. As already mentioned, administration of chimeric soluble VEGF receptors resulted in a marked reduction of retinal neovascularization in a mouse model of retinopathy of prematurity.39 Also, in collaboration with Tony Adamis and Joan Miller, we tested the effects of the anti-VEGF monoclonal antibody used in the cancer studies28 in a primate model of iris neovascularization induced by central retinal vein occlusion.48 Similar to the tumor models, we observed a substantial inhibition of blood vessel growth following administration of the antibody.48 These effects were not limited to models of retinal ischemia. As described in the next section, wet (neovascular) AMD became the primary clinical target of our anti-VEGF efforts in the eye.49 To this end, we engineered an affinity-matured antibody fragment (Fab) derived from the murine antibody parent of bevacizumab.50,51 Krzystolik et al.52 kindly agreed to support these efforts by testing this Fab, subsequently known as ranibizumab, in a primate model of choroidal neovascularization.52 These studies showed a dramatic inhibition of neovascularization and leakage following intravitreal administration of ranibizumab.52
An Anti-VEGF Therapy for the Eye
The development path of anti-VEGF agents for wet AMD and other intraocular neovascular disorders has been described previously.51 Briefly, developing an anti-VEGF therapy for wet AMD presented at that time a number of significant challenges.51 We initially considered testing the intravenous administration of bevacizumab, but the possibility of cardiovascular adverse events in elderly patients led us to discard this possibility in favor of the intraocular route of administration. However, one could not rule out that long-term injection of full-length antibodies in human eyes might result in complement-mediated or cell-dependent cytotoxicity that might be triggered by interaction of the antibody Fc portion with receptors in inflammatory or immune cells.53 We felt that removing the Fc would be prudent. As already noted, we created an affinity-matured Fab variant of bevacizumab to further enhance its binding affinity.50 Genentech initiated the first clinical trial in subjects with wet AMD in February 2000. After encouraging data from phase I and phase II studies,54,55 ranibizumab was tested in pivotal phase III trials. Examining in detail the phase III studies with ranibizumab and other VEGF inhibitors (bevacizumab and aflibercept) is beyond the scope of this article, which mainly focuses on the discovery and science of VEGF. A very recent review summarizes such clinical trials and discusses a decade of clinical experience with VEGF inhibitors.56 Suffice to say here that these agents have had a dramatic impact in ophthalmology. Patients with different variants of wet AMD receiving monthly intravitreal injections of ranibizumab experienced significantly improved visual acuity compared to sham-injected57 or verteporfin-treated58 patients. In addition, near vision, reading speed, and overall quality of life, were improved.59 Subsequent large randomized clinical trials have demonstrated the efficacy of ranibizumab and other VEGF inhibitors in several other vision-threatening diseases, including diabetic macular edema and retinal vein occlusion.56
A few years ago, Bressler et al.60 modeled visual acuity outcomes in patients with wet AMD in the United States population based on data from the ranibizumab phase III trials. Their analysis indicated that ranibizumab has the potential to reduce the rate of legal blindness from neovascular AMD over two years by 72%.60 In good agreement with these predictions, recent studies have documented a marked reduction in the incidence rate of legal blindness due to AMD in some countries following the introduction of intravitreal VEGF inhibitors in 2006.61,62 However, not all patients receive adequate treatment to experience maximal visual improvement. A recent multicountry, retrospective study of wet AMD patients treated with ranibizumab indicated that, especially in some countries, patients receive fewer injections and have poorer outcomes than those reported in clinical trials.63 Therefore, the cost and burden of chronic therapy in some cases limits benefit of anti-VEGF treatment. It is hoped that long-acting delivery technologies will address the gap in visual outcomes between clinical trials and “real life” clinical practice.56
Conclusions
I am gratified and humbled that work that I initiated almost 30 years ago during my years as a postdoctoral fellow eventually resulted in a therapy for wet AMD and other intraocular neovascular disorders. The magnitude of the benefit, particularly the visual acuity gains, vastly exceeded my expectations, considering that previous treatments only slowed down the rate of vision loss.64,65
Numerous trials currently are exploring a variety of novel therapeutic agents.66 These efforts give hope that combining VEGF inhibitors with agents that target additional pathways may go beyond the benefits achieved so far from targeting VEGF alone.
Acknowledgments
This manuscript is based in part on review articles cited in the text.2,12,49,56
Disclosure: N. Ferrara, None
References
- 1. Carrel A. Artificial activation of the growth in vitro of connective tissue. J Exp Med. 1913; 17: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrara N. VEGF, and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002; 2: 795–803. [DOI] [PubMed] [Google Scholar]
- 3. Ide AG,, Baker NH,, Warren SL. Vascularization of the Brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am J Roentgenol. 1939; 42: 891–899. [Google Scholar]
- 4. Algire GH,, Chalkley HW,, Legallais FY,, Park HD. Vascular reactions of normal and malignant tissues in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst. 1945; 6: 73–85. [Google Scholar]
- 5. Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc UK. 1948; 68: 137–180. [Google Scholar]
- 6. Ashton N. Observations on the choroidal circulation. Br J Ophthalmol. 1952; 36: 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wise GN. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956; 54: 729–826. [PMC free article] [PubMed] [Google Scholar]
- 8. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 9. Folkman J,, Klagsbrun M. Angiogenic factors. Science. 1987; 235: 442–447. [DOI] [PubMed] [Google Scholar]
- 10. Klagsbrun M,, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991; 53: 217–239. [DOI] [PubMed] [Google Scholar]
- 11. Senger DR,, Galli SJ,, Dvorak AM,, Perruzzi CA,, Harvey VS,, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219: 983–985. [DOI] [PubMed] [Google Scholar]
- 12. Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009; 29: 789–791. [DOI] [PubMed] [Google Scholar]
- 13. Senger DR,, Connolly DT,, Van de Water L,, Feder J,, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990; 50: 1774–1778. [PubMed] [Google Scholar]
- 14. Ferrara N,, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989; 161: 851–858. [DOI] [PubMed] [Google Scholar]
- 15. Leung DW,, Cachianes G,, Kuang WJ,, Goeddel DV,, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 16. Ferrara N,, Fujii DK,, Goldsmith PC,, Widdicombe JH,, Weiner RI. Transport epithelial characteristics of cultured bovine pituitary follicular cells. Am J Physiol. 1987; 252: E304–E312. [DOI] [PubMed] [Google Scholar]
- 17. Vila-Porcile E. [The network of the folliculo-stellate cells and the follicles of the adenohypophysis in the rat (pars distalis)]. Z Zellforsch Mikrosk Anat. 1972; 129: 328–369. [PubMed] [Google Scholar]
- 18. Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010; 21: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keck PJ,, Hauser SD,, Krivi G,, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989; 246: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 20. Houck KA,, Leung DW,, Rowland AM,, Winer J,, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992; 267: 26031–26037. [PubMed] [Google Scholar]
- 21. Park JE,, Keller G-A,, Ferrara N. The vascular endothelial growth factor isoforms (VEGF): Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993; 4: 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips HS,, Hains J,, Leung DW,, Ferrara N. Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology. 1990; 127: 965–967. [DOI] [PubMed] [Google Scholar]
- 23. Jakeman LB,, Winer J,, Bennett GL,, Altar CA,, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992; 89: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shibuya M,, Yamaguchi S,, Yamane A,, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase (flt) closely related to the fms family. Oncogene. 1990; 8: 519–527. [PubMed] [Google Scholar]
- 25. de Vries C,, Escobedo JA,, Ueno H,, Houck K,, Ferrara N,, Williams LT. The fms-like tyrosine kinase a receptor for vascular endothelial growth factor. Science. 1992; 255: 989–991. [DOI] [PubMed] [Google Scholar]
- 26. Terman BI,, Dougher-Vermazen M,, Carrion ME,, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992; 187: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 27. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25: 581–611. [DOI] [PubMed] [Google Scholar]
- 28. Kim KJ,, Li B,, Winer J,, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993; 362: 841–844. [DOI] [PubMed] [Google Scholar]
- 29. Presta LG,, Chen H,, O'Connor SJ,, et al. Humanization of an anti-VEGF monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997; 57: 4593–4599. [PubMed] [Google Scholar]
- 30. Ferrara N,, Hillan KJ,, Gerber HP,, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 31. Ferrara N,, Carver-Moore K,, Chen H,, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996; 380: 439–442. [DOI] [PubMed] [Google Scholar]
- 32. Carmeliet P,, Ferreira V,, Breier G,, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996; 380: 435–439. [DOI] [PubMed] [Google Scholar]
- 33. Shalaby F,, Rossant J,, Yamaguchi TP,, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995; 376: 62–66. [DOI] [PubMed] [Google Scholar]
- 34. Gerber HP,, Hillan KJ,, Ryan AM,, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999; 126: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 35. Park JE,, Chen HH,, Winer J,, Houck KA,, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994; 269: 25646–25654. [PubMed] [Google Scholar]
- 36. Davis-Smyth T,, Chen H,, Park J,, Presta LG,, Ferrara N. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J. 1996; 15: 4919–4927. [PMC free article] [PubMed] [Google Scholar]
- 37. Gerber HP,, Vu TH,, Ryan AM,, Kowalski J,, Werb Z,, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Med. 1999; 5: 623–628. [DOI] [PubMed] [Google Scholar]
- 38. Ferrara N,, Chen H,, Davis-Smyth T,, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med. 1998; 4: 336–340. [DOI] [PubMed] [Google Scholar]
- 39. Aiello LP,, Pierce EA,, Foley ED,, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995; 92: 10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klagsbrun M,, Soker S. VEGF/VPF: the angiogenesis factor found? Curr Biol. 1993; 3: 699–702. [DOI] [PubMed] [Google Scholar]
- 41. Shweiki D,, Itin A,, Soffer D,, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359: 843–845. [DOI] [PubMed] [Google Scholar]
- 42. Plate KH,, Breier G,, Weich HA,, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992; 359: 845–848. [DOI] [PubMed] [Google Scholar]
- 43. Aiello LP,, Avery RL,, Arrigg PG,, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. [see comments]. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 44. Adamis AP,, Miller JW,, Bernal MT,, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118: 445–450. [DOI] [PubMed] [Google Scholar]
- 45. Malecaze F,, Clemens S,, Simorer-Pinotel V,, et al. Detection of vascular endothelial growth factor mRNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994; 112: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 46. Lopez PF,, Sippy BD,, Lambert HM,, Thach AB,, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996; 37: 855–868. [PubMed] [Google Scholar]
- 47. Kvanta A,, Algvere PV,, Berglin L,, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37: 1929–1934. [PubMed] [Google Scholar]
- 48. Adamis AP,, Shima DT,, Tolentino MJ,, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996; 114: 66–71. [DOI] [PubMed] [Google Scholar]
- 49. Ferrara N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med. 2010; 16: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 50. Chen Y,, Wiesmann C,, Fuh G,, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999; 293: 865–881. [DOI] [PubMed] [Google Scholar]
- 51. Ferrara N,, Damico L,, Shams N,, Lowman H,, Kim R. Development of Ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26: 859–870. [DOI] [PubMed] [Google Scholar]
- 52. Krzystolik MG,, Afshari MA,, Adamis AP,, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002; 120: 338–346. [DOI] [PubMed] [Google Scholar]
- 53. Raghavan M,, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996; 12: 181–220. [DOI] [PubMed] [Google Scholar]
- 54. Rosenfeld PJ,, Schwartz SD,, Blumenkranz MS,, et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005; 112: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 55. Heier JS,, Antoszyk AN,, Pavan PR,, et al. Ranibizumab for treatment of neovascular AMD. A phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006. 113: 633–642. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N,, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy.Nat Rev Drug Discov.Published online ahead of print 18 January, 2016, doi:10.1038/nrd.2015.17. [DOI] [PubMed]
- 57. Rosenfeld PJ,, Brown DM,, Heier JS,, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- 58. Brown DM,, Kaiser PK,, Michels M,, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- 59. Frennesson C,, Nilsson UL,, Peebo BB,, Nilsson SE. Significant improvements in near vision, reading speed, central visual field and related quality of life after ranibizumab treatment of wet age-related macular degeneration. Acta Ophthalmol. 2010; 88: 420–425. [DOI] [PubMed] [Google Scholar]
- 60. Bressler NM,, Doan QV,, Varma R,, et al. Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularization: non-Hispanic white population in the United States with age-related macular degeneration. Arch Ophthalmol. 2011; 129: 709–717. [DOI] [PubMed] [Google Scholar]
- 61. Bloch SB,, Larsen M,, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012; 153: 209–213. [DOI] [PubMed] [Google Scholar]
- 62. Borooah S,, Jeganathan VS,, Ambrecht AM,, et al. Long-term visual outcomes of intravitreal ranibizumab treatment for wet age-related macular degeneration and effect on blindness rates in south-east Scotland. Eye (Lond). 2015; 29: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holz FG,, Tadayoni R,, Beatty S,, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015; 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Treatment of Age Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol. 1999; 117: 1329–1345. [PubMed] [Google Scholar]
- 65. Gragoudas ES,, Adamis AP,, Cunningham ET,, Jr, Feinsod M,, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351: 2805–2816. [DOI] [PubMed] [Google Scholar]
- 66. Pecen PE,, Kaiser PK. Current phase 1/2 research for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2015; 26: 188–193. [DOI] [PubMed] [Google Scholar]
- 67. Soker S,, Takashima S,, Miao HQ,, Neufeld G,, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998; 92: 735–745. [DOI] [PubMed] [Google Scholar]
- 68. Olsson AK,, Dimberg A,, Kreuger J,, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006; 7: 359–371. [DOI] [PubMed] [Google Scholar]
- 69. LeCouter J,, Moritz DR,, Li B,, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003; 299: 890–893. [DOI] [PubMed] [Google Scholar]
- 70. Yao J,, Wu X,, Zhuang G,, et al. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc Natl Acad Sci U S A. 2011; 108: 11590–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]