Abstract
Dilated cardiomyopathy is a major cause of heart failure that affects millions. Corin cleaves and biologically activates pro-atrial natriuretic peptide (pro-ANP) and pro-B-type natriuretic peptide (pro-BNP). High corin levels reduce the development of systolic dysfunction and heart failure in experimental dilated cardiomyopathy. Yet, patients with significant heart failure unexpectedly show low corin levels with high plasma ANP/BNP levels. Therefore, we examined the relationship between cardiac corin expression, ANP/BNP levels and the stages of heart failure. We used a well-established, dilated cardiomyopathy model to evaluate gene and protein expression as mice longitudinally developed Stages A-D heart failure. Cardiac systolic function (ejection fraction) continuously declined over time (P<0.001). Cardiac corin transcripts were decreased at early Stage B heart failure and remained low through Stages C-D (P<0.001). Cardiac corin levels were positively correlated with systolic function (r=0.96, P=0.003) and inversely with lung water (r=-0.92, P=0.001). In contrast, cardiac pro-ANP/BNP transcripts increased later (Stage C-D) and plasma levels rose only with terminal heart failure (Stage D, P<0.001). Immunoreactive plasma ANP and BNP levels were positively associated with plasma cyclic guanosine monophosphate (cGMP) levels (r=0.82, P=0.01 & r=0.8, P=0.02 respectively). In experimental dilated cardiomyopathy, corin levels declined early with progressive systolic dysfunction before the development of heart failure, while significant increases in plasma ANP, BNP and cGMP levels were found only in later Stage (C, D) heart failure. This dyssynchrony in expression of corin vs. ANP/BNP may impair cleavage-activation of pro-natriuretic peptides and thereby promote the transition from earlier to later stage heart failure.
Keywords: Corin, natriuretic peptides, ANP, BNP, dilated cardiomyopathy, heart failure
Introduction
Dilated cardiomyopathy (DCM) is characterized by progressive enlargement of the heart ventricles and depressed systolic function. DCM is one of the major causes of severe heart failure (HF) worldwide.1 HF is associated with pathologic accumulations of extracellular salt and water leading to lung edema, pleural effusions and other types of edema. Four clinical stages of development of HF have been recognized.1 Stage A occurs in individuals at risk, but without structural heart disease or HF symptoms. Stage B includes evidence of structural heart disease, but no findings of HF. Stage C is associated with HF and Stage D includes severe end-stage HF.1 HF is associated with increased blood levels of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP).2–5
Pro-ANP and pro-BNP are cleaved to their active forms by corin, a cardiac transmembrane serine protease.6 ANP and BNP activate the guanylyl cyclase/natriuretic peptide receptor A (NPRA) signaling cascade to generate cyclic guanosine monophosphate (cGMP).7, 8 In experimental DCM, increased corin expression has protective effects, positively modulating plasma cGMP levels, systolic function, myocardial fibrosis, HF and survival.9 Recent experimental data shows that ANP also prevents HF and the progression of experimental DCM.10 While the components of the corin-NPs-NPRA pathway appear to protect against experimental HF7, recent data suggest that this pathway is dysregulated in overt HF9, 11, 12. Levels of circulating immunoreactive ANP and BNP rise,2, 3, 5 while circulating levels of corin unexpectedly are depressed in patients with severe HF.13–16 In addition, an inverse association between cardiac corin and pro-ANP/pro-BNP transcript levels has been found in severe systolic HF in humans12 and, in experimental mouse models of HF.9, 17
In this study, we examined if dyssynchrony in corin and ANP/BNP expression develops in experimental DCM with advancing systolic dysfunction as animals progress through all four stages of HF development. Our data indicate that there is a an early, progressive decline in corin levels, which is a sensitive marker of early systolic dysfunction, that appears prior to the development of ventricular dilation, increases in NP levels and the onset of HF. While corin levels may prove to be an early biomarker of cardiac dysfunction, this early dyssynchrony in the expression of NP system components provides further evidence of NP system dysregulation, which may affect the progression of cardiomyopathy and the development of HF.
Methods
We analyzed DCM and wild-type control mice of age 4, 7, 13, 20 weeks in vivo and ex vivo. Experimental details are available in the online-only Data Supplement (please see http://hyper.ahajournals.org).
Statistical Analysis
Statistical analyses were performed using two-way ANOVA (unless otherwise indicated). Differences were considered to be significant if the two-tailed p ≤ 0.05. The number of animals (n or N) is indicated in the figure legends. Data are reported as mean ± SE or mean ± SD.
Results
Stages of DCM with progressive declines in systolic function and HF
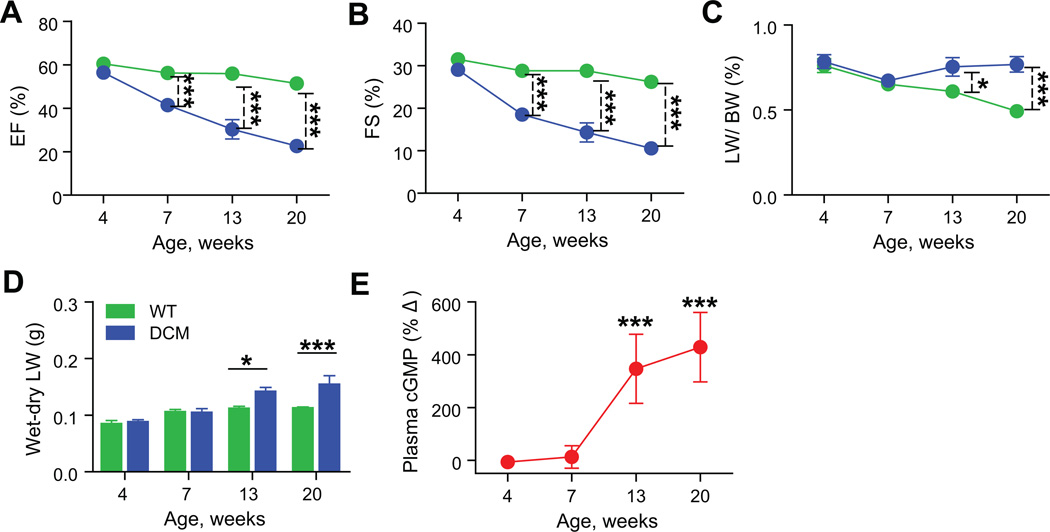
To evaluate longitudinal changes in the NP system, we used a well-characterized, translationally-relevant experimental mouse model of DCM, in which progressive systolic dysfunction leads to HF in the setting of preserved kidney function.9, 10, 18, 19 Ejection fraction (EF%) and lung edema (a criterion of human HF20) were assessed in DCM mice and control wild-type (WT) littermates at 4, 7, 13 and 20 weeks of age. The EF% declined in DCM mice with time (P<0.001; Figure 1A) as did fractional shortening (FS%, P<0.001; Figure 1B). The EF% at 4 weeks was not significantly different between DCM (56±1.3%) and control WT (60±1.8%) mice. Beginning at 7 weeks of age, EF% decreased to 41±0.9% in DCM group vs. 56±1.2% in WT controls (P<0.001). By 20 weeks of age group, EF% had declined from (51±2.2%) to 23±2.6% (P<0.001) and FS% dropped from (26±1.3%) to 11±1.3% (P<0.001), representing nearly a 3- fold decline in systolic function in DCM mice vs. WT mice. This pronounced decline in EF% was consistent with the accelerated mortality of DCM mice vs. control mice (median survival 18 vs. 72 weeks, P<0.0001).
Figure 1. Progressive declines in systolic function and development of lung edema in DCM mice.
(A) Changes in EF% and (B) FS% in mice with time. (C–D) Development of lung edema as assessed by lung to body weight ratio (LW/BW%) and the difference between wet and dry lung weights (n=7–14 per group); WT (green), DCM (blue). (E) Percent change (%Δ) in plasma cGMP levels (determined by ELISA) in DCM mice to WT littermates (red) (n=7–16 per group). Data represent mean±SD. *P<0.05, ***P<0.001.
The development of HF was associated with pleural effusions, increased lung water and histologic evidence of alveolar edema. There was no evidence of HF in DCM mice at 4 and 7 weeks of age, but 13 and 20 weeks DCM mice showed large pleural effusions and lung edema. Consistent with this observation, LW/BW ratios (%) and the wet-dry lung weights in DCM mice were significantly increased at 13 and 20 weeks of age (Figs. 1C & D). Histologic analysis of 20 week DCM mice showed lung congestion, alveolar and intra-alveolar edema (pink area), and accumulation of red blood cells and leakage of fluid into alveoli (solid arrow, Figure S1). Alveolar wall thickness appeared increased (broken arrow) in DCM mice when compared to WT controls (Figure S1). Circulating levels of cGMP are useful biomarkers of HF.21 Plasma cGMP levels were unchanged at 4 and 7 weeks in DCM and WT controls (Figure 1E). However, at 13 weeks and 20 weeks, DCM mice showed significant elevations of cGMP by comparison to WT controls (Figure 1E, P<0.001).
The progressive systolic dysfunction and the development of lung edema observed in these DCM mice correspond roughly to the four stages of HF. Stage A (4 weeks) corresponds to the risk of DCM without a decline in systolic function. Stage B (7 weeks) was associated with development of systolic dysfunction without signs of HF. Stage C (13 weeks) was associated with systolic dysfunction and HF while Stage D (20 weeks) was associated with end stage HF and mortality.
Cardiac corin levels are linked to declining contractile function in DCM mice
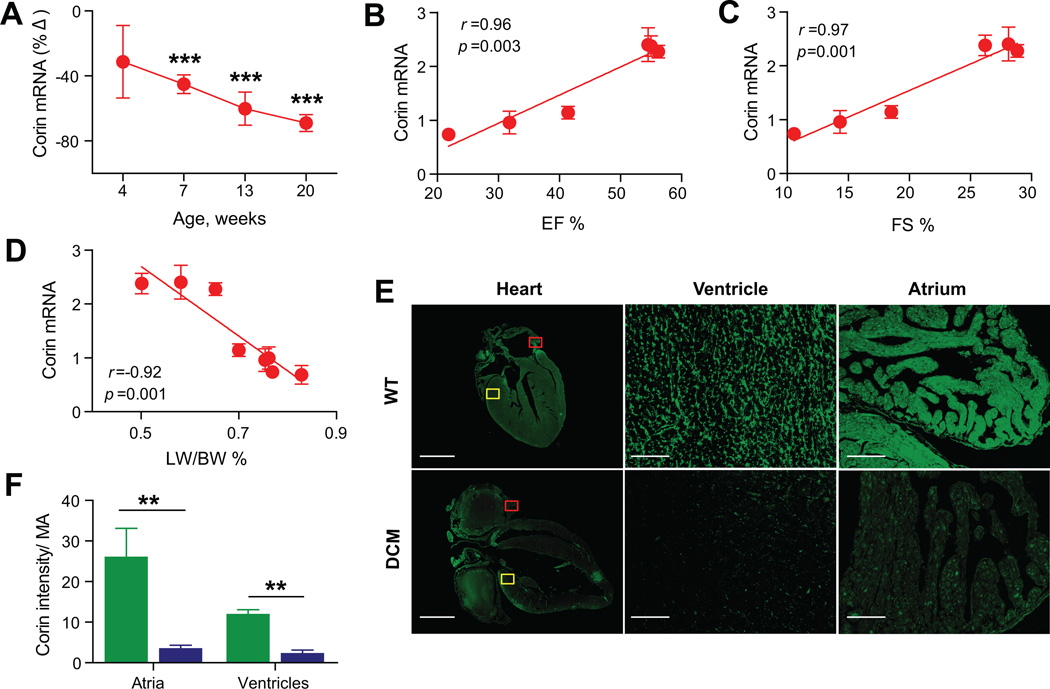
Although corin expression appears to affect the development of HF,9 there is little known about how corin expression changes during the progression of cardiomyopathy. Corin transcripts were significantly decreased from Stage B (7 weeks; −46±6%, P<0.001) to Stage C (13 weeks; −60±10%, P<0.001) to Stage D (20 weeks;-69±5%, P<0.001) (Figure 2A). Corin cardiac transcript levels (Stage B to D) showed a linear association with contractile function as assessed by EF% (r=0.96, P=0.003, Figure 2B) or FS% (r=0.97, P=0.001, Figure 2C). There was also a negative association between corin transcripts and the severity of lung edema assessed by the LW/BW ratio (r=-0.92, P=0.001, Figure 2D). Cardiac corin protein expression was assessed by immunofluorescence staining of paraffin embedded heart sections with anti-corin IgG22 (Figure 2E). Corin protein expression was significantly reduced in the atria and ventricles of DCM mice at Stage D (P<0.01) vs. WT (Figure 2F). Corin plasma levels in DCM were significantly decreased at Stage A (4 weeks, −45±28%, P<0.001) and were consistently lower (-37±20%) than WT mice through all stages of HF (P<0.0001).
Figure 2. Relationship between corin cardiac expression and systolic function.
(A) Percent change (%Δ) of corin cardiac transcript levels in DCM mice to WT littermates determined by qRT-PCR analysis. (B–C–D) Linear regression analysis in DCM and WT mice showed a significant association between EF% (B), FS% (C), LW/BW% (D) and corin cardiac transcripts (arbitrary units). N=7–14 per group. (E) Representative immunofluorescence images (scale bars, 2mm) of 20 week old WT and DCM hearts with magnified views of atrium and ventricle (100 µm) from areas indicated by red and yellow boxes, respectively. (F) Bar graph showing total immunofluorescence intensity/ total myocardium area (Intensity/MA) in WT and DCM mice (n=5 per group). WT (green), DCM (blue). Data represent mean±SE. **P <0.01, ***P <0.001.
Changes in ANP and BNP levels with progressive declines in systolic function and HF
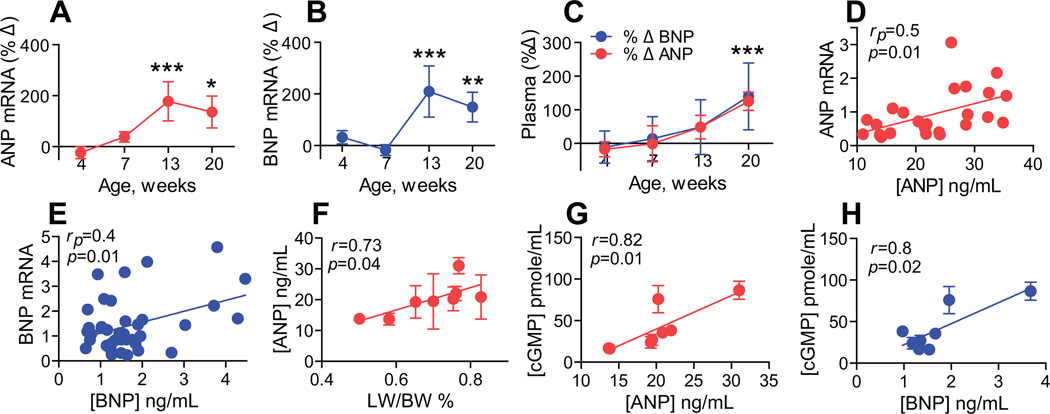
Cardiac pro-ANP and pro-BNP are natural substrates of corin.6 Pro-ANP and pro-BNP cardiac transcript levels were not significantly altered at Stage A (4 weeks) or Stage B (7 weeks) in DCM mice vs. controls (Figs. 3A & B). However, at Stage C (13 weeks) and Stage D (20 weeks), pro-ANP transcript levels were significantly increased by 178±76% (P<0.001) and 136±63% (P<0.05) in DCM mice (Figure 3A). In a similar fashion, pro-BNP transcript levels were increased by 209±99% (P<0.001) at Stage C and 149±58% (P<0.01) at Stage D in DCM mice vs. WT controls.
Figure 3. Changes in ANP, BNP levels during DCM progression.
(A–B) Percent change (%Δ) in cardiac transcript of pro-ANP (A) and pro-BNP (B) in DCM mice to WT littermates determined by qRT-PCR analysis (mean±SE). (C) Percent changes (%Δ) in plasma ANP (red) and BNP (blue), determined by ELISA in DCM mice to WT littermates (mean±SD). (D–E) Pearson correlation between ANP/BNP plasma levels and cardiac pro-ANP or pro-BNP transcript levels (arbitrary units). (F) Linear regression analysis of LW/BW% and ANP plasma levels in all DCM and WT groups. (G–H) Linear regression analysis of cGMP plasma levels and ANP (G) or BNP (H) plasma levels in DCM and WT mouse groups. N=7–16 per group, ***P<0.001, **P<0.01, *P<0.05.
Consistent with transcript levels, ANP and BNP plasma levels were unchanged in DCM and WT controls at Stage A and Stage B but were significantly elevated at Stage D (P<0.001) by comparison to littermate controls (Figure 3C). Plasma ANP levels and cardiac pro-ANP transcripts (rp=0.5, P=0.01, Figure 3D) were positively correlated, as were plasma BNP levels and cardiac pro-BNP transcripts (rp=0.4, P=0.01, Figure 3E). ANP but not BNP plasma levels were directly related to edema as assessed by LW/BW% (r=0.73, P=0.04, Figure 3F). Unlike corin levels, there was not a strong relationship between ANP levels and declining systolic function (r=0.04 P=0.08).
Plasma levels of cGMP in all experimental groups were positively associated with total levels of immunoreactive ANP (r=0.82, P=0.01, Figure 3G) and BNP (r=0.8, P=0.02, Figure 3H). There was a significant negative relationship between corin transcript levels and cGMP levels (r=-0.7, P=0.05).
Discussion
Recent studies show that cardiac corin and ANP are protective against HF development in experimental DCM.9, 10 Thus, it is reasonable to hypothesize that corin and the pro-NPs are coordinately regulated to forestall the development of HF as systolic function declines.7, 11 However, when measured at single time points during overt systolic HF in humans12–15 and in mice,9, 17 cardiac and plasma levels of corin were diminished and ANP/BNP were paradoxically increased. In this study we examined the longitudinal relationship between corin and NP expression as systolic dysfunction progressed in DCM. Corin expression was correlated with systolic function. Corin levels were depressed in Stage B in association with the decline in systolic function, even before the onset of HF or increases in levels of NPs. Corin levels remained depressed in Stage C, D while cGMP and circulating levels of NPs rose only with the development of HF.
This study used a well-characterized mouse model of progressive DCM9, 10, 18, 19 as an experimental tool to examine the longitudinal relationships between changes in contractile function, HF development and expression of corin, pro-ANP and pro-BNP. This DCM model shows significant similarities to the Stages of HF development in humans. At 4 weeks of age, animals are at the risk for developing HF, but systolic function is normal and corin levels are not yet significantly different from age-matched animals; this is compatible with Stage A in humans, who are at risk for HF, but have not yet developed signs or symptoms. DCM mice at 7 weeks show findings similar to Stage B, with objective evidence of a decline in their EF by a 1.5 fold, but without other findings. Animals at 13 weeks develop overt HF, consistent with Stage C. Animals at 20 weeks show Stage D HF with a nearly 3-fold decline in systolic function, limitation of activity and imminent demise.9, 10
Cardiac corin transcript levels were closely linked to contractile function and showed a ~50% decrease at Stage B. There was a further, but less significant (~60–70%) decline in corin levels with Stage C and D. In contrast, transcripts for pro-ANP and pro-BNP showed quite different patterns of expression. Pro-ANP and pro-BNP transcripts were unchanged during Stage A and Stage B HF but rose significantly with the onset of lung edema and the rise in plasma levels of cGMP accompanying Stage C and D HF. Thus, with the onset of DCM, cardiac transcripts for pro-ANP and pro-BNP were modulated in opposite directions from corin cardiac transcripts. Plasma ANP and BNP levels were closely correlated with cardiac transcript levels for pro-ANP and pro-BNP. A pattern of low corin and high ANP, BNP blood levels has been noted at single time points in patients admitted for treatment of acute systolic HF.12, 13, 15 Although coordinate regulation of pro-ANP/pro-BNP and corin genes by GATA-4 and other transcription factors has been predicted for hypertrophic conditions,11, 23 recent studies of systolic and diastolic overload show a divergence in the expression of these genes. 24
Plasma ANP and BNP levels are significantly elevated in human HF and have established roles as biomarkers in the diagnosis and prognosis of HF.2–5 In these longitudinal studies we found that ANP and BNP cardiac and plasma levels did not change with the development of asymptomatic systolic dysfunction (Stage B) but rose significantly with the development of Stage C and D HF, when there was objective evidence of fluid retention and rises in cGMP. In contrast, a significant decline in corin cardiac and plasma levels marked the onset of systolic dysfunction before the development of HF (Stage A and B). Thus, corin levels may permit detection of contractile dysfunction, during early incipient cardiomyopathy when immunoreactive ANP and BNP measurements are not changed. Cardiac corin was positively correlated with physiological markers of cardiomyopathy (impaired contractile or systolic function) and negatively associated with lung edema. However, ANP plasma levels, which reflected cardiac pro-ANP expression, were correlated only with lung edema and not with the cardiac contractile function.
Corin protein expression was significantly reduced in both the atria and ventricles of DCM mice at terminal stage of HF; this finding is consistent with reports of reduced cardiac corin transcript levels in hearts from humans with end-stage HF undergoing transplantation.12 Insufficient cardiac corin proteins levels might be responsible for impaired pro-ANP/pro-BNP cleavage reported at acute decompensated HF with diminished systolic functions in human13, 15, 25 and may in part explain the fact that the highest levels of circulating NPs are found in patients with the most severe HF. Although the present studies were conducted in male DCM mice on C57/B6 background, they are consistent with our previous findings in female DCM mice on a CD1 background, suggesting that the observed effects are not sex or strain-specific. Restoration of cardiac corin levels in DCM mice was shown to increase plasma cGMP levels, improve contractile function, reduce HF and prolong survival.9 Still, it remains unclear how cardiac corin deficiency plays such critical role in HF development. Corin-knockout mice generated by several labs showed a mild hypertensive phenotype, but HF has not been reported in these mice.26 However corin-deficient KitW-sh/W-sh mice developed rapidly progressive cardiac dilation and systolic dysfunction after aortic banding.27
In circulation biologically active ANP and BNP execute their cardiorenal and vascular effects through the cGMP signaling cascade after binding with the receptor NPRA.8, 28 Plasma ANP and BNP levels were significantly elevated at terminal Stage D HF corresponding to 20 weeks. Consistent with clinical reports for overt HF15, 20, 29 cGMP levels were elevated at Stage C and D of HF in DCM. While plasma cGMP levels are positively associated with plasma immunoreactive ANP or BNP, NP system activation appears insufficient to attenuate the decline in systolic function and transition to terminal HF. NP signaling is an important, but not sole determinant of circulating cGMP.29 cGMP is an intracellular second messenger for nitric oxide (NO)30 which is also elevated in chronic human HF.29 It has been suggested that cGMP is produced mainly by NO in humans with chronic HF requiring hospitalization, but as HF improves and the patient nears discharge, NO is primarily produced by ANP and BNP.29
To our knowledge this is the first longitudinal study to demonstrate lack of coordination, or dyssynchrony, in cardiac corin and pro-ANP/pro-BNP expression as systolic function declines and DCM progresses from Stage A to D HF. A failure of coordinated expression of cardiac levels of corin and the pro-NPs may lead to reduced activation of pro-NPs13, 15, 25 and to the apparent functional dysregulation of the NP system in HF.31 This may contribute to the decreased cleavage of pro-NPs reported in human HF13, 15, 25 lending support to the idea that HF is “a corin-deficient state” in which low corin levels may have a causative role.11 Still, the mechanisms responsible for the dyssynchronous regulation of corin and the NPs are unknown. It will be important to determine how dyssynchronous expression of the NP system affects the activity of the renin angiotensin aldosterone system which also modulates HF in DCM.8, 28 Although we and others have found a similar pattern of corin and NPs expression in human Stage D HF, it is necessary to replicate these longitudinal findings in humans and define their relevance to cardiomyopathies caused by ischemia and other etiologies. Nevertheless, these data suggest that corin levels may be valuable as a potential marker of systolic dysfunction, prior to the development of symptomatic HF.
Supplementary Material
Perspectives.
There is a critical need to identify patients at risk who may benefit from early interventions to prevent progressive systolic dysfunction and the development of HF. Corin levels are correlated with systolic function and low corin levels may be an early indicator of systolic dysfunction that precedes increases in ANP and BNP, and the development of overt HF.
Novelty and Significance.
What is new?
In experimental dilated cardiomyopathy we found:
Dyssynchrony between cardiac corin vs. ANP/BNP expression.
Corin expression levels correlated with declining heart systolic function.
ANP/BNP expression or plasma levels rise only at end stage of HF (Stage C, D).
What is relevant?
Corin is an early indicator of heart systolic dysfunction before development of heart failure.
Summary: In experimental dilated cardiomyopathy, corin levels decline with onset of systolic dysfunction before the development of heart failure and associated increases in atrial/ B-type natriuretic peptides; thus corin levels may detect patients at risk before the onset of heart failure.
Acknowledgment
We thankfully acknowledge the technical assistance of Aiilyan Houng and Nelson Houng.
Sources of Funding
This work was supported by NIH grants (HL58496, NS089707 to G.L.Reed and HL115036 to I. P. Gladysheva); and AHA grant (14SDG20510068 to D. Wang)
Footnotes
Disclosures
None
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Dardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Boerrigter G, Costello-Boerrigter LC, Burnett JC., Jr Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009;5:501–514. doi: 10.1016/j.hfc.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HN, Januzzi JL., Jr Natriuretic peptide testing in heart failure. Circulation. 2011;123:2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 4.Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, Mant J. The diagnostic accuracy of the natriuretic peptides in heart failure: Systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–1539. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep. 2014;16:415. doi: 10.1007/s11906-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 9.Gladysheva IP, Wang D, McNamee RA, Houng AK, Mohamad AA, Fan TM, Reed GL. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension. 2013;61:327–332. doi: 10.1161/HYPERTENSIONAHA.112.193631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Gladysheva IP, Fan TH, Sullivan R, Houng AK, Reed GL. Atrial natriuretic peptide affects cardiac remodeling, function, heart failure, and survival in a mouse model of dilated cardiomyopathy. Hypertension. 2014;63:514–519. doi: 10.1161/HYPERTENSIONAHA.113.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo DT, Horowitz JD, Sverdlov AL. Heart failure: A corin-deficient state? Hypertension. 2013;61:284–285. doi: 10.1161/HYPERTENSIONAHA.112.196253. [DOI] [PubMed] [Google Scholar]
- 12.Lee R, Xu B, Rame JE, Felkin LE, Barton P, Dries DL. Regulated inositol-requiring protein 1-dependent decay as a mechanism of corin RNA and protein deficiency in advanced human systolic heart failure. J Am Heart Assoc. 2014;3:e001104. doi: 10.1161/JAHA.114.001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibebuogu UN, Gladysheva IP, Reed GL. Is heart failure due to impaired cleavage and activation of atrial natriuretic peptide? J Am Coll Cardiol. 2009;53:A468–A469. [Google Scholar]
- 14.Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnet CS, Liu X, Body SC, Collard CD, Shernan SK, Muehlschlegel JD, Jarolim P, Fox AA. Plasma corin decreases after coronary artery bypass graft surgery and is associated with postoperative heart failure: A pilot study. J Cardiothorac Vasc Anesth. 2015;29:374–381. doi: 10.1053/j.jvca.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, Willenbrock R, Bader M. Rat corin gene: Molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1516–H1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- 18.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative creb transcription factor in the heart. J Clin Invest. 1998;101:2415–2426. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huggins GS, Lepore JJ, Greytak S, Patten R, McNamee R, Aronovitz M, Wang PJ, Reed GL. The creb leucine zipper regulates creb phosphorylation, cardiomyopathy, and lethality in a transgenic model of heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1877–H1882. doi: 10.1152/ajpheart.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Ma SJ, Zhang TH, Chen SC. Plasma levels of cyclic nucleotides in chronic congestive heart failure and their clinical implication. Int J Cardiol. 1993;40:7–15. doi: 10.1016/0167-5273(93)90225-6. [DOI] [PubMed] [Google Scholar]
- 22.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–142. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 24.Onitsuka K, Ide T, Arai S, Hata Y, Murayama Y, Hosokawa K, Sakamoto T, Tobushi T, Sakamoto K, Fujino T, Sunagawa K. Cardiac phase-targeted dynamic load on left ventricle differentially regulates phase-sensitive gene expressions and pathway activation. J Mol Cell Cardiol. 2013;64:30–38. doi: 10.1016/j.yjmcc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Jr, Ichiki T. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ Heart Fail. 2015;8:89–97. doi: 10.1161/CIRCHEARTFAILURE.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley CL, Stokes AJ. Corin-deficient w-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC, Jr, Krum H. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: Novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi M, Takeda S, Kurokawa S, Kubo T, Fukuda N, Izumi T. Cyclic GMP production by ANP, BNP, and NO during worsening and improvement of chronic heart failure. Jpn Heart J. 2003;44:713–724. doi: 10.1536/jhj.44.713. [DOI] [PubMed] [Google Scholar]
- 30.Deschepper CF. Cardioprotective actions of cyclic gmp: Lessons from genetic animal models. Hypertension. 2010;55:453–458. doi: 10.1161/HYPERTENSIONAHA.109.145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKie PM, Burnett JC., Jr Rationale and therapeutic opportunities for natriuretic peptide system augmentation in heart failure. Curr Heart Fail Rep. 2015;12:7–14. doi: 10.1007/s11897-014-0235-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.