Abstract
Various cytogenetic risk scoring systems may determine prognosis for patients with myelodysplastic syndromes (MDS). We evaluated four different risk scoring systems in predicting outcome after allogeneic hematopoietic cell transplantation (alloHCT). We classified 124 patients with MDS using the International prognostic scoring system (IPSS), the revised international prognostic scoring system (R-IPSS), Armand's transplantation-specific cytogenetic grouping (TSCG), and monosomal karyotype (MK) both at the time of diagnosis and alloHCT. After adjusting for other important factors, MK at diagnosis (compared to no MK) was associated with poor 3-year disease-free survival (DFS) (27% (95% CI, 12-42%) versus 39% (95% CI, 28-50%), p=0.02) and overall survival (OS) (29% (95% CI, 14-44%) versus 47% (95% CI, 36-59%), p=0.02). OS but not DFS was affected by MK at HCT. MK frequency was uncommon in low score R-IPPS and IPSS. Although IPSS and R-IPSS discriminated good/very good groups from poor/very poor groups, patients with intermediate risk scores had the worst outcomes and therefore these scores did not show a progressive linear discriminating trend. Cytogenetic risk score change between diagnosis and alloHCT was uncommon and did not influence OS. MK cytogenetics in MDS are associated with poor survival suggesting the need for alternative or intensified approaches to their treatment.
Keywords: Myelodysplastic syndrome, allogeneic hematopoietic cell transplantation, relapse, monosomal karyotype, cytogenetics, cytogenetic scoring systems
Introduction
Myelodysplastic syndromes (MDS) are heterogeneous diseases characterized by bone marrow dysplasia, cytopenias, and frequent evolution to acute myelogenous leukemia.[1] Several scoring systems, including the International Prognostic Scoring System (IPSS), utilize clinical and molecular features, including cytogenetics, to risk-stratify patients.[2] The IPSS was recently revised (R-IPSS).[3] This revision maintained bone marrow cytogenetics, marrow blast percentage, and cytopenias as the basis of the new system, with increased stratification within these categories. Cytogenetics has been a major component of all MDS scoring systems. The R-IPSS defines five cytogenetic subgroups: very good, good, intermediate, poor, and very poor [3, 4] whereas the IPSS only includes three cytogenetic patterns: good, intermediate, and poor.[2] These scoring systems are used prognostically and to aid clinical decision-making at initial presentation [5, 6]. Higher risk patients are often recommended for hematopoietic cell transplantation (alloHCT), the only potentially curative treatment for MDS [7-11]. The IPSS and R-IPSS scoring systems have been shown to predict alloHCT outcomes.[12-15] In addition to these scoring systems, Armand et al created and verified transplant-specific cytogenetic grouping (TSCG) (standard risk vs. adverse risk) that influenced the outcomes of alloHCT [16, 17]. Other cytogenetic groupings recognizing the monosomal karyotype (MK) are also found to impact on OS in MDS patients.[18, 19] The molecular/genetic prognostic landscape remains complex in MDS, and it is not clear which prognostic system, the IPSS, R-IPSS, TSCG, or presence/absence of MK, are the most clinically relevant in the setting of alloHCT.
To address this question, we compared the ability of these four cytogenetic risk stratification systems to predict alloHCT outcomes. Since some MDS patients referred for alloHCT may have evolution in their cytogenetic risk scores during pre-transplant therapy, we also evaluated the frequency of change within each cytogenetic risk score from diagnosis to alloHCT and whether these changes had any impact on alloHCT outcomes.
Patients and Methods
Patient Populations
Through the University of Minnesota Blood and Marrow Transplant (BMT) database, we identified adult MDS patients (≥ 18 years) who underwent their first alloHCT between 1995 and 2012. One hundred and twenty-four patients who had ≤10% myeloblasts in the bone marrow at the time of alloHCT (only 9 patients had >10% blasts at alloHCT) and had cytogenetic data at both diagnosis and alloHCT were included in the analysis. (By excluding patients with high blast counts at alloHCT, we were able to focus on the effect of the cytogenetic scoring systems on outcomes. Our prior analysis showed that high blast counts had a marked effect on outcome [20], making it difficult to control for the higher level blasts when evaluating the impact of cytogenetic scoring, particularly in a cohort of relatively limited number of patients.) Umbilical cord blood (UCB) and volunteer unrelated donors (URDs) were considered when there was no HLA-matched sibling available. Depending on the urgency of transplant or availability of study protocols UCB was at times prioritized over URD. UCB were selected using criteria that we have previously published [21, 22]. UCB grafts were matched at 4–6 of 6 HLA-A, -B (Ag level) and -DRB1 (allele level) to the recipient, and in patients receiving two UCB units, to each other.[23, 24] In addition to the HLA matching, stem cell count in UCB unit was considered in donor selection.
Definitions
MK was defined as the presence of any autosomal monosomy accompanied by either one additional autosomal monosomy or one structural chromosomal abnormality.[25] Cytogenetic classifications of IPSS, R-IPSS, and TSCG were described per published studies,[2-4] and are summarized in a supplemental table (Table 2). Relapse was defined as any recurrence of known hematologic, morphologic, or cytogenetic markers consistent with disease prior to transplant. GVHD data was captured prospectively by attending physicians at regular post-HCT intervals and graded using standard criteria with histopathologic confirmation when possible. [26-29] Graft source and matching was defined as matched (HLA 8/8 allele matched) versus mismatched (HLA <8/8 matched) bone marrow/peripheral blood stem cell (BM/PBSC) and matched (HLA 5/6 locus or 6/6 antigen matched) versus mismatched (HLA <5/6) umbilical cord blood (UCB).[23, 24] Conditioning regimen intensity was defined according to Bacigalupo et al.[30] Patients receiving acute myelogenous leukemia (AML)-type induction regimens or hypomethylating agents were defined as chemotherapy group.
Table 2. Cytogenetic risk scores by various systems at diagnosis and alloHCT.
| Scoring System | Cytogenetics Risk Score | At Diagnosis | At AlloHCT | Change in cytogenetic score between Diagnosis and AlloHCT | |
|---|---|---|---|---|---|
| IPSS | Good | 35 (29%) | 44 (37%) | Improved | 17 (15%) |
| Intermediate | 28 (23%) | 26 (22%) | No change | 91 (78%) | |
| Poor | 58 (48%) | 50 (42%) | Progression | 9 (8%) | |
|
| |||||
| R-IPSS | Very good | 2 (2%) | 1 (1%) | Improved | 16 (14%) |
| Good | 34 (28%) | 44 (37%) | No change | 91 (78%) | |
| Intermediate | 24 (20%) | 24 (20%) | Progression | 10 (9%) | |
| Poor | 32 (26%) | 25 (21%) | |||
| Very poor | 29 (24%) | 25 (21%) | |||
|
| |||||
| TSCS | Favorable | 63 (52%) | 71 (59%) | Improved | 13 (11%) |
| Adverse | 59 (48%) | 49 (41%) | No change | 102 (86%) | |
| Progression | 3 (3%) | ||||
|
| |||||
| MK | No | 83 (70%) | 90 (75%) | Improved | 9 (8%) |
| Yes | 36 (30%) | 30 (25%) | No change | 104 (90%) | |
| Progression | 3 (3%) | ||||
Abbreviations: AlloHCT, allogeneic hematopoietic cell transplantation; IPSS, International Prognostic Scoring System; R-IPSS, International Prognostic Scoring System-revised; MK, monosomal karyotype; TSCG transplant-specific cytogenetic grouping.
Disease-Related Variables
Diagnostic specimens were reviewed by hematopathologists at our institution and classified according to the current 2008 World Health Organization (WHO) MDS criteria.[31] Therapy-related MDS (t-MDS) was clinically defined as MDS following exposure to alkylating agents, topoisomerase II inhibitors, or radiotherapy within an appropriate timeframe. Clinical variables, histopathologic data, cytogenetic information and data on therapy were obtained via retrospective chart review. Two authors (B.T., M.D.) independently scored all available diagnostic and transplant cytogenetics; discrepancies were resolved after consensus review. Standard G-banding and FISH techniques were used for cytogenetic analysis, with at least 20 metaphase cells analyzed by G-banding and 200 interphase cells analyzed by FISH.
Conditioning Regimens
Conditioning regimens have been previously reported for myeloablative (MAC)/reduced intensity conditioning (RIC) related or URD sources and MA/RIC UCB donor sources. [32-34] Per institutional protocol, equine anti-thymocyte globulin (ATG) (15 mg/kg twice daily × 3 days) was provided to patients who had not received multi-agent chemotherapy within 3 months of HCT when using a UCB or URD or 6 months when using a matched-related donor (MRD).
Supportive Care
All patients received supportive care according to institutional guidelines including blood product transfusion, infection prophylaxis (bacterial, fungal, cytomegalovirus (CMV)/herpes simplex virus (HSV) and Pneumocystis jiroveci), and GVHD prophylaxis. CMV surveillance was performed weekly with pre-emptive treatment at the time of positive antigenemia or polymerase chain reaction (PCR) testing. For GVHD prophylaxis, the majority of patients received cyclosporine (CSA)-based regimens (targeting trough levels of 200-400 ng/mL) through day +180 with either short course methotrexate (MTX) in MA regimens or mycophenolate mofetil (MMF) through day +30 with RIC or UCB regimens. Granulocyte-colony stimulating factor was administered to all patients through neutrophil recovery. Chimerism was determined by quantitative PCR of informative polymorphic variable number tandem repeat or short tandem repeat regions in the recipient and donor as described.[23]
Data Collection and Analysis
Patient outcomes following alloHCT were prospectively collected and recorded in the University of Minnesota BMT database. Treatment protocols were approved by the University of Minnesota Institutional Review Board and registered at http://clinicaltrials.gov, and all patients gave informed consent before alloHCT. Factors considered in statistical analysis included the following: patient age, sex, Karnofsky performance status (KPS), recipient CMV serostatus, year of transplant, donor graft source, conditioning regimen intensity, GVHD prophylactic regimen, MDS diagnosis according to WHO criteria [35], available cytogenetics, t-MDS, all four cytogenetic risk scoring systems at both diagnosis and alloHCT, and blast percentage at diagnosis and transplant.
Statistical Methods
Unadjusted estimates of OS and DFS were calculated by Kaplan-Meier curves.[36] Comparisons were completed with the simple log rank test. Unadjusted estimates of non-relapse mortality (NRM) were analyzed using cumulative incidence treating relapse as a competing risk. Relapse was similarly analyzed using cumulative incidence treating mortality as a competing risk. Comparisons were completed with Gray's test. Cox regression was used to assess the independent effect of cytogenetic indices on OS and DFS.[37] and Fine and Gray proportional hazards regression [38]was used to assess the independent effect of indices on NRM and relapse. Martingale residuals were used to test against non-proportionality.[39] with tests for linear contrasts. After calculation of final regression models, the adjusted OS and DFS curves by MK were computed as average estimates of the pooled sample, weighted by the proportions of the variables in the regression models.[40] Similarly, the adjusted relapse and NRM curves by MK were estimated based on other significant risk factors in the regression models. [41] SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2 were used to perform all statistical analyses.
Results
Patients' demographic data are shown in Table 1. The median age was 55 years, and the majority of patients (62%) received alloHCT after RIC. Twenty-three percent of patients had t-MDS. Patients were grouped by four cytogenetic scoring systems at the time of diagnosis and at alloHCT (Table 2). Changes in the cytogenetic score between diagnosis and alloHCT occurred in 22%, 22%, 14%, and 10% of patients in the IPSS, R-IPSS, TSCG, and MK cytogenetic scoring systems, respectively (Table 2).
Table 1. Patient- and Transplantation-Characteristics.
| Variable | Total Study Group | |
|---|---|---|
| Year of Transplant | 1995-1999 | 16 (13%) |
| 2000-2006 | 40 (32%) | |
| 2007-2013 | 68 (55%) | |
| Age of Patients | < 50 | 41 (33%) |
| 50-60 | 47 (38%) | |
| >60 | 36 (29%) | |
| Median (range) (IQR) | 55 (18-72), (47-62) | |
| Patient Gender | Male | 83 (67%) |
| KPS | <90 | 25 (21%) |
| 90/100 | 97 (80%) | |
| Recipient CMV Serostatus | Negative | 57 (46%) |
| Positive | 67 (54%) | |
| Donor Type | RD/URD Match | 67 (54%) |
| RD/URD MM | 8 (7%) | |
| UCB 5+6/6 | 24 (19%) | |
| UCB 4/6 | 25 (20%) | |
| Conditioning | MAC | 47 (38%) |
| RIC: w/ ATG | 52 (42%) | |
| RIC: w/o ATG | 25 (20%) | |
| GvHD Prophylaxis | CSA/MMF | 75 (61%) |
| CSA containing | 31 (25%) | |
| Other | 18 (15%) | |
| Diagnosis | MDS-NOS | 24 (19%) |
| MDS - RA | 5 (4%) | |
| MDS - RAEB-1 | 34 (27%) | |
| MDS - RAEB-2 | 30 (24%) | |
| MDS - RARS | 4 (3%) | |
| MDS - RCMD | 21 (17%) | |
| RCMD - RS | 6 (5%) | |
| Months from Diagnosis to Transplant: | Median (range), (IQR) | 6 (1-371), (4-13) |
| Therapy-related MDS | No | 94 (77%) |
| Yes | 28 (23%) | |
| Blast at alloHCT | <=2% | 74 (60%) |
| >2-<5% | 33 (27%) | |
| 5-10% | 17 (14%) |
Abbreviations: AlloHCT, allogeneic hematopoietic cell transplantation; CMV, cytomegalovirus; CSA, cyclosporine; GVHD, graft-versus-host disease; IPSS, International Prognostic Scoring System; R-IPSS, International Prognostic Scoring System-revised; KPS, Karnofsky performance status; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MDS-U, MDS- unclassifiable; MRD, matched related donor; MMRD, mismatched related donor; MTX, methotrexate; RIC, reduced intensity conditioning; MMF, mycophenolate mofetil; MDS, myelodysplastic syndrome; NOS, not otherwise specified; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ringed sideroblasts; RD, related donor; RCMD, refractory cytopenias with multilineage dysplasia; RCMD/RS, RCMD with ringed sideroblasts; UCB, umbilical cord blood; URD, unrelated donor; WHO, World Health Organization
Patients with or without MK had no significant difference in patient-, disease-, or transplantation- related characteristics except for more therapy-related MDS in MK patients (Table 3). The frequency of receiving chemotherapy before alloHCT was also similar in these groups. IPSS cytogenetic risk score groups had similar characteristics except the poor risk group had a lower blast percentage, more MK, and more patients with therapy-related MDS (Table 3). MK was most common in very poor R-IPSS risk (28/29, 96%) followed by poor (6/30, 20%), intermediate (1/24, 4%), good (1/34, 2.9%), and very good cytogenetic risk groups (0/2, 0%). R-IPSS very poor group had also more patients with therapy-related MDS.
Table 3. Patient Characteristics by Monosomal Karyotype and IPSS at Diagnosis.
| IPSS | R-IPSS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | No MK | MK | P- value |
Good | Intermediate | Poor | P- value |
Good/ Very Good |
Intermediate | Poor | Very Poor | P- value |
|
| Age of Patient | < 50 | 26 (31%) | 13 (36%) | 0.82 | 6 (17%) | 14 (50%) | 20 (35%) | 0.03 | 6 (17%) | 11 (46%) | 15 (47%) | 8 (28%) | 0.04 |
| 50-60 | 32 (39%) | 14 (39%) | 13 (37%) | 8 (29%) | 25 (43%) | 13 (36%) | 8 (33%) | 11 (34%) | 14 (48%) | ||||
| >60 | 25 (30%) | 9 (25%) | 16 (46%) | 6 (21%) | 13 (22%) | 17 (47%) | 5 (21%) | 6 (19%) | 7 (24%) | ||||
| Age (Years) | Median (range) | 55 (19-72) | 52 (19-69) | 0.88 | 58 (27-72) | 52 (21-69) | 52 (19-72) | 0.11 | 59 (27-72) | 53 (28-69) | 51 (19-72) | 53 (22-69) | 0.17 |
| Patient Gender | Male | 55 (66%) | 24 (67%) | 0.97 | 23 (66%) | 17 (61%) | 40 (69%) | 0.75 | 23 (64%) | 16 (67%) | 23 (72%) | 18 (62%) | 0.86 |
| KPS | <90 | 19 (23%) | 5 (14%) | 0.29 | 6 (17%) | 7 (25%) | 11 (19%) | 0.73 | 7 (19%) | 6 (25%) | 7 (22%) | 4 (14%) | 0.80 |
| 90/100 | 64 (77%) | 30 (83%) | 29 (83%) | 21 (75%) | 46 (79%) | 29 (81%) | 18 (75%) | 25 (78%) | 24 (86%) | ||||
| Recipient CMV | Positive | 46 (55%) | 19 (53%) | 19 (54%) | 18 (64%) | 28 (48%) | 0.38 | 20 (56%) | 16 (67%) | 14 (44%) | 15 (52%) | 0.39 | |
| Donor Type | RD/URD Match | 42 (51%) | 23 (64%) | 0.44 | 17 (49%) | 14 (50%) | 35 (60%) | 0.19 | 18 (50%) | 12 (50%) | 16 (50%) | 20 (69%) | 0.22 |
| RD/URD MM | 6 (7%) | 2 (6%) | 2 (6%) | 4 (14%) | 2 (3%) | 2 (6%) | 4 (17%) | 1 (3%) | 1 (3%) | ||||
| UCB 5+6/6 | 19 (23%) | 4 (11%) | 11 (31%) | 3 (11%) | 10 (17%) | 11 (31%) | 3 (13%) | 6 (19%) | 4 (14%) | ||||
| UCB 4/6 | 16 (19%) | 7 (19%) | 5 (14%) | 7 (25%) | 11 (19%) | 5 (14%) | 5 (21%) | 9 (28%) | 4 (14%) | ||||
| Conditioning | MA | 32 (39%) | 13 (36%) | 0.97 | 11 (31%) | 11 (39%) | 24 (41%) | 0.78 | 11 (31%) | 8 (33%) | 16 (50%) | 11 (38%) | 0.61 |
| RIC: w/ ATG | 35 (42%) | 16 (44%) | 17 (49%) | 10 (36%) | 24 (41%) | 18 (50%) | 9 (38%) | 11 (34%) | 13 (45%) | ||||
| RIC: w/o ATG | 16 (19%) | 7 (19%) | 7 (20%) | 7 (25%) | 10 (17%) | 7 (19%) | 7 (29%) | 5 (16%) | 5 (17%) | ||||
| GvHD Prophylaxis | CSA/MMF | 52 (63%) | 21 (58%) | 0.57 | 23 (66%) | 18 (64%) | 32 (55%) | 0.77 | 24 (67%) | 17 (71%) | 17 (53%) | 15 (52%) | 0.49 |
| CSA containing | 21 (25%) | 8 (22%) | 8 (23%) | 7 (25%) | 15 (26%) | 8 (22%) | 4 (17%) | 11 (34%) | 7 (24%) | ||||
| Other | 10 (12%) | 7 (19%) | 4 (11%) | 3 (11%) | 11 (19%) | 4 (11%) | 3 (13%) | 4 (13%) | 7 (24%) | ||||
| Diagnosis | MDS-NOS | 12 (15%) | 10 (28%) | 0.30 | 6 (17%) | 5 (18%) | 13 (22%) | 0.04 | 6 (17%) | 5 (21%) | 13 (21%) | 5 (17%) | 0.05 |
| MDS - RA | 5 (6%) | 0 | 1 (3%) | 2 (7%) | 2 (3%) | 1 (3%) | 3 (13%) | 1 (3%) | 0 | ||||
| MDS - RAEB-1 | 21 (25%) | 11 (31%) | 7 (20%) | 6 (21%) | 19 (33%) | 18 (50%) | 4 (17%) | 2 (6%) | 6 (21%) | ||||
| MDS - RAEB-2 | 25 (30%) | 5 (14%) | 18 (51%) | 5 (18%) | 7 (12%) | 2 (6%) | 0 | 1 (3%) | 1(3%) | ||||
| MDS - RARS | 3 (4%) | 1 (3%) | 1 (3%) | 1 (4%) | 2 (3%) | 1 (3%) | 5 (21%) | 7 (22%) | 8 (28%) | ||||
| MDS - RCMD | 13 (16%) | 8 (22%) | 1 (3%) | 7 (25%) | 13 (22%) | 1 (3%) | 2 (8%) | 1 (3%) | 1 (3%) | ||||
| RCMD - RS | 4 (5%) | 1 (3%) | 1 (3%) | 2 (7%) | 2 (3%) | 7 (19%) | 5 (21%) | 12 (38%) | 8 (28%) | ||||
| Therapy-related MDS | yes | 13 (16%) | 13 (36%) | 0.01 | 16 (46%) | 5 (18%) | 6 (10%) | <0.01 | 1 (3%) | 4 (17%) | 13 (41%) | 19 (68%) | <0.01 |
| Blast at TX | <=2% | 46 (55%) | 27 (75%) | 0.07 | 21 (60%) | 15 (54%) | 38 (66%) | 0.53 | 22 (61%) | 13 (54%) | 18 (56%) | 21 (72%) | 0.61 |
| >2-<5% | 25 (30%) | 4 (11%) | 11 (31%) | 8 (29%) | 11 (19%) | 11 (31%) | 7 (29%) | 8 (25%) | 4 (14%) | ||||
| 5-10% | 12 (15%) | 5 (14%) | 3 (9%) | 5 (18%) | 9 (16%) | 3 (8%) | 4 (17%) | 6 (19%) | 4 (14%) | ||||
| Chemotherapy | No | 43 (52%) | 16 (44%) | 0.46 | 34 (97%) | 23 (85%) | 35 (61%) | <0.01 | 35 (97%) | 23 (96%) | 24 (80%) | 1 (3%) | <0.01 |
| Yes | 40 (48%) | 20 (56%) | 1 (3%) | 4 (15%) | 22 (39%) | 1 (3%) | 1 (4%) | 6 (20%) | 28 (97%) | ||||
| MK | No | 34 (97%) | 27 (96%) | 22 (39%) | <0.01 | 6.7 (1-372) | 7.4 (2-63) | 5.4 (2-73) | 5.2 (2-38) | 0.06 | |||
| Yes | 1 (3%) | 1 (4%) | 34 (61%) | 1 (3%) | 4 (17%) | 13 (41%) | 19 (68%) | <0.01 | |||||
| Months to AlloHCT | Median (range) | 6.7 (1-372) | 5.3 (2-39) | 0.14 | 6.3 (1-77) | 10.4 (2-81) | 5.3 (2-372) | 0.58 | 22 (61%) | 13 (54%) | 18 (56%) | 21 (72%) | 0.61 |
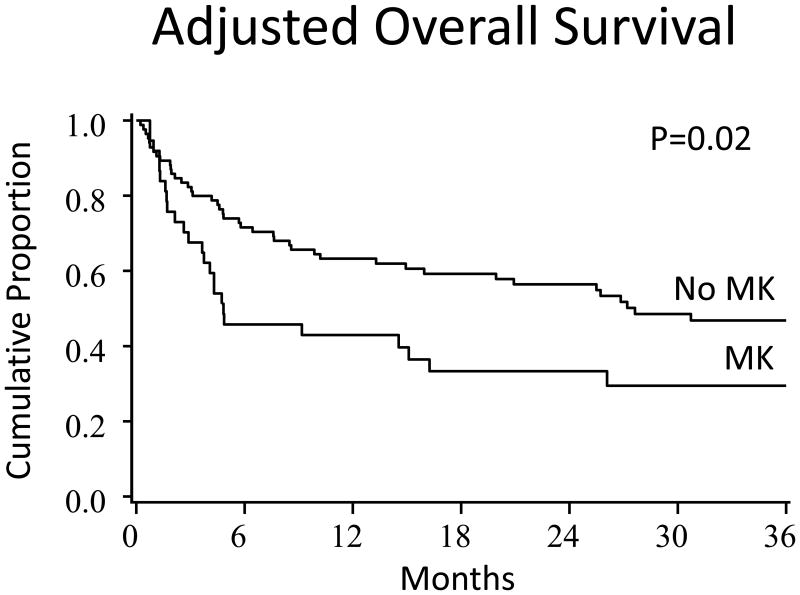
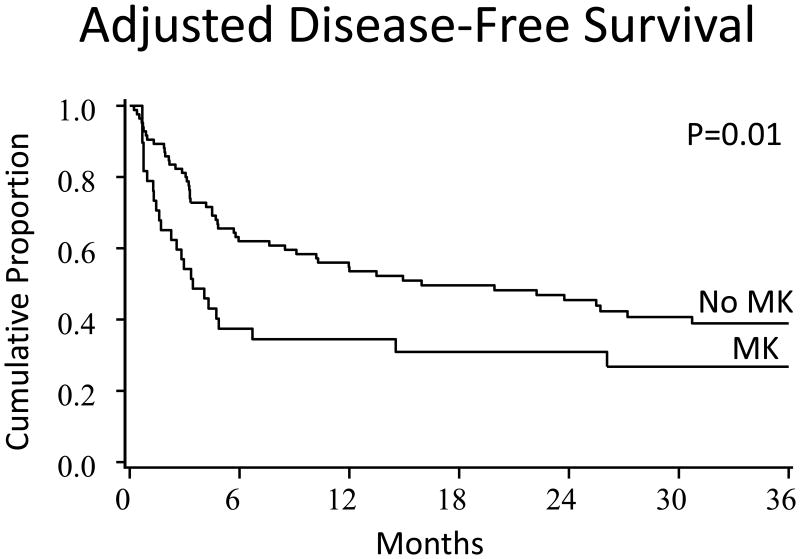
The adjusted Kaplan-Meier estimates of OS and DFS and cumulative incidence estimates of relapse and NRM by cytogenetic scoring system groupings are shown in Table 4. Similarly, multiple regression analysis, showing the relative risk of patients with MK as well as other confounding factors, is shown in Table 5. Patients with MK at diagnosis had lower survival (Figure 1 A and B) and higher relapse. Relapse/progression was the cause of mortality in 52% and 20% of patients with MK and without MK at diagnosis, respectively. In a different model, when MK at alloHCT was evaluated, it was associated with decreased OS (HR, 1.9; CI95%:1.1-3.3, p=0.03) but not relapse (HR, 1.7; CI95%:0.8-3.9, p=0.17). Patients with IPSS good cytogenetic risk score (either at diagnosis or alloHCT) had the best DFS, OS, and relapse rates whereas intermediate risk group had the highest relapse rate and the shortest survival. Similarly, patients with R-IPSS very good/good cytogenetic risk score (either at diagnosis or alloHCT) had the longest DFS and OS whereas intermediate risk group had the worst survival. Neither R-IPSS nor IPSS predicted outcomes in an expected linear fashion (p > 0.05) due to poor outcomes in the intermediate group. Outcomes by TSCG were similar between the adverse or favorable groups. In the regression analyses that focused on the effect of patients with and without MK, other risk factors for poor OS and higher relapse were MM RD/URD and RIC without ATG in multivariate regression analysis, respectively (Table 5). UCBT had no significant effect on relapse, TRM, DFS or OS in MVA.
Table 4. The effects of cytogenetic scoring system groupings on alloHCT outcomes adjusted for donor type, conditioning and baseline Karnofsky.
| Time | Cytogenetic scoring systems | Cytogenetic Risk Score | NRM at 3 years | P-value | Relapse at 3 years | P-value | DFS at 3 years | P-value | OS at 3 years | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| AT DIAGNOSIS | IPSS | Good | 22%(8-36%) | 0.01 | 21%(8-34%) | 0.28 | 59%(43-73%) | <0.01 | 67%(51-83%) | <0.01 |
| Intermediate | 55%(45-65%) | 35%(17-53%) | 11%(1-23%) | 16%(4-30%) | ||||||
| Poor | 42%(28-56%) | 26%(15-37%) | 32%(20-44%) | 37%(24-50%) | ||||||
| R-IPSS | Very Good/Good | 21%(7-35%) | <0.01 | 23%(9-37%) | 0.02 | 57%(41-73%) | <0.01 | 66%(50-82%) | <0.01 | |
| Intermediate | 59%(38-80%) | 36%(17-55%) | 8%(1-19%) | 19%(3-35%) | ||||||
| Poor | 47%(29-65%) | 10%(1-21%) | 41%(24-58%) | 44%(26-62%) | ||||||
| Very Poor | 35%(16-54%) | 39%(22-56%) | 24%(7-41%) | 27%(11-44%) | ||||||
| TSCG | Standard | 36%(24-48%) | 0.29 | 28%(17-39%) | 0.98 | 37%(25-49%) | 0.36 | 44%(31-57%) | 0.28 | |
| Adverse | 44%(31-57%) | 26%(15-37%) | 31%(19-43%) | 36%(24-48%) | ||||||
| MK | No | 40%(29-51%) | 0.96 | 23%(14-32%) | 0.02 | 39%(28-50%) | 0.01 | 47%(36-59%) | 0.02 | |
| Yes | 35%(19-51%) | 38%(23-43%) | 27%(12-42%) | 29%(14-44%) | ||||||
| AT ALLOHCT | IPSS | Good | 27%(13-41%) | 0.19 | 27%(14-40%) | 0.86 | 47%(32-62%) | 0.09 | 57%(42-72%) | <0.01 |
| Intermediate | 56%(36-76%) | 29%(12-46%) | 19%(4-34%) | 22%(6-38%) | ||||||
| Poor | 43%(29-57%) | 25%(13-37%) | 32%(19-45%) | 36%(22-50%) | ||||||
| R-IPSS | Very Good/Good | 27%(13-31%) | 0.23 | 29%(16-52%) | 0.84 | 45%(30-60%) | 0.02 | 56%(41-71%) | <0.01 | |
| Intermediate | 59%(39-79%) | 27%(10-44%) | 18%(3-33%) | 24%(6-42%) | ||||||
| Poor | 37%(17-57%) | 18%(2-34%) | 44%(23-65%) | 49%(29-69%) | ||||||
| Very Poor | 46%(26-66%) | 31%(14-47%) | 24%(6-42%) | 23%(6-40%) | ||||||
| TSCG | Standard | 40%(28-52%) | 0.51 | 27%(17-37%) | 0.73 | 36%(24-48%) | 0.27 | 42%(31-54%)53%) | 0.34 | |
| Adverse | 41%(27-54%) | 28%(16-40%) | 32%(19-45%) | 38%(24-52%) | ||||||
| MK | No | 41%(31-51%) | 0.85 | 25%(16-34%) | 0.17 | 36%(26-46%) | 0.10 | 43%(33-53%) | 0.08 | |
| Yes | 39%(21-60%) | 34%(18-50%) | 28%(12-44%) | 31%(14-48%) |
Table 5. Multiple regression analyses through 3 years for NRM, Relapse, DFS and OS.
| Factors* | N | RR of NRM (95% CI) | P-value | RR of Relapse (95% CI) | P-value | RR of Relapse/Death (95% CI) | P-value | RR of Death (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| MK at Diagnosis | |||||||||
| No | 83 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 36 | 1.0 (0.5-2.0) | 0.96 | 2.4 (1.1-5.3) | 0.02 | 2.0 (1.2-3.4) | 0.01 | 1.9 (1.1-3.3) | 0.02 |
| Donor Type | |||||||||
| Matched RD/URD | 67 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| MM RD/URD | 8 | 5.9 (2.4-14.5) | <0.01 | No events | <0.01 | 2.8 (1.2-6.4) | 0.02 | 3.7 (1.6-8.8) | <0.01 |
| 5+6/6 UCB | 24 | 0.9 (0.4-2.4) | 0.91 | 0.5 (0.2-1.5) | 0.26 | 0.7 (0.4-1.5) | 0.40 | 0.7 (0.3-1.6) | 0.45 |
| 4/6 UCB | 25 | 1.2 (0.5-2.6) | 0.88 | 0.9 (0.4-2.3) | 0.85 | 1.0 (0.5-1.8) | 0.96 | 1.2 (0.6-2.3) | 0.58 |
| Conditioning | |||||||||
| MAC | 47 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| RIC: w/ ATG | 52 | 1.1 (0.5-2.4) | 0.87 | 2.1 (0.8-5.5) | 0.13 | 1.6 (0.9-2.8) | 0.14 | 1.5 (0.8-2.8) | 0.20 |
| RIC: w/o ATG | 25 | 1.3 (0.6-2.9) | 0.48 | 3.4 (1.3-9.3) | 0.02 | 2.3 (1.2-4.4) | 0.01 | 2.2 (1.1-4.2) | 0.02 |
| KPS | |||||||||
| 90-100* | 97 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| <90 | 25 | 1.1 (0.6-2.2) | 0.73 | 1.5 (0.6-4.0) | 0.43 | 1.7 (1.0-3.0) | 0.06 | 1.6 (0.9-3.0) | 0.10 |
| Grade II-IV AGVHD (Time Dependent) | |||||||||
| No | 76 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 48 | 1.0 (0.5-2.0) | 0.99 | 0.7 (0.3-1.5) | 0.33 | 0.7 (0.4-1.1) | 0.12 | 0.9 (0.5-1.6) | 0.78 |
Tested for MK at Diagnosis (No versus Yes), Age (<50 versus 50-60 versus 60+), year of alloHCT (1995-1999 versus 2000-2006 versus 2007-2013), gender (male versus female), KPS (<90 versus 90/100), cmv serostatus (positive versus negative), donor type (RD/URD match versus RD/URD MM versus 5+6/6 UCB versus 4/6 UCB), conditioning (Myeloablative versus RIC with ATG versus RIC w/o ATG), GvHD prophylaxis (cyclosporine/mycophenolate mofetil versus cyclosporine/other versus other), diagnosis (RA/RARS versus RAEB versus RCMD versus unknown), treatment related MDS (No versus Yes) and Blasts at alloHCT (≤2% versus 3-4% versus 5-10%).
Figure 1. OS (A) and DFS (B) at 3 years by MK.
Changes in cytogenetic risk score between diagnosis and alloHCT appeared to have no impact on NRM or OS, regardless of scoring system. All three patients progressing to unfavorable risk from standard risk score by TSCG died (two after relapse). Prior chemotherapy had no effect on relapse, DFS or OS in univariate analysis.
Discussion
The Importance of MK in predicting MDS prognosis continues to emerge. Non-transplant studies have shown that MK has more a significant effect on survival compared with that found in other classification systems for complex cytogenetics in MDS.[18, 42, 43] The few recent studies evaluating the importance of MK in alloHCT have reported results similar to ours. In a specific cohort of MDS patients with chromosome 7 abnormalities, Van Gelder et al, using the European Group for Blood and Marrow transplantation (EBMT) database, showed that MK was more predictive of progression-free survival and OS after alloHCT than complex cytogenetics in 261 MDS or AML patients.[19] In fact, MK and marrow blast counts of >5% were the only prognostic factors for DFS in MDS patients receiving alloHCT.[44] Deeg et al reported that both MK and R-IPSS cytogenetic risk score were associated with relapse and survival after alloHCT in MDS patients.[15] In our study, MK was more predictive of alloHCT outcomes in MDS patients after alloHCT compared to other established scoring systems. Moreover, the presence of MK, both at diagnosis and at alloHCT, was predictive of survival after alloHCT; to our knowledge, ours is the first study to evaluate this. We also showed that MK frequency was correlated with R-IPSS cytogenetic risk score; the highest frequency of MK was found in the R-IPSS very poor cytogenetic risk group and was progressively less frequent in the more favorable risk groups, similar to the findings of Deeg et al.[15] Although some studies indicate that a complex karyotype is more prognostic than MK in the non-alloHCT MDS setting, [45, 46] we could not evaluate this because of the strong correlation between MK and R-IPSS very poor cytogenetic risk group (>3 cytogenetic abnormalities).
The IPSS and R-IPSS have been shown in previous studies to be associated with outcomes of alloHCT [12, 15, 47, 48]. Our study as in line with other studies in that it highlights the predictive potential of IPSS and R-IPSS primarily in the good risk cytogenetic groups that had the best expected outcomes after alloHCT. In addition, we noted a worsening trend for relapse, DFS and OS from the good/very good risk group toward the poor/ very poor risk group. However, the intermediate risk groups classified by both IPSS and R-IPSS had unexpectedly poor outcomes (the highest NRM and relapse yielding the lowest DFS and OS) in our cohort. When we compared factors among risk scoring groups in IPSS and R-IPSS scoring system such as blast percentage or inferior KPS, there was no significant difference to explain the poor outcome in the intermediate groups. In our study we found that the TSCG had no utility in predicting alloHCT outcomes.[16, 17] The difference between our study and other large studies [15, 48] may be due in part to the limited number of patients in our study. Moreover, our study cohort had the largest UCBT. UCBT has been used in MDS patients.[20, 49, 50] Although there is no study directly comparing UCBT with other graft sources for MDS, comparable results were shown in acute myelogenous leukemia.[51, 52] In our study, UCBT frequency was similar within the cytogenetic scoring systems, and was not found to be associated with relapse, TRM, DFS or OS.
Changes between diagnosis and alloHCT in cytogenetics risk score group in each scoring system occurred were uncommon. These cytogenetic changes had little influence on outcomes of alloHCT. In general, the value of cytogenetic risk scoring in alloHCT outcomes was similar between classification at diagnosis and at alloHCT. Although this was not one of the primary objectives of our retrospective analysis and the patient cohort was relatively small, this might indicate that outcomes of alloHCT were not affected significantly by therapy between diagnosis and alloHCT. The effect of therapy in MDS before alloHCT is still controversial, mainly due to reported all results are from retrospective studies.[7, 48, 53-57] In a large single center study, Oran et al showed that therapy prior to alloHCT and disease status at alloHCT were not found to be prognostic for any disease outcome.[44] OS at 5 years was 57% for patients who underwent alloHCT as a primary treatment for RAEB-t or secondary AML and 54% for those who underwent alloHCT in remission after induction chemotherapy (p=0.81).[53] In that study, achieving a CR before a standard alloHCT was not to be associated with a better prognosis posttransplantation; however, disease status had a significant impact in patients who progressed to AML. In contrast, other studies have indicated CR status is important for alloHCT outcomes.[47, 57-59] In our prior study, we highlighted the importance of blast percentage at the time of transplant in MDS patients [20]. Consequently, to focus this analysis on the impact of cytogenetic risk group instead of confounding characteristic of high blast burden, we excluded a limited number of patients who had >10% marrow blasts at alloHCT in this cohort. A recent EBMT study showed that in patients with high risk cytogenetic score by IPSS, relapse rate at 5-year was much higher if they were in CR (70%) versus not in CR (38%). However, relapse was lower in patients with low risk cytogenetic score in CR (38% vs. 18%). [48] These findings in others and our study may suggest that cytogenetic risk group may be more important than CR status in MDS- CR is a difficult end point to measure in MDS, regardless.
In conclusion, this study evaluates the ability of 4 different cytogenetic scoring systems used at diagnosis and alloHCT to predict outcome. We found that MK, particularly at diagnosis, is the cytogenetic risk scoring system most predictive of post alloHCT outcomes. Changes in the cytogenetics risk score between diagnosis and alloHCT occur only rarely and have limited effects on outcomes of alloHCT. Therefore, cytogenetic risk scoring at diagnosis or at alloHCT seemed to have similar power of prediction of alloHCT outcomes.
Supplementary Material
Footnotes
Disclosure: Authors have conflict of interest to disclose
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 3.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised International Prognostic Scoring System (IPSS-R) for myelodysplastic syndromes. Blood. 2012 doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schanz J, Tuchler H, Sole F, Mallo M, Luno E, Cervera J, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–9. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 6.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31:2662–70. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–87. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE, et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol. 2007;82:867–72. doi: 10.1002/ajh.20989. [DOI] [PubMed] [Google Scholar]
- 10.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 11.Appelbaum FR AJ. Allogeneic bone marrow transplantation for myelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia Leukemia. 1998;12 [PubMed] [Google Scholar]
- 12.Nevill TJ, Fung HC, Shepherd JD, Horsman DE, Nantel SH, Klingemann HG, et al. Cytogenetic abnormalities in primary myelodysplastic syndrome are highly predictive of outcome after allogeneic bone marrow transplantation. Blood. 1998;92:1910–7. [PubMed] [Google Scholar]
- 13.Sutton L, Chastang C, Ribaud P, Jouet JP, Kuentz M, Attal M, et al. Factors influencing outcome in de novo myelodysplastic syndromes treated by allogeneic bone marrow transplantation: A long-term study of 71 patients. Blood. 1996;88:358–65. [PubMed] [Google Scholar]
- 14.Diez Campelo M, Sanchez-Barba M, de Soria VG, Martino R, Sanz G, Insunza A, et al. Results of allogeneic stem cell transplantation in the Spanish MDS registry: Prognostic factors for low risk patients. Leukemia research. 2014 doi: 10.1016/j.leukres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Deeg HJ, Scott BL, Fang M, Shulman HM, Gyurkocza B, Myerson D, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120:1398–408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–64. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armand P, Deeg HJ, Kim HT, Lee H, Armistead P, de Lima M, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant. 2010;45:877–85. doi: 10.1038/bmt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing R, Li C, Gale RP, Zhang Y, Xu Z, Qin T, et al. Monosomal karyotype is an independent predictor of survival in patients with higher-risk myelodysplastic syndrome. Am J Hematol. 2014;89:E163–8. doi: 10.1002/ajh.23801. [DOI] [PubMed] [Google Scholar]
- 19.van Gelder M, de Wreede LC, Schetelig J, van Biezen A, Volin L, Maertens J, et al. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2013;27:879–88. doi: 10.1038/leu.2012.297. [DOI] [PubMed] [Google Scholar]
- 20.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–8. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–9. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 23.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 24.Ustun C, Bachanova V, Shanley R, MacMillan ML, Majhail NS, Arora M, et al. Importance of donor ethnicity/race matching in unrelated adult and cord blood allogeneic hematopoietic cell transplant. Leukemia & lymphoma. 2014;55:358–64. doi: 10.3109/10428194.2013.800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–7. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 26.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–9. [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 29.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British journal of haematology. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 30.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunning RD, Orazi A, Germing U, Le Beau MM, Porwit A, Baumann I, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008:88–107. [Google Scholar]
- 32.Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47:494–8. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ustun C, Wiseman AC, DeFor TE, Yohe S, Linden MA, Oran B, et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant. 2013;48:1415–20. doi: 10.1038/bmt.2013.124. [DOI] [PubMed] [Google Scholar]
- 34.Warlick ED, Tomblyn M, Cao Q, Defor T, Blazar BR, Macmillan M, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biol Blood Marrow Transplant. 2011;17:1025–32. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunning RD, Orazi A, Germing U, Le Beau LM, Porwit A, Baurman I, et al. In: Myelodysplastic syndromes/neoplasms, overview in WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JS, editors. Lyon: pp. 2008pp. 89–93. [Google Scholar]
- 36.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 37.Cox DR. Regression models and life tables. J Royal Stat Soc Bulletin. 1972;34:187–220. [Google Scholar]
- 38.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 39.Colett D. Modelling Survival Data in Medical Research. 2003 [Google Scholar]
- 40.Gail MH, Byar DP. Variance Calculations for Direct Adjusted Survival Curves, with Applications to Testing for No Treatment Effect. Biometrical J. 1986;28:587–99. [Google Scholar]
- 41.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Meth Prog Bio. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patnaik MM, Hanson CA, Hodnefield JM, Knudson R, Van Dyke DL, Tefferi A. Monosomal karyotype in myelodysplastic syndromes, with or without monosomy 7 or 5, is prognostically worse than an otherwise complex karyotype. Leukemia. 2011;25:266–70. doi: 10.1038/leu.2010.258. [DOI] [PubMed] [Google Scholar]
- 43.Gangat N, Patnaik MM, Begna K, Kourelis T, Knudson RA, Ketterling RP, et al. Evaluation of revised IPSS cytogenetic risk stratification and prognostic impact of monosomal karyotype in 783 patients with primary myelodysplastic syndromes. Am J Hematol. 2013;88:690–3. doi: 10.1002/ajh.23477. [DOI] [PubMed] [Google Scholar]
- 44.Oran B, Kongtim P, Popat U, de Lima M, Jabbour E, Lu XY, et al. Cytogenetics, Donor Type, and Use of Hypomethylating Agents in Myelodysplastic Syndrome with Allogeneic Stem Cell Transplantation. Biol Blood Marrow Tr. 2014;20:1618–25. doi: 10.1016/j.bbmt.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valcarcel D, Adema V, Sole F, Ortega M, Nomdedeu B, Sanz G, et al. Complex, Not Monosomal, Karyotype Is the Cytogenetic Marker of Poorest Prognosis in Patients With Primary Myelodysplastic Syndrome. Journal of Clinical Oncology. 2013;31:916–22. doi: 10.1200/JCO.2012.41.6073. [DOI] [PubMed] [Google Scholar]
- 46.Schanz J, Tuchler H, Sole F, Mallo M, Luno E, Cervera J, et al. Monosomal karyotype in MDS: explaining the poor prognosis? Leukemia. 2013;27:1988–95. doi: 10.1038/leu.2013.187. [DOI] [PubMed] [Google Scholar]
- 47.Nevill TJ, Shepherd JD, Sutherland HJ, Abou Mourad YR, Lavoie JC, Barnett MJ, et al. IPSS Poor-Risk Karyotype as a Predictor of Outcome for Patients with Myelodysplastic Syndrome following Myeloablative Stem Cell Transplantation. Biol Blood Marrow Tr. 2009;15:205–13. doi: 10.1016/j.bbmt.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Onida F, Brand R, van Biezen A, Schaap M, von dem Borne PA, Maertens J, et al. Impact of the International Prognostic Scoring System cytogenetic risk groups on the outcome of patients with primary myelodysplastic syndromes undergoing allogeneic stem cell transplantation from human leukocyte antigen-identical siblings: a retrospective analysis of the European Society for Blood and Marrow Transplantation-Chronic Malignancies Working Party. Haematologica. 2014;99:1582–90. doi: 10.3324/haematol.2014.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. The Lancet Oncology. 2011;12:1214–21. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato A, Ooi J, Takahashi S, Tsukada N, Kato S, Kawakita T, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with advanced myelodysplastic syndromes. Bone Marrow Transplant. 2011;46:257–61. doi: 10.1038/bmt.2010.91. [DOI] [PubMed] [Google Scholar]
- 51.Warlick ED, Peffault de Latour R, Shanley R, Robin M, Bejanyan N, Xhaard A, et al. Allogeneic Hematopoietic Cell Transplantation Outcomes in Acute Myeloid Leukemia: Similar Outcomes Regardless of Donor Type. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peffault de Latour R, Brunstein CG, Porcher R, Chevallier P, Robin M, Warlick E, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19:1355–60. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Nakai K, Kanda Y, Fukuhara S, Sakamaki H, Okamoto S, Kodera Y, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19:396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JE, Gooley TA, Schoch G, Anasetti C, Bensinger WI, Clift RA, et al. Stem cell transplantation for secondary acute myeloid leukemia: Evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89:2578–85. [PubMed] [Google Scholar]
- 55.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelodysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) British journal of haematology. 2000;110:620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 56.Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112:895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 57.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, et al. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94:542–9. doi: 10.3324/haematol.2008.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yakoub-Agha I, de La Salmoniere P, Ribaud P, Sutton L, Wattel E, Kuentz M, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18:963–71. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.