Abstract
Purpose
We performed video-oculography to evaluate vergence eye movement abnormalities in students diagnosed clinically with vergence disorders. We tested the efficiency of a novel rehabilitation method and evaluated its benefits with video-oculography cross-correlated with clinical tests and symptomatology.
Methods
A total of 19 students (20–27 years old) underwent ophthalmologic, orthoptic examination, and a vergence test coupled with video-oculography. Eight patients were diagnosed with vergence disorders with a high symptomatology score (CISS) and performed a 5-week session of vergence rehabilitation. Vergence and rehabilitation tasks were performed with a trapezoid surface of light emitting diodes (LEDs) and adjacent buzzers (US 8851669). We used a novel Vergence double-step (Vd-s) protocol: the target stepped to a second position before the vergence movement completion. Afterward the vergence test was repeated 1 week and 1 month later.
Results
Abnormally increased intertrial variability was observed for many vergence parameters (gain, duration, and speed) for the subjects with vergence disorders. High CISS scores were correlated with variability and increased latency. After the Vd-s, variability of all parameters dropped to normal or better levels. Moreover, the convergence and divergence latency diminished significantly to levels better than normal; benefits were maintained 1 month after completion of Vd-s. CISS scores dropped to normal level, which was maintained up to 1 year.
Conclusions and Translational Relevance:
Intertrial variability is the major marker of vergence disorders. The Vd-s research-based method leads to normalization of vergence properties and lasting removal of symptoms. The efficiency of the method is due to the spatiotemporal parameters of repetitive trials that stimulate neural plasticity.
Keywords: vergence, accommodation, binocular vision/stereopsis, rehabilitation, double-step task
Introduction
Dysfunctions in binocular vision affect quality of vision and quality of life as it is an important socioeconomic issue in modern society. Prolonged use of visual interfaces, such as smartphones and tablets, can increase such dysfunctions; particularly vergence disorders are highly prevalent, occurring in up to 38% of young adults.1
Among disorders of binocular function, vergence insufficiency is the inability to converge or diverge the eyes smoothly and effectively to the object of interest and/or inability to maintain the vergence angle.2 The prevalence of convergence insufficiency (CI) alone is approximately 5%.3 Diagnosis of binocular dysfunctions and vergence insufficiency is based mostly on clinical testing and clinical criteria that can vary with investigators.4–6 Recent standardization efforts of several clinical centers leads to a consensus for a primary survey of patients with a questionnaire designed to evaluate symptomatology.7
The evaluation of symptomatology is used widely in the literature,5,6,8–16 mostly with the questionnaire called Convergence Insufficiency Symptom Survey (CISS), which have been validated from childhood to adulthood.7,17 Despite a few limitations of this subjective test, clinical and global studies have suggested a symptomatic prevalence close to 30%.4,18
Vergence orthoptic training (also called vision therapy) helps to alleviate symptoms.2,19 Orthoptic rehabilitation typically includes convergence exercises. Pencil pushup is the cheapest and the most used exercise.6,12,16,20,21 Disparity vergence exercises using prism bar or a synoptophore also are common, as well as the use of stereograms. Interestingly, the decrease of symptomatology in children with vergence insufficiency appears later than the improvements measured with clinical tests, such as the near point of convergence (NPC) or the fusional vergence measured with a prism bar.22 Scheiman et al.3 investigated the treatment modalities for CI; the most common treatment modalities used by optometrists and ophthalmologists among pencil push up, home-based vision therapy orthoptics, and office-based therapy. The Convergence Insufficiency Treatment Trial (CITT) Investigator Group conducted a preliminary study assessing the effectiveness of home-based push-up therapy and office-based vision therapy/orthoptics for the treatment.23 This study established the placebo method used by the same group subsequently14 to demonstrate superiority of office-based therapy. Yet, there is no standard method from one study to the other or from one clinician to another, as pointed out by Rogers et al.24 Moreover, the use of prisms, synoptophores, and stereograms all involve a conflict between vergence and accommodation.
Objective eye movement recording could be of interest to characterize vergence disorders as well as to assess the effects of visual therapy. The very few existing studies will be summarized below.
The relation between orthoptic tests and eye movement parameters recorded over training sessions was first reported by Grisham et al.25 The vergence tracking rate measured with an eye monitor and vergence measured with prisms increase after a conventional orthoptic training. Van Leeuwen et al.26 also reported changes in vergence dynamics after orthoptic training. After 12 sessions of orthoptic therapy in children with vertigo associated with vergence disorders, Bucci et al.27 reported an improvement of the accuracy of convergence and divergence as well as a latency reduction.
More recently, Alvarez and Kim10 also performed vergence recordings before and after vergence rehabilitation. They used a haploscopic device (involving inevitably a conflict between vergence and accommodation) and reported an increase in the peak velocity of disparity-driven vergence after the end of vergence rehabilitation. Disparity-driven vergence was tested again with the haploscopic device. The latest work from this team provide reference data on the neural substrate of such vergence improvements, highlighting the role of the parietal, frontal, and cerebellar areas.28,29
In a laboratory study, Jainta et al.30 measured properties changes of vergence eye movements in children with vertigo while repeating convergence and divergence movements between light-emitting diode (LED) targets displayed in the real space. Indeed, such display of LED target requires synergetic vergence accommodation changes similar to eye movements usually performed in everyday life. After approximately 15 minutes of repetition, improvements were measured in the latency and the accuracy of vergence eye movements.
We developed a visual acoustic device (REMOBI) that allows one to diagnose vergence disorders and rehabilitate vergence. This apparatus uses targets in the real 3-dimensional (3D) space so that there is no conflict between vergence and accommodation (patent US8851669, WO2011073288). With use of the REMOBI device together with video-oculography, we aimed to quantify the properties differences of vergence eye movements in healthy subjects versus ones clinically diagnosed with vergence disorders, test the clinical validity of a research-based method for vergence rehabilitation, the so-called vergence double-step (Vd-s) protocol implemented on the REMOBI device,31 and cross-correlate eye movement parameters with orthoptic tests and symptomatology scores.
Material and Methods
The investigation adhered to the tenets of the Declaration of Helsinki and was approved by the local human experimentation committee, the “Comité de Protection des Personnes” (CPP) Ile de France VI (No: 07035), Necker Hospital in Paris, France. Informed written consent was obtained from each subject.
Subjects
We recruited 19 students (mean age, 23.1 ± 2.0), mostly in a technical secondary school specialized in optics (Fresnel, Paris, France). Subjects were divided into two groups according to orthoptic evaluation using standard criteria. On the basis of such testing, eight subjects were considered as healthy (23.3 ± 1.9 years; 4 male), while 11 other subjects were diagnosed as presenting with vergence disorders (22.9 ± 2.1 years; 4 male).
Orthoptic Examination
Orthoptic examination was done on separate days before the oculomotor tests with video-oculography (45 ± 46 days). Binocular vision was evaluated with the TNO random-dot stereo test and/or with the Titmus Fly test.32–34 Healthy subjects had scores <60 seconds of arc. Orthoptic vergence response and heterophoria were tested with the use of prisms. Also, heterophoria was tested with the Maddox test. The near point of convergence was measured, as well as the heterophoria at near viewing and at far distance.
Normative values from the study of von Noorden et al.19 were used for the orthoptic tests as reported by Yang et al.35 (NPC < 10 cm, stereo acuity ≤ 60″, far exophoria 0–2 prism diopters [pD], near exophoria inferior to 6 pD, far divergence 5–9 pD, near divergence 15–23 pD, far convergence 15–23 pD, and near convergence 18–24 pD). Symptomatology was evaluated using the CISS17,36 and CISS scores ≤20/60 were considered normal as reported by several investigators.4,6 Clinical results are summarized in Table 1.
Table 1.
Values of Orthoptic Tests for Healthy Subjects and for Subjects with Vergence Disorders, and Follow-Up of the Vergence Disorders Group 1 Month after the Rehabilitation of the Subjects with Vergence Disorders
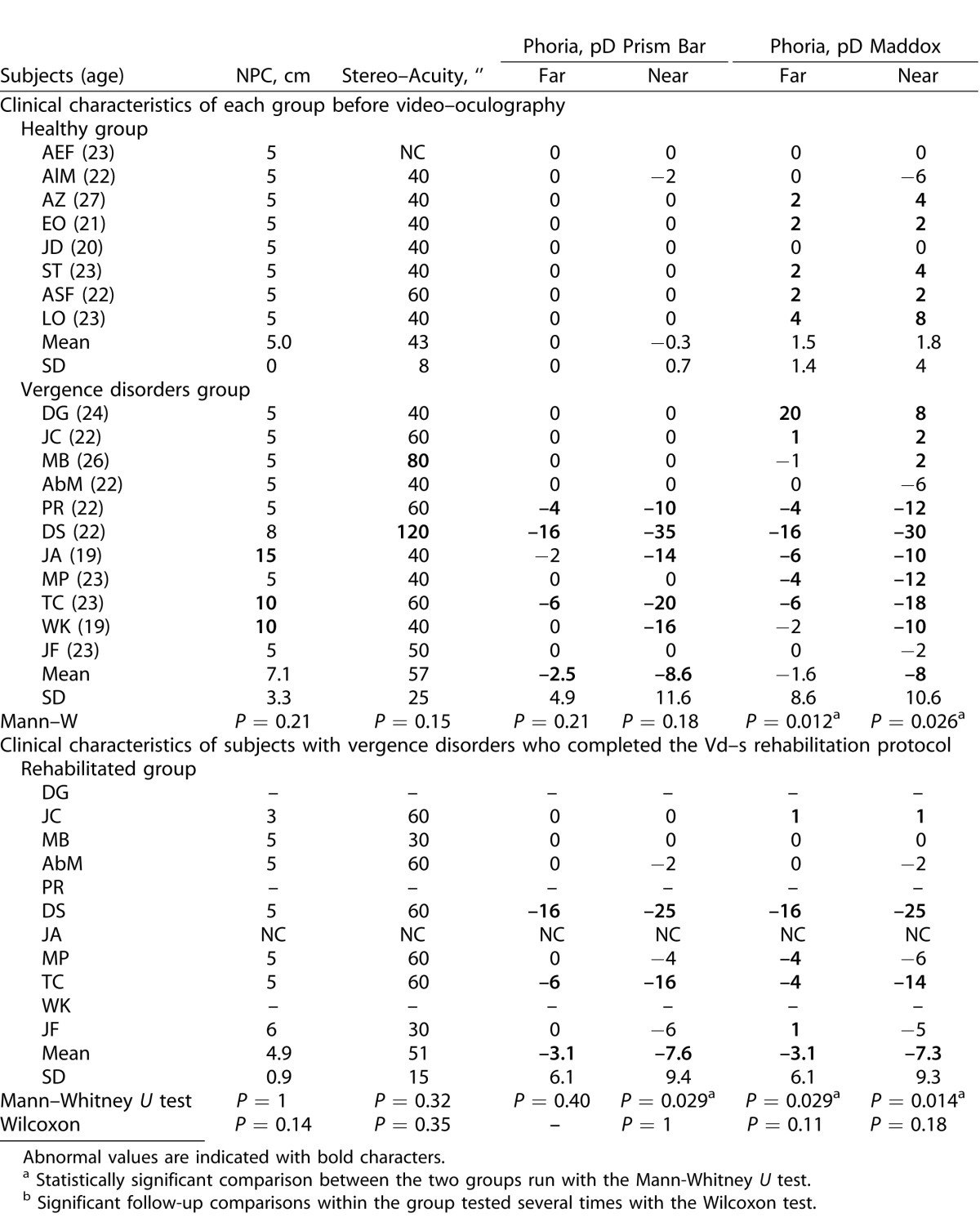
Table 1.
Extended.
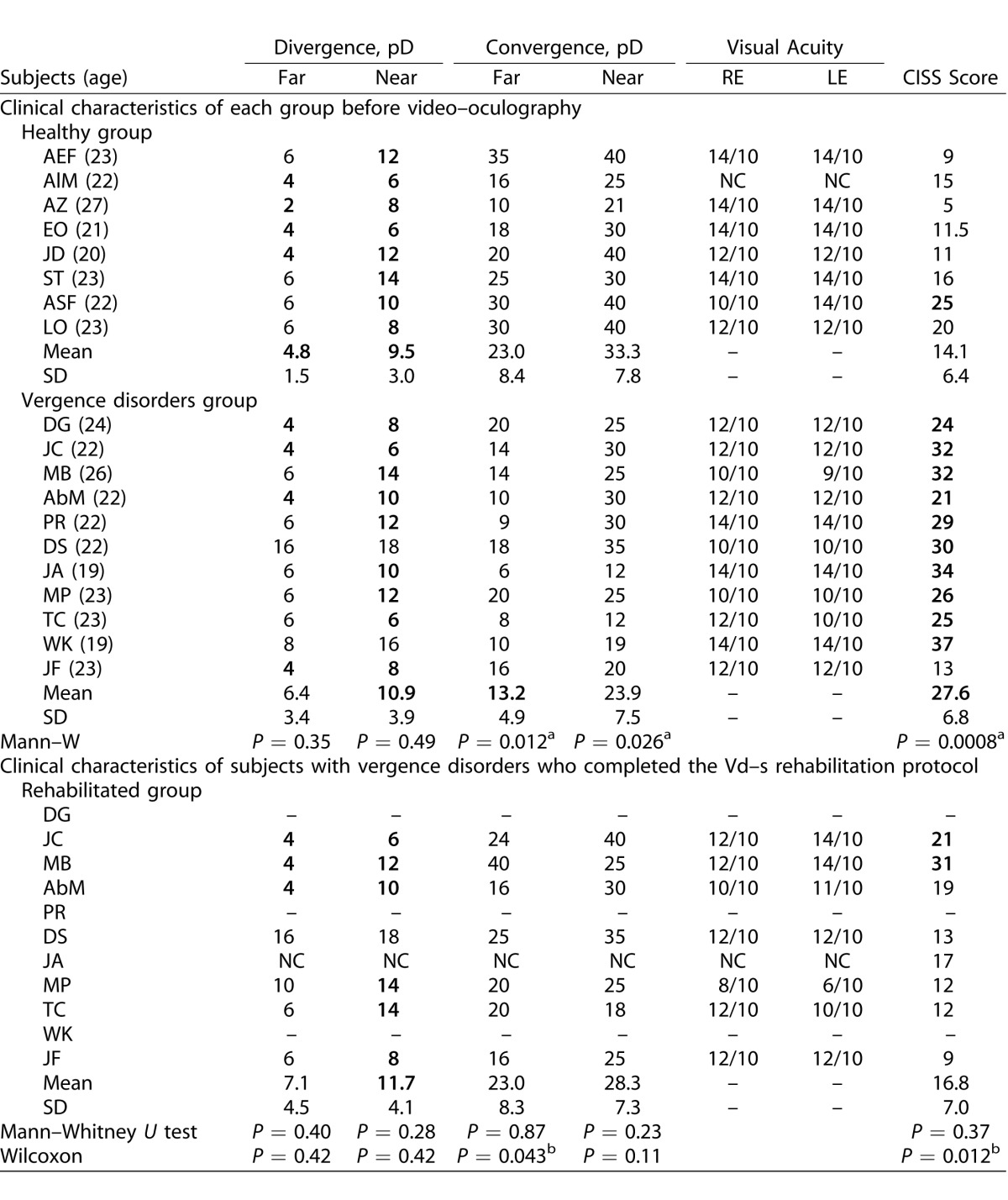
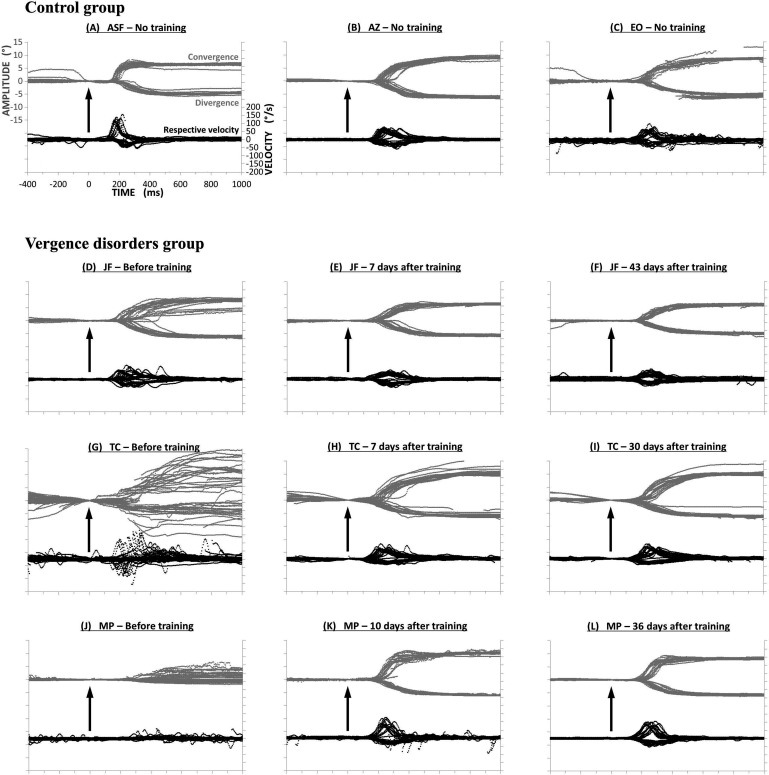
Subjects were divided into healthy versus vergence disorders groups by taking into consideration the following criteria: high CISS score (>20) and the presence of at least two abnormal values in orthoptic tests. Nevertheless, our population showed in general low values of divergence for either subgroup and raised the question whether the criteria reported by Von Noorden for divergence19 still are valid for young students nowadays. Moreover, two of the subjects were difficult to classify on clinical criteria alone as their final classification was based on recorded eye movement properties: subject ASF had a high CISS score but overall normal values in the orthoptic tests. Moreover, objective video-oculography of vergence confirmed normal or better to normal spatiotemporal properties. Therefore, we considered this subject as healthy despite a high CISS score (see Fig. 6A, described later). In contrast, subject JF had a very low CISS score and no major abnormality in the orthoptic tests; yet, the vergence test with video-oculography revealed inaccuracy particularly for convergence. Therefore, she was classified in the vergence disorder group and underwent the vergence rehabilitation protocol (see Fig. 6D, described later).
Figure 6.
Trajectories of vergence movements from three healthy subjects ASF (A), AZ (B) and EO (C), and three subjects with vergence disorders JF (D–F), TC (G–I), and MP (J–L). Convergence and divergence traces are superimposed at the top of each figure (in gray); their respective velocity traces are shown at bottom (in black). Vergence traces have small variability, that is, they are reproducible for healthy subjects (A–C). In contrast, variability is high for subjects JF (D) and TC (G), and subject MP shows quasi-paucity of divergence (J). After rehabilitation with the Vd-s protocol: variability decreased for all subjects. For each subject the benefits are stable over 1 week (E, H, K) and 1 month (F, I, L) after the end of rehabilitation.
Oculomotor Tests
Oculomotor tests were performed in mesopic light conditions; subjects sat in front of the trapezoid REMOBI device, which was placed at eye level. Stimuli of this visual display were red LEDs displayed at different distances always in the horizontal and the midsagittal planes (0°); LED characteristics were nominal frequency 626 nm, intensity 180 mCd, and diameter 3 mm. Adjacent to each LED was embedded a buzzer with the following characteristics: nominal frequency approximately 2048 Hz, sound pressure level ≥70 dB, diameter 12 mm. The oculomotor tasks will be described below.
Eye Movement Recording
Eye movements were recorded binocularly with a video-oculography EyeSeeCam system (University of Munich Hospital, Clinical Neuroscience, Munich, Germany, available in the public domain at http://eyeseecam.com/). The sampling rate of the EyeSeeCam system was 222 Hz. The optimal spatial resolution was approximately 0.01°.
Calibration
The standard EyeSeeCam five points calibration was applied using a matrix of laser dots that was presented at a viewing distance of 1.5 m with peripheral dots displayed at 8.5° rightward, downward, leftward, upward; subjects fixated each dot at their own pace, and the total calibration task lasted 10 seconds.
A further calibration task was performed before the vergence test; this task consisted of eight leftward and eight rightward randomly interleaved saccades to LEDs located at 10° and 20° from the midsagittal plane of REMOBI, at a distance of 1.5 m from the subjects' eyes.
Vergence Test
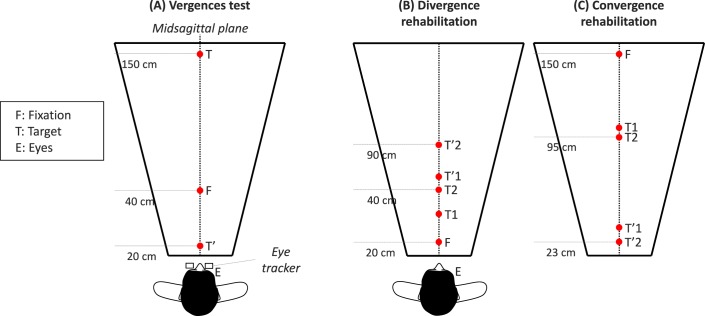
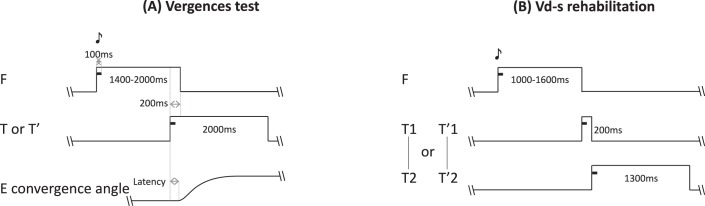
Each trial started by lighting the LED at 40 cm along the median plane (F, see Figs. 1A, 2A). After a variable fixation period of 1200 to 1800 ms, the target LED (T or T') was lit for 2000 ms. The fixation LED was switched off 200 ms after the onset of the target LED (overlap paradigm). The lighting of each LED (fixation or target) was accompanied by the corresponding sound buzzer for 100 ms. Such visual-acoustic stimulation aimed to reinforce deployment of attention resources to the LED. The vergence test contained 40 trials, half of them to the far target (T) and the other to the near target (T'). The far one called for a change in vergence angle from 9° to 2°, that is a divergence movement of 7°. The near target LED (T') called for a change of vergence from 9° to 17°, that is, a convergence of 8°. Convergence and divergence trials were interleaved pseudo-randomly. Between trials, a blanked period of 300 to 700 ms was applied. All eye movement data to be reported here came from this task.
Figure 1.
Spatial arrangement of targets in the vergence test ([A] subjects are looking successively at different LEDs; from F, the initial fixation LED, to T, the target LED for divergence; or T', the target LED for convergence). Double-step targets used for divergence and convergence rehabilitation ([B, C] from F to T1 or T2, the first target location; then to T'1 or T'2, the final target location). The two types of trials were randomly interleaved.
Figure 2.
(A) Temporal arrangement of the vergence test. Each trial starts with the fixation target that appears for a variable period of 1200 to 1800 ms; following this period the target LED lights are on for 2000 ms together with a paired buzzer that lasts only 100 ms; the fixation LED switches off 200 ms after the onset of the target LED (overlap period). (B) Temporal arrangement of the vergence double-step rehabilitation protocol. The trial starts with lighting on the fixation LED for a random period between 1000 and 1600 ms; the fixation LED is accompanied with a buzzer sound for the first 100 ms. Following the fixation period, the target LED lights are on at its first position together with a paired buzzer for 100 ms. After 200 ms the fixation LED switches off and at the same time the LED target steps to it final position (T2 or T'2) where it stays on for 1300 ms; a sound accompanies this second step also for the first 100 ms. A blank period of 300 to 400 ms separates trials.
V-ds Protocol
A 35-minute rehabilitation session was applied weekly, except for subject AbM, who performed all sessions within 1 week. The rehabilitation sessions contained 11 blocks sequenced as follows: 2 blocks of divergence, 3 blocks of convergence, 2 blocks of divergence, 3 blocks of convergence, and a final block of divergence. Each block contained 40 trials and lasted 2 to 3 minutes (divergence and convergence trials are illustrated, respectively, in Figs. 1B, 1C). A 1-minute pause was applied between blocks during which the subject was questioned on whether LEDs were seen single, double, or blurred at each location; subjects were allowed to stand up and relax while resting.
Typical convergence and divergence rehabilitation trials with the double-step paradigm are shown in Figure 2B. Briefly, the target for convergence or divergence appeared first to a location (T1 or T'1); after 200 ms, presumably before the accomplishment of the vergence eye movement, the target stepped to a second location (T2 or T'2). Given that the vergence latency is between 160 and 250 ms,37,43 and vergence execution lasts between 350 and 550 ms,34 it is almost certain that the second step of the target occurred before the initial vergence eye movement has been made. This double-step protocol for clinical purposes is part of the patent.
After Oculomotor Rehabilitation
To evaluate the effects of the rehabilitation, the vergence test described above was repeated at least 5 days after the end of the five sessions of the Vd-s protocol (8 ± 4 days). A final vergence test was repeated at least 1 month after the end of the rehabilitation (37 ± 8 days) together with orthoptic examination that was performed by the same orthoptist as before rehabilitation; the orthoptist did not have access to the video-oculography data.
Symptomatology was evaluated with CISS score 1 month after the end of rehabilitation, and several months later (7.7 ± 2.2 months).
Data Analysis
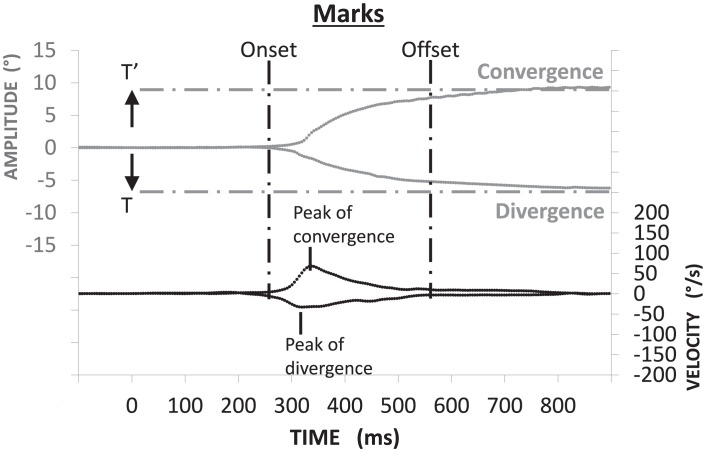
Calibration factors for each eye were extracted from the saccades recorded in the calibration task. From the individual calibrated eye position signals we derived the vergence signal by calculating the difference between the two eyes (i.e., left eye – right eye). The velocity of the vergence signal was computed using a symmetrical two-point differentiator combined to low-pass filtering with a Gaussian finite impulse response (FIR) filter (cut-off frequency 33 Hz). The beginning and end of vergence movements were defined as the time point when the eye velocity exceeded or dropped below 5°/s. These criteria are standard.37–41 The placement of the markers was verified carefully by an investigator scrutinizing the vergence signal on a computer (Fig. 3).
Figure 3.
Method for vergence analysis: convergence and divergence (in gray) were obtained by subtraction of right eye position from the left eye position (LE – RE). The corresponding velocity trace is shown below. Mark of onset and offset of vergence is based on velocity criteria: the time point when the eye velocity respectively exceeded or dropped below 5°/s. The horizontal dotted lines indicate the targets' position.
Some eye movements were rejected from the analysis due to blinks or to partial loss of signal during the recording, or to anticipation (i.e., below 80 ms) or to latencies longer than 800 ms. These criteria of exclusion are standard.38,39,42 Note that subject JA performed quasi-systematically a blink during the movements recorded after the Vd-s rehabilitation protocol (≥90% of divergence movements were concerned) and this behavior is known to diminish the dynamic performance of eye movements.43 For the remaining subjects (N = 18), 80 to 90% of the trials were used for the analysis; such rejection level (10 to 20%) is similar to that reported in a previous study of vergence in healthy young adults.38,39
Statistical Analysis
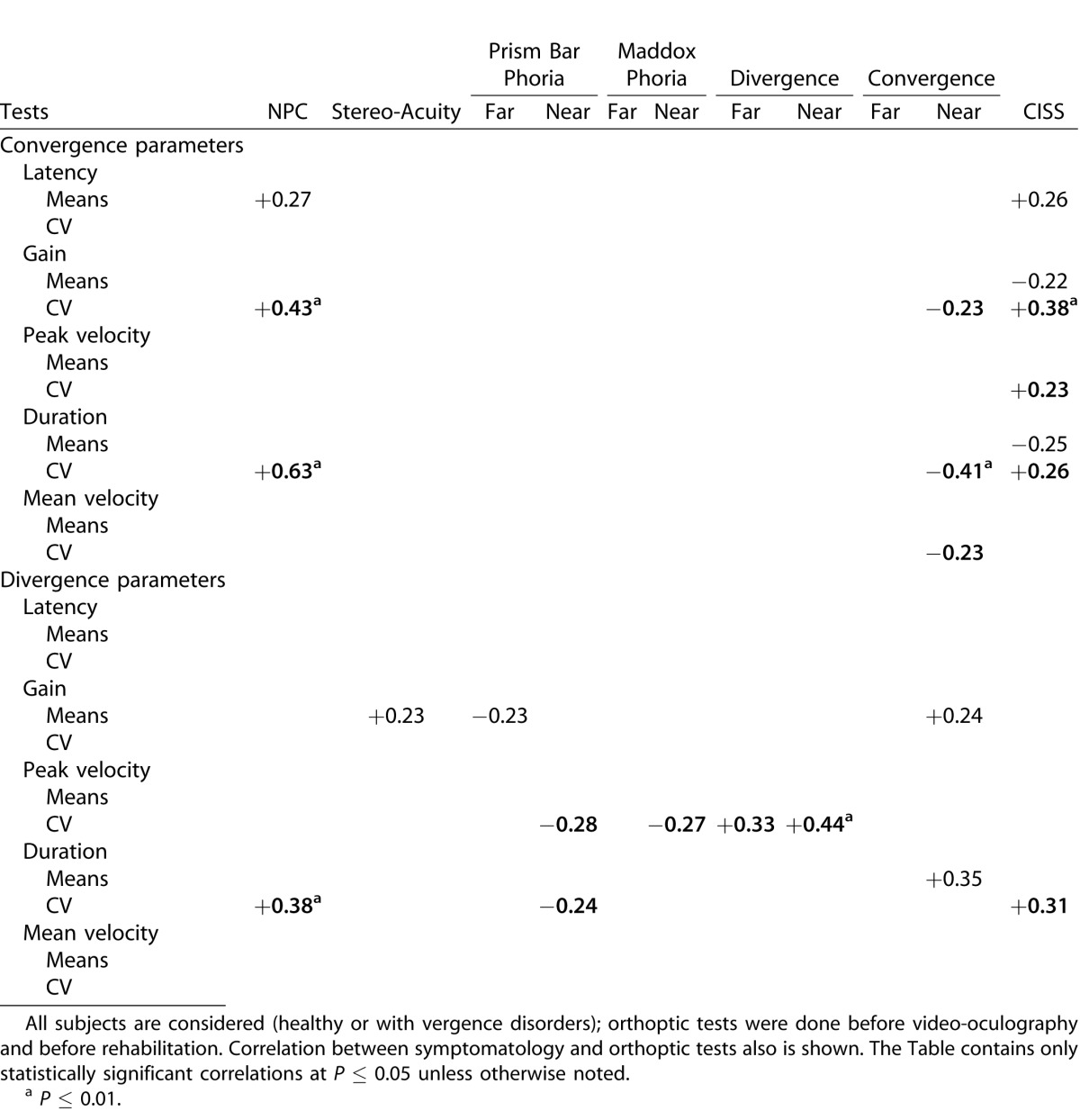
Due to the rather limited number of subjects and also to nonnormal distribution of some of the parameters, we applied nonparametric tests using the software “Statistica.” All statistics were done on individual means and on the coefficients of variation (CV), which was evaluated as the ratio of the standard deviation over the mean. This parameter describes the intertrial variability.44 Comparison of means or CV between the healthy and vergence disorders groups was done with the Mann-Whitney test.45 This comparison was done for the data obtained before the rehabilitation; the comparison was repeated 1 week and 1 month after completion of the Vd-s rehabilitation protocol. Follow-up of the vergence disorders group was done with the Wilcoxon test, comparing each parameter of eye movements for the three sessions taken two by two (before, 1 week, and 1 month after the end of the Vd-s protocol). We called rehabilitation effect the difference between before, and 1 week or 1 month after rehabilitation. Finally, all eye movement parameters shown (see Table 7, to be described later) were cross correlated with the values of orthoptic tests and CISS scores of symptomatology, using multiple linear regression analysis.46
Table 7.
Values of Correlation Coefficients between Orthoptic Tests and Convergence or Divergence Parameters Measured with Video-Oculography
Results
Effects of Vd-s Rehabilitation on Symptomatology
As mentioned in the Methods, the classification of subjects into two groups was overall in line with previously established norms4,6,19,35 even though two subjects were difficult to classify and our decision also was based on objective measurement of vergence eye movements.
Of major importance is to what extent our rehabilitation with the Vd-s protocol could alleviate symptoms. The results are shown in Table 1. Overall the healthy subjects showed a very low score of symptomatology (14.1 ± 6.4) relative to the group that was rehabilitated with the Vd-s (27.6 ± 6.8). The two exceptional cases (ASF and JF) already were mentioned in the Methods. At 1 and 8 months after rehabilitation with the Vd-s protocol, the symptomatology score dropped to 16.8 (±7.0) and 16.3 (±7.0), which is close to normal value. Table 1 shows the statistical comparison between the healthy group and the group with vergence disorders together with the probability values (Mann-Whitney U test). Before Vd-s rehabilitation, the score for subjects with vergence disorders was significantly higher than that of the healthy group (P = 0.0008), whereas after rehabilitation with the Vd-s the difference was no longer significant (P = 0.37).
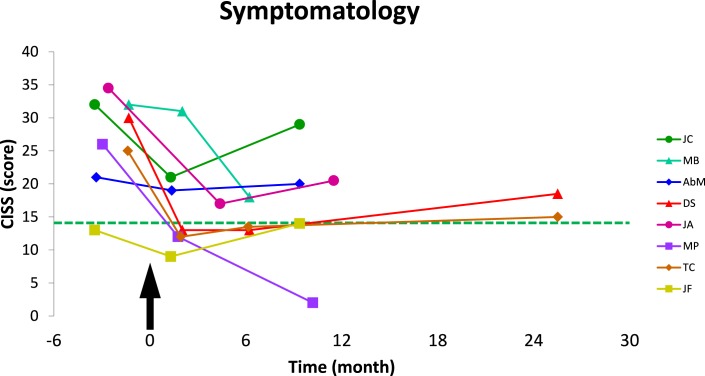
Follow-up comparison for the vergence disorders group before and after rehabilitation also was done with the Wilcoxon test and the probability values are shown in Table 1. The CISS score following the Vd-s protocol was significantly lower than before rehabilitation, and 1 and 8 months after the end of Vd-s protocol (respectively, P = 0.012 and P = 0.021). Figure 4 shows the changes in CISS for individual subjects, for comparison the group mean score of the healthy subjects is indicated by the horizontal line; all subjects showed decrease of their CISS score.
Figure 4.
Follow-up of the symptomatology scores (CISS) of subjects with vergence disorders are plotted over time. Arrow indicates the beginning; that is, the first day of Vd-s rehabilitation protocol. Dotted line shows the mean score of the healthy group. CISS score decreased after rehabilitation, and the results were stable over time.
Orthoptic Characteristics and Effects of the Vd-s Rehabilitation Protocol
Results of all orthoptic tests are shown in Table 1. Compared values of orthoptic tests between the two groups showed several significant differences: the Mann-Whitney U test showed significant differences for the Maddox values (at near and far, P = 0.026 and P = 0.012, respectively), but also for convergence at near (P = 0.026) and at far (P = 0.012). If one excludes the two cases ASF and JF for these comparisons, the overall group results still are the same.
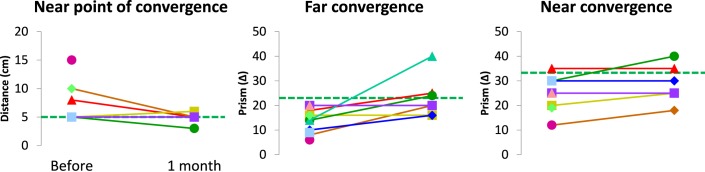
Follow-up comparison for the vergence disorders group before and after rehabilitation with the Vd-s protocol, showed statistically significant increases for convergence at far (P = 0.043). Individual data of NPC and convergence at near and far are shown in Figure 5.
Figure 5.
Individual values of orthoptic tests for the vergence disorders group: dotted lines show the mean values of the healthy group. Following the Vd-s rehabilitation, the near point of convergence decreased, and the convergence amplitude measured with prisms at far or near increased; the results are durable as the orthoptic examination is done 1 month after the end Vd-s rehabilitation.
In summary, the CISS is most sensitive for differentiating healthy subjects from those with vergence disorders and also for follow-up of the patients' evolution after the Vd-s rehabilitation protocol. In terms of orthoptic tests, convergence particularly at far is the second important parameter which differentiates the healthy from the vergence disorder group and also reflects the evolution after the Vd-s protocol. In contrast, divergence is systematically of low value for both groups. As it will be shown below, objective vergence eye movement with video-oculography corroborates the weakness of divergence for both groups with or without vergence disorders.
Eye Movement Data – Qualitative Results
Figure 6 shows vergence eye movement traces for convergence and divergence; traces are superimposed and offset at the time point zero corresponding to onset of the target LED, from which the latency is measured. Figures 6B and 6C show traces from two healthy subjects (AZ and EO), whose CISS score was 5 and 11.5, respectively. Figures 6G and 6J show traces from two subjects (TC and MP) with vergence disorders (respectively diagnosed for severe CI and high heterophoria); results from the same subjects are shown 1 week (Figs. 6H, 6K) and 1 month (Figs. 6I, 6L) after completion of the five sessions of Vd-s rehabilitation. An immediate observation is that the subjects with vergence disorders show abnormal vergence traces, namely high variability from one trial to the next and general hypermetria, particularly for subject MP. Also, the initiation is delayed relative to the healthy subjects. Following the five sessions of Vd-s protocol, all these aspects improved considerably and, in fact, became normal or better. Note the lasting nature of the effect seen 1 month later.
Figure 6A shows the data from subject ASF, who was considered normal, and those from subject JF, who was considered as patient is presented in Figure 6D. Indeed, normal accuracy and reproducible vergence traces are observed for subject ASF. In contrast, subject JF showed abnormal variability and lasting improvement after the Vd-s rehabilitation protocol.
In summary, qualitative analysis shows two major results: (1) clear differences in eye movement trajectories between the healthy subjects and those clinically diagnosed as having vergence disorders, and (2) vergence traces also reveal the benefits and the lasting improvement after the Vd-s rehabilitation protocol. Finally, in cases of incompatibility between symptomatology scores and orthoptic tests, objective vergence measures provide the solution.
Eye Movement Data – Quantitative Results
Latency
Table 2 shows the results on latency for healthy subjects and subjects with vergence disorders; for the latter, data are shown for recordings made before, 1 week, and 1 month after the 5 sessions of Vd-s protocol. Data are shown separately for convergence and divergence. Each Table shows the individual means and the corresponding coefficient of variation (i.e., intertrial variability). Group means also are shown as well as the P value of statistical comparison with the Mann-Whitney U test for between group comparisons and the Wilcoxon test for within group follow-up comparisons.
Table 2.
Individual and Group Means of Latency (in ms) Together with the Coefficient of Variation (SD/Mean) that Indicates Intertrial Variability
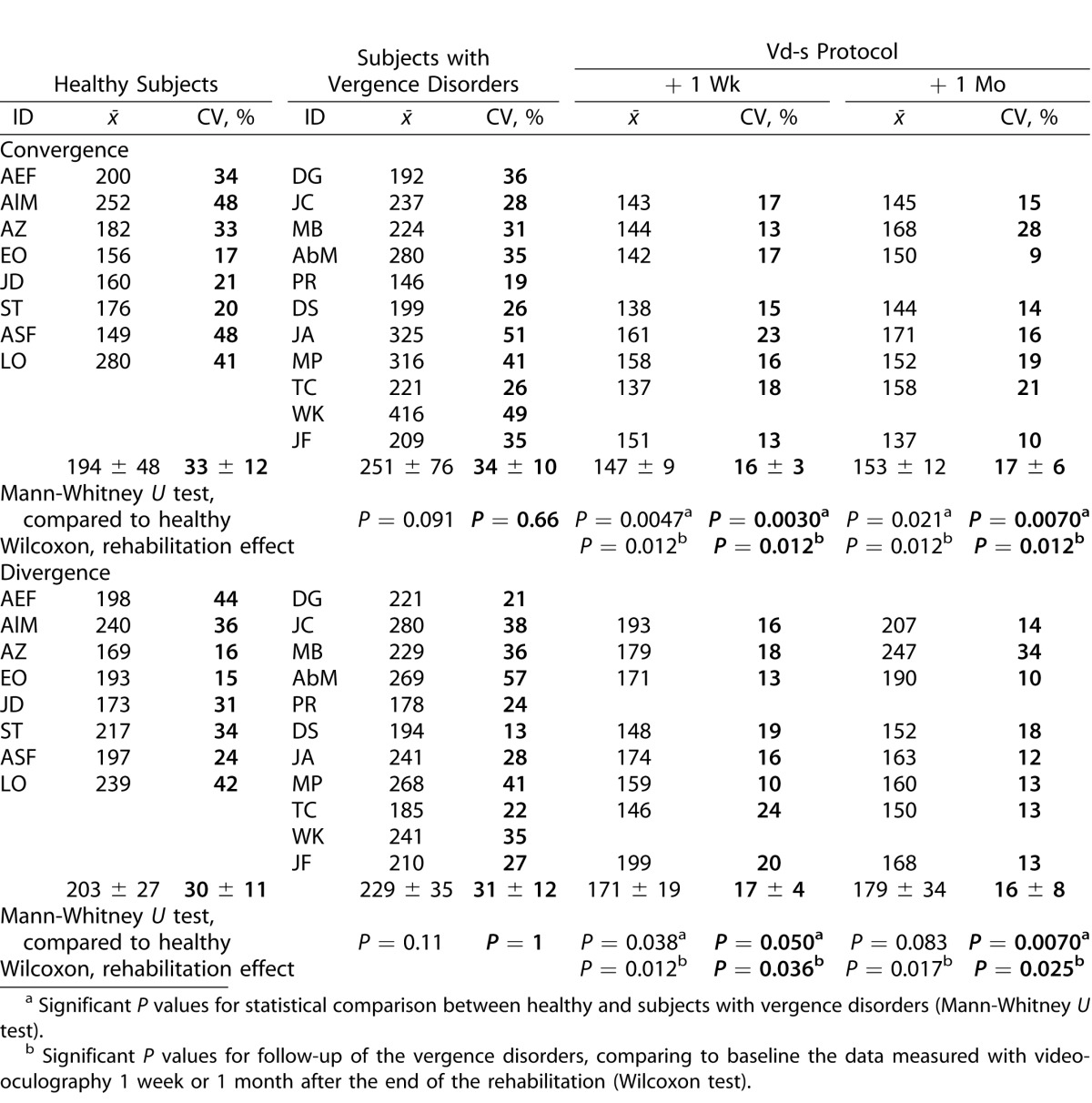
The first important result concerns latency that tends to be longer in subjects with vergence disorders, but the difference does not reach statistical significance, neither for convergence nor for divergence; intertrial variability is not significantly different either.
Nevertheless, follow-up of the vergence disorders group with the Wilcoxon test shows several statistically significant effects due to Vd-s rehabilitation protocol. Indeed, the latency for convergence or divergence decreased significantly relative to prerehabilitation values as well as their variability (all comparisons were significant at P ≤ 0.038); the effects were present 1 week and 1 month after the end of rehabilitation. The mean latency value of postrehabilitation was 147 ms (±9) then 153 ms (±12) for convergence, and 171 ms (±19) then 179 ms (±34) for divergence and the intertrial variability was inferior to 20%. Such performance indicates optimization in vergence initiation following the Vd-s protocol.
Indeed, relative to the values of the healthy subjects (comparison with the Mann-Whitney U test) the latency of convergence or divergence after the rehabilitation became significantly less variable (all comparisons were significant at P ≤ 0.05).
In summary, even though latency of convergence and divergence does not differ significantly for subjects with vergence disorders, rehabilitation with the Vd-s optimizes the initiation process reducing the latency of convergence and divergence to values even shorter than those from healthy subjects, and reduces intertrial variability.
Accuracy of Vergence
The results on accuracy are expressed in terms of gain values (e.g., amplitude of vergence divided by target excursion) and are shown in Table 3. Individual and group means are shown together with the intertrial variability (CV). Healthy subjects present accurate convergence (0.97 ± 0.17) but hypometria for divergence (0.76 ± 0.06). The intertrial variability is small (approximately 20% ± 9%); note that normal variability in the oculomotor field no matter what parameter is studied is approximately 20%, as reported by Yang et al.44
Table 3.
Individual and Group Means for Gain (Movement Amplitude/Target Amplitude) Together with CV
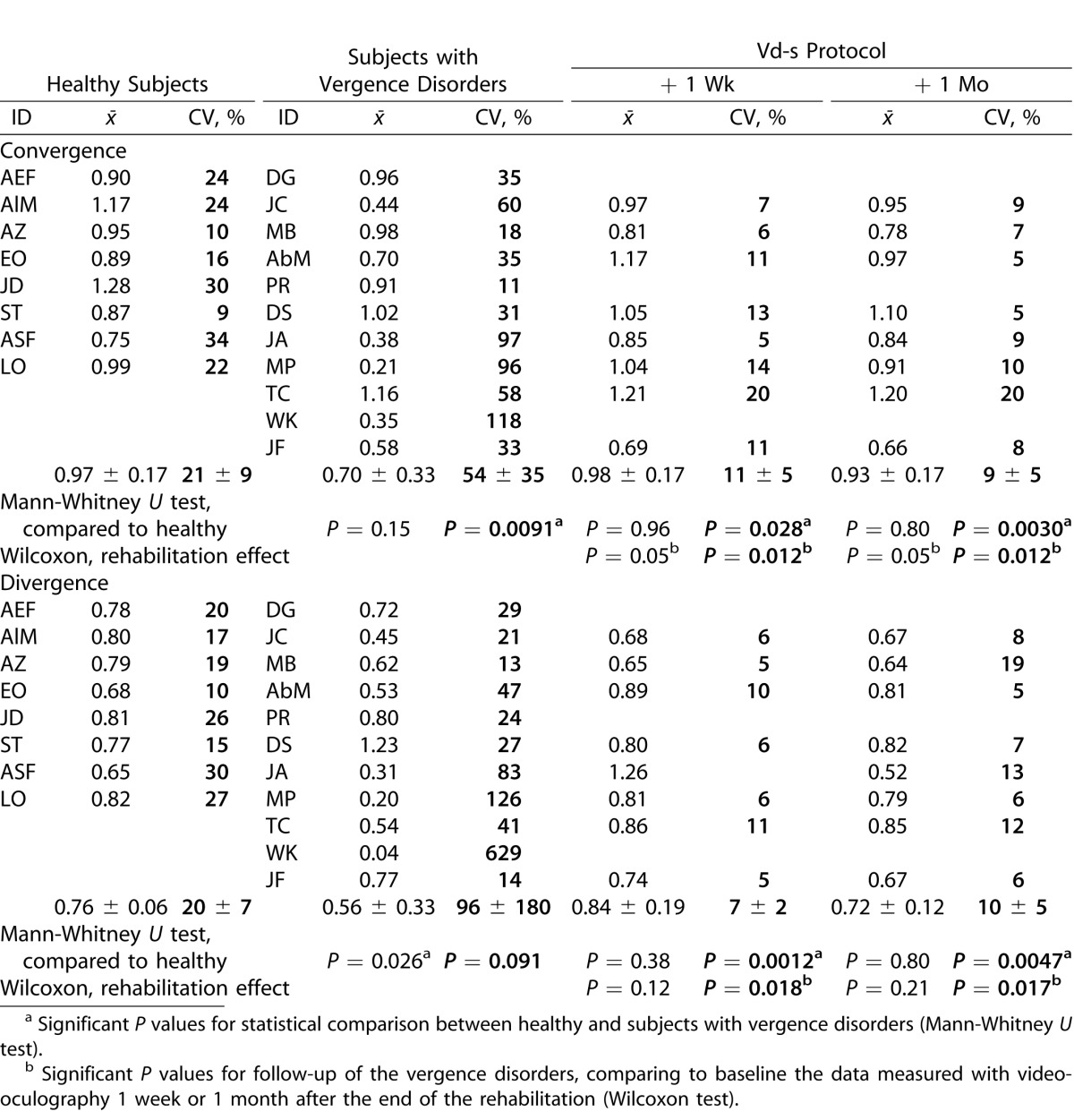
Relative to the healthy group, subjects with vergence disorders show lower mean gains (0.70 ± 0.33 and 0.56 ± 0.33, respectively, for convergence and divergence); divergence is significantly more hypometric for the vergence disorders group (Mann-Whitney U test, P = 0.026). The intertrial variability in subjects with vergence disorders is significantly higher than that of the healthy subjects. Variability reflects the difficulty in producing repetitively vergence eye movements. The intersubjects comparison shows significant higher variability of convergence for the vergence disorders groups (P = 0.0091) and a tendency for higher divergence variability (P = 0.091).
Follow-up of the vergence disorders group shows important effects of Vd-s. After the Vd-s rehabilitation protocol, mean values of convergence gain increased from 0.70 to value close to 1 (0.98 ± 0.17 and 0.93 ± 0.17); the changes were statistically significant 1 week and 1 month after the end of rehabilitation (Wilcoxon test, P < 0.05). Also, the variability for the vergence disorders group decreased significantly (P ≤ 0.012).
Relative to the healthy group, the variability became significantly smaller 1 week and 1 month after rehabilitation (Mann-Whitney U test, P = 0.028 and P = 0.0030, respectively). Thus, the results show lasting optimization of convergence.
For divergence, after the Vd-s rehabilitation protocol, the vergence disorders group show significant increase of the gain value (from 0.56 to 0.84 ± 0.19 and 0.72 ± 0.12, 1 week and 1 month after rehabilitation, respectively); however, the changes were not statistically significant (Wilcoxon test, P > 0.10) and the difference from healthy was not statistically significant either. In contrast, there was statistically significant reduction of the coefficient of intertrial variability, that dropped to 7 (± 2) and 10 (± 5). The difference from the before rehabilitation values is statistically significant (P ≤ 0.018 1 week and 1 month after rehabilitation).
Relative to the healthy group, the difference also is significant in the sense that after rehabilitation the variability decreased to supranormal levels (Mann-Whitney U test, P ≤ 0.005, for the comparison of healthy versus vergence disorders group 1 week and 1 month after rehabilitation).
In summary, the results show marked divergence hypometria for the healthy group but physiologic intertrial variability at approximately 20% for divergence and convergence. In contrast, the vergence disorders group shows increased hypometria particularly for divergence and abnormally large intertrial variability. The Vd-s rehabilitation protocol increased significantly the convergence gain and decreased significantly the intertrial variability, reducing it to supranormal values for convergence and divergence.
Vergence Dynamics: Peak Velocity, Duration, and Mean Velocity
Peak velocity data (individual and group means) are shown in Table 4. Comparison of the healthy and the vergence disorders groups shows a tendency for lower peak velocity for convergence for the latter group (Mann-Whitney U test, P = 0.062), and significantly higher intertrial variability (P = 0.0050).
Table 4.
Individual and Group Means of Peak Velocity Together with the CV
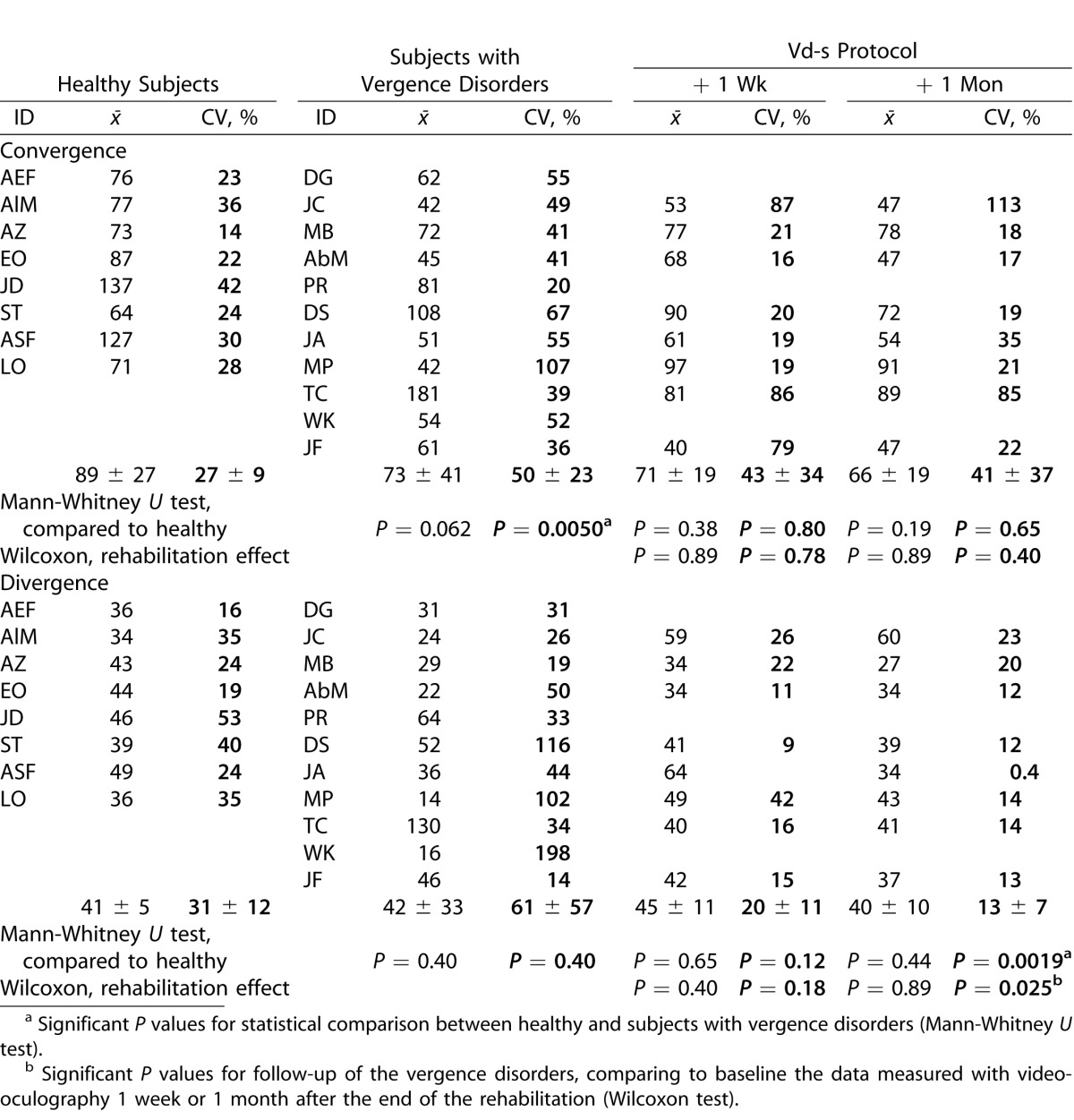
Follow-up of the vergence disorders group show that the Vd-s rehabilitation protocol did not significantly change the mean peak velocity neither for convergence nor for divergence. Yet, the intertrial variability decreased after Vd-s rehabilitation protocol and the change reached statistical significance for divergence when comparing variability before and 1 month following the end of Vd-s rehabilitation (Wilcoxon test, P = 0.025).
Also relative to the healthy group, the variability of divergence peak velocity became significantly lower for the vergence disorders group (Mann-Whitney U test, P = 0.0019). Thus, the results show, again, optimization after Vd-s rehabilitation even for parameters, such as peak velocity.
Concerning the duration, the group and individual means are shown in Table 5 for both groups of subjects. The differences in mean duration were not significant, neither between the healthy and vergence disorders groups nor for the variability.
Table 5.
Individual and Group Means of Duration Together with the CV
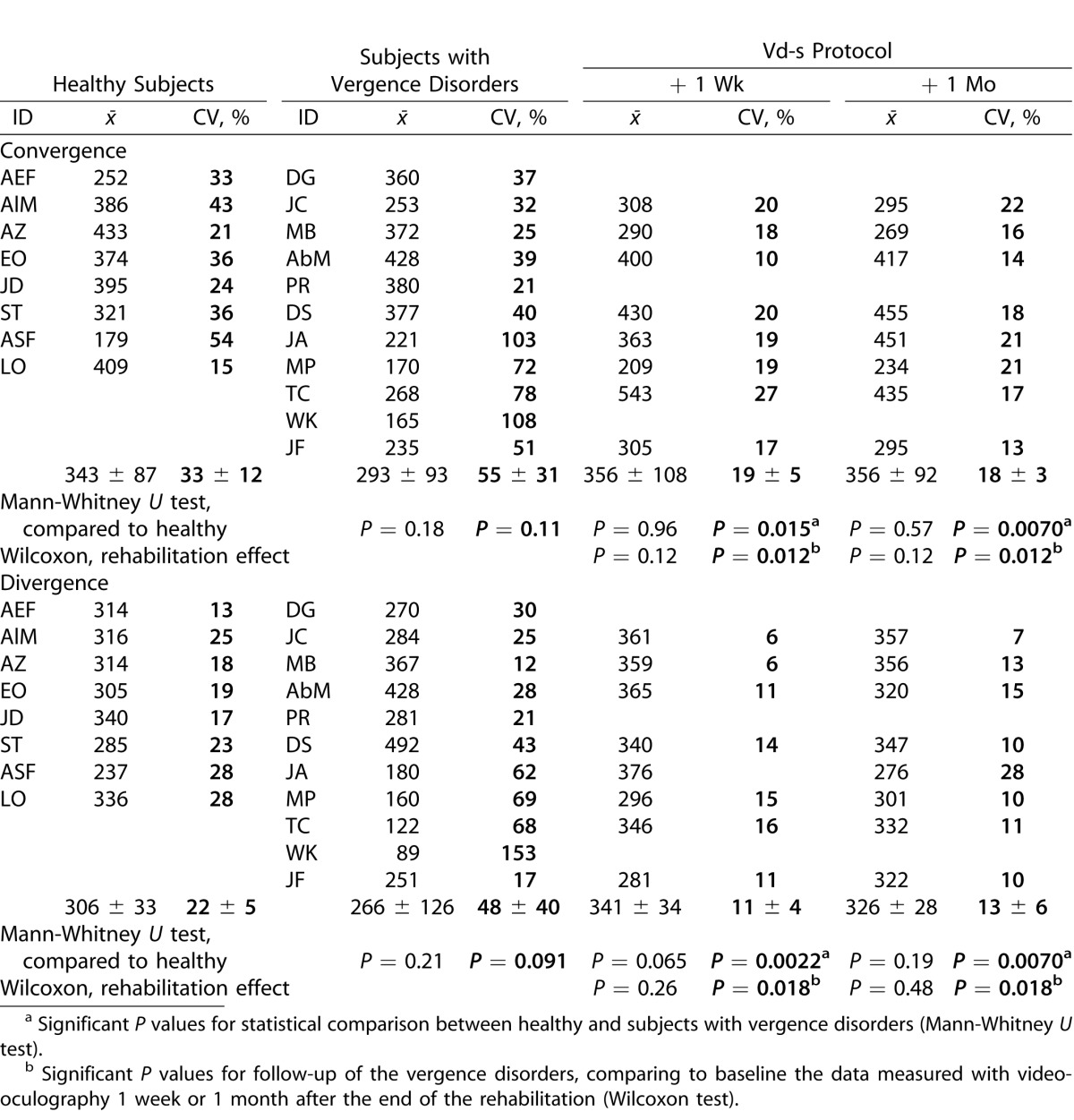
Follow-up of the vergence disorders group by comparing measurements before and after completion of Vd-s rehabilitation protocol did not show significant group mean differences for convergence and the same observation applies for divergence. Again, significant effects occurred for the intertrial variability: for convergence, the intertrial variability of the vergence disorders group after the Vd-s rehabilitation variability decreased significantly (Wilcoxon test, P = 0.012). The intertrial variability of divergence also was reduced significantly 1 week or 1 month after the Vd-s protocol (P < 0.02 for both comparisons).
Intertrial variability of divergence after the Vd-s protocol also was significantly lower than that of the healthy group for convergence and divergence (Mann-Whitney U test, P ≤ 0.02 1 week and 1 month after the completion of rehabilitation).
Given that some changes occurred in the amplitude of vergence eye movements (gain) and considering that peak velocity and duration depend on the amplitude, we also examined the mean velocity, which is the ratio of amplitude and duration (amplitude/duration). The results are shown in Table 6. Statistical results are similar to those for peak velocity and duration.
Table 6.
Individual and Group Means of Mean Velocity Together with the CV
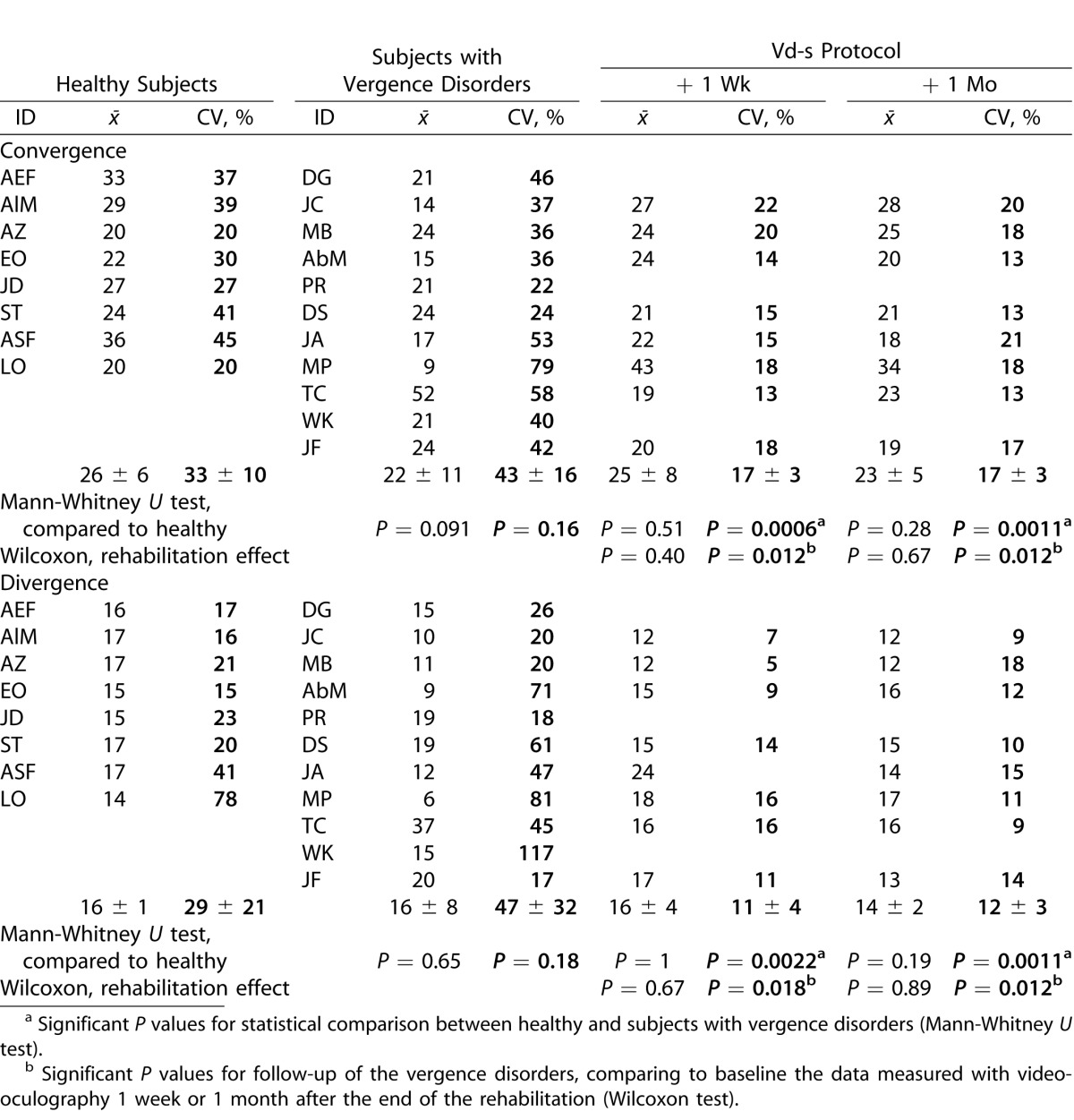
Before rehabilitation, no significant difference was observed between healthy subjects and those with vergence disorders, nor were there significant follow-up changes for the means of the latter group after rehabilitation.
Yet, follow-up showed a significant reduction of the intertrial variability 1 week and 1 month after the end of the Vd-s protocol (Wilcoxon test, P < 0.02 for convergence and divergence).
In summary, the results showed that the intertrial variability of all vergence parameters (latency, accuracy, peak velocity, mean velocity, and duration) is the parameter that differentiates most the healthy from the vergence disorders groups, and reflects best that rehabilitation benefits are stable over 1 month. Reduction of mean latency is the second major effect of such rehabilitation.
Correlation between Eye Movement Parameters, Orthoptic Tests, and Symptomatology
Multiple linear correlations were run between all parameters of eye movements and all values of orthoptic tests for the total group of subjects, healthy and vergence disorders groups before the Vd-s rehabilitation protocol. The results are shown in Table 7, which contains only the statistically significant correlations. Correlations were run separately for parameters of convergence and divergence eye movements.
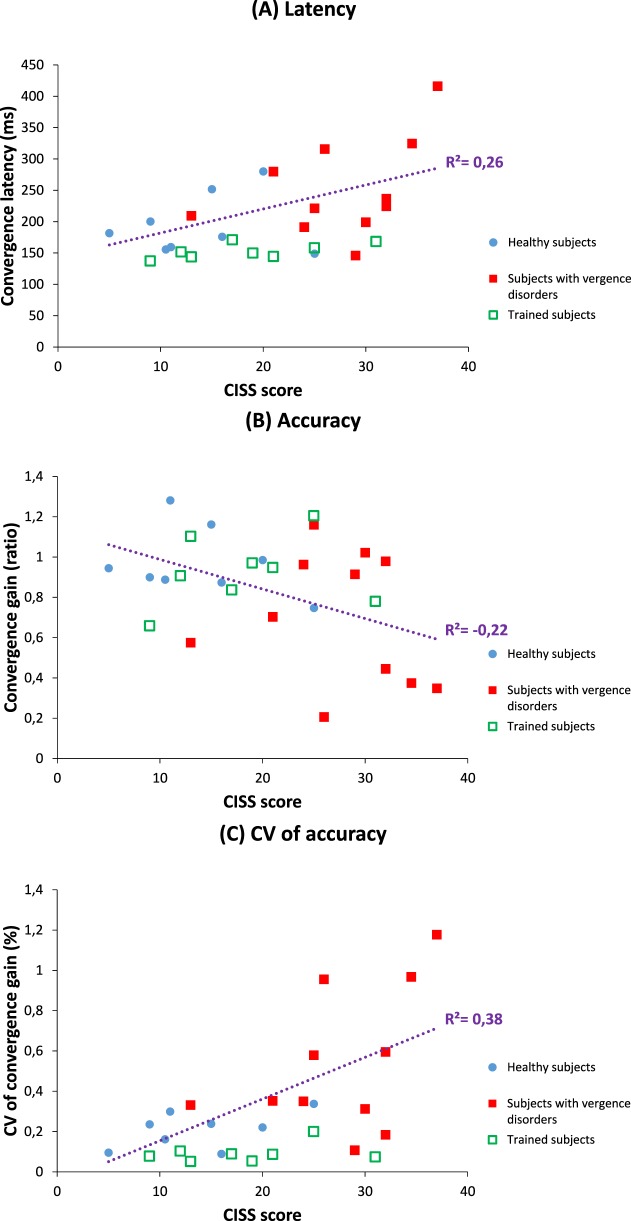
The main observation is that the NPC orthoptic measure correlates positively with many parameters of convergence eye movements measured with video-oculography (Table 7, the more remote the NPC, the longer the latency of convergence, and the higher the intertrial variability of the gain and duration for convergence. Another orthoptic measure correlated with the convergence properties is the convergence values at near measured with the bar of prisms: the lower the orthoptic value of convergence, the higher the intertrial variability for the gain, for the duration, and for the mean velocity of the convergence measured with video-oculography. The CISS score correlates with many parameters of the convergence measured with video-oculography; for instance, the higher the CISS score, the longer the latency of the convergence (Fig. 7A) and the lower its mean gain (Fig. 7B), also the higher the intertrial variability of the gain for convergence (Fig. 7C).
Figure 7.
Convergence latency is correlated positively with symptomatology measured by CISS score (A), and convergence gain is negatively correlated with symptomatology (B), while it is positively correlated with the variability of convergence gain (C). Data from healthy subjects are illustrated in blue circles, and for subjects diagnosed for vergence disorders, with full red squares (before rehabilitation) or empty green squares (after Vd-s rehabilitation); linear correlation model was run by considering only the data from the healthy and vergence disorder groups before rehabilitation. Values of subjects after rehabilitation are shown for illustrating the clear move towards normal levels.
For divergence, few meaningful correlations are indicated in Table 7: the higher the intertrial variability of the duration, the more remote the NPC (R2 = 0.38) and the higher the symptomatology (R2 = 0.31).
Discussion
Firstly, the study shows important differences in eye movement properties, namely in intertrial variability between healthy subjects and those clinically diagnosed for vergence disorders. Secondly, the results show important and lasting benefits of the Vd-s rehabilitation protocol in terms of latency, accuracy, and intertrial variability of all parameters of vergence; these results provided evidence for the clinical validity of the Vd-s method. Finally, eye movement parameters are correlated with some of the orthoptic tests. Most importantly, above all, eye movement properties correlate with symptomatology as measured with CISS, which decreased after Vd-s rehabilitation protocol. These results will be discussed below.
Classification of Subjects, Interest of Eye Movement Analysis
The orthoptic measures indicate small values of divergence measured with the bar prism test. This observation was corroborated with the objective eye movement recording: in healthy subjects, the gain of the divergence always was lower than that of convergence. Clinicians know that the divergence capacity is smaller. Yet, the clinical normative values reported by Von Noorden19 seem to be inappropriate for the young population nowadays.
Another important aspect is that diagnosis of vergence disorders on the basis of clinical tests might be difficult as incongruence between symptomatology and orthoptic tests may exist. Objective vergence movement recording and analysis used in our study provide a handy solution. Our observations are in line with the study of Brautaset and Jennings,16 who also reported no effects of orthoptic treatment on orthoptic measures of vergence-accommodation, but improvement measured with a refractometer.
Finally, an important aspect is that eye movement parameters are highly correlated with the symptomatology (CISS score), particularly with the latency, variability of the gain, and speed of convergence. These observations consolidate the interest of the CISS to evaluate the symptomatology and the relevance of eye movement recording. Such correlations are meaningful. Indeed, symptoms, such as fatigue, eyestrain, and occasionally double or blurred vision, all can be related to vergence insufficiency: long latency, variable gain, and speed all signify inappropriate vergence from one instant to the next, thus, leading to symptoms. Next, we will discuss the eye movement data.
Differences between Healthy and Vergence Disorders Groups
Previous studies of our team and others have reported abnormality of vergence eye movements recorded with video-oculography. For instance, Bucci et al.27,47 have shown longer latency, lower accuracy, and speed particularly for convergence in children suffering from vertigo associated with vergence disorders. In adults with vergence insufficiency, Alvarez et al.10,29 also reported abnormally low speed of vergence. In the present study, we focused on the intertrial variability of vergence parameters. To our knowledge, this aspect was rather neglected in prior studies. Our findings showed that the most important abnormality of vergence eye movements in students with vergence disorders is the abnormally high intertrial variability, indicating a difficulty in reproducing stereotyped vergence movements over time. It also is important that abnormal variability is seen for parameters, such as gain and velocity, but not for latency. Latency of eye movements is believed to reflect multiple cortical process, such as attention shift to target, target localization, release of oculomotor fixation, and decision to move the eyes; these processes can take place most likely in parallel.48,49 Our results show that latencies are not significantly longer nor more variable in students with vergence disorders. Our observation contrast with those of children reported by Bucci et al.47 Note, however, that in children cortical maturation is still ongoing and this could explain the latency deficit. For the adults studied here, we can conclude that vergence disorders did not alter significantly the capacity to initiate such movements with rather normal delays. Yet, as it will be discussed later, there is even capacity for shortening considerably the latency after Vd-s rehabilitation.
The measured vergence accuracy (gain) show marked hypometria of divergence even for the healthy group, and this calls for a reconsideration of what is the normal value of divergence. Perhaps prolonged work at near distance (with small screens) leads to a reduction of divergence capacity. There is an evidence for fewer divergence than convergence cells in the mesencephalic reticular formation than convergence cells.50–52 Our study also suggested that divergence is further weakened in the vergence disorder group; moreover the intertrial variability is higher for convergence and divergence.
Effect of Vd-s Rehabilitation Protocol
The major effects are: reduction of intertrial variability for all oculomotor parameters (amplitude, duration, accuracy, and mean velocity) for convergence and divergence. Variability of peak velocity for divergence also decreased significantly, and the latency of convergence and divergence was reduced considerably.
Reduction of Variability
Variability of eye movement parameters is approximately 20% in healthy adults.53 Variability increases in the elderly, as well as in patients with neurodegenerative disorders.44,54,55 The present study suggested that variability is a biomarker of the vergence disorder and the Vd-s rehabilitation protocol acts primarily on this parameter. Variability of the gain dropped to supranormal values; similarly variability of duration and of velocity was drastically reduced. Thus, the Vd-s rehabilitation protocol enabled patients to reproduce appropriate vergence trajectories over time. Given that the effects concern amplitude, duration, and speed parameters, one can argue that the Vd-s rehabilitation protocol acted on cortical and subcortical structures controlling these parameters. Indeed, speed and duration of vergence are known to depend on properties of the vergence generator located in the brainstem, that is, in the mesencephalic reticular formation.51
Recently, Horwood et al.56 reported benefits of 2 weeks of laboratory exercises using either naturalistic targets (e.g., haploscopic images) either targets with partial visual cues (e.g., haploscopic image deprived of blur, disparity or proximal cue). They showed that a simple instruction for effort can induce a placebo effect on vergence performances. They mentioned that high variability and voluntary overconvergence can be involved in vergence insufficiency. Our study introduced an objective way for diagnosing and rehabilitating vergence disorders. Using natural targets displayed in the real 3D space, vergence was initiated without specific voluntary effort due to neuroplasticity. Vergence properties, measured and analyzed, revealed benefits over time.
Optimization of Latency
The overlap paradigm (i.e., the target and initial fixation target are overlapping in time) has been used extensively for saccades or vergence and it is well established that the initiation of eye movements with such paradigm is relatively slow with latency beyond 200 ms.37 The novel observation is that latency of subjects with vergence disorders trained with the Vd-s rehabilitation protocol dropped from 251 to 147 and 153 ms for convergence, and from 229 to 171 and 179 ms for divergence. The results can be interpreted in the context of the hypothetical process involved during eye movement preparation: fixation release, localization of the target, attention shift, computation of eye movement parameters, and decision to move.49,57 The optimization of the latencies could be attributed to shortening of the time needed to accomplish one of several of these sub processes. In prior studies concerning children with vergence disorders,30,47 a reduction of convergence latency following orthoptic training has been reported; the reduction was 40% of the initial latency for convergence,47 which is similar to the present results. In contrast, for divergence the reduction was only 4% of the initial latency. Although the two studies are in line, the important decrease of latency we observed for divergence (25% of the initial latency) could be due to the efficiency of the Vd-s rehabilitation method in which temporal and spatial parameters of target location are controlled precisely to stimulate optimally cortical and subcortical neuroplasticity.
In real life, it is important to be able to trigger the movements promptly as our eyes are constantly moving from one space location to the other, almost every second. The persistence of the benefit (at least 1 week after) and the correlation between latency and symptomatology demonstrated that the Vd-s rehabilitation protocol, by improving the timing of eye movements perhaps more than the orthoptic methods, is a handy tool contributing to visual motor efficiency. Also, the Vd-s protocol uses an automatized and reproducible method; the REMOBI is the first device to provide a reliable solution.
Thus, our study provides solid evidence that eye movement preparation can be improved via neuroplasticity. Studies with brain imaging could be of interest in assessing the neural substrate of such neuroplasticity. Functional magnetic resonance imaging studies were applied, for example by Arnolduseen et al.,58 who investigated depth perception and the substrate of vergence eye movements; they reported an activation of area V1 associated with vergence eye movements. Alvarez et al.28,29 and Jazwal et al.59 focused on the natural coupling of accommodation and vergence, by comparing CI to healthy subjects. They demonstrated a relation between improvements of vergence eye movements due to orthoptic training and activity of the frontal eye field, posterior parietal cortex and cerebellar vermis.
Finally, it is worth considering cognitive and motivation aspects in vergence rehabilitation. Considering, for instance, activation of the frontal eye field, this area alone is involved with attentional orienting, visual awareness, conscious access, perceptual performance, and decision making; but the relation between eye movements and cognition was barely explored explicitly.60 One could argue that motivation reinforced by rehabilitation could lead to fast initiation of vergence. Using a remarkably large cohort, Scheiman et al.22 compared home-based therapy, office-based therapy, and an office-based placebo protocol (i.e., exercises not related to vergence); office based-therapy was shown to be superior than home-based and placebo therapy. However, the placebo protocol induced a substantial effect relative to the baseline. Indeed, placebo and motivation can influence the results. However, such cognitive aspects can be active for any type of rehabilitation, including the REMOBI. It is possible that the REMOBI device enhances some cognitive aspects, particularly with the use of visuo-acoustic stimulation that renders target localization and movement process rather automatic and effortless. Nevertheless, the effects we reported are indicative of oculomotor neuroplasticity, namely those on the variability of speed and accuracy of eye movements. Studies with brain imaging could be of interest to delineate further the neural substrate of such plasticity.
Conclusion
We report the critical abnormalities of vergence eye movements in subjects with vergence disorders and establish the correlation between symptomatology and eye movement parameters. Importantly, our study demonstrated the efficiency of the Vd-s rehabilitation protocol for solving these problems. The major problem was high variability of vergence spatio-temporal parameters, which is drastically improved by the Vd-s rehabilitation together with an optimization of the latency of convergence and divergence.
Acknowledgments
Disclosure: Z. Kapoula, None; A. Morize, None; F. Daniel, None; F. Jonqua, None; C. Orssaud, None, D. Brémond-Gignac, None
References
- 1. Montés-Micó R. Prevalence of general dysfunctions in binocular vision. Ann Ophthalmol. 2001; 33: 205–208. [Google Scholar]
- 2. Lavrich JB. Convergence insufficiency and its current treatment. Curr Opin Ophthalmol. 2010; 21: 356–360. [DOI] [PubMed] [Google Scholar]
- 3. Scheiman M,, Cooper J,, Mitchell GL,, et al. A survey of treatment modalities for convergence insufficiency. Optom Vis Sci. 2002; 79: 151–157. [DOI] [PubMed] [Google Scholar]
- 4. Horwood AM,, Toor S,, Riddell PM. Screening for convergence insufficiency using the CISS is not indicated in young adults. Br J Ophthalmol. 2014; 98: 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cacho-Martínez P,, García-Muñoz Á,, Ruiz-Cantero MT. Is there any evidence for the validity of diagnostic criteria used for accommodative and nonstrabismic binocular dysfunctions? J Optom. 2014; 7: 2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheiman M,, Mitchell GL,, Cotter S,, et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005; 82: E583–E595. [DOI] [PubMed] [Google Scholar]
- 7. Borsting EJ,, Rouse MW,, Mitchell GL,, et al. Convergence Insufficiency Treatment Trial Group. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003; 80: 832–838. [DOI] [PubMed] [Google Scholar]
- 8. Sreenivasan V,, Bobier WR. Reduced vergence adaptation is associated with a prolonged output of convergence accommodation in convergence insufficiency. Vision Res. 2014; 100: 99–104. [DOI] [PubMed] [Google Scholar]
- 9. Thiagarajan P,, Ciuffreda KJ. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. J Rehabil Res Dev. 2013, 50: 1223–1240. [DOI] [PubMed] [Google Scholar]
- 10. Alvarez TL,, Kim EH. Analysis of saccades and peak velocity to symmetrical convergence stimuli: binocularly normal controls compared to convergence insufficiency patients. Invest Ophthalmol Vis Sci. 2013; 54: 4122–4135. [DOI] [PubMed] [Google Scholar]
- 11. Borsting E,, Mitchell GL,, Kulp MT,, et al. CITT Study Group. Improvement in academic behaviors following successful treatment of convergence insufficiency. Optom Vis Sci. 2012; 89: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serna A,, Rogers DL,, McGregor ML,, Golden RP,, Bremer DL,, Rogers GL. Treatment of symptomatic convergence insufficiency with a home-based computer orthoptic exercise program. J Am Assoc Ped Ophthalmol Strab. 2011; 15: 140–143. [DOI] [PubMed] [Google Scholar]
- 13. Cooper J,, Feldman J. Reduction of symptoms in binocular anomalies using computerized home therapy—HTS™. Optometry. 2009; 80: 481–486. [DOI] [PubMed] [Google Scholar]
- 14. CITT group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008; 1260: 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arnoldi K,, Reynolds JD. A review of convergence insufficiency: what are we really accomplishing with exercises? Am Orthop J. 2007; 57: 123–130. [DOI] [PubMed] [Google Scholar]
- 16. Brautaset RL,, Jennings AJ. Effects of orthoptic treatment on the CA/C and AC/A ratios in convergence insufficiency. Invest Ophthalmol Vis Sci. 2006; 47 (7): 2876–2880. [DOI] [PubMed] [Google Scholar]
- 17. Rouse MW,, Borsting EJ, LynnMitchell G,, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthal Physiol Optics. 2004; 24: 384–390. [DOI] [PubMed]
- 18. Porcar E,, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997; 74: 111–113. [DOI] [PubMed] [Google Scholar]
- 19. Von Noorden GK. Binocular Vision and Ocular Motility. Theory and Management of Strabismus. St. Louis MO: Mosby; 2002. [Google Scholar]
- 20. Momeni-Moghaddam H,, Kundart J,, Azimi A,, Hassanyani F. The effectiveness of home-based pencil push-up therapy versus office-based therapy for the treatment of symptomatic convergence insufficiency in young adults. Middle East Afr J Ophthalmol. 2015; 22: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Passmore JW,, Maclean F. Convergence insufficiency and its management: an evaluation of 100 patients receiving a course of orthoptics. Am J Ophthalmol. 1957; 43: 448–456. [PubMed] [Google Scholar]
- 22. Scheiman M,, Kulp MT,, Cotter S,, et al. Vision therapy/orthoptics for symptomatic convergence insufficiency in children: treatment kinetics. Optom Vis Sci. 2010; 87: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kulp MT,, Borsting E,, Mitchell GL,, et al. Convergence Insufficiency Treatment Trial (CITT) Investigator Group. Feasibility of using placebo vision therapy in a multicenter clinical trial. Optom Vis Sci. 2008; 85: 255–261. [DOI] [PubMed] [Google Scholar]
- 24. Rogers DL,, Serna A,, McGregor ML,, et al. Treatment of symptomatic convergence insufficiency with a home-based computer orthoptic exercise program. J Am Assoc Ped Ophthalmol Strab. 2011; 15: 511–512. [DOI] [PubMed] [Google Scholar]
- 25. Grisham JD,, Bowman MC,, Owyang LA,, Chan CL. Vergence orthoptics: validity and persistence of the training effect. Optom Vis Sci. 1991; 68: 441–451. [DOI] [PubMed] [Google Scholar]
- 26. van Leeuwen AF,, Westen MJ,, van der Steen J,, de Faber JTH,, Collewijn H. Gaze-shift dynamics in subjects with and without symptoms of convergence insufficiency: influence of monocular preference and the effect of training. Vision Res. 1999; 39: 3095–3107. [DOI] [PubMed] [Google Scholar]
- 27. Bucci MP,, Kapoula Z,, Yang Q,, Brémond-Gignac D,, Wiener-Vacher S. Speed-accuracy of saccades vergence and combined eye movements in children with vertigo. Exp Brain Res. 2004; 157: 286–295. [DOI] [PubMed] [Google Scholar]
- 28. Alvarez TL,, Jaswal R,, Gohel S,, Biswal BB. Functional activity within the frontal eye fields posterior parietal cortex, and cerebellar vermis significantly correlates to symmetrical vergence peak velocity: an ROI-based, fMRI study of vergence training. Front Integr Neurosci. 2014; 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alvarez TL,, Alkan Y,, Gohel S,, Ward BD,, Biswal BB. Functional anatomy of predictive vergence and saccade eye movements in humans: a functional MRI investigation. Vision Res. 2010; 50: 2163–2175. [DOI] [PubMed] [Google Scholar]
- 30. Jainta S,, Bucci MP,, Wiener-Vacher S,, Kapoula Z. Changes in vergence dynamics due to repetition. Vision Res. 2011; 51: 1845–1852. [DOI] [PubMed] [Google Scholar]
- 31. Takagi M,, Oyamada H,, Abe H,, et al. Adaptive changes in dynamic properties of human disparity-induced vergence. Invest Ophthalmol Vis Sci. 2001; 42: 1479–1486. [PubMed] [Google Scholar]
- 32. Fawcett SL. An evaluation of the agreement between contour-based circles and random dot-based near stereoacuity tests. J Am Assoc Ped Ophthalmol Strab. 2005; 9: 572–578. [DOI] [PubMed] [Google Scholar]
- 33. Ohlsson J,, Villarreal G,, Abrahamsson M,, Cavazos H,, Sjöström A,, Sjöstrand J. Screening merits of the Lang II, Frisby, Randot, Titmus, and TNO stereo tests. J Am Assoc Ped Ophthalmol Strab. 2001; 5: 316–322. [DOI] [PubMed] [Google Scholar]
- 34. Walraven J,, Janzen P. Tno stereopsis test as an aid to the prevention of amblyopia. Ophthal Physiol Optics. 1993; 13: 350–356. [DOI] [PubMed] [Google Scholar]
- 35. Yang Q,, Jurion F,, Bucci MP,, et al. Six adult cases with a pseudo-vestibular syndrome related to vergence abnormalities. Neuroophthalmology. 2008; 32: 93–104. [Google Scholar]
- 36. Convergence Insufficiency Treatment Trial (CITT) Investigator Group. Validity of the convergence insufficiency symptom survey: a confirmatory study. Optom Vision Sci. 2009; 86: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Q,, Bucci MP,, Kapoula Z. The latency of saccades, vergence, and combined eye movements in children and in adults. Invest Ophthalmol Vis Sci. 2002; 43: 2939–2949. [PubMed] [Google Scholar]
- 38. Lang A,, Gaertner C,, Ghassemi E,, Yang Q,, Orssaud C,, Kapoula Z. Saccade-vergence properties remain more stable over short-time repetition under overlap than under gap task: a preliminary study. Front Hum Neurosci. 2014; 8: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang Q,, Kapoula Z. Saccade-vergence dynamics and interaction in children and in adults. Exp Brain Res. 2004; 156: 212–223. [DOI] [PubMed] [Google Scholar]
- 40. Coubard OA,, Kapoula Z. Saccades during symmetrical vergence. Graefe's Arch Clin Exp Ophthalmo. 2008; 246: 521–536. [DOI] [PubMed] [Google Scholar]
- 41. Takagi M,, Frohman EM,, Zee DS. Gap-overlap effects on latencies of saccades vergence and combined vergence-saccades in humans. Vision Res. 1995; 35: 3373–3388. [DOI] [PubMed] [Google Scholar]
- 42. Yang Q,, Vernet M,, Orssaud C,, Bonfils P,, Londero A,, Kapoula Z. Central crosstalk for somatic tinnitus: abnormal vergence eye movements. PLoS One. 2010; 5: e11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rambold H,, Sprenger A,, Helmchen C. Effects of voluntary blinks on saccades vergence eye movements, and saccade-vergence interactions in humans. J Neurophysiol. 2002; 88: 1220–1233. [DOI] [PubMed] [Google Scholar]
- 44. Yang Q,, Wang T,, Su N,, Liu Y,, Xiao S,, Kapoula Z. Long latency and high variability in accuracy-speed of prosaccades in Alzheimer's disease at mild to moderate stage. Dement Geriatr Cogn Disord Extra. 2011; 1: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dinneen LC,, Blakesley BC., Algorithm AS, 62: a generator for the sampling distribution of the Mann-Whitney U statistic. Appl Stat. 1973; 22: 269–273. [Google Scholar]
- 46. Pearson K,, Lee A. On the generalised probable error in multiple normal correlation. Biometrika, 1908; 6: 59–68. [Google Scholar]
- 47. Bucci MP,, Kapoula Z,, Yang Q,, Wiener-Vacher S,, Brémond-Gignac D. Abnormality of vergence latency in children with vertigo. J Neurol. 2004; 251: 204–213. [DOI] [PubMed] [Google Scholar]
- 48. Leigh RJ,, Zee DS. The neurology of eye movements. Oxford University Press. 2015.
- 49. Findlay JM,, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci. 1999; 22: 661–674. [DOI] [PubMed] [Google Scholar]
- 50. Mays LE,, Gamlin PD. A neural mechanism subserving saccade-vergence interactions. Studies Vis Inform Process. 1995; 6: 215–223. [Google Scholar]
- 51. Mays LE,, Porter JD,, Gamlin PD,, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986; 56: 1007–1021. [DOI] [PubMed] [Google Scholar]
- 52. Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984; 51: 1091–1108. [DOI] [PubMed] [Google Scholar]
- 53. Kapoula Z,, Yang Q,, Sabbah N,, Vernet M. Different effects of double-pulse TMS of the posterior parietal cortex on reflexive and voluntary saccades. Front Hum Neurosci. 2011; 5: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kapoula Z,, Yang Q,, Vernet M,, Dieudonné B,, Greffard S,, Verny M. Spread deficits in initiation, speed and accuracy of horizontal and vertical automatic saccades in dementia with Lewy bodies. Front Neurol. 2010; 1: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Beers RJ. The sources of variability in saccadic eye movements. J Neurosci. 2007; 27: 8757–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Horwood A,, Toor S. Clinical test responses to different orthoptic exercise regimes in typical young adults. Ophthalmic Physiol Opt. 2014; 34: 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang Q,, Kapoula Z. TMS over the left posterior parietal cortex prolongs latency of contralateral saccades and convergence. Invest Ophthalmol Vis Sci. 2004; 45: 2231–2239. [DOI] [PubMed] [Google Scholar]
- 58. Arnoldussen DM,, Goossens J,, van Den Berg AV. Dissociation of retinal and headcentric disparity signals in dorsal human cortex. Front Syst Neurosci. 2015; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jaswal R,, Gohel S,, Biswal BB,, Alvarez TL. Task-modulated coactivation of vergence neural substrates. Brain Connect. 2014; 4: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vernet M,, Quentin R,, Chanes L,, Mitsumasu A,, Valero-Cabré A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci. 2014; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]