Abstract
Objectives:
Despite the rising number of patients with osteoarthritis, no sufficient chondroprotective and prophylactic therapy for osteoarthritis has been established yet. The purpose of this study was to verify whether stimulation of the nicotinic acetylcholine receptor via nicotine has a beneficial effect on cartilage degeneration in the development of osteoarthritis and is capable of reducing the expression of proinflammatory cytokines and cartilage degrading enzymes in synovial membranes after osteoarthritis induction.
Methods:
Experimental osteoarthritis was induced in Lewis rats using a standardized osteoarthritis model with monoiodoacetate. A total of 16 Lewis rats were randomized into four groups: control, sham + nicotine application, osteoarthritis, and osteoarthritis + nicotine application. Nicotine (0.625 mg/kg twice daily) was administered intraperitoneally for 42 days. We analyzed histological sections, radiological images and the expression of the proinflammatory cytokines, such as interleukin-1β, tumor necrosis factor-α and interleukin-6, and of matrix metalloproteases 3, 9 and 13 and tissue inhibitors of metalloprotease-1 in synovial membranes via quantitative polymerase chain reaction.
Results:
Histological and x-ray examination revealed cartilage degeneration in the osteoarthritis group compared to control or sham + nicotine groups (histological control vs osteoarthritis: p = 0.002 and x-ray control vs osteoarthritis: p = 0.004). Nicotine treatment reduced the cartilage degeneration without significant differences. Osteoarthritis induction led to a higher expression of proinflammatory cytokines and matrix metalloproteases as compared to control groups. This effect was attenuated after nicotine administration. The differences of proinflammatory cytokines and matrix metalloproteases did not reach statistical significance.
Conclusion:
With the present small-scale study, we could not prove a positive effect of nicotinic acetylcholine receptor stimulation on osteoarthritis due to a conservative statistical analysis and the consecutive lack of significant differences. Nevertheless, we found promising tendencies of relevant parameters that might prompt further experiments designed to evaluate the potency of stimulation of this receptor system as an additional treatment approach for osteoarthritis.
Keywords: Osteoarthritis, monoiodoacetate, metalloproteases, cytokines, inflammation, nicotine
Introduction
Osteoarthritis (OA) is one of the leading diseases in modern orthopedic surgery. Multiple pathophysiological changes have been identified to be relevant for the pathogenesis of OA. This includes a proinflammatory immune reaction with an increased release of proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6.1,2 Furthermore, matrix metalloproteases (MMPs) are eminent pathogenic factors in the development of OA due to their importance to cartilage matrix turnover.3 Important MMPs are MMP-3, MMP-9 and MMP-13. They are often used in OA models as markers of cartilage metabolism and vitality.3–8 For example, the overexpression of MMP-13 in transgenic mice led to cartilage degeneration which is comparable with the degeneration found in human OA.9 On the other side, MMP-13-deficient mice had less cartilage destruction in a surgically induced OA model than wild-type mice.10
MMPs are physiologically inhibited by tissue inhibitors of metalloproteases (TIMPs) via a one-to-one stoichiometry.11 In osteoarthritic cartilage, the imbalance between the activities of MMPs and TIMPs is considered to be a reason of tissue degeneration.12 In vitro studies showed that there is a link between proinflammatory cytokines and the production of MMPs.5,13 Bondeson et al.5 verified decreased expressions of MMPs via reduction in IL-1β and TNF-α. Accordingly, the inhibition of these proinflammatory cytokines and MMPs could be of paramount importance to reduce the progression of OA.5,10 In several rat animal models, OA was induced using monoiodoacetate (MIA).14–18
Tracey et al.19 described an anti-inflammatory effect via stimulating the nicotinic acetylcholine receptor (nAchR). Nicotine is a potent agonist of this receptor and its anti-inflammatory effect has been described in several experimental animal models for inflammatory processes like sepsis, peritonitis, autoimmune myocarditis and rheumatoid arthritis.19–21 Interestingly, Felson et al.22 delineated in their study from 1989 that cigarette smoking seems to have a protective effect against OA. Moreover, Mnatzaganian et al.23 could show that smoking seems to reduce the rate of performed total joint replacements. In a recent study, Liu et al.24 investigated the effect of nicotine on MIA-induced OA. They verified that the activation of the nAchR with nicotine leads to an inhibition of the phosphorylation of signaling pathways and therefore has a protective effect of nicotine on MIA-induced OA.
The central purpose of this small-scale study was to evaluate whether nicotine is capable of reducing cartilage degeneration and thereby might decelerate the development of OA. Furthermore, this study was undertaken to investigate whether nicotine reduces the expression of proinflammatory cytokines, MMPs and effects the expression of TIMP-1 in the MIA model of OA.
Materials and methods
Study design
Male Lewis rats (220–240 g) from the breeding of Hannover Medical School were housed under specific pathogen-free conditions at a 12-h light–dark cycle and fed ad libitum. The Institutional Committee on Use and Care of Animals of the Hannover Medical School (MHH) and the Lower Saxony State Board on the Care and Use of Animals (LAVES; Oldenburg, Lower Saxony, TV 12/0686) approved all experiments. Rats were categorized into four groups as follows: untreated control (CTRL), OA, OA plus nicotine application (OA + NICOTINE) and sham-operated plus nicotine application (SHAM + NICOTINE) (Figure 1). In the CTRL group, both legs of the animals were used for radiological, histological and polymerase chain reaction (PCR) evaluation. In the other groups, the operated legs of the rats were used for the analysis. Rats of the CTRL group received no further treatment before being euthanatized. The experimental animals of OA, OA + NICOTINE and SHAM + NICOTINE groups were euthanatized after 42 days by heart removal under deep isoflurane anesthesia.
Figure 1.

Study design: 16 male Lewis rats were randomly divided into four groups.
Anesthesia and OA induction
For anesthesia, the induction chamber was ventilated with 5% isoflurane under an oxygen-flow rate of 2.5 L/min. The experimental animal remained in the induction chamber for 3 min. Then it was ventilated via a mask with 3% isoflurane under an oxygen flow rate of 2.0 L/min during the induction of OA and tissue harvesting.
OA was induced according to the protocol of Woo et al.25 We conducted a single unilateral injection with 4-mg MIA (Sigma Aldrich, St Louis, MO, USA) dissolved in 50 µL of 0.9% saline with a tuberculin syringe through the patellar ligament of the left knee joint. SHAM + NICOTINE rats were injected with an equivalent volume of saline. CTRL rats have not been injected.
Nicotine application
Nicotine application started directly on the day of the OA induction. Liquid nicotine was applied twice daily via intraperitoneal injection. The dose was 0.625 mg/kg nicotine (Sigma Aldrich) for 42 days. Rats from the OA + NICOTINE and SHAM + NICOTINE groups received nicotine treatment. The application dose was orientated on a study by Yu et al.26
Sample preparation, radiological and histological analysis
After euthanasia, a 3 × 3-mm piece of synovial membrane tissue of the prepared joint was cut and shock frozen for RNA extraction. Then legs were dissected and fixed in 4% buffered formalin (Roti®-Histofix 4%; Carl Roth, Karlsruhe, Germany) for radiological assessment and histological analysis.
We conducted x-ray images in two layers (anterior–posterior and lateral) which were analyzed independently by two investigators utilizing the Kellgren and Lawrence27 Score. The score included 0–4 points resulting in five grades of OA as follows: 0 points: no OA, 1 point: doubtful OA, 2 points: minimal OA, 3 points: moderate OA and 4 points: severe OA.
Fixed samples were decalcified in 5% formic acid for 55 h, dehydrated by ascending concentrations of ethanol (ETOH) and then embedded in paraffin. Frontal sections (5 µm) of the posterior third of the knee joint were cut and deparaffinized by descending concentrations of ETOH. After being hydrated with distilled water, the slides were stained with hematoxylin and eosin (H&E) or Safranin O fast green (SO/FG). H&E staining was carried out for routine diagnostic and synovial inflammation assessment and SO/FG staining was used for evaluation of cartilage degeneration. OA was scored after the osteoarthritis cartilage histopathology (OACH) assessment system.28 The sections were first allocated to 1 of 6 grades and then categorized into four stages. The multiplication of grade and stage produces the corresponding score value (Figure 2).28 Synovial inflammation was scored according to the thickness of the synovial lining cell layer, the cell density and the inflammatory infiltrate in the synovial membrane (Figure 3).29
Figure 2.
The OACH assessment system score. For histological assessment of OA, we used the OACH assessment system score.29 The sections were first allocated to one of the six grades. Depending on the horizontal expansion of OA, the sections were allocated to one of the four stages. The multiplication of grade and stages produces the corresponding score value.
Figure 3.
The synovitis score. To classify synovitis, we used the synovitis score.17 Each of the features received 0–3 points and the sum of all points produces the corresponding score value.
RNA purification and quantitative PCR
For RNA extraction, the sample of synovial membrane material was immersed in liquid nitrogen and grinded using pestle and mortar. The grinded samples were homogenized in 1000-µL Trizol (Ambion RNA; Life Technologies, Carlsbad, CA, USA) and centrifuged at 12,000g for 5 min. A 200-µL chloroform was added to the supernatant and centrifuged at 12,000g for 15 min. The aqueous phase was transferred and mixed with 500-µL isopropanol and then centrifuged at 12,000g for 10 min. The resulting RNA pellet was washed with 1000 µL of 75% ETOH in dimyristoylphosphatidylcholine (DMPC) water and centrifuged at 7500g for 5 min. The supernatant was then removed and the pellet was first dried and then dissolved in 20-µL DMPC water. Purity and intactness of the resulting total RNA were verified by spectrophotometry (Nanodrop 1000; Peqlab, Erlangen, Germany) and RNA electrophoresis. Reverse transcription was carried out with the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Quantitative PCR was performed in triplicates using 5-µL cDNA and was carried out with SsoFast™EvaGreen®Supermix (Bio-Rad Laboratories, Inc.) using the i-Cycler system (Bio-Rad Laboratories, Inc.). For PCR analysis, we used the BIOGazelle qbase+ (Biogazelle, Zwijnaarde, Belgium) program and the results were normalized using two reference genes (Ribosomal protein L32 (RPL32) and beta2 microtubulin). PCR was used to detect the gene expression rates of IL-1β, TNF-α and IL-6 and of MMP-3, MMP-9 and MMP-13. We also measured the expression of TIMP-1. For primer sequences, see Table 1.
Table 1.
Primer sequences used in our experiment.
| Target | Direction | Primer sequence (5′–3′) |
|---|---|---|
| MMP-3 MMP-3 |
Forward Reverse |
CGACGTCGGTGGCTTCAGTA TCACCTCCTCCCAGACCTTCA |
| MMP-13 MMP-13 |
Forward Reverse |
ATCCCCGCCTCATAGAAGA TGGGCCCATTGAAAAAGTA |
| MMP-9 MMP-9 |
Forward Reverse |
CCACCGAGCTATCCACTCAT GTCCGGTTTCAGCATGTTTT |
| TIMP-1 TIMP-1 |
Forward Reverse |
ACAGGTTTCCGGTTCGCCTAC CTGCAGGCAGTGATGTGCAA |
| IL-6 IL-6 |
Forward Reverse |
CCGGAGAGGAGACTTCACAG ACAGTGCATCATCGCTGTTC |
| TNF-α TNF-α |
Forward Reverse |
CTCCCAGAAAAGCAAGCAAC CGAGCAGGAATGAGAAGAGG |
| IL-1β IL-1β |
Forward Reverse |
TGCTGATGTACCAGTTGGGG CTCCATGAGCTTTGTACAAG |
| RPL32 RPL32 |
Forward Reverse |
AGATTCAAGGGCCAGATCCT CTACGATGGCTTTTCGGTTC |
| β-2-microglobulin β-2-microglobulin |
Forward Reverse |
TGACCGTGATCTTTCTGGTG ATCTGAGGTGGGTGGAACTG |
MMP: matrix metalloprotease; TIMP: tissue inhibitors of metalloprotease; IL: interleukin; TNF: tumor necrosis factor.
Data analysis
Statistical analysis consisted of Kruskal–Wallis test and multiple comparisons were corrected by Dunn’s test. Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). The values were expressed as mean ± standard deviation. A p value of <0.05 was accepted as statistically significant.
Results
In this study, the effect of nicotine on cartilage degeneration was evaluated radiologically and histologically. Furthermore gene expression analyses of proinflammatory cytokines, MMPs and TIMP-1 were performed to gain insight in potential reasons for the effect of nicotine application.
Radiological and histological analysis
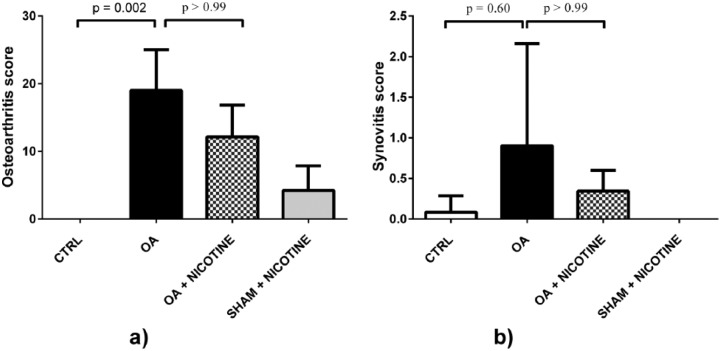
The radiological assessment of the dissected legs verified advanced joint degeneration after OA induction. The resected knees were highly affected up to deformation of femur and tibia. In contrast, experimental animals of the CTRL group showed no degenerative changes (Figure 4). Compared to CTRL group, the Kellgren and Lawrence score of the OA group was elevated with a value of 2.9 ± 1.5 (p = 0.004). In radiological analysis, nicotine-treated animals had less cartilage degeneration. A slightly lower Kellgren and Lawrence score of 1.4 ± 0.63 in the OA + NICOTINE group illustrates this (p > 0.99). The SHAM + NICOTINE group showed no statistically relevant differences to the CTRL group (Figure 5). The SO/FG samples of the OA group showed a high grade of cartilage degeneration. Joint destruction was characterized by delamination or complete cartilage depletion. In some cases, a fibrocartilaginous repair tissue could be detected over the bony surface (Figure 6). In none of the dissected knees H&E-stained sections indicated histologically visible synovial inflammation 42 days post-MIA induction (Figure 7). The OA group had a score of 19 ± 6 in the OACH assessment system. This value was increased compared to the baseline of 0.0 ± 0.0 of the CTRL group (p = 0.002). In histological analysis, nicotine-treated animals had less cartilage degeneration with a score of 12.2 ± 4.7, whereas the difference did not reach statistical significance (p > 0.99). No significant differences were found regarding SHAM + NICOTINE and CTRL group (p = 0.66; Figure 8). In no group the synovitis score was higher than 1, indicating that no synovitis was detectable (Figure 8).
Figure 4.
X-ray examinations and macroscopic appearance. (a) Representative x-ray images: A: CTRL; B: OA; C: OA + NICOTINE; and D: SHAM + NICOTINE. Whereas in OA (B) bone deformity and joint destruction were detectable (white arrow), nicotine-treated animals (C) showed only low-grade cartilage degeneration. (b) Representative macroscopic images: A: CTRL; B: OA; C: OA + NICOTINE; D: SHAM + NICOTINE; E: magnification of (B); and F: magnification of (C). In the OA group, bone deformity was detectable (red arrow in E). It is apparent from these pictures that nicotine alleviated joint destruction. The arrow (F) indicates fissures found in the OA + NICOTINE group.
Figure 5.

Kellgren and Lawrence score. Nicotine application led to a reduced score compared to the OA group. Statistical results of comparison between CTRL and OA group, and OA and OA + NICOTINE groups are highlighted.
Figure 6.
Safranin O fast green staining. Representative pictures of SO/FG staining: (a) CTRL, (b) OA, (c) OA + NICOTINE, (d) SHAM + NICOTINE and (e) zoomed region of picture (b). No degenerative changes were found in the control group. In the OA group, (b) severe cartilage affection was found, whereas in (c) nicotine-treated samples reduced cartilage degeneration could be detected. We did not find degenerative changes in the (d) SHAM + NICOTINE group. The star in picture (e) depicts reparative tissue above the eroded articular surface.
Figure 7.
(a)–(e) H&E staining. Representative pictures of H&E-stained histologic slides of synovial membranes: (a) and (b) CTRL, (c) OA, (d) OA + NICOTINE and (e) SHAM + NICOTINE. No synovitis was found after 42 days in any probe.
Figure 8.
Histological scoring. (a) OACH assessment system score. The graph shows semiquantitative scoring of SO/FG stained cuts 42 days after OA induction. Statistical results comparing CTRL and OA group and OA and OA + NICOTINE groups are shown. OA has been attenuated following nicotine application, (b) Synovitis score. The histologic assessment indicated no synovitis in any group.
Proinflammatory cytokines
In the OA group, slightly higher gene expression rates of the proinflammatory cytokines IL-1β, TNF-α and IL-6 compared to CTRL groups were found (TNF-α: p = 0.055; IL-1β: p = 0.147; and IL-6: p = 0.077). The administration of nicotine did not influence the expression of IL-1β as in the OA group expression was 1.3 ± 0.65 arbitrary units (a.u.) compared to 1.4 ± 0.45 in the OA + NICOTINE group (p > 0.99). The expression of TNF-α decreased by trend from 2.2 ± 0.9 to 0.9 ± 0.5 a.u. (p = 0.285). Additionally, nicotine application reduced the expression of IL-6 from 3.8 ± 3.2 to 1.4 ± 0.8 a.u., whereas the difference did not reach statistical significance (p > 0.99). The SHAM + NICOTINE group showed no statistically relevant differences to the CTRL group (Figure 9).
Figure 9.
Quantitative PCR of proinflammatory cytokines in synovial membrane. The results were normalized using RPL32 and β-2-microglobulin as stable reference genes. Gene expression rates are described as arbitrary units. Statistical results of comparison between CTRL and OA group, and OA and OA + NICOTINE groups are highlighted. (a) IL-1β: Gene expression of IL-1β was higher in the OA than in the CTRL group and tendentiously lower in the OA + NICOTINE compared to the OA group. (b) TNF-α: Expression of TNF-α was higher in the OA than in the CTRL group. There was a decrease in the gene expression of TNF-α in the OA + NICOTINE compared to the OA group. (c) IL-6: Expression of IL-6 was higher in the OA than in the CTRL group. There is a clear trend of decreasing the gene expression of IL-6 through nicotine application.
MMPs
Compared to CTRL group, the OA group had a slightly higher expression MMP-3 (p = 0.135). After nicotine application it was reduced by trend from 3.0 ± 2.1 to 1.3 ± 1.2 a.u. (p > 0.99). Gene expression rates of MMP-9 were significantly increased to 2.6 ± 0.9 in the OA group compared to 0.8 ± 0.5 a.u. of the CTRL group (p = 0.018). The expression of MMP-9 was slightly decreased to 1 ± 0.46 a.u. in the OA + NICOTINE group (p = 0.183). Simultaneously, the expression of MMP-13 was decreased in the OA + NICOTINE group compared to the OA group without significant difference (p = 0.975). Additionally, we found slightly increased TIMP-1 expression in the OA group compared to CTRL group (p > 0.99). In the OA + NICOTINE group, TIMP-1 expression was significantly lower compared to the OA group (p = 0.039). No significant differences were found regarding SHAM + NICOTINE and CTRL group (Figure 10).
Figure 10.
Quantitative PCR of MMPs and TIMP-1. Gene expression rates are described as arbitrary units: statistical results of comparison between CTRL and OA group, and OA and OA + nicotine groups are highlighted. (a) MMP-3: MMP-3’s expression was higher in the synovial membrane of the OA group than in the CTRL group. An impaired gene expression in the OA + NICOTINE group compared to the OA group could be detected. (b) MMP-9: Gene expression of MMP-9 was increased in OA but lower in the OA + NICOTINE group. (c) MMP-13: Gene expression of MMP-13 was elevated in the OA group and nicotine-treated animals showed a reduced expression of it. (d) TIMP-1: Gene expression of TIMP-1 was higher in the OA than in the CTRL group. These data illustrate that it has been reduced following nicotine application.
Discussion
OA is one of the leading diseases in modern medicine, especially in an aging population.2 Its pathogenesis includes the release of proinflammatory cytokines and MMPs.1 Moreover, an anti-inflammatory effect of nicotine is widely described.20,21,30,31 We therefore intended to investigate whether nicotine has a potential beneficial effect on cartilage degeneration and thereby on the development of OA. Furthermore, we set out to examine whether nicotine reduces the expression of proinflammatory cytokines and MMPs in the MIA model of OA.
This study has some limitations. In our study, we assessed the gene expression rates of proinflammatory cytokines, MMPs and TIMP-1 via PCR in the synovial membrane. This does not directly correlate with protein concentrations, but it shows the effects on cell metabolism level. Wang et al.8 and Kobayashi et al.13 also evaluated gene expressions of MMPs in their studies as relevant outcome parameter; however, the assessment of protein concentrations would have led to additional information. Additionally, the group sizes should be enhanced in following studies to reach statistically relevant differences. With this study, we concentrated on a late state after MIA injection. Proinflammatory cytokine and MMPs evaluation as well as the influence of nicotine on the early stages of OA development are of interest and cannot be answered by our study. Moreover, the oral median lethal dose (LD50) of nicotine in rats is with 50 mg/kg much higher than the oral LD50 in humans which is estimated to be <5 mg/kg.32 As humans tolerate less nicotine than rats, the dose of nicotine we used for our study will probably cause side effects in humans. Additionally, we do not know how much nicotine will be needed to evoke an anti-inflammatory effect in human OA. Based on these facts, nicotine itself might not be applicable on humans. This points up the importance of further research in this field.
The first major question of this study contemplated the effect of nicotine on the cartilage degeneration in OA. OA was induced utilizing the established MIA model of OA that has a high correlation to human OA.18 It has therefore been used to study osteoarthritic pain and the effect of several medicaments on cartilage destruction in OA.14,15,17,33,34 Interestingly, we could not detect a histologically visible inflammation 42 days after OA induction. Nevertheless, in a similar case, Beyreuther et al.15 and Bove et al.16 were not able to detect synovial inflammation 14 days post-MIA injection. Maybe the fact that the induction of OA using MIA is based on the inhibition of glycolysis in chondrocytes might not completely correlate to the pathophysiological process in men.14 We pharmacologically stimulated the nAChR with the potent agonist nicotine.19 In their study, Van Maanen et al.20 showed that intraperitoneally nicotine application to mice could alleviate clinical rheumatoid arthritis. In addition, Leib et al.21 showed that nicotine reduces inflammation in a mouse model of autoimmune Myocarditis and Claassen et al.35,36 verified an antiinflammatory effect of Nicotine after burn trauma. In clinical studies, the anti-inflammatory effect of nicotine has been verified in patients suffering from ulcerative colitis.37,38 Furthermore, Ying et al.39 could show that nicotine has a positive effect on the cell proliferation of OA chondrocytes in vitro. Recently, Liu et al.24 also investigated the effect of nicotine on cartilage degeneration in MIA-induced OA. They could detect the nAchR on chondrocytes. Additionally, on cell level, they could prove the anti-inflammatory effect of nicotine in chondrocytes via inhibiting the signaling cascade.24 This supports the basic assumptions of this study that nicotine is capable to attenuate the inflammatory response to OA directly in the joint and on cell level. And thereby, Liu et al.24 could show that the activation of the nAChR and the ensuing inhibition of the phosphorylation of signaling pathways play a pivotal role in the protective effect of nicotine on cartilage degeneration. In another previous study, Gu et al.40 gained additional information on glycosaminoglycan and collagen changes and an evaluation of degenerative changes via magnetic resonance imaging (MRI). Their study was characterized by a high number of used animals. Both most recent studies described in OA models an attenuated joint degradation after nicotine application. However, the present study solely provides an evaluation of MMPs. In this study, the development of OA has been verified radiologically and histologically. Both radiological and histological examinations revealed that the reaction of cartilage degeneration in the OA model was attenuated by trend after nicotine application.
The second major question intended to regard the effect of nicotine on proinflammatory cytokines and MMPs in the MIA model of OA. In this study, the OA-induced increased expression of both proinflammatory cytokines and MMPs was reduced after nicotine application, whereas differences did not reach significance. Concordantly with the literature we found increased expression of IL-1β and IL-6 in the OA group compared to the CTRL group. Likewise, tendentiously higher expression of TNF-α could be detected in the OA group. This is exemplified in the work undertaken by Orita et al.41 and Ashkavand et al.14 where IL-6, TNF-α and IL-1β were significantly increased in a MIA model. The gene expression rates of proinflammatory cytokines were decreased after nicotine application by trend. These findings are consistent with the fact that nicotine was capable of inhibiting TNF-α and IL-6 in a mouse model of autoimmune myocarditis.21
The increased gene expression rates of MMP-3, 9, 13 and TIMP-1 additionally indicated the development of OA. This is in accordance with Wang et al.42 where MMP-3 and MMP-13 were significantly upregulated in a MIA model. Additionally, we confirm the findings of another study that found increased MMP-9 expression in osteoarthritic cartilage.43 Moreover, our findings are in correspondence with a study of Naito et al.44 where TIMP-1 was increased in OA. In our study, gene expression rates of all MMPs and TIMP-1 were decreased by trend after nicotine application. Given the fact that we concurrently observed a reduction in cytokine expression of TNF-α and IL-6, it is possible that the inhibition of those cytokines leads to an inhibition of MMPs and TIMP-1 in the MIA model of OA. Bondeson et al. showed that there is a link between cytokine production and MMP production. They verified that the inhibition of each of the proinflammatory cytokines IL-1β and TNF-α on its own leads to an inhibition of MMPs. Moreover, in their study, the neutralization of both cytokines leads to a more intensive inhibition of all MMPs (1, 3, 9 and 13) regarded.5 Consequently it is possible that the reduction in gene expression of proinflammatory cytokines after nicotine is accompanied by a reduction in MMPs’ production and therefore could have a protective effect on cartilage destruction in OA. This correlates with the decreased cartilage destruction that we detected in histology and x-ray investigation.
Conclusion
This small-scale study provides indication that gene expression rates of proinflammatory cytokines and MMPs are increased due to osteoarthritic processes in the MIA model of OA. Nicotine application tendentiously reduced the gene expression rates of proinflammatory cytokines and MMPs and thereby led to macroscopically and microscopically visible protective effects on cartilage degeneration in OA. In the light of these results, this study therefore may prompt further studies in this research field. Self-evidently pure nicotine as used in our study cannot be compared to cigarette smoking. Contrary to pure nicotine, tobacco smoke contains toxic and carcinogenic substances.45 Still for nicotine itself adverse effects are described especially on wound healing.46 This emphasizes the importance that possible positive aspects of nicotine application have to be distinguished from its side effects via more specific agents. The findings of this study are worth exploring further and the challenge for future research will be to develop useful treatment approach for treating frequent orthopedic diseases like primary and secondary OA. Ongoing studies are needed to identify the exact mechanism of OA prevention of nicotine. Furthermore, the goal of these studies should be the development of a specific agonist with low toxicity.
Acknowledgments
We mourn Prof. Kerstin Reimers after an unexpected and untimely decease. Our condolences go to the bereaved. May she rest in peace.
Footnotes
Animal welfare: This study followed international, national and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from the Lower Saxony State Board on the Care and Use of Animals (LAVES,Oldenburg, Lower Saxony, TV 12/0686).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthrit Rheum 2001; 44: 1237–1247. [DOI] [PubMed] [Google Scholar]
- 2. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013; 5: 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malemud CJ, Islam N, Haqqi TM. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs 2003; 174: 34–48. [DOI] [PubMed] [Google Scholar]
- 4. Bau B, Gebhard PM, Haag J, et al. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthrit Rheum 2002; 46: 2648–2657. [DOI] [PubMed] [Google Scholar]
- 5. Bondeson J, Wainwright S, Lauder S, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006; 8: R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masuhara K, Nakai T, Yamaguchi K, et al. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthrit Rheum 2002; 46: 2625–2631. [DOI] [PubMed] [Google Scholar]
- 7. Mehraban F, Lark MW, Ahmed FN, et al. Increased secretion and activity of matrix metalloproteinase-3 in synovial tissues and chondrocytes from experimental osteoarthritis. Osteoarthr Cartilage 1998; 6: 286–294. [DOI] [PubMed] [Google Scholar]
- 8. Wang GW, Wang MQ, Wang XJ, et al. Changes in the expression of MMP-3, MMP-9, TIMP-1 and aggrecan in the condylar cartilage of rats induced by experimentally created disordered occlusion. Arch Oral Biol 2010; 55: 887–895. [DOI] [PubMed] [Google Scholar]
- 9. Neuhold LA, Killar L, Zhao W, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice 1. J Clin Invest 2001; 107: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthrit Rheum 2009; 60: 3723–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cawston TE, Galloway WA, Mercer E, et al. Purification of rabbit bone inhibitor of collagenase 1. Biochem J 1981; 195: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean DD, Martel-Pelletier J, Pelletier JP, et al. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage 1. J Clin Invest 1989; 84(2): 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthrit Rheum 2005; 52: 128–135. [DOI] [PubMed] [Google Scholar]
- 14. Ashkavand Z, Malekinejad HF, Amniattalab AF, et al. Silymarin potentiates the anti-inflammatory effects of Celecoxib on chemically induced osteoarthritis in rats. Phytomedicine 2012; 19: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 15. Beyreuther B, Callizot NF, Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther 2007; 9: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bove SE, Calcaterra SL, Brooker RM, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartilage 2003; 11: 821–830. [DOI] [PubMed] [Google Scholar]
- 17. Di Cesare ML, Bani DF, Bencini AF, et al. Therapeutic effects of the superoxide dismutase mimetic compound MnIIMe2DO2A on experimental articular pain in rats. Mediators Inflamm 2013; 2013: 905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guingamp C, Gegout-Pottie P, Philippe L, et al. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthrit Rheum 1997; 40: 1670–1679. [DOI] [PubMed] [Google Scholar]
- 19. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Maanen MA, Lebre MC, Van der, Poll Tom, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 2009; 60: 114–122. [DOI] [PubMed] [Google Scholar]
- 21. Leib C, Goser S, Luthje D, et al. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circ Res 2011; 109: 130–140. [DOI] [PubMed] [Google Scholar]
- 22. Felson DT, Anderson JJ, Naimark A, et al. Does smoking protect against osteoarthritis? Arthrit Rheum 1989; 32: 166–172. [DOI] [PubMed] [Google Scholar]
- 23. Mnatzaganian G, Ryan P, Reid C, et al. Smoking and primary total hip or knee replacement due to osteoarthritis in 54,288 elderly men and women. BMC Musculoskel Dis 2013; 14: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Wu DF, Song FF, et al. Activation of alpha7 nicotinic acetylcholine receptors prevents monosodium iodoacetate-induced osteoarthritis in rats. Cell Physiol Biochem 2015. [DOI] [PubMed] [Google Scholar]
- 25. Woo YJ, Joo YB, Jung YO, et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp Mol Med 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu H, Yang YH, Rajaiah R, et al. Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum 2011; 63(4): 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis 1. Ann Rheum Dis 1957; 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartilage 2006; 14: 13–29. [DOI] [PubMed] [Google Scholar]
- 29. Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006; 49: 358–364. [DOI] [PubMed] [Google Scholar]
- 30. Van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 2006; 130: 1822–1830. [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Yu MF, Ochani MF, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384–388. [DOI] [PubMed] [Google Scholar]
- 32. Karaconji IB. Facts about nicotine toxicity. Arh Hig Rada Toksikol 2005; 56(4): 363–371. [PubMed] [Google Scholar]
- 33. Fernihough J, Gentry CF, Malcangio MF, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 2004; 112: 83–93. [DOI] [PubMed] [Google Scholar]
- 34. Ivanavicius SP, Ball AD, Heapy CG, et al. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain 2007; 128: 272–282. [DOI] [PubMed] [Google Scholar]
- 35. Claassen L, Papst S, Reimers K, et al. Transdermal nicotine application attenuates cardiac dysfunction after severe thermal injury. Biomed Res Int 2015; 2015: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Claassen L, Papst S, Reimers K, et al. Inflammatory response to burn trauma: nicotine attenuates proinflammatory cytokine levels. Eplasty 2014; 14: e46. [PMC free article] [PubMed] [Google Scholar]
- 37. Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. New Engl J Med 1994; 330: 811–815. [DOI] [PubMed] [Google Scholar]
- 38. Sandborn WJ, Tremaine WJ, Offord KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1997; 126: 364–371. [DOI] [PubMed] [Google Scholar]
- 39. Ying X, Cheng S, Shen Y, et al. Nicotine promotes proliferation and collagen synthesis of chondrocytes isolated from normal human and osteoarthritis patients. Mol Cell Biochem 2012; 359(1–2): 263–269. [DOI] [PubMed] [Google Scholar]
- 40. Gu Q, Li D, Wie B, et al. Effects of nicotine on a rat model of early stage osteoarthritis. Int J Clin Exp Path 2015; 8(4): 3602–3612. [PMC free article] [PubMed] [Google Scholar]
- 41. Orita S, Ishikawa T, Miyagi M, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskel Dis 2011; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang XD, Kou XX, He DQ, et al. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS ONE 2012; 7: e45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Söder S, Roach HI, Oehler S, et al. MMP-9/gelatinase B is a gene product of human adult articular chondrocytes and increased in osteoarthritic cartilage. Clin Exp Rheumatol 2006; 24: 302–304. [PubMed] [Google Scholar]
- 44. Naito K, Takahashi M, Kushida K, et al. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology 1999; 38: 510–515. [DOI] [PubMed] [Google Scholar]
- 45. Hecht SS. Research opportunities related to establishing standards for tobacco products under the family smoking prevention and tobacco control act. Nicotine Tob Res 2012; 14: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xanthoulea S, Deliaert AF, Romano AF, et al. Nicotine effect on inflammatory and growth factor responses in murine cutaneous wound healing. Int Immunopharmacol 2013; 17: 1155–1164. [DOI] [PubMed] [Google Scholar]