Abstract
How do we know where we are looking? A frequent assumption is that the subjective experience of our direction of gaze is assigned to the location in the world that falls on our fovea. However, we find that observers can shift their subjective direction of gaze among different nonfoveal points in an afterimage. Observers were asked to look directly at different corners of a diamond-shaped afterimage. When the requested corner was 3.5° in the periphery, the observer often reported that the image moved away in the direction of the attempted gaze shift. However, when the corner was at 1.75° eccentricity, most reported successfully fixating at the point. Eye-tracking data revealed systematic drift during the subjective fixations on peripheral locations. For example, when observers reported looking directly at a point above the fovea, their eyes were often drifting steadily upwards. We then asked observers to make a saccade from a subjectively fixated, nonfoveal point to another point in the afterimage, 7° directly below their fovea. The observers consistently reported making appropriately diagonal saccades, but the eye movement traces only occasionally followed the perceived oblique direction. These results suggest that the perceived direction of gaze can be assigned flexibly to an attended point near the fovea. This may be how the visual world acquires its stability during fixation of an object, despite the drifts and microsaccades that are normal characteristics of visual fixation.
Keywords: eye movements, direction of gaze, afterimage
Introduction
As the eyes move around, the point in the visual world that lands on our fovea shifts. It is commonly assumed that our perceived direction of gaze is determined by the location in the world that falls directly on our fovea. Subjectively, the center of our gaze seems exceptionally well defined and we seldom feel that we don't know where we are looking within a visual scene. The conceptual equivalence of mental and physical gaze is ingrained to the point that the two concepts share almost all their terminology, even in scientific and technical usage. Terms such as “gaze,” “look at,” “fixate,” etc. do not distinguish between the subjective sense of visual targeting and the physical pointing of the eyes at something.
Whereas a large body of work exists regarding the perceived direction of visual targets in egocentric space (i.e., where a target is located with respect to the head or body), the location of visual fixation in retinotopic space has received less attention. One study that directly measured where a fixated target fell on the retina used adaptive optics (Putnam et al., 2005). These high resolution images specify the absolute position of the stimulus on the retina with an accuracy of about 5 arcsec (Roorda, 2011). Using this method, Putnam et al. (2005) showed that, for three observers, the center of fixation was offset from the location of highest foveal cone density by no more than 0.2° with an even smaller dispersion of about 0.05°. The offset was in different directions for each observer. These measurements corroborated earlier studies, which did not have access to the absolute position of the fixation direction, but could show that the retinal locations used to fixate targets had a dispersion of less than 4 arcmin (Steinman, 1965; Kowler & Blaser 1995). These results show that the retinal location chosen for fixation is typically quite stable and close to the physical center of the fovea.
However, this offset between the fixation target and the fovea is not constant but has a strong dynamic component as our physical gaze is typically far less steady than our mental experience would indicate. When we fixate on a visual target, our eyes constantly drift and jump, by up to 0.5°, around the point we feel we are fixating (Ortero-Millan, Troncosco, Macknik, Serrano-Pedraza, & Martinez-Conde, 2008; for a review, see Rolfs, 2009). Movements of this magnitude are easily seen when they occur in the external world (Murakami, 2003), yet we do not generally sense the visual motion caused by our eye movements of fixation. Instead, it requires special techniques (Verheijen, 1961; Murakami & Cavanagh, 1998; Murakami, 2003), or highly controlled laboratory conditions (Haddad & Steinman, 1973) for us to perceive the movements in our fixations.Thus, while our eyes are often jittering around the fixation, our subjective experience is more that our gaze is locked onto the fixation point. This difference between the subjective feeling of unwavering fixation and the objective measurement of rapid, random jumps and drifts (Rolfs, 2009) indicates that the subjective feeling of where we are looking is not determined simply by what falls directly on, or even slightly offset from, the fovea. It has been suggested that the perception of the motion caused by the eye movements of fixation may be suppressed based on the retinal motion signals themselves (Murakami & Cavanagh, 1998; Poletti, Listorti & Rucci, 2010; Arathorn, Stevenson, Yang, Tiruveedhula, & Roorda, 2013). However, the suppression of motion signals is not sufficient—we see our fixation not only as lacking in motion energy, but also as having a clearly fixed position (i.e., not jumping from point to point or being smeared). So some additional mechanism may be steadying the perceived position of the fixation along with the mechanisms that are suppressing its motion.
A much larger discrepancy in apparent direction of gaze happens just before a saccade. Hunt and Cavanagh (2009), following up on an earlier study by Deubel, Irwin, and Schneider (1999), asked observers to shift their gaze to a clock with a fast-moving hand and report the time on the clock when their eyes landed. Interestingly, the time they reported seeing on the clock when they landed corresponded to a time before their eyes had moved. This result suggested that observers sensed that they were looking directly at the clock before they had begun the eye movement: The perceived direction of gaze had shifted before their eyes did.
These effects, found during fixation or just before saccades, are suggestive but the interpretations are complicated by the speed of the events. To avoid this temporal uncertainty, we used long-lasting afterimages to measure whether the perceived direction of gaze could move independently of the fovea.
It is well known that voluntary saccades will make a small afterimage appear to move, following the eye position as it does (e.g., Bell, 1823; Hering, 1861; Mach, 1886/1959). However, it is also the case that this motion of the afterimage is slow compared to the motion of the eye (Grüsser, Krizič, & Weiss, 1987), again suggesting that the subjective direction of gaze is not obligatorily linked to the fovea. If an observer is asked to make an eye movement to the location of a small afterimage at more than 3° eccentricity (Kommerell & Täumer, 1972), the observers typically make a series of saccades, never succeeding in foveating the target, which flees further with each eye movement. However, for an afterimage at less than 2° in the periphery, observers almost always made smooth pursuit eye movements, a finding previously reported with mechanically stabilized stimuli by Robinson (1965) for targets offset by 0.1° and by Brune and Lücking (1969) for eccentricities of up to 1° to 2°. In one condition of Kommerell and Täumer's experiment (1972), some observers showed smooth pursuit to afterimages at up to 10° eccentricity.
These earlier studies were primarily investigations of eye movement mechanisms, and observers were asked neither where they felt they were looking, nor whether the target itself appeared to be moving.
A study by Pelz and Hayhoe (1995) also used afterimages but did ask observers whether the afterimage appeared steady or seemed to move. They induced whole scene afterimages and found that when observers were instructed to maintain their direction of gaze, their eyes showed slow drifts and occasional saccades but, despite these, observers did not report the afterimage as moving. When the instructions to fixate were relaxed and subjects were told to “inspect” or “watch the afterimage,” saccades of up to 5° were seen, but again without reports of movement of the afterimage. The authors did not remark upon observers' subjective direction of gaze within the afterimage scene, but it could be easily inferred that the eye movements corresponded to intentional and successful shifts in the subjective location of gaze. Indeed, it was the case that the observers' attempts to look around in the scene were felt to be successful so long as the afterimage didn't disappear or move (Hayhoe, personal communication, 2015).
In this paper, we ask observers to move their subjective direction of gaze to specified locations in the afterimage and report whether they felt they successfully looked directly at the intended target, and whether the afterimage appeared to move. Eye movements were analyzed for components that varied systematically with the target. We find that when observers are requested to move their gaze to a target at less than 3° from the fovea in an afterimage, they often report that they have subjectively fixated the target and, despite the smooth pursuit and small saccades that this often elicits, they report that the target is not moving. Eye movements showed a systematic bias, tending to drift in the direction of displacement between the fovea and the point of subjective gaze.
Experiment
Methods
Observers were six members of Harvard Vision Sciences Laboratory, all naïve to the purposes of the experiment. All provided written informed consent. All experimental procedures consent forms, and debriefing materials were approved by the Harvard Committee on Human Subjects in accordance with the Declaration of Helsinki. One observer (TC) performed the experiment without eye tracking. The remaining five observers had their eye movements monitored throughout their experiments. Eye movement data for one observer had to be discarded because time markers for experimental events were unreliably synced. Thus, results regarding the observers' reports are based on six observers, and results regarding eye movements are based on four observers.
Setup
The experiment room was entirely sealed from outside light, and all internal sources of light were either eliminated or covered with blackout curtain material. The observer sat at a chinrest 115 cm away from the stimulus field, a 48 × 96 in. piece of matte black foamboard. Stimuli were cut from white multipurpose copy paper (Brightness US92) and affixed to the foamboard with double-sided tape. Stimulus configuration is shown in Figure 1. For one observer (JF), the stimulus was rotated such that the “X” was to the right. Squares of white tape were affixed to the stimulus field in the default configuration used by Eyelink for calibration markers.
Figure 1.
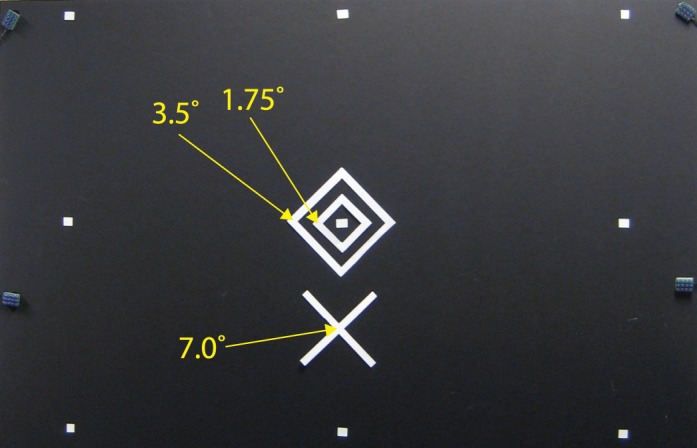
Stimulus used to generate afterimages. The yellow labels indicate degrees eccentricity from the center of the diamond configuration. The outermost white dots are markers used to calibrate the eye tracker. The four grayish tabs on the edges of the board are infrared markers used by the EyeLink to track head position. After calibration, the infrared camera of the eye tracker continued to function without visible light. A red, fixation dot (a laser pointer), aligned to the center of the diamond shapes, directed the observer's gaze to the center of the image. The stimulus was briefly illuminated to create an afterimage, and the red dot was extinguished. Observers were then asked to look at a specific corner of the diamond afterimage, to report if they succeeded and whether they saw any motion of the image. In some conditions they were then asked to make a saccade to the center of the lower X.
Stimulus illumination was provided by a studio photographer's flash setup: a Speedotron 105 light unit with 11.5 in. reflector, powered by a Speedotron 4803 power pack, which sent 4800 Ws to four MW24Q flash tubes. The flash was positioned directly behind the observer, with reflector oriented up and toward the rear of the room, so as to flood the viewed area with a wide field of reflected light. Flashes were full-strength, lasting 1.6 ms.
During prestimulus periods, a red laser pointer, attenuated by 99% through neutral density filters, provided a fixation point at the center of the stimulus configuration. During the afterimage phase of a trial, the laser beam was blocked by a wooden board. When necessary, a red LED flashlight was used in between trials to allow the experimenter and observer to make adjustments and reposition materials.
For the observer whose eyes were not tracked, the head was stabilized by a forehead attachment to the chinrest. For the others, the head was stabilized by cheek rest attachments. An Eyelink II head-mounted infrared eye tracker sampled the right eye at 250 Hz, and monitored head movements based on infrared markers mounted in the periphery of the stimulus field. The calibration routine was set to a fixed sequence of markers (all computer monitors were kept off during the experiment, making the typical randomized calibration method impractical).
The Eyelink hardware and PC were controlled from a Macintosh G4 desktop using the Psychophysics Toolbox and Eyelink Toolboxes in the Matlab programming environment, under the OS 9 operating system. The G4 received gaze data from the Eyelink PC, and received time markers from the experimenter and observer via USB keyboards.
Procedure
Prior to the experiment, the experimenter affixed the eye-tracking helmet and adjusted the cameras. The eye-tracking computer display and lights were then turned off and the observer sat in complete darkness for at least 7 min. The observer took position at the chinrest, and then the experimenter the calibrated the eyetracker, using a red LED flashlight to direct the observers' gaze to the fixed sequence of calibration markers in time with the Eyelink computer's audio prompts.
At the start of each trial, the experimenter uncovered the laser pointer, which was pointed at the center of the stimulus. The dim red fixation point was the only source of light in the room. The observer was instructed to gaze at the fixation point, and then Eyelink performed its drift-correction routine to recenter the recording. Following a countdown, the experimenter discharged the studio flash and pressed a keyboard key to insert a time marker into the eye-tracking data file. Immediately following the flash, the experimenter covered the laser pointer, returning the room to complete darkness. The experimenter then verbally instructed the observer to “gaze at” a location (or sequence of locations) on the afterimage shape. The observer waited until the afterimage had developed and stabilized, and then attempted to carry out the instructions, pressing a keyboard key to indicate completion of each step. The trial ended when the task sequence was complete, or when the observer indicated that the afterimage had faded or become too blurry for task performance. The observer then verbally described what he or she experienced throughout the trial, with specific prompts to indicate if their subjective gaze was successfully maintained upon the landmark, and whether the afterimage was seen to move.
Observers also performed a task in which the establishment of gaze upon a landmark was followed by a saccade to the “X.” They reported whether they felt their gaze landed appropriately, and whether they felt their eye movement was straight or angled.
Analysis
Reports for successful subjective fixation and perceived afterimage motion were recorded for each fixation target, per observer and in aggregate. Eye-tracking data for fixation periods were identified using time markers entered by the experimenter and observer. If the observer indicated a subjective fixation longer than two seconds, only the first two seconds of data were used. Otherwise, data were truncated at the next time marker (when the observer indicated cessation of afterimage, beginning of next instructed fixation, or diagonal saccade). Data from these fixation periods were divided into 100 ms sequential segments, and net direction of movement was calculated for each segment. Magnitude information was discarded to minimize the influence of large saccadic eye movements, which were infrequent. Analysis of subjective fixation trials excluded trials in which the observer reported afterimage motion. To pool data across different trial types for statistical analysis, data were rotated by the amount needed to align the attempted subjective fixation with the upward direction (e.g., data from trials with leftward fixations were rotated 90° clockwise).
Data from the saccade experiment were analyzed by the maximum-likelihood fits of mixed-effects models. In the full model, the dependent variable was the angle of the saccade, the independent variable was the angle between the subjective fixation origin and the target, and the random effect was observer identity. Both fixed and random effects contained intercept, slope and interaction terms. To perform model selection, likelihood ratio tests were used to compare among the nested versions of this model. None of the mixed model forms were found to have a significantly better fit than the reduced model with fixed effects only (e.g., full model df = 6, AIC = 169, log-likelihood = −78.6; fixed effects model df = 3, AIC = 165, log-likelihood = −79.6; likelihood ratio statistic = 1.76, Δdf = 3, p = 0.624). Parameter fits among the models were similar (e.g., full model intercept = 2.5° ± 2.8° (SE), slope = 0.32 ± 0.11; reduced model intercept = 2.0° ± 1.9°, slope = 0.27 ± 0.11). Thus, the results presented are based on parameter estimates and statistics from the reduced model containing no random effects terms.
Results
Subjective fixation reports
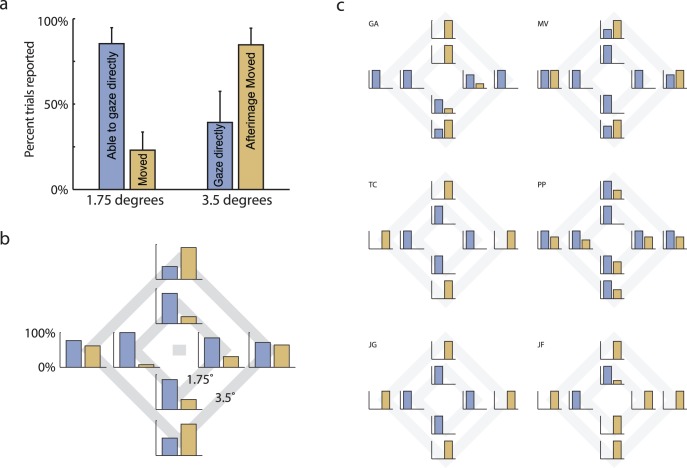
The observers' reports showed a very consistent pattern (Figure 2). When asked to look directly at corners of the inner diamond (1.75° distance), they almost always claimed that they had succeeded, and they reported very little if any motion of the afterimage while they were subjectively “fixating” outside their fovea. For the corners of the outer diamond (3.5° distance), however, the results were mixed. Some observers reported being able to look directly at these corners, but more frequently they found the diamond moving away as they attempted the fixation, leading to a continual chase. Occasionally, some observers reported both successful, nonfoveal subjective “fixation” and afterimage motion (they found it similar to tracking a moving object). Figure 2a shows the reports averaged across observers and locations for the inner and outer diamond corners. The percentages of trials with successful subjective fixations are shown in blue, and the percentages of trials with perceived motion are shown in brown.
Figure 2.
Subjective fixation reporting rates. (a) Reports of success in subjectively fixating at the requested corner (blue bars) and appearance of motion in the afterimage (brown bars) for the two eccentricities of target corners. These results are averaged over six observers and all four corners (up, down, right, left). Error bars indicate standard error of the means. (b) Observer group means broken down by target location, presented as an overlay over the stimulus Figure. (c) Individual observer reports, in the same format. The reports of “fixating” and “moving” were not mutually exclusive.
Figure 2b shows the responses for each location separately, and Figure 2c further separates the data for individual observers. Observers performed each condition two to three times unless they became uncomfortable or fatigued. A total of 103 trials were performed. Individually, each observer was quite consistent for any given position, such that they reported success either 100% or 0% of the time. Variability existed mainly across observers and across the cardinal directions. For example, some observers found horizontal locations easier to subjectively fixate than vertical ones, whereas other observers found the opposite.
Eye movements
What were the observers' eyes doing while they attempted to look directly at nonfoveal locations in the afterimage in total darkness? First of all, even when not attempting the nonfoveal fixation, observers' eyes were drifting and small saccades were common. These eye movements were larger than normal fixational eye movements, undoubtedly because there was no visual feedback to damp them (Pelz & Hayhoe, 1995).
Looking at the trials where observers attempted nonfoveal fixations, there were some clear differences between successful and nonsuccessful attempts. In trials where observers reported successful fixation, large saccades were quite rare. However, in trials where they reported failure to fixate, there was often a pattern of repeated saccades. This result accords with their subjective description of the trials, in which they perceived the afterimage to jump away, forcing them to repeatedly try and chase it.
Nonfoveal fixation attempts also showed changes in the drift component of eye movements. During the trial, there was often an added bias to the drift, in the direction of the selected target (Figure 3). To quantify this tendency, we selected trials in which observers reported that they had subjectively fixated the requested diamond corner in a nonmoving afterimage. We divided the “fixation” intervals into 100 ms epochs, and then subtracted each epoch's start point from its end point. The angle of each epoch's directional vector was then taken as a data point.
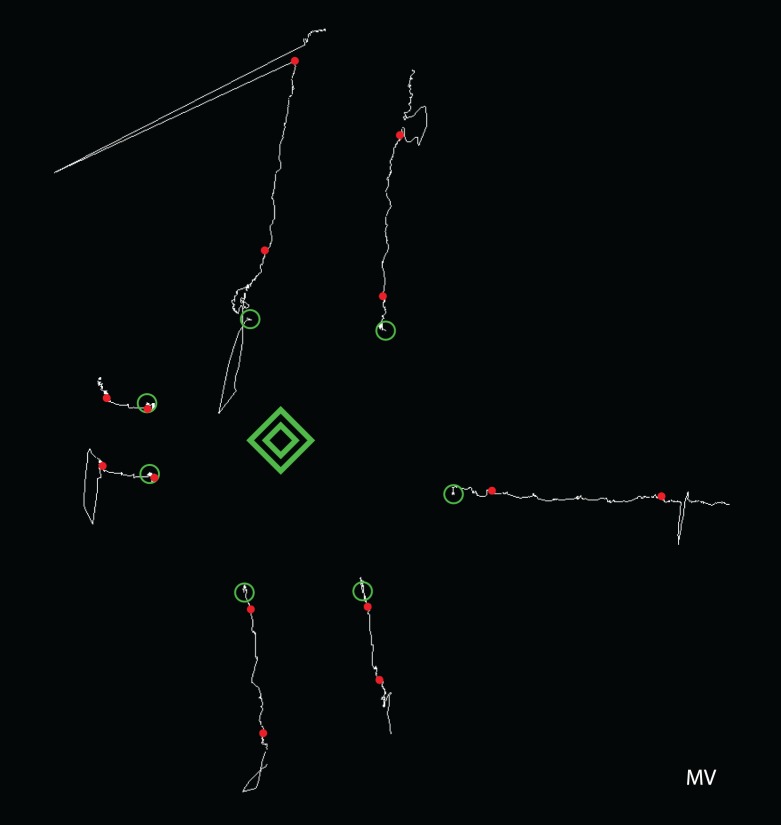
Figure 3.
Eye movement traces for one observer when subjectively fixating each of the four corners of the inner diamond. Traces start at the green circle, and red dots mark the points at which the observer indicated the beginning or end of the fixation period. Traces have been shifted for display. Scale: Outer diamond is 7° across.
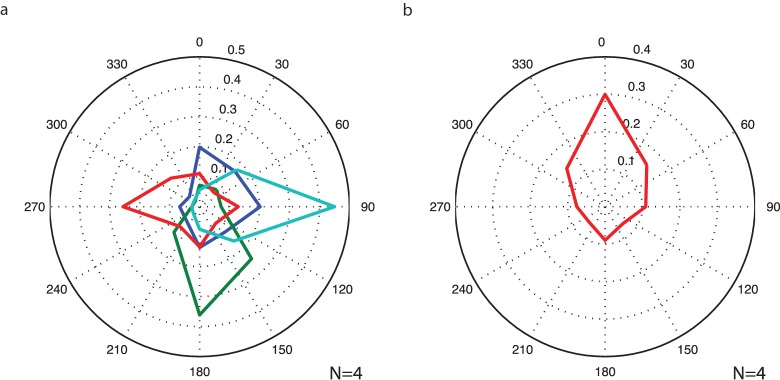
Overall, the data shows that the direction of drift was biased in the direction of the target the observer was attempting to look at. Figure 4 plots the polar frequency histogram for the directional data, first for each subjective fixation direction separately, then for data pooled across directions. The average drift was significantly biased (Rayleigh's test, z = 141, p ≪ 0.0001) and more likely to be pointed in the direction of fixational target than away (binomial test z = 26.6, p ≪ 0.0001).
Figure 4.
Distribution of eye movement directions during subjective fixation of retinotopically eccentric points. Histograms plot the direction of the movement vectors from sequential 100 ms epochs of eye-tracking data. (a) Fixation targets plotted separately (up, down, left, right = blue, green, red, aqua). (b) Pooled conditions. Data from each fixation condition are rotated as if fixating at a point above the fovea.
These results show that observers often reported looking directly at a stable, unmoving target (typically an inner corner) while at the same time their eyes were drifting in the direction of the target's offset relative to the fovea. In other words, the observer felt that he or she was fixating steadily on the nonfoveal target, but the oculomotor system was registering the displacement between fovea and target and was trying to reduce it.
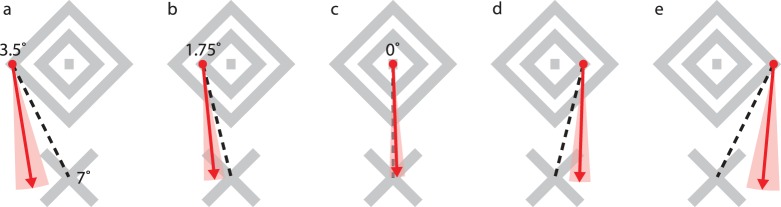
In our next test, we asked observers to perform a two-step task. As before, they directed their subjective gaze upon a landmark in the diamond afterimage. They then attempted to saccade to the “X” directly below the fovea. The first landmark could be the left or right corner of the inner or outer diamond, or the central origin point. Because observers varied in terms of the points they felt able to subjectively fixate, the total number of trials per condition (Figure 5a–e, left to right) was three, five, five, seven, and two. Further, JF fixated above or below and attempted to saccade to the target on the right; her data was rotated to align with the other observers.
Figure 5.
Direction of saccadic eye movements made when observers attempted to saccade to the X after first “fixating” upon a far lateral vertex, a near lateral vertex, or the foveal point. The saccadic angles expected from the observers' subjective experiences are depicted with dashed lines. Red vectors depict the maximum likelihood fit from a mixed effects modeling process; shaded areas depict 95% confidence intervals.
We examined how these voluntary saccades were experienced by the observer, and whether they would trigger eye movements corresponding to the perceived (diagonal) vector to the target or follow the real (downward) vector that would bring the fovea to the target.
Observers all reported that they performed appropriately diagonal saccades after gazing at a diamond corner, and felt that their gaze landed at the intended location. The afterimage would fade as they shifted gaze and reappear at a more distant location after a delay.
In contrast, the eye movement records seldom showed appropriately diagonal saccades. Instead, the saccades were closer to the vertical direction that actually linked the position of the fovea to the intended target. Figure 5 depicts saccadic directions for each subjective, starting point condition as fit by a mixed effects modeling process (details in methods section). For all diagonal saccade conditions, the appropriate (and perceived) saccadic vector lay well outside 95% confidence interval for the saccades performed. With a subjective starting point on the outer diamond (Figure 5a, 5e), the path to the target would be 27° from vertical. However, saccades were instead 9.2° ± 7.6° (95% CI) and 5.2° ± 7.6° from vertical for the left and right subjective starting points, respectively. For the inner diamond (Figures 5b, d) paths to the target would be 14° from vertical. However in these cases, the saccade directions were 1.8° ± 5.3° and 5.8° ± 5.3°. Further, the slope parameter of the fitted function for saccade angle as a function of the physical angle was 0.27° ± 0.11°(SE), which is significantly below the veridical slope of 1 (t = 6.2, df = 20, p ≪ 0.0001). Thus, for the diagonal saccade conditions, eye movements did not match the appropriate (and perceived) saccadic vector.
The model fit nearly vertical saccades for all conditions, with the vertical direction always within the 95% confidence bounds. However, there was some evidence of an incremental effect of lateralized subjective gaze. The slope of the fitted function was shallow but significantly different from zero (t = 2.3, df = 20, p < 0.05). This deviation from zero could be interpreted as an influence of the intended diagonal direction of the saccade. Alternatively, this could be a mechanical consequence of the lateral drift of the eye prior to saccade. During the preparatory step of holding one's subjective gaze on a leftward lateral target, the eye would have already drifted to the left by the time the saccade was executed. Thus an essentially downward saccade may have been biased to the right, toward a more neutral position.
Conclusions
Observers frequently reported being able to look directly at nonfoveal locations in an afterimage. With targets at 1.75° eccentricity, they most often saw the targets as steady even though their eyes were drifting, on average, toward the targeted location. For more eccentric targets, observers more frequently saw the target as jumping away. Some observers reported successful subjective fixations at 3.5° eccentricity, sometimes with and sometimes without accompanying motion. When asked to saccade from an eccentric “fixated” location to a second location, observers reported making the saccade along the perceived oblique vector (from the subjective fixation to target rather than from the actual foveal location to the target). However, their real saccades only sometimes showed this oblique direction, corresponding more closely to the actual vertical displacement between the fovea and the intended target.
These findings are related to, but distinct from, the rich literature regarding the perception of visual direction, exemplified by classic experiments such as Matin et al. (1982) and Bridgeman and Graziano (1989). These experiments concerned the perception of directions in egocentric (e.g., head-centered) reference frames. These studies specified models in which eye position information was used to transform visual input directions from their native retinotopic coordinate frame into an egocentric coordinate frame for external space. In this literature, there is little discussion of gaze direction within the retinotopic frame of reference. If anything, the eye-position-based models presuppose that perceived gaze direction is locked to a fixed point on the retina.
In our study, the reports of perceived fixation direction were made based on landmarks in the retinally-stabilized afterimage, and thus entirely within the visual input's native retinotopic reference frame. In this sense, eye position information is not theoretically necessary for our judgments. Nevertheless, eye position information may have some effect on the location of subjective gaze via some kind of bias or cue. It remains quite open-ended what inputs might influence where subjective gaze is located in the retinal image.
Our study parallels that of Pelz and Hayhoe (1995), who reported that observers, when asked to “inspect” a full scene afterimage, often made saccades of moderate sizes without seeing any movement in the afterimage. Our results here duplicate their finding as our observers often reported the afterimage as motionless despite drift, small and moderate size saccades. Pelz and Hayhoe's afterimages covered a larger spatial extent than ours and probably because of that additional anchoring, supported robust constancy of visual direction in the face of even larger saccades than for our observers. This is consistent with the cue-conflict interpretation of the afterimage stability. The large size of the retinally stabilized image may cause a down-weighting of inputs signaling eye movement, thus reinforcing the percept of an egocentrically unmoving afterimage.
What we have added to their earlier report is an analysis of exactly where observers claimed to be looking in the afterimage and how frequently the afterimage appeared to be moving. By asking observers to move their subjective fixation to specific points within the afterimage, we were able to analyze how these sustained subjective fixations related to the physical eye-movement data. Our finding of systematically biased drift in the direction of the targeted location supports Pelz and Hayhoe's (1995) interpretation regarding the random drifts in their data. They surmised that the movements involved a form of open loop pursuit, which occurs toward off-foveal targets in retinally stabilized stimuli. Our study confirmed that the drifts were systematic with respect to the subjective fixation of off-foveal targets, and that this corrective drift occurred in the direction of mismatch between the fovea and subjective point of fixation.
Our observers' consistent reports that they could move their subjective fixation within the afterimage suggest that the perceived direction of gaze within the retinal scene is not hard-wired to the fovea but may be flexibly assigned to nearby locations during fixation. This flexibility may not manifest itself so readily under normal visual conditions, where cues such as retinal slip are present, and eye position information would not be so severely suppressed. Nevertheless, it appears that the subjective location of gaze has the potential to move about under the right conditions.
This could have the advantage of providing an impression that our gaze is locked onto the target of central interest, despite the reality that our eyes wander during fixation so that the central target of interest is drifting and jumping around within the foveal region. Poletti, Listorti, and Rucci (2013) suggest that these movements are a fine scale exploration of the target that compensates for nonhomogeneous visual sensitivity within the fovea, and others suggest that they help maintain a strong transient response (cf., Martinez-Condé, Macknik, & Hubel, 2004; Rolfs, 2009). Whatever the case, we do not experience these displacements of the eye movements of fixation except following adaptation to dynamic noise (the jitter aftereffect, Murakami & Cavanagh, 1998) or when superimposing an afterimage on a visible image (Verheijen, 1961).
The possibility that the apparent direction of gaze during fixation is flexible is consistent with other evidence, described in the Introduction, that the direction of gaze can be disconnected from the fovea just before an eye movement (Deubel et al., 1999; Hunt & Cavanagh, 2009).
Other examples showing that the “direction of gaze” is not obligatorily tied to the fovea come from individuals who have lost vision in the fovea. They develop a preferred retinal locus, which they direct to targets of central interest for reading or visual scrutiny (e.g., Timberlake, Peli, Essock, & Augliere, 1987). Several studies suggest that many individuals with longstanding conditions do associate this eccentric gaze with the notion and sensation of looking directly at small targets (von Noorden & Mackensen, 1961; White and Bedell, 1990; Crossland, Culham, Kabanarou, & Rubin, 2005; however, see White & Bedell, 1990, regarding some caveats to these observations).
Evidence for a flexible assignment of direction of gaze also comes from the pseudofovea in the nondominant eye of some strabismic individuals (e.g., Arnoldi, 2011). Since the nondominant eye is not properly converged with the dominant eye, any target foveated by the dominant eye will fall on a nonfoveal location in the nondominant eye. This is the pseudo fovea and for some, when the dominant eye is closed, they report feeling that they are looking directly at a target with either the fovea or the pseudo fovea.
In all these cases, we face the problem that the subjective direction of gaze is just that—subjective. We have no independent evidence that verifies that our observers were really reporting where they were looking as opposed to reporting where they wanted to be looking or simply where they were attending.
One might, therefore, be concerned that our observers were merely confused between an attended point, and the point at which they felt they were looking. However, our observers included a number of vision researchers who had experience performing covert attention tasks, and were familiar with distinguishing between attending and looking. Some also noted the apparent contradiction involved in “looking” at off-foveal portions of an afterimage, and still re-confirmed their subjective feeling of looking at locations away from the fovea. Even for novice observers, it would be surprising for them to confuse covert attention with looking. The act of covertly attending is defined by the intention to attend to points away from the point at which one is looking.
Even though we are assuming that our observers could distinguish between looking at and attending to a target, we do want to suggest a close relationship between attention processing and the processes that determine the feeling of looking. Our suggestion is that, under normal circumstances, one of the main determinants of the subjective direction of gaze is the location of an attended item near the fovea. The attended stimulus near the fovea may be assigned a special status where it acts as the fixed center of the visual scene, stabilizing otherwise visible changes in position due to eye movements of fixation just as other mechanisms (Murakami & Cavanagh, 1998; Poletti et al., 2010; Arathorn et al., 2013) suppress the motion signals that these produce. Moreover, it is the offset between this subjective gaze location and the fovea that provides a basis for the error signal to drive pursuit.
Supplementary Material
Acknowledgments
This research was supported by NIH grant EY09258, an ANR Chaire d'Excellence, Dartmouth PBS, and an ERC Advanced grant to PC.
Commercial relationships: none.
Corresponding author: Patrick Cavanagh.
Email: Patrick.cavanagh@dartmouth.edu.
Address: Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, USA.
Contributor Information
Daw-An Wu, daw-an@caltech.edu, https://www.hss.caltech.edu/content/daw-j-wu.
Patrick Cavanagh, patrick.cavanagh@dartmouth.edu, http://pbs.dartmouth.edu/people/patrick-cavanagh.
References
- Arathorn D. W.,, Stevenson S. B.,, Yang Q.,, Tiruveedhula P.,, Roorda A. (2013). How the unstable eye sees a stable and moving world. Journal of Vision, 13 (10): 6 1–19, doi:10.1167/13.10.22 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldi K. (2011). Factors contributing to the outcome of sensory testing in patients with anomalous binocular correspondence. American Orthoptic Journal, 61, 128–136. [DOI] [PubMed] [Google Scholar]
- Bell G. (1823). On the motions of the eye, in illustration of the uses of the muscles and nerves of the orbit. Transactions of the Royal Society of London, 113, 166–186. [Google Scholar]
- Bridgeman B.,, Graziano J. A. (1989). Effect of context and efference copy on visual straight ahead. Vision Research, 29 (12), 1729–1736. [DOI] [PubMed] [Google Scholar]
- Brune F.,, Lücking C. H. (1969). Oculomotorik, Bewegungswahrnehmung und Raumkonstanz der Sehdinge [Translation: Oculomotoricity, movement perception and space constant of vision] Nervenartz, 40, 413–421. [PubMed] [Google Scholar]
- Crossland M. D.,, Culham L. E.,, Kabanarou M. D.,, Rubin G. S. (2005). Preferred retinal locus development in patients with macular disease. Ophthalmology, 112 (9), 1579–1585. [DOI] [PubMed] [Google Scholar]
- Deubel H.,, Irwin D. E.,, Schneider W. X. (1999). The subjective direction of gaze shifts long before the saccade. In Wolfgang Becker, Heiner Deubel, &Thomas Mergner (Eds.), Current oculomotor research: Physiological and psychological aspects (pp 65–70). New York: Plenum Press. [Google Scholar]
- Grüsser O.-J.,, Krizič A.,, Weiss L.-R. (1987). Afterimage movement during saccades in the dark. Vision Research, 27, 215–226. [DOI] [PubMed] [Google Scholar]
- Haddad G. M.,, Steinman R. M. (1973). The smallest voluntary saccade: Implications for fixation. Vision Research, 13, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Hering E. (1861). Beiträge zur Physiologie, 1, Zur Lehre vom Ortssinne der Netzhaut [Translation: Contributions to physiology, the doctrine of the local sense of the retina] Leipzig: Englemann. [Google Scholar]
- Hunt A. R.,, Cavanagh P. (2009). Looking ahead: The perceived direction of gaze shifts before the eyes move. Journal of Vision, 9 (9): 6 1–7, doi:10.1167/9.9.1 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommerell G.,, Täumer R. (1972). Investigations of the eye tracking system through stabilized retinal images. Bibliotheca Ophthalmologica Supplementa ad Ophthalmologica, 82, 288–297. [PubMed] [Google Scholar]
- Kowler E.,, Blaser E. (1995). The accuracy and precision of saccades to small and large targets. Vision Research, 35 (12), 1741–1754. [DOI] [PubMed] [Google Scholar]
- Mach E. (1959). Die Analyse der Empfindungen und das Verhältnis des physischen zum psychischen [The analysis of sensations and the relation of the physical to the psychical] ( Fischer J. Trans.) New York: Dover; (Original work published 1886) [Google Scholar]
- Martinez-Conde S.,, Macknik S. L.,, Hubel D. H. (2004). The role of fixational eye movements in visual perception. Nature Reviews Neuroscience, 5, 229–240. [DOI] [PubMed] [Google Scholar]
- Matin L.,, Picoult E.,, Stevens J. K.,, Edwards M. W.,, Young D.,, Macarthur R. (1982). Oculoparalytic illusion: Visual-field dependent spatial mislocalizations by humans partially paralyzed with curare. Science, 216 (4542), 198–201. [DOI] [PubMed] [Google Scholar]
- Murakami I. (2003). Illusory jitter in a static stimulus surrounded by a synchronously flickering pattern. Vision Research, 43, 957–969. [DOI] [PubMed] [Google Scholar]
- Murakami I.,, Cavanagh P. (1998). A jitter after-effect reveals motion-based stabilization of vision. Nature, 395, 798–801. [DOI] [PubMed] [Google Scholar]
- Ortero-Millan J.,, Troncosco X. G.,, Macknik S. L.,, Serrano-Pedraza I.,, Martinez-Conde S. (2008). Saccades and microsaccades during visual fixation, exploration and search: Foundations for a common saccade generator. Journal of Vision, 8 (14): 6 1–18, doi:10.1167/8.14.21 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Pelz J. B.,, Hayhoe M. M. (1995). The role of exocentric reference frames in the perception of visual direction. Vision Research, 35 (16), 2267–2275. [DOI] [PubMed] [Google Scholar]
- Poletti M.,, Listorti C.,, Rucci M. (2010). Stability of the visual world during eye drift. Journal of Neuroscience, 30 (33), 11143–11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M.,, Listorti C.,, Rucci M. (2013). Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Current Biology, 23, 1691–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N. M.,, Hofer H. J.,, Doble N.,, Chen L.,, Carroll J.,, Williams D. R. (2005). The locus of fixation and the foveal cone mosaic. Journal of Vision, 5 (7): 6 632–639, doi:10.1167/5.7.3 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Robinson D. A. (1965). The mechanics of human smooth pursuit eye movement. Journal of Physiology, 180 (3), 569–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49, 2415–2441. [DOI] [PubMed] [Google Scholar]
- Roorda A. (2011). Adaptive optics for studying visual function: A comprehensive review. Journal of Vision, 11 (5): 6 1–21, doi:10.1167/11.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. (1965). Effect of target size, luminance and color on monocular fixation. Journal of the Optical Society of America, 55 (9), 1158–1165. [Google Scholar]
- Timberlake G. T.,, Peli E.,, Essock E. A.,, Augliere R. A. (1987). Reading with a macular scotoma. II. Retinal locus for scanning text. Investigative Ophthalmology & Visual Science, 28, 1268–1274. [PubMed] [Article] [PubMed] [Google Scholar]
- Verheijen F. J. (1961). A simple after image method demonstrating the involuntary multidirectional eye movements during fixation. Optica Acta: International Journal of Optics, 8 (4), 309–312. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K.,, Mackensen G. (1961). Phenomenology of eccentric fixation. American Journal of Ophthalmology, 53, 642–660. [DOI] [PubMed] [Google Scholar]
- White J. M.,, Bedell H. E. (1990). The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology & Visual Science, 31 (6), 1149–1161. [PubMed] [Article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.