Abstract
An increasing body of studies has revealed neuroanatomical impairments in developmental dyslexia. However, whether these structural anomalies are driven by dyslexia (disorder-specific effects), absolute reading performance (performance-dependent effects), and/or further influenced by age (maturation-sensitive effects) remains elusive. To help disentangle these sources, the current study used a novel disorder (dyslexia vs. control) by maturation (younger vs. older) factorial design in 48 Chinese children who were carefully matched. This design not only allows for direct comparison between dyslexics versus controls matched for chronological age and reading ability, but also enables examination of the influence of maturation and its interaction with dyslexia. Voxel-based morphometry (VBM) showed that dyslexic children had reduced regional gray matter volume in the left temporo-parietal cortex (spanning over Heschl’s gyrus, planum temporale and supramarginal gyrus), middle frontal gyrus, superior occipital gyrus, and reduced regional white matter in bilateral parieto-occipital regions (left cuneus and right precuneus) compared with both age-matched and reading-level matched controls. Therefore, maturational stage-invariant neurobiological signatures of dyslexia were found in brain regions that have been associated with impairments in the auditory/phonological and attentional systems. On the other hand, maturational stage-dependent effects on dyslexia were observed in three regions (left ventral occipito-temporal cortex, left dorsal pars opercularis and genu of the corpus callosum), all of which were previously reported to be involved in fluent reading and its development. These striking dissociations collectively suggest potential atypical developmental trajectories of dyslexia, where underlying mechanisms are currently unknown but may be driven by interactions between genetic and/or environmental factors. In summary, this is the first study to disambiguate maturational stage on neuroanatomical anomalies of dyslexia in addition to the effects of disorder, reading performance and maturational stage on neuroanatomical anomalies of dyslexia, despite the limitation of a relatively small sample-size. These results will hopefully encourage future research to place greater emphasis on taking a developmental perspective to dyslexia, which may, in turn, further our understanding of the etiological basis of this neurodevelopmental disorder, and ultimately optimize early identification and remediation.
Keywords: dyslexia, developmental trajectories, etiology, brain morphometry, MRI
1. Introduction
Developmental dyslexia is a common neurological disorder, affecting about 7% of school-age children (Peterson & Pennington, 2012). It is characterized by neuroanatomical anomalies in focal regions, as evident from recent meta-analyses of voxel-based morphometry (VBM) studies (Linkersdorfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Richlan, Kronbichler, & Wimmer, 2013). These studies reported anomalies in bilateral temporo-parietal cortex (LTPC), posterior superior temporal and supramarginal gyri, left ventral occipito-temporal cortex (LvOTC) consisting of inferior temporal and fusiform gyri, as well as bilateral cerebellum. It is unclear, however, whether these cortical anomalies represent causal relationships from having dyslexia (disorder-specific effects), their absolute level of reading performance regardless of dyslexia or age (performance-dependent effects), and/or abnormal brain changes unfolding over time (maturation-sensitive effects). Since most previous studies adopted a cross-sectional design and included only two groups (usually children with dyslexia and their age-matched controls, where children with a wide range of age were collapsed), it is possible that important maturational, stage-dependent deviations in dyslexic children have been masked (Oliver, Johnson, Karmiloff-Smith, & Pennington, 2000).
Until now, only two studies (in the English language) have tried to disentangle these effects using a design that additionally included reading-level matched typically developing readers (reading-matched controls) (Hoeft, et al., 2007; Krafnick, Flowers, Luetje, Napoliello, & Eden, 2014). In these studies, the authors identified disorder-specific regions by showing that older dyslexic children (DYS-older) were anomalous compared to both chronological age-matched typically developing readers (age-matched controls, a.k.a. TD-older) and younger but reading-matched typically developing readers (reading-matched controls, a.k.a. TD-younger). Performance-dependent regions were identified by showing that DYS-older were anomalous compared to TD-older but similar to reading-matched TD-younger readers. Using this design, Hoeft and colleagues (2007) found a disorder-specific neuroanatomical region that overlapped with a similar disorder-specific region identified functionally in the left inferior parietal lobule (LIPL). Conversely, a recent VBM study conducted by Krafnick et al. (2014) did not find any significant disorder-specific effects in regions previously considered important for dyslexia, e.g., left temporo-parietal, occipito-temporal or bilateral cerebellar regions. They found, instead, that many of the deficits observed in previous studies of dyslexia could be explained by individual differences in reading performance. Curiously, they identified a small area in the right precentral region as the only region that showed disorder-specific effects. Inconsistent results between these two studies could be due to methodological differences such as sample size and the threshold adopted, but another possibility is the difference in the developmental stages that were examined, especially considering developmental stage influences neuroanatomy (Giedd & Rapoport, 2010) and participants of these two studies differed in age by an average of 4 years (y) (i.e., mean age of 13 and 9 y for Hoeft’s and Krafnik’s studies respectively). Indeed, previous studies have shown that maturational stages make a substantial difference in neuroanatomy, especially during the developmental stages that were investigated in the two studies (Clark, et al., 2014).
In order to dissociate disorder-specific, performance-dependent and maturation-sensitive effects at the neuroanatomical level, the current study adopted a novel design. In addition to matching groups based on age and reading-level, a fourth group consisting of younger dyslexic readers who were matched by age to the reading-matched controls (DYS-younger) was recruited. This design allowed developmental effects of the brain and its interaction with the disorder (i.e., dyslexia) to be taken into account. Therefore, this study addresses three critical questions concerning neural impairments in dyslexia that had remained unresolved:
-
(1)
Neuroanatomical signatures of disorder-specific deficits in dyslexia independent of performance and maturation. If reduced volume is a persistent hallmark of dyslexia from a young age, there should be significant differences in three independent comparisons between dyslexia and normal controls (i.e., DYS-older < TD-older and DYS-older < TD-younger as in previous studies, but additionally, DYS-younger < TD-younger). In other words, there will be main effects of group (dyslexia versus respective age-matched controls), but additionally, DYS-older would be anomalous compared to TD-younger. This pattern provides insights into the causal relationship between neuroanatomy and disorder and also the stability over maturation, at least within the age-range of the current study.
-
(2)
Neuroanatomical signatures of reading performance. If a region is purely associated with reading performance, it will show both main effects of disorder and maturation, but no significant difference between groups with comparable reading performance (i.e., DYS-older and TD-younger). This pattern provides information on the neural underpinning of an individual’s absolute level of reading irrespective of disorder and maturational stage.
-
(3)
Neuroanatomical signatures of maturation-sensitive effects in dyslexia. If neuroanatomical manifestations change with age, the affected regions will show significant interaction effect between disorder [DYS vs. TD] and maturation [younger vs. older]. This pattern could be used to reveal possible neuroanatomical perturbation to the typical developmental trajectory, which might be associated with factors such as abnormal gene expression during maturation. Such an abnormality may also be a defining feature of dyslexia, but one that is different from sustained disorder-specific impairment defined in (1) above.
2. Methods
2.1. Participants and Study Design
Twenty-four dyslexic children and the same number of typically developing children were selected from two projects, which shared the same goal of investigating neural differences between dyslexic and typical readers, same MRI acquisition parameters, and imaging data collection period, but differed in the relative age of participants (Table 1) (Lei, et al., 2011; Pan, Yan, Laubrock, Shu, & Kliegl, 2013). All children were healthy, right-handed native mandarin speakers, aged 10―15 years old. All participants had normal or corrected-to-normal vision and hearing, without a history of neurological or psychiatric disorders based on self and parental-reports. They were recruited from local primary and middle schools in Beijing. IQ of each participant was within normal range (primary school: Full Scale IQ above 80 on the Chinese version of Wechsler Intelligence Scale for Children [C-WISC] (Gong & Cai, 1993); middle school: standardized score above 80 on the Standard Progressive Matrices (Raven & Court, 1998)). The current study was approved by the Institutional Review Board at Beijing Normal University. All parents and children gave informed consents and assents.
Table 1.
Demographics of the four subgroups
| Group | DYS-younger [Mean (SD)] |
TD-younger [Mean (SD)] |
DYS-older [Mean (SD)] |
TD-older [Mean (SD)] |
DYS-younger vs. TD-younger (Age-matched control) |
DYS-older vs. TD-older (Age-matched control) |
DYS-older vs. TD-younger (Read-matched control) |
|---|---|---|---|---|---|---|---|
| N | 12 | 12 | 12 | 12 | --- | --- | --- |
| Age | 11 (0.9) | 11 (0.3) | 14.1 (0.4) | 14.1 (0.5) | 0.707 | 0.781 | < 0.001*** |
| Gender | 7 M, 5 F | 6 M, 6 F | 7 M, 5 F | 6 M, 6 F | 0.682 | 0.682 | 0.682 |
| Performance IQ | 100 (10) | 101 (10) | 110 (17) | 117 (9) | 0.800 | 0.188 | --- a |
| Character Recognition | 88 (8) | 125 (9) | 122 (11) | 139 (5) | < 0.001*** | < 0.001*** | 0.505 |
| Word List Reading | 66 (14) | 100 (13) | 98 (24) | 120 (23) | < 0.001*** | 0.036* | 0.862 |
| Reading Fluency | 151 (52) | 308 (66) | 383 (129) | 552 (120) | < 0.001*** | 0.003** | 0.086 |
| Phoneme Deletion | 13 (6) | 20 (4) | 19 (5) | 23 (2) | 0.002** | 0.023* | 0.454 |
| Rapid Automatically Naming | 22 (6) | 15 (2) | 14 (4) | 13 (3) | 0.002** | 0.215 | 0.599 |
| Morphological Production | 19 (4) | 25 (2) | 24 (4) | 27 (1) | < 0.001*** | 0.026* | 0.841 |
| Digit Recall | 16 (2) | 18 (2) | 18 (3) | 19 (1) | 0.038* | 0.055 | 0.728 |
Note:
Participants in younger and older subsamples were recruited from two projects, respectively. Because IQ measurements were different, it was not possible to compare IQ between younger and older subsamples.
DYS-younger, dyslexic children of younger subsample; DYS-older, dyslexic children of older subsample; TD-younger, control participants of younger subsample; TD-older, control participants of older subsample.
P < 0.05
P < 0.01
P < 0.001.
Criteria for dyslexia were met if the child performed at least 1 standard deviation (SD) below the norm (lowest 15 percentile) in the standardized single-character recognition task. This procedure has been proven diagnostic for dyslexia in previous studies in Mainland China (e.g., Lei, et al., 2011; Xue, Shu, Li, Li, & Tian, 2013; Zhang, et al., 2012). In order to reduce the overlap between the two groups, the scores of TD children recruited if above −0.5 SD (highest 70 percentile) in the screening task, which was conducted between Grades 4 to 6. Once these two groups were identified, younger and older subsamples were selected based on age. There were no significant differences in age, gender or handedness at the group level. Specifically, DYS-younger group and TD-younger group did not differ significantly in terms of age and gender (DYS-younger: N = 12, 7 females, age: mean [M] = 11.0 y; SD = 0.9; range = 10.0―12.3; TD-younger: N = 12, 6 females, age: M = 11.0 y; SD = 0.3; range = 10.6―11.3; age: P = 0.71; gender: P = 0.68). DYS-older and TD-older groups did not differ significantly either (DYS-older: N = 12, 7 females, age: M = 14.1 y; SD = 0.4; range = 13.2―14.7; TD-older: N = 12, 6 females, age: M = 14.1 y; SD = 0.5; range = 13.1―14.9; age: P = 0.78; gender: P = 0.68). Performance IQ was matched in the younger and older subsamples, respectively (DYS-younger vs. TD-younger: based on Picture Completion, Arrangement and Object Assembly of C-WISC: P = 0.80; DYS-older vs. TD-older: based on Raven’s Standard Progressive Matrices in the older subsample: P = 0.19). Since the IQ measurements were different between the younger and older subsamples, it was not possible to make direct IQ comparisons based on age. Overall, there were two independent dyslexic groups and their respective age-matched controls. Additionally, the older dyslexic (DYS-older) and younger typically developing children (TD-younger) were matched in reading measures at the group level (all P’s > 0.10; Table 1). Thus, the TD-younger also served as the reading-matched control for the DYS-older group.
2.2. Behavioral Measures
To assess children’s current reading abilities and underlying cognitive skills, a battery of tests was conducted within one week of their MRI scans. Ms and SDs of raw scores are summarized in Table 1. The battery included: a) Character recognition was used to estimate the literacy level of children (Xue, et al., 2013). Participants were instructed to read aloud 150 characters one-by-one until failing 15 items consecutively. Each correct answer was worthy of one score. b) Word list reading was used to measure the accuracy and speed of familiar real word reading (Zhang, et al., 2012), in which 180 two-character words with high frequency were arranged in a 9-column by 20-row matrix in one A4 paper. Children had to read aloud these words in sequence as accurately and rapidly as possible. The time used to complete the task was recorded and converted to an index that indicated how many words the individual could read correctly in one minute. c) Reading fluency was used to measure the efficiency of accessing the semantic information of written words (Lei, et al., 2011). There were 100 sentences (e.g., the sun rises in the west), gradually increasing in length. Children were required to read silently as fast as possible (within 3 minutes) and indicate the correctness of each sentence. The final score was calculated using the sum of characters in the sentences with correct responses. d) Phonological deletion was used to measure phonological awareness (Li, Shu, McBride-Chang, Liu, & Peng, 2012). In the task, the child had to pronounce loudly what would be left when a consonant or vowel was deleted from the given syllable, for example, say /u1/ in response to the syllable /shu1/ if /sh/ was deleted. This test consists of three sections (i.e., to delete initial, middle or final phonemes, respectively). There were three practice trials and 8―10 testing items in each section. The full score was 28. e) Rapid Automatized Naming was used to assess their efficiency of phonological representation retrieval from visual input (Lei, et al., 2011). Five numbers, 1, 2, 3, 5, 8 were repeated randomly five times in the task, and participants were instructed to name the items as accurately and rapidly as possible. The task was given twice and an average time was calculated as the final score. f) Morphological awareness was assessed by morphological production (Shu, McBride-Chang, Wu, & Liu, 2006). In each trial, one character was orally presented to the child in the context of a high frequency word. Participants were required to produce one new word in which the character has the same meaning as it in the example word and another one in which the character has a different meaning. In total, fifteen characters were given and the full mark was 30. g) Phonological working memory was assessed using Digit Recall. There were 21 sets of numbers arranged in length (from 2 to 8). The child had to repeat the digits in the original order immediately after listening. One correct repetition was worth one score with a full score being 21.
2.3. T1-Weighted Image Acquisition
Before formal MRI scanning, all the children were familiarized with the experiment procedure and the noise of an actual MRI environment using a mock scanner. They were also informed about the duration of each session and setting details. All images were acquired at Beijing Normal University’s Imaging Center for Brain Research (BICBR) using a 3.0 Tesla Siemens MAGNETOM Trio scanner. High resolution whole brain T1-weighted structural MR image (magnetization-prepared rapid acquisition with gradient echo [MPRAGE], repetition time [TR] = 2530 ms; echo time [TE] = 3.39 ms; inversion time [TI] = 1100 ms; Flip Angle = 7 degree; axial slices = 144; slice thickness = 1.33 mm; field of view [FOV] = 256 × 192 mm; matrix = 256 × 192 × 144; voxel size = 1 × 1 × 1.33 mm) was acquired for each child. After acquisition, all images were reviewed by a radiologist who was blind to the participants’ clinical details to determine any pathological deviations and ensure data quality.
2.4. Image Processing
VBM was performed with VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/), implemented in SPM8 (http://www.fil.ion.ucl.ac.ul/spm) and executed in MATLAB (version 7.11, MathWorks, Natick, MA). Since the participants were children, Template-O-Matic (TOM8) Toolbox was used to create customized Tissue Probability Maps in Montreal Neurological Institute (MNI) space specific to sample group characteristics including age and gender (Wilke, Holland, Altaye, & Gaser, 2008). Native T1 volumes were first segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid with a resampling at 1.5 × 1.5 × 1.5 mm with a trilinear interpolation. Then, GM and WM segments were registered to a custom template initially by applying affine-normalization and then by using Diffeomorphic Anatomical Registrations Through Expoentiated Lie Algebra (DARTEL) (Ashburner, 2007). Next, GM and WM segments were modulated by the ‘non-linear only’ modulation (http://www.neuro.uni-jena.de/vbm/segmentation/modulation/), which is conducted by multiplying normalized GM/WM segments with the Jacobian determinant acquired from non-linear spatial normalization. The modulated images were smoothed with an 8-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. The outputs were maps in which values in each voxel represented regional tissue volume of GM, WM or CSF. These individually modulated data were thresholded to create masks by minimizing partial volume effects, which were then used to restrict analyses to GM and WM regions. Typical to VBM analyses, only values of GM or WM volume greater than 0.25 were kept within these masks for further statistics.
2.5. Statistical Analyses
2.5.1. Whole Brain Voxel-Based Analyses
In order to identify the effects of disorder and its interaction with maturational stage, whole brain analysis of covariance (ANCOVA) with gender as a nuisance variable was conducted on regional GM and WM volume, respectively (using the full factorial option of SPM8). Family-Wise Error (FWE) correction with non-stationary of smoothness correction (uncorrected P-voxel < 0.001, corrected P-cluster < 0.05) was used to control multiple comparisons error (Hayasaka, Phan, Liberzon, Worsley, & Nichols, 2004). If no clusters survived under the conservation FWE correction, a less stringent threshold of P-voxel < 0.001 (uncorrected) with at least 200 continuous voxels (675 mm3) was applied for exploratory analyses. Brain regions that showed only the main effect of maturation were not interrogated in ROI analyses or discussed further. This was because age-related changes (e.g., decrease in GM and increase in WM within the second decade) are commonly observed across a widespread region of the brain, and the focus of this study was to distinguish anomalies related specifically to dyslexia.
For the purpose of presentation, significant clusters were displayed on a mean normalized GM or WM image of all subjects with MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Anatomical labeling of significant brain areas was performed with Xjview (http://www.alivelearn.net/xjview8/) for the aim of comparison with previous studies.
2.5.2. Region-of-Interest (ROI) Analyses
ROI analyses were conducted in significant clusters from whole brain analyses to confirm the direction of the effects and to examine the specific hypotheses of the current study. The value of each voxel in the cluster was extracted and averaged. All the subsequent analyses were conducted with PASW Statistics 18.0 (SPSS inc., Chicago, IL, USA). A false discovery rate of 5% was used to control multiple comparisons error for the number of regions.
In order to identify dyslexia-specific anomalies in regional brain volumes that exist independent of maturation and reading performance, three post-hoc contrasts were performed (DYS-older vs. TD-older, DYS-younger vs. TD-younger, and DYS-older vs. TD-younger [reading-matched controls]) in the clusters showing main effects of disorder. Additionally, comparisons between older and younger subsamples were also conducted in DYS and TD separately (i.e., DYS-older vs. DYS-younger; TD-older vs. TD-younger) to test whether these regions also displayed maturational (i.e., age) effects. The same analysis was also used to detect performance-dependent effect, in which there were significant effects of disorder and maturation, while there were no significant differences between reading-matched pairs. Finally, to reveal the source of the disorder-by-maturation interaction, post hoc independent t-tests were performed. In another words, the difference in slopes of regional brain volume across different age groups between dyslexic and typically developing children were compared ([DYS-older - DYS-younger] vs. [TD-older - TD-younger]).
2.5.3. Non-Parametric Statistics
Considering the small sample size of the current study (12 subjects for each of the four groups), we repeated analyses in ‘2.5.2. ROI Analysis’ using non-parametric statistics to confirm the reliability of our findings. Permutation testing (5000 permutations) was applied using R software (version 3.2.2; https://www.r-project.org/) and the package ‘asbio’ (A Collection of Statistical Tools for Biologists; https://www.r-project.org/) in each cluster identified in the previous parametric whole-brain model. Function ‘perm.fact.test’ was used to conduct permutation testing of the main effect of disorder and interaction effect in the ANOVA, and function ‘MC.test’ was used in post-hoc contrasts. A false discovery rate of 0.05 was used to control multiple comparisons error.
3. Results
3.1. Behavioral Profiles
As expected, reading performances and underlying cognitive skills were significantly impaired in dyslexia (Table 1). In the age-matched comparisons, DYS-younger performed significantly worse than TD-younger in all reading measures (character recognition: P < 0.001; word list reading: P < 0.001; reading fluency: P < 0.001) and underlying cognitive skills (phoneme deletion: P = 0.002; RAN: P = 0.002; morphological production: P < 0.001; digit recall: P = 0.038). DYS-older also performed worse than TD-older, especially in character recognition (P < 0.001) and reading fluency (P = 0.003). As for the underlying component processes, the lower performance of older dyslexics was found in phoneme deletion (P = 0.023) and morphological production (P = 0.026). In the reading-matched comparison, DYS-older showed no significant difference from TD-younger in any raw scores (i.e., not standardized for age) of these measures (all Ps > 0.05). Additionally, there was maturation-related improvement in both dyslexics and controls, i.e., older children performed better than their younger counterparts in raw scores of all measures (all Ps < 0.05) except trends in digit recall (TD-older vs. TD-younger: P = 0.057; DYS-older vs. DYS-younger: P = 0.123).
3.2. Brain Results
3.2.1. Whole-Brain Results
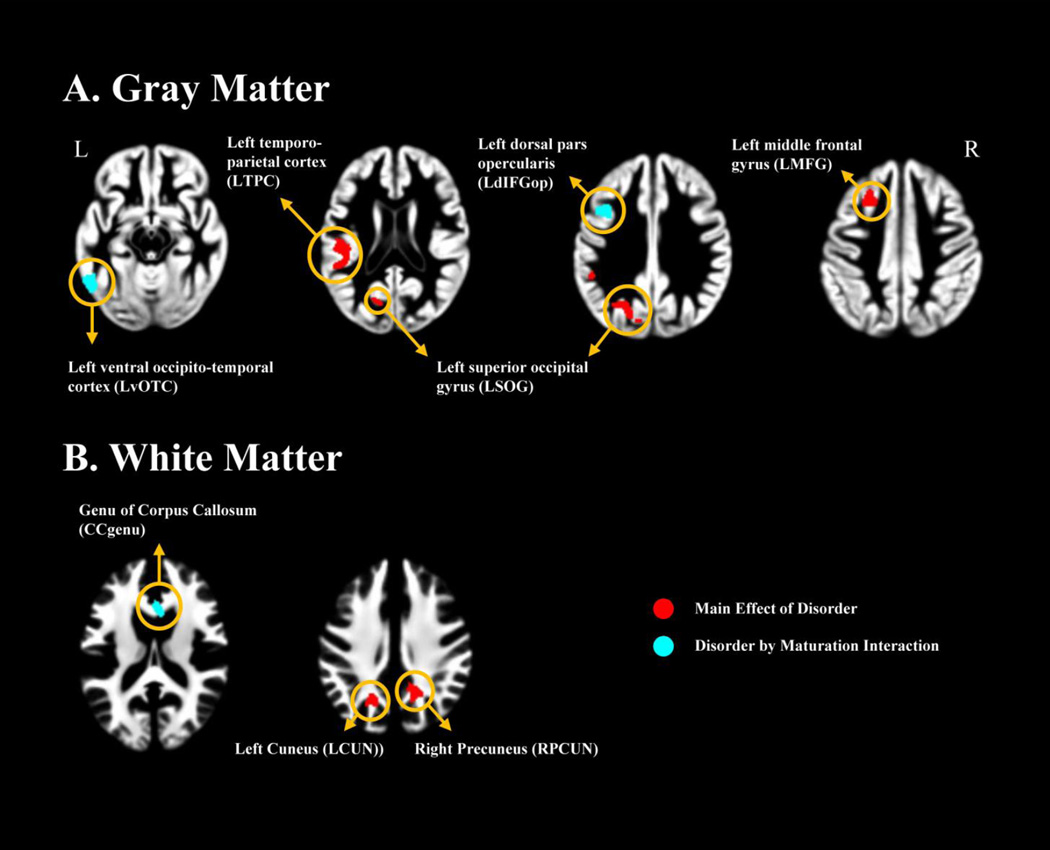
As the first step, whole brain ANCOVA of regional GM volume controlling for gender showed a main effect of disorder in three clusters (P-cluster < 0.05, corrected) (Table 2, Fig. 1A). One was located in the LTPC spanning over Heschl’s gyrus, planum temporale and supramarginal gyrus. The other two were located in the left middle frontal gyrus (LMFG) and the left superior occipital gyrus (LSOG) respectively. Two clusters displayed significant disorder-by-maturation interaction, one in the LvOTC, and the other at the junction between left inferior frontal sulcus and precentral sulcus (i.e., dorsal part of left pars opercularis; hereafter: LdIFGop) (Table 2, Fig. 1A).
Table 2.
Peak coordinates and details of clusters from whole brain voxel-based analyses
| Matter | Brain area | P-corrected | Size (voxels) | MNI coordinates (peak) |
|||
|---|---|---|---|---|---|---|---|
| Z value | x | y | z | ||||
| Gray matter | Main Effect of Disorder | ||||||
| Left supramarginal gyrus, left superior temporal gyrus, left rolandic operculum left postcentral gyrus |
0.008 | 866 | 4.27 | −50 | −45 | 28 | |
| Left superior occipital gyrus, left middle occipital gyrus, left calcarine fissure and surrounding cortex |
0.032 | 648 | 4.05 | −20 | −75 | 21 | |
| Left middle frontal gyrus, left superior frontal gyrus | 0.047 | 228 | 4.07 | −27 | 18 | 43 | |
| Disorder × Maturation Interaction | |||||||
| Left inferior temporal gyrus, left occipital gyrus | 0.006 | 606 | 4.79 | −54 | −57 | −15 | |
| Left inferior frontal gyrus (opercular part), left middle frontal gyrus, left inferior frontal gyrus (triangular part) |
0.049 | 269 | 4.78 | −41 | 12 | 33 | |
| White matter | Main Effect of Disorder | ||||||
| Right precuneus, right median cingulate and paracingulate gyri, right superior occipital gyrus |
0.043 | 343 | 4.08 | 17 | −57 | 37 | |
| Left superior occipital gyrus, left precuneus, left middle occipital gyrus, left superior parietal gyrus |
0.046 | 310 | 4.11 | −18 | −61 | 36 | |
| Disorder × Maturation Interaction | |||||||
| Genu of corpus callosum | --- a | 505 | 3.65 | 6 | 21 | 7 | |
Note:
Note: voxel-level P < 0.001, uncorrected.
Figure 1.
Results of whole brain ANCOVA. All significant at P-cluster < 0.05 with FWE correction, except CCgenu: P-voxel < 0.001 (uncorrected) with 505 continuous voxels. The GM clusters are presented on the mean normalized GM image of all subjects at the upper panel (A) and the WM clusters are presented on the mean normalized WM image of all subjects at the lower panel (B). Three GM clusters (left temporo-parietal cortex, LTPC; left middle frontal gyrus, LMFG; left superior occipital gyrus, LSOG) and two WM clusters (left cuneus, LCUN; right precuneus, RPCUN) showed main effect of disorder (red). Two clusters (left ventral occipito-temporal cortex, LvOTC; left dorsal pars opercularis, LdIFGop) and one cluster located in the genu of the corpus callosum showed disorder-by-maturation interaction effect (cyan).
ANCOVA was also conducted in regional WM volume with gender as a nuisance variable. For the main effect of disorder, two clusters in bilateral parieto-occipital cortices: left cuneus (LCUN) and right precuneus (RPCUN) survived the FWE correction with non-stationary cluster extent of P-cluster < 0.05 (Table 2; Fig. 1B). For the disorder-by-maturation interaction, no region survived the correction. For exploratory purposes, we applied a less stringent uncorrected threshold of P-voxel < 0.001 (uncorrected) with at least 200 continuous voxels (675 mm3). A cluster (505 voxels, 1704 mm3) was identified in the genu of the corpus callosum (CCgenu; Table 2; Fig. 1B). Since CCgenu has been associated with working memory and fluent reading, and also showed shrinkage in dyslexia (Casanova, et al., 2010; Edwards, Sherr, Barkovich, & Richards, 2014; Hynd, et al., 1995), this locus was also included in further ROI analyses.
3.2.2. Dyslexia-Specific Abnormalities
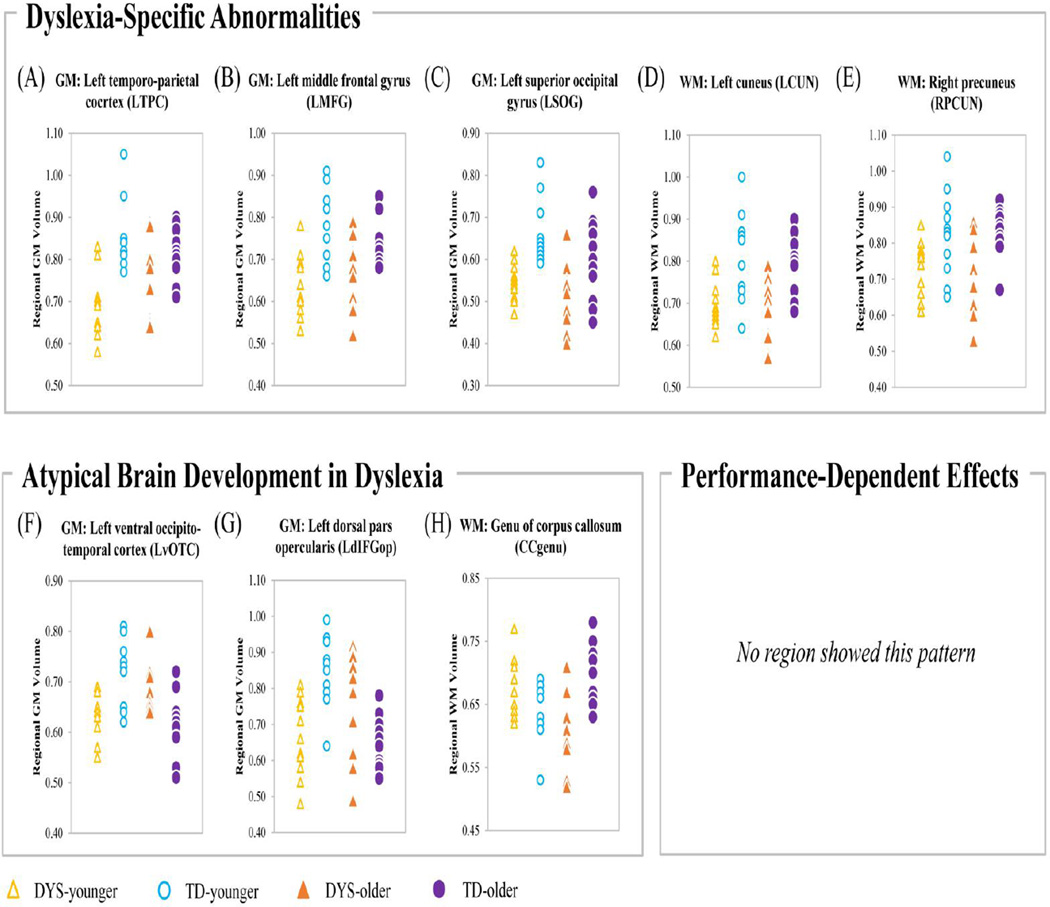
ROI analyses were first performed in the five clusters showing main effects of disorder. According to the logic presented in the Introduction, if one region shows similar effects in all three comparisons (i.e., DYS-older vs. TD-older, DYS-younger vs. TD-younger, DYS-older vs. TD-younger), it is more likely to be disorder-specific. Here, we found that dyslexic readers showed reduced volume in the three GM ROIs compared to typical readers in both older and younger subsamples (LTPC: DYS-older < TD-older: P = 0.012; LTPC: DYS-younger < TD-younger: P < 0.001; LMFG: DYS-older < TD-older: P = 0.008; LMFG: DYS-younger < TD-younger: P < 0.001; LSOG: DYS-older < TD-older: P = 0.023; LSOG: DYS-younger < TD-younger: P < 0.001). Furthermore, dyslexic readers showed reduced GM Volume in these regions compared with reading-matched controls (DYS-older < TD-younger; LTPC: P = 0.002; LMFG: P = 0.001; LSOG: P < 0.001; Fig. 2A, B, C).
Figure 2.
Raw regional volumes are plotted for each of the ROIs (gold = DYS-younger; blue = TD-younger; orange = DYS-older; purple = TD-older).
The WM clusters in bilateral parieto-occipital areas displayed a similar pattern. That is, dyslexic children had significantly decreased volume in the older age-range (DYS-older < TD-older; RPCUN: P = 0.001; LCUN: P = 0.003) as well as the younger age-range (DYS-younger < TD-younger; RPCUN: P = 0.014; LCUN: P = 0.004). Additionally, dyslexics showed significantly less volume compared with reading-matched controls (DYS-older < TD-older: RPCUN: P = 0.009; LCUN: P = 0.003; Fig. 2D, E).
Most of the clusters remained significant even when age, gender and nonverbal IQ were controlled for (LTPC: DYS-younger < TD-younger: P < 0.001; DYS-older < TD-younger: P = 0.002; LMFG: DYS-older < TD-older: P = 0.013; DYS-younger < TD-younger: P = 0.001; DYS-older < TD-younger: P = 0.001; LSOG: DYS-older < TD-older: P = 0.011; DYS-younger < TD-younger: P < 0.001; DYS-older < TD-younger: P < 0.001; LCUN: DYS-older < TD-older: P = 0.002; DYS-younger < TD-younger: P = 0.005; DYS-older < TD-younger: P = 0.003; RPCUN: DYS-older < TD-older: P = 0.007; DYS-younger < TD-younger: P = 0.019; DYS-older < TD-younger: P = 0.006). The only exception was that the comparison between DYS-older and TD-older in LTPC, which was no longer significant and showed a marginal effect (P = 0.053). Same as before, there was either no significant difference between younger and older groups for either the dyslexic (DYS-older vs. DYS-younger) or TD groups (TD-older vs. TD-younger; all Ps > 0.05). Taken together, these findings reveal persistent regional impairments across maturation, even when compared to reading-matched controls, and suggest causal involvement of these regions in dyslexia.
3.2.3. Performance-Dependent Effects
As per the logic outlined in Introduction, if a region is performance-dependent, there should be main effects of both disorder and maturation, but no significant difference between dyslexics and reading-matched controls (i.e., DYS-older vs. TD-younger). However, in this study no significant maturational effects were found within regions showing an effect of disorder (Fig. 2 lower right panel). Thus, no regions were purely associated with reading performance, at least during the age-range we examined.
3.2.4. Atypical Brain Development in Dyslexia
We compared both regional GM and WM volume of the three regions showing disorder-by-maturation interaction between younger and older children within dyslexic and control groups separately to explore the nature of the interaction effect. Interestingly, we found that the maturational effects were opposing in the dyslexic and controls: while the control group showed reduced GM in older compared with younger children (TD-older < TD-younger; LdIFGop: P < 0.001; LvOTC: P < 0.001), dyslexic children showed increased GM in older compared with the younger subsample (DYS-older > DYS-younger; LdIFGop: P = 0.046; LvOTC: P = 0.003). Furthermore, while DYS-younger showed less GM than TD-younger (DYS-younger < TD-younger; LdIFGop: P < 0.001; LvOTC: P < 0.001; Fig. 2F, G), an opposite effect was found in the older subsample (DYS-older > TD-older; LdIFGop: P = 0.015; LvOTC: P = 0.003).
For white matter, the interaction effect in the CCgenu was different from that in GM clusters. That is, while older typically developing children showed larger WM volume than the younger children (TD-older > TD-younger: P = 0.018), dyslexics showed the opposite pattern (DYS-older < DYS-younger: P = 0.004). In other words, CCgenu showed opposite effects compared to GM regions in its relationship between dyslexics and typically developing children across the two maturational stages examined. Dyslexic children of primary school age had significantly larger volume in this region than typically developing children (P = 0.035), whereas middle school aged dyslexic children showed significantly smaller CCgenu than their typical peers (P = 0.002) (Fig. 2H).
3.2.5. Confirmatory Analyses using Non-Parametric Statistics
ROI analyses were repeated with a non-parametric permutation test. All significant clusters related to dyslexia-specific and maturation-sensitive impairments remained significant (all P’s < 0.05, corrected) and were in line with the specific hypotheses described before (Table S1).
4. Discussion
Our study is the first study to disambiguate the effects of disorder, performance and maturation in dyslexic children. By adopting a novel disorder-by-maturation factorial design that also enabled us to compare dyslexics with reading-level matched controls, we confirmed that children with dyslexia have maturation- and performance-independent tissue loss in networks of regions that closely align with speech/phonological (LTPC) and the dorsal attention pathways (LMFG, LSOG and bilateral parieto-occipital areas). Additionally, three previously reported reading-related regions (LdIFGop, LvOTC and CCgenu) showed an interaction between disorder and maturational stage. In accordance with the possible predictions of the nature of abnormalities laid out in the introduction, this finding suggests that neuroanatomical manifestations of children with dyslexia might follow abnormal developmental trajectories (Clark, et al., 2014; Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012). We first discuss persistent deficits specific to dyslexia found in the current study, followed by the neurodevelopmental insights gained from this study. Cross-linguistic issues are touched upon at the end.
4.1. Persistent Neural Impairments in Developmental Dyslexia
Although increasing evidence has demonstrated the neurobiological substrate of dyslexia, the issue of causality remains hotly debated (Goswami, 2015). By revealing the same reduction of GM/WM volume across two age subsamples and the reading-matched comparison, the present study provides strong support for the existence of etiological neuroanatomical alterations that are often implicated as auditory/phonological areas and dorsal visual attention systems.
Anomaly in the left temporo-parietal region is the most consistent finding in dyslexia research (Gabrieli, 2009). In accordance with previous findings, we found that dyslexic children displayed persistently reduced GM in this region, regardless of age (maturational stage) and absolute level of reading performance. The peak was located in the left supramarginal gyrus and extended into the superior temporal cortex, including Heschl’s gyrus and planum temporale. Functional imaging studies suggest these regions are involved in phonological processing, including fine-grained phonological representations (Chang, et al., 2010; Mesgarani, Cheung, Johnson, & Chang, 2014), integration of audiovisual input and grapheme to phoneme conversion (Erickson, Heeg, Rauschecker, & Turkeltaub, 2014), and phonological working memory (Leff, et al., 2009), all of which are closely associated with reading development (Ramus & Szenkovits, 2008). In line with these findings, dysfunction in these regions is also associated with phonological processing deficits in dyslexia (Gabrieli, 2009; Hoeft et al., 2006; B. A. Shaywitz et al., 2002; Tanaka et al., 2011; Simos et al., 2013). In particular, our finding in LTPC is compatible with Hoeft and colleagues’ study, where hypoactivation of LIPL (including supramarginal gyrus) during a rhyming task and reduced GM of similar regions were found when comparing dyslexics with both age-matched and reading-matched controls (Hoeft, et al., 2006; Hoeft, et al., 2007). Furthermore, phonological processing-related impairments have been shown to precede the onset of reading instruction at both neuro-functional and anatomical levels (Black, et al., 2012; Raschle, Chang, & Gaab, 2011; Raschle, Zuk, & Gaab, 2012), which might be caused by specific genetic anomalies (for a review, see Giraud & Ramus, 2013). Combining previous findings with our current study, we deduce that impairment in the LTPC might occur at an early stage and persist throughout reading development.
Besides the LTPC, dyslexic children also displayed persistent GM reduction in LMFG, LSOG and WM reduction in bilateral parieto-occipital regions (cuneus and precuneus) irrespective of age. Anatomically, these regions are located within the fronto-parietal attention network, which are mainly connected by the superior longitudinal fasciculus (Thiebaut de Schotten, et al., 2011). The spatial dorsal attention network has been proposed to be associated with dyslexia and reading (S. E. Shaywitz & Shaywitz, 2008; Vidyasagar & Pammer, 2010; Vogel, Miezin, Petersen, & Schlaggar, 2012). Consistent with this hypothesis, behavioral studies have shown selective visual-attention deficits in dyslexia across cultures (Facoetti, Paganoni, Turatto, Marzola, & Mascetti, 2000; Kronbichler, et al., 2008; Zhao, Qian, Bi, & Coltheart, 2014). As for the underlying neural basis, functional MRI studies support evidence of reduced activation in parietal lobe during visual attention tasks in dyslexia (Peyrin, Demonet, N’Guyen-Morel, Le Bas, & Valdois, 2011; Peyrin, et al., 2012). Most recently, decreased resting-state functional connectivity between left inferior parietal sulcus and LMFG were found in dyslexic children, regardless of whether they received remediation or not (Koyama, et al., 2013). These results suggest that individuals with dyslexia can be characterized by a persistent functional connectivity anomaly (S. E. Shaywitz & Shaywitz, 2008). Although concrete evidence is lacking and clarification of the precise mechanism requires further research, increasing studies suggest a functional disruption of the dorsal visual attention pathway in dyslexia, for which the current study provides support at the neuroanatomical level.
4.2. Anomalous Neuroanatomical Development in Developmental Dyslexia
The underlying neural mechanisms of dyslexia are complex and are likely associated with interactions among disorder, performance (level of reading proficiency) and maturational stage. However, most neuroanatomical studies have only focused on the “average” deficits in dyslexics collapsed across maturational stages and compared with chronological age-matched controls. Thus, findings of those studies are prone to confounding factors such as natural brain maturation. In our study, three previously reported reading related regions (i.e., LvOTC, LdIFGop and CCgenu) showed opposing maturation-related differences between dyslexics and controls. These findings are consistent with the consensus that brain structure undergoes continuing changes (Giedd & Rapoport, 2010), and that relations between brain and cognitive abilities change dynamically (Erus, et al., 2014; Schnack, et al., 2014; B. A. Shaywitz, et al., 2007; Yeatman, et al., 2012). These interactions further suggest that, similar to other neurodevelopmental disorders such as attention deficit hyperactivity disorder and autism (Ecker, et al., 2014; Shaw, et al., 2007), dyslexia might also be characterized by atypical developmental trajectory of specific brain areas.
Typically, GM development follows an inverted U-shape where peaks coincide at different times depending on the anatomical location (Giedd & Rapoport, 2010; Raznahan, et al., 2011). Functional neuroimaging studies have also shown an inverted U-shape in the developmental trajectory of neural responses to words (Ben-Shachar, Dougherty, Deutsch, & Wandell, 2011; Maurer, et al., 2006) in regions such as the LvOTC. Thus, our findings that TD displayed a decrease while DYS showed an increase in LvOTC GM may reflect different developmental tuning curves. In line with our findings that dyslexic children in the younger subsample showed anomalous decrease in LvOTC compared to TD controls, reduced tissue volume has been reported in preschool children at risk for dyslexia, suggesting this alteration exists even before formal reading instruction begins (Raschle, et al., 2011). On the other hand, in the older subsample, the reverse relationship was observed (GM volume was elevated in dyslexics when typical readers were already descending in volumes). It is possible that dyslexic children show anomalous and possibly a protracted period of GM increase compared to TD controls of similar age. To some extent, this addresses why this ventral area often shows conflicting results in previous literature. First, when a study includes children with a large age-range, the effects may wash out and show non-significant effects (e.g., Brambati, et al., 2004). Second, if participants are recruited before the age at which this shift occurs, dyslexic children are likely to display decreased volume compared with controls (e.g., Kronbichler, et al., 2008). Conversely, those who predominantly focus on adults would find GM volume to be increased in dyslexics (e.g,. Silani, et al., 2005). Our finding in the LvOTC not only supports the existence of a shift of abnormality at a neuroanatomical level, it also provides some indication that this shift occurs at some point within ages 10 to 15. This might be associated with the fact that while typically developing children begin to focus more on fluency and comprehensive skill, children with dyslexia are still putting effort in individual word recognition. However, this time window is somewhat inconsistent with Kronbichler’s results where he found a decrease in dyslexic children aged 14 to 16 years of age (Kronbichler, et al., 2008). Speculatively, the point at which the shift occurs might be affected by several factors including region-specificity, disorder severity, reading experience and linguistic specificity. To directly address this issue, future studies are warranted to establish empirical evidence of the developmental trajectory and influencing factors in both dyslexics and typical readers.
Similar to the LvOTC, LdIFGop also showed the interaction effect. This area is considered an important part of the articulatory system (Hickok & Poeppel, 2007) and is involved in phonological processing (Fiez, Tranel, Seager-Frerichs, & Damasio, 2006), motor programming and articulatory coding (Price, 2012). It also plays a compensatory role when the circuit involved in naming is disrupted (Seghier, Bagdasaryan, Jung, & Price, 2014). Findings of functional neuroimaging studies suggest that dyslexics’ increased reliance on an articulation-based strategy is a result of compensation for poor performance in reading across development (Brunswick, McCrory, Price, Frith, & Frith, 1999; B. A. Shaywitz, et al., 2002). Although there is less evidence at the neuroanatomical level, an association between phonological awareness and cortical thickness in this region has been identified (but see Lu, et al., 2007). Together, the abnormal age-related GM change in LdIFGop may reflect a compensatory mechanism, i.e., strategies employed by dyslexics that are different from typical developing children (B. A. Shaywitz, et al., 2002; B. A. Shaywitz, et al., 2007).
Finally, we found a maturational-sensitive effect of dyslexia in WM volume of the CCgenu. This structure, as a bridge connecting bilateral homotopic frontal regions, plays an important role in working memory and fluent reading (Casanova, et al., 2010; Edwards, et al., 2014; Short, et al., 2013). This is especially observed in individuals with agenesis of the corpus callosum, who show intact general cognitive processing skills but impairments in phonological processing, a characteristic also seen in developmental dyslexia (Paul, Corsello, Kennedy, & Adolphs, 2014). The increased WM of CCgenu in TD children is in line with results of neurodevelopmental research (Giedd & Rapoport, 2010; Raznahan, et al., 2011). Furthermore, atypical trajectory of fractional anisotropy in white matter fibers (i.e., left superior longitudinal fasciculus and inferior longitudinal fasciculus) that manifests initially higher but decreases with age in children with poor reading skill has been shown in a recent longitudinal study (Yeatman, et al., 2012). While WM change across the lifespan can be driven by multiple biological processes and environmental factors (Yeatman, Wandell, & Mezer, 2014), further research is required to clarify maturation of this structure and its fine-grained function in reading acquisition and deficits.
4.3. Universal Principles Underlying Neural Manifestations of Chinese Dyslexia
There is a long-lasting history discussing similarities and differences in the manifestations of dyslexia between alphabetic languages (e.g., English) and non-alphabetic languages (e.g., Chinese) (Peterson & Pennington, 2012). At a more general level, the question of whether universal principles underlie reading and reading development across languages (Bolger, Perfetti, & Schneider, 2005; Tan, Laird, Li, & Fox, 2005) is still hotly debated. Chinese is a logographic writing system that does not require phoneme-grapheme conversion, and hence, is very different from alphabetic scripts (Siok, Perfetti, Jin, & Tan, 2004). While increasing evidence suggests that cognitive skills, such as phonological processing, that are repeatedly found to be critical in English-speaking samples also largely contribute to reading development in Chinese children (Goswami, et al., 2011; Lei, et al., 2011; Shu, et al., 2006; Shu, Peng, & McBride-Chang, 2008; Zhang, et al., 2012), most available neuroanatomical evidence suggests that there might be different neural circuitries underlying Chinese dyslexia, which are driven by the linguistic specificities such as visuo-spatial complexity and heavy memory burden while learning to read (Liu, et al., 2013; Siok, Niu, Jin, Perfetti, & Tan, 2008). Since longitudinal research is still lacking and all the previous studies only recruited groups of dyslexia and age-matched controls, the different sources of neuroanatomical differences were largely unknown. Against this background, the current study adopted a novel research design and for the first time identified disorder-specific effects in regions that have been associated with auditory/phonological and visual attentional processing in Chinese dyslexia. The locations of these clusters were similar to those found in prior studies of English, e.g., LIPL (Hoeft, et al., 2007). Moreover, we also found manifestations that were sensitive to maturational stages, which are in line with recent longitudinal studies (Clark, et al., 2014; Yeatman, et al., 2012).
In general, all writing systems, including Chinese, represent spoken language and seem to share a common central reading network including left posterior superior temporal gyrus, dorsal aspects of the inferior frontal gyrus and occipito-temporal area (Bolger, et al., 2005). In fact, auditory/phonological and visual/orthographic information processing are two main important aspects for reading in any language, and breakdown of any aspect will impede reading development, irrespective of writing systems (Goswami, et al., 2011; Shu, et al., 2008; Siok & Fletcher, 2001; Zhang, et al., 2012; Zhao, et al., 2014). In alphabetic languages, there is a consensus that dyslexia is associated with phonological processing deficits and the underlying neural basis is located in the left temporo-parietal cortex (Gabrieli, 2009; Linkersdorfer, et al., 2012; Richlan, et al., 2013). The dorsal attention deficit hypothesis has also received support from imaging studies (Eden, et al., 1996; S. E. Shaywitz & Shaywitz, 2008; Vidyasagar & Pammer, 2010). Therefore, the persistent neuroanatomical tissue loss in the LTPC, LMFG, LSOG and bilateral parieto-occipital regions identified in the current study suggests some impairments in Chinese dyslexic children are similar to those in English.
The disorder-by-maturation interaction effects in LvOTC, LdIFGop, and CCgenu provide a glimpse into the abnormal development of high-order brain regions. More recently, a longitudinal study found abnormal cortical thickness changes in a group of Norwegian dyslexic children (7 dyslexics vs. 10 controls) (Clark, et al., 2014). The authors also found early and persistent neuroanatomical abnormalities only in lower-level auditory areas, rather than putative reading network (e.g., left fusiform gyrus), which might be affected more by external factors like reading instruction. Although our study differed in experimental design (i.e., longitudinal design vs. factorial design containing reading-level matched controls), language background (i.e., Norwegian has a shallow orthography while Chinese is very deep) and regions showing specific effects (e.g., persistent deficit: left Heschl’s gyrus in theirs vs. LTPC, LSOG, LMFG, and bilateral parieto-occipital cortex in our study), both found sustained and maturation-sensitive deficits in the dyslexic brain. This consistency reflects a possible universal principle underlying neural manifestations of dyslexia. While impairments of regions involved in basic sensory processes may impede typical reading acquisition from an early stage (Clark, et al., 2014), the development of high-level reading areas may be affected more by multiple factors such as the statistical properties of writing system (e.g., orthographic complexity; Frost, 2012). In the other words, central neural deficits may be shared across different writing systems and further shaped by language-related variations (Richlan, 2014).
4.4. Limitations and Future Directions
First, considering the relatively small sample size and cross-sectional nature of the data, the current findings should be interpreted with caution. Nevertheless, we believe that the design adopted here is informative in disentangling different resources of neural manifestations in dyslexia. To confirm and extend our findings, longitudinal studies with much larger cohorts of subjects should be conducted. Such research can further answer questions including the precise timing and trajectory of neuroanatomical growth (i.e., the precise timetable) and how abnormal trajectories occur in children with dyslexia. Secondly, factors like gender (Altarelli, et al., 2013) and intervention (Krafnick, Flowers, Napoliello, & Eden, 2011) also influence neuroanatomical manifestations of dyslexia. It is therefore important to explore the developmental trajectories of the reading network in dyslexic boys and girls respectively, as well as different outcomes of intervention applied at different time points. Thirdly (and finally), since GM and WM features are strongly heritable and typical brain maturation depends on finely controlled expression of specific genes, appropriate environments and experiences (Tau & Peterson, 2010), examining how genetic programming, brain maturation, environmental factors, and their interactions lead to atypical development of reading abilities is critically important (Wright & Zecker, 2004).
5. Conclusion
In summary, the current study disambiguates for the first time the syndrome-specific, performance-dependent and disorder-by-maturation related abnormalities in dyslexic brain. The findings support that dyslexia is a neurological disorder with both persistent neural deficits and anomalous developmental trajectories in specific areas. As a starting point, more research should be conducted to build causal models of dyslexia in order to further understand the mechanisms at the biological, cognitive and behavioral levels.
Supplementary Material
Highlights.
This study disentangled different sources of anomalies in dyslexic children.
Persistent volume decreases were found in phonology and attention related regions.
There were areas that showed maturation-sensitive impairments in dyslexia.
Dyslexia is characterized by both persistent and altered neural deficits.
Acknowledgments
The authors would like to thank all the children and their parents participating in this study, as well as Mengmeng Su, Shuang Song and other lab members for their work on data collection.
Funding
This research was supported by the National Key Basic Research Program of China (2014CB846103), the Natural Science Foundation of China (31271082, 81461130018), the Natural Science Foundation of Beijing (7132119), and Fundamental Research Fund for the Central Universities to Hua Shu. Fumiko Hoeft is supported in part by the NIH grants K23HD054720 (PI: F. Hoeft), R01HD078351 (PI: F. Hoeft), UCSF Academic Senate Pilot Grant for Junior Investigators (PI: F. Hoeft), UCSF - Center for Creativity (CCC) Neuroscience Fellowship (PI: F. Hoeft), UCSF Dyslexia Center (PI: F. Hoeft), Dennis & Shannon Wong DSEA 88 Wong Family Foundation (F. Hoeft), R01HD067254/R01HD044073 (PI: L. Cutting, Vanderbilt U), R01HD065794 (PI: K. Pugh, Haskins Labs), P01HD001994 (PI: J. Rueckl, Haskins Labs), R01MH104438 (PI: C. Nordahl, UC Davis MIND Inst), R01MH103371 (PI: D. Amaral, UC Davis MIND Inst), Flora Family Foundation (PI: R. Hendren), UCSF Catalyst Award (PI: R. Hancock), and UCSF Resource Allocation Program (RAP) Digital Health Award (PI: R. Hancock).
Abbreviations
- age-matched
chronological age-matched
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- CCgenu
genu of corpus callosum
- DYS-older
dyslexic children of older subsample
- DYS-younger
dyslexic children of younger subsample
- GM
gray matter
- LCUN
left cuneus
- LIPL
left inferior parietal lobule
- LdIFGop
left dorsal pars opercularis
- LMFG
left middle frontal gyrus
- LSOG
left superior occipital gyrus
- LTPC
left temporo-temporal cortex
- LvOTC
left ventral occipito-temporal cortex
- reading-matched
reading level-matched
- ROI
region-of-interest
- RPCUN
right precuneus
- TD-older
typically developing children of older subsample
- TD-younger
typically developing children of younger subsample
- VBM
voxel-based morphometry
- WM
white matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Altarelli I, Monzalvo K, Iannuzzi S, Fluss J, Billard C, Ramus F, Dehaene-Lambertz G. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. Journal of Neuroscience. 2013;33:11296–11301. doi: 10.1523/JNEUROSCI.5854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. The Development of Cortical Sensitivity to Visual Word Forms. Journal of Cognitive Neuroscience. 2011;23:2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Kesler S, Hulme C, Lyytinen H, Glover GH, Serrone C, Raman MM, Reiss AL, Hoeft F. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 2012;59:3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Hum Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122(Pt 10):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Elnakib A, Giedd J, Rumsey JM, Williams EL, Switala AE. Corpus Callosum Shape Analysis with Application to Dyslexia. Translational Neuroscience. 2010;1:124–130. doi: 10.2478/v10134-010-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13:1428–1432. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014 doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Shahidiani A, Feng Y, Daly E, Murphy C, D’Almeida V, Deoni S, Williams SC, Gillan N, Gudbrandsen M, Wichers R, Andrews D, Van Hemert L, Murphy DGM. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. J Neural Transm. 2014;121:1157–1170. doi: 10.1007/s00702-014-1207-1. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion In dyslexia revealed by functional brain imaging. Nature. 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ. Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain. 2014;137:1579–1613. doi: 10.1093/brain/awt358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson LC, Heeg E, Rauschecker JP, Turkeltaub PE. An ALE meta-analysis on the audiovisual integration of speech signals. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, Davatzikos C, Gur RC. Imaging Patterns of Brain Development and their Relationship to Cognition. Cerebral Cortex. 2014 doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Paganoni P, Turatto M, Marzola V, Mascetti GG. Visual-spatial attention in developmental dyslexia. Cortex. 2000;36:109–123. doi: 10.1016/s0010-9452(08)70840-2. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Tranel D, Seager-Frerichs D, Damasio H. Specific reading and phonological processing deficits are associated with damage to the left frontal operculum. Cortex. 2006;42:624–643. doi: 10.1016/s0010-9452(08)70399-x. [DOI] [PubMed] [Google Scholar]
- Frost R. Towards a universal model of reading. Behav Brain Sci. 2012;35:263–279. doi: 10.1017/S0140525X11001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE. Dyslexia: A New Synergy Between Education and Cognitive Neuroscience. Science. 2009;325:280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Ramus F. Neurogenetics and auditory processing in developmental dyslexia. Curr Opin Neurobiol. 2013;23:37–42. doi: 10.1016/j.conb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Gong Y, Cai T. Wechsler intelligence scale for children, Chinese revision (C-WISC) China: Map Press Hunan; 1993. [Google Scholar]
- Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat Rev Neurosci. 2015;16:43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Goswami U, Wang HLS, Cruz A, Fosker T, Mead N, Huss M. Language-universal Sensory Deficits in Developmental Dyslexia: English, Spanish, and Chinese. Journal of Cognitive Neuroscience. 2011;23:325–337. doi: 10.1162/jocn.2010.21453. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JDE. Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. Journal of Neuroscience. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd GW, Hall J, Novey ES, Eliopulos D, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M. Dyslexia and corpus callosum morphology. Arch Neurol. 1995;52:32–38. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Castellanos FX, Milham MP. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS One. 2013;8:e55454. doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. An Investigation into the Origin of Anatomical Differences in Dyslexia. Journal of Neuroscience. 2014;34:901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Napoliello EM, Eden GF. Gray matter volume changes following reading intervention in dyslexic children. Neuroimage. 2011;57:733–741. doi: 10.1016/j.neuroimage.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 2008;29:613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Pan J, Liu HY, McBride-Chang C, Li H, Zhang YP, Chen L, Tardif T, Liang WL, Zhang ZX, Shu H. Developmental trajectories of reading development and impairment from ages 3 to 8 years in Chinese children. Journal of Child Psychology and Psychiatry. 2011;52:212–220. doi: 10.1111/j.1469-7610.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- Li H, Shu H, McBride-Chang C, Liu HY, Peng H. Chinese children’s character recognition: Visuo-orthographic, phonological processing and morphological skills. Journal of Research in Reading. 2012;35:287–307. [Google Scholar]
- Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One. 2012;7:e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, You WP, Wang WJ, Guo XJ, Peng DL, Booth J. Altered brain structure in Chinese dyslexic children. Neuropsychologia. 2013;51:1169–1176. doi: 10.1016/j.neuropsychologia.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, Steinhausen HC, Brandeis D. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33:749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343:1006–1010. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Johnson MH, Karmiloff-Smith A, Pennington B. Deviations in the emergence of representations: A neuroconstructivist framework for analysing developmental disorders. Developmental Science. 2000;3:1–23. [Google Scholar]
- Pan J, Yan M, Laubrock J, Shu H, Kliegl R. Eye-voice span during rapid automatized naming of digits and dice in Chinese normal and dyslexic children. Developmental Science. 2013;16:967–979. doi: 10.1111/desc.12075. [DOI] [PubMed] [Google Scholar]
- Paul LK, Corsello C, Kennedy DP, Adolphs R. Agenesis of the corpus callosum and autism: a comprehensive comparison. Brain. 2014;137:1813–1829. doi: 10.1093/brain/awu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin C, Demonet JF, N’Guyen-Morel MA, Le Bas JF, Valdois S. Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain Lang. 2011;118:128–138. doi: 10.1016/j.bandl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Lallier M, Demonet JF, Pernet C, Baciu M, Le Bas JF, Valdois S. Neural dissociation of phonological and visual attention span disorders in developmental dyslexia: FMRI evidence from two case reports. Brain Lang. 2012;120:381–394. doi: 10.1016/j.bandl.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F, Szenkovits G. What phonological deficit? The Quarterly Journal of Experimental Psychology. 2008;61:129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court JH. Raven’s progressive matrices and vocabulary scales. Oxford Psychologists Press; 1998. [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How Does Your Cortex Grow? Journal of Neuroscience. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Frontiers in Human Neuroscience. 2014;8:347. doi: 10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum Brain Mapp. 2013;34:3055–3065. doi: 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE. Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence. Cerebral Cortex. 2014 doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Bagdasaryan J, Jung DE, Price CJ. The importance of premotor cortex for supporting speech production after left capsular-putaminal damage. Journal of Neuroscience. 2014;34:14338–14348. doi: 10.1523/JNEUROSCI.1954-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, Zelterman D, Lacadie C, Shaywitz SE. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Short SJ, Elison JT, Goldman BD, Styner M, Gu HB, Connelly M, Maltbie E, Woolson S, Lin WL, Gerig G, Reznick JS, Gilmore JH. Associations between white matter microstructure and infants’ working memory. Neuroimage. 2013;64:156–166. doi: 10.1016/j.neuroimage.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, McBride-Chang C, Wu S, Liu HY. Understanding Chinese developmental dyslexia: Morphological awareness as a core cognitive construct. Journal of Educational Psychology. 2006;98:122–133. [Google Scholar]
- Shu H, Peng H, McBride-Chang C. Phonological awareness in young Chinese children. Developmental Science. 2008;11:171–181. doi: 10.1111/j.1467-7687.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Siok WT, Fletcher P. The role of phonological awareness and visual-orthographic skills in Chinese reading acquisition. Dev Psychol. 2001;37:886–899. [PubMed] [Google Scholar]
- Siok WT, Niu ZD, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum Brain Mapp. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends in Cognitive Sciences. 2010;14:57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex. 2012;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: A toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wright BA, Zecker SG. Learning problems, delayed development, and puberty. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9942–9946. doi: 10.1073/pnas.0401825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Shu H, Li H, Li WL, Tian XM. The Stability of Literacy-Related Cognitive Contributions to Chinese Character Naming and Reading Fluency. Journal of Psycholinguistic Research. 2013;42:433–450. doi: 10.1007/s10936-012-9228-0. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci U S A. 2012;109:E3045–3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932. doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Zhang LJ, Shu H, Xi J, Wu H, Zhang Y, Li P. Universality of categorical perception deficit in developmental dyslexia: an investigation of Mandarin Chinese tones. Journal of Child Psychology and Psychiatry. 2012;53:874–882. doi: 10.1111/j.1469-7610.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Qian Y, Bi HY, Coltheart M. The visual magnocellular-dorsal dysfunction in Chinese children with developmental dyslexia impedes Chinese character recognition. Sci Rep. 2014;4:7068. doi: 10.1038/srep07068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.