Abstract
Purpose
Intraocular vascular diseases are leading causes of adult vision loss, and in the mid-1900s, I. C. Michaelson postulated that the retina releases a soluble, diffusible factor that causes abnormal vascular growth and leakage. What became known as “Factor X” eluded investigators for decades.
Methods
The field of cancer research, where Judah Folkman pioneered the concept of angiogenesis, provided the inspiration for the work honored by the 2014 Champalimaud Vision Award. Recognizing that tumors recruit their own blood supply to achieve critical mass, Dr Folkman proposed that angiogenic factors could be therapeutic targets in cancer. Napoleone Ferrara identified vascular endothelial growth factor (VEGF) as such an angiogenic agent: stimulated by hypoxic tumor tissue, secreted, and able to induce neovascularization. VEGF also was a candidate for Factor X, and the 2014 Champalimaud Laureates and colleagues worked individually and collaboratively to identify the role of VEGF in ocular disease.
Results
The Champalimaud Laureates correlated VEGF with ocular neovascularization in animal models and in patients. Moreover, they showed that VEGF not only was sufficient, but it also was required to induce neovascularization in normal animal eyes, as VEGF inhibition abolished ocular neovascularization in key animal models.
Conclusions
The identification of VEGF as Factor X altered the therapeutic paradigms for age-related macular degeneration (AMD), diabetic retinopathy, retinal vein occlusion, and other retinal disorders.
Translational Relevance
The translation of VEGF from discovery to therapy resulted in the most successful applications of antiangiogenic therapy to date. Annually, over one million patients with eye disease are treated with anti-VEGF agents.
Keywords: age-related macular degeneration (AMD), angiogenesis, António Champalimaud Vision Award, Factor X, vascular endothelial growth factor (VEGF)
On behalf of the laureates, I want to thank the Champalimaud Foundation; in particular, its President Leonor Beleza, who leads the foundation with such wisdom and grace, and the members of the Champalimaud Vision Award Jury. Of course, I also would like to acknowledge the vision and generosity of its founder, António Champalimaud. The Champalimaud Foundation challenges us to conquer the unknown, much like the Portuguese explorers who set sail from her shores. The Champalimaud Vision Award recognizes contributions in vision research, and in alternate years, it is given to groups delivering care in developing countries. However, the Foundation also supports an internal research program in neuroscience and cancer research, as well as a clinical cancer care in its Centre for the Unknown in Lisbon. It is well worth a visit.
I want to thank the other Champalimaud Laureates for allowing me to represent them. We all are honored by the award, and recognize that the group involved in translating the discovery of vascular endothelial growth factor (VEGF) to therapy is very much larger, and includes many other investigators, a few of whom I will mention. However, there also were many postdoctoral fellows, students, and clinicians, as well as industry scientists, and of course patients, whose important contributions I wish to acknowledge.
In the 1990s, the treatment of many retinal diseases was rather grim. For neovascular age-related macular degeneration (AMD), all we really had was a destructive laser treatment, which cauterized the vessels but also caused destruction of the neurosensory retina, leading to scotomas, or blind spots, and often decreased vision. This was not very rewarding, either for the patient or the treating clinician. Diabetic retinopathy was better controlled with laser photocoagulation, but even so, there were drawbacks and side effects. Treatment for retinal vein occlusion (RVO) also was of limited benefit.
Early in the 1990s, there was a confluence of researchers, especially in Boston, who were interested in ocular neovascularization (Fig. 1). Together and separately, we investigated models of ocular neovascularization in human disease. We explored findings from tumor angiogenesis in ocular disease. Among these investigators, there was Dr Judah Folkman, who was, indeed, the “Father of Angiogenesis”; and Harold Dvorak, who identified a soluble factor known as vascular permeability factor (VPF);1 Napoleone Ferrara (Genentech), who isolated VEGF and recognized its role in angiogenesis;2 and Evangelos Gragoudas and Donald D'Amico (Massachusetts Eye and Ear), who provided great clinical insight and leadership. At Harvard Medical School, there were two primary groups investigating VEGF and ocular angiogenesis. One was led by Lloyd Paul Aiello and George King at Joslin Diabetes Center, who later were joined by Lois Smith and Eric Pierce of Boston Children's Hospital. Another group was led by Patricia D'Amore and her graduate student David Shima, along with Anthony Adamis and me, at Boston Children's Hospital and Massachusetts Eye and Ear. Of course, we had many collaborators, postdoctoral fellows, and students who were instrumental in carrying out this work.
Figure 1.
Researchers involved in the discovery of VEGF as Factor X and translation to therapy. Top row (left to right): Judah Folkman, Harold Dvorak, Napoleone Ferrara, Evangelos Gragoudas, Donald D'Amico, Lloyd Paul Aiello. Bottom row (left to right): George King, Lois Smith, Eric Pierce, Patricia D'Amore, David Shima, Anthony Adamis, and Joan Miller.
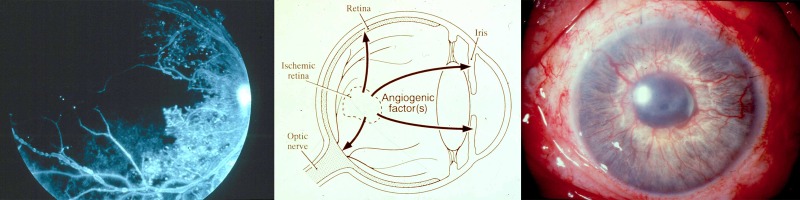
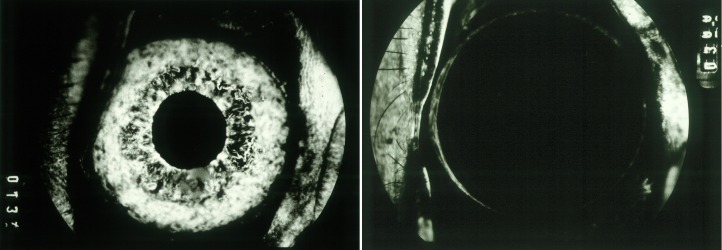
The search for a secreted “Factor X” that stimulated ocular neovascularization dates back at least to 1948, when Michaelson postulated that a soluble and diffusible growth factor was responsible for retinal vascular growth in development and disease.3 Clinicians recognized that damaged or ischemic retina secreted a factor that could leak out into other parts of the eye and cause new blood vessel growth, either in the retina, optic nerve, or on the iris (Fig. 2). However, the exact identity of Factor X would remain elusive for several decades.
Figure 2.
The search for Factor X. Left: Fluorescein angiogram of the retina in a patient with diabetic retinopathy (JWM patient seen at Massachusetts Eye and Ear). Black area represents nonperfused, ischemic retina. Center: Proposed action of unidentified angiogenic factor(s) in ocular neovascularization. Image courtesy of Anthony Adamis, MD; reproduced with permission. Right: Color photo of iris neovascularization in a patient with neovascular glaucoma (JWM patient seen at Massachusetts Eye and Ear).
In the 1970s, Folkman's laboratory identified tumor angiogenesis factor,4 and Folkman published his seminal theory of tumor angiogenesis in the November 1971 issue of New England Journal of Medicine: that angiogenesis, or the recruitment and growth of new blood vessels, was required for tumor growth.5 However, Folkman's theory was met with skepticism.
In the 1980s, Pat D'Amore, Bert Glaser, Arnall Patz, and others looked for an angiogenic factor in the retina and vitreous. Fibroblast growth factors 1 and 2 (FGF-1 and FGF-2) were identified as angiogenic factors and potential candidates for Factor X.6–10 Importantly, however, FGF did not meet all the criteria for Factor X; namely, it is not secreted. The identity of Factor X remained elusive.
In 1983, Harold Dvorak and Don Senger at Harvard Medical School isolated VPF from ascites fluid and tumor cells, and they demonstrated that it was a very potent permeability factor: 50,000 more potent than histamine.1 In 1989, Napoleone Ferrara and others cloned, sequenced, and characterized VEGF,2 which turned out to be the same molecule as VPF.11 VEGF was a secreted endothelial cell mitogen and an angiogenesis factor that was regulated by hypoxia (a condition known to stimulate neovascularization in certain retinopathies). Ferrara identified high-affinity tyrosine kinase receptors for VEGF,12 and demonstrated that heterozygous Vegf knockouts were embryonically lethal.13
Intrigued by these findings, our groups set off in two directions to investigate the role of VEGF in ocular disease. First, Adamis et al.14 grew retinal pigment epithelium (RPE) cells in culture and showed that they indeed produced VEGF (Fig. 3), the first demonstration that ocular cells made this factor. They also demonstrated that this production was regulated by hypoxia (Fig. 4).15,16
Figure 3.
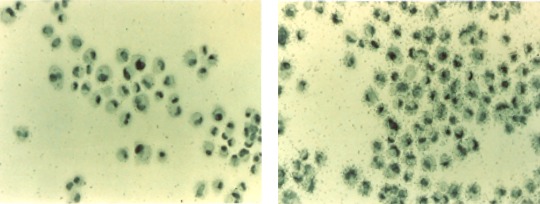
Left: Human retinal pigmented epithelial (hRPE) cells hybridized in situ with a VEGF sense riboprobe (control), showing nonspecific cellular labeling and low background levels. Right: hRPE hybridized in situ with a VEGF antisense riboprobe, showing strong labeling of all hRPE cells and indicating VEGF expression. Reprinted with permission from Adamis AP, Shima DT, Yeo KT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631–638. Copyright 1993 Elsevier.14
Figure 4.
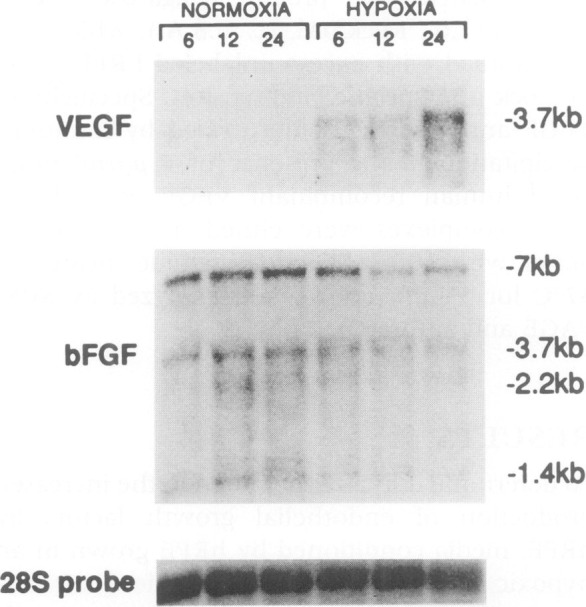
Northern analysis of total RNA extracted from rRPE cells grown under normoxic and hypoxic conditions for 6, 12, and 24 hours, probed for VEGF (upper), bFGF (middle), and 28S RNA (lower). Reproduced with permission from Shima DT, Adamis AP, Ferrara N, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–193.15
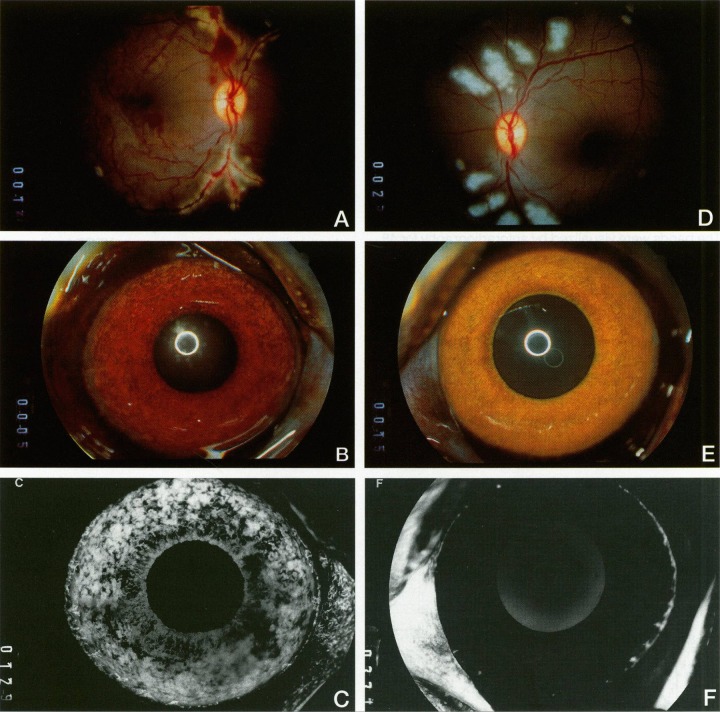
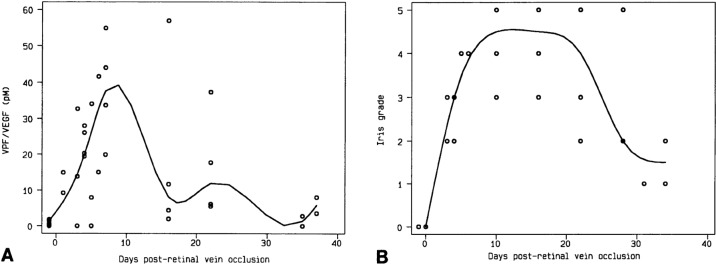
At the same time, we collected ocular fluid samples from a model of ischemic retinopathy in a nonhuman primate, in which experimental retinal ischemia following laser vein occlusion leads to iris neovascularization (Fig. 5). When we looked at VEGF/VPF levels with Dvorak, we saw that the protein was virtually nonexistent before neovascularization appeared, but rose very quickly to high levels that correlated closely with increasing severity of iris neovascularization, and levels decreased as the iris neovascularization regressed (Fig. 6).17 This was the first correlation of increased VEGF levels with ocular neovascularization in vivo. We also demonstrated that it was the ischemic retina that produced VEGF, specifically VEGF 121 and VEGF 165 (Fig. 7), which are the secreted isoforms.18
Figure 5.
Experimental iris neovascularization. (A) Fundus photograph immediately following laser vein occlusion. (B) Color photograph showing new vessels on the surface of the iris, which appear 4 to 7 days after laser vein occlusion. (C) Fluorescein angiography showing iris neovascularization with abundant leakage of fluorescein (grade 3). (D) Fundus photograph immediately following sham laser, aimed adjacent to the retinal vessels and producing retinal injury but preserving normal vasculature. (E) Color photograph of iris 12 days after sham laser, which appears normal. (F) Fluorescein angiography of the iris in (E), showing normal iris vessels with no fluorescein leakage (grade 0). Reproduced with permission from Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogensis in a primate model. Am J Pathol. 1994;145:574–584. Copyright 1994 Elsevier.17
Figure 6.
Correlation of VEGF levels in the aqueous (A) and grade of iris neovascularization (B) of four monkey eyes after laser vein occlusion. VEGF levels and neovascularization are represented as a scatterplot with best fit curves. Reproduced with permission from Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogensis in a primate model. Am J Pathol. 1994;145:574–584. Copyright 1994 Elsevier.17
Figure 7.
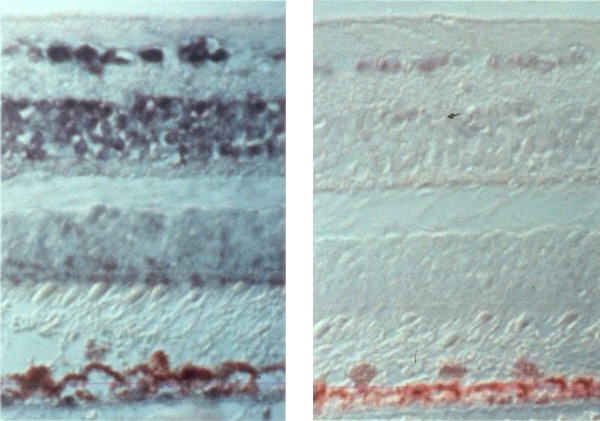
In situ localization of VEGF mRNA in ischemic retinas. Left: Cellular localization of VEGF mRNA expression by hybridization with an antisense VEGF riboprobe 13 days after laser vein occlusion. Right: Sense (control) riboprobe hybridized in 13-day ischemic retina. Adapted with permission from Shima DT, Gougos A, Miller JW, et al. Cloning and mRNA expression of vascular endothelial growth factor in ischemic retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci. 1996;37:1334–1340.18
In collaboration with D'Amico and Folkman, Adamis and I collected vitreous samples from patients, and found that VEGF levels were significantly increased in eyes with proliferative diabetic retinopathy.19 In a much larger study,20 Lloyd Paul Aiello, working with George King and Napoleone Ferrara, collected samples from patients with diabetic retinopathy and RVO, and found that increased VEGF levels were associated with active neovascular disease (such as proliferative diabetic retinopathy and iris neovascularization), whereas VEGF levels were low in eyes with quiescent disease or in normal control eyes (Fig. 8).
Figure 8.
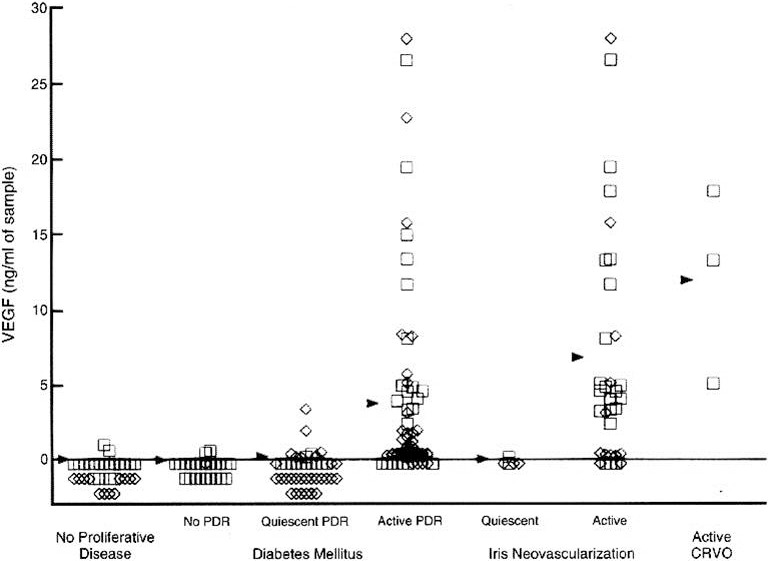
VEGF concentrations in the aqueous (squares) and vitreous (diamonds) of patients undergoing intraocular procedures. Arrowheads indicate mean values. Reproduced with permission from Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. Copyright 1994 Massachusetts Medical Society.20
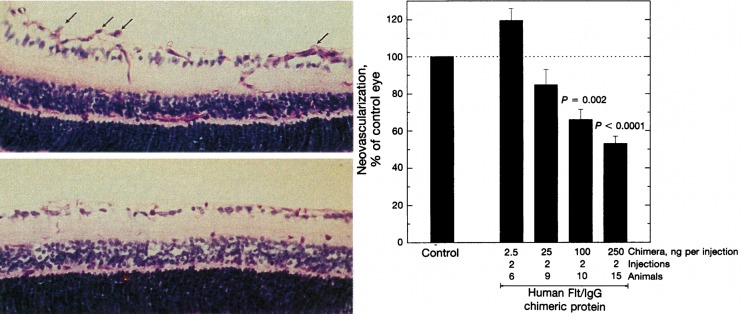
Aiello then went on, collaborating with Lois Smith and Eric Pierce, to inhibit VEGF using soluble VEGF receptors in a murine model of retinopathy of prematurity, and found that blocking VEGF in this fashion led to a pronounced reduction in retinal neovascularization, the first time that VEGF inhibition was shown to decrease neovascularization in the eye (Fig. 9).21
Figure 9.
Inhibition of neovascularization using soluble VEGF receptor-IgG chimeric proteins in a mouse model of retinal ischemia. Left: Retinal neovascularization extending into the vitreous is indicated by arrows in control-treated mice (upper panel), and absent in mice treated with human Flt-IgG chimeric proteins (lower panel). Right: Dose-dependent inhibition of retinal vascularization with human Flt-IgG chimeric proteins. Reproduced with permission from Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. Copyright 1995 National Academy of Sciences.21
Adamis and I and our group, working with Ferrara, used the ischemic retinopathy and iris neovascularization model to test the A461 full-length anti-VEGF antibody (essentially the precursor to Avastin), and we found that intravitreal injection of the antibody prevented any iris neovascularization, whereas a control antibody had no effect (Fig. 10).22 We also injected VEGF into normal eyes in our primate model, and found that this recapitulated all that we saw in neovascular eye disease, such as new blood vessels in the iris, neovascular glaucoma, retinal ischemia, and microangiopathy (Fig. 11).23,24
Figure 10.
Inhibition of iris neovascularization using anti-VEGF antibody. Left: Early-phase fluorescein angiogram of an eye treated with anti-gp120 mAb (control), showing new iris vessels filling with fluorescein dye. Right: Early-phase fluorescein angiograms of the contralateral eye, treated with anti-VEGF mAb, showing no neovascularization. Reproduced with permission from Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. Copyright 1996 American Medical Association.22
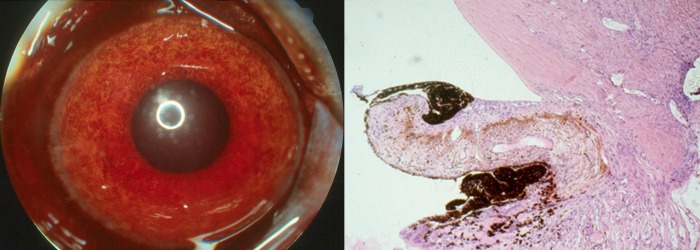
Figure 11.
VEGF induces iris neovascularization and neovascular glaucoma. Left: Color iris photograph of an eye that received four 1.25-μg injections of VEGF, showing diffuse iris neovascularization and ectropion uveae (JWM image). Right: Histopathologic examination of the anterior chamber angle of an eye that received ten 1.25-μg injections of VEGF, showing a dense anterior fibrovascular membrane, ectropion uveae, and trabecular meshwork scarring. Reproduced with permission from Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114:964–970. Copyright 1996 American Medical Association.23
At this point, in 1996, we were eager to develop a clinical treatment for patients. We had extensive evidence that VEGF had a key role in ischemic retinal disease in animal models and in humans. For neovascular AMD, there was perhaps less direct evidence for a causal role of VEGF, but there was definitely an unmet need, and Genentech (San Francisco, CA, USA) had a clear opportunity with several inhibitors of VEGF. Genentech, however, had many drugs in their pipeline at that time, and were perhaps influenced by Roche (Basel, Switzerland), who had supported clinical trials in AMD using α-interferon, which had negative outcomes.25,26 Genentech, thus, was less driven to pursue ophthalmic targets. Genentech continued to develop anti-VEGF drugs, primarily for cancer indications, but its ophthalmology program lagged somewhat. After several employees left to form Eyetech Pharmaceuticals (New York, NY) and develop pegaptanib (Macugen), the newly competitive environment led to renewed interest at Genentech for AMD therapies.
In the meantime, our group continued preclinical work with Ferrara et al. at Genentech, demonstrating that VEGF, indeed, was upregulated in the model of laser-induced choroidal neovascularization (CNV), which was relevant to AMD. Since we were unsure whether an intravitreally injected antibody could reach CNV, we tested whether intravenous delivery of anti-VEGF antibodies might be effective, and demonstrated that it could, indeed, reach the CNV (Fig. 12) and persist for several days.27
Figure 12.
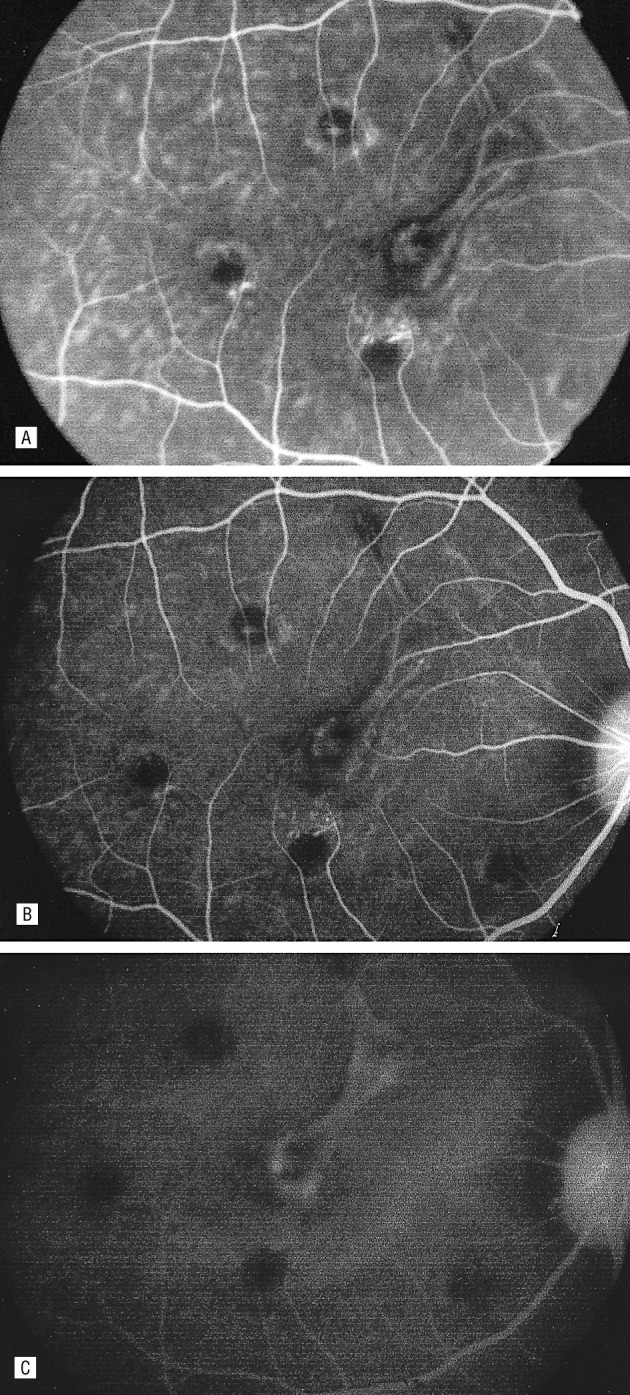
Fluorescein angiogram of one eye after intravenous injection with fluoresceinated anti-VEGF antibody, demonstrating localization to experimental choroidal neovascularization. Hyperfluorescence noted 1 minute after injection (A), 20 minutes after injection (B), and with leakage noted one hour after injection. Reproduced with permission from Tolentino MJ, Husain D, Theodosiadis P, et al. Angiography of fluoresceinated anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch Ophthalmol. 2000;118:78–84. Copyright 2000 American Medical Association.27
As described by Ferrara,28 the development of anti-VEGF agents continued, ultimately resulting in two molecules: bevacizumab (Avastin), the full-length antibody, and ranibizumab (Lucentis), the antibody fragment. Our group investigated intravitreal injection of the anti-VEGF antibody fragment (essentially Lucentis) in the laser-induced CNV model, and showed it could prevent neovascularization (Fig. 13), suggesting that this approach might be effective for neovascular AMD.29
Figure 13.
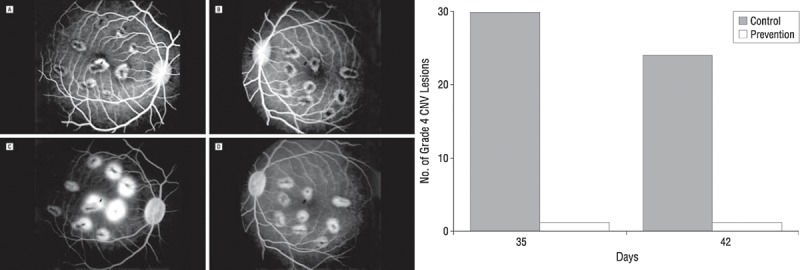
VEGF blockade prevents CNV. Left: Fluorescein angiogram of control (A, C) and prevention (B, D) eyes with laser-induced lesions. (A) Early frame of the control eye, which received intravitreal vehicle injection. (B) Early frame of the prevention eye, injected with humanized monoclonal anti-VEGF antibody (rhuFab VEGF). (C) Late frame of the control eye (vehicle). (D) Late frame of the prevention eye (rhuFab VEGF). Late frames demonstrate presence of grade 4 lesions in the control eye but not in the prevention eye. Right: Total number of grade 4 CNV lesions in the control group (shaded bars) versus prevention group (empty bar) 2 weeks (day 35) and 3 weeks (day 42) after laser induction. Injections of anti-VEGF or vehicle preceded laser induction of CNF. Reproduced with permission from Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. Copyright 2002 American Medical Association.29
As I hinted earlier, the founding of Eyetech Pharmaceuticals led to some interesting outcomes. Its founders included former Genentech employees, Marty Glick and John McLaughlin, and three familiar ophthalmologists: David Guyer, Samir Patel, and Tony Adamis. They led the development of pegaptanib (Macugen) into clinical trials, and Phase 3 data showed its efficacy in slowing vision loss in all forms of neovascular AMD.30 This was the first demonstration of efficacy of an antiangiogenic therapy in ophthalmology, and, thus, noteworthy. The vision outcomes with pegaptanib were not better than those achieved with verteporfin photodynamic therapy (Visudyne), a treatment developed earlier by our group,31 but were applicable to a broader range of neovascular AMD subtypes.
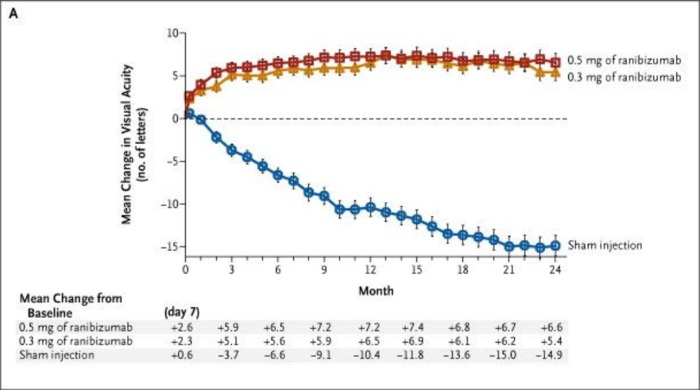
On the other hand, the Phase 3 results using ranibizumab for neovascular AMD were dramatic (Fig. 14). One-year results from the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA) trial were presented in 2005, and 2-year results were published the following year,32 and showed for the first time that sustained vision improvement was possible, in patients given intravitreal injections with anti-VEGF therapy (0.3 mg or 0.5 mg ranibizumab), compared to gradual vision loss in sham-injected patients over the same course of time. Similar results obtained from after 1 year of treatment in the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) study,33 which compared verteporfin photodynamic therapy with the same dosing of ranibizumab as the MARINA trial.
Figure 14.
MARINA 2-year results: Mean changes in visual acuity from baseline to 24 months in patients injected with ranibizumab (0.3 and 0.5 mg monthly) versus sham injections. Reproduced with permission from Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. Copyright 1996 Massachusetts Medical Society.32
The same year that the ranibizumab results were presented (in fact, at the same meeting), Philip Rosenfeld presented optical coherence tomography (OCT) data showing improvement in OCT findings with stable vision in a patient with neovascular AMD after intravitreal injection of bevacizumab (Fig. 15).34 This was dramatic and unexpected—we did not think that full-length antibody would be effective—but this stunning finding led to widespread off-label use of Avastin for neovascular AMD, which continues to this day.
Figure 15.
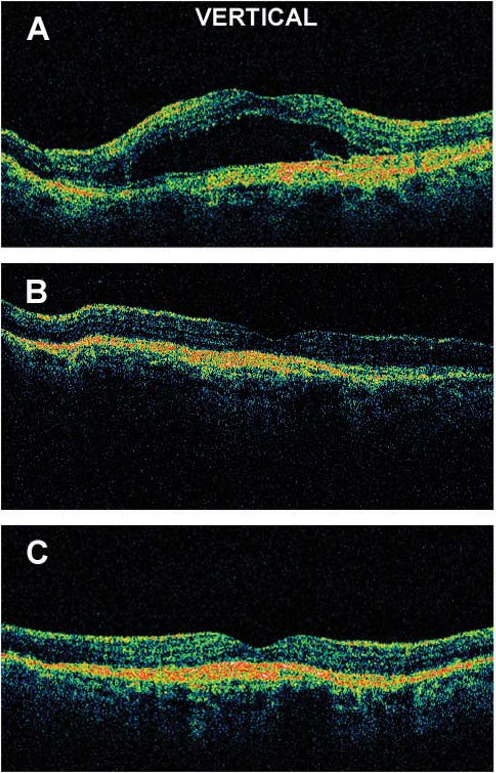
Neovascular AMD showing improved OCT after intravitreal injection of approximately 1.0 mg Avastin. (A) Baseline, (B) 1 week postinjection, (C) 4 weeks postinjection. Reproduced with permission from Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. Copyright 2005 Slack, Inc.34
Today, clinicians have multiple anti-VEGF agents to use: pegaptanib, bevacizumab (off label), ranibizumab, and aflibercept (Eylea; Table 1). With these drugs, more than 90% of patients avoid moderate vision loss, and approximately a third of patients achieve vision of 20/40 or better. More recently, the CATT, IVAN, and other trials have demonstrated similar vision outcomes with a variety of anti-VEGF agents.35,36
Table 1.
Anti-VEGF Therapy for AMD
What about anti-VEGF therapy beyond AMD? The earliest evidence of efficacy in the eye was achieved in a model of ischemic retinopathy, essentially a model of RVO.22 Therefore, it made sense to investigate anti-VEGF therapy for RVO. With the results of numerous Phase III studies of bevacizumab/ranibizumab and aflibercept, BRAVO,37 CRUISE,38 COPERNICUS,39,40 GALILEO,41 and VIBRANT,42 we can conclude that anti-VEGF therapy is effective for RVO (Fig. 16); both branch retinal vein (BRVO) and central retinal vein (CRVO) occlusion.
Figure 16.
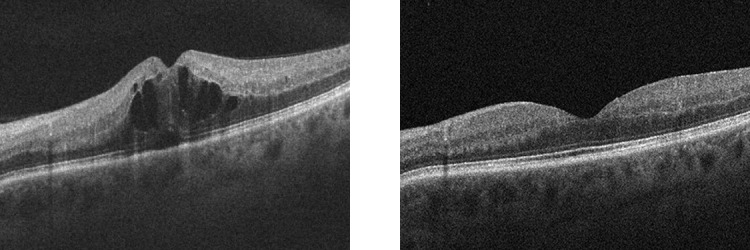
OCT scans of a patient with RVO (JWM patient seen at Massachusetts Eye and Ear). The intraretinal cystic changes and subretinal fluid (left) completely resolved after treatment with anti-VEGF therapy (right).
Additionally, we had shown that VEGF injected into a normal eye causes breakdown of the blood retinal barrier (Figs. 17, 18), seen as fluorescein leaking out of retinal vessels and changes in the retinal vasculature that are reminiscent of the vascular aberrations in diabetic retinopathy and other ischemic retinopathies,24 as well as endothelial cell hyperplasia.43 Therefore, it is not surprising that anti-VEGF therapy also is successful for diabetic macular edema (DME), showing benefit in Phase III DRCRnet clinical trials,44,45 the RIDE/RISE trials,46 and more recently in the Protocol T study comparing ranibizumab, aflibercept, and bevacizumab.47
Figure 17.
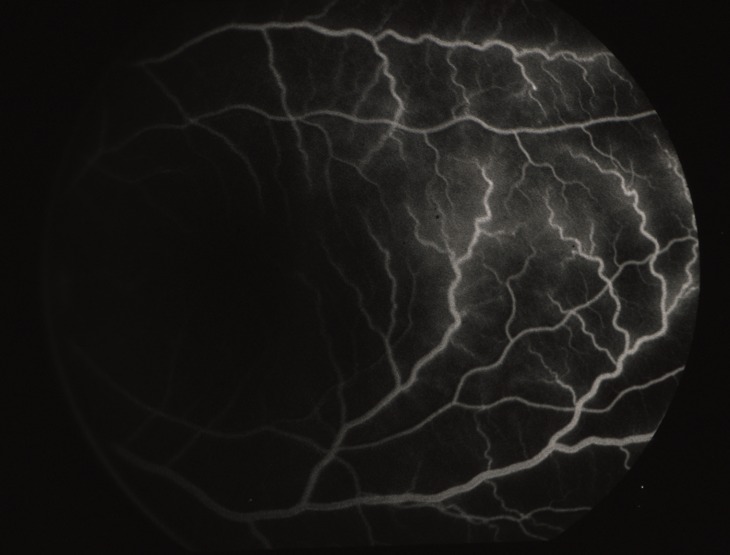
VEGF-induced blood-retinal breakdown. Fluorescein angiogram shows dilation, tortuosity, and leakage of temporal retinal vessels, 4 days after a single injection of 1.25 μg VEGF mixed with nonneutralizing antibody. Reproduced with permission from Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–1828. Copyright 1996 Elsevier.24
Figure 18.
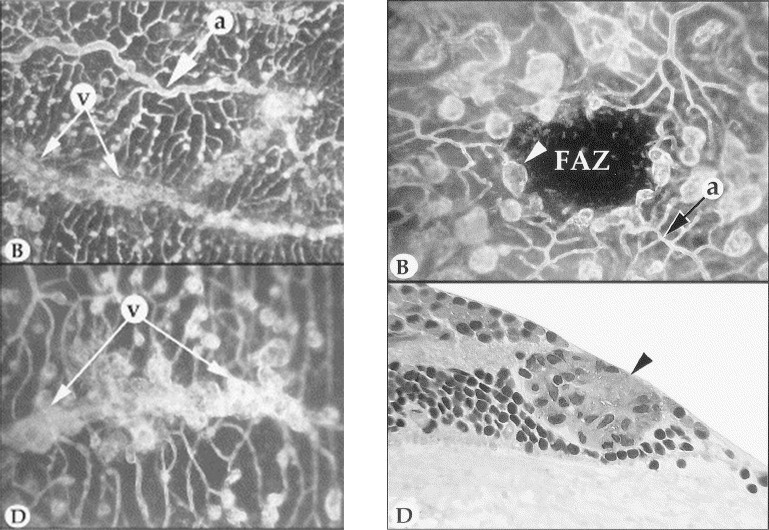
Left: Preretinal neovascularization in peripheral retina. Artery (“a”) and veins (“v”) in the peripheral retinal of an eye injected with VEGF. The artery displays tortuosity and dilation, with microaneurysmal-like formations at the levels of precapillary arterioles and capillaries. In the flat perspective (lower left), saccular neovascular buds obscure the parent veins (paired arrows). Right: Upper right shows a macula of a VEGF-injected eye with grotesquely dilated and saccular capillaries and veins. White arrowhead indicates microaneurysmal-like structure, and black arrow indicates arteriole. FAZ, foveal avascular zone. Lower: Section taken through the microaneurysmal-like structure (indicated by white arrowhead in the upper panel), showing endothelial cell hyperplasia (black arrowhead) and basement membrane material occluding the lumen. Reproduced with permission from Tolentino MJ, McLeod DS, Taomoto M, Otsuji T, Adamis AP, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002;133:373–385. Copyright 2002 Elsevier.43
The story of VEGF, from discovery to therapy, shows that we not only solved for Factor X, but also that we were able to develop treatments that dramatically improved outcomes for patients for AMD, diabetic retinopathy, RVO, and other retinal disorders. Today, we recognize the contributions of the 2014 Champalimaud Vision Award Laureates and their respective teams, but also the contributions of many other physicians, scientists, funding agencies, industry partners, and especially the patients who enrolled in the clinical trials.
However, there is more to be done, and the research continues. We must improve our phenotyping and develop biomarkers for AMD, diabetic retinopathy, and RVO. We must develop treatments for early AMD. Moreover, I believe that we must target other pathways beyond VEGF, particularly in DME and RVO.
I would like to conclude with a quote from Leonor Beleza: “Now we start afresh every day with the sense that today is already past and tomorrow is the present. We honour [Antonio Champalimaud's] philosophy of life: A search for excellence…outcomes…rigour…and knowledge.”
Acknowledgments
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, Colorado, May 5, 2015. Previously presented in part as The Harvard Angiogenesis Story: The Paul Henkind Memorial Lecture at the 34th annual meeting of the Macula Society in Boca Raton, Florida, March 11, 2011 (Miller JW. The Harvard Angiogenesis Story. Surv Ophthalmol. 2014;59:361–364).
Disclosure: J.W. Miller, Alcon Research Council (C), Amgen (C), KalVista (C), Maculogix (C), Valeant via Massachusetts Eye and Ear (P,R), ONL Therapeutics (C,P).
References
- 1. Senger DR,, Galli SJ,, Dvorak AM,, Perruzzi CA,, Harvey VS,, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219: 983–985. [DOI] [PubMed] [Google Scholar]
- 2. Leung DW,, Cachianes G,, Kuang WJ,, Goeddel DV,, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 3. Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 1948; 68: 137–180. [Google Scholar]
- 4. Gimbrone MA,, Jr,, Leapman SB,, Cotran RS,, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972; 136: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 6. D'Amore PA,, Glaser BM,, Brunson SK,, Fenselau AH. Angiogenic activity from bovine retina: partial purification and characterization. Proc Natl Acad Sci U S A. 1981; 78: 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glaser BM,, D'Amore PA,, Lutty GA,, Fenselau AH,, Michels RG,, Patz A. Chemical mediators of intraocular neovascularization. Trans Ophthalmol Soc U K. 1980; 100: 369–373. [PubMed] [Google Scholar]
- 8. Glaser BM,, D'Amore PA,, Michels RG,, et al. The demonstration of angiogenic activity from ocular tissues. Preliminary report. Ophthalmology. 1980; 87: 440–446. [DOI] [PubMed] [Google Scholar]
- 9. Glaser BM,, D'Amore PA,, Michels RG,, Patz A,, Fenselau A. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol. 1980; 84: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sivalingam A,, Kenney J,, Brown GC,, Benson WE,, Donoso L. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1990; 108: 869–872. [DOI] [PubMed] [Google Scholar]
- 11. Keck PJ,, Hauser SD,, Krivi G,, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989; 246: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 12. de Vries C,, Escobedo JA,, Ueno H,, Houck K,, Ferrara N,, Williams LT. The fms-like tyrosine kinase a receptor for vascular endothelial growth factor. Science. 1992; 255: 989–991. [DOI] [PubMed] [Google Scholar]
- 13. Ferrara N,, Carver-Moore K,, Chen H,, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996; 380: 439–442. [DOI] [PubMed] [Google Scholar]
- 14. Adamis AP,, Shima DT,, Yeo KT,, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993; 193: 631–638. [DOI] [PubMed] [Google Scholar]
- 15. Shima DT,, Adamis AP,, Ferrara N,, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995; 1: 182–193. [PMC free article] [PubMed] [Google Scholar]
- 16. Shima DT,, Deutsch U,, D'Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995; 370: 203–208. [DOI] [PubMed] [Google Scholar]
- 17. Miller JW,, Adamis AP,, Shima DT,, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994; 145: 574–584. [PMC free article] [PubMed] [Google Scholar]
- 18. Shima DT,, Gougos A,, Miller JW,, et al. Cloning and mRNA expression of vascular endothelial growth factor in ischemic retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci. 1996; 37: 1334–1340. [PubMed] [Google Scholar]
- 19. Adamis AP,, Miller JW,, Bernal MT,, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118: 445–450. [DOI] [PubMed] [Google Scholar]
- 20. Aiello LP,, Avery RL,, Arrigg PG,, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 21. Aiello LP,, Pierce EA,, Foley ED,, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995; 92: 10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adamis AP,, Shima DT,, Tolentino MJ,, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996; 114: 66–71. [DOI] [PubMed] [Google Scholar]
- 23. Tolentino MJ,, Miller JW,, Gragoudas ES,, Chatzistefanou K,, Ferrara N,, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996; 114: 964–970. [DOI] [PubMed] [Google Scholar]
- 24. Tolentino MJ,, Miller JW,, Gragoudas ES,, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996; 103: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 25. Engler CB,, Sander B,, Koefoed P,, Larsen M,, Vinding T,, Lund-Andersen H. Interferon alpha-2a treatment of patients with subfoveal neovascular macular degeneration. A pilot investigation. Acta Ophthalmol. 1993; 71: 27–31. [DOI] [PubMed] [Google Scholar]
- 26. Ross C,, Engler CB,, Sander B,, Bendtzen K. IFN-alpha antibodies in patients with age-related macular degeneration treated with recombinant human IFN-alpha2a. J Interferon Cytokine Res. 2002; 22: 421–426. [DOI] [PubMed] [Google Scholar]
- 27. Tolentino MJ,, Husain D,, Theodosiadis P,, et al. Angiography of fluoresceinated anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch Ophthalmol. 2000; 118: 78–84. [DOI] [PubMed] [Google Scholar]
- 28. Ferrara N. Discovery of VEGF-A, a Key Regulator of Intraocular Neovascularization:The Champalimaud Award Lecture. Transl Vis Sci Technol . In press.
- 29. Krzystolik MG,, Afshari MA,, Adamis AP,, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002; 120: 338–346. [DOI] [PubMed] [Google Scholar]
- 30. Gragoudas ES,, Adamis AP,, Cunningham ET,, Jr,, Feinsod M,, Guyer DR. Group VISiONCT. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351: 2805–2816. [DOI] [PubMed] [Google Scholar]
- 31. Miller JW,, Schmidt-Erfurth U,, Sickenberg M,, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117: 1161–1173. [DOI] [PubMed] [Google Scholar]
- 32. Rosenfeld PJ,, Brown DM,, Heier JS,, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- 33. Anchor Study Group, Brown DM,, Kaiser PK, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- 34. Rosenfeld PJ,, Moshfeghi AA,, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36: 331–335. [PubMed] [Google Scholar]
- 35. CATT Research Group Martin DF,, Maguire MG,, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. IVAN Study Investigators, Chakravarthy U, Harding SP,. et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012; 119: 1399–1411. [DOI] [PubMed] [Google Scholar]
- 37. Campochiaro PA,, Heier JS,, Feiner L,, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 38. Brown DM,, Campochiaro PA,, Singh RP,, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 39. Boyer D,, Heier J,, Brown DM,, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012; 119: 1024–1032. [DOI] [PubMed] [Google Scholar]
- 40. Brown DM,, Heier JS,, Clark WL,, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013; 155: 429–437. [DOI] [PubMed] [Google Scholar]
- 41. Holz FG,, Roider J,, Ogura Y,, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013; 97: 278–284. [DOI] [PubMed] [Google Scholar]
- 42. Campochiaro PA,, Clark WL,, Boyer DS,, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015; 122: 538–544. [DOI] [PubMed] [Google Scholar]
- 43. Tolentino MJ,, McLeod DS,, Taomoto M,, Otsuji T,, Adamis AP,, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002; 133: 373–385. [DOI] [PubMed] [Google Scholar]
- 44. Diabetic Retinopathy Clinical Research Network. Elman MJ,, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010; 117: 1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elman MJ,, Bressler NM,, Qin H,, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011; 118: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen QD,, Brown DM,, Marcus DM,, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 47. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR,. et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]