Abstract
Despite considerable progress in reducing cigarette smoking prevalence and enhancing smoking cessation treatments, most smokers who attempt to quit relapse. The current randomized clinical trial evaluated the efficacy of an adjunctive behavioral smoking cessation treatment based on learning theory. Adult daily smokers were randomly assigned to standard treatment (N=47) with nicotine patch and individual counseling or to standard treatment plus a “practice quitting” program involving seven sessions of escalating prescribed abstinence periods (N=46) prior to a target stop smoking date. Practice quitting was designed to extinguish smoking in response to withdrawal symptoms. Retention in treatment was excellent and the treatment manipulation increased the interval between cigarettes across practice quitting sessions on average by 400%. The primary endpoint, seven-day point-prevalence abstinence four weeks post-quit, was not significantly affected by practice quitting (31.9% in the standard treatment condition, 37.0% in the practice quitting condition). Practice quitting increased latency to a first lapse among those who quit smoking for at least one day and prevented progression from a first lapse to relapse (smoking daily for a week) relative to standard treatment, however. Practice quitting is a promising adjunctive treatment in need of refinement to enhance adherence and efficacy.
Keywords: smoking cessation, behavioral treatment, randomized clinical trial
Graphical abstract
Introduction
Smoking remains the leading preventable cause of death among adults in the U.S. [Centers for Disease Prevention and Control (CDC), 2008; U.S. Department of Health and Human Services, 2004]. Although more than half of current U.S. smokers attempt to quit each year, cessation failure and relapse remain the most likely outcomes of quit attempts (CDC, 2011). Currently available treatments, such as varenicline or combination nicotine replacement therapies offered with counseling, roughly triple success rates relative to placebo treatment, but still help fewer than 50% of smokers achieve abstinence lasting six-months or longer (Cahill, Stevens, Perera, & Lancaster, 2013; Fiore et al., 2008). Quitline counseling interventions with broad reach are also effective, but help fewer than 20% of smokers achieve lasting abstinence (Stead, Hartmann-Boyce, Perera, & Lancaster, 2013). As such, despite considerable progress, we still have an urgent need for novel smoking cessation interventions. Developing low-risk, low-cost adjunctive interventions that can supplement current first-line treatments may be a way to achieve the long-elusive goal of developing treatments that work for a majority of smokers. The aim of the current randomized clinical trial was to gather initial efficacy data on such an adjunctive smoking cessation preparation intervention based on contemporary learning theory.
Smoking is a learned behavior supported by both classical and operant conditioning (McCarthy, Baker, Minami, & Yeh, 2011). Many conditioned stimuli (e.g., lighters, ashtrays, internal states of distress or craving) elicit smoking motivation and serve as triggers for continued or renewed smoking (see Baker et al., 2004; McCarthy et al., 2011 for reviews). Internal signals of withdrawal may be particularly potent triggers of drug motivation and use (Baker et al., 2004). That is, avoidance and escape of withdrawal distress may be critical to the maintenance of smoking behavior (Baker et al., 2004). Self-reported withdrawal symptoms have been shown to emerge in just a few hours of abstinence (Hendricks, Ditre, Drobes, & Brandon, 2006). More subtle signs of withdrawal that may not enter awareness may nonetheless prompt smoking behavior (Baker et al., 2004). In support of this negative reinforcement model of smoking motivation, most returns to smoking begin within the first few weeks of a quit attempt (Brandon, Tiffany, Obremski, & Baker, 1990; Brown et al., 2008; Piasecki, Fiore, McCarthy, & Baker, 2002), when withdrawal is often most intense (Hughes, 2007; Shiffman, Patten, et al., 2006). A wealth of laboratory and clinical evidence supports the role of withdrawal in maintaining smoking behavior (Allen, Bade, Hatsukami, Center, 2008; Welsch et al., 1999; West, Hajeck, & Belcher, 1989; Zhou et al., 2009). Existing treatments may also work, in part, by reducing withdrawal and craving (McCarthy et al., 2008; Piper et al. 2008; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006) and thereby reducing smoking motivation.
If smoking is indeed maintained by avoidance or escape of withdrawal or similar affective states, then extinction of this avoidance behavior may be a promising adjunctive treatment approach, as others have suggested (Otto, Powers, & Fischmann, 2005; Otto, Safren, & Pollack, 2004). Extinction treatments are among the most effective psychotherapies developed to date and are the first-line treatments for many anxiety disorders (Barlow, Gorman, Shear, & Woods, 2000; Craske & Mystkowski, 2006; Foa et al., 2005; Mineka & Thomas, 1999; NICE, 2011). Recently, the concept of facilitating extinction through exposure to feared internal stimuli with prevention of drug use responding has been applied to treatment for substance use disorders. A pilot investigation of exposure to internal distress (elicited by hyperventilation) among individuals addicted to opiates indicated a medium-sized reduction in the percentage of positive drug screens in women (Cohen’s d=.61), but not men (Pollack et al., 2002). By this same logic, an intervention that incrementally exposes smokers to withdrawal and associated symptoms may enhance a smokers’ ability to endure withdrawal which may, in turn, increase the likelihood of maintaining abstinence after quitting. Evidence supporting the utility of such an approach is beginning to accumulate. A series of single-subject designs has supported the utility of combining graded interoceptive exposure to withdrawal induced by smoking deprivation and exposure to interoceptive cues of anxiety in smokers high in anxiety (Feldner, Smith, Monson, & Zvolensky, 2013; Zvolensky, Yartz, Gregor, Gonzales, & Bernstein, 2008). An open trial of a treatment targeting anxiety sensitivity in Argentinian smokers has also generated promising evidence that interventions involving graded exposure to withdrawal prior to quitting may facilitate successful quitting (Zvolensky, Bogiaizian, Lopez Salazar, Farris, & Bakshaie, 2011). Thus, there is emerging evidence that exposure to interoceptive cues associated with substance use may facilitate extinction of substance use.
Extinction is complicated and fragile, however, as demonstrated by extensive animal and human research (see Vervliet, Craske, & Hermans, 2013 for a review). Although the exact nature of the learning during extinction that leads to observable changes in behavior is not yet clear, it seems as though new, inhibitory learning is critical (Bouton, 2004; Vervliet et al., 2013). Extinction is highly stimulus- and context-dependent (Vervliet et al., 2013). For this reason, it is important to conduct extinction training in the contexts (including internal states) in which a return of smoking is most likely to occur, in the presence of the conditioned stimuli most likely to elicit smoking responses (Otto, O’Cleirigh, Pollack, 2007; Vervliet et al., 2013). The laboratory and the clinic are therefore not the optimal contexts for this form of treatment. In vivo extinction in smokers’ real-world settings is more likely to facilitate robust behavior change, in addition to being less burdensome and more feasible as an adjuvant treatment. For this reason, the current randomized trial involves extinction of smoking behavior in the presence of withdrawal (induced by prescribed abstinence) in smokers’ natural environments on different days of the week. In this way, the current study is similar to an independent pilot study (N=16) of a multicomponent treatment that included four sessions of exposure to withdrawal and smoking cues (e.g., lighters, ashtrays) during escalating periods of abstinence of one to four hours (Brown et al., 2008).
The current study differs from this earlier uncontrolled pilot study, however, in isolating the additive benefit of practice quitting as an adjunct to standard nicotine patch and brief counseling treatment, increasing the dose of exposure to seven sessions, and tailoring the duration of prescribed abstinence based on smokers’ past success. The current study evaluates the additive benefit of a behavioral intervention designed to systematically expose smokers to periods of abstinence and withdrawal prior to a target quit day. The aim of the treatment was to prepare smokers to quit by weakening or inhibiting associations between smoking and diverse internal and external real-world, personally relevant stimuli and contexts. Smokers were randomized to receive either standard treatment (nicotine patch and smoking cessation counseling) alone or standard treatment plus ‘practice quitting.’ Periods of abstinence were tailored to the individual based on their previous longest period between cigarettes to optimize progress and gradually increase exposure to withdrawal. Rest days were offered between practice-quitting sessions in an effort to enhance the acceptability of the treatment and also to enhance the retrieval strength of new extinction learning (Vervliet et al., 2013). Practice quitting was scheduled to take place from overnight into the next morning, to optimize exposure to interoceptive withdrawal cues likely to be strongest after overnight abstinence (when blood nicotine levels typically fall). The seven practice quits occurred once on every day of the week in an effort to extend the contexts in which abstinence, and associated exposure to withdrawal symptoms, occurred.
We assessed the feasibility and acceptability of the practice quitting intervention by examining attrition and abstinence adherence rates. The primary outcome of interest was seven-day point-prevalence abstinence four weeks after the quit attempt. We hypothesized that practice quitting would enhance cessation success compared to the standard treatment control. Building on evidence that past duration of abstinence from tobacco predicts future success at quit attempts (e.g., Hyland et al., 2006; Vangeli, Stapleton, Smit, Borland, & West, 2011), we also expected that longer successful practice quitting intervals prior to the quit day would predict improved cessation outcomes. We examined whether practice quitting enhanced success above and beyond standard treatment across cessation milestones (Shiffman, Scharf, et al., 2006), predicting greater initial cessation success and longer time to lapse or relapse after quitting among those in the practice quitting condition.
Method
Participants
Participants were adult, daily smokers from central New Jersey recruited through direct mail and flyers. Inclusion criteria for this study required participants be over age 18, English-literate, motivated to quit smoking (at least 6 on a 10-point scale), and smoking at least 10 cigarettes per day for at least 6 months with at least 8 parts per million carbon monoxide (CO) in their expired breath at baseline. Study exclusion criteria included: contraindications to nicotine patch use (heart attack or heart surgery in the past three months; irregular heartbeat; heart disease; severe skin problems; or past negative reactions to the nicotine patch); a history of bipolar disorder or psychosis diagnosis; current use of other stop-smoking treatments; cohabitating with a study participant; past-month use of marijuana, illegal drugs, or other forms of tobacco; an inability or unwillingness to complete study activities; or pregnancy, breastfeeding, or unwillingness to prevent pregnancy for the duration of the study.
Procedures
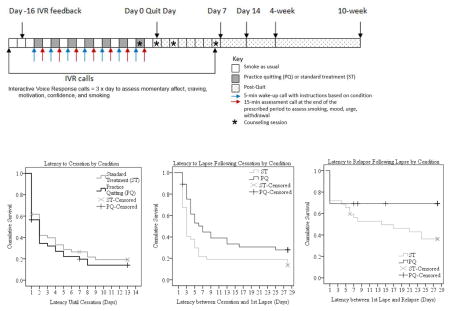
Study procedures were approved by an Institutional Review Board. Interested volunteers completed initial eligibility screening by telephone and then were invited to a group orientation session at which the study procedures were described in detail. At the orientation, participants provided written informed consent and completed baseline questionnaires, CO testing, and training in the completion of interactive voice response (IVR) surveys using cellular telephones. Cellular telephones were provided to those who did not have a phone to use for study calls. Post-enrollment study procedures are summarized in Table 1.
Table 1.
Timeline of study procedures.
| Day | Treatment Manipulation | IVR | Counseling | Patch | Follow-up |
|---|---|---|---|---|---|
| −17 | Normal smoking | Begin | |||
| −16 | Normal smoking | Feedback | |||
| −15 | Normal smoking | ||||
| −14 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −13 | Normal smoking | ||||
| −12 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −11 | Normal smoking | ||||
| −10 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −9 | Normal smoking | ||||
| −8 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −7 | Normal smoking | ||||
| −6 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −5 | Normal smoking | ||||
| −4 | Practice quitting vs. self-monitoring (ST) | Feedback | |||
| −3 | Normal smoking | ||||
| −2 | Practice quitting vs. self-monitoring (ST) | Feedback | Session 1 | Instructions | |
| −1 | Normal smoking | ||||
|
| |||||
| 0 | Target quit day | Feedback | Session 2 | Start use | |
|
| |||||
| 2 | Feedback | Session 3 | |||
| 7 | End | Session 4 | |||
| 28 | Follow-up* | ||||
| 42 | End use | ||||
| 70 | Follow-up | ||||
Those reporting smoking in the past 7 days were randomized to receive either advice only or very low nicotine cigarettes
Interactive voice response survey calls lasting two to three minutes were programmed to occur three times per day beginning the Sunday following enrollment and ending 24 days later. An IVR feedback call was scheduled for the day after IVR recording began to review participant adherence to the IVR protocol and trouble-shoot problems completing the calls promptly.
Participants were randomized to a study condition with a 1:1 ratio by a research assistant at the end of the IVR feedback call. Randomization was blocked on gender and achieved through use of a computer-generated list of conditions prepared at the outset of the study by the Principal Investigator (PI). Randomization was blind to neither participants nor researchers, given the behavioral nature of the intervention.
Standard Treatment: Counseling and Nicotine Patch
All participants received standard treatment. Brief (15–20-minute) cessation counseling was offered two days before the target quit day set by investigators, on the target quit day, two days later, and one week post-quit. The first three sessions were offered by telephone and the last occurred at the last office visit. The counseling manual and quit planning booklet provided to all participants was based on the Tobacco Dependence Treatment Handbook (Abrams et al., 2003) and You Can Quit Smoking (US Public Health Service, 2000), a worksheet developed to accompany the Public Health Service Guideline on Treating Tobacco Use and Dependence (Fiore et al., 2008). All subjects received the same counseling and all were encouraged to draw on past experience in change efforts to plan the current quit attempt. Counselors were bachelor’s or master’s level research staff who completed roughly 12 weeks of training including observing and conducting practice sessions until reaching criterion prior to providing counseling to study participants. Counselors were supervised by the PI who reviewed case notes and provided feedback to counselors at least bi-weekly, occasionally monitored therapist behavior during live calls, and held regular group supervision and training meetings.
All participants were provided a six-week supply of 21-mg nicotine patches with instructions to begin use on the target quit day. These were sent by mail to participants so they would arrive shortly before the quit day to reduce the likelihood that participants would wear patches during practice quit attempts. A six-week duration was selected based on meta-analyses in the Clinical Practice Guideline (Fiore et al., 2008).
Treatment Manipulation: Practice Quitting (PQ) vs. Standard Treatment (ST)
The treatment manipulation began two days following the IVR feedback call. On this day, participants received a live phone call from a study staffer at the participant’s anticipated wake-up time. All participants were instructed not to smoke before receiving this wake-up call. At this call, the participants randomized to the standard treatment control condition received instructions to smoke normally, but monitor their smoking, urges to smoke, and triggers to smoke closely that day in preparation for the upcoming quit day. These participants were also told to anticipate a live 15-minute survey call two hours later. Those randomized to the practice quitting condition were instructed to stop smoking for a period of time equal to 150% of their maximal inter-cigarette interval at baseline and to expect a live 15-minute survey call at the end of this period. This procedure was repeated six more times before the target quit day (every other day over the next 12 days). Control subjects were asked to monitor their smoking and then complete survey calls at fixed times 4, 6, 6, 8, 10, and then 12 hours later, while practice quitting subjects were asked to abstain for increasing intervals of time (described below) and then complete survey calls at the end of each practice quitting period. Participants in the two conditions were asked to complete an equal number of calls and visits and had equal contact time with study staff.
Practice-quitting subjects were asked to abstain for 150% of their maximal period of abstinence to date if they achieved their target abstinence duration on the previous call, or to abstain for 125% of their maximal period of abstinence to date if they did not achieve their abstinence target on the last practice day. As such, participants in the practice quitting group were asked to go beyond their maximal period of abstinence achieved in the study period, but this was lowered from 50% longer to only 25% longer if participants did not achieve the 50% goal. Participants were only considered to have achieved their target abstinence if they abstained from smoking between going to bed the previous night and the end of the target abstinence interval on the day of the practice quitting session. Individuals who reported smoking between bedtime and the scheduled live wake-up call in the morning were considered to have failed to achieve the target period of abstinence, which was intended to extend overnight abstinence longer into the day with each practice quitting period. Limits were placed on the absolute increases in expected abstinence across sessions so that no one was asked to extend abstinence by more than four hours over the duration from the previous session and no one was asked to practice quitting for more than one full waking day.
Follow-Up
Telephone follow-up interviews were conducted 28 days following the target quit day in order to assess tobacco, alcohol, and tobacco treatment use since the last contact. Adverse events were assessed and additional items regarding withdrawal symptom severity, affect, quitting motivation, self-efficacy, and perceived control over mood and urges to smoke were administered during the 10-minute telephone interview.
An additional follow-up was conducted 10-weeks post-quit but will not be analyzed here. Those who reported any smoking between days 21 and 28 post-quit were randomized to a lapse recovery intervention and either given advice to stay engaged in quitting or sent a six-week supply of very-low-nicotine-content cigarettes to smoke in place of conventional cigarettes for the next six weeks. In light of this re-randomization, it is not appropriate to compare 10-week abstinence rates solely as a function of withdrawal exposure condition.
Compensation
Participants could earn up to $465 in compensation for devoting up to 17 hours to study activities (excluding travel to and from office visits) over 12.5 weeks. This corresponds to a maximum of $27.35 per hour. Participants were paid $40 for the two-hour orientation, $10 for the IVR feedback call, $20 per day for the pre-quit wake-up and live survey calls, $50 for the one-week post-quit office visit, $10 for each follow-up interview, and $20 for a follow-up visit. A $50 bonus was offered for completion of at least 80% of scheduled IVR calls, plus $0.10 per minute for each minute of personal cell phone time used for study activities. Fifty dollars were deducted from compensation if participants failed to return a study cell phone. On average, participants earned $291.17 (SD=$128.83, Range=$0-$450.10) in compensation.
Measures
Demographics
Participants completed a pen-and-paper questionnaire assessing age, gender, self-identified race, ethnicity, educational attainment, marital status, income, and employment status at orientation.
Smoking, Quitting, and Health History
At baseline, participants provided information about the number of years they smoked, number of past quit attempts, longest duration of past abstinence from tobacco, and current motivation and confidence regarding quitting. Participants also rated their self-assessed health (Idler & Benyamini, 1997).
Fagerström Test of Cigarette Dependence (FTCD)
This is a widely-used six-item self-report measure of physical dependence on cigarettes (Fagerström, 2012; Heatheron, Koslowksi, Frecker, & Fagerström, 1991) scored on a scale from 0 to 10, with higher scores indicating greater dependence. The FTCD was administered at orientation. The FTCD has moderate internal consistency in general (Cronbach’s α = .61; Heatherton et al, 1991) but had very low internal consistency in this sample (Cronbach’s α = .29).
Wisconsin Inventory of Smoking Dependence Motives (WISDM-37; Smith et al., 2010)
This 37-item scale administered at orientation assesses cigarette dependence by measuring 11 domains of smoking dependence motives (such as craving or taste and sensory properties of cigarette smoke). Prior analyses have identified primary and secondary motives tapped by this measure. The primary dependence motives reflect automaticity in smoking, loss of control over smoking, cigarette craving, and nicotine tolerance. Secondary motives include less central motives, such as smoking for weight control or cognitive or affective enhancement. Internal consistency was adequate for both the primary (Cronbach’s α = .73) and secondary motive scales (Cronbach’s α = .67) in the current sample.
Depressive Symptoms
The Center for Epidemiologic Studies-Depression (CES-D) scale (Radloff, 1977) is a 20-item scale administered at orientation. Total scores range from 0 to 60, with scores greater than or equal to 16 indicating clinical levels of depressive symptoms. Internal consistency was excellent for the current sample (Cronbach’s α = .91). The CES-D was administered to monitor changes in participants’ affective distress to trigger further assessment and referral for mental health services, when appropriate.
Treatment Adherence
Participants in both conditions were asked how many cigarettes they had smoked since going to bed the night before, when they smoked their most recent cigarette, and what the longest period they went without smoking was at each of the seven live survey calls conducted at the end of the practice quitting or self-monitoring periods pre-quit. These data were used to code adherence to the treatment manipulation (i.e., smoking instructions).
Smoking Cessation Outcomes
Daily tobacco use was assessed using a timeline follow-back calendar method in which participants were asked at each study contact through follow-up to report on their tobacco, alcohol, and stop-smoking treatment use for each day since the last study contact (Brown et al., 1998). During the peri-cessation period (two weeks pre- and one week post-quit) participants also reported recent smoking at each IVR call.
The primary outcome in the current study is complete self-reported seven-day point-prevalence abstinence between days 21 and 28 of the quit attempt. This four-week abstinence outcome was based on participant self-report and was not biochemically verified. This decision was informed by the general consistency between self-reported and biochemically verified abstinence (SRNT Subcommittee on Biochemical Verification, 2002), the relatively low level of face-to-face contact with treatment staff, and the fact that participants were informed at enrollment that they would be eligible for randomization to additional treatment with very-low-nicotine-content cigarettes if they reported smoking at the four-week follow-up. These factors reduce the likelihood of abstinence over-reporting (SRNT Subcommittee on Biochemical Verification, 2002).
Secondary outcomes included duration of abstinence during the pre-quit practice quitting sessions, as a manipulation check. Maximum abstinence duration (coded in minutes) was assessed via interview at live survey telephone calls every other day in the two weeks preceding the target quit day. In addition, we assessed latency to key cessation milestones (Shiffman, Scharf, et al., 2006) including initial cessation (abstaining for at least one full calendar day during the first two weeks of the scheduled quit attempt), first lapse (a first puff following cessation), and relapse (a return to smoking seven days in a row following a first lapse). Cessation, lapse, and relapse latencies in the first week post-quit were coded based on both IVR and retrospective smoking calendar data. Events occurring after the end of IVR recording one week post-quit-day were coded based on smoking calendar data only.
Analysis Plan
We ran descriptive analyses characterizing the sample and checking for baseline differences in the two treatment conditions. We tested treatment effects on the primary four-week abstinence outcome using logistic regression. Secondary mediation analyses were conducted in Mplus 7.2 (Muthen & Muthen, 1998–2015) by looking at indirect effects of treatment on four-week abstinence through abstinence duration mean and slope variables across the seven pre-quit practice quitting PQ (or ST control) sessions. Survival analyses of cessation milestones were conducted using IBM SPSS Statistics 20.0 software (IBM, Corp., Armonk, NY).
Results
Sample Characteristics
Sample characteristics are shown in Table 2. The sample was fairly evenly split in terms of gender. Most participants identified as White although a substantial proportion identified as African American. Income levels were modest and a small proportion of participants were college graduates. The sample was middle-aged, on average (M=47.13, SD=14.27). Smoking history and dependence variables suggest the sample smoked heavily and was at least moderately dependent on tobacco. The treatment conditions were well balanced in terms of baseline characteristics, except that those in the standard-treatment condition had significantly higher depressive symptom ratings than did those in the practice quitting condition. Given that CES-D scores were not related to any abstinence outcome and that we did not specify CES-D as an a priori covariate, we will present results unadjusted for baseline CES-D scores. Including prognostic covariates can enhance estimates of treatment effects in binary logistic and Cox regression survival models (Hauck, Anderson, & Marcus, 1998), but in this case CES-D scores were not prognostic of any outcome (all ps>0.36) and so results were not adjusted for CES-D scores.
Table 2.
Demographic Characteristics of the Analytical Sample (N=93).
| Standard Treatment (n=47) | Practice Quitting (n=46) | χ2(N=93) (phi) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | |||
| Gender | Male | 23 | 48.9 | 22 | 47.8 | 0.01 (0.01) |
| Female | 24 | 51.1 | 24 | 52.2 | ||
| Race | White | 32 | 68.1 | 29 | 63.0 | 1.42 (0.12) |
| African American | 11 | 23.4 | 15 | 32.6 | ||
| Other race | 4 | 8.5 | 2 | 4.3 | ||
| Ethnicity | Hispanic | 3 | 6.4 | 2 | 4.3 | 0.19 (0.05) |
| Marital status | Married | 15 | 31.9 | 15 | 32.6 | 7.76 (0.27) |
| Never married | 16 | 34.0 | 9 | 19.6 | ||
| Separated, divorced, widowed | 16 | 34.0 | 17 | 37.0 | ||
| Cohabitating, unmarried | 0 | 0 | 5 | 10.9 | ||
| Annual Household Income | Less than $20,000 | 14 | 29.8 | 8 | 24.6 | 3.84 (0.20) |
| $20,000 to $49,999 | 15 | 31.9 | 14 | 25.4 | ||
| $50,000 to $99,999 | 8 | 17.0 | 15 | 20.6 | ||
| $100,000 or more | 10 | 21.3 | 9 | 27.0 | ||
| Education | High school or less | 17 | 36.2 | 13 | 28.3 | 1.84 (0.14) |
| Some college | 20 | 42.6 | 26 | 56.5 | ||
| College graduate | 10 | 21.3 | 7 | 15.2 | ||
| Standard Treatment | Practice Quitting | t(91) | |||
|---|---|---|---|---|---|
| M | SD | M | SD | (Cohen’s d) | |
| Age in years | 47.13 | 14.27 | 48.41 | 10.36 | −0.50 (0.10) |
| Years smoked | 28.11 | 13.35 | 28.76 | 11.23 | −0.26 (0.05) |
| Smoking heaviness index | 3.45 | 0.97 | 3.43 | 1.03 | 0.06 (0.01) |
| Number of past quit attempts | 4.02 | 3.47 | 5.46 | 14.81 | −0.64 (0.13) |
| Longest duration of past abstinencea | 3.43 | 2.27 | 3.24 | 2.72 | 0.36 (0.07) |
| Time since last quit attempt (months) | 40.11 | 92.18 | 56.63 | 71.94 | −0.92 (0.20) |
| FTCD | 5.74 | 1.54 | 5.70 | 1.58 | 0.15 (0.03) |
| WISDM-37 Primary Smoking Motives | 5.43 | 1.05 | 5.21 | 1.04 | 1.03 (0.21) |
| WISDM-37 Secondary Smoking Motives | 4.02 | 1.09 | 3.67 | 0.81 | 1.75 (0.36) |
| Baseline CO | 21.49 | 12.08 | 21.72 | 8.06 | −0.11 (0.02) |
| Baseline inter-cigarette interval (minutes) | 131.70 | 79.43 | 119.24 | 66.11 | 0.82 (0.17) |
| Self-assessed healthb | 2.96 | 0.96 | 3.20 | 0.88 | −1.28 (0.27) |
| CES-D | 17.21 | 11.36 | 12.26 | 9.68 | 2.26* (0.46) |
| Motivation to quit smokingc | 4.77 | 0.58 | 4.72 | 0.54 | 0.48 (0.10) |
| Willingness to work hard at quittingc | 4.89 | 0.31 | 4.89 | 0.32 | 0.04 (0.01) |
| Confident could quit for 24 hoursc | 3.53 | 1.23 | 3.50 | 1.23 | 0.13 (0.03) |
| Confident could quit for goodc | 3.81 | 0.90 | 3.63 | 1.00 | 0.91 (0.19) |
p<0.05.
Assessed on an ordinal scale where 1= <1 day, 2=1–7 days, 3=8–14 days, 4=15–30 days, 5=1–3 months, 6=3–6 months, 7=6–12 months, and 8= >1year abstinent.
Participants were asked to rate their general health on a scale from 1 to 5 where 1=poor, 2=fair, 3=good, 4=very good, and 5=excellent.
Rated from 1=not at all to 5=extremely.
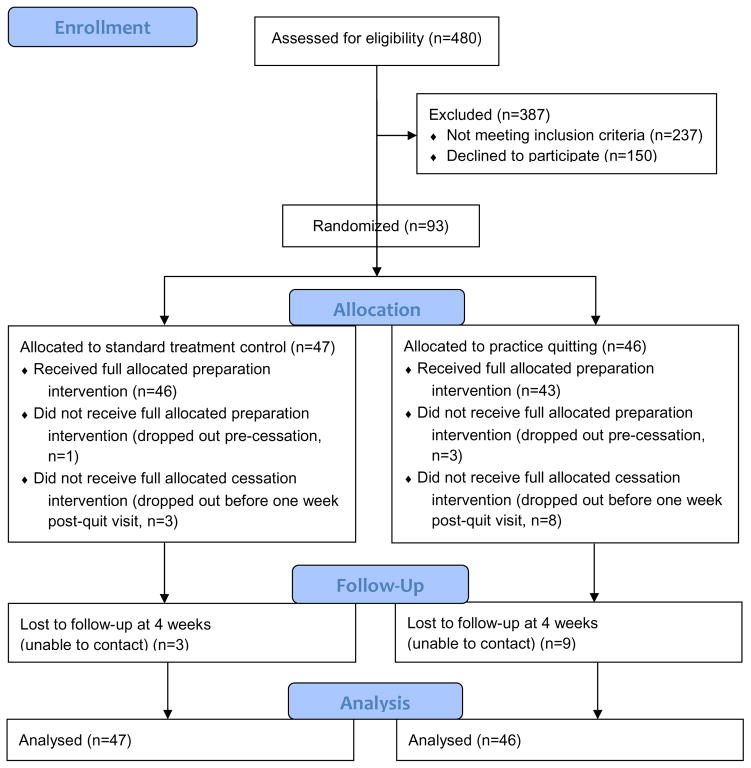
Attrition
Retention in the quit preparation phase was excellent in both conditions (see Figure 1 flow diagram), with only one ST participant (2.1%) lost prior to the target quit day and only three (6.5%) lost in the PQ condition. An additional two participants (4.3%) in the ST condition and one participant (2.2%) in the PQ condition were lost in the cessation phase that ended one week post-quit, and thus did not receive the full dose of cessation counseling. Three ST participants (6.4%) and nine PQ participants (19.6%) were not reached for the four-week follow-up. This varied by gender. Four-week follow-up loss rates were low and roughly equal across conditions for women, with two (8.3%) lost in the ST condition and one (4.2%) lost in the PQ condition, χ2(N=48)=0.36, p=.55. Among men, however, significantly more in the PQ group (n=7, 31.8%) than in the ST (n=1, 4.3%) group were lost to follow-up,χ2(N=45)=4.50, p=.03. Four of the seven men lost to follow-up in the PQ condition were lost prior to the quit date. Thus, gender was examined as a potential moderator in analyses of treatment outcome.
Figure 1.
CONSORT 2010 Flow Diagram.
Treatment Adherence
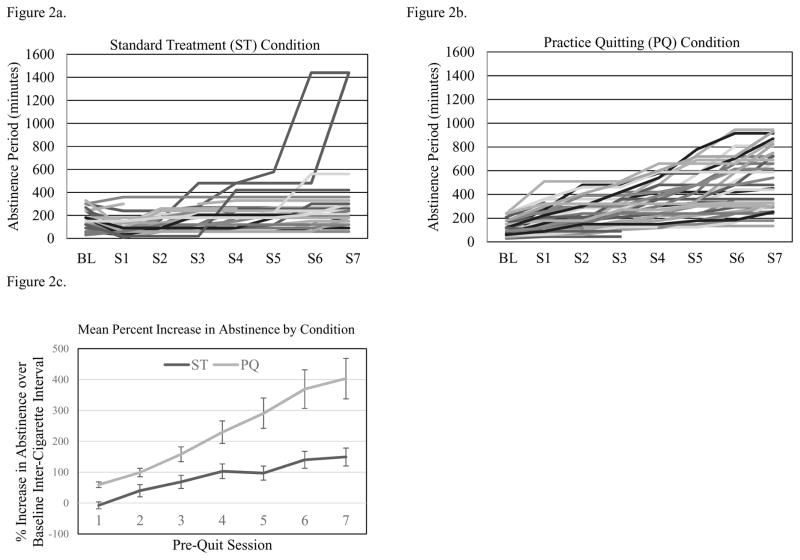
Plots of abstinence duration during the seven pre-quit ST or PQ sessions are shown for every participant in each condition in Figures 2a and 2b. Mean increase in abstinence duration from baseline inter-cigarette interval is shown by condition in Figure 2c. Two participants in the ST condition quit prior to the target quit day and others showed slight increases in abstinence duration across pre-quit monitoring sessions. Both conditions showed increases in abstinence duration across pre-quit sessions, but this was significantly greater in the PQ condition than in the ST condition.
Figure 2.
Abstinence duration (minutes) for individual subjects in the control and practice quitting conditions.
Adherence to specific instructions was less optimal, with a high of 58.7% of participants achieving target abstinence periods (occurring at the first practice quitting call, when the average prescribed abstinence duration was 178.86 minutes, SD=99.16) and a low of 30.4% achieving the target duration at later practice quitting sessions (when abstinence targets grew by as much as four hours between sessions). An even smaller proportion (ranging from a low of 14.6% to a high of 26.2%) reported abstinence from the night before to the end of the target waking period at each practice session, as most participants did not refrain from smoking between bedtime and the scheduled wake-up call (indicating that participants typically awoke prior to the wake-up time confirmed at the previous contact). Despite this, participants in the practice quitting condition increased the duration of abstinence over the previous maximum by at least 22% on average through the fourth practice session, and then by diminishing amounts (16.9%, 17.8%, and 7.6% over the last three sessions, when abstinence duration increments were truncated among the most successful practice quitters so as not to exceed a full waking day).
Logistic Regression Model
In intent-to-treat (ITT) analyses in which all missing values were coded as smoking, 34.4% of the full sample of 93 subjects reported no smoking in the fourth week of the quit attempt. This did not vary significantly by treatment condition; 31.9% of standard treatment and 37.0% of practice quitting participants achieved seven-day abstinence four weeks post-quit in this intent-to-treat analysis. In a binary logistic regression analysis predicting four-week abstinence, practice quitting was associated with a modest and non-significant 1.25 odds ratio (B=.22, SE=.44, 95% CI=0.53, 2.95) compared to standard treatment. At this magnitude of effect, we would need to treat 20 people with the practice quitting intervention to get one more quitter at four weeks than we would get in the standard-treatment condition.
We also examined treatment effects on abstinence when missing abstinence values were imputed. We imputed 10 datasets using all baseline variables and pre-quit abstinence duration data as predictors of missing abstinence values. In the pooled analysis of the imputed datasets, treatment condition remained weakly and non-significantly related to four-week point-prevalence abstinence (B=.41, SE=.45, OR=1.5, 95% CI=.62, 3.64).
In both ITT and imputed analyses, gender did not significantly moderate these results (ITT: B=.71, SE=.88, p=.42; imputed: B=.28, SE=.97, p=.78). Gender was also unrelated to abstinence rates (ITT: B=−.66, SE=.64, p=.30; imputed: B=−.56, SE=.64, p=.38).
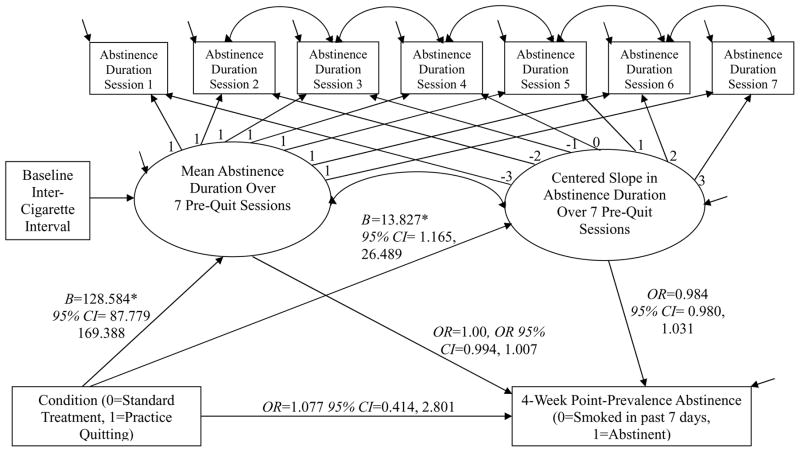
Mediation Model
Despite the lack of significant effects of treatment condition on four-week abstinence, we conducted a mediation analysis. This was done in part to verify that the treatment manipulation worked, and also to estimate associations between abstinence-duration increases and subsequent point-prevalence abstinence. The results of the excellent-fitting mediation model using full maximum likelihood estimation are shown in Figure 3. The treatment manipulation induced a significant two-hour increase on average in mean abstinence duration in PQ vs. ST across the seven pre-quit sessions. The practice quitting manipulation also induced a modest but significant 14-minute average increase in abstinence duration per session, relative to the ST control condition and uncorrected for attrition. These increases were not significantly associated with four-week point-prevalence abstinence, however. Neither the indirect effects nor the residual direct effect of treatment on abstinence were statistically significant in this model.
Figure 3.
Mediation model of practice quitting effects on 4-week point-prevalence abstinence. Unconditional model χ2(24)=25.250, p=0.392, RMSEA=0.024, CFI=.998 Indirect effect of condition on abstinence through mean abstinence duration=0.032, 95% CI= −0.835, 0.899 Indirect effect of condition on abstinence through abstinence slope=0.103, 95% CI= −0.234, 0.439 * p<.05
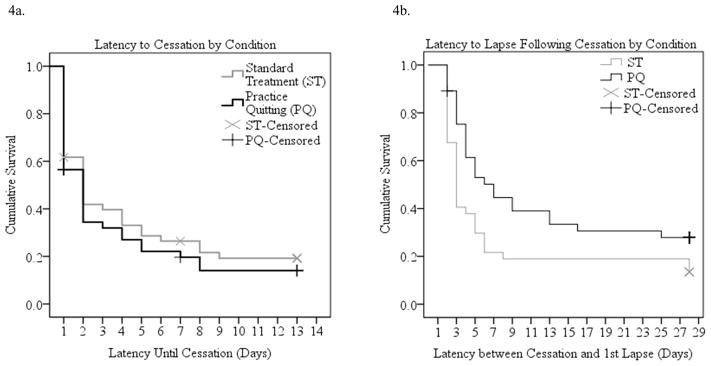
Survival Analyses
Initial cessation was achieved by 37 participants in each group (78.7% in the ST control group, 80.4% in the PQ group). Median time to cessation was two days in both the PQ and ST control conditions and the differences in survival curves were not significant (Mantel-Cox Log-Rank Chi-Square=.582, p=.445 in Kaplan Meier survival analysis) (Figure 4a). The odds of achieving cessation were not significantly greater in the PQ condition than in the ST control condition in a Cox regression analysis (OR=1.16, 95% CI=.73, 1.83). Gender was not related to cessation (OR=.84, 95% CI=.44, 1.60) and did not moderate treatment effects on cessation latency (OR=1.45, 95% CI=.58, 3.63).
Figure 4.
Cessation milestones by condition.
Among those who abstained for at least one day, 32 (86.5%) of the 37 ST control participants and 26 (70.3%) of the 37 PQ participants lapsed within the first month of the quit attempt. Median latency to a first lapse after quitting was three days (95% CI=2.58, 5.42) in the ST control group and seven days (95% CI=1.81, 16.19) in the PQ group. The survival curves were significantly different in the two groups (Mantel Cox Log Rank Chi-Square=5.078, p=.024, Figure 4b). In a Cox regression, practice quitting reduced the odds of a first lapse significantly (OR=0.58, 95% CI=0.35, 0.98). Gender was unrelated to lapse (OR=1.22, 95% CI=.61, 2.44) and did not moderate treatment effects (OR=1.08, 95% CI=.38, 3.09).
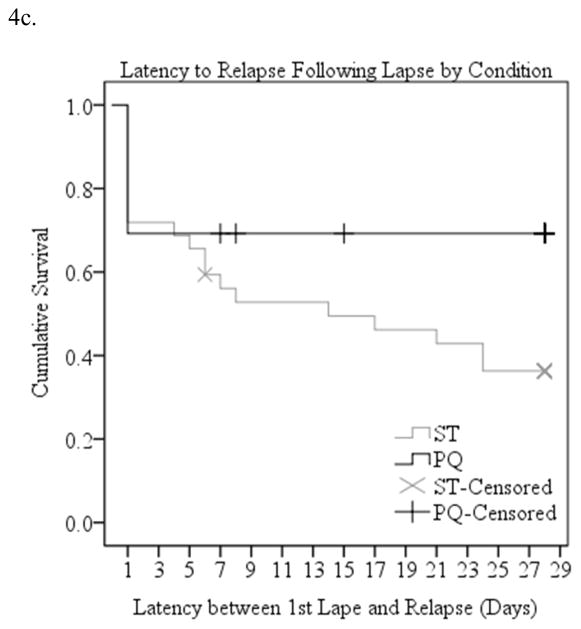
Twenty (62.5%) of the 32 ST control participants and eight (30.8%) of the 26 PQ participants who lapsed ultimately relapsed to daily smoking for a week within the first month of the quit attempt. Median latency to relapse following a first lapse was 14 days in the standard treatment condition and could not be computed in the practice quitting group because there were so few relapses. The shape of the survival distributions differed significantly in the two conditions (Mantel Cox Log Rank Chi-Square=4.40, p=.036, Figure 4c). In a Cox regression analysis, there was a marginal reduction in the odds of relapse in the practice quitting condition (OR=0.46, 95% CI=0.20, 1.04, p=.063). Gender was unrelated to relapse (OR=.84, 95% CI=.44, 1.60) and did not interact with treatment (OR=1.45, 95% CI=.58, 3.27).
Discussion
The current randomized clinical trial tested the efficacy of a tailored behavioral adjuvant smoking cessation intervention. The two-arm trial tested the additive value of asking smokers to practice quitting for progressively longer periods in the weeks leading up to an assisted quit attempt. All smokers received standard counseling and nicotine replacement therapy and were instructed to monitor pre-quit smoking closely for two weeks. Results suggested that asking smokers to practice abstinence prior to a target quit day is feasible, acceptable to smokers, and efficacious in preventing first lapses and relapse.
The current findings support the feasibility and acceptability of a withdrawal-exposure intervention for smoking cessation, but also point to the need for refinement of abstinence targets and timing. Roughly 38% of eligible smokers enrolled in the study despite its demands and retention in the pre-quit preparation phase of the trial was excellent (greater than 93%), despite the challenging nature of the intervention which encouraged increasing abstinence duration above and beyond a prior personal best, without nicotine replacement. On average, retained smokers in the practice quitting condition increased the maximal interval between cigarettes fivefold over the course of seven practice quitting sessions. Most participants in this condition exhibited growth in abstinence duration in the practice quitting period. The tailored abstinence targets set for participants appeared to be too ambitious, however, as only 30–59% of participants abstained for the prescribed period at each session. The instruction to abstain from morning smoking also appears to have been very difficult for participants to follow, with no more than a quarter of participants succeeding in extending overnight abstinence by the prescribed amount.
Refraining from smoking the first cigarette of the day is particularly important because this cigarette is thought to be the most reinforcing and the inability to delay morning smoking is a robust indicator of nicotine dependence (Baker et al., 2007; Muscat, Stellman, Caraballo, & Richie, 2009). Interoceptive signals of withdrawal and craving may be strongest after overnight abstinence and refraining from smoking in this context may be particularly helpful in weakening the stimulus-response associations that help maintain smoking behavior (McCarthy et al., 2011). Although participants in the practice quitting condition quintupled their periods of abstinence on average, they may not have done this at the optimal time and this may have reduced the potency of practice quitting. Finding ways to improve adherence to this potentially important component of the intervention may enhance the efficacy of the practice quitting intervention.
Practice quitting had a modest (NNT=20) and non-significant effect on the primary outcome, seven-day point-prevalence self-reported abstinence four weeks post-quit, and did not seem to improve initial cessation rates, relative to standard treatment. Smokers in the practice quitting group maintained initial abstinence prior to lapsing roughly twice as long (median survival seven days) compared to the standard treatment group (median three days) and had significantly reduced odds of lapsing, however. In addition, practice quitting decreased full-blown relapse post-lapse, relative to standard treatment. Of those who lapsed, 30.8% progressed to relapse in the practice quitting condition, while twice as many (62.5%) relapsed in the standard treatment condition. Thus, although the effects of the intervention on the primary four-week abstinence outcome were modest and non-significant, practice quitting appeared to delay first lapses and to prevent returns to daily smoking within the first four weeks of an attempt to quit permanently. These effects are impressive, given the limited schedule of practice quitting used in this study (seven sessions over two weeks) and the extensive learning histories (an average of 28 years smoking) we hoped to inhibit through extinction learning. Relapse is likely to be a problem, however, as it is in extinction-based anxiety treatments (Vervliet et al., 2013), and it is therefore essential to conduct longer-term follow-ups in future studies.
Refinement of the protocol may enhance the efficacy of the intervention. For example, improving adherence to abstinence prescriptions during practice quit periods may enhance efficacy. In addition, moderation analyses may help identify subgroups of smokers particularly responsive to practice quitting. There was considerable heterogeneity in the pattern and slope of practice quitting abstinence in this sample. Identifying individual differences associated with these patterns may foster matching to this adjunctive treatment in future trials. Additional research is also needed to explore mediators of practice quitting effects on lapse and relapse risk, such as enhanced distress- or craving-coping skills, improved self-efficacy, or habituation to withdrawal or craving. Investigation of these mechanisms of change may suggest ways to enhance the intervention. Even with suboptimal adherence, practice quitting was associated with reduced lapse and relapse risk in this study. If we can refine the intervention to be even more acceptable to smokers (so that a higher proportion accept and adhere to the treatment) and to be delivered remotely via quitline or website, this approach may be a clinically useful adjunct to standard treatments with broad reach.
Limitations
The findings should be considered in light of the following limitations. First, the sample comprised adult treatment-seeking smokers who met inclusion criteria and were willing to participate in a randomized controlled trial involving intensive IVR assessment and offering substantial compensation. Our results may not generalize to other samples or contexts, particularly given that only 19% of individuals screened were eligible and enrolled in the study. We collected limited information about the physical and mental health status of participants, so the degree to which results will generalize to medically or mentally ill smokers is unknown. In addition, neither participants nor researchers were blind to condition assignment, so it is not possible to rule out expectancy or bias as explanations for results. An adjunctive practice quitting intervention would necessarily be transparent to smokers in clinical practice, however, and positive expectancies may augment this the low-risk, low-cost, and potentially highly disseminable adjunctive intervention. In addition, the tailoring algorithm used was not successful, as indicated by the low rates of adherence to prescribed periods of abstinence. Tailoring prescribed abstinence based on earlier smoking behavior also introduces variability in the dose of the intervention developed. The aim of tailoring was to set targets that would be more achievable for individual smokers. Although we missed this mark, we note that most smokers in the practice quitting condition were able to extend abstinence, albeit by different amounts and at different rates. Another limitation was that all abstinence outcomes were based on self-report rather than biochemical verification. Future research could use low-cost carbon monoxide testing with web-chat verification of smoking status without substantially increasing participant burden. This, along with relaxation of inclusion criteria, may enhance the reach of the practice quitting intervention. Future research should also track longer-term outcomes and assess treatment efficacy at 6- or 12-months post-quit to be included in future meta-analyses.
Conclusions
A smoking cessation preparation intervention designed to train smokers for abstinence had modest, non-significant effects on point-prevalence abstinence, but had promising effects on lapse and relapse processes above and beyond standard smoking cessation treatment. Practice quitting is a feasible and acceptable intervention that may help smokers delay or prevent lapses and relapses during assisted smoking cessation attempts. This behavioral intervention rooted in learning theory has the potential to develop into a useful and highly disseminable adjunct to standard treatment.
Highlights.
Acceptance of a behavioral “practice quitting” smoking cessation preparation intervention was excellent
Asking smokers to practice quitting for progressively longer periods of time results in improved abstinence duration
Practice quitting had a modest, non-significant effect on point-prevalence abstinence
In smokers who quit, practice quitting improved survival prior to a first lapse and prevented progression from lapse to relapse
Acknowledgments
This work was supported by the National Institute on Drug Abuse Grant 1R21DA026511-01A1 awarded to Dr. McCarthy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
The authors wish to thank the staff of the Rutgers Smoking Cessation Laboratory and the Institute for Health, Health Care Policy and Aging Research, and Timothy B. Baker, Ph.D., Saul Shiffman, Ph.D., and Maxine Stitzer, Ph.D., for their contributions to this research.
All authors contributed significantly to the design, implementation, analysis, and reporting of this research. All authors have read and approved the final manuscript.
Footnotes
Disclosures
Dr. McCarthy has received nicotine replacement therapy at discounted rates from GlaxoSmithKline in the past. GlaxoSmithKline played no role in the current study. None of the other authors have conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Danielle E. McCarthy, Email: demccart@rci.rutgers.edu.
Krysten W. Bold, Email: krysten.bold@yale.edu.
Haruka Minami, Email: hminami@fordham.edu.
Vivian M. Yeh, Email: vivyeh@deloitte.com.
References
- Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The tobacco dependence treatment handbook: A guide to best practices. New York, NY: Guilford Press; 2003. [Google Scholar]
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine and Tobacco Research. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, … Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine and Tobacco Research. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive behavioral therapy, imipramine, or their combination for panic disorder. JAMA. 2000;283:2592–2450. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. doi: http://dx.doi.org/10.1016/0306-4603(90)90013-N. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. doi: http://dx.doi.org/10.1037/0893-164X.12.2.101. [Google Scholar]
- Brown RA, Palm KA, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, … Gifford EV. Distress tolerance treatment among early-lapse smokers: Rationale, program description, and preliminary findings. Behavior Modification. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews. 2013;(5) doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting Smoking Among Adults—United States, 2001–2010. Morbidity and Mortality Weekly Report. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Craske MG, Mystkowski JL. Exposure therapy and extinction: Clinical studies. In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and learning: From basic processes to clinical implications. Washington, DC, US: American Psychological Association; 2006. pp. 217–233. http://dx.doi.org/10.1037/11474-011. [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Smith RC, Monson CM, Zvolensky MJ. Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent multiple-baseline design. Behavior Therapy. 2013;44:514–528. doi: 10.1016/j.beth.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, … Wewers ME. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. Retrieved from http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, … Tu X. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. http://dx.doi.org/10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Hauck WW, Anderson S, Marcus SM. Should we adjust for covariates in nonlinear regression analyses of randomized trials? Controlled Clinical Trials. 1998;19:249–256. doi: 10.1016/S0197-2456(97)00147-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hyland A, Borland R, Li Q, Yong H, McNeill A, Fong GT, … Cummings KM. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15:iii83–iii94. doi: 10.1136/tc.2005.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of 27 community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- McCarthy DE, Baker TB, Minami HM, Yeh VM. Applications of contemporary learning theory in the treatment of drug abuse. Invited chapter. In: Schachtman TR, Reilly S, editors. Associative Learning and Conditioning Theory: Human and Non-Human Applications. New York: Oxford University Press; 2011. pp. 235–269. [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Thomas C. Mechanisms of change in exposure therapy for anxiety disorders. In: Dagleish T, Power MJ, editors. Handbook of Cognition and Emotion. New York: John Wiley and Sons; 1999. pp. 747–764. [Google Scholar]
- Muscat JE, Stellman SD, Caraballo RS, Richie JP. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- NICE. Common Mental Health Disorders: Identification and Pathways to Care. NICE Care Guideline. 2011:123. Available at www.nice.org.uk/CG123 [NICE guideline]
- Otto MW, O’Cleirigh CM, Pollack MH. Attending to emotional cues for drug abuse: Bridging the gap between clinic and home behaviors. Science & Practice Perspectives. 2007;3:48–55. doi: 10.1151/spp073248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Powers MB, Fischmann D. Emotional exposure in the treatment of substance use disorders: conceptual model, evidence, and future directions. Clinical Psychology Review. 2005;25:824–839. doi: 10.1016/j.cpr.2005.05.002. doi: http://dx.doi.org/10.1016/j.cpr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Otto MW, Safren SA, Pollack MH. Internal cue exposure and the treatment of substance use disorders: Lessons from the treatment of panic disorder. Journal of Anxiety Disorders. 2004;18:69–87. doi: 10.1016/janxdis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, … Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Penava SA, Bolton E, Worthington JJ, III, Allen GL, Farach FJ, Jr, Otto MW. A novel cognitive-behavioral approach for treatment-resistant drug dependence. Journal of Substance Abuse Treatment. 2002;23:335–342. doi: 10.1016/s0740-5472(02)00298-2. doi: http://dx.doi.org/10.1016/S0740-5472(02)00298-2. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, … Balabanis Natural history of nicotine withdrawal. Addiction. 2006;101:1822–1832. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CG, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74(2):276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, Baker TB. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine and Tobacco Research. 2010;12:489–499. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2013;(8):Art. No.:CD002850. doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of smoking: A report of the surgeon general. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- U.S. Public Health Service. Consumer Guide. U.S. Public Health Service; 2000. You Can Quit Smoking. http://www.surgeongeneral.gov/tobacco/quits.htm. [Google Scholar]
- Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: A systematic review. Addiction. 2011;106:2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: State of the art. Annual Review of Clinical Psychology. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacol,ogy. 1999;7:354–361. doi: 10.1037/1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome in an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. doi: http://dx.doi.org/10.1017/S0033291700005705. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors. 2009;34:365–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zwolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakshaie J. An anxiety sensitivity reduction smoking-cessation program for Spanish-speaking smokers (Argentina) Cognitive and Behavioral Practice. 2011;21:350–363. doi:1077-7229/13/350-36331.00/0. [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008;22:346–365. doi: 10.1891/0889-8391.22.4.346. [DOI] [Google Scholar]