Abstract
Background
Although cognitive deficits in patients with schizophrenia are rooted early in development, the impact of psychosis on the course of cognitive functioning remains unclear. In this study a nested case-control design was used to examine the relationship between emerging psychosis and the course of cognition in individuals ascertained as clinical high-risk (CHR) who developed psychosis during the study (CHR+T).
Method
Fifteen CHR+T subjects were administered a neurocognitive battery at baseline and post-psychosis onset (8.04 months, S.D. = 10.26). CHR+T subjects were matched on a case-by-case basis on age, gender, and time to retest with a group of healthy comparison subjects (CNTL, n = 15) and two groups of CHR subjects that did not transition: (1) subjects matched on medication treatment (i.e. antipsychotics and antidepressants) at both baseline and retesting (Meds-matched CHR+NT, n = 15); (2) subjects unmedicated at both assessments (Meds-free CHR+NT, n = 15).
Results
At baseline, CHR+T subjects showed large global neurocognitive and intellectual impairments, along with specific impairments in processing speed, verbal memory, sustained attention, and executive function. These impairments persisted after psychosis onset and did not further deteriorate. In contrast, CHR+NT subjects demonstrated stable mild to no impairments in neurocognitive and intellectual performance, independent of medication treatment.
Conclusions
Cognition appears to be impaired prior to the emergence of psychotic symptoms, with no further deterioration associated with the onset of psychosis. Cognitive deficits represent trait risk markers, as opposed to state markers of disease status and may therefore serve as possible predictors of schizophrenia prior to the onset of the full illness.
Keywords: Clinical high risk, linear mixed-effects models, nested case-control study, neurocognition, prodromal, psychosis
Introduction
Cognitive deficits have long been considered core features of schizophrenia and other psychotic disorders (Elvevag & Goldberg, 2000; Green et al. 2004; Keefe & Harvey, 2012). Deficits in processing speed and verbal memory, for example, pervasively endure throughout the lifespan and are major contributors of the profound disability that is associated with the illness (Green & Harvey, 2014). However, the role of impaired cognition in the onset of psychosis is not yet fully understood and is an issue of central importance in the possible prevention of illness. A key unresolved etiological question is whether cognitive deficits represent long-standing traits that are part of the lifelong vulnerability to schizophrenia or, alternately, whether the emergence of psychotic symptoms causes a noticeable drop in cognitive functioning (McGlashan, 2006). Although considerable data suggest that impaired cognition is in fact neurodevelopmental in nature (Cornblatt et al. 1999; Zipursky et al. 2013; Bora, 2015) with problems detectable early in life (Cannon et al. 2000), there is a persistent view in the literature that cognition follows a neurodegenerative course through the progression of psychotic illness (Bilder et al. 1992; Gold, 1998), Since neurocognition provides a window into brain functioning, understanding the course of cognitive functioning in schizophrenia may provide an opportunity of reducing risk for later psychosis (Cornblatt et al. 2003; Lencz et al. 2006; Pukrop et al. 2007; Michel et al. 2014). Therefore, the goal of the current report was to prospectively examine the course of neurocognition before and after the transition to psychosis in a group of individuals initially ascertained as clinical high-risk (CHR, i.e. putative prodrome to psychosis).
Although it is well-documented that cognitive deficits are rooted early in neurodevelopment (Cannon et al. 2000; Fusar-Poli et al. 2012a), earlier cross-sectional studies with patients with first-episode psychosis (FEP) found less severe neuropsychological deficits compared to chronically ill patients (Schwartzman & Douglas, 1962; Bilder et al. 1992), suggesting that deterioration may have occurred after the onset of psychotic symptoms. The cross-sectional design, however, makes it difficult to tease apart true progressive changes after illness onset, as the recruitment of chronic patients may be biased toward participants with poorer neurocognition (Keshavan et al. 2005). Similarly, older patients with a chronic course of schizophrenia are more likely to be recruited from services that provide ongoing treatments for poor outcomes and disability. More recent cohort studies have found evidence of altered neurocognitive trajectories in individuals that developed schizophrenia relative to those who did not develop the schizophrenia (Reichenberg et al. 2002, 2010; Caspi et al. 2003; Meier et al. 2014). Meier et al. (2014) for example, demonstrated a decline in cognitive performance in individuals who developed schizophrenia repeatedly tested from childhood through adulthood (at age 38) after illness onset.
While these findings suggest neurocognitive deterioration over the course of illness progression, determining the exact timing of the decline is relatively difficult. Cognitive decline could have occurred during the prodromal period, during the first episode, or after the onset of psychosis (Seidman et al. 2006; Bora, 2014). In addition, inconsistent neuropsychological test batteries over the course of a longitudinal study further complicates determining whether or not the onset of psychosis, per se, causes further deterioration in cognitive performance (Bora, 2014). Moreover, the impact of the onset and development of psychosis, in and of itself, on the course of cognition in the earliest stages of the illness remains unclear.
Looking to overcome these problems and address the specific issue of whether the onset of psychosis causes further deterioration in cognition, recent studies have prospectively followed individuals that are earlier in the course of illness and are at CHR for developing psychosis. These adolescents and young adults with increasing attenuated positive symptoms are in a critical phase of illness progression and developmental brain maturation processes that may contribute to the pathogenesis of the illness. To date, several CHR studies (Keefe et al. 2006; Wood et al. 2007; Hawkins et al. 2008; Becker et al. 2010; Jahshan et al. 2010; Woodberry et al. 2013) have examined the course of cognitive functioning in individuals transitioning to psychotic disorders (i.e. converters) during prospective follow-up. For example, Wood et al. (2007) found that individuals who progressed to full-blown psychosis showed a decline over the follow-up period on measures of visual memory and attentional set-shifting. Similarly, Woodberry et al. (2013) also found a progressive impairment in verbal memory in those who transitioned to psychosis. In contrast, Becker et al. (2010) reported no deterioration after the onset of psychosis, with large and stable impairments for converters in verbal memory and processing speed.
These inconsistencies may be related to several factors. First, many studies do not account for certain factors that are known to impact the neurodevelopment trajectory of the illness, like gender (Walder et al. 2013) and age (Glahn et al. 2013). Second, the duration of time between assessments could partially explain differences between those who develop psychosis and those who did not (Becker et al. 2010). Participants not developing psychosis during the study are tested at regular and pre-determined intervals (e.g. 12 and 24 months). In contrast, the development of psychosis and the post-psychosis neurocognitive assessment can occur at any time between regularly scheduled assessments resulting in different practice effects. Lastly, the impact of medication treatment with antipsychotics and antidepressants on cognition in CHR subjects who develop psychosis is unclear. This last issue is an important point of consideration given that previous work from our group (Bowie et al. 2012) found that antipsychotic treatment was associated with worse neurocognitive performance in high-risk subjects over a short amount of time. Furthermore, cumulative exposure to anti-psychotic treatment in schizophrenia may be significantly associated with changes in brain structure and function over time (Fusar-Poli et al. 2013). This is particularly problematic in high-risk samples as true positives are treated with a higher proportion of antipsychotics (Cannon et al. 2008; Ruhrmann et al. 2010), further confounding the relationship between the onset of psychosis and neurocognition. For example, while Hawkins et al. (2008) found a post-psychosis decline in motor speed, treatment with olanzapine was initiated in the CHR subjects who developed psychosis prior to the post-conversion assessment making it difficult to attribute the decline to the onset of psychosis.
In order to determine whether the neurocognitive deficits that characterize schizophrenia are stable traits, present prior to the onset of psychosis, or manifest due to emergence of psychosis, the course of neurocognition was examined in individuals initially ascertained as CHR who transitioned to psychosis over the course of the prospective study (i.e. converters). The neurocognitive performance of the CHR converters was examined pre-psychosis (i.e. baseline assessment) and shortly after the onset of psychosis and compared to CHR subjects who did not develop psychosis and healthy control subjects. A nested case-control design was conducted to avoid the possible confounding effects of age, gender, time between assessments, and medication treatment. Based on recent meta-analytic data that FEP patients show no deterioration in neurocognitive performance relative to pre-morbid levels (Bora & Murray, 2014) we hypothesized that cognitive deficits in true positives (CHR subjects who developed psychosis) would be apparent prior to the onset of psychosis and would remain stable after post-psychosis onset with no deterioration despite the emergence of psychosis.
Method
Design
In this nested case-control study, the CHR subjects who transitioned to psychosis (CHR+T) were assessed before and after the onset of psychosis. CHR+T subjects were matched to two groups of CHR subjects who did not transition to psychosis (CHR+NT). The first group of CHR+NT subjects were matched for age, gender, baseline severity of positive symptoms, time to retest, and medication status (antipsychotic and antidepressant) at baseline and retest. The second group of CHR+NT subjects were matched for the same demographic and clinical variables, but were unmedicated at both baseline and retest. Healthy controls were also included to assess practice effects. The nested design allowed us to address: (1) cognitive changes over time in subjects who developed psychosis (CHR+T); and (2) practice effects of matched CHR+NT subjects, unrelated to psychosis, medication, age, gender, and time to retest.
Participants
This paper reports retest data for participants recruited during Phase 1 (2000–2006) and Phase 2 (2006–2012) of the Recognition and Prevention (RAP) Program, an ongoing longitudinal investigation initiated in 1998 and funded by the National Institute of Mental Health in 2000. Patient referrals were made to the RAP Program by affiliated outpatient and inpatient psychiatry departments, local mental health providers, school psychologists or counselors, or were self-referred. All procedures were approved by the Institutional Review Board at the North Shore-LIJ Health System. Written informed consent (with assent from participants aged <18 years) was obtained from all participants.
Forty-five participants meeting criteria for Clinical High-Risk, Positive (CHR+) derived from the Scale of Prodromal Symptoms (SOPS; Miller et al. 1999, 2002, 2003) were included in this nested sub-sample. Inclusion criteria were based on the presence of one or more moderate, moderately severe, or severe (scores of 3, 4, or 5) SOPS rated attenuated positive symptoms (scale 0–6). A score of 6 (severe and psychotic) on any item was an exclusion factor for the CHR group. In this paper, subjects in the CHR+ group are broadly comparable to those considered ‘prodromal’ in most other studies in North America and internationally (Correll et al. 2010).
Recruitment in Phase 1 and Phase 2 yielded a total of 240 CHR+ participants. The current nested sub-sample included 15 CHR+T subjects out of a total of 23 CHR+T subjects who developed a psychotic disorder. Eight CHR+T subjects were excluded for not having both a pre- and post-psychosis onset neuropsychological assessment. Matches for the CHR+T subjects were selected from a total pool of 217 CHR+NT subjects. Of the 217 CHR+NT subjects, 188 had a baseline and at least one follow-up visit. Forty CHR+NT subjects were unmedicated at both testing points and a total of 148 were medicated at either the baseline or retest assessment. The sample selection process is outlined in Supplementary Fig. S1.
The 15 CHR+T subjects in the current study were tested at both time 1 (baseline, before psychosis) and time 2 (retest, post-psychosis). Mean time to conversion was 12.34 months (S.D. = 16.06, median = 8.31). The mean time between conversion and the post-conversion retest was 8.14 months (S.D. = 10.19, median = 3.55). Diagnoses at the last follow-up evaluation included: schizophrenia (n = 6), psychosis not otherwise specified (n = 5), bipolar I disorder, most recent episode manic, severe with psychosis (n = 3), delusional disorder, persecutory type (n = 1).
CHR+T subjects were matched on a case-by-case (1:1 ratio) with two groups of CHR+NT subjects: (1) Meds-matched CHR+NT subjects (n = 15) matched on medication treatment (i.e. antipsychotics, anti-depressants) at both baseline and retesting; (2) Meds-free CHR+NT subjects (n = 15) unmedicated at both assessments. The CHR+T subjects were also matched in a 1:1 ratio with healthy comparison subjects (CNTL; n = 15). All four subject groups were therefore matched on gender, age (±1-year window), and time to retest (±4-month window). All subjects on medication at testing were receiving stable doses for at least 2 weeks prior to the assessment.
CNTLs were recruited through announcements in local newspapers and within the medical center. Inclusion criteria required participants to be between the ages of 12 and 22 years. Exclusion criteria for all participants included: (1) schizophrenia-spectrum diagnosis; (2) non-English speaking; (3) a medical or neurological disorder; (3) estimated IQ < 70. Healthy controls with a first-degree relative with a diagnosed Axis I psychotic disorder were also excluded.
Baseline clinical assessment
Axis I diagnoses were assessed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Version (K-SADS-E; Orvaschel & Puig-Antich, 1994). Prodromal symptoms were assessed by the Structured Interview for Prodromal Syndromes (SIPS) and the companion SOPS (Miller et al. 1999). Conversion to psychosis was defined as the presence of a psychotic level positive symptom (score of 6 on the SOPS). The K-SADS-E was used to confirm diagnoses in those participants whose symptoms developed into full psychotic disorders. Social and role functioning was assessed using the GF: Social and GF:Role scales (Cornblatt et al. 2007). The GF:Social scale assesses peer relationships, peer conflict, age-appropriate intimate relationships, and involvement with family members. The GF:Role scale rates performance and amount of support needed in one’s specific role (i.e. school, work).
Baseline neurocognitive assessment
Patients were administered a comprehensive battery of tests that took approximately 3.5 h to complete at study entry. Testers were at the master’s level or above and trained in the administration and scoring of all tests. Estimated full-scale IQ scores were derived from the Vocabulary and Block Design subscales of the Wechsler Intelligence Scale for Children, 3rd edition (WISC-III; Wechsler, 1991) for subjects aged <16 years and from the same subscales of the Wechsler Adult Intelligence Scale, Revised (WAIS-R; Wechsler, 1981) for subjects aged ≥16 years.
In addition to the intelligence tests, the baseline and retest batteries included neuropsychological tests that assessed six cognitive domains (see Table 1): processing speed, verbal memory, executive function, working memory, sustained attention, and language. Domain construction was based on: (1) rational criteria derived from previous findings in patients with schizophrenia that demonstrated separable neurocognitive factors (Green et al. 2004) and; (2) previous work with subjects at CHR that demonstrated the content validity of the domains (see Seidman et al. 2010; Carrión et al. 2011, 2013 for more details). Prior to neurocognitive domain construction, raw test scores were log-transformed to reduce skewness and improve the distribution. Extreme values (±3.5 S.D.) were Winsorized to reduce the impact of outliers.(Dixon & Tukey, 1968) Test scores were then transformed into standard Z scores using the age-stratified means and S.D.s of a larger group of CNTLs (n = 114) to control for age-related change in cognitive performance. When applicable, tests were reverse-scored so that lower scores reflected worse performance. Domain scores were computed by averaging each subject’s Z scores on tests assessing the same neurocognitive domain. Z scores for each domain were then re-standardized using the mean and S.D. of the domain scores of the healthy comparison group. A composite of global neurocognitive performance was calculated by averaging the neurocognitive domains.
Table 1.
Neurocognitive domains, individual tests, and dependent measures
| Verbal memory | |
| California Verbal Learning Test (CVLT) | |
| Total for trials 1–5 | Words recalled in trials 1–5 |
| Long delay free recall | Recognition errors |
| Working memory | |
| Wechsler Intelligence Scale for Children – III/Wechsler | |
| Adult Intelligence Scale – R (WISC-III/WAIS-R) | |
| Digit span forward and backward | Digit sequences recalled |
| Letter-number span | Number of correct trials |
| Executive function | |
| Wisconsin Card Sorting Test (WSCT), version 2 | |
| Perseverative errors | Percentage of perseverative errors; |
| Categories completed | Number of correctly completed categories |
| Conceptual level responses | Number of consecutive correct responses in ≥3 runs |
| Controlled Oral Word Association Test (COWAT) | Words produced in 1 min |
| Sustained attention | |
| Continuous Performance Test – Identical Pairs (CPT-IP) | d′ (for all stimulus sets) |
| 2, 3, and 4 digits | |
| Processing speed | |
| Trails Making Test, Part A and B | Time to complete trails |
| WISC-III/WAIS-R digit symbol coding | Symbols accurately coded in 2 min |
| Animal naming test | |
| Language | |
| Wide Range Achievement Test – III (WRAT-III) Reading | Total score for words read correctly |
| WAIS-R/WISC-III vocabulary | Number of words orally defined |
Statistical analyses
All analyses were conducted using SPSS 16.0 (SPSS Inc., USA). Comparisons of demographic and clinical characteristics were performed with Student’s t tests for continuous variables, Pearson’s χ2 or Fisher’s exact tests for categorical variables, and Kolmogorov–Smirnov Z for one ordinal variable (two-tailed, p < 0.05). Linear mixed-effects models for repeated measures were used to compare the neurocognitive performance of the four subjects groups, as well as the change in performance from baseline to retest. Linear mixed effect modeling enabled the use of all available measurements and is robust in the presence of unbalanced designs (i.e. missing observations, inconsistent time intervals) and non-independent correlated data, providing unbiased estimates of covariance parameters (Verbeke & Molenberghs, 2000; Mallinckrodt et al. 2001; Gueorguieva & Krystal, 2004; McCulloch et al. 2008). Performance on each neurocognitive test was used as the primary dependent variable. Fixed effects were group (CHR+T, Meds-matched CHR+NT, Meds-free CHR+NT, and CNTLs) and time as (baseline and retest) and the interaction between group and time. The subjects were entered as a random effect. Restricted maximum-likelihood estimation and Type III tests of fixed effects were used, with a heterogeneous autoregressive covariance structure. Post-hoc pairwise comparisons were performed with Bonferroni corrections. Cohen’s d was calculated as the mean difference from the mixed model divided by the pooled standard deviation [d = (V2 – V1)/σ pooled] and can be interpreted using the following categories (Cohen, 1988): small = 0.20, medium = 0.50, large = 0.80. A main effect of time along with a group × time interaction would support evidence of a decline specific to the converters. Failing to find worsening in neurocognitive performance for the converters after the onset of psychosis, along with a significant difference between the four groups would suggest a pre-existing cognitive impairment for those who go on to develop a full-blown psychotic disorder. The linear mixed-effects models were also used to examine changes in clinical symptoms (SOPS total positive, negative, disorganized, and general symptom severity levels) from baseline to retest. Partial correlations (adjusted for group status) were conducted to examine the relationships between changes in neurocognitive performance and clinical symptoms over the follow-up period, with the alpha level adjusted using a Bonferroni correction for the number of tests in this analysis.
Results
Demographic and clinical characteristics
Table 2 summarizes baseline demographic and clinical characteristics for the CHR+T subjects along with the two groups of CHR+NT subjects and CNTLs. The four groups were well-matched, with no differences in baseline age, education level, gender ratio, handedness, race, ethnicity, and time to the retest assessment. The CNTL group had significantly better functioning as seen on the GAF, GF:Social and GF:Role compared to all three CHR groups, while the three CHR groups had comparable levels of functioning at baseline. The CHR+T group was retested on average 8.04 months (S.D. = 10.26) after the onset of psychosis.
Table 2.
Demographic and clinical characteristics
| Characteristic | CNTL (n = 15) | Meds-free CHR+NT (n = 15) | Meds-matched CHR+NT (n = 15) | CHR+T (n = 15) | p value |
|---|---|---|---|---|---|
| Age, years, mean (S.D.) | 17.84 (1.92) | 17.40 (2.18) | 17.60 (1.50) | 17.56 (1.35) | 0.50 |
| Years of education, mean (S.D.) | 12.00 (1.81) | 11.20 (1.74) | 11.20 (1.37) | 11.20 (1.57) | 0.32 |
| Gender, n (%) | |||||
| Female | 3 (20.0) | 3 (20.0) | 3 (20.0) | 3 (20.0) | 0.24 |
| Male | 12 (80.0) | 12 (80.0) | 12 (80.0) | 12 (80.0) | |
| Handedness, right, n (%) | 13 (82.4) | 12 (85.2) | 15 (86.7) | 11 (90.9) | 1.00 |
| Race, n (%) | |||||
| White | 8 (64.7) | 10 (66.7) | 9 (60.0) | 8 (53.3) | 0.76 |
| Ethnic origin | |||||
| Hispanic, n (%) | 2 (11.8) | 7 (25.9) | 2 (13.3) | 0 (0.0) | 0.22 |
| GAF, mean (S.D.) | 82.47 (11.70) | 46.07 (6.29) | 41.67 (9.03) | 43.00 (8.38) | <0.001 |
| Global functioning scale, mean (S.D.) | |||||
| Social | 8.73 (1.10) | 5.53 (1.46) | 5.80 (1.70) | 4.40 (1.35) | <0.001 |
| Role | 8.80 (1.08) | 6.07 (2.46) | 4.60 (2.67) | 4.53 (2.33) | <0.001 |
| DSM-IV diagnoses, n (%) | |||||
| Mooda | – | 15 (55.6) | 9 (60.0) | 5 (45.5) | 0.76 |
| Anxietyb | – | 16 (59.3) | 11 (73.3) | 7 (63.6) | 0.66 |
| Substancec | – | 3 (11.1) | 0 (0.0) | 2 (18.2) | 0.27 |
| Baseline medication, n (%) | |||||
| No medication | 15 (100.0) | 15 (100.0) | 4 (0.27) | 4 (0.27) | |
| Antipsychoticsd | 0 (0.0) | 0 (0.0) | 5 (33.3) | 5 (33.3) | |
| Antidepressantse | 0 (0.0) | 0 (0.0) | 6 (40.0) | 6 (40.0) | |
| Retest medication, n (%) | |||||
| No medication | 15 (100.0) | 15 (100.0) | 4 (27.0) | 4 (27.0) | |
| Antipsychotics | 0 (0.0) | 0 (0.0) | 6 (40.0) | 6 (40.0) | |
| Antidepressants | 0 (0.0) | 0 (0.0) | 5 (33.3) | 5 (33.3) | |
| Time to retest, months, mean (S.D.) | 18.67 (10.89) | 20.641 (19.91) | 21.29 (19.30) | 20.49 (20.53) | 0.85 |
CNTL, Healthy comparison subjects; CHR+NT, CHR + subjects who did not transition to psychosis; CHR+T, CHR+ subjects who did transition to psychosis; GAF, Global Assessment of Functioning; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
Compared to previous descriptions of CHR+ subjects recruited into the RAP Program (e.g. Carrión et al. 2011, 2013; Olvet et al. 2015), the mean age of the present cohort is slightly older; however, this is due to matching of the comparison groups to the CHR+T subjects.
DSM-IV-defined diagnosis of depression, dysthymia, or depressive disorder not otherwise specified (NOS).
DSM-IV-defined diagnosis of panic disorder, post-traumatic stress disorder, obsessive-compulsive disorder, generalized anxiety disorder, anxiety disorder NOS, or phobias including simple phobias and social phobia.
DSM-IV-defined diagnosis of alcohol, amphetamine, cannabis, cocaine, hallucinogen, nicotine, opioid, or polysubstance related disorder.
Antipsychotics included risperidone, quetiapine, aripiprazole, and olanzapine.
Antidepressants included escitalopram, citalopram hydrobromide, fluoxetine, paroxetin, and bupropion.
Medication treatment at baseline and retest in the CHR+T and Meds-matched CHR+NT groups were comparable. For both groups at the baseline and retest assessment, 11 out of the 15 subjects (73%) were taking medication. At baseline, five (33.3%) subjects were prescribed atypical antipsychotics and six (40.0%) anti-depressants. At the retest/post-psychosis assessment, six (40.0%) subjects were prescribed atypical anti-psychotics and five antidepressants (33.3%).
Changes in clinical symptoms
At baseline, CNTLs were significantly different from all three CHR groups, with lower SOPS positive, negative, disorganized, and general symptom levels. The three CHR groups had comparable positive and general symptom levels; however, CHR+T subjects showed significantly worse negative and disorganized symptoms (see Table 2).
As shown in Table 3, the linear mixed-models for repeated measures found significant group differences for all four SOPS symptom scales (all p < 0.001). There were also differential changes across time in symptom levels, as reflected by significant interactions between visit and group (see Table 3). The two groups of CHR+NT subjects showed substantial improvements over time and showed less severe symptoms at retest relative to baseline assessment. On the other hand, CHR+T subjects showed consistent SOPS symptom levels over time, with a worsening in positive symptom levels at retest. Post-hoc comparisons showed that compared to the healthy controls, CHR subjects showed consistently higher levels of SOPS positive, negative, disorganized, and general symptoms. The Meds-free CHR+NT and Meds-matched CHR+NT groups had comparable SOPS symptom levels on all four subscales; however, the CHR+T subjects had higher levels of SOPS positive, negative, disorganized, and general symptoms compared to the two non-converter groups.
Table 3.
SOPS (positive, negative, disorganized, general) symptom levels by group at baseline and post-psychosis onset/retest
| SOPS score | Visit | CNTL | Meds-free CHR+NT | Meds-matched CHR+NT | CHR+T |
p values
|
||
|---|---|---|---|---|---|---|---|---|
| Group | Visit | Group × visit | ||||||
| Positive | Time 1 | 1.67 (0.55) | 9.60 (0.81) | 10.00 (.87) | 12.20 (1.29) | <0.001 | <0.001 | <0.001 |
| Time 2 | 1.17 (0.42) | 5.67 (0.71) | 4.53 (.82) | 14.39 (1.47) | ||||
| Negative | Time 1 | 1.80 (0.43) | 12.33 (1.15) | 13.87 (1.67) | 19.92 (1.72) | <0.001 | <0.001 | 0.001 |
| Time 2 | 1.87 (0.66) | 6.73 (1.39) | 9.73 (1.23) | 16.75 (2.15) | ||||
| Disorganized | Time 1 | 0.87 (0.25) | 4.07 (0.57) | 5.40 (0.96) | 9.61 (1.18) | <0.001 | <0.001 | 0.011 |
| Time 2 | 0.53 (0.30) | 2.32 (0.52) | 2.87 (0.68) | 7.49 (1.10) | ||||
| General | Time 1 | 1.47 (0.52) | 8.27 (1.13) | 11.40 (1.15) | 10.09 (1.06) | <0.001 | <0.001 | 0.053 |
| Time 2 | 0.82 (0.40) | 5.78 (1.09) | 6.93 (1.32) | 8.53 (1.17) | ||||
SOPS, Scale of Prodromal Symptoms; CNTL, healthy comparison subjects; CHR+NT, CHR+ subjects who did not transition to psychosis; CHR+T, CHR+ subjects who did transition to psychosis.
SOPS total scores are presented along with the standard error of the mean in parentheses and are estimated marginal means derived from the linear mixed models.
Relationship between changes in clinical symptoms and neurocognitive performance
Partial correlations between changes in clinical symptoms and neurocognitive performance over time indicated that increases in positive symptom severity levels were related to improvements in processing speed, sustained attention, working memory, and global cognition (see Supplementary Table S1). Similar relationships were seen also seen for working memory and negative symptoms as well as with global cognition and disorganized symptoms, suggesting that increases in symptom severity over the short follow-up period did not translate into declines in neurocognition. However, these effects did not withstand correction for multiple comparisons.
Neurocognitive performance
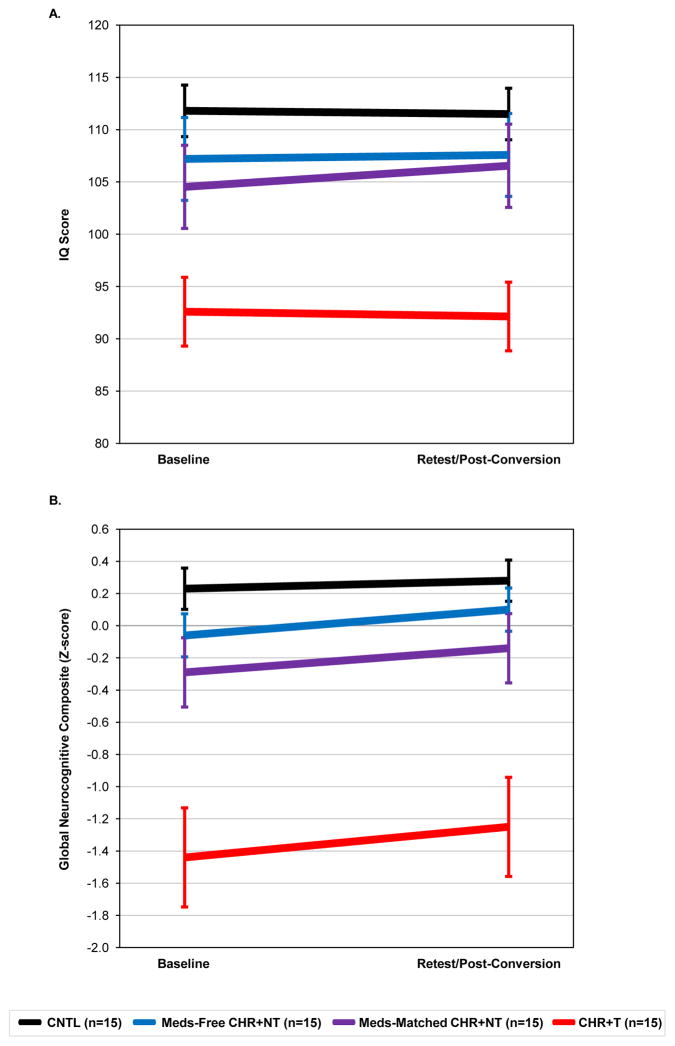
As shown in Fig. 1, each group demonstrated stable neurocognitive and intellectual performance from baseline to retest. However, compared to the healthy controls and CHR subjects that did not transition to psychosis, CHR+T subjects showed a consistent impairment in global neurocognitive and intellectual performance (see Fig. 1a, b) from baseline (before psychosis) to retest (after psychosis).
Fig. 1.
(a) Global neurocognitive and (b) intellectual performance (±S.E.) at baseline and retest assessment (post-conversion or matched testing based on duration from baseline) for all four groups. Global neurocognitive performance was calculated by averaging the six neurocognitive domains.
Estimated marginal means of IQ estimates and each neurocognitive domain derived from the linear mixed-models are shown in Table 4. Significant group effects were found for all the neurocognitive domains, including processing speed, verbal memory, executive function, sustained attention, working memory, and language. Post-hoc pairwise comparisons demonstrated that the CHR+T group had significantly lower global neurocognitive and intellectual scores, as well as lower performance on all six neurocognitive domains compared to CNTLs. The CHR+T group also had worse performance compared to the Meds-free CHR+NT group on every measure, except executive function and language. The two non-converter groups demonstrated similar performance, without significant differences.
Table 4.
Intellectual (IQ) performance and neurocognitive Z scores by group at baseline and post-psychosis onset/retest
| Variable | Visit | CNTL | Meds-free CHR+NT | Meds-matched CHR+NT | CHR+T |
p values
|
||
|---|---|---|---|---|---|---|---|---|
| Group | Visit | Group × visit | ||||||
| IQa | Time 1 | 111.80 (2.89) | 107.20 (3.89) | 104.53 (4.22) | 92.60 (3.48) | 0.002 | 0.82 | 0.97 |
| Time 2 | 111.50 (2.34) | 107.59 (4.84) | 106.55 (5.06) | 92.13 (3.52) | ||||
| Global neurocogitive composite | Time 1 | 0.23 (0.13) | −0.06 (0.13) | −0.29 (0.23) | −1.44 (0.32) | <0.001 | 0.11 | 0.85 |
| Time 2 | 0.28 (0.14) | 0.10 (0.15) | −0.14 (0.25) | −1.25 (0.33) | ||||
| Verbal memory | Time 1 | 0.37 (0.23) | −0.13 (0.17) | −0.30 (0.27) | −1.82 (0.33) | 0.001 | 0.34 | 0.15 |
| Time 2 | −0.16 (0.38) | −0.13 (0.38) | 0.40 (0.31) | −1.26 (0.31) | ||||
| Processing speed | Time 1 | 0.25 (0.26) | −0.51 (0.27) | −0.64 (0.34) | −1.98 (0.37) | <0.001 | 0.03 | 0.17 |
| Time 2 | 0.53 (0.33) | 0.41 (0.38) | −0.52 (0.52) | −1.88 (0.39) | ||||
| Sustained attention | Time 1 | 0.23 (0.24) | −0.25 (0.28) | −0.30 (0.29) | −1.60 (0.31) | 0.001 | 0.01 | 0.73 |
| Time 2 | 0.67 (0.22) | 0.14 (0.22) | −0.2 (0.26) | −1.24 (0.38) | ||||
| Executive function | Time 1 | 0.25 (0.25) | −0.29 (0.23) | −0.26 (0.32) | −1.44 (0.47) | 0.05 | 0.85 | 0.29 |
| Time 2 | 0.55 (0.47) | −0.51 (0.37) | −0.75 (0.72) | −0.87 (0.45) | ||||
| Working memory | Time 1 | 0.06 (0.25) | 0.22 (0.26) | 0.17 (0.32) | −1.23 (0.37) | 0.002 | 0.35 | 0.80 |
| Time 2 | −0.20 (0.29) | 0.18 (0.25) | 0.21 (0.32) | −1.50 (0.34) | ||||
| Language | Time 1 | 0.66 (0.22) | 0.21 (0.26) | 0.00 (0.31) | −0.92 (0.35) | 0.02 | 0.82 | 0.74 |
| Time 2 | 0.55 (0.26) | 0.10 (0.14) | 0.33 (0.28) | −0.88 (0.48) | ||||
CNTL, Healthy comparison subjects; CHR+NT, CHR+ subjects who did not transition to psychosis; CHR+T, CHR+ subjects who did transition to psychosis.
Scores are presented as z scores (standard error of the mean) and are estimated marginal means derived from the linear mixed models. A main effect of time along with a group × time interaction would support evidence of a decline specific to the converters. Failing to find worsening in neurocognitive performance for the converters after the onset of psychosis, along with a significant difference between the four groups would suggest a pre-existing cognitive impairment for those who go on to develop a full-blown psychotic disorder.
Estimated full-scale IQ scores were derived from the vocabulary and block design subscales of the Wechsler Intelligence Scale for Children – Third Edition for subjects aged <16 years and from the Wechsler Adult Intelligence Scale – Revised for subjects aged ≥16 years.
All subject groups demonstrated similar and small improvements (i.e. practice effects) in performance in many of the domains, with only significant improvements in sustained attention (p < 0.01) and processing speed (p = 0.03). Trend level improvements were also seen for the global neurocognitive composite (p = 0.11). Notably, group × visit interactions were not significant for any domain (see Table 3). Table 5 shows effect size (Cohen’s d) estimates for changes in intellectual and neurocognitive performance from baseline to retest for all four subject groups. CHR+T subjects showed similar, small effect sizes compared to the healthy controls and other CHR+NT comparison groups. One exception was in the processing speed and attention domains, with healthy controls and Meds-free CHR+NT subjects showing moderate to large improvements, while CHR+T and Meds-matched CHR+NT groups showing smaller improvements over time (see Table 5).
Table 5.
Effect sizes (Cohen’s da) of neurocognitive change over time for the four subject groups
| Variable | CNTL | Meds-free CHR+NT | Meds-matched CHR+NT | CHR+T |
|---|---|---|---|---|
| IQ | 0.03 | 0.02 | 0.10 | 0.04 |
| Global neurocognitive composite | 0.09 | 0.30 | 0.13 | 0.15 |
| Verbal memory | 0.32 | 0.00 | 0.45 | 0.37 |
| Processing speed | 0.22 | 0.66 | 0.06 | 0.07 |
| Sustained attention | 0.50 | 0.35 | 0.05 | 0.07 |
| Executive function | 0.16 | 0.15 | 0.17 | 0.30 |
| Working memory | 0.21 | 0.04 | 0.03 | 0.16 |
| Language | 0.10 | 0.11 | 0.22 | 0.02 |
CNTL, Healthy comparison subjects; CHR+NT, CHR+ subjects who did not transition to psychosis; CHR+T, CHR+ subjects who did transition to psychosis.
Cohen (1988) recommended the following categories for interpreting effect sizes: small = 0.2, medium = 0.5, and large = 0.8.
Discussion
In order to understand the temporal and mechanistic nature of cognitive change following the onset of psychosis, we prospectively assessed the neurocognitive performance of a group of clinically at-risk adolescents and young adults before and on average 8 months after the emergence of full-blown psychosis. True positives, CHR subjects who developed psychosis after the baseline testing, showed large neurocognitive and intellectual impairments at baseline, prior to the onset of psychosis, compared to CHR subjects who did not transition to psychosis. These impairments persisted over the course of the short follow-up period, with no further deterioration seen after the onset of psychosis. Moreover, the same seems to be true for antipsychotic and antidepressant treatment, at least in the short-term. On the other hand, false-positives, subjects ascertained as CHR but who did not transition over the follow-up period, demonstrated mild to no impairments in neurocognitive and intellectual performance independent of medication treatment, suggesting that cognitive impairment during the prodrome is related to the underlying vulnerability to illness, consistent with the neurodevelopmental model (Weinberger, 1987). Taken together, our results indicate that cognition is impaired prior to the onset of psychosis and that the onset of psychosis, in and of itself, does not have a detrimental or ‘neurotoxic’ effect on the course of neurocognition. Thus, cognitive deficits represent trait risk markers, as opposed to state markers of disease status and may serve as possible predictors of schizophrenia prior to the onset of the full illness.
At baseline, individuals at CHR who later converted to psychosis showed a global neurocognitive impairment that mirrored performance levels seen in patients with FEP (Addington & Addington, 2002; Gonzalez-Blanch et al. 2007). True prodromal subjects showed a deficit in overall neurocognitive performance that was approximately 1.5 S.D.s below that of the healthy controls. These impairments persisted after the onset of full-blown psychosis and did not decline further. Rather, converters to psychosis actually demonstrated small improvements that were most likely due to practice effects over the follow-up period that were highly comparable to those seen in both non-converter groups. In addition to a global neurocognitive impairment, converters also demonstrated stable intellectual deficits that were significantly lower than levels seen in the healthy comparison and matched non-converter groups. In fact, the level of estimated IQ levels seen in the converter group is in line with a large body of evidence from cohort studies that have associated lower intellectual performance with a higher risk for developing schizophrenia (David et al. 1997).
Converters also showed large impairments of ≥1.5 S.D.s below healthy control levels in specific areas of neurocognition, including sustained attention, verbal memory, processing speed, and executive function. Deficits in these domains have been well-documented at different stages of psychotic illness and have been described as among the core cognitive deficits in schizophrenia (Green et al. 2004). Performance in sustained visual attention as measured by the CPT-IP has been found to be heritable, reliable, and stable (independent of clinical state), representing a promising endophenotype for molecular genetics research in schizophrenia (Cornblatt & Malhotra, 2001). Impairments in verbal learning and memory have been shown to make an independent contribution to the prediction of psychosis in CHR subjects (Lencz et al. 2006). Becker et al. (2010) also found stable deficits in verbal memory as assessed by the CVLT (Trials 1–5, free recall total correct) in CHR subjects before and after the onset of psychosis compared to healthy controls. Moreover, in a meta-analytic review of neurocognitive deficits in first-episode psychosis, Mesholam-Gately et al. (2009) found that performance on measures of verbal memory, the CVLT included, were among the poorest compared to healthy levels. Finally, processing speed plays a central role in a variety of high-order cognitive abilities such as language and reading as well as functional outcomes in the earliest phases of the illness (Carrión et al. 2011; Meyer et al. 2014). Moreover, due to the varying task demands (e.g. flexibility, cognitive control, visual scanning, and motor abilities) used in processing speed measures, deficits in this domain are mostly likely reflective of dysfunction in spatially distributed and interconnected brain regions that are linked to the underlying pathophysiology of the illness (Dickinson et al. 2007).
Indeed, these domain-specific deficits most likely reflect a dysfunction of complex integrative neural systems that subserve the neuropsychological measures. Our findings are consistent with mounting evidence of the presence of neurofunctional (Fusar-Poli et al. 2007) and neuroanatomical (Fusar-Poli et al. 2012b) abnormalities prior to the onset of psychosis in subjects at CHR. Abnormal functional connectivity has been found within brain networks that underlie domain-specific performance in working memory, executive function, and processing speed tasks, for example, that include the dorsolateral prefrontal cortex and anterior cingulate cortex, along with hippocampus and subcortical regions. Future prospective cohorts with larger CHR converter groups (e.g. North American Prodrome Longitudinal Study), can examine the relationship between neurocognition along the pathway towards psychosis and changes in brain morphology that have been documented in CHR individuals (Pantelis et al. 2003; Cannon et al. 2015).
Limitations
Our findings should be interpreted in light of the following potential limitations. First, our data cannot rule out cognitive deterioration at other periods along the trajectory of the disease. The current report only addresses one critical window on the pathway to illness, the prodrome to shortly after post-psychosis. Decline may occur in childhood or closer to onset of the prodrome (Harvey, 2014). Moreover, the relatively short time frame (8 months) between transition and retest does not rule out further deterioration in the long-term course of the established illness. Cognitive deterioration may occur years later, possibly exacerbated by prolonged medication treatment and repeated hospitalizations. However, recent meta-analyses have not found support for deterioration in older patients with a chronic course of schizophrenia (Szöke et al. 2008).
Second, it is possible that cognitive functions other than those studied here (e.g. visual memory) do not deteriorate until psychosis manifests, or become more impaired as the illness becomes more chronic. Third, the current study only used a combination of the block design and vocabulary sub-tests to estimate a full-scale IQ. This combination of a verbal and performance measure did, however, demonstrate excellent stability across time in all four comparison groups.
Finally, our ability to detect subtle differences between the groups and to relate individual differences in cognitive course to clinical outcome may have been hindered by the small sample of converters. However, the size of the transitioned group is in line with previous studies that have retested CHR subjects before and after the onset of psychosis (e.g. n = 17, Becker et al. 2010; n = 16, Wood et al. 2007; n = 10, Woodberry et al. 2013). Furthermore, the neurocognitive effect sizes from baseline to retest were consistent for each group. Despite the small sample size, the effect size of cognitive change over time was consistent across groups, with almost all subjects groups showing small improvements (85% of the results) in performance over time. These sample sizes are most likely due to the difficulty in obtaining repeated neurocognitive assessments on individuals who transition to psychosis during the course of a prospective study, especially after the onset of psychosis. Nevertheless, this limitation should not necessarily diminish the interpretation of the primary result, that is, converters as a group did not show decline in neurocognitive function after the onset of psychosis.
In addition, our study design has a number of strengths compared with previous research. Converters were well-matched to three separate comparison groups on a number of variables known to influence cognitive performance. Confounding by age, gender, baseline positive symptoms, medication at testing, and time to retest are unlikely to explain the key findings. The nested-case control design also minimizes selection bias as cases and controls were sampled from the same cohort ensuring the comparability of the groups.
In summary, the current study does not provide evidence of cognitive deterioration shortly after the emergence of full-blown psychosis. On the contrary, large cognitive deficits are apparent in true positives pre- and post-psychosis onset, with no signs of decline, and therefore appear to be stage-invariant vulnerability traits. Our findings provide further support for the important role of cognition in the neurodevelopmental processes leading to psychotic illness that may ultimately serve as a target for preventive intervention.
Supplementary Material
Acknowledgments
We thank the study participants and entire staff of the RAP Program for their time and effort from the very onset of these studies. In addition, the authors would like to thank Pradeep Nagachandran, M.D., Samantha Diaz, Ph.D., and Miranda Farabaugh, MA, for their assistance in carrying out this study. This work was supported by grants from the National Institute of Mental Health (NIMH): MH 61523 (Dr Cornblatt), the Zucker Hillside Hospital Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543 (John M. Kane, M.D.).
Footnotes
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715001233.
Declaration of Interest
Drs. Carrión, Auther, and Ms. Olsen and McLaughlin report no financial relationships with commercial interests. Dr Cornblatt was the original developer of the CPT-IP and has been an advisor for Hoffmann-La Roche. Dr Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Bristol-Myers Squibb, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, MedAvante, Medscape, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Supernus, and Takeda. He has received grant support from the American Academy of Child and Adolescent Psychiatry BMS, Janssen/J&J, National Institute of Mental Health (NIMH), Novo Nordisk A/S, Otsuka, Takeda and the Thrasher Foundation.
References
- Addington J, Addington D. Cognitive functioning in first-episode schizophrenia. Journal of Psychiatry and Neuroscience. 2002;27:188–192. [PMC free article] [PubMed] [Google Scholar]
- Becker HE, Nieman DH, Wiltink S, Dingemans PM, van de Fliert JR, Velthorst E, de Haan L, van Amelsvoort Ta, Linszen DH. Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psychological Medicine. 2010;40:1599–1606. doi: 10.1017/S0033291710000048. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophrenia Bulletin. 1992;18:437–448. doi: 10.1093/schbul/18.3.437. [DOI] [PubMed] [Google Scholar]
- Bora E. Developmental lag and course of cognitive deficits from the premorbid to postonset period in schizophrenia. American Journal of Psychiatry. 2014;171:369. doi: 10.1176/appi.ajp.2013.13091283. [DOI] [PubMed] [Google Scholar]
- Bora E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychological Medicine. 2015;45:1–9. doi: 10.1017/S0033291714001263. [DOI] [PubMed] [Google Scholar]
- Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophrenia Bulletin. 2014;40:744–755. doi: 10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, McLaughlin D, Carrión RE, Auther AM, Cornblatt BA. Cognitive changes following antidepressant or antipsychotic treatment in adolescents at clinical risk for psychosis. Schizophrenia Research. 2012;137:110–117. doi: 10.1016/j.schres.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophrenia Bulletin. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R North American Prodrome Longitudinal Study C. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. American Journal of Psychiatry. 2011;168:806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, Correll CU, Cornblatt Ba. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Kaplan Z, Knobler H, Davidson-Sagi N, Davidson M. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophrenia Research. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Green MF, Walker EF, Mittal VA. Etiology and Neurocognition. In: Blaney PH, Millon T, editors. Oxford Textbook of Psychopathology. Oxford University Press; New York, NY: 1999. pp. 298–332. [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophrenia Bulletin. 2003;29:633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. American Journal of Medical Genetics. 2001;105:11–15. [PubMed] [Google Scholar]
- Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. Journal of Child Psychology and Psychiatry. 2010;51:390–431. doi: 10.1111/j.1469-7610.2010.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychological Medicine. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Tukey JW. Approximate behavior of the distribution of Winsorized t (Trimming/Winsorization 2) Technometrics. 1968;10:83–98. [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14:1–21. [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz R-D, Vita A, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Archives of General Psychiatry. 2012a;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophrenia Bulletin. 2012b;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neuroscience and Biobehavioral Reviews. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Kent JW, Sprooten E, Diego VP, Winkler AM, Curran JE, McKay DR, Knowles EE, Carless Ma, Göring HHH, Dyer TD, Olvera RL, Fox PT, Almasy L, Charlesworth J, Kochunov P, Duggirala R, Blangero J. Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging. Proceedings of the National Academy of Sciences USA. 2013;110:19006–19011. doi: 10.1073/pnas.1313735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J. Intellectual decline in schizophrenic patients. American Journal of Psychiatry. 1998;155:995–996. doi: 10.1176/ajp.155.7.995b. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Blanch C, Crespo-Facorro B, Alvarez-Jimenez M, Rodriguez-Sanchez JM, Pelayo-Teran JM, Perez-Iglesias R, Vazquez-Barquero JL. Cognitive dimensions in first-episode schizophrenia spectrum disorders. Journal of Psychiatric Research. 2007;41:968–977. doi: 10.1016/j.jpsychires.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Green MF, Harvey PD. Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition. 2014;1:e1–e9. doi: 10.1016/j.scog.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RSE, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Harvey PD. What is the evidence for changes in cognition and functioning over the lifespan in patients with schizophrenia? Journal of Clinical Psychiatry. 2014;75 (Suppl 2):34–38. doi: 10.4088/JCP.13065su1.08. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Keefe RSE, Christensen BK, Addington J, Woods SW, Callahan J, Zipursky RB, Perkins DO, Tohen M, Breier A, McGlashan TH. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophrenia Research. 2008;105:1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24:109–120. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. In: Geyer MA, Gross G, editors. Novel Antischizophrenia Treatments (Handbook of Experimental Pharmacology) Springer; Berlin, Heidelberg, Germany: 2012. pp. 11–37. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Berger G, Zipursky RB, Wood SJ, Pantelis C. Neurobiology of early psychosis. British Journal of Psychiatry. 2005;48:s8–s18. doi: 10.1192/bjp.187.48.s8. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. Journal of Biopharmaceutical Statistics. 2001;11:9–21. doi: 10.1081/BIP-100104194. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. 2. Wiley-Interscience; Hoboken, NJ: 2008. [Google Scholar]
- McGlashan TH. Is active psychosis neurotoxic? Schizophrenia Bulletin. 2006;32:609–613. doi: 10.1093/schbul/sbl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RSE, Fisher HL, Harrington H, Houts R, Poulton R, Moffitt TE. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. American Journal of Psychiatry. 2014;171:91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyer EC, Carrión RE, Cornblatt BA, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Heinssen R, Seidman LJ group N. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophrenia Bulletin. 2014;40:1452–1461. doi: 10.1093/schbul/sbt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C, Ruhrmann S, Schimmelmann BG, Klosterkotter J, Schultze-Lutter F. A stratified model for psychosis prediction in clinical practice. Schizophrenia Bulletin. 2014;40:1533–1542. doi: 10.1093/schbul/sbu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. American Journal of Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Carrión RE, Auther AM, Cornblatt BA. Self-awareness of functional impairment in individuals at clinical high-risk for psychosis. Early Intervention Psychiatry. 2015;9:100–107. doi: 10.1111/eip.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version. Center for Psychological Studies, Nova Southeastern University; Fort Lauderdale, FL: 1994. [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophrenia Research. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. American Journal of Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkotter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Archives of General Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Schwartzman AE, Douglas VI. Intellectual loss in schizophrenia. I. Candian Journal of Psychiatry. 1962;16:1–10. doi: 10.1037/h0083239. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology. 2006;28:225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA North American Prodrome Longitudinal Study G. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöke A, Trandafir A, Dupont M-E, Méary A, Schürhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. British Journal of Psychiatry. 2008;192:248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer Series in Statistics. 2000;xxii:568. [Google Scholar]
- Walder DJ, Holtzman CW, Addington J, Cadenhead K, Tsuang M, Cornblatt B, Cannon TD, McGlashan TH, Woods SW, Perkins DO, Seidman LJ, Heinssen R, Walker EF. Sexual dimorphisms and prediction of conversion in the NAPLS psychosis prodrome. Schizophrenia Research. 2013;144:43–50. doi: 10.1016/j.schres.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Revised. The Psychological Corporation; New York, NY: 1981. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Brewer WJ, Koutsouradis P, Phillips LJ, Francey SM, Proffitt TM, Yung AR, Jackson HJ, McGorry PD, Pantelis C. Cognitive decline following psychosis onset: data from the PACE clinic. British Journal of Psychiatry. 2007;51:s52–57. doi: 10.1192/bjp.191.51.s52. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, McFarlane WR, Giuliano AJ, Verdi MB, Cook WL, Faraone SV, Seidman LJ. Change in neuropsychological functioning over one year in youth at clinical high risk for psychosis. Schizophrenia Research. 2013;146:87–94. doi: 10.1016/j.schres.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophrenia Bulletin. 2013;39:1363–1372. doi: 10.1093/schbul/sbs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.