Abstract
In 1927 Otto Warburg established that tumours derive energy primarily from the conversion of glucose to lactic acid and only partially through cellular respiration involving oxygen. In the 1950s he proposed that all causes of cancer reflected different mechanisms of disabling cellular respiration in favour of fermentation (now termed aerobic glycolysis). The role of aberrant glucose metabolism in cancer is now firmly established. The shift away from oxidative phosphorylation towards the metabolically expensive aerobic glycolysis is somewhat counter-intuitive given its wasteful nature. Multiple control processes are in place to maintain cellular efficiency and it is likely that these mechanisms are disrupted to facilitate the shift to the reliance on aerobic glycolysis. One such process of cell control is mediated by the nuclear receptor superfamily. This large family of transcription factors plays a significant role in sensing environmental cues and controlling decisions on proliferation, differentiation and cell death for example, to regulate glucose uptake and metabolism and to modulate the actions of oncogenes and tumour suppressors. In this review we highlight mechanisms by which nuclear receptors actions are altered during tumorigenic transformation and can serve to enhance the shift to aerobic glycolysis. At the simplest level, a basic alteration in NR behaviour can serve to enhance glycolytic flux thus providing a basis for enhanced survival within the tumour micro-environment. Ameliorating the enhanced NR activity in this context may help to sensitize cancer cells to Warburg targeted therapies and may provide future drug targets.
Keywords: cancer, nuclear receptors, energy regulation, Warburg effect
Nuclear Receptors Respond to Environmental Signals
The Nuclear receptor (NR) super-family of transcription factors have wide-ranging actions. NRs sense environmental, systemic and local factors by binding a wide range of lipophilic molecules. They respond by regulating transcriptomes influencing fundamental processes such as proliferation and differentiation. Ligands for NRs are frequently derived from dietary derived factors and metabolism, and regulate processes such as glycolysis, oxidative phosphorylation and fatty acid synthesis (reviewed in Refs. 1–3). To achieve these actions NR bind with a variety of co-activators, co-repressors and histone modifying enzymes to form large DNA associated complexes that regulate chromatin structure and gene transcription (reviewed in Ref. 4).
Functionally, the 48 members of the human NR super-family fall into three main groups. Firstly, are those with high-affinity for ligand, such as steroidal receptors (e.g. AR and ERα) and seco-steroidal receptors such as VDR and the RARs. The VDR and RARs respond to dietary factors including vitamin D3 and retinoids and do so at the low nM range, although there is evidence for low affinity binding to other dietary compounds, for example the VDR can bind certain bile acids.5 The second group including the PPARs, FXRs, and LXRs have low binding affinities, but for a wider range of lipophilic molecules. These NRs respond to µM concentrations of dietary derived factors such as fatty acids and glucose. The classical steroid NRs are predominately in the cytoplasm in the absence of ligand and are shuttled in when activated. By contrast the VDR and RARs, and many other NRs that bind ligand with low affinity are bound to chromatin in the absence and presence of ligand; the addition of ligand re-distributes the receptor and changes the gene regulation function, generally to activation. Thereby regulating gene expression in the presence and absence of ligands allows distinct responses based on the local microenvironment, cellular milieu or other factors.
The final group, the orphan NR, are receptors for which either ligands have not yet been identified or contain no ligand binding domain, examples include NR4A1/NUR77 and ERRs. These orphan receptors frequently utilize co-factors in place of a true ligand, so can be regulated as the other two classes are but through changes in protein bioavailability.6,7
It has emerged that this receptor superfamily is centrally placed to regulate many pathways relating to energy metabolism and that analyses of their function has been central to the development of the field of Molecular Endocrinology.8 These features pivotally position the NR superfamily to mediate cellular response to changes in nutrient availability and systemic and inter-cellular signaling. Coupled with these functions, it is also clear that their activity is frequently altered in cancer, and surprisingly, as a family, their expression is significantly distorted more than predicted by chance.9 Together, therefore, by physiological and pathophysiological function the NRs appear to be intimately placed within the signaling cascades that are central to the Warburg effect.
The Central Cellular Role of ATP Production and the Warburg Effect
A primer on ATP production
Given that ATP is the fundamental energy unit of the cell, its generation is vital to maintain processes such as transporting molecules against concentration gradients, and protein and nucleic acid synthesis. Additionally the growing cell needs to make a choice over whether to divert glucose away from ATP production either to de novo fatty acid synthesis for the generation of cellular structures, or aromatic amino acids to aid in protein synthesis. The synthesis of ATP is therefore a tightly controlled process within the cell and there are many points during the generation of ATP on which signaling cascades converge to bring about changes to ATP flux. Glucose metabolism provides the most efficient method of generating energy within the cell; other compounds such as proteins and fatty acids may be utilized but give reduced efficiency in the net generation of ATP. Glucose is therefore initially used as a substrate for glycolysis, and its breakdown products are, under normal aerobic conditions, also substrates for the citric acid cycle and oxidative phosphorylation (OXPHOS). For further information the reader is directed to some detailed reviews.10–12
Given this role for glucose, there are a host of glucose receptors present on the cell surface and the number and type of transporter vary greatly depending on the tissue and cell type and with disease status. Hexokinase is the first of several regulatory enzymes to process glucose and modifies it through phosphorylation to glucose-6-phosphate (G-6-P), the substrate for the rest of glycolysis and the pentose phosphate shunt (PPS). As G-6-P is not able to bind to glucose transporters, and can be converted to storage molecules such as glycogen, hexokinase has a key regulatory role by depleting the local levels of free glucose in the cell. For every one molecule of glucose, the immediate enzymatic reactions that ensue result in a net increase of two molecules each of ATP and pyruvate. Under normal aerobic conditions pyruvate is imported the mitochondria and is metabolized to Acetyl-CoA, a precursor of citrate, which is central to the citric acid cycle and fatty acid synthesis. Under these conditions maximum chemical potential (about 36 ATPs) is extracted via the metabolism of citrate into NADH and FADH2 and finally ATP using the electron transport chain. Under anaerobic conditions, however, such as heavy muscle use, pyruvate entry to the mitochondria is prevented and is rapidly utilized (×100 faster than under aerobic conditions during OXPHOS) to generate a small amount of ATP (a net increase of just two ATPs) via lactate dehydrogenase (LDH) metabolism to lactic acid. This process is extremely inefficient at extracting chemical potential from the glucose; <5% of the total possible ATP is formed.
The Warburg effect–A metabolic shift
Production of ATP via “fermentation” as described by Otto Warburg, (now termed aerobic glycolysis) is a key feature of many cancer cells.13 Tumor initiation and progression requires selection for the most aggressive and resilient cells to power and sustain proliferation and survival. Pathways such as glycolysis, oxidative phosphorylation and fatty acid synthesis are de-regulated to meet the requirements for ATP and precursors for de novo biomass.14 Thus, the strong selection pressures within the tumor micro-environment selects for clones that can generate ATP rapidly at the expense of efficiency whilst also providing the necessary nutrients for rapid cellular division.13,15,16 The observation that tumours produce massive amounts of the aerobic glycolysis waste product, lactic acid was central to the concept of deregulated metabolism17,18 and that cancer was even termed “disorder of metabolism.”19 Although cancer is now more accurately defined in terms of genomics, it remains clear that there are substantial changes to metabolic pathways as result of genetic and epigenetic changes. This hallmark of cancer, now known as the Warburg effect, is so widespread and palpable that it has been used to identify primary and metastatic lesions through radio labeled glucose analogues combined with PET scanning for the last 20 years.20
Specifically, the Warburg Effect describes what happens in cancer cells when, although oxygen is plentiful, the cell shifts in preference of generating ATP away from the efficient oxidative phosphorylation and towards the rapid aerobic glycolysis. Although wasteful of glucose, this has significant benefits for the tumour cell. Aerobic glycolysis produces ATP far quicker than the slow route of oxidative phosphorylation and results in the generation of crucial precursors for biomass production such as NADPH which is not produced at such levels via oxidative phosphorylation.16 It is hypothesized that cancer cells utilize the rapid generation of ATP and the increase in de novo fatty acid synthesis to grow and divide quickly. The quickest dividing cells are by definition the ones to form the bulk of the tumor, in so long as their growth can be sustained.
Selection for induction of the Warburg shift is therefore likely, but whether this shift is a cause of cancer (due to the accelerated mutation rate in uncontrollably dividing cells) or a consequence downstream of other initiation events has not been experimentally validated. Irrespective of the cellular origins of these adaptations, the advantages to the cell are obvious; this shift not only allows rapid generation of ATP, fatty acids and nucleotides whilst glucose is abundant, but the generation of lactic acid ensures a tumour micro-environment protective against immune attack.21 Furthermore, glucose flux modeling has indicated that mere presence of glucose elevated above a molecular tipping point turns the cell to be energetically in favour of aerobic glycolysis over oxidative phosphorylation, even in normal cells suggesting a role for cytosolic glucose sensors.22 Glucose flux and intracellular concentrations in the cancer cell could therefor be the trigger for the Warburg effect to occur.
NR Signaling Impacts on Glucose Metabolism
Increased glucose uptake In cancer
The ultimate gatekeepers for the glucose avarice of tumor cells are the family of transport proteins that regulate the import of glucose. There are three classes of SLC2A/GLUT transport proteins and are grouped based on their sequence homology.23 In normal biology several GLUTs are expressed in insulin and insulin growth factor (IGF) sensitive tissues and respond to the presence of both Insulin and the IGFs.24 The class I GLUTs (1–4) appear especially important in cancer progression.25 GLUT1 expression predicts survival in bladder cancer patients26 and non-small cell lung tumour,27 presence of thyroid cancer28 and marks advanced breast cancer stages and breast cancer cells with high proliferative potential.29 GLUT1 is also upregulated in colorectal cancers displaying KRAS or BRAF mutations. Intriguingly, however, this stems from glucose deprivation driving mutations in one of the two oncogenes as a way of redressing the glucose levels.30 When cells with wild-type KRAS were deprived of glucose surviving cells showed a significant mutation rate in the KRAS allele and GLUT1 expression was elevated.
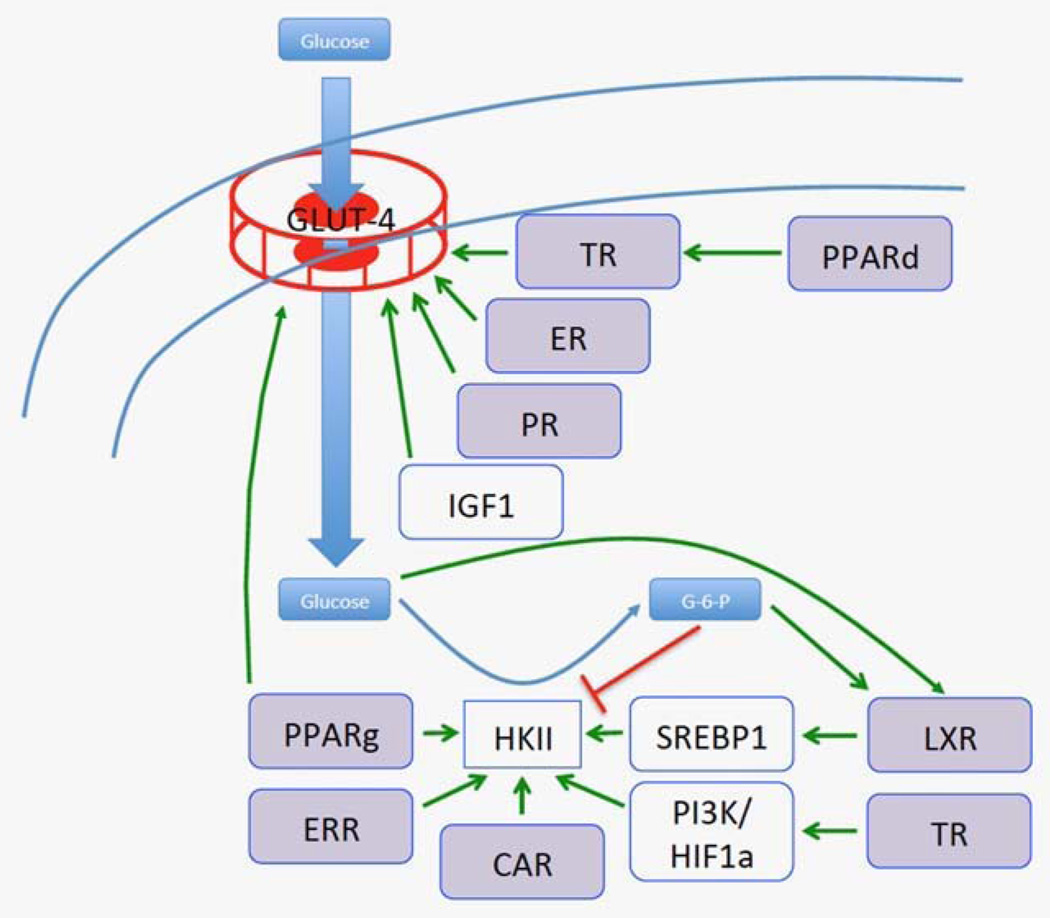
Reflecting the importance of GLUTs, their expression is tightly controlled, for example by repression by wild-type but not mutated p53.25 Class I GLUT expression and activity is controlled by a range of NRs (Fig. 1). All class 1 GLUTs show tissue specific expression to some extent and are frequently over expressed in a range of tumour types. GLUT-4 expression, for example, is altered in breast cancer31,32 and is translocated to the plasma membrane in an ER dependent manner.33 PR as well can act alone and synergistically with ER to elevate GLUT4 expression and increase glycolytic flux.34
Figure 1.
The interface between nuclear receptor signaling the Warburg effect. Multiple nuclear receptors (TR, ER, PR, PPARs, ERR, LXR, CAR) regulate expression of glucose transporters (such as GLUT4) and the downstream metabolic enzymes that handle its metabolism. Interestingly glucose has been to shown to activate LXR receptors directly.
The TR binds and regulates expression of GLUT1, GLUT3, and GLUT4 in several cell types35–38 both directly and indirectly through TR-mediated activation of PI3K and stabilization of HIF1α and mTORC1.39 There is evidence that constitutive over-production of the ligand T3 is caused by a point mutation in the TSH gene40 and that this excess T3 may over stimulate transcription of its downstream targets. Indeed, a range of cancer patients have significantly elevated levels of circulating T3, T4 and TSH and that the levels of these factors correlated with development of carcinogenesis.41 Furthermore, Itoh et at. found that TRα1 mutant knock in mice were less able to utilize glucose in the brain,42,43 but this mechanism in cancer has not been assessed.
Under normal conditions, GLUT4 imports glucose in adipocytes, in a T3 regulated manner.44 PPARδ regulates expression of TR directly and also combines with insulin signaling to induce uptake and storage of glucose in adipose tissue.45 However, PPARδ is unable to upregulate GLUT4 directly, as demonstrated by the observation that GLUT246 but not GLUT445 is induced in mice treated with the PPARδ agonist GW501516. GLUT4 is, however, directly regulated by PPARγ through a validated PPRE in its promoter, ingestion of the PPARγ agonist pioglitazone by obese Zucker rats led to significant increase in expression of this transporter.47 The VDR is also able to upregulate GLUT1 and GLUT4 expression in response to calcitriol in normal tissue, this upregulation was significantly more in diabetic models.48
Combinatorial NR gene regulation
Often multiple receptors bind at compound response elements. Most frequently there is kinetic competition for the common heterodimer and transactivation partner RXR. Other interactions include competitive binding; TRα can bind the PPRE at the AOX promoter and prevent access for the PPARδ/RXR heterodimer49 thus antagonizes PPAR induced reporter gene expression.50 TRα also appears to be a dominant regulator of PPAR-γ genes at some loci.51 Another type of interaction occurs at the CYP7A1 promoter where a compound PPARα-LXRα response element exists. Stimulation of either factor leads to gene expression, but stimulation of both prevents expression as the PPAR-LXR dimer binds instead of PPAR-RXR or LXR-RXR52 This probably occurs throughout the genome; LXR and PPAR bind several degenerate response elements in direct competition with each other and with ChIP-Seq PPAR has been shown to bind to approximately 75% of all LXR sites.53
Given that Class I GLUTs are frequently dependent upon IGF signaling, the role for NR regulation in this process is complex. Whilst there appears to be a linear path for activation of GLUT gene transcription by many NRs, they also stabilize IGF activity through the induction IGF binding proteins (IGFBPs).54–57 Conversely, the transcriptional co-repressors NCOR1 and NCOR2/SMRT are frequently elevated in different tumour types56,58–60 and prevents expression of many NR targets, including IGF1.61 However, these co-repressors are themselves under transcriptional control of multiple NRs including the ER62 and the VDR,63 allowing tight feed-back regulation to balance the pro-proliferative and anti-glycolytic function of NR co-repressors, as has been described in breast cancer. These observations suggest that tumours selectively target portions of the NR transcriptome to repress or enhance depending upon suitability for advancing tumour growth.
Glucose retention by hexokinase II
Glucose is efficiently retained within the cancer cell through enhanced heoxokinase activity. Hexokinase II (HKII) converts glucose to G-6-P and is a rate-limiting step for of ATP generation. HKII is particularly interesting as, in contrast to the other HK isoenzymes, it is over expressed in many cancers; it has a very high affinity for glucose, both catalytic domains rather than just one are active and it is tethered to the mitochondria allowing access to ATP and avoiding its product-inhibitor G-6-P (reviewed in Ref. 64).
Several NR converge on the regulation of HKII expression (Fig. 1), both directly through PPARγ,65 CAR,66 ERR67,68 and indirectly69,70 for example, through LXR activation of SREBP1.71 NRs probably also contribute to its expression through their effects on PI3K activity. Despite HKII responding directly to glucose through elevated gene expression,72 a characterized glucose cis-element within its promoter has not been identified. LXR is an intriguing candidate for this role. It is a glucose responsive transcription factor73 and frequently binds to PPAR compound elements53 (discussed above in Combinatorial NR gene regulation section) of which several have been identified in the HKII promoter. It will be of interest to determine whether LXR shares a compound element with the characterized PPAR response element in the HKII promoter and whether LXR antagonists prevent glucose mediated induction of HKII expression.
Enhancement of glucose metabolism
Free glucose (or G-6-P) can bind LXRs and induce transactivation of LXR targets genes involved in cholesterol, fatty acid and carbohydrate metabolism.73 LXRs are expressed widely and in both normal and tumorigenic breast74 and prostate cells.75,76 Considering the huge intake of glucose in cancer cells, mere is a significant amount of substrate for the LXR to interact with. However, as many NR are regulated by co-repressors and co-activators,77 the mere presence of ligand may not be sufficient to induce gene expression.
The co-repressors NCOR1 and NCOR2/SMRT limit signaling of NRs including LXRs, PPARs, VDR and RARs.56,58–60,78–82. This distortion results in selective skewing of the transcriptome (reviewed in Ref. 4). It remains a tantalizing possibility that LXRα signaling is similarly distorted to sustain the capacity of glucose to signal and facilitate further the Warburg effect. Indeed there is evidence this may occur; LXR has higher basal mRNA levels in prostate cancer cells than non-malignant counterparts and have diminished sensitivity to natural LXR ligands.60 This is in agreement with data from the SAGE genie anatomical viewer which indicates LXRα shows approximately sevenfold mRNA elevation in tumour compared to non-malignant matched tissue. LXR agonism has significant anti-tumour function through inhibition of Akt activity in a cholesterol-mediated manner,83 so whether it acts with oncogenic or tumour suppressor behaviour is unclear. Nonetheless epigenetic mechanism mediated via distorted co-repressor interactions may be central to the selective distortion of LXRs actions.
Glucose also induces FXR mRNA and protein expression and cooperates with FXR ligands to additively regulate several FXR targets involved in triglyceride and bile acid homeostasis.84 This is counter to the actions of insulin which inhibit FXR expression and FXR mRNA is lowered in two rat models of diabetes.84 Again, there is reasonable evidence in malignancy that the normally well integrated actions of FXR are selectively disrupted and an oncogenic subset of the transcriptome is maintained in a transcriptionally responsive manner.59,85–87
These examples highlight the fact that there are several GLUT transporters controlled by NRs that are deregulated in cancer, which lead to increased levels of substrate for the metabolic pathways. A significant association occurs between expression of these GLUT family members and the selective and enhanced functions of key NR such as TR and LXR. In addition to enhancing transport of glucose, NRs can enhance the rate at which conversion to G-6-P by HKII occurs. The mere presence of excessive glucose within the cell appears sufficient for the cell to switch to aerobic glycolysis as a preferential form of energy generation.22 NR deregulation may therefore aid in the shift to aerobic glycolysis solely because of elevated glycolytic flux. This is an attractive hypothesis as it supports the idea that mitochondrial dysfunction is not necessarily a pre-requisite for the Warburg shift.88
Downstream of the initial step of sequestration in glucose metabolism comes the key conversion of pyruvate to lactic acid. This reaction is controlled by the opposing actions of the LDH-A (forward) and LDH-B (reverse) isoforms.89 Loss of LDH-B is an early event in breast cancer through promoter DNA methylation90 and LDH-A expression, which drives conversion of pyruvate to lactate, is significantly enhanced in many cancer types. MYC and hypoxia91 increase transcriptional expression of LDH-A. ChIP assays also revealed that ERRα binds to response elements within the promoters of both LDH-A and LDH-B isoforms in human thyroid cancer tissue and induces LDH gene expression.92
The involvement of the nuclear co-activator, PGC-1α, adds a layer of subtlety to the transcriptional relationship between ERRα and LDH. In skeletal cells, under oxidative stress induced by exercise, PGC-1α is able to differentially regulate the two major LDH isoforms. Using ERRα as a direct intermediaory, PGC-1a increases the ratio of LDH-B/LDH-A.93 Direct stimulation of PPAR-β/δ may also support LDH-B expression in tumours as these NRs are able to induce expression of LDH-B via AMPK and MEK2.94 LDH-B may also be driven indirectly by PPAR-γ through transcriptional activation of MEF2. Conversely, Estradiol (E2) induces expression of LDH-A in the rat via its control over the CREB transcription factor.95 A contribution to the effects of selective ER modulators (SERMs) is likely to be antagonizing lactate production.
Enhanced glucose metabolism influences the tumor microenvironment
A major corollary of enhanced glucose metabolism is the influence of the epithelial tumour cells over its microenvironment. The excretion of lactic acid causes acidification of the local area. The roles of Carbonic anhydrase XII (CA12) and monocarboxylate transporters (MCT) in this process of acidification are increasingly apparent. CA12 catalyzes conversion of CO2 to bicarbonate thus acidifying the local region. MCTs are major transporters of lactate and other proton donating moieties. In breast cancer CA12 expression is tightly linked to ERα levels and estradiol stimulates its expression. Using chromatin conformation capture and ChIP assays, Barnett et al. established that a distal enhancer becomes bound by ERα and recruits RNA-polymerase and co-activators to the promoter of the CA12 gene.96 Small molecules targeting these acidification factors are currently under intensive research (reviewed in Refs. 97 and 98)
Secondary Effects of NRs in the Warburg Effect
The “Warburg kinase” AKT and HIF1a
AKT is a potent oncogenic kinase, and controls the expression and localization of several glucose transporters and hexokinase activity (reviewed in Ref. 70). AKT is amplified in breast and colon cancer99 and deregulation is common in breast, prostate, pancreatic and gastric cancers.100–102 AKT activation probably leads to HIF1α stabilization via mTORC,103–106 even in the absence of hypoxia, further enhancing the aerobic glycolysis phenotype. Furthermore AKT deactivation of cell cycle checkpoints leads to rapid proliferation, an increased demand for ATP, and thus depletion of the cellular ATP/AMP ratio. This has the significant effect of deactivating AMPK, the “brake” that can limit the activity of PI3K (the upstream effector of AKT and mTORC).107 Thus aberrant AKT imposes a positive feedback loop on cell growth by inducing factors that elevate glucose and allow its metabolism to generate ATP whilst repressing factors that control normal cellular checkpoints. PTEN can negatively regulate AKT signaling and is also frequently mutated in several solid cancers.108–112
NR regulation of AKT and HIF1a
Crucially, AKT and HIF1a are activated and controlled in multiple ways and the roles of several NRs in their regulation are significant. IGF1 which is stabilized by IGFBPs that in turn are downstream of multiple NRs including VDR,113 ER 114 and RXR115,116 can induce AKT activity along with insulin itself. The PV mutation in the TRβ causes hyper-activation of AKT by excessive phosphorylation.117 T3 can induce PI3K signaling via TR118–120 and TRIP230, a THR co-factor, interacts with ARNT and HIF1α on the promoters of hypoxia inducible genes.121 RARβ is downregulated through the PI3K/AKT pathway122 and all-trans retinoic acid can activate the PI3K/AKT pathway via RARs in MEF’s and COS-7 cells.123 FXR is also documented to enhance AKT signaling,124,125 which can establish positive feedback as AKT can activate PKC, which in turn phosphorylates FXR and cause recruitment of PPARgC1.126 Interestingly, the co-repressor NCOR1 binds to and represses key members of the AKT/PI3K pathway and is repressed in thyroid cancers, presumably resulting increased AKT signaling.117
In addition to being regulated by NRs, activation of the AKT pathway can lead to the deregulation of several NRs. Perhaps surprisingly NUR77127 which is a potent inducer of HIF1α128 is inactivated by AKT signaling, although this may be cell type dependent.129 More predictably however is the AKT mediated inhibition of RARα130 and RAR mediated cell cycle arrest and differentiation. It is certainly possible therefore that AKT contributes to retinoid therapy resistance.
Under normal conditions, metabolic requirement and hypoxia are major factors governing the rate of metabolism and therefore processes such as glycolytic flux and synthesis of fatty acids. NRs alter these cellular decisions by altering their own transcription targets and influencing the activity of several signaling pathways such as AKT/PI3K. If AKT can cause the switch to aerobic glycolysis by stabilizing HIF-1, then the switch is in part anticipatory of hypoxia rather than reactive. Switching to the glycolytic pathway increases the amount of lactic acid released by cells thereby causing an acidic environment around them. This selects for cells resistant to an acidic environment in the rapidly dividing tumor, prevents proper immune invasion and is damaging to surrounding normal tissue, thereby giving further mechanisms of selection for aggressive growth of the tumor.131
Impact on Cancer Diagnosis and Therapy
Highlighted in this review are nuclear receptors that impinge on multiple aspects of the glycolytic pathway in cancer; some support whilst other inhibit the Warburg effect, and their activity is either enhanced or suppressed to allow the shift to continue. Many NRs respond to dietary derived factors and environmental cues and thus represent a large repertoire of targets against which novel therapies can be directed, and many of which have been attractive targets for differentiation therapy. Stimulation of the NRs PPARd and the PPAR co-factor PGC1a could have a significant impact upon the ability of cancer cells to generate lactate from pyruvate because of their enhancement of the lactacte to pyruvate enzymatic reaction.
Summary
NRs integrate endocrine signals and those from the microenvironment, to control cellular metabolism and growth. Several NRs play key roles in the progression of tumours, either through activation of their oncogenic properties, or through silencing of their tumor suppressor ones. The current review presents evidence that they are acutely involved in the shift from oxidative phosphorylation to aerobic glycolysis, and therefore play a central role in the Warburg effect. The Warburg effect is now understood to be far more than the enhancement of ATP generation, although this is still a major component.
Given their central role in interpreting cellular signals, and the wide-array of transcriptional targets they control, NRs are well placed to allow the tumour to generate ATP and the essential biomass precursors that result from diverting glucose utilization to alternative pathways. Understanding of how glucose transport mechanisms become enhanced in cancer remains incomplete but is partially explained by a combination of oncogene activation132 and tumour suppressor gene inactivation.25 There is significant evidence from the studies outlined here that multiple NRs converge on several high capacity/affinity GLUT transporters to bring about gene expression changes. Changes to NR co-factor expression, ligand accessibility and the actual expression of NRs themselves are frequent events in many tumour types and lead to a shift in the activity of their transcriptional targets. NRs therefore provide multiple additional mechanisms through which elevation of GLUT expression to pathological levels is achieved by tumours. In parallel there is a growing appreciation of how NR transcriptomes can be modulated pharmacologically and this therefore represents an exciting area of research to target the distorted glucose metabolism that is prevalent in malignancy.1,7,133–135
The integration between NRs, oncogenes, tumour suppressor genes and cellular metabolism underlines the importance of normal and distorted NR functions in tumour progression and their continued suitability for clinical research and drug development.
Table 1.
Illustrative examples of NR regulation of key genes that appear to play central roles in the Warburg effect in cancer cells
| NR | Target Gene | Direction | Reference |
|---|---|---|---|
| CAR | HKII | Up | 65–68 |
| ER | LDH-A | Up | 95 |
| GLUT4 | Up | ||
| ERRa | LDHA | Up | 92 |
| LDHB | Up | 92 | |
| HKII | Up | 66,67 | |
| LXR | HKII | Up | 71 |
| PGC1a | LDHA | Down | 92 |
| LDHB | Up | 92 | |
| PPARd | GLUT2 | Up | 45 |
| LDHB | Up | 93 | |
| PPARg | GLUT4 | Up | 46 |
| HKII | Up | 64 | |
| PR | GLUT4 | Up | |
| TR | GLUT1 | Up | 34–37 |
| GLUT3 | Up | 34–37 | |
| GLUT4 | Up | 43 | |
| VDR | GLUT1 | Up | 47 |
| GLUT4 | Up | 48 |
Acknowledgments
M.J.C. acknowledges the support of NucSys, a European Community FP6-Marie Curie Research Training Network, the Biotechnology and Biological Sciences Research Council [BB/D523651/1] and support in part from National Institute of Health grants [R01 CA095367-06 and 2R01-CA-095045-06]. M.J.C. also acknowledges support, in part, of the NCI Cancer Center Support Grant to the Roswell Park Cancer Institute [CA016056]. J.L.T. acknowledges support of Yorkshire Cancer Research [LPP064] and the Breast Cancer Research Action Group.
Grant sponsor: NucSys, a European Community FP6-Marie Curie Research Training Network, the Biotechnology and Biological Sciences Research Council; Grant number: BB/D523651/1; Grant sponsor: National Institute of Health grants; Grant numbers: R01 CA095367-06 and 2R01-CA-095045-06; Grant sponsor: NCI Cancer Center Support Grant to the Roswell Park Cancer Institute; Grant number: CA016056, LPP064; Grant support: Breast Cancer Research Action Group
References
- 1.Hollenberg AN. Metabolic health and nuclear-receptor sensitivity. New Engl J Med. 2012;366:1345–1347. doi: 10.1056/NEJMcibr1114529. [DOI] [PubMed] [Google Scholar]
- 2.Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Lamia KA, Evans RM. Nuclear receptors, metabolism, and the circadian dock. Cold Spring Harb Symp Quant Biol. 2007;72:387–394. doi: 10.1101/sqb.2007.72.058. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia S, Maguire O, Campbell MJ. Transcription factor co-repressors in cancer biology: roles and targeting. Int J Cancer. 2010;126:2511–2519. doi: 10.1002/ijc.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 6.Close AF, Rouillard C, Buteau J. NR4A orphan nuclear receptors in glucose homeostasis: a minireview. Diabetes Metab. 2013;39:478–484. doi: 10.1016/j.diabet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Baek SH, Kim KI. Emerging roles of orphan nuclear receptors in cancer. Annu Rev Physiol. 2014;76:177–195. doi: 10.1146/annurev-physiol-030212-183758. [DOI] [PubMed] [Google Scholar]
- 8.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long MD, Thorne JL, Russell J, et al. Cooperative behavior of the nuclear receptor superfamily and its deregulation in prostate cancer. Carcinogenesis. 2014;35:262–271. doi: 10.1093/carcin/bgt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amoedo ND, Valencia JP, Rodrigues MF, et al. How does the metabolism of tumour cells differ from that of normal cells. Biosci Rep. 2013;33:865–873. doi: 10.1042/BSR20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (review) Oncol Lett. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cori CF, Cori GT. The carbohydrate metabolism of tumors. II. Changes in the sugar, lactic acid, and co-combing power of blood passing through a tumor. J Biol Chem. 1925;65:397–405. [Google Scholar]
- 19.Warburg O. Origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 20.Hoh C. Cancer detection with whole-body PET using 2-[18F]fluoro-2-deoxy-d-glucose. J Comput Assist Tomogr. 1993;17:582–589. doi: 10.1097/00004728-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez A, Liu J, Zhou Y, et al. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58. doi: 10.1186/1752-0509-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joost HG, Bell GI, Best JD, et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol. 2002;282:E974–E976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- 24.Macdougald OA, Lane MD. Transcriptional regulation of gene-expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 25.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 26.Younes M, Juarez D, Lechago LV, et al. Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res. 2001;21:575–578. [PubMed] [Google Scholar]
- 27.Younes M, Brown RW, Stephenson M, et al. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Haber RS, Weiser KR, Pritsker A, et al. GLUT1 glucose transporter expression in benign and malignant thyroid nodules. Thyroid. 1997;7:363–367. doi: 10.1089/thy.1997.7.363. [DOI] [PubMed] [Google Scholar]
- 29.Younes M, Brown RW, Mody DR, et al. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 30.Yun JY, Rago C, Cheong I, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godoy A, Ulloa V, Rodriguez F, et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614–627. doi: 10.1002/jcp.20606. [DOI] [PubMed] [Google Scholar]
- 32.Binder C, Binder L, Marx D, et al. Deregulated simultaneous expression of multiple glucose transporter isoforms in malignant cells and tissues. Anticancer Res. 1997;17:4299–4304. [PubMed] [Google Scholar]
- 33.Garrido P, Moran J, Alonso A, et al. 17beta-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology. 2013;154:1979–1989. doi: 10.1210/en.2012-1558. [DOI] [PubMed] [Google Scholar]
- 34.Medina RA, Meneses AM, Vera JC, et al. Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology. 2003;144:4527–4535. doi: 10.1210/en.2003-0294. [DOI] [PubMed] [Google Scholar]
- 35.Santalucia T, Palacin M, Zorzano A. T3 strongly regulates GLUT1 and GLUT3 mRNA in cerebral cortex of hypothyroid rat neonates. Mol Cell Endocrinol. 2006;251:9–16. doi: 10.1016/j.mce.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Ulisse S, Jannini EA, Pepe M, et al. Thyroid hormone stimulates glucose transport and GLUT1 mRNA in rat Sertoli cells. Mol Cell Endocrinol. 1992;87:131–137. doi: 10.1016/0303-7207(92)90241-w. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein SP, Haber RS. Glucose transport stimulation by thyroid hormone in ARL 15 cells: partial role of increased GLUT1 glucose transporter gene transcription. Thyroid. 1993;3:135–142. doi: 10.1089/thy.1993.3.135. [DOI] [PubMed] [Google Scholar]
- 38.Torrance CJ, Usala SJ, Pessin JE, et al. Characterization of a low affinity thyroid hormone receptor binding site within the rat GLUT4 gene promoter. Endocrinology. 1997;138:1215–1223. doi: 10.1210/endo.138.3.4982. [DOI] [PubMed] [Google Scholar]
- 39.Moeller LC, Cao X, Dumitrescu AM, et al. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nuclear Receptor Signal. 2006;4:e020. doi: 10.1621/nrs.04020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holzapfel HP, Bergner B, Wonerow P, Paschke R. Expression of G(alpha)(s) proteins and TSH receptor signalling in hyperfunctioning thyroid nodules with TSH receptor mutations. Eur J Endocrinol. 2002;147:109–116. doi: 10.1530/eje.0.1470109. [DOI] [PubMed] [Google Scholar]
- 41.Deokant BS, Sinha MP. Alteration of thyroid hormone profile as a biomarker of carcinogenesis. Global Journal of Bio-science and Biotechnology. 2012;1:40–44. [Google Scholar]
- 42.Cheng SY. Isoform-dependent actions of thyroid hormone nuclear receptors: lessons from knockin mutant mice. Steroids. 2005;70:450–454. doi: 10.1016/j.steroids.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Itoh Y, Esaki T, Kaneshige M, et al. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone alpha or beta receptor gene. Proc Natl Acad Sci USA. 2001;98:9913–9918. doi: 10.1073/pnas.171319498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Casanova B, Pulido N, et al. Stimulation of glucose transport by thyroid hormone in 3T3-L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J Endocrinol. 2000;164:187–195. doi: 10.1677/joe.0.1640187. [DOI] [PubMed] [Google Scholar]
- 45.Lee CH, Olson P, Hevener A, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao M, Long Y, Tong Y, et al. Activation of PPARdelta up-regulates the expression of insulin gene transcription factor MafA and ameliorates glucose-induced insulin secretion impaired by palmitate. Mol Cell Biochem. 2012;366:183–189. doi: 10.1007/s11010-012-1296-9. [DOI] [PubMed] [Google Scholar]
- 47.Hallakou S, Doare L, Foufelle F, et al. Pioglita-zone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–1399. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 48.Nasri H, Behradmanesh S, Maghsoudi AR, et al. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J Renal Injury Prevent. 2014;3:31–34. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunter J, Kassam A, Winrow CJ, et al. Crosstalk between the thyroid hormone and peroxisome proliferator-activated receptors in regulating peroxisome proliferator-responsive genes. Mol Cell Endocrinol. 1996;116:213–221. doi: 10.1016/0303-7207(95)03717-9. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto T, Kaneko A, Kakizawa T, et al. Inhibition of peroxisome proliferator signaling pathways by thyroid hormone receptor. Competitive binding to the response element. J Biol Chem. 1997;272:7752–7758. doi: 10.1074/jbc.272.12.7752. [DOI] [PubMed] [Google Scholar]
- 51.Araki O, Ying H, Furuya F, Zhu X, Cheng SY. Thyroid hormone receptor beta mutants: Dominant negative regulators of peroxisome proliferator-activated receptor gamma action. Proc Natl Acad Sci USA. 2005;102:16251–16256. doi: 10.1073/pnas.0508556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gbaguidi GF, Agellon LB. The inhibition of the human cholesterol 7alpha-hydroxylase gene (CYP7A1) promoter by fibrates in cultured cells is mediated via the liver x receptor alpha and peroxisome proliferator-activated receptor alpha heterodimer. Nucleic Acids Res. 2004;32:1113–1121. doi: 10.1093/nar/gkh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boergesen M, Pedersen TA, Gross B, et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol. 2012;32:852–867. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sachdev D, Yee D. The IGF system and breast cancer. Endocr Relat Cancer. 2001;8:197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- 55.Thorne JL, Maguire O, Doig CL, et al. Epigenetic control of a VDR-governed feed-forward loop that regulates p21((waf1/cip1)) expression and function in non-malignant prostate cells. Nucleic Acids Res. 2011;39:2045–2056. doi: 10.1093/nar/gkq875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doig CL, Singh PK, Dhiman VK, et al. Recruitment of NCOR1 to VDR target genes is enhanced in prostate cancer cells and associates with altered DNA methylation patterns. Carcinogenesis. 2013;34:248–256. doi: 10.1093/carcin/bgs331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh PK, Doig CL, Dhiman VK, et al. Epigenetic distortion to VDR transcriptional regulation in prostate cancer cells. J Steroid Biochem Mol Biol. 2013;136:258–263. doi: 10.1016/j.jsbmb.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abedin SA, Banwell CM, Colston KW, et al. Epigenetic corruption of VDR signalling in malignancy. Anticancer Res. 2006;26:2557–2566. [PubMed] [Google Scholar]
- 59.Abedin SA, Thorne JL, Battaglia S, et al. Elevated NCOR1 disrupts a network of dietary-sensing nuclear receptors in bladder cancer cells. Carcinogenesis. 2009;30:449–456. doi: 10.1093/carcin/bgp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battaglia S, Maguire O, Thome JL, et al. Elevated NCOR1 disrupts PPARalpha/gamma signaling in prostate cancer and forms a targetable epigenetic lesion. Carcinogenesis. 2010;31:1650–1660. doi: 10.1093/carcin/bgq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furuya F, Guigon CJ, Zhao L, et al. Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol Cell Biol. 2007;27:6116–6126. doi: 10.1128/MCB.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frasor J, Danes JM, Funk CC, et al. Estrogen down-regulation of the corepressor N-CoR: Mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc Nail Acad Sci USA. 2005;102:13153–13157. doi: 10.1073/pnas.0502782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunlop TW, Vaisanen S, Frank C, et al. The genes of the coactivator TIF2 and the corepressor SMRT are primary 1alpha,25(OH)2D3 targets. J Steroid Biochem Mol Biol. 2004;89:257–260. doi: 10.1016/j.jsbmb.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 64.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s Stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panasyuk G, Espeillac C, Chauvin C, et al. PPARgamma contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong B, Saha PK, Huang W, et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci USA. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Onishi A, Peng GH, Poth EM, et al. The orphan nuclear hormone receptor ERRbeta controls rod photoreceptor survival. Proc Natl Acad Sci USA. 2010;107:11579–11584. doi: 10.1073/pnas.1000102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tennessen JM, Baker KD, Lam G, et al. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13:139–148. doi: 10.1016/j.cmet.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 70.Robey RB, Hay N. Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Defour A, Dessalle K, Castro Perez A, et al. Sirtuin 1 regulates SREBP-1c expression in a LXR-dependent manner in skeletal muscle. PloS One. 2012;7:e43490. doi: 10.1371/journal.pone.0043490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rempel A, Mathupala SP, Perdersen PL. Glucose catabolism in cancer cells: regulation of the Type II hexokinase promoter by glucose and cyclic AMP. FEBS Lett. 1996;385:233–237. doi: 10.1016/0014-5793(96)00399-7. [DOI] [PubMed] [Google Scholar]
- 73.Mitro N, Mak PA, Vargas L, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 74.Vigushin DM, Dong Y, Inman L, et al. The nuclear oxysterol receptor LXRalpha is expressed in the normal human breast and in breast cancer. Med Oncol. 2004;21:123–131. doi: 10.1385/MO:21:2:123. [DOI] [PubMed] [Google Scholar]
- 75.Wang JH, Tuohimaa P. Calcitriol and TO-901317 interact in human prostate cancer LNCaP cells. Gene Regul Syst Biol. 2008;2:97–105. doi: 10.4137/grsb.s562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thorne JL, Long M, Russell J, et al. Cooperative behavious of the Nuclear Receptor Superfamily ands its deregulation in Prostate Cancer. Carcinogenesis. 2014;35:262–271. doi: 10.1093/carcin/bgt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu C, Markan K, Temple KA, et al. The nuclear receptor corepressors NCoR and SMRT decrease PPARgamma transcriptional activity and repress 3T3-L1 adipogenesis. J BiolChem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 78.Banwell CM, O’Neill LP, Uskokovic MR, et al. Targeting 1alpha,25-dihydroxyvitamin D3 anti-proliferative insensitivity in breast cancer cells by co-treatment with histone deacetylation inhibitors. J Steroid Biochem Mol Biol. 2004;89:245–249. doi: 10.1016/j.jsbmb.2004.03.081. [DOI] [PubMed] [Google Scholar]
- 79.Banwell CM, Singh R, Stewart PM, et al. Anti-proliferative signalling by 1,25(OH)2D3 in prostate and breast cancer is suppressed by a mechanism involving histone deacetylation. Recent Results Cancer Res. 2003;164:83–98. doi: 10.1007/978-3-642-55580-0_5. [DOI] [PubMed] [Google Scholar]
- 80.Banwell CM, Maccartney DP, Guy M, et al. Altered nuclear receptor corepressor expression attenuates vitamin d receptor signaling in breast cancer cells. Clin Cancer Res. 2006;12:2004–2013. doi: 10.1158/1078-0432.CCR-05-1218. [DOI] [PubMed] [Google Scholar]
- 81.Gommersall LM, Khanim FL, Peehl DM, et al. Epigenetic repression of transcription by the Vitamin D3 receptor in prostate cancer cells. J Steroid Biochem Mol Biol. 2004;89:251–256. doi: 10.1016/j.jsbmb.2004.03.080. [DOI] [PubMed] [Google Scholar]
- 82.Khanim FL, Gommersall LM, Wood VH, et al. Altered SMRT levels disrupt vitamin D3 receptor signalling in prostate cancer cells. Oncogene. 2004;23:6712–6725. doi: 10.1038/sj.onc.1207772. [DOI] [PubMed] [Google Scholar]
- 83.Pommier AJ, Alves G, Viennois E, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 84.Duran-Sandoval D, Mautino G, Martin, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–898. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 85.Swales KE, Korbonits M, Carpenter R, et al. The farnesoid x receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 86.Kim I, Morimura K, Shah Y, et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Journe F, Durbecq V, Chaboteaux C, et al. Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast Cancer Res Treat. 2009;115:523–535. doi: 10.1007/s10549-008-0094-2. [DOI] [PubMed] [Google Scholar]
- 88.Calabrese C, Iommarini L, Kurelac I, et al. Respiratory complex I is essential to induce a Warburg profile in mitochondria-defective tumor cells. Cancer Metab. 2013;1:11. doi: 10.1186/2049-3002-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dawson DM, Goodfriend TL, Kaplan NO. Lactic dehydrogenases: functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science. 1964;143:929–933. doi: 10.1126/science.143.3609.929. [DOI] [PubMed] [Google Scholar]
- 90.Brown NJ, Higham SE, Perunovic B, et al. Lactate dehydrogenase-B is silenced by promoter methylation in a high frequency of human breast cancers. PloS One. 2013;8:e57697. doi: 10.1371/journal.pone.0057697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirebeau-Prunier D, Le Pennec S, Jacques C, et al. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PloS One. 2013;8:e58683. doi: 10.1371/journal.pone.0058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Summermatter S, Santos G, Perez-Schindler J, et al. Skeletal muscle PGC-1 alpha controls whole-body lactate homeostasis through estrogen-related receptor alpha-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA. 2013;110:8738–8743. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gan ZJ, Burkart-Hartman EM, Han DH, et al. The nuclear receptor PPAR beta/delta programs muscle glucose metabolism in cooperation with AMPK and MEF2. Gene Dev. 2011;25:2619–2630. doi: 10.1101/gad.178434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Qin C, Burghardt R, et al. Hormonal regulation of lactate dehydrogenase-A through activation of protein kinase C pathways in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2004;320:625–634. doi: 10.1016/j.bbrc.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 96.Barnett DH, Sheng S, Charn TH, et al. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 97.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 98.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J Cell Physiol. 2011;226:299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 99.Luo J, Manning BD, Cantley LC. Targeting the PI3K–Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 100.Paweletz CP, Charboneau L, Bichsel VE, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 101.Paweletz CP, Liotta LA, Petricoin EF., III New technologies for biomarker analysis of prostate cancer progression: Laser capture microdissection and tissue proteomics. Urology. 2001;57:160–163. doi: 10.1016/s0090-4295(00)00964-x. [DOI] [PubMed] [Google Scholar]
- 102.Stal O, Perez-Tenorio G, Akerberg L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003;5:R37–R44. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brugarolas JB, Vazquez F, Reddy A, et al. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 104.Skeen JE, Bhaskar PT, Chen CC, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 106.Semenza GL, Roth PH, Fang HM, et al. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 107.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. MolCell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 108.Gray IC, Stewart LM, Philips SM, et al. Mutation and expression analysis of the putative prostate tumour-suppressor gene PTEN. Br J Cancer. 1998;78:1296–1300. doi: 10.1038/bjc.1998.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Modur V, Nagarajan R, Evers BM, et al. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 110.Aveyard JS, Skilleter A, Habuchi T, et al. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer. 1999;80:904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cairns P, Evron E, Okami K, et al. Point mutation and homozygous deletion of PTENJ MMAC1 in primary bladder cancers. Oncogene. 1998;16:3215–3218. doi: 10.1038/sj.onc.1201855. [DOI] [PubMed] [Google Scholar]
- 112.Yang J, Ren Y, Wang L, et al. PTEN mutation spectrum in breast cancers and breast hyperplasia. J Cancer Res Clin Oncol. 2010;136:1303–1311. doi: 10.1007/s00432-010-0781-3. [DOI] [PubMed] [Google Scholar]
- 113.Thimmaiah KN, Easton JB, Germain GS, et al. Identification of N1O–substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- 114.Foulstone EJ, Zeng L, Perks CM, et al. Insulin-like growth factor binding protein 2 (IGFBP-2) promotes growth and survival of breast epithelial cells: novel regulation of the estrogen receptor. Endocrinology. 2013;154:1780–1793. doi: 10.1210/en.2012-1970. [DOI] [PubMed] [Google Scholar]
- 115.Lee KW, Ma LQ, Liu BR, et al. Insulin-like growth factor binding protein-3 (IGFBP-3) induces apoptosis via rapid RXR alpha-dependent TR3 mitochondrial targeting in cancer cells. Pediatr Res. 2003;53:154. [Google Scholar]
- 116.Dailly YP, Linkhart TA, Mohan S, et al. The human IGFBP-6 promoter is regulated by a novel complex retinoic acid response element (RARE) activated by RAR alpha, RXR alpha receptors and a CACC box binding protein in human osteoblasts. J Bone Miner Res. 2000;15:S195. [Google Scholar]
- 117.Furuya F, Guigon CJ, Zhao L, et al. Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol Cell Biol. 2007;27:6116–6126. doi: 10.1128/MCB.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furuya F, Hanover JA, Cheng SY. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc Natl Acad Sci USA. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verga Falzacappa C, Petrucci E, Patriarca V, et al. Thyroid hormone receptor TRbetal mediates Akt activation by T3 in pancreatic beta cells. J Mol Endocrinol. 2007;38:221–233. doi: 10.1677/jme.1.02166. [DOI] [PubMed] [Google Scholar]
- 120.Furuya F, Lu C, Guigon CJ, et al. Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors. Steroids. 2009;74:628–634. doi: 10.1016/j.steroids.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beischlag TV, Taylor RT, Rose DW, et al. Recruitment of thyroid hormone receptor/retinoblastoma-interacting protein 230 by the aryl hydrocarbon receptor nuclear translocator is required for the transcriptional response to both dioxin and hypoxia. J Biol Chem. 2004;279:54620–54628. doi: 10.1074/jbc.M410456200. [DOI] [PubMed] [Google Scholar]
- 122.Lefebvre B, Brand C, Flajollet S, et al. Down-regulation of the tumor suppressor gene retinoic acid receptor beta2 through the phosphoinositide 3-kinase/Akt signaling pathway. Mol Endocrinol. 2006;20:2109–2121. doi: 10.1210/me.2005-0321. [DOI] [PubMed] [Google Scholar]
- 123.Masia S, Alvarez S, de Lera AR, et al. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 124.Renga B, Mencarelli A, Vavassori P, et al. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Rizzo G, Passeri D, De Franco F, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gineste R, Sirvent A, Paumelle R, et al. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol. 2008;22:2433–2447. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 127.Pekarsky Y, Hallas C, Palamarchuk A, et al. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA. 2001;98:3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoo YG, Yeo MG, Kim DK, et al. Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1 alpha. J Biol Chem. 2004;279:53365–53373. doi: 10.1074/jbc.M408554200. [DOI] [PubMed] [Google Scholar]
- 129.Masuyama N, Oishi K, Mori Y, et al. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276:32799–32805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 130.Srinivas H, Xia D, Moore NL, et al. Akt phosphorylates and suppresses the transactivation of retinoic acid receptor alpha. Biochem J. 2006;395:653–662. doi: 10.1042/BJ20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 132.Flier JS, Mueckler MM, Usher P, et al. Elevated levels of glucose-transport and transporter messenger-RNA are induced by Ras or Src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 133.Deblois G, Giguere V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13:27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 134.Battaglia S, Maguire O, Campbell MJ. Transcription factor co-repressors in cancer biology: roles and targeting. Int J Cancer. 2010;126:2511–2519. doi: 10.1002/ijc.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Campbell MJ, Carlberg C, Koeffler HP. A role for the PPARgamma in cancer therapy. PPAR Res. 2008;2008:314974. doi: 10.1155/2008/314974. [DOI] [PMC free article] [PubMed] [Google Scholar]