Abstract
Deceased donors are labeled increased risk for disease transmission (IRD) if they meet certain criteria. New PHS guidelines were recently implemented; the impact of these changes remains unknown. We aimed to quantify the impact of the new guidelines on the proportion of deceased donors labeled IRD, as well as demographic and clinical characteristics. We used Poisson regression with an interaction term for era (new versus old guidelines) to quantify changes. Under the new guidelines, 19.5% donors were labeled IRD, compared to 10.4%, 12.2%, and 12.3% in the 3 most recent years under the old guidelines (IRR = 1.45, p<0.001). Increases were consistent across OPOs: 44/59 had an increase in the percent of donors labeled IRD, and 25 OPOs labeled >25% of their donors IRD under the new guidelines (versus 5 OPOs under the old). African-Americans were 52% more likely to be labeled IRD under the new guidelines (RR=1.52, p=0.01). There has been a substantial increase in donors labeled IRD under the new PHS guidelines; it is important to understand the mechanism and consequences to ensure an optimal balance of patient safety and organ utilization is achieved.
INTRODUCTION
While all potential deceased donors are tested for infectious diseases such as HIV and hepatitis C virus (HCV) by serology, donors who are infected just before death, during the window period (WP) of the serologic test used, may test negative and subsequently transmit the infection to recipients of their organs (1). Based on guidelines from the Centers for Disease Control and Prevention (CDC) and Public Health Service (PHS), donors are categorized as at increased risk for disease transmission (IRD) if they meet certain criteria thought to increase the risk of undetected HIV or HCV infection (2).
These guidelines seek to improve organ safety by reducing HIV, HBV, and HCV transmission through transplantation, without unnecessarily decreasing organ availability, given significant clinical need in the setting of a severe organ shortage (3). While the risk that an IRD is infected during the WP is higher relative to a non-IRD, the absolute risk is still very low (published risks ranging from < 1 in 1000 for hepatitis C to <1 in 10,000 for HIV) (4, 5), and many patients are predicted to derive substantial survival benefit (6). While the IRD label is intended to reduce infectious transmission, there are potential downsides. For example, UNOS mandates centers obtain special informed consent when an IRD organ is transplanted (7), and some patients are unwilling to accept IRD offers (8, 9). Furthermore, these organs are perceived to carry increased legal risk (10, 11), and a study in 2010 found that IRD organs were discarded at 1.5 times the rate of comparable non-IRDs (12).
In 1994, the CDC published guidelines delineating increased risk categories; donors falling into one or more of these were classified as IRD (Table 1A) (2). In 2013, they published new guidelines which modified the 1994 categories, including the addition of persons on hemodialysis in the year preceding death (increased risk for HCV infection only, Table 1), and more detailed specification of criteria under which a person with a sexually transmitted infection (STI) should be considered IRD (3). Other significant changes were the inclusion of risk factors for HIV, HBV, and HCV (versus HIV only in 1994), a shortened time frame for many categories to better reflect the risk of recent infection (from 5 years to 12 months), a clear definition of incarceration, and the elimination of the hemophiliac and cutaneous exposure categories. During the transition period, between August 27, 2013 and January 31, 2014, those filling out the UNOS deceased donor form could choose whether to designate a donor as IRD using either the old or new guidelines; as of February 1, 2014, use of the new guidelines was required (13).
Table 1.
Summary of (a) 1994 and (b) 2013 Guidelines for Reducing Transmission Through Organ Transplantation:
| 2013 Guidelines: Increased Risk for HIV, HBV, and HCV |
1994 Guidelines: Increased Risk for HIV Only |
|---|---|
| Men who have had sex with men (MSM) in the preceding 12
months |
Men who have had sex with men (MSM) in the preceding 5
years |
| People who have injected drugs by intravenous, intramuscular or subcutaneous route for nonmedical reasons in the preceding 12 months |
Persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years. |
| No Equivalent | Persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates |
| People who have had sex in exchange for money or drugs in the preceding 12 months |
Men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years. |
| Women who have had sex with a man with a history of MSM behavior in the preceding 12 months |
Persons who have had sex in the preceding 12 months with any person described in items above or with a person known or suspected to have HIV infection. |
| People who have had sex with a person who had sex in exchange for money or drugs in the preceding 12 months | |
| People who have had sex with a person who injected drugs by intravenous, intramuscular or subcutaneous route for nonmedical reasons in the preceding 12 months | |
| People who have had sex with a person known or suspected to have HIV, HBV or HCV infection in the preceding 12 months | |
| No Equivalent | Persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane |
| People who have been in lockup, jail, prison or a juvenile
correctional facility for more than 72 consecutive hours in the preceding 12 months |
Inmates of correctional systems |
| People who have been newly diagnosed with, or have been treated for, syphilis, gonorrhea, Chlamydia or genital ulcers in the preceding 12 months |
Persons whose history, physical examination, medical
records, or autopsy reports reveal other evidence of HIV infection or high-risk behavior, such as diagnosis of AIDS, unexplained weight loss, night sweats, blue or purple spots on the skin or mucous membranes typical of Kaposi’s sarcoma, unexplained lymphadenopathy lasting > 1 month, unexplained temperature > 100.5 (38.6 C) for > 10 days, unexplained persistent cough and shortness of breath, opportunistic infections, unexplained persistent diarrhea, male-to-male sexual contact, sexually transmitted diseases, or needle tracks or other signs of parenteral drug abuse. |
| No Equivalent | Persons who cannot be tested for HIV infection because of refusal, inadequate blood samples (e.g., hemodilution that could result in false-negative tests), or any other reasons. |
| No Equivalent | Persons with a repeatedly reactive screening assay for HIV-1 or HIV-2 antibody regardless of the results of supplemental assays |
| People who have been on hemodialysis in the preceding 12 months (Increased risk for HCV only) |
No Equivalent |
| A child who is 18 months of age and born to a mother known to be infected with, or at increased risk for, HIV, HBV or HCV infection |
Children less than or equal to 18 months of age who are born to mothers with or at risk for HIV infection or who have been breast fed within the past 12 months should not be accepted as donors regardless of their HIV test results |
| A child who has been breastfed within the preceding 12 months and the mother is known to be infected with, or at increased risk for HIV infection |
The impact of the new guidelines on the landscape of IRDs is unknown. The goals of our study were to 1) quantify the impact of the new guidelines on the percent of donors labeled IRD, and 2) characterize changes in the demographic and clinical characteristics of IRDs resulting from the new guidelines.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Study Population
Our study population included 80,007 deceased donors who were recovered for transplantation between July 1, 2004 (when the IRD designation was added to the deceased donor recovery form) and July 1, 2014, as captured by the OPTN and reported by the SRTR. The IRD designation was determined from the following question on the deceased donor registration form: "Does the donor meet CDC guidelines for "High Risk" for an organ donor?"
Changes in Proportion of Donor Volume from IRDs
The percent of donor volume from IRDs was calculated over 3 eras: 1) July 1 2004-August 26, 2013 (old guidelines only), 2) August 27 2013-January 31 2014 (transition period, IRD designation could be based on either old or new guidelines at the discretion of the reporting center), and 3) February 1 2014-July 1 2014 (new guidelines only). Initial exploration showed that the percent of donors labeled IRD began to increase in 2009; as such, our goals were twofold: 1) to quantify whether there was a statistically significant increase in the risk of a donor being labeled IRD under the new guidelines, and 2) to quantify whether any increases under the new guidelines were in excess of those that would have been expected based on the existing trend. To do this, we included donors recovered between January 1, 2009 and July 1, 2014 and used Poisson regression to model the risk of being labeled IRD as a function of time in years, including an indicator variable for the new guidelines (to estimate the relative risk of being labeled IRD under the new guidelines) as well as an interaction term between year and the new guidelines (to estimate the amount IRD labeling increased under the new guidelines in excess of what would be expected based on existing trends.)
Characteristics of IRDs under Old and New Guidelines
In order to identify potential changes in the phenotype of IRDs resulting from the new guidelines, the following demographic and clinical characteristics were examined: gender, age, race (White, African-American, Hispanic, Asian/Other), BMI, and cause of death (anoxia, stroke, head trauma, CNS tumor, other). The characteristics of donors recovered under the new guidelines (February 1 2014-July 1 2014) were compared to those recovered exactly one year earlier under the old guidelines (February 1 2013-July 1 2013) to minimize the influence of secular trends or seasonality in donor characteristics. In order to analyze associations between each characteristic and being categorized as IRD, we built modified Poisson regression models, as previously described (14); each model included the characteristic of interest, time period (old or new guidelines) and an interaction term between these to quantify whether donors with that characteristic were more likely to be classified as IRD under the new guidelines. Thus each interaction term represents a change in the characteristic among IRDs under the new guidelines in excess of what would be expected based on any changes among non-IRDs; in other words, if males were 1.5 times more likely to be labeled IRD under both the old and new guidelines, the interaction term would be 1.0 (males were no more likely to be labeled IRD under the new guidelines than they were under the old ones.) An interaction term > 1 would indicate that males were more likely to be labeled IRD under the new guidelines compared to the old ones.
Geographic Distribution of IRDs
The proportion of OPO donor volume from IRDs was calculated by dividing total number of IRDs by the total number of donors in the OPO from February 1 to July 1, 2013 (old guidelines), and from February 1 to July 1, 2014 (new guidelines). Similarly, proportion of transplant volume from IRDs was calculated by dividing the total number of transplants performed using IRD organs by the total number of organs transplanted in the OPO under the old and new guidelines. Percent of volume from IRDs was categorized as <10%, >10-15%, >15-20%, >20-25%, and >25% (roughly corresponding to quintiles). Maps were generated to depict geographic patterns in changes to IRD volume. The maps were built using ARC GIS version 10.2.2 (ESRD, Redlands, CA). Histograms were generated to depict the percent change in volume for each OPO under the new guidelines.
Statistical Analyses
All statistical analyses were performed using STATA 13 SE (College Station, TX).
RESULTS
Proportion of Donors Labeled IRDs
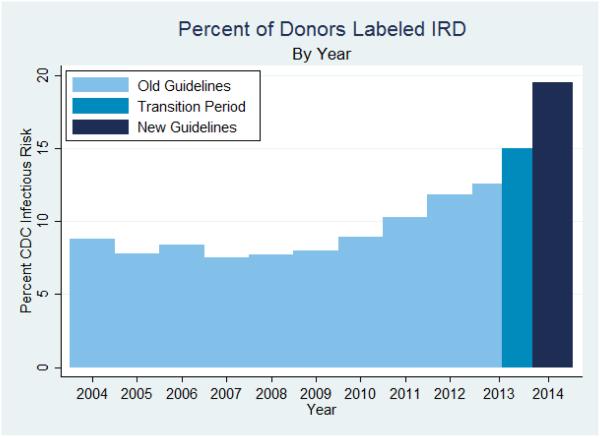
Over the 5 month period when the new guidelines were implemented (February 1 2014-July 1 2014), 19.5% of donors were labeled IRD, compared to 12.3% in the comparable 5 month period one year earlier (February 1 2013-July 1 2013, Table 2). Beginning in 2009, the percent of donors labeled IRD had already began to rise, from 7.9% to 11.8% in 2012 (Figure 1), so we wanted to understand how much more they rose in association with the guidelines. In a model assuming a continuation of the existing rise, we found that the percent labeled IRD was still 45% higher than what would have been expected based on the previously existing trend (August 27th 2013-July 1 2014) (IRR 1.45, 95% CI: 1.43-1.46, p <0.001).
Table 2.
Changes in Characteristics of Deceased Donors Labeled IRD Under Old and New Guidelines
| Old Guidelines | New Guidelines | Interaction |
p-
value** |
|||
|---|---|---|---|---|---|---|
| Donor Characteristic |
Non-IRD
N=2998 |
IRD
N=424 |
Non-IRD
N=2804 |
IRD
N=680 |
RR (95% CI) | |
| Overall (%) | 87.7 | 12.3 | 80.5 | 19.5 | ||
| Male (%) | 58.9 | 64.4 | 59.1 | 66.9 | 1.07 (0.85-1.36) | 0.6 |
| Age (%) | ||||||
| <18 months | 2.1 | 3.1 | 2.0 | 1.0 | 0.44 (0.18-1.04) | 0.06 |
| 18 mnths-18 yrs | 8.7 | 4.3 | 8.7 | 3.7 | 0.96 (0.53-1.74) | 0.9 |
| 18-40 yrs | 32.1 | 61.1 | 31.0 | 60.3 | Reference | |
| 41-50 yrs | 20.6 | 16.3 | 20.2 | 18.2 | 1.18 (0.87-1.61) | 0.3 |
| 51-60 yrs | 22.2 | 10.9 | 23.4 | 13.4 | 1.24 (0.86-1.79) | 0.2 |
| >60 yrs | 14.2 | 4.5 | 14.9 | 3.4 | 0.81 (0.44-1.49) | 0.5 |
| Race (%) | ||||||
| White | 64.4 | 72.6 | 66.0 | 67.4 | Reference | |
| African American | 18.0 | 13.4 | 16.5 | 18.1 | 1.52 (1.11-2.10) | 0.01 |
| Hispanic | 14.0 | 12.7 | 13.6 | 12.1 | 1.08 (0.76-1.52) | 0.7 |
| Other | 3.6 | 1.2 | 3.9 | 2.5 | 2.13 (0.81-5.65) | 0.2 |
| BMI (mean) | 27.7 | 26.7 | 28.0 | 26.7 | 0.99 (0.98-1.01) | 0.7 |
| ECD (%) | 26.2 | 9.2 | 26.4 | 11.6 | 1.34 (0.91-1.97) | 0.1 |
| Serum Creatinine (mean) |
1.5 | 1.5 | 1.4 | 2.0 | 1.11 (1.05-1.17) | <0.001 |
| Cause of Death (%) | ||||||
| Anoxia | 28.9 | 45.5 | 30.5 | 47.9 | Reference | |
| CBV/Stroke | 37.5 | 21.0 | 37.5 | 19.1 | 0.99 (0.73-1.34) | 0.9 |
| Head Trauma | 30.7 | 31.6 | 28.9 | 30.6 | 1.06 (0.82-1.36) | 0.6 |
| CNS Tumor/Other | 2.8 | 1.9 | 3.1 | 2.4 | 1.20 (0.53-2.72) | 0.7 |
P-value for Interaction between characteristic and era (old guidelines, February 1-July 1 2013 new guidelines, February 1-July 1 2014
RR = Relative Risk
IRD = Increased Risk for Disease Transmission
Figure 1.
Percent of Donors Labeled IRD, by Year
Change in IRD Phenotype under New Guidelines
Under both old and new guidelines, IRDs were more likely to be younger, male, and have anoxia as the cause of death, and less likely to be classified as ECD (for kidneys) (Table 2, left columns). In models including interactions between each patient characteristic and time period, we found that African-Americans were 52% more likely to be labeled IRD under the new guidelines compared to their risk of being labeled under the old ones.(RR interaction = 1.52, 95% CI: 1.11-2.10, p = 0.01, Table 2, right columns). There were no statistically significant differences in likelihood of being labeled IRD by gender, age, BMI, ECD status, or cause of death under the new guidelines.
Geographic Patterns
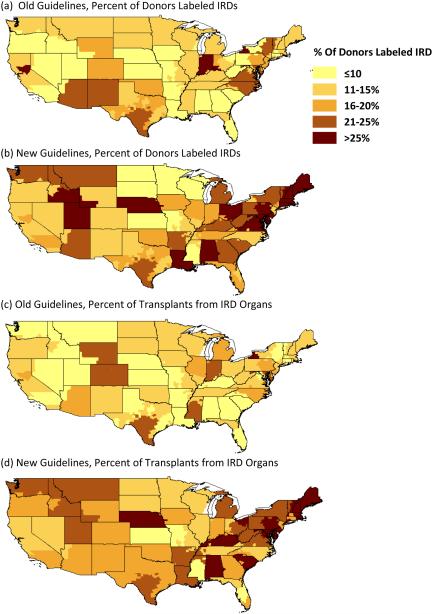
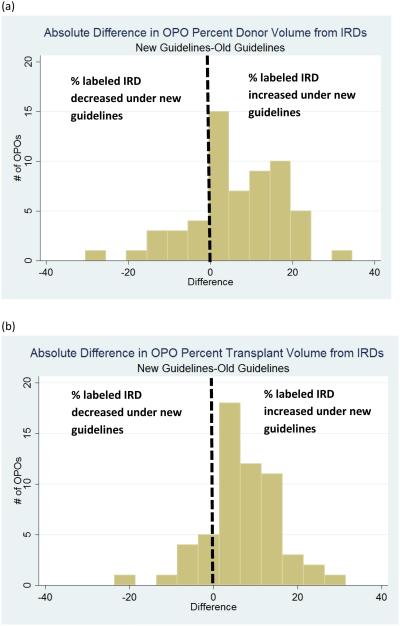
Under the old guidelines (February 1, 2013 to July 1, 2014), 24 OPOs (40.7%) labeled ≤ 10% of their donors IRD, compared to only 5 OPOs (8.5%) under the new guidelines (Figure 2a and 2b). Only 5 OPOs labeled >25% of their donors IRDs under the old guidelines; this rose to 25 OPOs after the new guidelines were implemented. Patterns were similar for the percent of transplant volume from IRDs: 23 OPOS (39.7%) derived ≤ 10% of their transplant volume from IRDs under the old guidelines, compared to only 4 OPOs (6.9%) under the new guidelines (Figure 2c and 2d). Only 1 OPO derived > 25% of its transplant volume from IRDs under the old guidelines; this rose to 9 under the new guidelines. Regarding the absolute difference in percent of donors labeled IRD within each OPO, 44 OPOs increased under the new guidelines (Figure 3a). The average absolute difference was 11.3%, and 6 OPOs experienced a difference > 20%. Similarly, 48 OPOs had an increase in percent of transplant volume from IRDs under the new guidelines, with an average absolute difference of 9.7% (Figure 3b).
Figure 2.
Percent of Donors Labeled IRD Under (a) Old Guidelines*, and (b) New Guidelines; Percent of Transplant Volume from Donors Labeled IRD Under (c) Old guidelines*, and (d) New guidelines, by OPO
Figure 3.
Histogram of Absolute Difference in OPO (a) Percent of Donors Labeled IRD, and (b) Percent of Transplants from Organs Labeled IRD Under New Guidelines
DISCUSSION
In a comprehensive national study of deceased organ donors, we found a substantial increase in the percent of donors labeled IRD under the new PHS guidelines that could not be explained by existing trends. Over the 5 month period the new guidelines were in place, 19.5% of donors were labeled IRD, compared to only 12.3% under the old guidelines one year earlier, representing a 45% rise that was statistically significant (p <0.001). Furthermore, the majority of OPOs saw an increase in both the percent of donors labeled IRD and the percent of transplant volume comprised of donors labeled IRDs. African-Americans were more likely to be labeled IRDs under the new guidelines.
Alternative explanations for the observed increase include an increase in reporting of IRDs following the new guidelines, or an increase in the number of deaths among persons meeting the IRD criteria. The slow rise in IRD labeling we observe beginning in 2009 seems consistent with either increased awareness of IRDs (likely resulting from highly publicized cases of HIV and HCV transmission just prior), or secular trends such as the reported increases in deaths from heroin overdose(15). Per the CDC deaths from heroin overdoses increased 39% between 2012 and 2013, and it is possible this contributed to the increase in percent of donors labeled IRD; however, we feel it is unlikely to explain a change of the magnitude we observed in 2014.
Though many IRD categories are similar between the old and new guidelines (Table 1), we hypothesize that both explicitly defining the STI category and the addition of the hemodialysis category (defined as "People who have been on hemodialysis in the preceding 12 months") may have contributed to the increase. If the old guidelines were followed exactly regarding STIs, more patients would have been labeled IRD compared to the new guidelines; however, it is possible there were differences in interpretation. Under the old guidelines, the inclusion of STIs was as follows: "Persons whose history, physical examination, medical records, or autopsy reports reveal other evidence of HIV infection or high-risk behavior, such as…sexually transmitted diseases." In contrast, under the new guidelines, the criterion is phrased: "People who have been newly diagnosed with, or have been treated for, syphilis, gonorrhea, chlamydia or genital ulcers in the preceding 12 months. According to recent CDC estimates, there are 21.5 million persons living with chlamydia, gonorrhea, syphilis, and herpes simplex virus-2 in the United States, and an additional 4.5 million new infections annually (16); thus many donors could potentially fall into this category.
IRDs carry a higher risk of infectious transmission relative to their non-IRD counterparts, ranging from <1 in 10,000 for HIV to < 1 in 1000 for HCV (4, 5). Data show that for almost all patients, an IRD transplant would significantly improve survival compared to remaining on the waitlist (6). However, regardless of the low absolute risks, patients may be reluctant to accept an organ specifically labeled as imparting any increased risk of stigmatized diseases such as HIV and hepatitis, and may instead choose to wait for a “risk-free” organ (8, 9). Providers may also be reluctant to use organs with this label: in 2007 a highly publicized case of HIV and HCV transmission from a single IRD led to widespread changes in practice, with many reporting decreased use of IRD organs (11). In a national survey of nephrologists, the majority stated they would recommend decline or not be comfortable making a recommendation if a patient asked for advice regarding an offer from a donor labeled IRD (19).
The IRD label may increase the administrative and processing burden to the OPO and center. The OPTN mandates that all centers obtain special informed consent when an IRD organ is transplanted, which may increase the time needed to place the organ (7). Furthermore, both sets of PHS guidelines recommend additional testing at time of recovery for donors labeled IRD, as well as additional recipient testing post-transplant for IRD organ recipients (between 1 and 3 months and again at 12 months for HIV, HBV, and HCV) (2, 3). Currently up to 25% of centers do not perform any post-transplant serologies and up to 43% do not perform nucleic acid testing (NAT) on IRD recipients (20). While post-transplant screening of recipients who have received transplants from donors with true infectious risk is an important patient safety measure, labeling a high number of donors IRD may be burdensome to centers.
In light of the fact that almost 20% of donors are labeled IRD, further studies are needed to elucidate the mechanism underlying the rise in IRD labeling, and, once several years of data are available, characterize discard rates under the new guidelines. We recommend that the OPTN begin capturing the reasons a given donor receives this designation. This information could be used to assess the discriminatory ability of each IRD category and guide future revisions to these guidelines, which currently are primarily informed by studies in non-donor populations.
Our study has several limitations that merit consideration. Since the new guidelines were implemented in February 2014, there were only 5 months of data available under the new guidelines, compared to 9 years under the old ones. However, the fact we observed a substantial, and statistically significant, increase in such a small time-frame suggests the rise was real; the risk during the transition period with an increased rise thereafter suggests that our findings might even be conservative, and that the proportion of deceased donors labeled IRD may even increase further. Because our study was observational, we could only examine associations between the new guidelines and the rise in IRD volume, so we cannot exclude the possibility that the increase was due to something other than the new guidelines.
Under the new CDC/PHS guidelines for risk of undetected HIV or hepatitis infection, there was a significant increase in the percent of donors labeled IRD, with an average of 19.5%, and, in 9 OPOs, more than 25% of deceased donors carrying this designation. While it is important to alert patients and providers to potential risks, no organ is entirely risk free, and broadening the definition of IRD may reduce the label’s effectiveness and increase the burden for centers. It is critical to understand the mechanism and consequences of the increase in IRDs to ensure an optimal balance of patient safety and organ utilization is achieved.
ACKNOWLEDGEMENTS
This work was supported by grant numbers K24DK101828 and F30DK095545 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Grossi PA, Fishman JA. Donor-derived infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S19–26. doi: 10.1111/j.1600-6143.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers MSR, Lawton KE, Moseley RR. Guidlines for Preventing Transmission of Human Immunodeficiency Virus Through Transplantation of Human Tissue and Organs. Morbidity and Mortality Weekly Report. 1994;43(RR-8):1–18. [PubMed] [Google Scholar]
- 3.Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128(4):247–343. doi: 10.1177/003335491312800403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, et al. Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant. 2011;11(6):1176–87. doi: 10.1111/j.1600-6143.2010.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, et al. Risk of window period hepatitis-C infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant. 2011;11(6):1188–200. doi: 10.1111/j.1600-6143.2011.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow EK, Massie AB, Muzaale AD, Singer AL, Kucirka LM, Montgomery RA, et al. Identifying Appropriate Recipients for CDC Infectious Risk Donor Kidneys. Am J Transplant. 2013 doi: 10.1111/ajt.12206. [DOI] [PubMed] [Google Scholar]
- 7.OPTN/HRSA Identification of Transmissible Diseases. 2014:173–174. [Google Scholar]
- 8.Ros RL, Kucirka LM, Govindan P, Sarathy H, Montgomery RA, Segev DL. Patient attitudes toward CDC high infectious risk donor kidney transplantation: inferences from focus groups. Clin Transplant. 2012;26(2):247–53. doi: 10.1111/j.1399-0012.2011.01469.x. [DOI] [PubMed] [Google Scholar]
- 9.Reese PP, Tehrani T, Lim MA, Asch DA, Blumberg EA, Simon MK, et al. Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection. Clin J Am Soc Nephrol. 2010;5(5):917–23. doi: 10.2215/CJN.08251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doe v, University of Chicago Medical Center . 2014 IL App (1st) 121593. Illinois Official Report; Chicago: 2014. pp. 1–16. [Google Scholar]
- 11.Kucirka LM, Ros RL, Subramanian AK, Montgomery RA, Segev DL. Provider response to a rare but highly publicized transmission of HIV through solid organ transplantation. Arch Surg. 2011;146(1):41–5. doi: 10.1001/archsurg.2010.303. [DOI] [PubMed] [Google Scholar]
- 12.Duan KI, Englesbe MJ, Volk ML. Centers for Disease Control 'high-risk' donors and kidney utilization. Am J Transplant. 2010;10(2):416–20. doi: 10.1111/j.1600-6143.2009.02931.x. [DOI] [PubMed] [Google Scholar]
- 13.Alcorn JB. Summary of Actions Taken at OPTN/UNOS Executive Committee Meeting (August 27th 2013). UNOS.2013. pp. 1–14. [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Rudd RA, Paulozzi LJ, Bauer MJ, Burleson RW, Carlson RE, Dao D, et al. Increases in heroin overdose deaths - 28 States, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849–54. [PMC free article] [PubMed] [Google Scholar]
- 16.CDC Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States. 2013.
- 17.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 18.USRDS USRDS Annual Data Report: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities 2013. 2013.
- 19.Kucirka LM BM, Gupta N, Chow EC, Choi M, Jaar B, Shafi T, Segev DL. Knowledge, Attitudes, and Counseling Practices for CDC Infectious Risk Donor Organs: A National Survey of Nephrologists. American Society of Nephrology; Baltimore: 2014. [Google Scholar]
- 20.Theodoropoulos N, Ladner DP, Ison MG. Screening recipients of increased-risk donor organs: a survey of transplant infectious diseases physician practices. Transplant infectious disease : an official journal of the Transplantation Society. 2013;15(5):545–9. doi: 10.1111/tid.12121. [DOI] [PubMed] [Google Scholar]