Abstract
Heart failure with preserved ejection fraction (HFpEF) is the most common form of heart failure (HF) in older adults. The primary chronic symptom in patients with HFpEF, even when well compensated, is severe exercise intolerance. Cardiac and peripheral functions contribute equally to exercise intolerance in HFpEF, though the latter has been the focus of fewer studies. Of note, multiple studies with exercise training have shown that exercise intolerance can improve significantly in the absence of improvements in exercise cardiac output, indicating a role of peripheral, non-cardiac adaptations. In addition, clinical drug trials performed to date in HFpEF, all of which have focused on influencing cardiovascular function, have not been positive on primary clinical outcomes and most have not improved exercise capacity. Mounting evidence indicates that sarcopenic obesity, characterized by the coexistence of excess fat mass and decreased muscle mass, could contribute to the pathophysiology of exercise intolerance in older HFpEF patients and may provide avenues for novel treatments.
Keywords: Heart failure with preserved ejection fraction, exercise intolerance, skeletal muscle, peak oxygen consumption, sarcopenia, obesity, aging, exercise training
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF in older adults, particularly women, and is increasing in prevalence as the population ages [1]. The prevalence of HFpEF is rising, with morbidity, mortality, and healthcare costs on par with HF with a reduced ejection fraction (HFrEF) [1–4]. The primary symptom in patients with HFpEF, even when well compensated, is severe exercise intolerance, and is associated with their reduced quality of life [5–11]. However, HFpEF pathophysiology is poorly understood; most drug trials in HFpEF focused on improving cardiovascular function have not resulted in an increase in exercise tolerance [12,13], the key symptom in this common, important disorder among the elderly.
Our data and those of others’ indicate that in older HFpEF patients, abnormalities in skeletal muscle and increased adiposity are major contributors to exercise intolerance [10,14,15]. Often overlooked regarding HFpEF is that approximately 85% of elderly patients with the disorder are overweight or obese and that the HFpEF epidemic has largely paralleled the obesity epidemic [16**]. Furthermore, normal aging is associated with characteristic changes in body composition, including decreases in lean body mass and muscle strength, and increases in adiposity [17–19]. Sarcopenic obesity, the coexistence of excess fat mass and decreased muscle mass, is a concern in the aged society. In this review, we will focus on relationship between the sarcopenic obesity on pathophysiology of exercise intolerance in elderly HFpEF patients.
Pathophysiology of Exercise Intolerance in HFpEF
Exercise intolerance can be objectively measured as reduced peak exercise oxygen consumption (VO2) by expired gas analysis, a technique that is valid and reproducible, including in elderly patients with HFpEF [20]. According to the Fick equation, VO2 is equal to the product of cardiac output (CO) and arterial–venous oxygen content difference (A-VO2 Diff); therefore, the reduced peak VO2 in patients with HFpEF may be caused by decreased CO or by decreased oxygen delivery to or impaired oxygen utilization by the exercising skeletal muscles.
The cause of decreased peak VO2 in elderly HFpEF patients has been attributed to reduced peak CO secondary to blunted chronotropic, lusitropic, inotropic, and vasodilator reserve [6,7], to reductions in both peak CO and A-VO2 Diff [8*,9,11], or primarily to reduced peak A-VO2 Diff secondary to impaired skeletal muscle oxidative metabolism [5]. Haykowsky and colleagues found that compared with age-matched healthy controls [8*], the change in A-VO2 Diff from rest to peak exercise was the strongest independent predictor of the reduced peak VO2 in HFpEF patients [8*]. Furthermore, in a separate study, these investigators found that improved peak A-VO2 Diff accounted for the most of the improvement in peak VO2 following exercise training [21*]. This is supported by the work of Fujimoto et al who showed that exercise training in HFpEF patients was not associated with any significant change in CO [22]. Recently Dhakal et al., found that directly measured AVO2 diff was the major determinant of exercise capacity in HFpEF and that these patients have abnormally low peripheral oxygen extraction during exercise compared to HFrEF subjects and normal controls [23].
Findings of another study indicate that improvements in peak VO2 with exercise training in HFpEF are not associated with altered endothelial function or arterial stiffness, suggesting that skeletal muscle hypoperfusion, skeletal muscle atrophy, and/or abnormal muscle metabolism play an important role in the severe exercise intolerance experienced by HFpEF patients and its improvement with exercise training [21*]. Supporting this is the results of a recent meta-analysis of 6 randomized controlled trials of exercise training in patients with HFpEF revealed exercise training improved peak VO2 and quality of life without any significant change in resting diastolic or systolic function [24*]. This may explain why drug trials in HFpEF to date, focused on influencing cardiovascular function have not improved exercise intolerance [12;13].
Aging, Obesity, and HFpEF
Aging is a systemic process affecting all organ systems and associated with significant alterations in body composition. Typically fat mass increases with age and peaks around age 60–75 years [17], whereas muscle mass and strength starts to decline progressively with a more accelerated loss after the age of 60 [18]. Visceral fat and intramuscular fat tend to increase, while subcutaneous fat in other regions of the body (abdomen, thigh, calves) decreases [19]. This change in body composition is worsened by concomitant disorders such as HF [25]. Recent studies have suggested underlying aging changes are important contributors to the HFpEF epidemic [14,26]. Obesity is one of the strongest risk factors for development of HFpEF, particularly among older women; overweight / obesity is present in about 85% of older HFpEF patients [27]. In obese older adults, the presence of excess fat mass and the age-related decrease of lean body mass exaggerate the above mentioned alterations [28]. Both regional and total adipose tissue has adverse consequents on organ function, overall system function, and outcomes. In addition to its effects on cardiovascular function, increased adiposity markedly impairs physical function and skeletal muscle composition and function.
Sarcopenic Obesity
Sarcopenic obesity, the coexistence of excess fat mass and decreased muscle mass, is not just a combination of two conditions, but is more related to cardio-metabolic and functional abnormalities, and is a concern in the aged society [29,30]. According to Baumgartner et al. criteria; sarcopenic-obesity characterizes individuals having 1) an appendicular skeletal muscle index (legs and arms muscle mass/height (m2) <2 standard deviation in comparison to a young adult reference group aged between 20 and 30 years old and 2) a percentage of body fat above the 60th percentile for the same gender and age [31].
Sarcopenic Obesity-Pathophysiology and Consequences
Aging is associated with a decline in a variety of neural, hormonal and environmental trophic signals to muscle. Physical inactivity, hormonal changes, pro-inflammatory state, malnutrition, loss of alpha-motor units in the central nervous system, and altered gene expression accelerate the loss of muscle mass and mass-specific strength [32].
Inflammation
Aging is associated with a systemic pro inflammatory state, and associated with increased levels of cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α [33,34]. Furthermore, adipose tissue is an active metabolic tissue that secretes hormones and proteins. In adipose tissue, either adipocytes directly or infiltrating macrophages produces pro-inflammatory cytokines, such as IL-6 and TNF-α [35]. These cytokines have direct catabolic effects on skeletal muscle: TNF-α impairs muscle protein synthesis [36,37] and increases muscle protein degradation [38,39] while IL-6 increases muscle protein degradation [40]. It has also been demonstrated that TNF-α impairs endothelial function with a decreased blood and nutrient supply to skeletal muscle, thus reducing exercise endurance. Recently, the transforming growth factor- β related cytokine myostatin was identified as an important mediator of cardiac-induced muscle wasting in HFrEF [41]. Of note, genetically modified mice with enhanced myostatin expression in the myocardium showed skeletal muscle rarefaction, indicating that cardiac myostatin elaboration is sufficient to induce skeletal muscle wasting [41]. Thus, a pro-inflammatory state may be one of the key factors in creating a vicious cycle of decreased muscle strength among older adults.
Oxidative Stress
Aging is associated with a chronic state of oxidative stress, which activates profibrotic signaling pathways [42,43], and one of the major contributing factors to skeletal muscle decline [44]. Skeletal muscle continues to produce reactive oxygen species (ROS) during contractile activity. These phenomena happen together with age-related decline of the muscle enzymatic scavenger systems, thus producing an alteration of mitochondrial DNA and abnormalities in the electron transport system [45].
Altered endocrine function
A recent study showed that sarcopenic obesity is associated with decreased growth hormone (GH) secretion and has lower testosterone [46,47]. Low levels of these anabolic hormones have been reported positively associated with low muscle strength [48,49]. It is known that insulin like growth factor (IGF-1) plays an important role in muscle growth and repair [50]. Clinical and experimental studies showed that low testosterone resulting in lower protein synthesis and a loss of muscle mass [51].
Obesity
Obesity has multiple adverse consequences for skeletal muscle, including inflammation, oxidative stress, and insulin resistance (IR). Along with visceral fat accumulation, loss of skeletal muscle, which is the largest insulin-responsive target tissue, produces IR. Adding to this, increases in visceral fat may lead to higher secretion of pro-inflammatory adipokines that further promote IR as well as potentially direct catabolic effects on muscles [52,53]. Moreover, it has been hypothesized that muscle fat infiltration causes IR in obese individuals [53]. IR promotes muscle catabolism, mitochondrial dysfunction, and impairs protein synthesis in skeletal muscle.
Fatty infiltration of skeletal muscle is associated with reduced strength [54,55] and functional status [56], muscle dysfunction [55] and decreased contractility and motor unit recruitment [55], and interference in normal cellular signaling [57]. Furthermore, increased intermuscular fat is associated with reduced mitochondrial mass, biogenesis, and oxidative metabolism [58]. Increased adiposity is often associated with high circulating free fatty acids, which inhibit GH production and decrease plasma IGF-I [59,60]. Thus, a vicious circle between skeletal muscle loss and fat gain changes in body composition may lead to more sarcopenia and then to further metabolic problems and inflammation [61]. In addition, Paulus and Tschope recently proposed that co morbidities and especially obesity induce a systemic inflammatory state, which induces oxidative stress in the coronary microvascular endothelium [62**].Obesity is associated with several impairments in the microcirculation, including rarefaction and impaired endothelial function[63].
Lack of physical activity
Sedentary life-style is an important risk factor for obesity [64]. Obese persons also tend to be less physically active and this might contribute to the loss of muscle mass and strength at any age [65,66]. Furthermore, muscle atrophy leads to reduction in metabolic rate both at rest and during physical activity, thus further aggravating the sedentary state, all of which can cause obesity. However, the adverse effects of obesity on pathogenesis and outcomes appear to not be merely due to deconditioning.
Sarcopenic Obesity and Exercise Intolerance
Sarcopenic obesity can lead to multiple alterations in skeletal muscle; decreased muscle quality, with reduced relative number of type II fibers [67,68] and decreased capillary density [69], decreased muscle mass and strength, increased muscle catabolism and impaired muscle protein synthesis, increased accumulation of intermuscular fat [70], and alterations in mitochondrial mass, biogenesis and oxidative metabolism with mitochondrial dysfunction [71,72]; all associated with reduced strength [73], endurance, peak VO2, and mobility [74–76]. Both decreased muscle mass and decreased oxidative capacity of skeletal muscle with aging contribute to the observed 1% annual decline in maximal aerobic capacity [77]. In healthy persons, peak VO2 declines at the rate of 3 to 8% per decade after the age of 30 years, but adjustment for muscle mass substantially mitigates this decline.
These skeletal muscle abnormalities, an imbalance between increased muscle catabolism and attenuated muscle anabolism, likely are significant contributors to the genesis of symptoms of exercise limitation. These alterations influence both peripheral and ventilatory muscles, are present at rest, and deteriorate during exercise. This suggests that loss of muscle mass is a significant contributor to associated inflammation and may play an important role in the age-related process that leads to sarcopenia. These findings mirror the role of skeletal muscle abnormalities in HFpEF, which is closely associated with aging.
Sarcopenic Obesity and Exercise Intolerance in HFpEF
It is noteworthy that abnormalities in skeletal muscle mass and function are frequently present in patients with mild or moderate chronic HFrEF and likely may contribute to fatigue and exercise intolerance [78]. Of note, in animal models of HFrEF, these occur independent of physical activity, indicating that they are not merely due to deconditioning that likely also occurs in symptomatic HF. The multinational SICA-HF study shows that muscle wasting is a frequent co-morbidity among patients with chronic HFrEF and associated with worse exercise capacity in treadmill performance and in walking exercise tests [79].
As most of these studies have been performed in patients with HFrEF, the specific changes of skeletal muscle in patients with HFpEF are less clear. Progressive decline in muscle mass, strength and function that occurs with normal human aging is associated with increased frailty and HFpEF [25,80]. Rather than being simply a result of deconditioning, recent data suggest that frailty and muscular abnormalities may directly contribute to the HFpEF syndrome, a finding similar to HFrEF, where skeletal muscle abnormalities appears to be independent of physical activity and deconditioning [81]. Non-cardiac co-morbidities are highly prevalent in HFpEF [82*] and these co-morbidities can produce systemic pro inflammatory state [62**] and further accelerate the process of muscle wasting as seen in HFrEF.
Using dual energy x-ray absorptiometry, Haykowsky and colleagues found percent body fat and percent leg fat were significantly increased, whereas percent body lean and leg lean mass were significantly reduced in older HFpEF patients versus healthy controls [83*]. Moreover, the slope of the relation of peak VO2 with percent leg lean mass was markedly reduced in the HFpEF versus healthy control group [83*]. This suggests abnormalities of skeletal muscle mass and quality contribute to exercise intolerance in HFpEF. These investigators extended these results by directly characterizing thigh muscle composition using phase-contrast MRI, which showed abnormal fat infiltration into the thigh skeletal muscle and that this was associated with reduced peak exercise VO2 in HFpEF [84**] indicating presence of abnormal skeletal muscle composition.
Increased intermuscular fat may contribute to reduced peak VO2 in patients with HFpEF by way of a number of mechanisms as described previously. Heinonen et al, [85] using positron emission tomography, found that adipose tissue blood flow adjacent to the active muscles increased sevenfold during continuous isometric knee-extension exercise in non-obese younger healthy sedentary women. Thus, increased thigh intermuscular fat in older patients with HFpEF may “steal” blood that would normally be delivered to the active muscles during exercise and, thereby, reduces perfusive oxygen delivery to the thigh muscle. Adipose within skeletal muscle is also metabolically active, and can potentially impair oxidative metabolism and mitochondrial function. Together, these findings support the concept that altered skeletal muscle composition contributes to exercise intolerance in older patients with HFpEF.
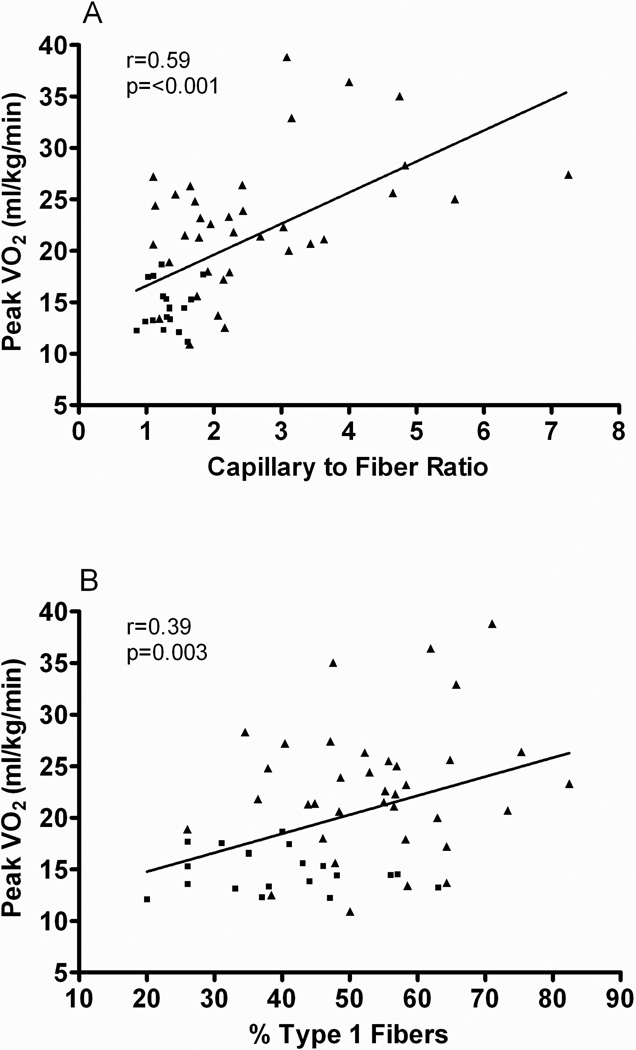
Kitzman et al. also showed that compared with healthy subjects, older HFpEF patients had a shift in skeletal muscle fiber type distribution with a reduced percentage of slow twitch type I fibers and reduced type I-to-type-II fiber ratio and reduced capillary-to-fiber ratio [86**]. Furthermore, both capillary-to-fiber ratio and percentage of type I fibers were significant, independent predictors of peak VO2 (Figure 1) [86**]. Several investigators have reported that HFrEF patients have decreased oxidative type I fibers compared with healthy control subjects [87–89], and that this is related to peak VO2 [90]. Compared with type II fibers, type I fibers, which were found to be reduced in HFpEF, have greater oxidative capacity and mitochondrial density and contribute disproportionately to the ability to perform sustained aerobic exercise. Recently, Bowen et al showed that in a Dahl salt-sensitive rats, HFpEF is associated with significant molecular, mitochondrial, and histological alterations in the diaphragm and soleus. [91**]. Importantly, exercise training was able to prevent skeletal muscle contractile dysfunction in both the diaphragm and soleus, and this was associated with preserved mitochondrial function. Collectively, therefore, these findings have important implications for better understanding the pathophysiology of HFpEF [86**, 91**].
Figure 1.
Relationship of capillary-to-fiber ratio (A) and percentage of type I muscle fibers (B) with peak O2 uptake (VO2) in older patients with heart failure with preserved ejection fraction (■) and age-matched healthy control subjects (▲).
From Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014;306:H1364-70, with permission from The American Physiological Society.
In aging and in HF, muscle blood flow (perfusive and diffusive O2 delivery) assumes an important role in limiting VO2 kinetics [92]. Therefore, the reduced capillary-to-fiber ratio in HFpEF patients, also known to be associated with sarcopenic obesity, would be expected to result in a decreased diffusive capacity for O2 transport to active skeletal muscle during exercise and limit exercise capacity [93]. Potential causes for the skeletal muscle abnormalities in HFpEF might include neuroendocrine activation, sympathetic overdrive, oxidative stress, inflammation, abnormal Ca2+ cycling and excitation-contraction coupling, and deconditioning [90]. Of note, the reduced microvascular density in skeletal muscle in older HFpEF patients parallels a similar finding in cardiac muscle as reported by Mohammed et al [94**]. This suggests the possibility of a common systemic factor that serves as a trigger for HFpEF, and adds further support to HFpEF as a likely systemic disorder [95*].
Loss of skeletal muscle mass and increased adiposity in old HFpEF patients are concerning because this can exacerbate physical inactivity. Even if they are not primarily caused by physical inactivity, many of these changes are further worsened by sedentary behaviors, so the symptoms and limitation are propagated as the older person limits their physical exertions. This may be another example of vicious cycles which of characteristerizes geriatric syndromes [96].
Future Perspectives
Understanding and addressing the role of sarcopenic obesity in HFpEF presents a major opportunity, because drug trials in HFpEF to date, focused on influencing cardiovascular function, have not improved exercise intolerance. Thus, a focus on sarcopenic obesity in HFpEF, while contrary to the traditional paradigm, could yield novel, potentially modifiable therapeutic targets.
Aerobic and resistance exercise programs
There is strong evidence that exercise training in patients with sarcopenic obesity in older adults improves physical function [97]. The results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study [98] demonstrated that an intervention of structured physical activity improves physical performance scores in a group of 70- to 89-year-old participants. In as little as 12 weeks, resistance training two or three days a week can result in muscle hypertrophy and increased cross-sectional area of both type I and type II fibers essential for both muscular endurance and strength [99]. Davidson et al, conducted a 6-month investigation to determine the independent and combined effects of resistance and aerobic training on functional limitations in abdominally obese older men and women [100]. The greatest loss in fat mass occurred in the combined training, and gain in skeletal muscle occurred in the resistance training and combined training groups at 6 months. Hence, functional improvements are enhanced with the addition of resistance training to exercise programs.
In patients with HFrEF, exercise interventions, primarily aerobic exercise training, have resulted in beneficial molecular and functional changes in skeletal muscle including improved oxidative function and higher mitochondrial number and density [16,87–90,101,102], increased IGF-1 expression [103] and reduced local oxidative stress and inflammation [104]. These molecular changes correlate with increased exercise performance manifested by longer exercise duration and higher peak VO2, as well as improved endothelial function [103,105]. As most of these studies have been performed in patients with HFrEF, the specific effect of exercise interventions in patients with HFpEF are unclear.
Exercise Therapy in HFpEF
Kitzman and colleagues reported the first single-center, medically supervised, randomized controlled trial comparing the effects of 16 weeks of endurance exercise training versus attention control in 53 older (mean age = 70 years) patients with HFpEF. The exercise training increased peak VO2, ventilatory anaerobic threshold, 6-minute walk distance, and physical quality-of-life scores [15]. Similar results for these end-points were seen in a multicenter study of 64 HFpEF patients randomized to 3 months of combined exercise training and strength training [106]. In a second, separate, randomized, attention-controlled, single- blind trial of 4 months upper and lower extremity endurance exercise training reported by Kitzman et al. exercise training resulted in a significant increase in peak VO2 without altering carotid arterial stiffness or brachial artery flow mediated dilation [107].
Taken together, exercise training with or without resistance training appears to be an effective non-pharmacologic therapy in clinically stable patients with HFpEF to improve exercise tolerance and possibly quality of life. Future studies should build on these data to understand optimal modality of exercise and ways to enhance the favorable response further..
Dietary intervention
Protein supplementation, in combination with resistance exercise, enhances muscle protein synthesis and improves body composition by increasing lean mass in relation to fat mass [108]. Supplementation with leucine, which appears to be the most potent branched-chain amino acid for stimulation of protein synthesis, has also been proposed for the prevention of sarcopenia [109]. To date, evidence is relatively sparse about the comparison of macronutrient manipulation and conventional caloric restriction in terms of weight loss and effects on body composition and especially on the phenotype of sarcopenic obesity, and is absent regarding older heart failure patients.
Caloric restriction
Intentional weight loss via caloric restriction has the potential to reduce excess adipose and improve many of the related abnormalities above. However, weight loss is controversial in patients with HF. Many studies suggest that overweight and obesity in established HF is associated with improved survival, whereas underweight and normal weights are associated with worsened survival. More recently, a U-shaped curve relating survival to body weight has shown excess mortality at the extremes, morbid obesity and cachexia. These trends are seen in HFpEF as well [110]. In small studies with variable controls, bariatric surgery appears to improve symptoms of HF in patients with morbid obesity and HF and potentially prevent overt HF. However, dietary weight loss in HF patients remains controversial, and no studies have reported examining this.
However, in non-HF patients, improvements in body composition and physical function have been reported consistently in patients without HF, including older persons and women. A meta-analysis of 18 randomized controlled trials of exercise studies with or without diet components indicated that 3–18 month programs that included aerobic and strengthening exercise (2–3 days per week) with caloric restriction (typically 750 kcal deficit/day), induced the greatest change in functional performance measures compared with exercise or diet alone [111]. Importantly, caloric restriction results in significant loss of skeletal muscle along with adipose, which could have adverse long term consequences in HF. Few studies showed compared to diet or the aerobic exercise [112], the combination of diet–exercise improvements in physical performance test and peak VO2. Thus, while there is promise for multimodal exercise coupled with diet for counteracting sarocpenic obesity and improving mobility, physical function and exercise capacity in older, obese adults in general, this is untested in HF [97].
Hormonal therapy
Studies of Rudman et al. showed that GH administration results in increased lean body mass and decreased fat-to-muscle ratio in older subjects [113]. Administration of these hormones to aged animals restores cellular protein synthesis, increases lean body mass, decreases adiposity, improves endothelial function, improves endurance and contractile function. Likewise, in patients with HIV-associated lipodystrophy, GH administration also increased lean mass and decreased adiposity [114]. Finally, in a recent randomized trial in patients with HFrEF, GH replacement increased peak VO2 and exercise duration, and improved quality of life [115]. The pleiotropic actions of GH and IGF-I exerted in the heart, skeletal muscle, and vascular bed could account for the effects of GH on enhancing exercise capacity.
A meta-analysis of modestly sized randomized, placebo-controlled trials showed that testosterone supplementation in patients with HFrEF is associated with an increase of ≈54 m on the 6min walk test, as well as improvements in peak VO2 and NYHA class [116].
Therapeutically, injection of a myostatin-blocking antibody in mice with preexisting HF preserved muscle mass [117]. Thus, myostatin inhibition might be a medically relevant avenue for the treatment of muscle wasting in HF. This needs to be verified in larger cohorts of patients with HF. However, a number of clinical trials that target myostatin in older patients with sarcopenia associated with other chronic disorders are ongoing. The results of these may inform future trials in older patients with HFpEF.
Conclusion
HFpEF is a true geriatric syndrome, with complex, multi-factorial pathophysiology and clinical heterogeneities. In addition to cardiac dysfunction, abnormalities in peripheral function including vascular, skeletal muscle, and adipose tissue may contribute substantially to pathogenesis and outcomes. A revised paradigm that incorporates the full range of abnormalities and targets non-cardiac as well as cardiac mechanisms, and searches for common linkages between these, may lead to improved understanding and advances in treatment. Clinical trials aimed specifically at addressing sarcopenia and excess adiposity in patients with HFpEF could help to ameliorate physical functional impairments and disability in patients with this important and increasingly prevalent syndrome in older patients.
Acknowledgments
Supported in part by NIH grant R01AG18915, P30AG021332, R01AG045551
Dalane W. Kitzman has received compensation from GlaxoSmithKline, Relypsa, DC Devices, AbbVie, Regeneron, and Westat for service as a consultant; grant support from Novartis; and claims stock ownership in Gilead Sciences and Relypsa.
Footnotes
Conflict of Interest
Bharathi Upadhya declares that he has no conflict of interest.
Mark J. Haykowsky declares that he has no conflict of interest.
Joel Eggebeen declares that he has no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Reference List
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction: Prevalence, Therapies, and Outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, et al. Costs for Heart Failure With Normal vs Reduced Ejection Fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 5.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. This study showed that change in A-VO2 Diff from rest to peak exercise was the strongest independent predictor of the reduced peak VO2 in HFpEF patients
- 9.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 11.Maeder MT, Thompson BR, Brunner-La Rocca H-P, Kaye DM. Hemodynamic Basis of Exercise Limitation in Patients With Heart Failure and Normal Ejection Fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Haykowsky MJ. Mechanisms of exercise training in heart failure with preserved ejection fraction: central disappointment and peripheral promise. Am Heart J. 2012;164:807–809. doi: 10.1016/j.ahj.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Kitzman DW. Understanding results of trials in heart failure with preserved ejection fraction: remembering forgotten lessons and enduring principles. J Am Coll Cardiol. 2011;57:1687–1689. doi: 10.1016/j.jacc.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. doi: 10.1016/j.jchf.2014.05.017. This study showed that higher BMI has an independent, linear association with subclinical myocardial injury
- 17.Ding J, Kritchevsky SB, Newman AB, Taaffe DR, Nicklas BJ, Visser M, et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr. 2007;85:405–410. doi: 10.1093/ajcn/85.2.405. [DOI] [PubMed] [Google Scholar]
- 18.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 19.Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, Bergamo-Andreis IA, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Bensimhon DR, Leifer E, Ellis SJ, Fleg JL, Keteyian SJ, Pina LI, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure. Am J Cardiol. 2008;102:712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. This study suggests the novel finding of peripheral mechanisms contribute to the improved exercise capacity after exercise training in HFpEF
- 22.Fujimoto N, Prasad A, Hastings JL, Bhella PS, Shibata S, Palmer D, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, et al. Mechanisms of exercise intolerance in heart hailure with preserved ejection fraction: the role of abnormal peripheral pxygen extraction. Circ Heart Fail. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001825. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. Recent more updated comprehensive meta-analysis of exercise training in HFpEF patients showed that exercise training improved functional capacity, without affecting cardiac function
- 25.Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging. 2008;12:470–478. doi: 10.1007/BF02982708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottdiener J, Bartz T, DeFilippi C, Kop W, Kitzman D, Barasch E, et al. Echocardiographic and biomarker phenotype of heart failure with preserved ejection fraction (HFPEF) in older individuals in comparison to hypertension without heart failure (HTN), elderly with risk factors, and healthy aging. Importance of myocyte injury, fibrosis, LV hypertrophy, and diastolic load. J Am Coll Cardiol. 2012;59:E852. [Google Scholar]
- 27.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 28.Waters DL, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27:401–421. doi: 10.1016/j.cger.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 32.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, et al. Pentraxin 3 Is a New Inflammatory Marker Correlated With Left Ventricular Diastolic Dysfunction and Heart Failure With Normal Ejection Fraction. J Am Coll Cardiol. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Kalogeropoulos A, Georgiopoulou V, Psaty B, Rodondi N, Smith A, Harrison D, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 37.Williamson DL, Kimball SR, Jefferson LS. Acute treatment with TNF-alpha attenuates insulin-stimulated protein synthesis in cultures of C2C12 myotubes through a MEK1-sensitive mechanism. Am J Physiol Endocrinol Metab. 2005;289:E95–E104. doi: 10.1152/ajpendo.00397.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- 39.Tan Y, Peng X, Wang F, You Z, Dong Y, Wang S. Effects of tumor necrosis factor-alpha on the 26S proteasome and 19S regulator in skeletal muscle of severely scalded mice. J Burn Care Res. 2006;27:226–233. doi: 10.1097/01.BCR.0000203378.85736.38. [DOI] [PubMed] [Google Scholar]
- 40.Fujita J, Tsujinaka T, Ebisui C, Yano M, Shiozaki H, Katsume A, et al. Role of interleukin-6 in skeletal muscle protein breakdown and cathepsin activity in vivo. Eur Surg Res. 1996;28:366. doi: 10.1159/000129477. [DOI] [PubMed] [Google Scholar]
- 41.Breitbart A, Auger-Messier M, Molkentin JD, Heineke J. Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol. 2011;300:H1973–H1982. doi: 10.1152/ajpheart.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai D, Santana L, Vermulst M, Tomazela D, Emond M, MacCoss M, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cucoranu I, Clempus R, Dikalova A, Phelan P, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 44.Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 45.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontold A Biol Sci Med Sci. 2008;63:536–541. doi: 10.1093/gerona/63.5.536. [DOI] [PubMed] [Google Scholar]
- 47.Allan CA, Strauss BJ, McLachlan RI. Body composition, metabolic syndrome and testosterone in ageing men. Int J Impot Res. 2007;19:448–457. doi: 10.1038/sj.ijir.3901552. [DOI] [PubMed] [Google Scholar]
- 48.Ceda GP, Dall'Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28:96–100. [PubMed] [Google Scholar]
- 49.Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol. 2005;63:152–160. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 50.Cappol AR, Bdandeen-Roche K, Wand GS, Volpato So, Fried LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clinc Endocrinol Me. 1101;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 51.Galvao DA, Taaffe DR, Spry N, Newton RU. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:340–346. doi: 10.1038/sj.pcan.4500975. [DOI] [PubMed] [Google Scholar]
- 52.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 53.Goodpaster FH, Brown FF. Skeletal muscle lipid and its association with insulin resistance: what is the role for exercise? Exerc Sport Sci Rev. 2005;33:150–154. doi: 10.1097/00003677-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 55.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 56.Sipila S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14:433–442. doi: 10.1111/j.1475-097x.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 57.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85:662–677. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 58.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Dam PS, Smid HE, de Vries WR, Niesink, Bolscher E, Waasdorp EJ, et al. Reduction of free fatty acids by acipimox enhances the growth hormone (GH) responses to GH-releasing peptide 2 in elderly men. J Clin Endocrinol Metab. 2000;85:4706–4711. doi: 10.1210/jcem.85.12.7087. [DOI] [PubMed] [Google Scholar]
- 60.Weltman A, Weltman JY, Veldhuis JD, Hartman ML. Body composition, physical exercise, growth hormone and obesity. Eating and weight disorders : EWD. 2001;6:28–37. [PubMed] [Google Scholar]
- 61.Roth SM, Metter EJ, Ling S, Ferrucci Luigi. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- 62. Paulus W, Tschope C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. Novel study explains the new paradigm of HFpEF development, which identifies a systemic proinflammatory state induced by comorbidities as the cause of myocardial structural and functional alterations.
- 63.Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology. 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 64.LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr Opin Clin Nutr Metab Care. 2006;9:540–546. doi: 10.1097/01.mco.0000241662.92642.08. [DOI] [PubMed] [Google Scholar]
- 65.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 66.Lee JS, Auyeung TW, Kwok T, Lau EM, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older chinese men and women: a cross-sectional study. Gerontology. 2007;53:404–410. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 67.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 68.Borges O, Essen-Gustavsson B. Enzyme activities in type I and II muscle fibres of human skeletal muscle in relation to age and torque development. Acta Physiol Scand. 1989;136:29–36. doi: 10.1111/j.1748-1716.1989.tb08626.x. [DOI] [PubMed] [Google Scholar]
- 69.Coggan AR, Spina RJ, King DS. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 70.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. The American Journal of Clinical Nutrition. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, et al. Skeletal Muscle Mitochondrial Energetics Are Associated With Maximal Aerobic Capacity and Walking Speed in Older Adults. J Gerontol A Bio Sci Med Sci. 2013;68:447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Science USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–136. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 74.Nicklas B, Leng I, Delbono O, Kitzman DW, Marsh A, Hundley WG, et al. Relationship of physical function to vastus lateralis capillary density and metabolic enzyme activity in elderly men and women. Aging Clin Exper Res. 2008;20:302–309. doi: 10.1007/bf03324860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 76.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. The American Journal of Clinical Nutrition. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evans WJ. Effects of Exercise on Body Composition and Functional Capacity of the Elderly. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50A:147–150. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 78.Georgiadou P, Adamopoulos S. Skeletal muscle abnormalities in chronic heart failure. Curr Heart Fail Rep. 2012;9:128–132. doi: 10.1007/s11897-012-0090-z. [DOI] [PubMed] [Google Scholar]
- 79.Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 80.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 81.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 82. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population With Heart Failure and Preserved Versus Reduced Ejection Fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. This study highlights importance of comorbidities on overall prognostic impact in HFpEF
- 83. Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. Novel finding of abnormalities in skeletal muscle perfusion and/or metabolism and its contribution to the severe exercise intolerance in older HFpEF patients.
- 84. Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. Important finding of abnormal fat infiltration into the thigh skeletal muscle and its association with reduced peak exercise capacity in HFpEF
- 85.Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. Jvf Applied Physiology. 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 86. Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. This study showed that older HFpEF patients have significant abnormalities in skeletal muscle
- 87.Drexler H, Riede J, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 88.Larsen A, Lindal S, Aukrust P, Toft I, Aarsland T, Dickstein K. Effect of exercise training on skeletal muscle fibre characteristics in men with chronic heart failure. Correlation between skeletal muscle alterations, cytokines and exercise capacity. Int J Cardiol. 2002;83:25–32. doi: 10.1016/s0167-5273(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 89.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–145. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 90.Middlekauff HR. Making the Case for Skeletal Myopathy as the Major Limitation of Exercise Capacity in Heart Failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scott Bowen T, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015 doi: 10.1002/ejhf.239. [Epub ahead of print] This study is the first to demonstrate that HFpEF induces significant molecular, mitochondrial, histological, and functional alterations in the diaphragm and soleus, which were attenuated by exercise training
- 92.Rossiter HB. Exercise: Kinetic Considerations for Gas Exchange, Comprehensive Physiology. John Wiley & Sons, Inc.; 2010. [DOI] [PubMed] [Google Scholar]
- 93.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.009625. [Epub ahead of print] This is the first autopsy study in HFpEF patients. The most novel finding was that of considerably reduced microvascular density that was independent of CAD and in adjusted analyses appeared to account for the increased fibrosis.
- 95. Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: the systemic nature of heart failure with preserved ejection fraction. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014420. [Epub ahead of print] This editorial offers an additional perspective to help reassure cardiologists uncomfortable with a paradigm conceptualizing the most common form of HF as anything other than a purely cardiac disorder.
- 96.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 97.Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, March AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese, older adults: a randomized controlled trial. Am J Clin Nutri. 2015 doi: 10.3945/ajcn.114.105270. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.The LS, I. Pahor M, Blair SN, Espeland MA, Fielding RA, Gill TM, et al. Effects of a Physical Activity Intervention on Measures of Physical Performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 99.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 100.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169:122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 101.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb F, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29 doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Toth M, Matthews DE, Ades PA, Tischler MD, Van Buren P, Previs M, et al. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288:E685–E692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 103.Hambrecht R, Schulze PC, Gielen S, Linke A, Mobius-Winkler S, Erbs S, et al. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. E J Cardiovasc Prev Rehabil. 2005;12:401–406. doi: 10.1097/01.hjr.0000173106.68485.b7. [DOI] [PubMed] [Google Scholar]
- 104.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 105.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edelmann F, Gelbrich G, Dungen H, Frohling S, Wachter R, Stahrenberg R, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 107.Kitzman D, Brubaker P, Herrington D, Morgan T, Stewart K, Hundley W, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerksick C, Harvey T, Stout J, Campbell B, Wilborn C, Kreider R, et al. International Society of Sports Nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2008;5:17. doi: 10.1186/1550-2783-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kraemer WJ, Hatfield DL, Volek JS, Fragala MS, Vingren JL, Anderson JM, et al. Effects of amino acids supplement on physiological adaptations to resistance training. Med Sci Sports Exerc. 2009;41:1111–1121. doi: 10.1249/MSS.0b013e318194cc75. [DOI] [PubMed] [Google Scholar]
- 110.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients With Preserved Ejection Fraction / Clinical Perspective. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santarpia L, Contaldo F, Pasanisi F. Body composition changes after weight-loss interventions for overweight and obesity. Clin Nutr. 2013;32:157–161. doi: 10.1016/j.clnu.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 112.Villareal D, Chode S, Parimi N, Sinacore D, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, et al. Effects of Human Growth Hormone in Men over 60 Years Old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 114.Sivakumar T, Mechanic OJ, Fehmie DA, Paul BT. Growth hormone axis treatments for HIV-associated lipodystrophy: a systematic review of placebo-controlled trials. HIV Medicine. 2011;12:453–462. doi: 10.1111/j.1468-1293.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 115.Cittadini A, Marra AM, Arcopinto M, Bobbio E, Salzano A, Sirico D, et al. Growth Hormone Replacement Delays the Progression of Chronic Heart Failure Combined With Growth Hormone Deficiency: An Extension of a Randomized Controlled Single-Blind Study. JCHF. 2013;1:325–330. doi: 10.1016/j.jchf.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 116.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–321. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 117.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]