Abstract
In this work we have shown that astrovirus infection induces apoptosis of Caco-2 cells, since fragmentation of cellular DNA, cleavage of cellular proteins which are substrate of activated caspases, and a change in the mitochondrial transmembrane potential occur upon virus infection. The human astrovirus Yuc8 polyprotein capsid precursor VP90 is initially processed to yield VP70, and we have shown that this processing is trypsin independent and occurs intracellularly through four cleavages at its carboxy-terminal region. We further showed that VP90-VP70 processing is mediated by caspases, since it was blocked by the pancaspase inhibitor benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone (z-VAD-fmk), and it was promoted by the apoptosis inducer TNF-related apoptosis-inducing ligand (TRAIL). Although the cell-associated virus produced in the presence of these compounds was not affected, the release of infectious virus to the cell supernatant was drastically reduced in the presence of z-VAD-fmk and increased by TRAIL, indicating that VP90-VP70 cleavage is important for the virus particles to be released from the cell. This is the first report that describes the induction and utilization of caspase activity by a virus to promote processing of the capsid precursor and dissemination of the viral particles.
Apoptosis is a cell suicide mechanism that plays a central role in development and homeostasis in diverse multicellular organisms (35), and it is also considered to represent a natural cell defense mechanism against pathogens, including viruses, to limit their replication and spread (10, 17). Viruses, however, have evolved mechanisms to evade the apoptotic response through the synthesis of antiapoptotic factors that prevent or delay this response until viral replication is no longer compromised (10, 17). When it is induced early during the infection, it is believed that the apoptotic process may facilitate the spread of viruses with short replication cycles (29), for instance, by packaging particles into apoptotic bodies to favor virus dissemination (36).
Caspases, a family of cysteinyl proteases whose activity is induced during apoptosis, are key effectors of this process (7); they recognize and cleave substrates at very characteristic motifs (7, 34). Their activation occurs through a cascade-type system in which the initially activated caspase activates the downstream executioner caspases, which are responsible for cleavage of the cellular targets (7, 34). The proteins of a number of viruses, such as human influenza virus, adenovirus, coronavirus, hepatitis C virus, and calicivirus, have been found to be cleaved by caspases (1, 9, 15, 31, 40), and in some cases these cleavages have been shown to interfere with virus morphogenesis (6, 40). On the other hand, caspase activity has been reported to be necessary for the efficient replication of Aleutian mink disease parvovirus and avian influenza virus, through promoting the relocation of viral proteins into different cell compartments (2, 39).
Human astroviruses (HAstV) are recognized as the second major cause of viral gastroenteritis around the world (14). Eight astrovirus serotypes have been identified in humans (HAstV-1 to HAstV-8), which differ mainly in the amino acid sequence of the carboxy-terminal half of the capsid polyprotein precursor (24, 38). The astroviral genome has three open reading frames (ORFs 1a, 1b, and 2) (19), each encoding a polyprotein which is processed during infection (13, 22, 23). ORF1a and ORF1b code for precursors of the viral nonstructural proteins, which are believed to be mainly processed by the viral serine protease (11, 23), while ORF2 codes for the precursor of the viral capsid proteins.
In HAstV strain Yuc8, VP90, the primary product of ORF2, is initially cleaved at its carboxy-terminal region to yield VP70, which is found in purified viral particles (22). The VP70-containing virus is not or only poorly infectious and requires trypsin to activate its infectivity (22). During trypsin activation, VP70 is initially processed into polypeptides VP41 and VP28, which are further cleaved in a sequential manner to yield a fully infectious virus composed of proteins VP34, VP27, and VP25 (22).
In this work, which was carried out to understand the processing of the capsid precursor of HAstV and its role in virus morphogenesis, we found that astrovirus Yuc-8 induces apoptosis in Caco-2 cells and utilizes the activated caspases for processing VP90 to VP70 and to regulate the timing of virus release.
MATERIALS AND METHODS
Virus and cells.
Colon carcinoma Caco-2 cells from the American Type Culture Collection were used in this work. Cells were cultured in a 10% CO2 atmosphere at 37°C with minimal essential medium (Eagle's salts) (MEM) supplemented with glutamine and 15% fetal bovine serum (FBS) (Gibco-BRL). Viral stocks of HAstV serotype 8, strain Yuc8 (24), were prepared as described (23), with 200 μg of trypsin/ml to activate virus infectivity and maintaining trypsin at 3 μg/ml during and after infection. Yuc8 was titrated in Caco-2 cells by an immunoperoxidase assay, essentially as described for rotavirus (16), but the cells were stained at 18 h postinfection and a rabbit hyperimmune serum to Yuc8 was used as the primary antibody.
Sera and reagents.
A polypeptide containing amino acid residues 666 to 782 of Yuc8 ORF2 was synthesized in Escherichia coli as a fusion with glutathione S-transferase (GST) with the pGEX4T vector (Pharmacia). This protein, named E4, was used to generate a hyperimmune rabbit serum, as previously described (22). Sera to recombinant proteins E2 (amino acid residues 209 to 341 of ORF2) and 1a-3 (amino acid residues 401 to 638 of ORF1a) have been described previously (22, 23). Monoclonal antibodies to PARP (poly-[ADP-ribose]-polymerase) and to lamins A and C were from Cell Signaling and StressGene, respectively; benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) and TNF-related apoptosis-inducing ligand (TRAIL) were purchased from Biomol. Res. Lab. MitoTracker Red was from Molecular Probes. The in situ cell death detection kit (Roche), based on the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay, was used to determine the fragmentation of DNA, following the instructions of the manufacturer.
Cell infection and virus purification.
Caco-2 cells were infected with astrovirus Yuc8 essentially as described (22, 23). All virus samples were incubated before titration with 200 μg of trypsin per ml for 1 h at 37°C to activate virus infectivity, and trypsin was maintained at 3 μg/ml during the adsorption period. When used, soybean trypsin inhibitor (Sigma) was maintained in the culture medium at 400 μg/ml during, as described (23), or after the adsorption period in addition to 2% FBS. z-VAD-fmk and TRAIL were used at 50 μM and 1 μg/ml, respectively. To radioactively label the viral proteins, 50 μCi of Express-[35S]-labeling mix (NEN Life Science) per ml in methionine-free MEM was added at 12 h postinfection, and the cells were incubated at 37°C for 12 additional h and then harvested in TNS buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.5% sodium dodecyl sulfate, 20 μg of phenylmethylsulfonyl fluoride per ml, and 100 μg of leupeptin/ml). Virus purification was carried out by isopycnic cesium chloride gradient centrifugation, as previously described (22).
Immunoassays.
Immunoprecipitation of 35S-labeled proteins was carried out as described with the anti-E2 polyclonal serum (22). For the immunoblot analysis, proteins in TNS buffer were separated in 11 or 12% sodium dodecyl sulfate-urea-polyacrylamide gels, transferred to a nitrocellulose membrane (Millipore), and revealed with the indicated antibodies, as described (22). For the immunofluorescence assays, Caco-2 cells were infected as mentioned above and fixed with paraformaldehyde at 24 or 36 h postinfection. After permeabilization of the cells with 0.05% Triton X-100 in 1% bovine serum albumin-phosphate-buffered saline and blocking with 1% bovine serum albumin in phosphate-buffered saline, the cells were incubated with the indicated primary antibody for 1 h at room temperature. After this, the secondary antibody, either anti-mouse or anti-rabbit immunoglobulin G conjugated with Alexa Fluor 488 or Alexa Fluor 568 (Molecular Probes), respectively, was added. When used, MitoTracker Red was added at 200 nM (from a 1 mM stock solution in dimethyl sulfoxide), and the cells were incubated at 37°C for 15 min before fixation with formaldehyde, as described (28). The cells were analyzed with a Bio-Rad MRC-600 confocal microscope.
RESULTS
Processing of VP90 to VP70 is trypsin independent.
In Caco-2 cells infected with HAstV serotype 8 strain Yuc8, VP90, the primary translation product of ORF2, is initially processed at its carboxy terminus to yield a protein of 70 kDa, and this processing has been suggested to occur intracellularly (22).
Trypsin, which is usually included in cell culture medium during the growth of human astroviruses, has been suggested to cleave the primary translation product of HAstV-2 ORF2 (8, 30). To evaluate the possibility that the processing of Yuc8 VP90 was mediated by trypsin, Caco-2 cells were infected with Yuc8, and 1 h after adsorption, the virus inoculum was removed and cell medium containing either trypsin, a trypsin inhibitor, or a trypsin inhibitor plus FBS was added. The cells were radioactively labeled for 12 h, starting at 12 h postinfection, and the cell-associated viral proteins were analyzed by immunoprecipitation.
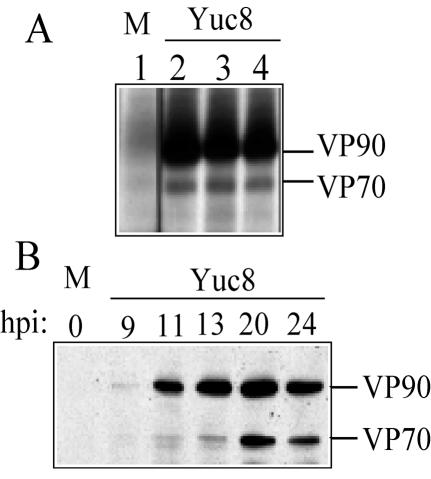
Both VP90 and VP70 were detected at the same level in the three conditions tested, regardless of whether trypsin activity was present during the infection period (Fig. 1A), indicating that the processing of VP90 to VP70 is trypsin independent. On the other hand, analysis of the kinetics of synthesis of VP90 and its processing to VP70 by Western blot showed that VP90 was initially detected at 9 h postinfection and clearly observed at 11 h postinfection, while its cleavage product, VP70, was clearly detected 2 h later (Fig. 1B). At 24 h postinfection, when the replication cycle of the virus has been completed, based on one-step growth curves determined for the Yuc8 strain (this work; data not shown), the processing of VP90 is limited (Fig. 1) (22). The lack of trypsin activity on the cell-associated fraction was confirmed by the fact that the primary trypsin cleavage products of VP70, VP41 and VP28, were not detected in these experiments (not shown).
FIG. 1.
Processing of Yuc8 capsid precursor polyprotein is trypsin independent. (A) Mock- (M) and Yuc8-infected Caco-2 cells were maintained in the presence of trypsin (lane 2), soybean trypsin inhibitor (lane 3), or trypsin inhibitor plus fetal bovine serum (lane 4). Cells were labeled for 12 h with the [35S]-Express labeling protein mix, starting at 12 h postinfection; cell lysates were collected, immunoprecipitated with anti-E2 antibodies, and analyzed by polyacrylamide gel electrophoresis. The mock-infected cells were treated as in lane 2. (B) Unlabeled Yuc8-infected cells were washed with phosphate-buffered saline and lysed at the indicated times (hours postinfection). Proteins were separated by polyacrylamide gel electrophoresis and detected by immunochemiluminescence with anti-Yuc8 serum as the primary antibody.
VP90 assembles into virions and is processed to VP70 through four cleavages.
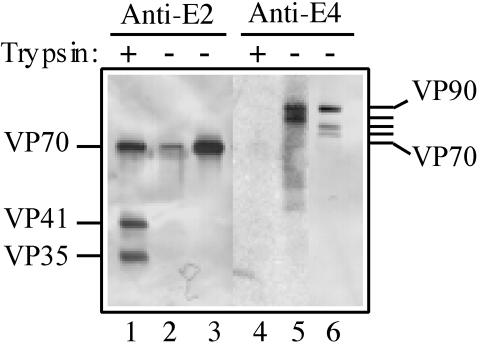
To evaluate if VP90 is assembled into virus particles or if its cleavage to VP70 is required for capsid assembly, viral particles grown in either the presence (3 μg/ml) or absence (trypsin inhibitor plus FBS) of trypsin were purified by isopycnic cesium chloride centrifugation, and the purified particles were analyzed for their protein composition with antibodies to recombinant proteins E2 and E4 (see Fig. 3). Antibodies to E2 recognize the region of VP90 between amino acid residues 209 and 341 and should thus recognize both VP70 and VP90, while antibodies to E4 are directed to the 116 carboxy-terminal amino acids of VP90 and should recognize this protein but not VP70.
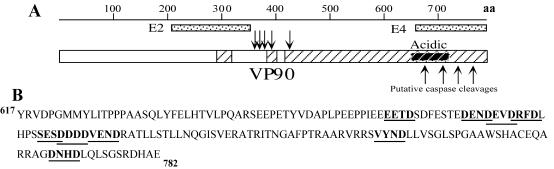
FIG. 3.
VP90 contains caspase cleavage motifs in its acidic domain. (A) Diagram of VP90, with the conserved and hypervariable regions depicted as open and striped boxes, respectively, shown to scale. The conserved acidic region located in the carboxy-terminal hypervariable region is shown as a black-striped box. The localization of recombinant proteins E2 and E4 (dotted boxes) and the trypsin-dependent cleavage sites on VP70 (downward arrows) (22) are indicated. (B) Sequence of the carboxy-terminal 165 amino acid residues of VP90, showing motifs that could putatively be cleaved by caspases (bold letters and underlined) (7, 34). The sequence numbering corresponds to Yuc8 VP90. The putative caspase cleavage sites on VP90 are indicated (panel A, upward arrows).
The viral particles from cultures maintained in the presence of trypsin yielded only one band in the gradient, with an apparent density of 1.36 g/cm3. The virions in this band contained VP70 and its amino-terminal trypsin cleavage products VP41 and VP35, as detected by anti-E2 antibodies (Fig. 2, lane 1), as previously reported (22). Antibodies to E4 recognized no protein in these particles (Fig. 2, lane 4). On the other hand, the virus from cultures maintained in the absence of trypsin produced two bands in the gradient, a major band at 1.36 g/cm3 and a minor one at 1.18 g/cm3. The 1.36-g/cm3 virions were formed by VP70 (detected by anti-E2 serum; Fig. 2, lane 3), and small amounts of three proteins of 75, 78, and 90 kDa (detected by anti-E4 serum; Fig. 2, lane 6). The fraction of 1.18 g/cm3 contained a small amount of VP70 (detected by anti-E2 serum; Fig. 2, lane 2) as well as VP90, and a protein of 82 kDa (detected by anti-E4 serum; Fig. 2, lane 5). Antibodies to E2 did not recognize proteins of 75 to 90 kDa in either of the gradient fractions because they were present in very low amounts in the purified virions (not detectable by staining with Coomassie brilliant blue). These results indicate that processing at the carboxy-terminal region of VP90 to yield VP70 may involve at least three intermediate cleavage products of 82, 78, and 75 kDa.
FIG. 2.
Purified Yuc8 particles are formed mainly of VP70 but may contain minor amounts of VP90. Viral particles were purified by CsCl gradient centrifugation from the total lysate of cultures infected in the usual manner (Materials and Methods) but maintained in the presence of trypsin (lanes 1 and 4) or soybean trypsin inhibitor plus FBS (lanes 2, 3, 5, and 6), after infection. Particles with an apparent density of 1.36 g/cm3 (lanes 1, 3, 4, and 6) were obtained from both culture conditions, while particles with an apparent density of 1.18 g/cm3 (lanes 2 and 5) were only obtained in the absence of trypsin. The protein content of each gradient fraction was determined by electrophoresis and immunoblot analysis with the indicated sera.
In addition, the fact that VP90 was found in viruses grown in the absence of trypsin indicates that this precursor polyprotein is able to assemble into virions. However, the fact that it was only found in small amounts suggests either that this protein is poorly assembled into viral particles or that the particles formed by this protein are unstable and disassemble during the purification step (see below).
Astrovirus infection induces apoptosis in Caco-2 cells.
VP90 contains motifs at its carboxy-terminal region similar to those recognized and cleaved by caspases in cellular proteins (e.g., DXXD and XEXD; Fig. 3), and acidic amino acid residues in this region of VP90 are highly conserved among astroviruses (Table 1), despite the fact that this region has been found to be hypervariable among HAstV strains belonging to different serotypes (24, 38). Since these putative caspase cleavage motifs are located in a region where cleavages to produce VP70 are expected to occur, we investigated whether astrovirus Yuc8 induces apoptosis and the possible role of caspases on the processing of VP90.
TABLE 1.
Putative caspase cleavage sites in the capsid precursor polyprotein among astrovirusesa
| Species specificity and strain | Sequence | Accession no. |
|---|---|---|
| Human | ||
| 8 | 664..PPIEEEETDSDFESTEDENDEVDRFDLHPSSESDDDDVENDRATLL...709 | AF260508 |
| 5 | 665...ALFSAEETDSDFESTEDETDEVDRFDLHLSSESDDDDVENNRVTLL...710 | U15136.1 |
| 6 | 669...HVNDEEETEYETESDEDETDEVDRFDLCCTSDSEDD-IENNRVTLL...713 | Z46658.1 |
| 1 | 670...QFKEDIETDTDIESTEDEDDEADRFDIIDTSDEEDGNETDRVTLL...714 | L23513.1 |
| 3 | 675...SRVFLEETDYEDEEDEDEDDEADRFDLHSSYGSEPEDDDENNRVTLL...721 | AF117209.1 |
| 2 | 677...CENVSEETETEDEEDEDEDDEADRFDLHSPYSSEPEDSDENNRVTLL...723 | A45695 |
| 7 | 672...VLELPTETDTESEEDEDEDDEADRFDLHSSYGSEPEDDDENNRVTLL...718 | AF292078 |
| 4 | 651...NIGLEEDQTDNWQEPDEDVQTSTEESDYETDSLEGESDDEDSNTCREL...698 | S45048 |
| Feline | 691...EDVSDEETDTETESNEDEDDEVDRFDLHDSSGSEPEDDDVENDRVTLL...738 | AF056197.1 |
| Porcine | 658...EPPAEEDVGDNETEDESDDEDLDHFDLHDSSGSEPEDEDVENNRVTLL705 | AB037272 |
| Ovine | 643...GALADTSFNLDSLRAD658................712SSSEDDLSDDDDFECL...727 | NC002469 |
| Turkey | 621...FLDHLRTVQFANLDDSQPAPYDSDDDDLSDVTSLFEQADLGDETDFKF...667 | AF206663 |
| Chicken | 557..QMQDVTDTTTHTTSVNVYF575.....637SDPDSDDDISLAGSVIGDEFDSVDHL...662 | AB033998 |
Putative caspase cleavage motifs are indicated in bold.
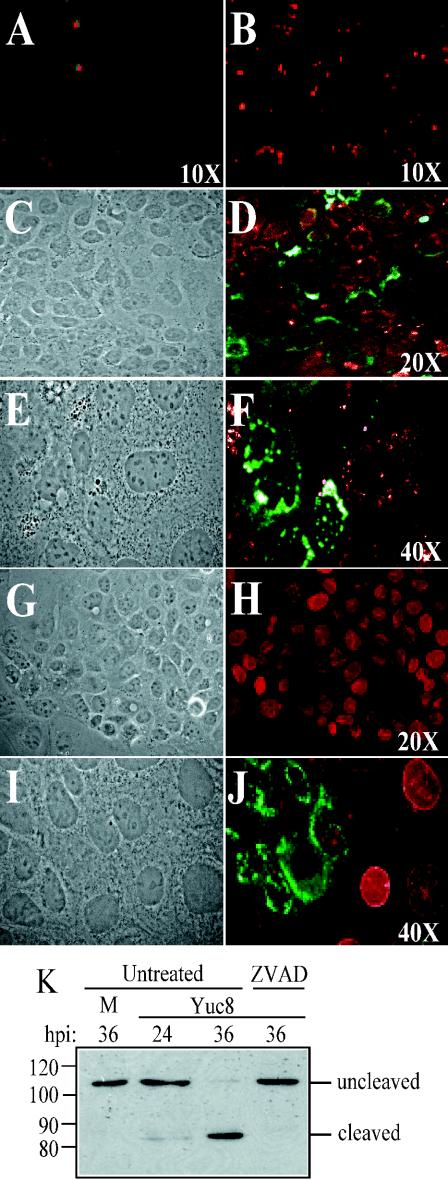
DNA fragmentation, an apoptosis indicator, was enhanced during astrovirus infection, as judged by a larger number of TUNEL-positive cells in Yuc8-infected compared to noninfected cells (Fig. 4A and B); although TUNEL-positive cells increased by more than fivefold in infected cultures, not every infected cell was TUNEL positive. Induction of apoptosis is accompanied by a drop in the mitochondrial transmembrane potential (18, 32). Thus, to confirm the apoptotic state of the astrovirus-infected cells, we evaluated changes in mitochondrial transmembrane potential with the MitoTracker Red reagent, based on its ability to be fixed in normal but not in apoptotic cells (28). As observed in Fig. 4D and 4F, the reagent was efficiently fixed in noninfected cells but did not label Yuc8-infected cells, indicating changes in the mitochondrial transmembrane potential in infected cells.
FIG. 4.
Yuc8 infection induces apoptosis in Caco-2 cells. Uninfected (A, G, and H) and Yuc8-infected cells (B to F and I and J) were processed 24 h postinfection for fluorescence microscopy. Fixed cells were analyzed for DNA fragmentation by the TUNEL assay (A and B). To measure mitochondrial membrane permeability, cells treated with MitoTracker Red (revealed in red) before fixation were stained with anti-Yuc8 antibodies (in green) (D and F). On the other hand, mock- (H) and Yuc8- (J) infected cells were stained with anti-lamin A and C (in red) and anti-Yuc8 antibodies (in green). Panels C, E, G, and I represent phase contrast images of the fluorescent images shown in panels D, F, H, and J, respectively. (K) Yuc8-infected cells maintained either in regular conditions (untreated) or in the presence of z-VAD-fmk and harvested at different times (hours) postinfection, as indicated. Proteins were analyzed by immunoblot with anti-PARP antibodies. M, mock-infected cells. The uncleaved and cleaved forms of PARP (at the right) and the positions of molecular size markers (at the left, in kilodaltons) are indicated.
To further confirm that Yuc8-infected cells were in apoptosis, the integrity of lamin A and PARP, two cellular proteins known to be cleaved by executioner caspases 6 and 3, respectively, was evaluated. Lamin A, a nuclear protein, was poorly or not detected in Yuc8-infected cells (Fig. 4J, in green) as opposed to uninfected cells (Fig. 4H and J), suggesting that caspase-6 had been activated. Similarly, cleavage of PARP was observed in Yuc8-infected cells, and this cleavage was prevented by the pancaspase inhibitor z-VAD-fmk (7) (Fig. 4K) and the specific caspase-3 inhibitor N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO) (not shown), suggesting againthat the executioner caspases 3 and 6 were activated upon virus infection. Altogether, these data indicate that astroviruses induce apoptosis in infected cells and suggested the possibility that virus-induced caspases could be responsible for the processing of VP90.
Caspase inhibitor prevents processing of VP90 and virus release.
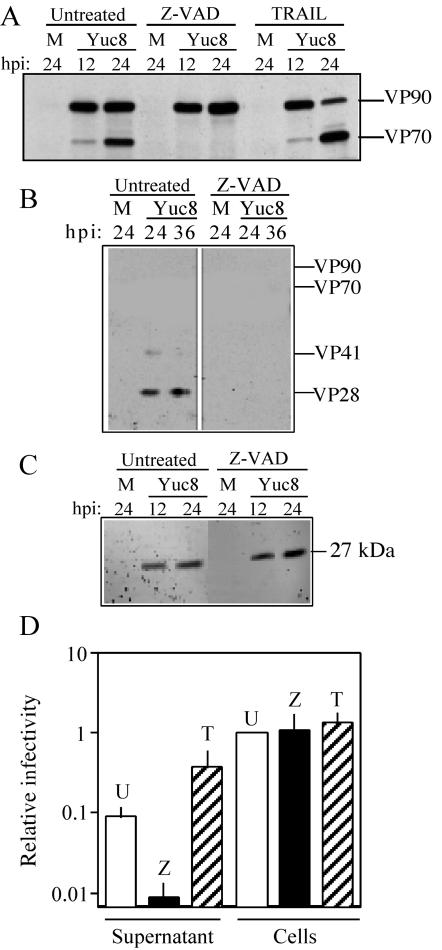
The role of caspases in virus replication was studied with the pancaspase inhibitor z-VAD-fmk. In a regular astrovirus infection (with trypsin at 3 μg/ml present in the cell culture medium), intracellular VP90-VP70 processing was clearly observed at 24 h postinfection (Fig. 1A and 5A), and at this time the virus was already detected in the cell medium, as judged by the presence of capsid proteins VP41 and VP28, initial trypsin cleavage products of VP70 (Fig. 5B). In contrast, when z-VAD-fmk was present during virus infection, the intracellular processing of VP90 was blocked (Fig. 5A) and the capsid proteins were not detected in the cell supernatant (Fig. 5B), suggesting that processing of VP90 is required either for assembly of the virus particles or for exit of the virions from the cells. The inhibitory effect of z-VAD-fmk was specific for the processing of the astrovirus capsid precursor polyprotein, since processing of the nonstructural polyprotein nsp1a, evaluated by the presence of the 27-kDa viral nonstructural protein (11, 23), was not affected (Fig. 5C).
FIG. 5.
Processing of VP90 to VP70 and virus release are modulated by caspases. Yuc8-infected cells were left untreated or treated with either z-VAD-fmk or TRAIL immediately after the adsorption period. At the indicated times postinfection (hours), cell-associated (A, C, and D) and cell supernatant (B and D) fractions were analyzed for viral proteins (A, B, and C) or infectious virus (D). Analysis of viral proteins was carried out by electrophoresis and immunoblot with anti-Yuc8 (A and B) and anti-nonstructural protein (C) antibodies. Virus infectivity (D) was determined after treatment of the supernatant and cell-associated fractions with trypsin from cultures that were untreated (U) or treated with z-VAD-fmk (Z) or TRAIL (T). These data were normalized to that for untreated cell-associated infectivity (relative infectivity of 1), which showed viral titers between 107 and 108 FFU/ml. Data are from six experiments, and the standard deviation is shown. M, mock-infected cells. The migration of viral Yuc8 proteins is marked at the right of each panel.
In the presence of z-VAD-fmk, virus infectivity in the cell supernatant at 24 h postinfection was about 10-fold lower than that found in the absence of the inhibitor (Fig. 5D). On the other hand, the infectious titer of the cell-associated virus fraction (after trypsin treatment) was essentially the same in the presence and absence of the inhibitor (Fig. 5D). It is important to note that the virus released represents only a small fraction of the total infectious particles (around 5 to 10%), which explains why the 10-fold reduction in its titer had no significant impact in the titer of the intracellular infectious particles (Fig. 5D and 6B). These observations demonstrate that the pancaspase inhibitor z-VAD-fmk abolishes the processing of VP90 to VP70 and strongly suggest that it prevents virus release from cells, indicating that these events are closely related.
FIG. 6.
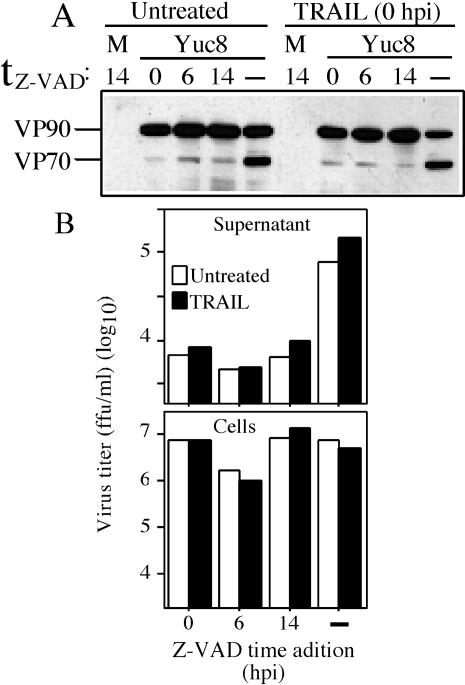
Astrovirus release is dependent on caspase activity and not on cell lysis. Yuc8-infected cells were left untreated or treated with TRAIL immediately after the adsorption period (0 h postinfection), as indicated. z-VAD-fmk was added to these cells at 0, 6, or 14 after addition of TRAIL. No z-VAD-fmk was added to control cells (dash). at 20 h postinfection, the cell-associated proteins were analyzed by immunoblot with anti-Yuc8 antibodies (A), and virus infectivity was determined in the supernatant as well as in the cell-associated fraction after treatment with trypsin (B). The data presented in this figure are representative of three independent experiments yielding similar results. M, mock-infected cells.
Apoptosis inducers promote processing of VP90 and virus release.
TRAIL has been reported to induce programmed cell death, via death receptors, in a number of cell lines, including Caco-2 cells (37). This was confirmed in our conditions by an increased number of TUNEL-positive cells in the presence of TRAIL (not shown). Thus, this ligand was used to evaluate the role of caspases in virus replication. As opposed to z-VAD-fmk treatment, treatment of cells with TRAIL increased the processing of VP90, with VP70 becoming more abundant than VP90 (Fig. 5A). This effect was observed even when TRAIL was added to the cell culture at 6 or 8 h postinfection (not shown), the time at which VP90 synthesis was still undetectable (see Fig. 1). If processing of VP90 to VP70 is required for virus to exit the cell, one would predict that increased cleavage of VP90 would be reflected as a greater amount of infectious virus released into the cell supernatant. As expected, treatment of infected cells with TRAIL resulted in an extracellular virus titer about four- to fivefold higher than that found in untreated cells (Fig. 5D). As in the case of z-VAD-fmk treatment, the titer of cell-associated virus (usually around 107 to 108 FFU/ml after incubation of the samples with trypsin) was essentially the same in untreated and TRAIL-treated cells (Fig. 5D). The lack of apparent reduction in the titer of intracellular virus can be explained, as mentioned above, by the fact that only a small fraction of the total virus is found in the supernatant. In contrast to VP70, VP90 was never detected in the medium from cells treated with TRAIL, even at late times postinfection (up to 48 h), reinforcing the conclusion that processing of the capsid precursor is needed for virus secretion.
Caspase activity and not cell lysis is responsible for virus release.
It has been reported that permeabilization of the plasma membrane is induced at late apoptosis (21). To discard the possibility that release of the VP70-containing virus was related to this permeabilization effect rather than to cleavage of VP90, we evaluated the processing of VP90 and virus release in cells treated with both TRAIL and z-VAD-fmk. It has been shown in various cell lines, including HT29 (colon adenocarcinoma cells), that the cell death response to death ligands such as tumor necrosis factor and TRAIL can be enhanced by the presence of caspases inhibitors such as z-VAD-fmk and CmrA from poxvirus, so that neither cytolysis nor plasma membrane permeabilization is blocked by blocking caspase activity (5, 21, 27). Under these conditions, caspase inhibitors can sensitize the cells to die by necrosis or by a caspase-independent apoptosis mechanism. Based on these observations, Yuc8-infected Caco-2 cells (which are expected to behave like HT29 cells, since both were derived from colon adenocarcinomas) were treated with TRAIL and then z-VAD-fmk was added 0, 6, or 14 h later, and processing of VP90 and virus release were analyzed.
In the absence of z-VAD-fmk, TRAIL induced processing of VP90 and release of virus, as described before (Fig. 6A and 6B); however, when z-VAD-fmk was added, even 14 h after TRAIL treatment, processing of VP90 and release of virus were both inhibited, while intracellular virus was maintained (Fig. 6A and B). The apoptotic state of the cells after 14 h of TRAIL treatment was suggested by an increase in the number of TUNEL-positive cells (not shown). In addition, the morphological changes observed in cells treated with the combination of TRAIL and z-VAD-fmk were more severe than those found in cells treated with TRAIL or z-VAD-fmk alone, suggesting that indeed cell death occurred in the absence of caspases activity.
Thus, in cells in which cell death was promoted but caspase activity was blocked, processing of VP90 and astrovirus particle release were diminished, confirming that VP90 needs to be processed for virus to exit the cell.
DISCUSSION
In this work we have shown that astrovirus infection induces apoptosis of Caco-2 cells because the number of TUNEL-positive cells increased upon infection, PARP and lamin A, substrates of executioner caspases 3 and 6, respectively, were cleaved and this cleavage was inhibited by the pancaspase inhibitor z-VAD-fmk, and the mitochondrial transmembrane potential was affected as a result of virus infection. We further showed that the activity of the induced caspases is involved in VP90-VP70 processing of the polyprotein capsid precursor, most likely by cleaving it, since cleavage of the precursor was inhibited by z-VAD-fmk and promoted by the apoptosis inducer ligand TRAIL. Activated caspases 3 and 6 could cleave VP90 at motifs similar to those recognized on PARP (DEVD) and lamina A (VEID). In fact, the motif EETD/S found in VP90, including amino acid residues 669 to 673, whose cleavage would yield a protein of about 70 kDa, is very similar to the cleavage site recognized on procaspase-3 at amino acid residue 175 (IETD175/S) (4).
The carboxy-terminal region of the capsid precursor polyprotein of HAstV has been shown to be hypervariable among virus strains belonging to different serotypes (24, 38); however, the acidic character of this region, particularly the putative caspase recognition sequence DEVDRFD located between amino acid residues 683 and 689 (based on the Yuc8 sequence), is highly conserved. On the other hand, although the carboxy-terminal region of VP90 is quite different between human and animal astrovirus strains (less than 10% similar) (20), caspase cleavage motifs are also present in this region of the capsid precursor of feline, sheep, porcine, and avian astroviruses (Table 1). In particular, the DEVDRFD motif is also present in a feline isolate. These similarities suggest a general mechanism for cleavage of the capsid precursor polyprotein of human and animal astrovirus strains.
Astrovirus particles purified by CsCl gradients were found to be composed mostly of VP70, although small amounts of VP90 could be detected. In addition, minor quantities of three proteins of 82, 78, and 75 kDa, which seemed to be intermediate products of VP90-VP70 processing, were observed, suggesting that at least four cleavages occur in the carboxy-terminal region of VP90 to yield VP70. In this regard, it is of interest that proteins of 82 and 75 kDa, sharing the amino terminus with the capsid precursor polyprotein, were also observed during infection with HAstV-1 (12). The putative intermediate cleavage polypeptides were only detected in viruses grown in the presence of FBS, indicating that VP90-VP70 processing can be partially blocked by the presence of serum. This blocking effect could be related to the ability of growth factors present in serum to delay apoptosis and thus activation of the caspases involved in cleavage of the capsid precursor. Potential caspase cleavage motifs on VP90 whose cleavage would generate polypeptides of about 82, 77, and 75 kDa (putative processing intermediaries of VP90) are indicated in Fig. 3.
In the presence of z-VAD-fmk, cleavage of VP90 to VP70 was shown to be drastically reduced, and the titer of virus released was also significantly decreased. However, if the lysate of z-VAD-fmk-treated cells was incubated with trypsin, intracellular infectious viruses could be recovered to a titer similar to that obtained from untreated cells (Fig. 5), strongly suggesting that VP90 is able to assemble into viral particles. The fact that small amounts of VP90 and the intermediary cleavage products of 82, 78, and 75 kDa were detected in cesium chloride-purified virions supports this conclusion. However, the VP90 particles do not seem to be as stable as the VP70 particles during the purification protocol, since no virions could be purified from cultures maintained in the presence of z-VAD-fmk. These observations are in agreement with a recent report where it was shown that the 87-kDa polyprotein precursor protein of HAstV-2 assembles into virus-like particles when expressed in monkey cells with a vaccinia virus expression vector (8). However, as in our case, no 87-kDa particles could be purified from those cells. These observations strongly suggest that VP90 is able to assemble into virus particles which may become structurally stabilized when the precursor is cleaved to VP70.
The fact that infectious viruses could be recovered from z-VAD-fmk-treated cells (in which VP90 is not processed) when the cell lysate is treated with trypsin indicates that this protease cleaves VP90 to yield a VP70-like protein that is subsequently processed into the final products of the capsid, VP41 to VP25, which enable the virus to be infectious (22). This possibility seems likely, since the intracellular virus-like particles formed by the HAstV-2 87-kDa protein were cleaved by trypsin into final products indistinguishable from those generated when HAstV-2 virus particles were treated with this protease (8, 30).
The results obtained in this work indicate that the VP90-VP70 cleavage is associated with the release of the virus from the cells' interior because VP70 or its trypsin cleavage products but not VP90 were observed in the medium of infected cultured cells; VP90 was never observed even at late time in cells treated with TRAIL; the caspase inhibitor z-VAD-fmk, which blocks the processing of VP90, drastically reduced the release of infectious particles, while TRAIL, which promotes VP90 processing, enhanced its release; virus release was limited in cells treated with both TRAIL and z-VAD-fmk, conditions in which cell death (probably through necrosis or by a caspase-independent mechanism) seem to occur (5, 21, 27), while the processing of VP90 is blocked; and the inhibitory effect of z-VAD-fmk on virus release was observed even when this inhibitor was added late after triggering apoptosis (14 h after TRAIL treatment), a time at which VP90 starts to be processed. In addition, recent data from our laboratory have shown that VP90 to VP70 processing and virus release are both blocked in conditions where caspase-3 is active (indicative of an active apoptotic process), while they proceed in the absence of caspase-3 activity (unpublished data). Altogether, these data strongly support the hypothesis that the release of virus and processing of VP90 to VP70 are two closely related events and that the apoptotic process itself is not a determinant of virus release.
In summary, we propose that VP90 assembles inside cells to form precursor virus particles, which are then processed by caspases to produce VP70-containing viruses through at least four cleavages. VP90-VP70 processing promotes the release of viral particles from the cell. Finally, VP70 particles become infectious viruses when trypsin cleaves VP70 extensively into the final structural proteins VP34, VP27, and VP25 (22). Since both VP90- and VP70-containing particles become infectious after trypsin treatment, processing of the capsid precursor to VP70 would seem to be relevant for virus to be released but not to be assembled or to gain infectivity.
Most viruses fight the antiviral apoptotic cell response by synthesizing factors that block or delay apoptosis until their replication is not compromised, such as human immunodeficiency virus, poxviruses, baculovirus, and adenovirus (17). However, it has been shown that viruses can benefit from these responses (2, 3, 25, 26, 33, 39). In particular, cleavage of the parvoviral NS1 protein by caspases is required for nuclear localization of NS1 and permissive replication of the virus (2), and activation of caspase-3 was shown to be essential for efficient avian influenza virus propagation (39), although in this case the target of the caspase, whether viral or cellular, was not identified. In the present work we have shown that HAstV-8 has evolved a mechanism, which seems to be general for astroviruses, to take advantage of the apoptotic response to complete the viral replication cycle. The astrovirus-induced apoptosis regulates the processing of VP90 to VP70 and the timing of infectious virus release. It remains to be determined what astrovirus product is responsible for inducing apoptosis, the caspase responsible for the cleavage, and the role of VP90-VP70 processing in virus formation and release from the cell. This is the first report that describes the induction and hijacking of caspase activity to promote the processing of a capsid precursor polyprotein and the release and dissemination of viral particles in consequence.
Acknowledgments
We thank Xochitl Alvarado for assistance in confocal microscopy and Gabriela Aguirre for assistance in laboratory techniques. We also acknowledge enriching discussions with Enrique Salas-Vidal and Susana Castro-Obregón. Finally, we also thank Charles M. Rice and members of our laboratory for critical reading of the manuscript.
This work was partially supported by grants MENSE31739 and G37621N from the National Council for Science and Technology-Mexico, IN200999 and IN227602 from DGAPA-UNAM, and 55003662 and 55000613 from the Howard Hughes Medical Institute.
REFERENCES
- 1.Al-Molawi, N., V. A. Beardmore, M. J. Carter, G. E. Kass, and L. O. Roberts. 2003. Caspase-mediated cleavage of the feline calicivirus capsid protein. J. Gen. Virol. 84:1237-1244. [DOI] [PubMed] [Google Scholar]
- 2.Best, S. M., J. F. Shelton, J. M. Pompey, J. B. Wolfinbarger, and M. E. Bloom. 2003. Caspase cleavage of the nonstructural protein NS1 mediates replication of Aleutian mink disease parvovirus. J. Virol. 77:5305-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best, S. M., J. B. Wolfinbarger, and M. E. Bloom. 2002. Caspase activation is required for permissive replication of Aleutian mink disease parvovirus in vitro. Virology 292:224-234. [DOI] [PubMed] [Google Scholar]
- 4.Bratton, S. B., G. Walker, S. M. Srinivasula, X. M. Sun, M. Butterworth, E. S. Alnemri, and G. M. Cohen. 2001. Recruitment, activation and retention of caspase-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 20:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauwels, A., B. Janssen, A. Waeytens, C. Cuvelier, and P. Brouckaert. 2003. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat. Immunol. 4:387-393. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. J., and S. Makino. 2002. Murine coronavirus-induced apoptosis in 17CI-1 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and BID-cleavage. Virology 302:321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton, R. M., E. P. Pastrana, and A. Sanchez-Fauquier. 2003. Vaccinia virus recombinant expressing an 87-kilodalton polyprotein that is sufficient to form astrovirus-like particles. J. Virol. 77:9094-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eleouet, J. F., E. A. Slee, F. Saurini, N. Castagne, D. Poncet, C. Garrido, E. Solary, and S. J. Martin. 2000. The viral nucleocapsid protein of transmissible gastroenteritis coronavirus (TGEV) is cleaved by caspase-6 and -7 during TGEV-induced apoptosis. J. Virol. 74:3975-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, H., and G. McFadden. 2001. Viruses and apoptosis:meddling with mitochondria. Virology 288:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Geigenmuller, U., T. Chew, N. Ginzton, and S. M. Matsui. 2002. Processing of nonstructural protein 1a of human astrovirus. J. Virol. 76:2003-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geigenmuller, U., N. H. Ginzton, and S. M. Matsui. 2002. Studies on intracellular processing of the capsid protein of human astrovirus serotype 1 in infected cells. J. Gen. Virol. 83:1691-1695. [DOI] [PubMed] [Google Scholar]
- 13.Geigenmuller, U., E. Mendez, and S. M. Matsui. 2002. Studies on the molecular biology of human astroviruses, p. 571-584. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science B.V., New York, N.Y.
- 14.Glass, R., J. Noel, D. Mitchell, J. Herrmann, N. Blacklow, L. Pickering, P. Dennehy, G. Ruiz-Palacios, M. Guerrero, and S. Monroe. 1996. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch. Virol. Suppl. 12:287-300. [DOI] [PubMed] [Google Scholar]
- 15.Grand, R., K. Schmeiser, E. Gordon, X. Zhang, P. Gallimore, and A. Turnell. 2002. Caspase-mediated cleavage of adenovirus early region 1A proteins. Virology 301:255. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay, S., and G. Kannourakis. 2002. A time to kill: viral manipulation of the cell death program. J. Gen. Virol. 83:1547-1564. [DOI] [PubMed] [Google Scholar]
- 18.Heiskanen, K. M., M. B. Bhat, H. W. Wang, J. Ma, and A. L. Nieminen. 1999. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J. Biol. Chem. 274:5654-5658. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, B., S. Monroe, E. Koonin, S. Stine, and R. Glass. 1993. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA 90:10539-10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonassen, C. M., T. O. Jonassen, Y. M. Saif, D. R. Snodgrass, H. Ushijima, M. Shimizu, and B. Grinde. 2001. Comparison of capsid sequences from human and animal astroviruses. J. Gen. Virol. 82:1061-1067. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer, G., B. Dallaporta, and M. Resche-Rigon. 1998. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60:619-642. [DOI] [PubMed] [Google Scholar]
- 22.Mendez, E., M. T. Fernandez, S. Lopez, M. Méndez-Toss, and C. F. Arias. 2002. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J. Virol. 76:7996-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez, E., M. P. Salas-Ocampo, M. E. Munguia, and C. F. Arias. 2003. Protein products of the open reading frames encoding nonstructural proteins of human astrovirus serotype 8. J. Virol. 77:11378-11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendez-Toss, M., P. Romero-Guido, M. E. Munguia, E. Mendez, and C. F. Arias. 2000. Molecular analysis of a serotype 8 human astrovirus genome. J. Gen. Virol. 81:2891-2897. [DOI] [PubMed] [Google Scholar]
- 25.Mori, S., M. Murakami, T. Takeuchi, T. Kozuka, and T. Kanda. 2002. Rescue of AAV by antibody-induced Fas-mediated apoptosis from viral DNA integrated in HeLa chromosome. Virology 301:90-98. [DOI] [PubMed] [Google Scholar]
- 26.Olsen, C. W., J. C. Kehren, N. R. Dybdahl-Sissoko, and V. S. Hinshaw. 1996. bcl-2 alters influenza virus yield, spread and hemagglutinin glycosylation. J. Virol. 70:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perfettini, J. L., and G. Kroemer. 2003. Caspase activation is not death. Nat. Immunol. 4:308-310. [DOI] [PubMed] [Google Scholar]
- 28.Poot, M., Y. Z. Zhang, J. A. Kramer, K. S. Wells, L. J. Jones, D. K. Hanzel, A. G. Lugade, V. L. Singer, and R. P. Haugland. 1996. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J. Histochem. Cytochem. 44:1363-1372. [DOI] [PubMed] [Google Scholar]
- 29.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Fauquier, A., A. Carrascosa, J. Carrascosa, A. Otero, R. Glass, J. Lopez, C. S. Martin, and J. Melero. 1994. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology 201:312-320. [DOI] [PubMed] [Google Scholar]
- 31.Satoh, S., M. Hirota, T. Noguchi, M. Hijikata, H. Handa, and K. Shimotohno. 2000. Cleavage of hepatitis C virus nonstructural protein 5A by a caspase-like protease(s) in mammalian cells. Virology 270:476-487. [DOI] [PubMed] [Google Scholar]
- 32.Scaffidi, C., S. Fulda, A. Srinivassan, C. Friesen, F. Li, K. J. Tomaselli, K. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95(APO-1/Fas) signaling pathway. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scallan, M. F., T. E. Allsopp, and J. F. Fazakerley. 1997. bcl-2 Acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J. Virol. 71:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459-470. [DOI] [PubMed] [Google Scholar]
- 35.Steller, H. 1995. Mechanisms and genes of cellular suicide. Science 267:1445-1449. [DOI] [PubMed] [Google Scholar]
- 36.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Q., X. Wang, A. Hernandez, M. R. Hellmich, Z. Gatalicia, and B. M. Evers. 2002. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J. Biol. Chem. 277:36602-36610. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Q. H., J. Kakizawa, L. Y. Wen, M. Shimizu, O. Nishio, Z. Y. Fang, and H. Ushijima. 2001. Genetic analysis of the capsid region of astroviruses. J. Med. Virol. 64:245-255. [DOI] [PubMed] [Google Scholar]
- 39.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 22:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhirnov, O. P., T. E. Konakova, W. Garten, and H. D. Klenk. 1999. Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J. Virol. 73:10158-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]