Abstract
While breast cancer patients benefit from the use of HER2 inhibitors, many fail therapy and become resistant to treatment, indicating a critical need to prevent treatment failure. A number of studies have emerged that highlight the catabolic process of autophagy in breast cancer as a mechanism of resistance to chemotherapy and targeted inhibitors. Furthermore, recent research has begun to dissect how autophagy signaling crosstalks with apoptotic signaling. Thus, a possible strategy in fighting resistance is to couple targeting of apoptotic and autophagy signaling pathways. In this review, we discuss how cellular response by autophagy circumvents cell death to promote resistance of breast cancers to HER2 inhibitors, as well as the potential avenues of therapeutic intervention.
Keywords: HER2, breast cancer, resistance, autophagy, apoptosis, trastuzumab, lapatinib
HER2-Targeted Therapy
Two clinically approved inhibitors, trastuzumab and lapatinib, are most commonly used in human epidermal growth factor receptor-2-positive (HER2+) breast cancer, an aggressive subtype that comprises nearly 25% of breast cancer cases.1,2 More recently, pertuzumab, a monoclonal antibody to HER2 that inhibits receptor dimerization, was also approved for use in early breast cancer with trastuzumab and docetaxel.3
Trastuzumab is a monoclonal antibody that binds to the extracellular region of HER2 and is specific for this epidermal growth factor receptor (EGFR)-family member. It is widely used in primary treatment of HER2+ breast cancers in conjunction with chemotherapy. Additionally, given with chemotherapy or on its own, trastuzumab has also been indicated for patients with metastatic disease who have already received multiple courses of chemotherapy.4,5
Lapatinib is a dual EGFR and HER2 inhibitor, which binds to the intracellular kinase domains of these growth factor receptors and is now widely used in breast cancer treatment. The effectiveness of lapatinib is exemplified by the numerous (~200) clinical trials that have been initiated or completed.6 This drug was initially approved for use in combination with capecitabine for the treatment of patients with HER2+ advanced or metastatic breast cancer, who may also have received prior therapy that includes anthracycline, taxane, and trastuzumab. Lapatinib is also indicated in neo-adjuvant treatment of HER2+ patients who receive chemotherapy prior to surgery and has received accelerated approval for use in patients with triple-positive—ER+, PR+, and HER2+—metastatic disease.6
A newer antibody-drug formulation of trastuzumab called ado-trastuzumab emtansine (T-DM1) links trastuzumab to the microtubule inhibitor emtansine (DM1).7 Release of internalized DM1 results in cytotoxicity of HER2+ breast cancer cells. Recent clinical trial data reports improved progression-free survival rates in patients with metastatic breast cancer who underwent Phase II clinical trials with T-DM1. Published trial data indicates significant progression-free survival in at least three patient populations: those with metastatic HER2+ breast cancer who received no prior chemotherapy for metastatic disease (NCT000679341);8 those with locally advanced or metastatic HER2+ breast cancer when given T-DM1 and pertuzumab (NCT00875979);9 and those with advanced metastatic HER2+ breast cancer when T-DM1 was compared to capecitabine given with lapatinib (NCT00829166).10
Given the success of HER2 inhibitors in the clinic, it is clear that breast cancer patients benefit from the use of these agents. However, many fail therapy due to de novo resistance as well as the development of acquired resistance. Acquired resistance can arise from a number of causes, including altered signaling from other HER receptors and receptor tyrosine kinases, enhanced PI3K/Akt signaling, overexpression of proteins that mask epitope binding sites on HER2 such as MUC4, and HER2 mutations and truncations.11 It has also been recently shown that intrinsic mechanisms of resistance to HER2 inhibitors, particularly trastuzumab, are due to different isoforms of HER2 caused by alternative splicing.12,13 Alternative splicing may become aberrant in a cancer environment to provide a growth and/or proliferative gain in comparison to nonmalignant cells. Specific isoforms contributing to drug resistance have already been identified for estrogen receptor (ER), HER2, CD44, and other tumor suppressors.12
In HER2+ breast cancer, the oncogenic isoform Δ16HER2, which lacks exon 20 encoding for 16 amino acids, results in a stable, constitutively active form of HER2 that shows enhanced signaling, transforming capacity, and metastatic potential that reduces trastuzumab binding when tested in vitro, promoting resistance.12–14 This isoform also results in increased stable HER2 homodimers, further displaying its oncogenic potential. An additional HER2 isoform that has also been linked to trastuzumab resistance is a cytosolic, truncated form of HER2 (p95HER2) that lacks the extracellular domain and is therefore unrecognizable by trastuzumab.11 However, it is interesting to note that some of these truncations may simply require a different course of treatment, which actually results in better responses. For example, Δ16HER2 isoforms may not respond to trastuzumab, but respond well to Src inhibitors, since the expression of this isoform results in enhanced Src signaling.11 Resistance from these various mechanisms is purported to contribute to primary treatment failure, which necessitates the need for alternative therapeutics for recurrence. Although the use of trastuzumab has significantly increased disease-free survival rates from 67% (chemotherapy alone) to 85% and overall survival from 87% to 91% in HER2+ breast cancer, recurrence remains to be an issue, with as many as 70% of patients acquiring resistance to trastuzumab and progressing to metastatic disease within a year of treatment.15–17 Because treatment failure is a primary consideration for breast cancer patients, identifying the diverse mechanisms that drive resistance and developing a first-line preventative treatment scheme that combines multiple pharmaceutical agents beyond HER2 receptor targeting will be required for significant progress in alleviating treatment failure and the move toward curative solutions.
Combination Therapy During First-line Treatment to Prevent HER2-inhibitor Resistance in Breast Cancer
Combination therapy (ie, a drug cocktail) is commonly used to treat diseases such as HIV, and a major benefit is a reduction in resistance to treatment. Currently, combination therapy is being applied to cancer as a means to avoid or overcome treatment failure by minimizing the potential for patients to develop resistance to cancer treatments,18,19 which is a common event. However, application of practical, long-term curative solutions for treatment resistance in breast cancer is still in an early stage. Encouragingly, cancer patient populations, including breast cancer, have begun to benefit from this strategy regardless of the subtype. An example is the recent FDA approval of the mechanistic target of rapamycin (mTOR) inhibitor everolimus in combination with the aromatase inhibitor exemestane in the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer who have recurrent disease or have failed treatment with other aromatase inhibitors—letrozole or anastrozole (NCT01231659).20
Early studies during the development of trastuzumab showed that the antibody combined with chemotherapy showed significant increases in overall survival and disease-free progression.15,16,21 However, recent studies have shown that trastuzumab combined with other nonchemotherapeutic agents may also show increased therapeutic potential. For example, one study showed that the hormone therapeutic agent anastrozole combined with trastuzumab showed a non-statistically significant increase in overall survival and a statistically significant longer progression-free survival from 2.4 months (anastrozole alone) to 4.8 months in postmenopausal HER2/hormone receptor co-positive metastatic breast cancer patients.16 Since some HER2+ cases show disease progression after treatment with chemotherapy and/or trastuzumab, combination therapy of lapatinib with chemotherapy, as well as lapatinib with capecitabine, has also been utilized in efforts to prevent HER2-inhibitor resistance, with improved progression-free survival when combined with trastuzumab.21 For trastuzumab-refractory HER2+ metastatic breast cancer, combination therapeutics of pilaralisib (a pan-class I PI3K inhibitor) with trastuzumab and paclitaxel are currently being studied for efficacy and safety.16
These recent and current studies of combination therapy are promising in their ability to significantly extend progression-free survival and overall survival, such as the case with the addition of pertuzumab to trastuzumab and docetaxel for previously untreated HER2+ metastatic breast cancer.16 As such, we are at the forefront of implementing polytherapy in cancer. However, due to the heterogeneity of breast cancers and the variety of breast cancer subtypes, the identification of all possible useable combinations is far from complete or optimal. Being able to identify useable methods and strategies to target each mechanism of resistance for each breast cancer subtype, or a method which is able to effectively target mechanisms of resistance universally throughout all breast cancer subtypes, will be critical to find effective and potentially more personalized treatments on an individualized patient basis.
The current clinical strategy is to pair targeted inhibitors with traditional treatment methods including radiotherapy, chemotherapy, hormone therapy, and HER2 targeted therapy. While many studies have identified compensatory signaling molecules and pathways that become activated to promote cell survival in response to treatment, such as PI3K/Akt pathways, a significant question is how to determine, on a per patient basis, which molecules to inhibit and add to a combination regimen. Many obstacles hamper the progress of individualized or personalized treatments, including the absence of reliable biomarkers and testing strategies, tumor heterogeneity, and the impact of cancer stem cells within tumor cell populations. An additional impediment to the development of personalized treatments is the length of time and/or lack of clinical trials stratified for specific patient populations with specific molecular alterations. Moreover, the issues that impede progress in treatment are interdependent. For example, stratified clinical trials cannot be performed without adequate biomarkers and testing strategies for each biomarker. Consequently, personalized treatments for breast cancer will require significant coordination of basic science and clinical efforts.
Targeting Autophagy is an Emerging Strategy in Breast Cancer Treatment
An emerging therapeutic strategy is to harness the catabolic process of autophagy in cancer treatment.22,23 Autophagy is a stress-induced cellular process, which is typically stimulated by nutrient deprivation in efforts to conserve cellular energy and maintain homeostasis.24–28 Upon stress stimuli, AMP cellular cargo that may be targeted for degradation, such as misfolded proteins and whole organelles, becomes sequestered by a double-membraned vesicle, referred to as the autophagosome.29 The genesis and circularization of the autophagosome is stimulated by upstream signaling pathways that sense stress, such as mTOR inhibition and AMPK activation and involves numerous proteins to complex and facilitate each step of the process.30 Once enclosed, the autophagosomes fuse with lysosomes, which contain hydrolases that degrade the engulfed cargo. This broken down cargo can then be taken up and recycled by the cell as precursor materials for the generation of new proteins, or may be exocytosed from the cell. Because autophagy can help restore homeostasis and clear the cell of misfolded and aggregated proteins and other potentially cytotoxic material, it can be thought of as a metabolic survival mechanism.26,28,31 However, targeting autophagy is proving to be complicated because this process is both death-inducing and survival-promoting, presenting a therapeutic paradox whether to inhibit or promote autophagy in cancer.
In a cancer environment, autophagy can be considered as tumor-inhibiting since activation of many oncoproteins such as mTOR, Akt1, and Bcl-2 results in autophagy inhibition.30,32 Therefore, inhibition of these oncoproteins results in growth suppression and can also lead to autophagy stimulation. Because autophagy is a degradative process, one can imagine that the degradation of protein aggregates and dysfunctional mitochondria is death-inducing in cancer cells.31 In addition, there is evidence that autophagy can lead to cell death independently of apoptosis, called autophagic cell death, which also provides evidence for its ability to act in a tumor inhibitory fashion, though this typically results from excessive levels of autophagy.30,33,34 Conversely, autophagy can also be considered as having a tumor-promoting role as a cell survival mechanism by degrading material, which can result in the generation of metabolic precursors needed for the cancer cells to survive. Likewise, since cancer cells are often faced with numerous stressful stimuli (e.g. hypoxia, ER stress, limited availability of nutrients), autophagy is often upregulated in cancer cells, which are then able to sustain survival despite stressful cellular conditions.33 With an upregulation of autophagy, cells have increased metabolism and stress tolerance, thus allowing their survival.31 Likewise, most of the therapeutic regimens to treat HER2+ breast cancer, including chemotherapy, radiotherapy, and HER2 inhibitors, can each contribute to autophagy stimulation by preventing cells from having access to essential metabolic and growth factors, and possibly preventing apoptosis induced by these different therapeutic agents.32,33 Likely individual cancer subtypes or even individual tumors will need to be evaluated in order to gain clarity.
Most cancer treatments, including those for breast cancer, rely on activation of apoptotic signaling to promote cell death. Interestingly, intracellular apoptosis and autophagy signaling are coupled, and a complicated relationship exists between apoptosis and autophagy, as these two pathways are linked as well as polarized. On one hand, the activation of apoptosis can lead to the activation35 or suppression of autophagy.36 On the other, the inverse may also occur where inactivation of apoptosis also results in either suppression35,37,38 or activation of autophagy.39,40 One potential link between these two signaling processes is the sharing of intermediary proteins that regulate both autophagy and apoptosis—the so-called crosstalk between these two processes—including proteins such as Beclin-1 and Bcl-2. A more detailed discussion of this “crosstalk” is offered later.
Various stimuli as well as activation of upstream signaling proteins can determine whether the fate of these molecules will shift in favor of activating either autophagy or apoptosis. Complicating matters, activating autophagy can result in cell death or cell survival, as can suppressing autophagy, and cell death can occur by multiple mechanisms including apoptosis and necrosis.41 Moreover, different cell types between human cancers, as well as different cell subtypes within the same cancer, respond differentially to the signals that regulate the activity of these two pathways.24 Taken together, the result is a large number of possible outcomes for how a cell uses these pathways to die or survive, as well as how a cell responds to the signaling pathways that govern apoptosis and autophagy.25
Despite autophagy’s paradoxical roles, significant progress has been made in deciphering its role in specific cancers and in breast cancer, autophagy has been shown to be both tumor-promoting26,27,42–47 and tumor-inhibitory28,48,49 depending on the cellular context. This paradox is evident in HER2+ breast cancers. Monoallelic deletion of the autophagy gene BECN-1, which encodes the Beclin-1 protein, is frequently found in HER2+ breast cancers.32,33 Since Beclin-1 is important for the initial phases of autophagy, BECN-1 deletion would imply a decrease in basal autophagy despite cancer progression, suggesting that autophagy exhibits a tumor-suppressive role in the early stages of cancer progression. However, those tumors with loss of one allele of BECN-1 are more sensitive to HER2-targeted therapy, implying that autophagy inhibition in conjunction with HER2 inhibition is effective at promoting tumor regression and that autophagy stimulation could alter the success of HER2 treatments.50
This analysis suggests a dual role for autophagy where during early stages of tumor development, autophagy inhibits tumorigenesis.51 Conversely, once a tumor has developed, breast cancer cells likely utilize autophagy for survival.51 Thus, inhibiting autophagy is predicted to make treatment more effective, suggesting that in the latter context autophagy is tumor-promoting. Likewise, several reports indicate that activation of autophagy contributes to trastuzumab and lapatinib resistance in breast cancer.26,43,52,53 However, at least one study in colon cancer suggests the opposite, that inhibiting autophagy promotes lapatinib resistance.54 The authors of this study note that lapatinib co-treatment with the Bcl-2 inhibitor obatoclax induced cytotoxicity via toxic forms of autophagy, since Bcl-2 inhibition activates Beclin-1 to induce autophagy, and the Beclin-1-induced autophagy may have contributed to cell death independent of inducing apoptosis. While this may be due to model variation (eg, use of BT474, SK-BR3, and JIMT-1 breast vs HCT-116 colon cancer cell lines), additional studies are needed to clarify these findings and to determine whether autophagy is universally tumor-inhibitory in colon cancer as opposed to breast cancer.
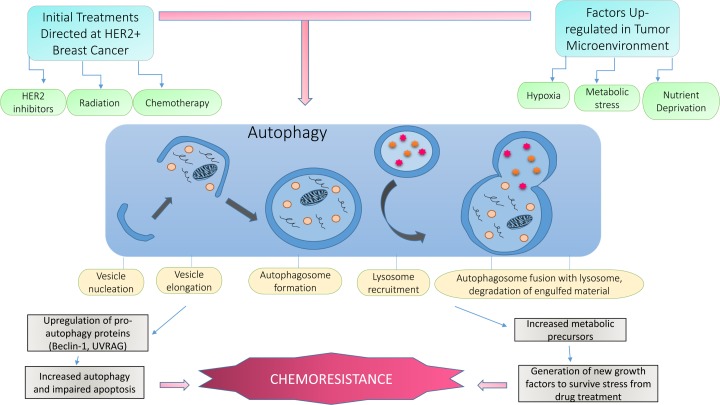
Despite the paradoxical role of autophagy in human cancer, in breast cancer it is generally well accepted that once a tumor has developed, tumor cells will undergo autophagy in response to HER2 inhibitor treatment, including chemotherapy and radiotherapy. In general terms, upregulated autophagy is thought to allow cancer cells to survive the stress induced from the therapy, thereby promoting drug resistance.33 In this context, autophagy acts as a survival mechanism against HER2 inhibition by trastuzumab and lapatinib.55 Figure 1 depicts a selection of the many possible stimulants of autophagy in the tumor microenvironment and how current HER2 therapeutics result in the regulation of autophagy.
Figure 1.
The upregulation of autophagy in HER2-positive breast cancer cells. This diagram depicts the normal process of autophagy and how typical inducers of autophagy are heightened within the tumor microenvironment. Although normal cells respond to cellular stress by undergoing autophagy or apoptosis, in cancer cells these stressors are constantly present, resulting in increased autophagy dependence for survival. Hypoxia, nutrient and growth factor deprivation, limited space due to increased growth demands, and metabolic stress are a few of these internal autophagy stimulators. In addition, most, if not all, cancer therapeutics including chemotherapy, radiation, and targeted HER2 therapies induce autophagy in breast cancer cells because they act as chemical stressors. These continuous stress signals cause breast cancer cells to upregulate this process for survival. Since these cells are autophagy-“addicted” for survival, they are better able to adapt to the targeted therapies they are exposed to, resulting in resistance to HER2 inhibitors.
Recent preclinical studies and early clinical trials indicate that treatment of breast cancer cells or patients with the autophagy inhibitor chloroquine or its derivative hydroxychloroquine may boost tumor cell death response when given with HER2-targeted inhibitors.33,43,52,56–58 Moreover, autophagy is also implicated in endocrine resistance of breast cancer, suggesting that understanding how to harness this pathway for treatment will be beneficial across breast cancer subtypes.44 Further deconvolution of how to target specific molecular aspects of autophagy signaling and how to combine autophagy-based therapies with additional targeted treatments into a cancer drug combination has the potential to lead to therapies that reduce or eliminate resistance in breast cancer. It is becoming increasingly apparent that autophagy’s involvement in cancer drug resistance is context-dependent, including the cancer type, subtype, stage, and history of treatment. Consequently, it is critical to dissect the mechanisms by which these pathways are regulated to ensure that the expected outcome is achieved during breast cancer treatment for a specific cell subtype or patient profile.
Relationship Between Signaling Pathways that Regulate HER2-inhibitor Resistance and Autophagy
To date, most of the research on HER2 resistance has focused on signaling molecules that compensate for the loss of HER2 activation in breast cancer cells that have been challenged with trastuzumab or lapatinib. Many of these compensatory pathways are known for their ability to drive cell survival. Reactivation of Akt signaling, either through loss of PTEN (phosphatase and tensin homolog) or activation of PI3K, is implicated in both intrinsic and acquired resistance to HER2 inhibitors.59–63 Moreover, compensatory activation of Akt has been shown to predict the response to HER2 inhibition, with high levels of Akt activity correlating with poorer response.59 Alterations in PTEN (PTEN) and PI3K (PIK3CA) are frequent in breast cancer, with mutations in the PTEN–PI3K–Akt axis occurring in ~30% of breast cancers and ~40% of breast cancers exhibiting non-mutational silencing of PTEN.64 Many studies indicate that mTOR activation and subsequent regulation of p70S6 kinase via Akt signaling contributes to resistance, and a number of promising clinical trials have been initiated based on this premise.65 More recent analysis suggests that combined PI3K–Akt–mTOR inhibition is highly promising.66 However, we must carefully consider how targeting of these major pathways impact autophagy because Akt and mTOR negatively regulate autophagy.67–69 Consequently, targeting of these pathways would promote autophagy, which could lead to the acquisition of resistance.
In addition to being an upstream activating kinase for mTOR, evidence suggests that Akt directly phosphorylates Beclin-1 to modulate its interaction with 14-3-3, sequestering Beclin-1 in an inactive state.69 Additional evidence puts Akt upstream of UVRAG (UV radiation resistance-associated gene) to negatively regulate the expression of UVRAG, which is a tumor-suppressor protein that complexes with Beclin-1 to activate autophagy during autophagosome formation.70,71 Several studies have also shown a direct interaction between mTOR and the autophagy pathway where mTOR phosphorylates the autophagy kinase ULK1.67,68 This mTOR-directed phosphorylation event in ULK1 negatively regulates autophagy by disrupting ULK1 association with AMPK. These studies are in line with Akt and mTOR as oncogenic drivers of breast cancer and are consistent with the tumor-inhibitory functions of autophagy during tumor initiation. Yet, given the high propensity for breast cancers to upregulate Akt as a compensatory mechanism that drives resistance, additional investigation is required to understand whether Akt’s pro-survival/anti-apoptotic functions could be uncoupled from Akt’s role in the negative regulation of autophagy.
Similarly, AMPK, which has clear implications in cancer, positively regulates autophagy. Like Akt and mTOR, AMPK regulates autophagy through the direct phosphorylation of ULK1, allowing ULK1 to complex with Beclin-1 and other proteins to facilitate autophagosome formation.67,68 Additionally, AMPK is a negative regulator of mTOR activity, providing an indirect secondary pathway toward activation of autophagy.72,73 As activation of AMPK signaling has been proposed as an avenue for cancer therapeutics to inhibit mTOR oncogenic signaling,74 these findings support a tumor-inhibitory role for autophagy, since the proposed AMPK activation from treatment would result in ULK1 activation and autophagy initiation.
More recently, it has been determined that downstream effectors of Akt and AMPK also feed into autophagy signaling. The cell cycle protein p27 is downstream of Akt and AMPK.75–84 Reduced p27 correlates with ErbB2/Neu overexpression, resistance, and poor clinical outcome.85–87 Moreover, increased p27 expression has been shown to increase sensitivity to trastuzumab in resistant HER2+ breast cancer cells.88 Akt directly phosphorylates p27 on multiple Threonine (T) residues, including T198, which have been shown to promote nuclear export and sequestration of p27 in the cytoplasm, resulting in the inactivation of the protein in human cells. Phosphorylation of p27 on T198 is additionally directed by AMPK, which promotes p27-directed autophagy.89 As follows, modulation of p27 levels and activity could be a potential area for breast cancer treatment strategy, and further delineating the mechanism of p27 regulation is warranted.
Autophagy Proteins and Crosstalk with Apoptotic Proteins in HER2-inhibitor-resistant Breast Cancer
Avoidance of apoptosis plays a clear role in overcoming cell death associated with HER2 inhibitor treatment. Studies show that the anti-apoptosis Bcl-2 family members and the pro-apoptotic BH3 family members are implicated in resistance, as Bcl-2 is upregulated and Bax is downregulated in trastuzumab-resistant HER2+ breast cancer cells.90 Likewise, Bcl-xL is regulated by Src,91 which is implicated in trastuzumab resistance via a PTEN-dependent mechanism.92
Bcl-2 is an anti-apoptotic protein that is often upregulated in various cancers, including breast cancer. This protein can act as an indirect negative regulator of autophagy, as Bcl-2 binds and sequesters Beclin-1, thus preventing it from inducing autophagy.93 In situations where Bcl-2 is overexpressed, it is possible that lower levels of apoptosis and autophagy would co-occur; however, this is again cell-type- and context-dependent. Conversely, the Bcl-2 family member Bax is pro-apoptotic, yet also regulates autophagy suppression through caspase-mediated cleavage of Beclin-1, which prevents Beclin-1 from initiating autophagy.37 This suggests that apoptosis activation results in autophagy suppression, though, again, this is context-dependent. Complicating matters further, the Bcl-2 family, which is classically recognized as apoptosis regulators, has also been found to regulate a noncanonical form of autophagy that results in cell death independent of apoptosis.40 So not only can suppression of apoptosis result in suppression of autophagy, but it may also result in autophagy initiation that leads to cell death.
Other proteins implicated in autophagy and apoptotic crosstalk include ATG5 and ATG12, which aid in autophagosome maturation as well as caspase activation in apoptosis: UVRAG, which is involved in different stages of autophagy as well as inhibiting apoptosis via preventing Bax mitochondrial translocation; and the well-studied tumor suppressor p53, which promotes apoptosis and also regulates expression of the damage-regulated autophagy mediator (DRAM) gene that encodes lysosomal proteins involved in autophagy progression.94
Several studies highlight autophagy as a mechanism of resistance by using cell culture model systems where HER2+ breast cancer cells have been made resistant to lapatinib or trastuzumab through continual growth in media containing these drugs.26,43,52 These studies suggest that inhibiting autophagy could be used to resensitize breast cancer cells to HER2 inhibition. Additional studies using these experimental resistance models as well as patient-derived trastuzumab-resistant cell lines indicate that the autophagy protein ATG12 is upregulated57 and that downregulation of ATG12 overcomes resistance.52 Likewise, a second autophagy protein, ATG5, facilitates lapatinib resistance.27
Mechanistically, ATG12 conjugates with ATG5 to act as an E3-like enzyme system that lipidates LC3 proteins, thereby regulating LC3 association with autophagy-generated vesicle membranes. Consequently, loss of ATG12 function could translate to a defect in autophagosome formation and impaired autophagy, which would be beneficial for preventing resistance. Because ATG12 works in concert with ATG5, it is highly likely that the balance between ATG12 and ATG5 levels will differentially impact resistance, as these proteins have individual functions that couple to apoptotic signaling when disassociated from one another.95–97
Evidence links the autophagy-related proteins ATG12 and ATG5 to apoptosis through interaction with the Bcl-2 family of proteins,54,95–97 indicating crosstalk between these two pathways. Specifically, ATG12 has been shown to be required for caspase activation as well as for inhibiting members of the Bcl-2 family. One of the most well studied points of convergence between autophagy and apoptosis is the Beclin-1/Bcl-2 interaction. Further evidence demonstrates crosstalk between Beclin-1 and Bcl-2 and Bcl-xL, where Beclin-1 is impaired through binding to either Bcl-2 or Bcl-xL protein, preventing autophagy initiation.35,93 Moreover, pro-apoptotic Bax has been shown to inactivate Beclin-1 through its cleavage.37
There are several molecular pathways and proteins of convergence that have dual implications in both autophagy and apoptosis, including the mTOR/Akt signaling pathway, death-associated protein kinase (DAPK), p53, ATG5/12, the Beclin-1/Bcl-2 interaction, and UVRAG.94–97 The mTOR pathway, which negatively regulates autophagy as previously discussed, concurrently has implications in apoptosis through Akt activation that can suppress pro-apoptotic genes by negatively regulating p53, FOXO, and JNK1, all of which have pro-apoptotic functions.94 Akt can also phosphorylate Bad, facilitating dissociation from Bcl-2 and preventing Bax/Bak-mediated apoptosis.
DAPK is a tumor suppressor that is frequently hypermethylated and silenced in many cancers and regulates apoptosis by positively regulating JNK activation upstream, while it also phosphorylates Beclin-1 to stimulate autophagy.51,98 Interestingly, DAPK has also been shown to induce cell death during ER stress that is both apoptotic and autophagic in nature, acting as a strong point of crosstalk between the two pathways.94,98 Another tumor suppressor, p53, has well-established regulation of apoptosis through its transcriptional control of the Bcl-2 family. However, recent studies show p53 has dual implications in autophagy. In its nuclear form, p53 regulates gene expression of damage- regulated autophagy modulator (DRAM), which is involved in autophagy initiation. On the other hand, cytoplasmic p53 can suppress autophagy, since loss of p53 can stimulate autophagy upregulation. Because of its dual roles depending on its cellular localization, this serves as one example of apoptosis activation simultaneously resulting in autophagy activation (via nuclear p53) or in autophagy inhibition through cytoplasmic p53.94,99
Finally, UVRAG is a fairly recent protein studied for its paired involvement in autophagosome maturation and apoptosis. Though UVRAG is a tumor suppressor through its ability to upregulate autophagy, thus resulting in autophagic cell death, it can also inhibit apoptosis by binding Bax, preventing Bax’s mitochondrial translocation for apoptosis initiation.100 To summarize, Table 1 illustrates several of the identified proteins implicated in both autophagy and apoptosis, which have altered expression in breast cancer. Taken together, it is clear that significant crosstalk between apoptotic and autophagy molecules is present in breast cancer cells, but how this crosstalk translates mechanistically in resistant model systems remains to be clarified.
Table 1.
Common proteins implicated in both autophagy and apoptosis in HER2 positive breast cancer. This table shows multiple points of crosstalk between autophagy and apoptosis through several distinct molecules, as well as their typical expression levels in breast cancer. Furthermore, because these processes are not mutually exclusive, some proteins may support or inhibit both processes, while others may promote or inhibit one or the other. Overall, this shows that the loss of one individual protein’s expression is not sufficient to result in an imbalance of these processes; yet when several of these proteins become imbalanced, it typically results in dysregulation of both autophagy and apoptosis in HER2-positive breast cancer.
| PROTEIN | PROTEIN EXPRESSION LEVEL IN HER2+ BREAST CANCER | ROLE IN APOPTOSIS | ROLE IN AUTOPHAGY |
|---|---|---|---|
| ATG 5 | High (50, 93) | May enhance apoptosis when silenced (93–95) | Pro-autophagy: Increases autophagosome formation (93–95) |
| ATG 12 | High (50, 93) | May enhance apoptosis when silenced (93–95) | Pro-autophagy: Increases autophagosome formation (93–95) |
| Bax (BH3 family) | Low (49) | Pro-apoptosis: Low expression results in decreased apoptosis (35) | Anti-autophagy: Blocks Beclin-1 from initiating autophagy in the nucleation phase (35) |
| Bcl-2 | High (31) | Anti-apoptosis: Blocks mitochondrial depolarization and cytochrome c release (91) | Anti-autophagy: Sequesters and inactivates Beclin-1, preventing autophagy initiation (91) |
| Beclin-1 | Low (31) | Tumor suppressor: Monoallelic deletion in breast cancer (31) | Pro-autophagy: Involved in nucleation phase of autophagy (48) |
| DAPK | Unknown in HER2+, low mRNA expression in ER+ breast cancer (49) | Tumor suppressor: Upregulates p53 tumor suppressor; stimulates JNK-mediated apoptosis (92) | Pro-autophagy: Phosphorylates Beclin-1 to initiate autophagosome formation (92) |
| UVRAG | Unknown | Tumor suppressor: Can inhibit apoptosis through Bax interaction (98) | Pro-autophagy: Enhances autophagosome formation and maturation (92) |
Clinical Targeting of Apoptosis and Autophagy
Currently, there are several ongoing clinical trials for breast cancer, investigating the therapeutic potential of different autophagy inhibitors and agents that target apoptotic pathways, used independently and in conjunction with chemotherapeutic agents. Table 2 shows the current clinical trials testing autophagy inhibition to improve breast cancer treatment. It is important to note that autophagy inhibition as a therapeutic is not limited to HER2+ breast cancer, as indicated by the various clinical breast cancer subtypes in Table 2. The most regularly used autophagy inhibitors in humans are chloroquine and its derivative hydroxychloroquine, which are more commonly known as antimalarial drugs. The mechanism of action of these agents is to suppress lysosomal acidification, thereby preventing autophagosome fusion with the lysosome and subsequent degradation. Recent clinical trials for these autophagy inhibitors are discussed in this section. Furthermore, while many apoptotic molecules are implicated in breast cancer, here we focus the discussion primarily on the anti-apoptotic protein Bcl-2 because inhibitors that target this protein have already been tested in clinical trials to some extent.
Table 2.
Recent and current clinical trials looking at direct or indirect targeting of autophagy. The clinical trials depicted will determine whether the selected inhibitors will improve clinical outcomes, which include increased sensitization of cancer cells to surgical intervention, chemotherapy, and other approved therapeutics.
| CLINICAL TRIAL | PHASE | BREAST CANCER TYPE | INHIBITOR INVOLVED | DETAILS OF TRIAL | STATUS |
|---|---|---|---|---|---|
| NCT01292408 | II | Anti-inflammatory invasive breast adenocarcinoma | Hydrochloroquine | Autophagy inhibitor between tumor biopsy and tumor excision | Recruitment status unknown |
| NCT01446016 | II | Metastatic | Chloroquine plus taxane like chemotherapy | For patients that have failed anthracycline-based chemotherapy | Recruitment phase |
| NCT01023477 | I/II | Ductal carcinoma in situ | Chloroquine | Inhibitor to decrease autophagy given before tumor excision | Recruitment phase |
| NCT02333890 | II | Invasive breast cancer | Chloroquine | Inhibitor to decrease autophagy given before tumor excision and before chemotherapy treatment | Recruitment phase |
| NCT00063934 | I/II | Metastatic and locally invasive breast cancer | Oblimersen plus chemotherapy | Bcl-2 inhibitor to increase apoptosis | Terminated |
| NCT02070094 | I/II | HER2+ | AT-737 plus T-DM1 | Bcl-2 inhibitor to allow greater sensitivity to T-DM1 treatment | Withdrawn |
To investigate the use of hydroxychloroquine in breast cancer, an ongoing clinical trial aims to look at hypoxia and autophagy markers on patient biopsies treated with hydroxychloroquine before and after surgery (NCT01292408).56 Once completed, this will be one of the first studies involving chloroquine analogs in autophagy inhibition in breast cancer, and may be informative for current as well as future clinical trials looking at the role or autophagy in other types of cancer. One primary outcome measure for this study is to evaluate differences in endogenous hypoxia markers (eg, CA9, PAI-1, VEGF).101 Since the tumor microenvironment is often hypoxic, this induces stress on the tumor cells to upregulate autophagy, which in turn makes these tumor cells more resistant to chemotherapy and radiation, and thus minimizing the effectiveness of those treatments (illustrated in Fig. 1). The results of this study are predicted to show that hydroxychloroquine effectively reduces autophagy in tumor cells and make them more likely to undergo cell death in the hypoxic environment since the main survival mechanism of the tumor cells is eliminated.
Recently a Phase I/II clinical trial was attempted using a combination of hydroxychloroquine and Ixabepilone, a chemotherapeutic agent that stabilizes microtubules, in patients with metastatic breast cancer. The goal of this study was to show a decrease in tumor growth and higher tumor response compared to chemotherapy alone (NCT00765765).56 Major aims of this trial included investigating the role of autophagy inhibition on metastatic breast cancer to determine the overall survival and duration of response to treatment. However, due to slow patient accrual, the study could not be seen to completion. One reason for the lack of accrual is that the exclusion criteria were too stringent to identify enough metastatic patients to complete the study.
Two studies testing chloroquine have also been initiated and are currently in the recruitment phase. The first is a Phase II study investigating the use of chloroquine in combination with taxane or taxane-like chemotherapy in metastatic breast cancer patients who have previously failed anthracycline-based chemotherapy (NCT01446016).56 The second is a Phase I/II study to test whether administration of chloroquine will reduce the ability of ductal carcinoma in situ (DCIS) to survive and become invasive. Participants will receive either a standard dose of chloroquine (Phase I) or a low dose of chloroquine (Phase II) for one month prior to surgical removal of the lesion (NCT01023477).56,102 A third trial that recently started recruiting is a Phase II randomized, double-blind study to evaluate chloroquine usage on breast tumor cell proliferation and apoptosis as primary outcomes (NCT02333890).56 The purpose of this study is to determine whether chloroquine administration will prevent breast cancer growth in patients currently not being treated with neoadjuvant chemotherapy prior to surgical intervention. Secondary outcomes of this study include evaluation of circulating chloroquine metabolites as well as autophagic markers in tumor tissue and its surrounding stroma, which will be measured by immunohistochemistry and gene expression analysis.
Bcl-2 is an anti-apoptotic protein that can bind to pro-apoptotic proteins and is also involved in the formation of several autophagic complexes.41 Since Bcl-2 has been shown to be overexpressed in several tumor types, including breast cancer, the use of Bcl-2 inhibitors is expected to show a decrease in tumor growth by relieving the inhibition of pro-apoptotic proteins and consequently promoting apoptosis.55 Although a promising idea, clinical trials with Bcl-2 inhibitors have met with difficulties. A clinical trial investigating the Bcl-2 inhibitor Oblimersen in combination with chemotherapy on metastatic and locally invasive breast cancer was recently terminated due to the entire cohort of treated patients experiencing serious adverse events that necessitated a permanent halt on medication (NCT00063934).56 These patients were receiving docetaxel and doxorubicin intravenously, as well as the oligonucleotide-based Bcl-2 inhibitor Oblimersen. Although Phase I of the trial determined the highest effective dosage on patients, it is possible that the combination therapy resulted in accelerated drug toxicity, which caused various adverse events on the patients, including neutropenia, anemia, and thrombocytopenia. Future studies may find that other Bcl-2 inhibitors may work better in combination with chemotherapy. A 2014 clinical trial that was withdrawn before enrollment could occur due to lack of funding would have been the first to look at the effectiveness of a combination therapy of T-DM1 and the Bcl-2 inhibitor, ABT-737, on HER2+ breast cancer patients (NCT02070094).56 If it were to be completed, this study had the potential to show that Bcl-2 inhibition may have allowed HER2+ tumor cells to be more sensitive to T-DM1 treatment, resulting in an increased response in patients.
Despite the lack of current clinical trials testing autophagy inhibition as a viable therapeutic in HER2+ breast cancer specifically, it is expected that positive results from the trials focusing on other breast cancer subtypes will translate into future trials utilizing autophagy inhibition for the treatment of HER2+ breast cancer in conjunction with HER2 inhibitors. Although the majority of the listed clinical trials are not yet focused on specific subtypes for testing autophagy inhibition, it is promising that increased patient response in these trials should translate well into HER2+ breast cancer predicted response, since current data establishes autophagy as a tumor-promoting mechanism in this specific subtype.
Conclusions and Future Perspectives
Based on the current assessment of basic research and clinical trial evidence, it is clear that targeting autophagy and apoptosis could be a viable method for therapeutic intervention for HER2+ breast cancer. Appropriate delineation of the mechanisms that regulate autophagy and its ability to interact with apoptotic signaling proteins is required to appropriately design combinatorial methods toward these processes to prevent and overcome resistance to HER2-targeted therapy. With future studies focused on elucidating the crosstalk between autophagy and apoptosis, better inhibitors of autophagy and anti-apoptotic molecules can be designed to not only eliminate a vital survival mechanism of cancer cells but also promote tumor cell death. With continued success in providing evidence to support the effectiveness of autophagy inhibition in breast cancer patients, it is expected that future clinical trials will be performed using current inhibitors as well as novel, more selective, and improved autophagy-modulating agents.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1161 words, excluding any confidential comments to the academic editor.
FUNDING: Research in the Yeh Lab is supported by R01-CA187305–01A1 from the NCI and a grant from the Concern Foundation. JZ is supported in part by GM072643 from the NIGMS. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Wrote and edited the paper: JZ, EY. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Carey LA. Breast cancer: HER2—a good addiction. Nat Rev Clin Oncol. 2012;9(4):196–197. doi: 10.1038/nrclinonc.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger MS, Locher GW, Saurer S, et al. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988;48(5):1238–1243. [PubMed] [Google Scholar]
- 3.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Macrinici V, Romond E. Clinical updates on EGFR/HER targeted agents in early-stage breast cancer. Clin Breast Cancer. 2010;10(suppl 1):E38–E46. doi: 10.3816/CBC.2010.s.006. [DOI] [PubMed] [Google Scholar]
- 6.2011. Available at: http://www.cancer.gov/cancertopics/druginfo/fda-lapatinib.
- 7.LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17(20):6437–6447. doi: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- 8.Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Dieras V, Harbeck N, et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol. 2014;32(14):1437–1444. doi: 10.1200/JCO.2013.52.6590. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett JT, Arteaga CL. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biol Ther. 2011;11(9):793–800. doi: 10.4161/cbt.11.9.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K, Fry EA. Aberrant splicing of estrogen receptor, HER2, and CD44 genes in breast cancer. Genet Epigenet. 2015;7:19–32. doi: 10.4137/GEG.S35500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castagnoli L, Iezzi M, Ghedini GC, et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014;74(21):6248–6259. doi: 10.1158/0008-5472.CAN-14-0983. [DOI] [PubMed] [Google Scholar]
- 14.Huynh FC, Jones FE. MicroRNA-7 inhibits multiple oncogenic pathways to suppress HER2Delta16 mediated breast tumorigenesis and reverse trastuzumab resistance. PLoS One. 2014;9(12):e114419. doi: 10.1371/journal.pone.0114419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Rugo HS. Human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer: how the latest results are improving therapeutic options. Ther Adv Med Oncol. 2015;7(6):321–339. doi: 10.1177/1758834015599389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bock C, Lengauer T. Managing drug resistance in cancer: lessons from HIV therapy. Nat Rev Cancer. 2012;12(7):494–501. doi: 10.1038/nrc3297. [DOI] [PubMed] [Google Scholar]
- 19.Glickman MS, Sawyers CL. Converting cancer therapies into cures: lessons from infectious diseases. Cell. 2012;148(6):1089–1098. doi: 10.1016/j.cell.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2013. Available at: http://www.cancer.gov/cancertopics/druginfo/fda-everolimus#Anchor-Breast.
- 21.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15(24):7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee SJ, Pandey S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol Ther. 2011;11(2):216–228. doi: 10.4161/cbt.11.2.13798. [DOI] [PubMed] [Google Scholar]
- 23.Saleem A, Dvorzhinski D, Santanam U, et al. Effect of dual inhibition of apoptosis and autophagy in prostate cancer. Prostate. 2012;72(12):1374–1381. doi: 10.1002/pros.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu WK, Coffelt SB, Cho CH, et al. The autophagic paradox in cancer therapy. Oncogene. 2012;31(8):939–953. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- 25.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS One. 2009;4(7):e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon JH, Ahn SG, Lee BH, Jung SH, Oh SH. Role of autophagy in chemoresistance: regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNA-PKcs and PARP-1. Biochem Pharmacol. 2012;83(6):747–757. doi: 10.1016/j.bcp.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 29.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159(6):1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozpolat B, Benbrook DM. Targeting autophagy in cancer management—strategies and developments. Cancer Manag Res. 2015;7:291–299. doi: 10.2147/CMAR.S34859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarzynska JM. The importance of autophagy regulation in breast cancer development and treatment. Biomed Res Int. 2014;2014:710345. doi: 10.1155/2014/710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain K, Paranandi KS, Sridharan S, Basu A. Autophagy in breast cancer and its implications for therapy. Am J Cancer Res. 2013;3(3):251–265. [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma K, Le N, Alotaibi M, Gewirtz DA. Cytotoxic autophagy in cancer therapy. Int J Mol Sci. 2014;15(6):10034–10051. doi: 10.3390/ijms150610034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiuri MC, Le Toumelin G, Criollo A, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in beclin-1. EMBO J. 2007;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo S, Garcia-Arencibia M, Zhao R, et al. Bim inhibits autophagy by recruiting beclin 1 to microtubules. Mol Cell. 2012;47(3):359–370. doi: 10.1016/j.molcel.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo S, Rubinsztein DC. Apoptosis blocks beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17(2):268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Li Q, Lee JY, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11(11):1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6(12):1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 41.Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaachouay H, Ohneseit P, Toulany M, Kehlbach R, Multhoff G, Rodemann HP. Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol. 2011;99(3):287–292. doi: 10.1016/j.radonc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Li X, Feng J, Chang Y, Wang Z, Wen A. Autophagy facilitates the lapatinib resistance of HER2 positive breast cancer cells. Med Hypotheses. 2011;77(2):206–208. doi: 10.1016/j.mehy.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Rev Anticancer Ther. 2011;11(8):1283–1294. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moussay E, Kaoma T, Baginska J, et al. The acquisition of resistance to TNFalpha in breast cancer cells is associated with constitutive activation of autophagy as revealed by a transcriptome analysis using a custom microarray. Autophagy. 2011;7(7):760–770. doi: 10.4161/auto.7.7.15454. [DOI] [PubMed] [Google Scholar]
- 46.Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5(3):400–403. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- 47.Sun WL, Chen J, Wang YP, Zheng H. Autophagy protects breast cancer cells from epirubicin-induced apoptosis and facilitates epirubicin-resistance development. Autophagy. 2011;7(9):1035–1044. doi: 10.4161/auto.7.9.16521. [DOI] [PubMed] [Google Scholar]
- 48.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112(12):1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100(25):15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, et al. Expression status of the autophagy-regulatory gene ATG6/BECN1 in ERBB2-positive breast carcinomas: bypassing ERBB2-induced oncogenic senescence to regulate the efficacy of ERBB2-targeted therapies. Genes Chromosomes Cancer. 2011;50(4):284–290. doi: 10.1002/gcc.20846. [DOI] [PubMed] [Google Scholar]
- 51.Avalos Y, Canales J, Bravo-Sagua R, Criollo A, Lavandero S, Quest AF. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014;2014:603980. doi: 10.1155/2014/603980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, et al. The anti-malarial chloroquine overcomes primary resistance and restores sensitivity to trastuzumab in HER2-positive breast cancer. Sci Rep. 2013;3:2469. doi: 10.1038/srep02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh ES, Abt MA, Hill EG. Regulation of cell survival by HUNK mediates breast cancer resistance to HER2 inhibitors. Breast Cancer Res Treat. 2015;149(1):91–98. doi: 10.1007/s10549-014-3227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8(21):2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maycotte P, Thorburn A. Targeting autophagy in breast cancer. World J Clin Oncol. 2014;5(3):224–240. doi: 10.5306/wjco.v5.i3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Available at: clinicaltrials.gov.
- 57.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, et al. Autophagy-related gene 12 (ATG12) is a novel determinant of primary resistance to HER2-targeted therapies: utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment. Oncotarget. 2012;3(12):1600–1614. doi: 10.18632/oncotarget.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez-Martin A, Oliveras-Ferraros C, del Barco S, Martin-Castillo B, Menendez JA. mTOR inhibitors and the anti-diabetic biguanide metformin: new insights into the molecular management of breast cancer resistance to the HER2 tyrosine kinase inhibitor lapatinib (Tykerb) Clin Trans Oncol. 2009;11(7):455–459. doi: 10.1007/s12094-009-0384-0. [DOI] [PubMed] [Google Scholar]
- 59.Chandarlapaty S, Sakr RA, Giri D, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18(24):6784–6791. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9(6):1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 61.Serra V, Scaltriti M, Prudkin L, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30(22):2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallardo A, Lerma E, Escuin D, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106(8):1367–1373. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 64.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 65.Gayle SS, Arnold SL, O’Regan RM, Nahta R. Pharmacologic inhibition of mTOR improves lapatinib sensitivity in HER2-overexpressing breast cancer cells with primary trastuzumab resistance. Anticancer Agents Med Chem. 2012;12(2):151–162. doi: 10.2174/187152012799015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham J. PI3K/AKT/mTOR pathway inhibitors: the ideal combination partners for breast cancer therapies? Expert Rev Anticancer Ther. 2015;15(1):51–68. doi: 10.1586/14737140.2015.961429. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy. 2011;7(8):924–926. doi: 10.4161/auto.7.8.15860. [DOI] [PubMed] [Google Scholar]
- 69.Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through beclin 1 phosphorylation. Science. 2012;338(6109):956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang W, Ju JH, Lee KM, Nam K, Oh S, Shin I. Protein kinase B/Akt1 inhibits autophagy by down-regulating UVRAG expression. Exp Cell Res. 2013;319(3):122–133. doi: 10.1016/j.yexcr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Liang C, Feng P, Ku B, Oh BH, Jung JU. UVRAG: a new player in autophagy and tumor cell growth. Autophagy. 2007;3(1):69–71. doi: 10.4161/auto.3437. [DOI] [PubMed] [Google Scholar]
- 72.Kimura N, Tokunaga C, Dalal S, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8(1):65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 73.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67(1):1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Cappellini A, Tabellini G, Zweyer M, et al. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia. 2003;17(11):2157–2167. doi: 10.1038/sj.leu.2403111. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Xia D, Luo JD, Wang P. Exogenous p27KIP1 expression induces antitumour effects and inhibits the EGFR/PI3K/Akt signalling pathway in PC3 cells. Asian J Androl. 2009;11(6):669–677. doi: 10.1038/aja.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Jonasch E, Alexander A, et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin Cancer Res. 2009;15(1):81–90. doi: 10.1158/1078-0432.CCR-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le XF, Pruefer F, Bast RC., Jr HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle. 2005;4(1):87–95. doi: 10.4161/cc.4.1.1360. [DOI] [PubMed] [Google Scholar]
- 79.Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8(10):1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 80.Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK. Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 2002;62(22):6764–6769. [PubMed] [Google Scholar]
- 81.Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 82.Viglietto G, Motti ML, Bruni P, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8(10):1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 83.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62(14):4132–4141. [PubMed] [Google Scholar]
- 84.Yang H, Zhao R, Yang HY, Lee MH. Constitutively active FOXO4 inhibits Akt activity, regulates p27 Kip1 stability, and suppresses HER2-mediated tumorigenicity. Oncogene. 2005;24(11):1924–1935. doi: 10.1038/sj.onc.1208352. [DOI] [PubMed] [Google Scholar]
- 85.Newman L, Xia W, Yang HY, et al. Correlation of p27 protein expression with HER-2/neu expression in breast cancer. Mol Carcinog. 2001;30(3):169–175. doi: 10.1002/mc.1025. [DOI] [PubMed] [Google Scholar]
- 86.Spataro VJ, Litman H, Viale G, et al. Decreased immunoreactivity for p27 protein in patients with early-stage breast carcinoma is correlated with HER-2/neu overexpression and with benefit from one course of perioperative chemotherapy in patients with negative lymph node status: results from International Breast Cancer Study Group Trial V. Cancer. 2003;97(7):1591–1600. doi: 10.1002/cncr.11224. [DOI] [PubMed] [Google Scholar]
- 87.Philipp-Staheli J, Payne SR, Kemp CJ. p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res. 2001;264(1):148–168. doi: 10.1006/excr.2000.5143. [DOI] [PubMed] [Google Scholar]
- 88.Kute T, Lack CM, Willingham M, et al. Development of herceptin resistance in breast cancer cells. Cytometry A. 2004;57(2):86–93. doi: 10.1002/cyto.a.10095. [DOI] [PubMed] [Google Scholar]
- 89.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9(2):218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 90.Crawford A, Nahta R. Targeting Bcl-2 in herceptin-resistant breast cancer cell lines. Curr Pharmacogenomics Person Med. 2011;9(3):184–190. doi: 10.2174/187569211796957584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karni R, Jove R, Levitzki A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene. 1999;18(33):4654–4662. doi: 10.1038/sj.onc.1202835. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17(4):461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Oral O, Akkoc Y, Bayraktar O, Gozuacik D. Physiological and pathological significance of the molecular cross-talk between autophagy and apoptosis. Histol Histopathol. 2015;18:11714. doi: 10.14670/HH-11-714. [DOI] [PubMed] [Google Scholar]
- 95.Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280(21):20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 96.Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44(5):698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 97.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 98.Gozuacik D, Bialik S, Raveh T, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15(12):1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 99.Tasdemir E, Chiara Maiuri M, Morselli E, et al. A dual role of p53 in the control of autophagy. Autophagy. 2008;4(6):810–814. doi: 10.4161/auto.6486. [DOI] [PubMed] [Google Scholar]
- 100.Yin X, Cao L, Kang R, et al. UV irradiation resistance-associated gene suppresses apoptosis by interfering with BAX activation. EMBO Rep. 2011;12(7):727–734. doi: 10.1038/embor.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rademakers SE, Span PN, Kaanders JH, Sweep FC, van der Kogel AJ, Bussink J. Molecular aspects of tumour hypoxia. Mol Oncol. 2008;2(1):41–53. doi: 10.1016/j.molonc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9(12):2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]