Abstract
Objectives
The objectives of this study were to determine the prevalence of carriage of ESBL-producing Enterobacteriaceae (ESBL-E) in a representative sample of the general adult Dutch community, to identify risk factors and to gain understanding of the epidemiology of these resistant strains.
Methods
Adults enrolled in five general practices in Amsterdam were approached by postal mail and asked to fill in a questionnaire and to collect a faecal sample. Samples were analysed for the presence of ESBL-E. ESBL genes were characterized by PCR and sequencing. Strains were typed using MLST and amplified fragment length polymorphism (AFLP) and plasmids were identified by PCR-based replicon typing. Risk factors for carriage were investigated by multivariate analysis.
Results
ESBL-E were found in 145/1695 (8.6%) samples; 91% were Escherichia coli. Most ESBL genes were of the CTX-M group (blaCTX-M-1 and blaCTX-M-15). MLST ST131 was predominant and mainly associated with CTX-M-15-producing E. coli. One isolate with reduced susceptibility to ertapenem produced OXA-48. In multivariate analyses, use of antimicrobial agents, use of antacids and travel to Africa, Asia and Northern America were associated with carriage of ESBL-E, in particular strains with blaCTX-M-14/15.
Conclusions
This study showed a high prevalence of ESBL-E carriage in the general Dutch community. Also, outside hospitals, the use of antibiotics was a risk factor; interestingly, use of antacids increased the risk of carriage. A major risk factor in the general population was travel to countries outside Europe, in particular to Asia, Africa and Northern America.
Introduction
Resistance to β-lactam antibiotics due to ESBLs has become a common problem worldwide.1 The prevalence of this resistance mechanism has increased rapidly, even in countries known for prudent antibiotic use.2
In a previous study we showed that over 10% of Dutch community patients with gastrointestinal complaints carry ESBL-producing Enterobacteriaceae (ESBL-E) in their gastrointestinal tract.3 This is remarkable, because the Netherlands is a country with low antibiotic use in humans and has among the lowest resistance rates in clinical isolates in Europe.2 This triggered us to perform the present study, which focused on the prevalence and molecular epidemiology of carriage of ESBL-E in the general population and on risk factors for carriage.
Methods
Study design and data collection
For this cross-sectional study we approached all adult persons (individuals aged ≥18 years), excepting those who were terminally ill, present in the databases of five general practices (∼10 000 persons), affiliated to the Academic General Practice Network (AGPN), VU University Medical Center, Amsterdam. In the Netherlands, citizens are registered with a general practitioner, regardless of health status. The database therefore is a representative sample of the general population. Individuals were approached by postal mail with a questionnaire, an informed consent form and a container for a faecal sample or perineal swab (according to their preference). Samples were returned in transport medium (Copan Italia, Brescia, Italy) between June 2011 and November 2011.
The questionnaire asked about sampling date, sample type (perineal swab or faecal sample), age, gender, profession, country of birth of the participant and his/her parents, years living in the Netherlands, admission to a (foreign) hospital, healthcare institution or long-term care facility and travel to foreign countries, all in the previous 12 months. Data on antimicrobial, antacid and corticosteroid use and comorbid conditions in the past 12 months were extracted from the database of the AGPN. Please see the Supplementary data (available at JAC Online) for items included in the questionnaire and data extracted from the AGPN database. Fifty ESBL-positive participants were asked for participation of their household members, with the same questionnaires and request for samples.
The medical ethics committee (METc ID NL29769.029.09) of the VU University Medical Center approved the study (NTR Trial ID NTR2453).
ESBL detection
Samples were inoculated in selective enrichment broth (Trypticase soy broth with ampicillin). After overnight incubation (37°C) an aliquot was inoculated on EbSA-ESBL screening agar (Cepheid Benelux, Apeldoorn, The Netherlands) and on blood agar.4,5 Growth on the blood agar plate indicated the sample was suitable for analysis. Three colonies of each distinct morphotype on the EbSA-ESBL agar were characterized. ESBL production was confirmed by combination disc diffusion test with both cefotaxime and ceftazidime, with and without clavulanic acid (Rosco, Taastrup, Denmark), interpreted according to the Dutch national guideline.6 Species identification and antibiotic susceptibility testing were performed with Vitek 2 (bioMérieux, Marcy-l'Étoile, France). MIC breakpoints were according to EUCAST.7 Reduced susceptibility (MIC ≥0.25 mg/L) to ertapenem (Etest, bioMérieux) indicated the possible presence of a carbapenemase.
ESBL- and carbapenemase-encoding genes were characterized by PCR and sequencing (BaseClear, Leiden, The Netherlands).8–11 Sequences were analysed with Bionumerics software (version 6.6; Applied Maths, Sint-Martens-Latem, Belgium) and compared with sequences in the NCBI (http://www.ncbi.nlm.nih.gov/BLAST) and Lahey database (http://www.lahey.org/studies/).
Molecular typing
E. coli strains were typed by MLST (http://mlst.warwick.ac.uk/mlst/mlst/dbs/Ecoli). Clonal complexes were assigned using eBURST v3 (http://eburst.mlst.net/).
A subset of E. coli strains was typed by amplified fragment length polymorphism (AFLP).12
Plasmids were identified by PCR-based replicon typing, as described by Carattoli and adapted by Boot et al.13
Analysis of risk factors and statistical methods
For a case–control analysis of risk factors, cases were carriers of ESBL-E and controls were persons free of ESBL-E. Statistical analyses were performed with Statistical Package for the Social Sciences, version 20.0 (SPSS, Chicago, IL, USA). Possible risk factors were analysed by univariate and multivariate logistic regression. ORs and 95% CIs were calculated.
Results
Participants
Of 7000 persons approached, 1695 (24.2%) returned the questionnaire with a completed consent form and a specimen. Participants lived in the region of Amsterdam. Age and gender characteristics are given in Table 1.
Table 1.
Participant characteristics and main risk factors for ESBL-E carriage in univariate analysis
| Risk factor | Cases | Controls | OR | 95% CI |
|---|---|---|---|---|
| Age, median (range); N=129 and 1393 | 48 (20–90) | 50 (18–95) | NA | NA |
| Female, n (%); N=129 and 1393 | 75 (58.1) | 852 (61.2) | 0.9 | 0.6–1.3 |
| Use of antibiotics, n (%); N=129 and 1393 | 33 (25.6) | 195 (14.0) | 2.1 | 1.4–3.2 |
| PPIs or H2 blockers, n (%); N=129 and 1393 | 28 (21.7) | 166 (11.9) | 2.0 | 1.3–3.2 |
| Travel to, n (%)a | ||||
| Africa; N=111 and 1245 | 19 (17.1) | 88 (7.1) | 2.7 | 1.6–4.7 |
| Latin America/Caribbean; N=109 and 1264 | 12 (11.0) | 112 (8.9) | 1.3 | 0.7–2.4 |
| Northern America; N=106 and 1255 | 23 (21.7) | 133 (10.6) | 2.3 | 1.4–3.8 |
| Asia; N=118 and 1310 | 39 (33.1) | 247 (18.9) | 2.1 | 1.4–3.2 |
| Australia/New Zealand; N=102 and 1222 | 0 (0) | 18 (1.5) | NA | NA |
NA, not applicable; PPIs, proton-pump inhibitors.
aNumber of patients who travelled to WHO region/total number of patients with exclusion of those patients that also travelled to one of the other WHO regions or did not travel outside the Netherlands.
Prevalence of carriage of ESBL-E and ESBL gene characterization
ESBL-E were detected in 145 of 1695 samples (8.6%, 95% CI 7.3%–10.0%): 132 (91.0%) Escherichia coli, 11 (7.6%) Klebsiella pneumoniae, 1 Enterobacter cloacae (0.7%) and 1 Serratia plymuthica (0.7%). The presence of genes encoding ESBL was confirmed in all phenotypically ESBL-producing strains (Table 2); these genes comprised mainly blaCTX-M-15 and blaCTX-M-1. Co-resistance to other antibiotics was common: 33% of strains were multiresistant as defined by Magiorakos et al.14 (Table S1, available as Supplementary data at JAC Online). No strains with reduced susceptibility to imipenem or meropenem were found; one E. coli strain had reduced susceptibility to ertapenem (MIC 0.75 mg/L); this strain carried blaOXA-48 and blaCTX-M-14. No difference was found in detection rate for faecal samples compared with perineal swabs (OR 1.0, 95% CI 0.7–1.4).
Table 2.
ESBL-encoding genes
| ESBL family | ESBL gene/type | n |
|---|---|---|
| CTX-M-1 | blaCTX-M-15 | 59 |
| blaCTX-M-15 + blaTEM-52 | 1 | |
| blaCTX-M-1 | 25 | |
| blaCTX-M-1 + blaSHV-12 | 1 | |
| blaCTX-M-3 | 4 | |
| CTX-M-2 | blaCTX-M-2 | 2 |
| CTX-M-9 | CTX-M-9 group | 1 |
| blaCTX-M-14a | 19 | |
| blaCTX-M-9 | 4 | |
| blaCTX-M-27 | 5 | |
| CTX-Mb | blaCTX-M | 2 |
| TEM and SHV | blaSHV-12 | 5 |
| blaTEM-52 | 6 | |
| blaTEM-52 + blaSHV-12 | 1 | |
| Other | blaCTX-M-21 | 1 |
| blaCTX-M-22 | 3 | |
| blaCTX-M-32 | 3 | |
| blaCTX-M-55 | 3 | |
| Total | 145 |
aOne isolate also encoded OXA-48.
bExact subtype of two CTX-M genes remained unresolved by sequencing.
The prevalence of carriage of ESBL-E in participants not using antibiotics (N = 1294) was 7.4% (95% CI 6.0%–8.9%).
MLST
MLST showed 47 different STs, 6 of which represented new types. Types ST131 (21 isolates; 15.9%), ST10 (18 isolates; 13.6%) and ST38 (9 isolates; 6.8%) were most frequent. ST10 was the main clonal complex, including 26 isolates (19.7%) (Figure S1). MLST ST131 was mainly associated with CTX-M-15-producing E. coli.
Household members
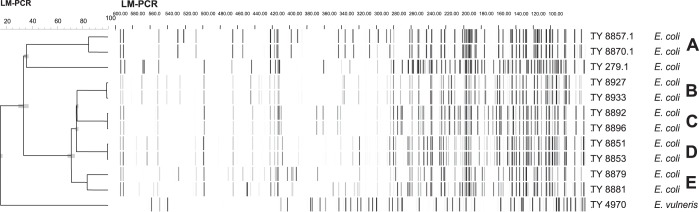
Fifty carriers volunteered 41 household members, of which five (12.2%, 95% CI 4.9%–26.0%) were carriers of ESBL-E. Figure 1 and Table 3 present the distribution of ESBL genes and plasmids within households. Three of five clusters of isolates from single households had identical AFLP patterns and shared the same ESBL genes and plasmids. In cluster A, two E. coli strains shared an ESBL gene and an Inc1 plasmid, but each contained an additional plasmid, resulting in one band difference in the AFLP pattern. E. coli strains in cluster E also belonged to the same CTX-M-1 family, however had different ESBL genes, did not share plasmids, and the AFLP pattern was different.
Figure 1.
AFLP household members. TY numbers are used for numbering laboratory strains; Escherichia vulneris (DSM 4564) and TY 279.1 (ATCC 35218) are reference strains used for AFLP. LM-PCR, ligation-mediated PCR.
Table 3.
Household members: strains and plasmids
| Cluster | Straina | Gene(s) | Plasmidb |
|||||
|---|---|---|---|---|---|---|---|---|
| Inc I1 | FrepB | ColE | FIA | Y | B/O | |||
| A | TY 8857.1 | blaCTX-M-1 | + | − | − | − | + | − |
| TY 8870.1 | blaCTX-M-1 | + | + | − | − | − | − | |
| B | TY 8927 | blaCTX-M-14/18 | − | + | + | − | − | − |
| TY 8933 | blaCTX-M-14/18 | − | + | + | − | − | − | |
| C | TY 8892 | blaCTX-M-15 | − | + | − | + | + | − |
| TY 8896 | blaCTX-M-15 | − | + | − | + | + | − | |
| D | TY 8851 | blaCTX-M-15 | − | + | + | + | − | − |
| TY 8853 | blaCTX-M-15 | − | + | + | + | − | − | |
| E | TY 8879 | blaCTX-M-3/TEM-1 | + | + | − | + | + | + |
| TY 8881 | blaCTX-M-1/TEM-1 | − | − | − | − | − | − | |
aLaboratory strain numbers.
bPlasmids R, ColEtp, FIIs, FIB, P, A/C, U, HI1, L/M, HI2, W, T, N, X, F/C and K were not detected.
Risk factors
For the case–control analysis, we included 1522 (129 ESBL-E carriers and 1393 non-carriers) of the 1695 participants who sent in a sample with the questionnaire and for whom data from the electronic database of the AGPN were available. Table 1 shows the main risk factors, with their univariate ORs and 95% CIs. Table S2 shows the full list of potential risk factors with univariate ORs. Countries were classified according to the format of the United Nations Department of Economic and Social Affairs into regions and major areas.15 Europe was chosen as the reference category.
Table 4 shows the multivariate analysis of the main potential risk factors. Travel to the different continents, antimicrobial use and antacid use (use of proton-pump inhibitors or H2 blockers) were identified as relevant factors and were therefore included in the multivariate analysis. The full list of factors with multivariate ORs and 95% CIs can be found in Table S3.
Table 4.
Main risk factors included in multivariate analysis
| Risk factor | Multivariate OR | 95% CI |
|---|---|---|
| Age (continuous variable) | 1.0 | 1.0–1.0 |
| Female | 0.9 | 0.6–1.5 |
| Use of antibiotics | 2.2 | 1.4–3.7 |
| PPIs or H2 blockers | 1.9 | 1.1–3.3 |
| Travel to | ||
| Africaa | 2.2 | 1.1–4.6 |
| Latin America/Caribbeana | 0.7 | 0.3–1.9 |
| Northern Americaa | 2.7 | 1.6–4.8 |
| Asiaa | 2.1 | 1.3–3.6 |
| Australia/New Zealanda | NA | NA |
NA, not applicable; PPIs, proton-pump inhibitors.
aCountries grouped according to WHO major area codes, reference = Europe (inclusive of persons who only travelled in the Netherlands or did not travel).
Travel
In the multivariate analysis, travel to Northern America, Africa and Asia remained associated with an increased risk of acquisition of ESBL-E relative to travel in Europe (Table 4). Detailed analysis by region, sub-region and country is given in Table S3. These analyses showed a statistically robust increase in the risk for Northern Africa (OR 2.9, 95% CI 1.1–7.7) and Eastern Africa (OR 5.5, 95% CI 1.1–27.4) and that the risk of travelling to Northern America was increased more than 3-fold and limited to the USA (OR 3.1, 95% CI 1.7–5.7). The risk associated with travel to Asia was highest for South-Central Asia (OR 5.5, 95% CI 2.2–14.2), for India in particular (OR 4.7, 95% CI 1.4–16.0).
Antimicrobial and antacid use
Both in univariate (Table 1) and multivariate (Table 4) analysis the use of antibiotics or antacids increased the risk of carriage of ESBL-E ∼2-fold.
Other factors
We explored other potential risk factors (Table S2) by adding them separately, i.e. one at a time, into the multivariate analysis shown in Table 4. Factors that stood out in the multivariate analysis were all travel related: working as airline cabin crew, admission to a foreign hospital, being born in Africa or having a father or mother born in Africa or Asia. We also performed an analysis for the risk associated with these travel-related factors, restricted to those participants who did travel outside the Netherlands. These were 1270 persons: 112 cases and 1158 controls. This restricted analysis suggested that working for an airline (multivariate OR 4.3, 95% CI 0.5–34.3) may pose an extra risk, since the OR in the restricted analysis did not change substantially. The OR associated with admission to a foreign hospital was halved in the restricted analysis, with a wide CI (multivariate OR 3.0, 95% CI 0.3–27.1). The OR associated with being born outside the Netherlands or having a father or a mother born outside the Netherlands was slightly different in the restricted analysis; the highest risk was having a mother born in Asia (multivariate OR 2.4, 95% CI 1.0–5.8), indicating that this risk was independent of the possible association with travel.
Association of travel with specific ESBL genes
The association of travel with carriage of strains with specific ESBL genes is shown in Table 5. blaCTX-M-1 is typically found in poultry in the Netherlands, while blaCTX-M-14 and blaCTX-M-15 are found in humans worldwide. blaCTX-M-15 and blaCTX-M-14 were associated with travel (Africa, Asia and Northern America); blaCTX-M-1 was not. The carbapenemase OXA-48 was found in an E. coli strain from a participant who visited Egypt and the USA; he was born in the Netherlands and the country of origin of both parents was Southern Asia. He had no other risk factors.
Table 5.
Association of genes with travel to different regions (univariate)
| ESBL gene(s) | No ESBL | OR | 95% CI | |
|---|---|---|---|---|
| ESBL blaCTX-M-1 (N = 26) | ||||
| Europe (reference) | 20 | 901 | ||
| Africa | 0 | 88 | NA | |
| Latin America/Caribbean | 1 | 112 | 0.5 | 0.1–3.7 |
| Northern America | 1 | 133 | 0.4 | 0.1–3.2 |
| Asia | 1 | 247 | 0.2 | 0.0–1.5 |
| Australia/New Zealand | 0 | 19 | NA | |
| ESBL blaCTX-M-14 and -15 (N = 79)a | ||||
| Europe (reference) | 19 | 901 | ||
| Africa | 18 | 88 | 5.5 | 3.0–9.9 |
| Latin America/Caribbean | 8 | 112 | 1.7 | 0.8–3.6 |
| Northern America | 15 | 133 | 3.1 | 1.7–5.7 |
| Asia | 25 | 247 | 3.0 | 1.8–5.1 |
| Australia/New Zealand | 0 | 18 | NA |
NA, not applicable.
aCTX-M-14 and -15 are grouped together because of their similar epidemiological distribution.
Discussion
We showed a faecal carriage rate of ESBL-E of >8% in the general adult population in Amsterdam. This confirms and extends our previous finding of a 10% carriage rate in patients who visit their general practitioner with gastrointestinal complaints.3 Main risk factors were antibiotic use, use of gastric acid-suppressing medication and travel to Africa, Asia or the USA. Additional risk factors were having a mother born in Asia and possibly working as cabin crew for an airline. While the findings that antibiotic use and travel to Asia and Africa increase the risk of carriage of ESBL-E are not unexpected, the association with antacid use and the >3-fold increased risk associated with travel to the USA have, to the best of our knowledge, not been clearly shown before.16 An interesting finding was that carriage of Enterobacteriaceae producing CTX-M-14 or CTX-M-15 was associated with travel to Africa, Asia or Northern America/the USA, while carriage of strains producing CTX-M-1 was not.
A strength of our study is that it aimed at the carriage rate in the general adult population, because we did not select patients upon a visit to their general practitioner or on admission to hospital, but used the general practitioner's databases to draw a sample from the general population. This was possible because in the Netherlands health insurance is obligatory and inhabitants are registered with a general practitioner. A second advantage of our approach is that we did not select for persons attending a travel clinic, which introduces strong bias towards countries that require vaccination or malaria prophylaxis. The weakness of our study was the participation rate of ∼25%. Participants had been informed that we were screening for resistant strains and of a possible relation with antibiotic use. This could have introduced self-selection bias for those participants that were concerned, because of previous antibiotic use, and could have affected the prevalence rate, rendering it higher. Therefore, we also determined the prevalence of carriage of resistant strains in participants who had not used antibiotics. This was also high, nearly 7.5%, and confirmed the high prevalence of ESBL-E in the Dutch population. Participants were unaware of other interests, such as types of ESBL, travel, ethnicity or acid-suppressing medication. Finally, our study was restricted to Amsterdam, a large, cosmopolitan city with inhabitants from many different origins and possibly a high propensity for international travel. Such selection does not invalidate the analysis of risk factors, because this selection is likely to be the same in all participants; numerically, it may have the effect of making the ORs closer to unity.
Several reports describe increasing rates of faecal carriage of ESBL-E in the community (reviewed by Woerther et al.16). The review by Woerther et al.16 shows that in Europe percentages of carriage of ESBL increased between 2002 and 2011, with the highest figures in Spain, where carriage rates of >7% were already noted in 2007.16 Overall, rates are quite similar to those we measured, albeit that our carriage rate of 8.6% is the highest determined so far in Europe. Possibly, this is due to our sensitive detection method, with an enrichment step.17 In other regions of the world, especially in South-East Asia and China, ESBL-E carriage rates can be as high as nearly 70%.16 The majority of ESBL-positive isolates in our study were E. coli and the predominant CTX-M allele was blaCTX-M-15, although a substantial proportion, almost one-fifth, of strains produced CTX-M-1. The predominance of CTX-M-15 is in concordance with the epidemiology in the community worldwide and comparable to what we found in our study in patients with gastrointestinal complaints.3,16 In the present study, carriage of CTX-M-15- and 14-producing ESBL-E was associated with visiting a foreign country, while carriage of CTX-M-1-producing strains was not. Possibly, Enterobacteriaceae producing ESBLs of this allele are acquired in the Netherlands, since CTX-M-1 is the main ESBL type found in E. coli on chicken meat.18,19 A large proportion of the ESBL-producing E. coli appeared to be related to ST131. This more virulent clone could lead to more adverse outcomes in case of infection.20
Risk factors for faecal carriage of ESBL-E in Europe have been investigated especially in healthcare settings. Because of our focus on the community we will limit our discussion to studies in community settings. Only a few European studies are available.16,21,22 With the exception of a study in Germany and one in the Netherlands, no risk factors for carriage were identified, possibly due to the limited size of the studies. The German study showed an association of ESBL-E carriage with travel to Greece and Africa and with ownership of a pet, while antibiotic use was not a risk factor.21 The Dutch study found ownership of a horse to be the only risk factor.22 In the present study, previous antimicrobial use increased the risk of ESBL-E carriage ∼2-fold. This finding is interesting because it is biologically plausible, but has not been shown in other European community studies. Possibly, the low level of antibiotic use, compared with other countries, makes this risk factor stand out in our country. Noteworthy is the relationship between use of acid-suppressing medication and ESBL-E carriage. Two clinical studies showed an association between antacid use and colonization with ESBL-E, one conducted among hospitalized patients in Israel and the other in the USA.23,24 The authors of these studies noticed the role of acid suppression, but did not discuss it. Our study indicates that antacids play a role as risk factors for acquisition of ESBL-E in the community too. Gastric acid suppression by bicarbonate has been shown to lower the infective dose of Vibrio cholerae in seminal studies conducted in the 1960s on inmates in correctional facilities that received oral doses of V. cholerae.25 An association has been shown between gastric pH and non-typhoidal salmonellosis.26,27 The risk of antacid use points to ingestion as a main route of acquisition of ESBL-E. Possibly, the use of antacids or antibiotics while abroad may pose an additive risk for acquisition of ESBL. Our study, however, did not have the statistical power to test this hypothesis.
Several studies have shown that travellers to foreign countries can be colonized with ESBL-E upon return.16 Travel, especially to Asia, but also to Africa, may be the most important risk factor also in the general population. An intriguing finding is the risk associated with travel to the USA, which was not identified before. Studies on travel so far, however, have used travel clinics as sources of participants. This means that participants mainly travelled to countries for which vaccinations or malaria prophylaxis is needed and not to countries in Northern America. Such studies, therefore, cannot detect risk associated with travel to the USA. It would be interesting to investigate the prevalence of ESBL-E in the community in the USA. Like in the Netherlands, ESBL-E might be present in the food chain. Different studies reported that >90% of chicken meat in our country is contaminated with ESBLs and we showed that raw vegetables may be contaminated as well.19,28–30 Also in the USA the use of antimicrobials is high in the food industry.31 Our finding that carriage of strains producing CTX-M-1 was not associated with travel, and therefore was probably acquired in the Netherlands, further points to the possibility of contaminated food as a source of ESBL. It would be interesting to investigate whether the decrease in antibiotic use in animals that was noted recently will reflect in a decrease in CTX-M-1 carriage in humans in the future.32 Like Leistner et al.,33 we found that having an Asian mother is a risk factor for ESBL-E. Admission to a foreign hospital did not stand out as a risk for ESBL-E carriage after adjustment for travel. Hence, in the general population travel seems to be the major risk factor, irrespective of hospitalization.
Several studies describe person-to-person transmission of resistant strains. In three households in our study, the ESBL-carrying E. coli strains were genetically identical and carried the same plasmids and ESBL genes, pointing to person-to-person transmission. The presence of different strains and plasmids in two households suggests that acquisition of ESBL-E within households is not due only to strain transmission.
In summary, this study shows that ESBL-E carriage is prevalent in the Dutch community, a worrying finding in a country with low resistance rates in healthcare facilities. Risk factors included use of antimicrobial agents, use of antacids and visits to foreign countries, in particular Asia, Africa and, surprisingly, the USA. That the use of antacids posed a risk points to ingestion as a mode of acquisition of ESBL-E. Our findings, combined with previous studies that show an abundant presence of ESBL-E in the food chain, warrant more attention to the potential risk to public health of resistant microorganisms in food and water.
Funding
This research was funded by ZonMw, project number 125020011.
The funding source had no involvement in: study design; collection, analysis and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Transparency declarations
None to declare.
Author contributions
N. a. N. and C. M. J. E. V.-G. conceived the study. A. M. K., M. H., J. A. J. W. K., P. H. M. S. and P. J. M. E. critically reviewed the study design and participated in the study. C. M. J. E. V.-G., N. a. N. and E. A. R. did the literature review. E. A. R. collected the data. C. M. J. E. V.-G., A. M. K. and E. A. R. analysed the data. E. A. R. wrote the first draft of the article. C. M. J. E. V.-G., N. a. N., M. H., J. A. J. W. K., P. H. M. S. and P. J. M. E. interpreted data, contributed to report writing and critically reviewed the article. All authors read and approved the final draft of the article.
Supplementary data
Acknowledgements
Part of the results of this study were presented at the Fifty-second Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 2012 (poster presentation P C2-104).
This research could only be performed thanks to the collaboration of the general practices affiliated to the Academic General Practice Network (AGPN), VU University Medical Center. We are grateful for the help and expertise of Alex Koek and Martine Rijnsburger, and the assistance of Eman Abdelrehim, Czikjain Heijblom, Marte van Keulen and Henrieke Snetselaar with the laboratory work (VU University Medical Center). We thank Kim van der Zwaluw and Martijn van Luit for the MLST experiments and analysis (Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands).
References
- 1.Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8: 159–66. [DOI] [PubMed] [Google Scholar]
- 2.European Antimicrobial Resistance Surveillance Network (EARS-Net). http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/database.aspx.
- 3.Reuland EA, Overdevest IT, Al Naiemi N et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect 2013; 19: 542–9. [DOI] [PubMed] [Google Scholar]
- 4.Naiemi NA, Murk JL, Savelkoul PH et al. Extended-spectrum β-lactamases screening agar with AmpC inhibition. Eur J Clin Microbiol Infect Dis 2009; 28: 989–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overdevest IT, Willemsen I, Elberts S et al. Laboratory detection of extended-spectrum-β-lactamase-producing Enterobacteriaceae: evaluation of two screening agar plates and two confirmation techniques. J Clin Microbiol 2011; 49: 519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen Stuart J, Leverstein van Hall M, al Naiemi N. NVMM Guideline: Laboratory Detection of Highly Resistant Microorganisms (HRMO), Version 2.0. Chapter 5. 2012. http://www.nvmm.nl/richtlijnen/hrmo-laboratory-detection-highly-resistant-microorganisms.
- 7.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. http://www.eucast.org/clinical_breakpoints/.
- 8.Mulvey MR, Bryce E, Boyd DA et al. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob Agents Chemother 2005; 49: 358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naiemi NA, Duim B, Savelkoul PH et al. Widespread transfer of resistance genes between bacterial species in an intensive care unit: implications for hospital epidemiology. J Clin Microbiol 2005; 43: 4862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirel L, Walsh TR, Cuvillier V et al. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 2011; 70: 119–23. [DOI] [PubMed] [Google Scholar]
- 11.Voets GM, Fluit AC, Scharringa J et al. A set of multiplex PCRs for genotypic detection of extended-spectrum β-lactamases, carbapenemases, plasmid-mediated AmpC β-lactamases and OXA β-lactamases. Int J Antimicrob Agents 2011; 37: 356–9. [DOI] [PubMed] [Google Scholar]
- 12.Savelkoul PH, Aarts HJ, de Haas J et al. Amplified-fragment length polymorphism analysis: the state of an art. J Clin Microbiol 1999; 37: 3083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boot M, Raadsen S, Savelkoul PH et al. Rapid plasmid replicon typing by real time PCR melting curve analysis. BMC Microbiol 2013; 13: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]
- 15.WHO. United Nations Department of Economic and Social Affairs. http://esa.un.org/unpd/wpp/General/Files/Definition_of_Regions.pdf.
- 16.Woerther PL, Burdet C, Chachaty E et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26: 744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murk JL, Heddema ER, Hess DL et al. Enrichment broth improved detection of extended-spectrum-β-lactamase-producing bacteria in throat and rectal surveillance cultures of samples from patients in intensive care units. J Clin Microbiol 2009; 47: 1885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluytmans JA, Overdevest IT, Willemsen I et al. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 2013; 56: 478–87. [DOI] [PubMed] [Google Scholar]
- 19.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 2011; 17: 873–80. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JR, Johnston B, Clabots C et al. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States . Clin Infect Dis 2010; 51: 286–94. [DOI] [PubMed] [Google Scholar]
- 21.Valenza G, Nickel S, Pfeifer Y et al. Extended-spectrum-β-lactamase-producing Escherichia coli as intestinal colonizers in the German community. Antimicrob Agents Chemother 2014; 58: 1228–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huijbers PM, de Kraker M, Graat EA et al. Prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in humans living in municipalities with high and low broiler density. Clin Microbiol Infect 2013; 19: E256–9. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Ami R, Schwaber MJ, Navon-Venezia S et al. Influx of extended-spectrum β-lactamase-producing Enterobacteriaceae into the hospital. Clin Infect Dis 2006; 42: 925–34. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa K, Gattu S, Marchaim D et al. Epidemiology and risk factors for isolation of Escherichia coli producing CTX-M-type extended-spectrum β-lactamase in a large U.S. Medical Center. Antimicrob Agents Chemother 2013; 57: 4010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornick RB, Music SI, Wenzel R et al. The Broad Street pump revisited: response of volunteers to ingested cholera vibrios. Bull N Y Acad Med 1971; 47: 1181–91. [PMC free article] [PubMed] [Google Scholar]
- 26.Giannella RA, Broitman SA, Zamcheck N. Salmonella enteritis. I. Role of reduced gastric secretion in pathogenesis. Am J Dig Dis 1971; 16: 1000–6. [DOI] [PubMed] [Google Scholar]
- 27.Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 1972; 13: 251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overdevest I, Willemsen I, Rijnsburger M et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis 2011; 17: 1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen Stuart J, van den Munckhof T, Voets G et al. Comparison of ESBL contamination in organic and conventional retail chicken meat. Int J Food Microbiol 2012; 154: 212–4. [DOI] [PubMed] [Google Scholar]
- 30.Reuland EA, Al Naiemi N, Raadsen SA et al. Prevalence of ESBL-producing Enterobacteriaceae in raw vegetables. Eur J Clin Microbiol Infect Dis 2014; 33: 1843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Boeckel TP, Brower C, Gilbert M et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA 2015; 112: 5649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MARAN 2015: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2014. http://www.wageningenur.nl/upload_mm/2/2/2/0ab4b3f5-1cf0-42e7-a460-d67136870ae5_NethmapMaran2015.pdf.
- 33.Leistner R, Meyer E, Gastmeier P et al. Risk factors associated with the community-acquired colonization of extended-spectrum β-lactamase (ESBL) positive Escherichia coli. an exploratory case–control study. PLoS One 2013; 8: e74323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.