Abstract
Intrauterine growth retardation (IUGR) induces metabolic syndrome, which is often characterized by insulin resistance (IR), in adults. Previous research has shown that microRNAs (miRNAs or miRs) play a role in the target genes involved in this process, but the mechanisms remain unclear. In the present study, we examined miRNA profiles using samples of skeletal muscles from both IUGR and control rat offspring whose mothers were fed either a protein-restricted diet or a diet which involved normal amounts of protein during pregnancy, respectively. miR-29a was found to be upregulated in the skeletal muscles of IUGR offspring. The luciferase reporter assay confirmed the direct interaction between miR-29a and peroxisome proliferator-activated receptor δ (PPARδ). Overexpression of miR-29a in the skeletal muscle cell line C2C12 suppressed the expression of its target gene PPARδ, which, in turn, influenced the expression of its coactivator, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α). Thus, PPARδ/PGC-1α-dependent signals together reduced insulin-dependent glucose uptake and adenosine triphosphate (ATP) production. Overexpression of miR-29a also caused a decrease in levels of glucose transporter 4 (GLUT4), the most important glucose transporter in skeletal muscle, which partially induced a decrease insulin-dependent glucose uptake. These findings provide evidence for a novel micro-RNA-mediated mechanism of PPARδ regulation, and we also noted the IR-promoting actions of miR-29a in skeletal muscles of IUGR.
Keywords: intrauterine growth retardation, miR-29a, C2C12 cells, peroxisome proliferator activated receptor-δ, insulin resistance
Introduction
Epidemiological and clinical studies in different countries and involving different ethnic groups have demonstrated that intrauterine growth restriction (IUGR) is associated with an increased risk of developing metabolic syndrome (MS), type 2 diabetes mellitus (T2DM), obesity, hypertension and dyslipidemia in adult life (1–4). Insulin resistance (IR) is a common pathological and physiological mechanism of MS. Skeletal muscle is one of the important peripheral tissues to be affected by insulin. In a model of IUGR mediated by various causes (e.g. calorie restriction, protein restriction, hypoxic condition in rodents), IUGR has been shown to alter insulin signaling in rodent offspring, leading to the development of IR in the skeletal muscles (5–7). However, the mechanisms that regulate IR in skeletal muscles remain unclear.
Recently, it has been demonstrated that microRNAs (miRNAs or miRs) affect skeletal muscles and play important roles in many aspects of muscle biological processes, such as muscle growth, development and regeneration (8,9). miRNAs are known to act as powerful post-transcriptional regulators, which act either through the inhibition of protein translation or via mRNA degradation, by partially binding to the 3′UTR of their target mRNAs (10). Considering that miRNAs are stringently regulated in skeletal muscles in order to maintain normal biological processes, it is conceivable that alterations of miRNA expression levels induce functional disorders and, therefore, influence the development of disease. Indeed, certain studies have revealed that various miRNAs are involved in the pathology of several muscle diseases and in metabolism: for example, myogenesis (11), exercise, atrophy, aging and dystrophy (8).
Concerning IUGR, several studies on miRNA expression have been undertaken. These studies mostly compared miRNA expression profiles or several specific miRNAs in a variety of tissues, such as glomeruli (12), skeletal (13), placental (14), and lung tissues from different IUGR models, where IUGR was caused by protein restriction (15), caloric restriction (14) or seminutrient restriction (13). However, specific miRNA signatures which are associated with the IR of skeletal muscles from subjects with IUGR are less common.
In the present study, we investigated the possible involvement of miRNAs in IUGR by using a miRNA microarray analysis of skeletal muscles from the offspring of control and IUGR rats. One of the key altered miRNAs was miR-29a, which suppressed the expression of its target gene, peroxisome proliferator-activated receptor δ (PPARδ), and influenced the expressions of other related genes, such as peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and glucose transporter 4 (GLUT4), as well as decreasing insulin-stimulated glucose uptake and adenosine triphosphate (ATP) production in the skeletal muscle cell line C2C12. These findings provide information on a novel micro-RNA-mediated mechanism of PPARδ regulation and suggest that miR-29a affects insulin resistance in the skeletal muscles of rats with IUGR.
Materials and methods
Animal procedures, collection of skeletal muscle and isolation of total RNA
Animal procedures were carried out as described previously (16). Briefly, 20 virgin, 7- to 8-week-old Sprague- Dawley (SD) rats weighing 180±20 g were purchased from the Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, China). All animals were housed at 21–23°C, 65–69% humidity with a 12-h light/dark cycle and had free access to food and tap water. Following 10 days of habituation, female rats were mated overnight with a male and copulation was verified the next morning by the presence of spermatozoa in vaginal smears. After conception, pregnant dams were housed individually and fed isocaloric diets containing either normal (20%) levels of protein (control) or a protein-restricted (PR) diet containing 8% protein until delivery. The composition of the diets has been described previously (17). After delivery, each mother rat fed 8 pups (any extra pups were removed at random) and was fed with normal rat chow. Six pups born from mothers who received the PR diet formed the IUGR group and 6 pups from mothers fed a normal diet formed the control group. Following weaning, 3 or 4 same sex offspring rats from the same group were housed in 1 cage until the end of the experiment, and 18-month-old control (CON) and IUGR offspring rats were sacrificed by decapitation. Six pairs of gastrocnemius muscle samples taken from the right posterior limb from the 2 groups were rapidly removed, frozen in liquid nitrogen and stored at −80°C. All experiments were approved by the Animal Care and Use Committee of Southeast University (Nanjing, China).
Total RNA was isolated from samples from both groups using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The concentration and quality of RNA was measured using a NanoDrop spectrophotometer (Thermo Scientific Inc., Nepean, ON, Canada), and RNA integrity was checked using gel electrophoresis. To minimize the effects of inconsistency and meet the sample demands for microarray profiling, measurements were taken to guarantee that an equal amount of RNA from each sample was taken. Equal aliquots of RNA samples from each group were pooled (n=5), and miRNA isolation was carried out from the pooled total RNA using an miRNeasy mini kit (Qiagen, Copenhagen, Denmark) according to the manufacturer's instructions.
Microarray analysis of miRNA expression
miRNA expression profiling was performed on the two pooled total RNA groups mentioned above. miRNA was labeled using the miRCURY™ Hy3™/Hy5™ power labeling kit (Exiqon, Vedbaek, Denmark) and hybridized on the miRCURY LNA array (v.18.0; Exiqon). All procedures were carried out according to the manufacturer's instructions. This array contains 3,100 capture probes, covering all human, mouse and rat miRNA annotated in miRBase 18.0. Following the washing steps, the slides were subsequently canned with the Agilent Scanner G2505C (Agilent Technologies, Santa Clara, CA, USA).
Scanned images were then imported into GenePix Pro 6.0 software (Axon Instruments, Union City, CA, USA) for grid alignment and data extraction. Replicated miRNAs were averaged, and miRNA with intensities ≥30 in all samples were chosen for calculating the normalization factor. Expressed data were normalized using median normalization. After normalization, differentially expressed miRNA was identified through fold-change filtering. Finally, hierarchical clustering was performed to examine distinguishable miRNAexpression profiling among the samples.
Reverse transcription-quantitative PCR (RT-qPCR) for analysis of miRNA expression
In order to verify the microarray results, the expression levels of abnormal genes were analyzed by RT-qPCR. Briefly, total RNA (3 ng) of skeletal muscle from CON and IUGR rats was reverse transcribed using an iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and the miRNA-specific reverse-transcription primers provided with the SYBR PrimerScript miRNA RT-PCR kit (Takara, Shiga, Japan). For reverse transcription, the iCycler™ Thermal cycler (Applied Biosystems, Foster City, CA, USA) was used under the following conditions: 16°C for 30 min; 42°C for 30 min and 85°C for 5 min and a quick cooling on ice. miRNA-specific (1.33 µl) cDNA from this reaction was amplified with TaqMan Universal PCR master mix and the respective specific probe provided in the SYBR PrimerScript miRNA RT-PCR kit (Takara). All primers were synthesized by Shengneng Bicolor Biotech (Shanghai, China). RT-qPCR was performed in an ABI 7500 RT-PCR system (Applied Biosystems). Amplification was performed at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. As an internal control, U6 primers were used for RNA template normalization. All reactions were performed in triplicate and included no template or reverse transcription controls for each gene. All mRNA levels were normalized to the values of U6, and the results expressed as fold changes of the threshold cycle (Ct) value relative to controls using the 2−ΔΔCt method. miRNA levels in the IUGR group were calculated relative to the CON group, for which values were arbitrarily set to 1 in order to obtain estimates of relative abundance, as has been previously described (16).
Prediction of miR-29a targets in silico
Since it has previously been reported that miR-29a is one of the most upregulated miRNAs in skeletal muscles from IUGR rats and that it plays an important role in diabetes mellitus, which is closely related to diabetes (18), we focused on miR-29a in subsequent experiments. To explore the potential mechanism by which miR-29a exerts its effects in IUGR, we applied two bioinformatics algorithms (from TargetScan and PicTar) to identify its potential target genes. TargetScan (www.targetscan.org) and PicTar (www.pictar.org) are the most commonly software links for predicting microRNA target genes. The intersection of the two results was performed by Gene Ontology (GO) analysis of the molecular function, biological process and using Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathway enrichment analysis.
Luciferase reporter assay
Firstly, the 3′UTR of rat PPARδ containing the putative target sites for miR-29a was synthesized. We also synthesized the control sequences containing several mutated bases within the complementary binding sites. We then cloned the corresponding genes into the pGL3-promoter vector (Promega, Madison, WI, USA). The 293T cells (purchased from the Cell Bank of the Chinese Academy of Sciences Shanghai Institute of Cell Biology) were plated at 1×105 in a 12-well plate. After 24 h, the pGL3 reporters containing wild-type PPARδ binding sites for miR-29a (WT) or the mutated PPARδ binding sites (MUT) were transiently co-transfected with either the pre-miR-29a plasmid or the negative control (pre-miR-NC; both from GenePharma, Shanghai, China) using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Renilla luciferase was used to normalize the cell number and transfection efficiency. Cells were harvested 48 h after transfection. Luciferase activity was measured by the Dual Luciferase assay kit according to the manufacturer's instructions on a Luminometer (both from Promega). Each assay was analyzed 3 times.
Transfecting C2C12 cells with miR-29a
The mouse myoblast cell line C2C12, which was purchased from the Cell Bank of the Chinese Academy of Sciences Shanghai Institute of Cell Biology, was maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Wisent, St. Bruno, QC, Canada) and 10 U/ml penicillin/streptomycin. Lentivirus miR-29a expression vector was synthesized by GenePharma. When the cells reached a confluence of 70–80%, the medium was replaced with DMEM supplemented with 2% horse serum (Gibco, St. Louis, MO, USA) and 10 U/ml penicillin/streptomycin to promote myoblast differentiation into myotubes. Six days later, the differentiated C2C12 cells had fused into myotubes. Subsequently, C2C12 myoblasts transfected with miR-29a-GFP-pGLPZ or GFP-pGLPZ lenti-virus (both from GenePharma) were set as the miR-29a and mock-transfected groups, respectively. C2C12 myoblasts which had not been transfected with lentivirus were considered the normal group. After transfection (48 h), the miR-29a expression levels in each cell line were verified by RT-qPCR and visualized using a fluorescence microscope (Zeiss, Germany).
RT-qPCR analysis of mRNA expression
The total RNA from each sample was extracted using TRIzol reagent (Invitrogen)-validated primers, which were designed for each target mRNA; these were synthesized by Shengneng Bicolor Biotech (Shanghai, China). The PCR primers used were as follows: GLUT4 forward, 5′-ACATACCTGACAGGGCAAGG-3′ and reverse, 5′-CGCCCTTAGTTGGTCAGAAG-3′; PGC-1α forward, 5′-TCTGAAAGGGCCAAACAGAG-3′ and reverse, 5′-GTAAATCACACGGCGCTCTT-3′; PPARδ forward, 5′-GGATTTTAGAGTGGGTGTTTTTTA-3′ and reverse, 5′-CACACCCGATTCCATGTTGAG-3′; β-actin forward, 5′-GAGACCTTCAACACCCCAGCC-3′ and reverse, 5′-GGAGAGCATAGCCCTCGTAG-3′. RT-qPCR analysis was performed on an ABI 7500 RT-PCR system under the following conditions: initial denaturation for 10 min at 95°C, followed by 40 cycles of 15 sec denaturation at 95°C, 30 sec annealing at the optimal primer temperature and 36 sec extension at 72°C. The expressions of GLUT4, PPARδ and PGC-1α and the housekeeping gene β-actin were assessed simultaneously in individual samples. Each sample was assayed in duplicate. Negative controls (no template or selected untranscribed RNA) were also run to ensure the absence of contamination. Analysis was performed using the 2−ΔΔCt method with β-actin as the housekeeping gene.
Western blot analysis
Western blot analysis was performed to determine protein expression of GLUT4, PPARδ and PGC-1α. Briefly, cells were washed with ice-cold PBS and lysed with RIPA Lysis Buffer (Beyotime, Shanghai, China) for 20 min on ice. Plasma membrane (PM) proteins were extracted using Eukaryotic Membrane Protein Extraction Reagent (Pierce, Rockford, IL, USA). Western blot analysis was performed as described previously (16). Briefly 30 µg protein lysates was electrophoresed on 10% SDS-PAGE and transferred to PVDF membranes. The following primary antibodies at the indicated dilutions were used (GLUT4, 1:1,000, ab564; PPARδ, 1:400, ab23673; PGC-1α, 1:1,000, ab54481; β-actin, 1:1,000, ab6276). All primary mouse polyclonal antibodies were purchased from Abcam (Cambridge, MA, USA). Enhanced chemiluminescence (Amersham, Piscataway, NJ, USA) reagent was applied to the blots and they were exposed to autoradiography film. Film was analyzed by densitometry to determine the quantity of protein expressed in each group, using Bio-Rad Quantity One software (Bio-Rad). β-actin was used as an internal control. Results are expressed as optical density.
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose (2-NBDG) uptake assay
Differentiated C2C12 cells were pre-incubated in 0.05% glucose containing Krebs Ringer Bicarbonate buffer (pH 7.4 with 2% BSA). After this pre-incubation at 37°C for 30 min, cells were incubated in the presence or absence of insulin (100 nM) (Peptide Institute, Osaka, Japan) for 10 min and then further treated with insulin and 500 µM 2-NBDG (Invitrogen) for 2 h at 37°C in glucose-free Krebs-Ringer Bicarbonate buffer containing 2% BSA. Upon termination of incubation, the cells were washed with cold PBS, and 2-NBDG uptake into cells was assayed by fluorescence- activated cell sorting (FACS) analysis (BD Biosciences, San Jose, CA, USA). Results were expressed as fluorescence intensity (arbitrary units) of the insulin-treated group as compared to the normal group. Each sample was analyzed three times.
ATP assay
ATP content was measured using a luciferase-based luminescence assay kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer's instructions. Briefly, the cell lysates were extracted with cell lysis reagent. ATP concentrations were determined with a single-tube luminometer (Turner Biosystems, Sunnyvale, CA, USA) and normalized to protein concentration. Each sample was analyzed three times.
Statistical analysis
Data are presented as the means ± SEM. Analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was tested by ANOVA for three parametric groups. Student's t-test was used for two parametric groups. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
Differential miRNA expression in CON and IUGR skeletal muscle samples
We successfully established an IUGR rat model by feeding the rats a protein-restricted diet during pregnancy (16). The miRNA expression profiles for the two groups were analyzed by employing a highly sensitive, high-throughput and specific miRCURY LNA microarray platform. After normalization, obtained average values for each miRNA were used for statistical analysis. The threshold values we used to screen up- and downregulated miRNAs are represented as fold-change >2.00 and fold-change <0.50. A total of 3,100 miRNAs were detected using miRNA microarray in the two groups. In the microarray-based experiments, we identified 56 overexpressed and 68 downregulated miRNAs in IUGR samples. Based on these differentially expressed miRNAs, a tree with a clear distinction between the IUGR and CON groups was generated by cluster analysis (data not shown).
Validation of microarray results using RT-qPCR
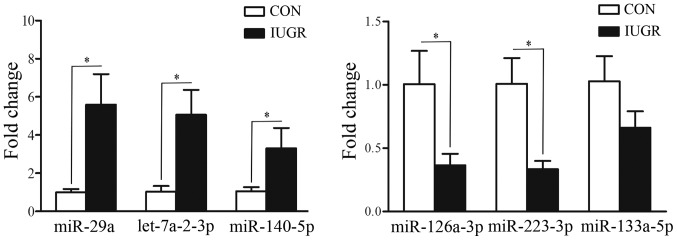
To validate the altered expression of miRNAs as detected by miRNA microarray, miR-29a, let-7a-2-3p, miR-140-5p, miR-126a-3p, miR-223-3p and miR-133a-5p were selected for confirmation by RT-qPCR. The majority of the RT-qPCR results correlated with those obtained by microarray analysis. As shown in Fig. 1, the expression levels of miR-29a, let-7a-2-3p and miR-140-5p were upregulated in the IUGR group (P=0.0001, P=0.0053 and P=0.0033, respectively), whereas the expression levels of miR-126a-3p and miR-223-3p were downregulated (P=0.0355 and P=0.0261, respectively) in the IUGR group. miR-133a-5p followed the same trend, but changes were not deemed to be significant. This aspect is deserving of further study in the future.
Figure 1.
RT-qPCR validation for specific miRNAs with altered expression. The six differentially expressed miRNAs (miR-29a, let-7a-2-3p, miR-140-5p, miR-126a-3p, miR-223-3p and miR-133a-5p) in the intrauterine growth retardation (IUGR) rat muscle samples are depicted; these were detected by the miRNA microarray and RT-qPCR. *P<0.05 vs. CON group.
Prediction and validation of PPARδ regulated by miR-29a
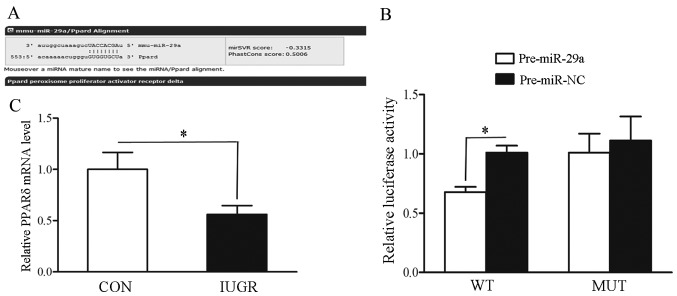
Since miR-29a was the most markedly upregulated miRNA in muscle samples from the IUGR group and it plays an important role in diabetes mellitus, which is closely related to IUGR, as has been reported previously (19), we focused on miR-29a in subsequent experiments. We found a putative target site for miR-29a in the 3′-UTR of PPARδ gene by applying TargetScan bioinformatics algorithms (Fig. 2A). Subsequently, the luciferase reporter assay confirmed that miR-29a directly regulates the expression of PPARδ by binding to the complementary sites within the 3′-UTR of the PPARδ gene (Fig. 2B). Finally, we noted that the mRNA expression level of PPARδ was significantly decreased in skeletal muscles from IUGR compared with those from control rats (P<0.05) (Fig. 2C).
Figure 2.
Prediction and validation of peroxisome proliferator-activated receptor δ (PPARδ) regulated by miR-29a. (A) The miR-29a targets were predicted using TargetScan (www.targetscan.org) and PicTar (www.pictar.org). (B) The impact of miR-29a overexpression on pGL3-MTA1 luciferase activity in 293T cells is shown. *P<0.05 vs. pre-miR-29a cells. (C) mRNA expression of PPARδ in the intrauterine growth retardation (IUGR) and control (CON) rat muscle samples was detected by using RT-qPCR. WT, wild-type; mut, mutant. *P<0.05 vs. CON group.
Establishment of miR-29a overexpression in stable C2C12 cells
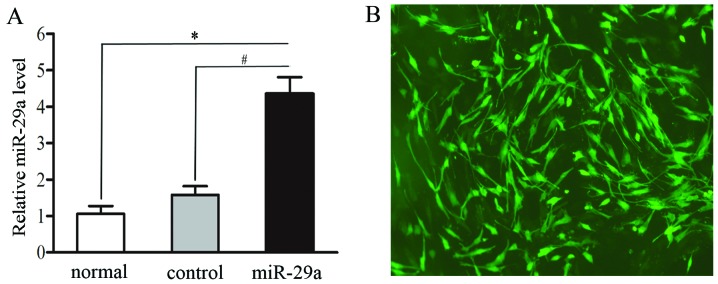
In order to study the biological significance of miR-29a induction, C2C12 cells without the lentivirus (normal group), C2C12 cells transfected with GFP-pGLPZ lentivirus (mock control group) or miR-29a-GFP-pGLPZ (miR-29a group) developed into myotubes after being treated with 2% horse serum for six days. After transfection (48 h), miR-29a expression levels in each cell line were detected by RT-qPCR and visualized using a fluorescence microscope. As expected, compared to non-transfected and mock-transfected cells, cells transfected with miR-29a exhibited significantly higher levels of miR-29a (2.30-fold increase, P=0.0016) (Fig. 3A).
Figure 3.
Establishment of miR-29a overexpression in C2C12 cells. (A) miR-29a expression in untransfected C2C12 myoblasts (normal group), C2C12 myoblasts transfected with GFP-pGLPZ lentivirus (mock control group) or miR-29a-GFP-pGLPZ lentivirus (miR-29a group) was assessed by RT-qPCR. *P<0.05 vs. normal cells. #P<0.05 vs. control cells. (B) Expression of miR-29a in C2C12 cells as represented by the GFP reporter under a fluorescence microscope.
Expression analysis of transcripts regulated by miR-29a
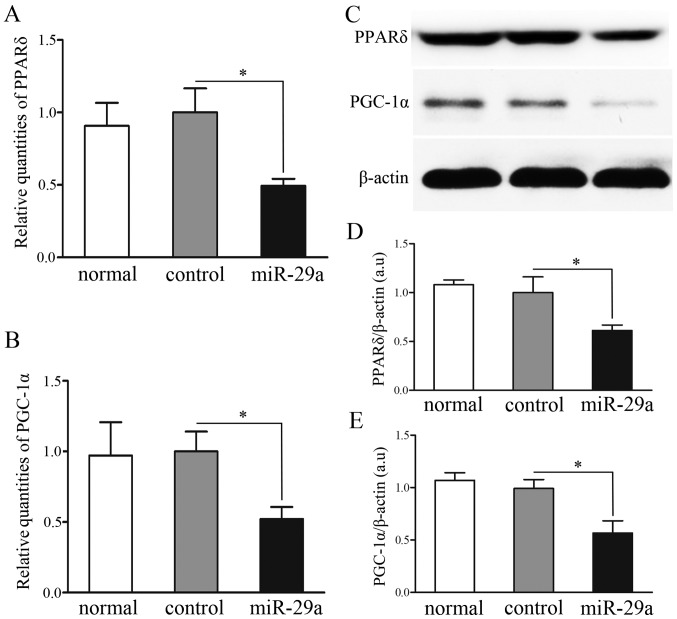
In the majority of tissues, PPARδ regulates a variety of material metabolisms with PGC-1α, one of its most important coactivators. In the C2C12 cells, overexpression of miR-29a decreased PPARδ and PGC-1α at the mRNA level, as compared to non- and mock-transfected cells (P<0.05) (Fig. 4A and B). Furthermore, western blot analysis revealed that expression of PPARδ and PGC-1α protein was significantly lower in C2C12 cells transfected with miR-29a than in the non- and mock-transfected cells (P<0.05) (Fig. 4C–E).
Figure 4.
Expression analysis of transcripts regulated by miR-29a. (A and B) Relative levels of peroxisome proliferator-activated receptor δ (PPARδ) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in untransfected C2C12 cells (normal group), C2C12 cells transfected with GFP-pGLPZ lentivirus (control group) or miR-29a-GFP-pGLPZ (miR-29a group), as measured by RT-qPCR. (C-E) protein levels of PPARδ and PGC-1α assessed in normal, control and miR-29a group were measured by western blot analysis. *P<0.05 vs. control cells.
Overexpression of miR-29a attenuates 2-NBDG uptake following insulin treatment and ATP production
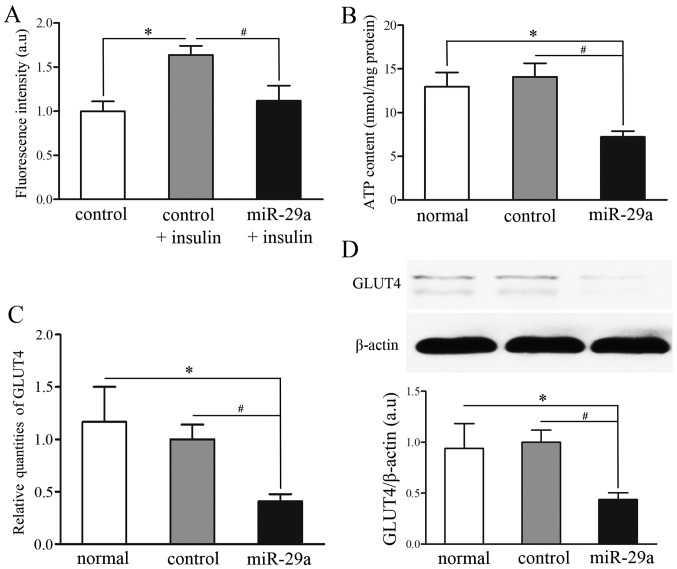
Disorders of glucose transport and energy production are known to be cellular manifestations to IR (20). To study the involvement of miR-29a and its impact on IR, a 2-NBDG uptake assay and an ATP production assay were performed in untransfected C2C12 cells (normal group), C2C12 cells transfected with GFP-pGLPZ lentivirus (control group) or miR-29a-GFP-pGLPZ (miR-29a group). The control group was divided into two subgroups, with or without insulin (100 nM) stimulation. Insulin treatment induced an increase in fluorescence intensity in both the control group and miR-29a group; fluorescence intensity in the miR-29a and insulin-treated group was significantly decreased (P<0.05) (Fig. 5A). Moreover, mitochondrial activity and energy production, as detected by the ATP luminescence kit, decreased more in the miR-29a group than the normal and control groups (P<0.05) (Fig. 5B). These data demonstrate that miR-29a overexpression induced IR in C2C12 cells.
Figure 5.
Overexpression of miR-29a attenuates 2-NBDG uptake and glucose transporter 4 (GLUT4) expression following insulin treatment as well as ATP production. (A) 2-NBDG uptake assays were performed in C2C12 cells transfected with GFP-pGLPZ lentivirus (control group) or miR-29a-GFP-pGLPZ lentivirus (miR-29a group). During 2-NBDG uptake assay, the control group was divided into two subgroups, with or without insulin (100 nM) stimulation. *P<0.05 vs. control cells without insulin stimulation. #P<0.05 vs. miR-29a cells with insulin stimulation. (B) ATP analysis was performed on each group using an ATP luciferase-based luminescence assay. (C and D) The expression of GLUT4 mRNA and protein during insulin stimulation was detected in each group. *P<0.05 vs. normal cells. #P<0.05 vs. control cells.
Overexpression of miR-29a downregulates GLUT4, an important glucose transporter
During insulin stimulation, one of the most important glucose transporter molecules is GLUT4 (21). In the present study, we noted a significant reduction in the mRNA and protein expression of GLUT4 in C2C12 cells transfected with miR-29a-GFP-pGLPZ (miR-29a group) compared with C2C12 cells which had not been transfected (normal group) and C2C12 cells transfected with GFP-pGLPZ lentivirus (control group) (P<0.05) (Fig. 5C and D). These results illustrated that overexpression of miR-29a attenuated insulin-induced glucose uptake which is mediated by regulation of GLUT4.
Discussion
Uteroplacental insufficiency and subsequent IUGR leads to IR, which is associated with metabolic disease, diabetes mellitus and angiocardiopathy in adulthood. A basic mechanism of metabolic diseases is IR, and skeletal muscle is a primary site of IR (22). Although several reports have described the status and role of miRNAs in diabetic tissues, very few studies have focused on the IUGR skeletal muscle (23). In the present study, we used an IUGR model induced by a protein-restricted diet during pregnant, which has also been widely used in other studies (15). In our previous study, we noted IR in the skeletal muscles of 18-month-old IUGR offspring rats (16), reflecting similar clinical findings in IUGR patients, thus verifying the validity of the model (24). In the present study, the skeletal muscles from 18-month-old IUGR offspring rats were selected. We pooled miRNAs from five control and five IUGR samples into two groups and compared the differences between them, in order that the random individual differences between subjects were eliminated. Although the sample pooling led to some loss of detailed information, this was not of great importance in our study.
In this study, we noted that the expression levels of 56 miRNAs were higher in the IUGR samples than in the CON samples, whereas 68 miRNAs demonstrated downregulated expression in the IUGR samples (data not shown). Six representative differentially expressed miRNAs (miR-29a, let-7a-2-3p, miR-140-5p, miR-126a-3p, miR-223-3p and miR-133a-5p) were chosen for validation using the RT-qPCR method and five independent samples. In general, in the present study the results of RT-qPCR were in concordance with the normalized microarray data. One exception was miR-133a-5p, which followed the same trend, but the changes were not deemed to be significant. Such discrepancies may be attributed to the greater individual differences among the samples. Furthermore, it should be noted that the purification process of the stem-loop RT-qPCR assay cannot completely remove long RNA nucleotides, and therefore we cannot exclude the possibility that the precursors are also quantified.
In the present study, miR-29a exhibited the highest fold change, and it should also be noted that in previous studies miR-29a has been shown to be critical in inhibiting insulin-stimulated glucose uptake in 3T3-L1 adipocytes (18), and it has been noted that it counteracts the effects of insulin on phosphoenolpyruvate carboxykinase (PEPCK) gene expression by primarily targeting phosphoinositide 3-kinase (PI3K) and abrogating downstream insulin signaling in HepG2 cells (25). A previous study showed that miR-29a expression was much higher in models using muscles of starved mother starved pups (SMSP) than in those using control mother control pups (CMCP) and, importantly, the progression and outcome of this model were similar to those of our model (13). Such studies have demonstrated that miR-29a plays various roles in different cells under different conditions, and we thus focused on miR-29a for further investigation in this study.
Using the popular website TargetScan for miRNA target gene prediction, we found that PPARδ harbored a binding site for miR-29a between 1470 and 1476 nucleotides on its 3′UTR. A luciferase reporter assay confirmed that PPARδ was the target gene of miR-29a, which was consistent with the results of the bioinformatics analysis. Our data demonstrated that PPARδ levels correspondingly decreased in miR-29a-transfected C2C12 cells. Thus, our findings provide further support for the theory that PPARδ is the target gene for miR-29a.
It is well known that PPARδ is involved in many different biological activities such as the metabolism of lipids, lipoproteins and glucose. The skeletal muscle is an organ that plays a key role in such processes (26). One of the best-described cofactors of PPARδ is a transcriptional coactivator (27). Targeting of PPARδ with miR-29a suggests that this miRNA contributes to the regulation of the corresponding metabolisms. Compared with the control group, in the miR-29a-transfected group, we noted significantly reduced mRNA and protein levels of PPARδ and PGC-1α in C2C12 cells. A previous study stated that overexpression of miR-29a in primary hepatocytes also decreased the protein levels of PGC-1α (28). It has also been reported that activation of PPARδ in the skeletal muscle led to increased levels of PGC-1α protein (29). Thus, it was speculated that one possible explanation for why C2C12 cells exhibit decreased levels of PGC-1α was most likely attributable to repression of PPARδ by miR-29a.
Since the skeletal muscle is a key organ for glucose uptake and lipid oxidation, increased insulin sensitivity in the skeletal muscle is beneficial to the control of glucose and lipid homeostasis (30). In the present study, we noted that overexpression of miR-29a significantly inhibited glucose uptake in the cell and impaired insulin sensitivity. GLUT4, the major insulin-stimulated glucose transporter, is expressed predominantly in skeletal muscle, cardiac muscle and adipose tissue, and is largely responsible for insulin-stimulated glucose transport into these tissues (31). In this study, reduced GLUT4 translocation was linked with decreased glucose uptake. The protein expression of GLUT4 was consistent with the pattern observed at the mRNA level. IR in type 2 diabetes is linked to decreased insulin-stimulated glucose transport in adipose tissue and skeletal muscle, and the downregulation of major insulin-responsive glucose transporter GLUT (32). Krämer et al (33) reported that exposure of human skeletal muscle cells and C2C12 cells to a PPARδ agonist increased glucose uptake in a PPARδ-dependent manner. Thus, we suggest that reduced glucose transport noted in our study is due not only partly to decreased GLUT4 but also partly to the direct effects of decreased PPARδ.
Furthermore, we found that ATP was significantly decreased in the miR-29a-transfected C2C12 cells compared to normal or control cells. This is likely due to energy being transferred from glucose to ATP through aerobic respiration or anaerobic respiration in cells and PGC-1α is a master regulator of mito chondrial biogenesis (34). Therefore, we speculate that miR-29a is important for efficient glucose transport activity and ATP production.
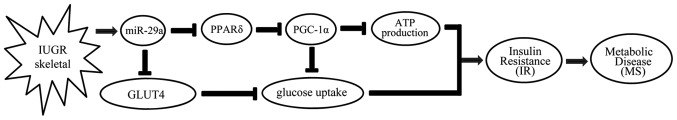
In conclusion, in the present study we noted that miR-29a was upregulated in skeletal muscle from IUGR offspring compared to control offspring. Overexpression of miR-29a suppressed the expression of its target gene, PPARδ, then reduced the expression of PGC-1α, which is an important coactivator of PPARδ. Thus, we posit that PPARδ/PGC-1α-dependent signals together reduce insulin-dependent glucose uptake and ATP production. Overexpression of miR-29a also caused a decrease in levels of GLUT4, the most important glucose transporter in skeletal muscle, which partly induced decreased insulin-dependent glucose uptake. As a result, metabolic disease occurred, which is secondary to IR in the skeletal muscles (Fig. 6). Taken together, the results provide evidence for a novel micro-RNA-mediated mechanism of PPARδ regulation, and are suggestive of the IR-promoting actions of miR-29a in skeletal muscles of IUGR rats. A further elucidation of miR-29a leading to skeletal dysfunction and other potential mechanisms is thus required, as well as in vitro and in vivo studies.
Figure 6.
Proposed role of miR-29a in skeletal muscles in the intrauterine growth retardation (IUGR) model. miR-29a is upregulated in skeletal muscle from IUGR offspring compared to control offspring. Overexpression of miR-29a in C2C12 cell line suppressed the expression of its target gene peroxisome proliferator-activated receptor δ (PPARδ), which, in turn, influenced the expression of its coactivator, such as peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α). Thus, PPARδ/PGC-1α-dependent signals together reduced insulin- dependent glucose uptake and ATP production. Overexpression of miR-29a also decreased glucose transporter 4 (GLUT4), the most important glucose transporter in skeletal muscle, and partly induced decreased insulin-dependent glucose uptake. As a result, metabolic disease/syndrome (MS) occurred, secondary to insulin resistance (IR) in the skeletal muscles.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grants no. 81300654, 81100592 and 81270800).
References
- 1.Henriksen T. Foetal nutrition, foetal growth restriction and health later in life. Acta Paediatr Suppl. 1999;88:4–8. doi: 10.1111/j.1651-2227.1999.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 2.Grella PV. Low birth weight and early life origins of adult disease: insulin resistance and type 2 diabetes. Clin Exp Obstet Gynecol. 2007;34:9–13. [PubMed] [Google Scholar]
- 3.Finken MJ, Keijzer-Veen MG, Dekker FW, Frölich M, Hille ET, Romijn JA, Wit JM, Dutch POPS-19 Collaborative Study Group Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia. 2006;49:478–485. doi: 10.1007/s00125-005-0118-y. [DOI] [PubMed] [Google Scholar]
- 4.Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 5.Gatford KL, Simmons RA, De Blasio MJ, Robinson JS, Owens JA. Review: placental programming of postnatal diabetes and impaired insulin action after IUGR. Placenta. 2010;31(Suppl):S60–S65. doi: 10.1016/j.placenta.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg M, Thamotharan M, Oak SA, Pan G, Maclaren DC, Lee PW, Devaskar SU. Early exercise regimen improves insulin sensitivity in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2009;296:E272–E281. doi: 10.1152/ajpendo.90473.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neitzke U, Harder T, Schellong K, Melchior K, Ziska T, Rodekamp E, Dudenhausen JW, Plagemann A. Intrauterine growth restriction in a rodent model and developmental programming of the metabolic syndrome: a critical appraisal of the experimental evidence. Placenta. 2008;29:246–254. doi: 10.1016/j.placenta.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Juvvuna PK, Kukreti H, McFarlane C. Mega roles of microRNAs in regulation of skeletal muscle health and disease. Front Physiol. 2014;5:239. doi: 10.3389/fphys.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minetti GC, Feige JN, Bombard F, Heier A, Morvan F, Nürnberg B, Leiss V, Birnbaumer L, Glass DJ, Fornaro M. Gαi2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Mol Cell Biol. 2014;34:619–630. doi: 10.1128/MCB.00957-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 11.Liu N, Bassel-Duby R. Regulation of skeletal muscle development and disease by microRNAs. Results Probl Cell Differ. 2015;56:165–190. doi: 10.1007/978-3-662-44608-9_8. [DOI] [PubMed] [Google Scholar]
- 12.Sene LB, Mesquita FF, de Moraes LN, Santos DC, Carvalho R, Gontijo JA, Boer PA. Involvement of renal corpuscle microRNA expression on epithelial-to-mesenchymal transition in maternal low protein diet in adult programmed rats. PLoS One. 2013;8:e71310. doi: 10.1371/journal.pone.0071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raychaudhuri S. MicroRNAs overexpressed in growth-restricted rat skeletal muscles regulate the glucose transport in cell culture targeting central TGF-β factor SMAD4. PLoS One. 2012;7:e34596. doi: 10.1371/journal.pone.0034596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PY, Ganguly A, Rubbi L, Orozco LD, Morselli M, Ashraf D, Jaroszewicz A, Feng S, Jacobsen SE, Nakano A, Pellegrini M. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics. 2013;45:565–576. doi: 10.1152/physiolgenomics.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Lin Y, Tian B, Miao J, Xi C, Liu C. Maternal protein restriction alters VEGF signaling and decreases pulmonary alveolar in fetal rats. Int J Clin Exp Pathol. 2014;7:3101–3111. [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Y, Gu P, Liu K, Huang P. Maternal protein restriction in rats leads to reduced PGC-1α expression via altered DNA methylation in skeletal muscle. Mol Med Rep. 2013;7:306–312. doi: 10.3892/mmr.2012.1134. [DOI] [PubMed] [Google Scholar]
- 17.Ozanne SE, Martensz ND, Petry CJ, Loizou CL, Hales CN. Maternal low protein diet in rats programmes fatty acid desatu rase activities in the offspring. Diabetologia. 1998;41:1337–1342. doi: 10.1007/s001250051074. [DOI] [PubMed] [Google Scholar]
- 18.He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21:2785–2794. doi: 10.1210/me.2007-0167. [DOI] [PubMed] [Google Scholar]
- 19.Thorn SR, Rozance PJ, Brown LD, Hay WW., Jr The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med. 2011;29:225–236. doi: 10.1055/s-0031-1275516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksen EJ, Prasannarong M. The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol. 2013;378:15–22. doi: 10.1016/j.mce.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Govers R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv Clin Chem. 2014;66:173–240. doi: 10.1016/B978-0-12-801401-1.00006-2. [DOI] [PubMed] [Google Scholar]
- 22.Bouzakri K, Koistinen HA, Zierath JR. Molecular mechanisms of skeletal muscle insulin resistance in type 2 diabetes. Curr Diabetes Rev. 2005;1:167–174. doi: 10.2174/1573399054022785. [DOI] [PubMed] [Google Scholar]
- 23.McClelland AD, Kantharidis P. microRNA in the development of diabetic complications. Clin Sci (Lond) 2014;126:95–110. doi: 10.1042/CS20130079. [DOI] [PubMed] [Google Scholar]
- 24.Jaquet D, Vidal H, Hankard R, Czernichow P, Levy-Marchal C. Impaired regulation of glucose transporter 4 gene expression in insulin resistance associated with in utero undernutrition. J Clin Endocrinol Metab. 2001;86:3266–3271. doi: 10.1210/jcem.86.7.7677. [DOI] [PubMed] [Google Scholar]
- 25.Pandey AK, Verma G, Vig S, Srivastava S, Srivastava AK, Datta M. miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol Cell Endocrinol. 2011;332:125–133. doi: 10.1016/j.mce.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Coll T, Rodrïguez-Calvo R, Barroso E, Serrano L, Eyre E, Palomer X, Vázquez-Carrera M. Peroxisome proliferator-activated receptor (PPAR) beta/delta: a new potential therapeutic target for the treatment of metabolic syndrome. Curr Mol Pharmacol. 2009;2:46–55. doi: 10.2174/1874467210902010046. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Schindler J, Svensson K, Vargas-Fernández E, Santos G, Wahli W, Handschin C. The coactivator PGC-1α regulates skeletal muscle oxidative metabolism independently of the nuclear receptor PPARβ/δ in sedentary mice fed a regular chow diet. Diabetologia. 2014;57:2405–2412. doi: 10.1007/s00125-014-3352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Liu C, Qiao A, Cui Y, Zhang H, Cui A, Zhang S, Yang Y, Xiao X, Chen Y, et al. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. J Hepatol. 2013;58:535–542. doi: 10.1016/j.jhep.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- 32.Kellerer M, Lammers R, Häring HU. Insulin signal transduction: possible mechanisms for insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:97–106. doi: 10.1055/s-0029-1212082. [DOI] [PubMed] [Google Scholar]
- 33.Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A. Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem. 2007;282:19313–19320. doi: 10.1074/jbc.M702329200. [DOI] [PubMed] [Google Scholar]
- 34.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]