Abstract
Herpes simplex virus 1 causes a shutoff of cellular protein synthesis through the degradation of RNA that is mediated by the virion host shutoff (Vhs) protein encoded by the UL41 gene. We reported elsewhere that the Vhs-dependent degradation of RNA is selective, and we identified RNAs containing AU-rich elements (AREs) that were upregulated after infection but degraded by deadenylation and progressive 3′-to-5′ degradation. We also identified upregulated RNAs that were not subject to Vhs-dependent degradation (A. Esclatine, B. Taddeo, L. Evans, and B. Roizman, Proc. Natl. Acad. Sci. USA 101:3603-3608, 2004). Among the latter was the RNA encoding tristetraprolin, a protein that binds AREs and is known to be associated with the degradation of RNAs containing AREs. Prompted by this observation, we examined the status of the ARE binding proteins tristetraprolin and TIA-1/TIAR in infected cells. We report that tristetraprolin was made and accumulated in the cytoplasm of wild-type virus-infected human foreskin fibroblasts as early as 2 h and in HEp-2 cells as early as 6 h after infection. The amounts of tristetraprolin that accumulated in the cytoplasm of cells infected with a mutant virus lacking UL41 were significantly lower than those in wild-type virus-infected cells. The localization of tristetraprolin was not modified in cells infected with a mutant lacking the gene encoding infected cell protein 4 (ICP4). TIA-1 and TIAR are two other proteins that are associated with the regulation of ARE-containing RNAs and that normally reside in nuclei. In infected cells, they started to accumulate in the cytoplasm after 6 h of infection. In cells infected with the mutant virus lacking UL41, TIA-1/TIAR accumulated in the cytoplasm in granular structures reminiscent of stress granules in a significant percentage of the cells. In addition, an antibody to tristetraprolin coprecipitated the Vhs protein from lysates of cells late in infection. The results indicate that the Vhs-dependent degradation of ARE-containing RNAs correlates with the transactivation, cytoplasmic accumulation, and persistence of tristetraprolin in infected cells.
For the past two decades, a rich literature has indicated that the reduction of host and viral protein synthesis observed in cells infected with herpes simplex virus 1 (HSV-1) is associated with the virion host shutoff (Vhs) protein, the product of the UL41 gene (28, 29, 36, 38). The consequence of this activity is that cellular protein synthesis is shut off, whereas viral proteins, by virtue of the enhanced transcription of viral DNA, are made and accumulate in the infected cell. Vhs mediates the degradation of RNA, and it has been reported that sequences at the 5′ end of mRNA are degraded more rapidly than those at the 3′ end of the transcript (23). Our studies on the degradation of cellular RNAs in infected cells emerged from observations that during the course of viral infection several cellular RNAs were induced but the corresponding proteins were not made (15, 42). The failure of the cell to synthesize the proteins in at least several specific instances could be related to the ICP27-dependent export of unprocessed RNAs containing introns and to Vhs-dependent deadenylation and 3′-to-5′ degradation of the RNA (15, 42).
A more detailed examination of cellular RNAs that are induced and degraded after infection revealed that they contain AU-rich elements (AREs) in their 3′ untranslated domains. AREs are frequently found in mRNAs that encode proto-oncogenes, nuclear transcription factors, and cytokines and characteristically confer a short half-life to the transcript (11). Further analyses of the RNAs that are upregulated after HSV-1 infection led to the identification of two RNAs that lack AREs and which are not degraded by any of the mechanisms described above. At least one of the RNAs was translated, and the gene product, GADD45β, was made and accumulated in infected cells. The conclusion of these studies was that Vhs-dependent degradation is selective, which raises questions regarding the mechanism by which Vhs mediates a general decrease in the synthesis of cellular proteins and at the same time mediates the selective degradation of the ARE-containing RNAs identified in the earlier report.
As indicated above, the presence of AREs in the 3′ untranslated domain confers instability to mammalian mRNAs (5). Current evidence suggests that in uninfected cells, as a first step, AREs promote both deadenylation and decapping processes (18, 30). A subsequent degradation of the mRNA body occurs in the 3′-to-5′ direction and is mediated by the exosome, a complex of exonucleases, after the recognition of AREs by AU-rich binding proteins (10). Several AU-rich binding proteins have actually been shown to modulate mRNA turnover (5). Among these, AUF-1, KSRP, TIA-1 (T-cell internal antigen 1), TIAR (TIA-1-related protein), and tristetraprolin (TTP) promote mRNA instability. In contrast, HuR stabilizes RNAs, probably due to its inability to recruit the exosome to ARE-containing RNAs (10). TTP is the product of an immediate-early response gene (Zfp-36) and the prototype of a group of CCCH tandem zinc finger proteins. It is expressed transiently in response to extracellular stimuli. TTP was shown to bind to ARE within the mRNAs of several genes, including tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor, interleukin-3, and the cyclooxygenase COX-2 (6, 37, 40, 46). This binding causes the destabilization of the mRNA and decreased secretion of the protein (6). TTP mRNA is widely distributed among tissue types, with particularly high levels of expression in the spleen, lymph nodes, and thymus (31). It has been described to rapidly translocate from the nucleus to the cytosol upon stimulation with serum or other mitogens (45). But TTP was later shown to be confined to the cytoplasm in peripheral blood leukocytes (16) and primary macrophages (8). It has also been reported that TTP, along with other AU-rich binding proteins, is recruited to stress granules (SGs), which are cytoplasmic microdomains where untranslated mRNAs accumulate during stress (2, 25). SGs represent a site of triage for poly(A)+ RNA during stress responses, and the SG-associated RNAs are in a dynamic equilibrium with polyribosomes (2). The actual formation of SGs occurs upon autoaggregation of the prion-like domain of TIA-1/TIAR proteins. The TIA-1/TIAR proteins are also nucleocytoplasmic shuttling proteins and bind to RNA.
In the studies described in this report, we examined the status of TTP and TIA-1/TIAR during HSV-1 infection. Two studies based on different microarray analyses suggested that TTP is upregulated in infected cells (27, 41). In light of the apparent connection between TTP, TIA-1/TIAR, and the degradation of ARE-containing RNAs, we were interested in validating the upregulation of TTP RNA in infected cells to determine whether TTP is made in these cells and whether both TIA-1/TIAR and TTP are activated by translocation to the cytoplasm. In this report, we show that TTP mRNA is upregulated in cells infected with wild-type virus and that the RNA is also upregulated, but to a lesser degree, in cells infected with a mutant lacking the gene encoding Vhs, ICP4, or ICP27. While it was present in the nucleus in mock-infected cells, the TTP protein was detected in the cytoplasm as early as 2 h after infection. The levels of TTP protein reflected the levels of accumulated mRNAs. TIA-1 and TIAR, two other AU-rich binding proteins, were also activated during the infection, as evidenced by their accumulation in the cytoplasm. While SGs were not observed after infection with wild-type HSV-1, a significant portion of the cells infected by the ΔUL41 mutant virus showed cytoplasmic granular structures containing TIA-1/TIAR. Finally, we also report that the TTP protein interacted physically with Vhs, consistent with evidence that in infected cells the 3′-to-5′ degradation of RNAs is Vhs dependent.
MATERIALS AND METHODS
Cells and viruses.
SK-N-SH, HEp-2, and HeLa cells obtained from the American Type Culture Collection were propagated in Dulbecco's modified Eagle's minimal essential medium supplemented with 5% newborn calf serum. Telomerase-transformed primary human foreskin fibroblasts (HFFs) (7), a kind gift of Thomas E. Shenk (Princeton University), were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HSV-1(F) is the prototype HSV-1 strain used in our laboratory (13). The ΔUL41 mutant virus, R2621, was reported elsewhere (35). The HSV-1(KOS) Δα4 mutant, a kind gift of N. DeLuca (University of Pittsburgh), lacks both copies of the α4 gene and was grown in a Vero-derived cell line (E5) expressing the α4 gene. The Δα27 mutant virus (vBSΔ27) and complementing cells (Vero 2.2) (39) were a kind gift of Saul Silverstein (Columbia University).
Isolation of total and cytoplasmic RNAs.
Total RNAs were extracted with the aid of TRIZOL reagent (Life Technologies, Rockville, Md.) used according to the manufacturer's instructions. DNase treatment (Life Technologies), phenol-chloroform extraction, and ethanol precipitation (Fisher Scientific, Houston, Tex.) were performed to remove possible DNA contamination. Cytoplasmic RNAs were isolated with the aid of an RNeasy mini kit used according to the protocol suggested by the manufacturer (Qiagen, Valencia, Calif.).
Real-time PCR.
Real-time PCRs were performed in an ABI Prism 7000 sequence detection system with SYBR green chemistry as previously described (41). The following primers were used: TTP forward (5′-CGCGCTACAAGACTGAGCTATG-3′) and TTP reverse (5′-CATGGGCAAACTGGCACTT-3′).
Northern blot analyses.
Eight micrograms of cytoplasmic RNA was loaded into a denaturing formaldehyde gel and transferred onto a nylon membrane. For TTP mRNA detection, a fragment containing the entire coding sequence, amplified by PCR from HSV-1-infected HFF cDNA, was used as probe. Prehybridization and hybridization were carried out at 42°C in ULTRAhyb buffer (Ambion, Austin, Tex.) supplemented with 200 μg of denatured salmon sperm DNA per ml (Stratagene, La Jolla, Calif.). The membranes were rinsed as suggested by ULTRAhyb's manufacturer and exposed to film for signal detection.
Antibodies.
A goat polyclonal antibody directed against TTP, a rabbit polyclonal antibody directed against TTP, and a goat polyclonal antibody raised against TIA-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The murine monoclonal antibody 3E6 (anti-TIA-1/TIAR) was a kind gift of Paul Anderson (Brigham and Women's Hospital, Boston, Mass.) (44). A goat anti-rabbit antibody coupled with fluorescein isothiocyanate (FITC) was obtained from Sigma (St. Louis, Mo.). An Alexa fluor 594-labeled goat anti-mouse antibody was obtained from Molecular Probes (Eugene, Oreg.). A polyclonal rabbit anti-Vhs antiserum was kindly provided by Duncan W. Wilson (Albert Einstein College of Medicine, Bronx, N.Y.) (32).
Immunoblots.
For immunoblots, cells were collected, rinsed once with cold phosphate-buffered saline (PBS), and lysed in RIPA buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, 5 mM EDTA, and 1× protease inhibitor cocktail [Sigma]). The samples were kept on ice for 1 h, and insoluble material was pelleted in an Eppendorf 5415C microcentrifuge at maximum speed for 10 min at 4°C. The fractionation of infected and mock-infected cells into nuclear and cytoplasmic extracts was performed as previously described (1). The protein concentration was determined, and 50 to 100 μg of proteins was separated in an SDS-polyacrylamide gel and electrically transferred to a nitrocellulose membrane. The membranes were blocked with 5% nonfat dry milk in PBS, allowed to react with the anti-TTP or anti-TIA-1 goat polyclonal antibody overnight at 4°C, rinsed, and then exposed to the secondary antibody at room temperature for 1 h. The antibodies were diluted in PBS containing 1% bovine serum albumin and 0.05% Tween 20. The secondary antibody was an alkaline phosphatase (AP)-conjugated anti-goat antibody (Sigma). All rinses were done with PBS containing 0.05% Tween 20. For development of the AP-conjugated secondary antibody, the immunoblots were incubated with AP buffer (100 mM Tris [pH 9.5], 100 mM NaCl, 5 mM MgCl2) containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
Indirect immunofluorescence staining.
HEp-2 cells and HFFs cultured on four-well slides were infected with HSV-1(F) or the Δα4 or ΔUL41 mutant virus or were mock infected. As a positive control for SG formation, mock-infected cells were treated with 0.5 mM arsenite for 1 h (26). The cells were briefly rinsed in PBS, incubated for 10 min in 2% paraformaldehyde in PBS, and immediately immersed in methanol for 20 min at −20°C. After three rinses in PBS, the cells were incubated overnight at 4°C with a rabbit antibody raised against TTP or with the murine antibody 3E6, which recognizes both TIAR and TIA-1. After three rinses in PBS containing 0.2% Tween 20, the cells were incubated for 60 min at room temperature with a secondary anti-rabbit or anti-mouse antibody conjugated to FITC or Alexa fluor 594, respectively. The cells that were incubated with the anti-TTP antibody were counterstained with propidium iodide (Sigma). Some slides were incubated with both the TTP and TIA-1/TIAR antibodies described above. After three final rinses, the cells were mounted with Glycergel (Dako, Carpinteria, Calif.) and examined under a laser scanning confocal microscope (Zeiss LSM 410).
Immunoprecipitation assay.
SK-N-SH cells in 25-cm2 flasks were either infected with 10 PFU of HSV-1(F) or mock infected. The cells were harvested 16 h after infection and rinsed twice with PBS. The pellets were resuspended in 200 μl of lysis buffer (20 mM Tris [pH 8.0], 1 mM EDTA, 1% NP-40, 400 mM NaCl, 2 mM dithiothreitol, 0.1 mM NaVO4, 10 mM NaF, 1× protease inhibitor cocktail0, chilled on ice for 40 min, and centrifuged at 1,000 rpm for 2 min. The cell lysate (150 μl) was diluted with an equal volume of low-salt lysis buffer (20 mM Tris [pH 8.0], 1 mM EDTA, 1% NP-40, 16 mM NaCl, 2 mM dithiothreitol) and incubated with 5% rabbit preimmune serum at 4°C for 1 h. The mixture was then incubated with 50 μl of protein A-Sepharose for 1 h at 4°C and centrifuged at 3,000 rpm for 3 min to remove nonspecifically bound proteins. The supernatant fluid was incubated with an anti-TTP polyclonal antiserum at 4°C overnight and then was mixed with 20 μl of protein A-Sepharose at 4°C for 1 h. Antigen-antibody complexes were rinsed three times with rinse buffer (50 mM Tris [pH 7.4], 10 mM MgCl2, 5 mM dithiothreitol) and pelleted by centrifugation at 3,000 rpm for 3 min. The antigen-antibody complexes were disrupted by boiling the pellet in sample buffer for 5 min. Supernatant fluids containing precipitated interaction proteins were recovered after centrifugation for 1 min and were analyzed in 10% denaturing polyacrylamide gels. The blots were probed with antibodies directed against Vhs.
RESULTS
TTP mRNA is upregulated in HSV-1-infected cells.
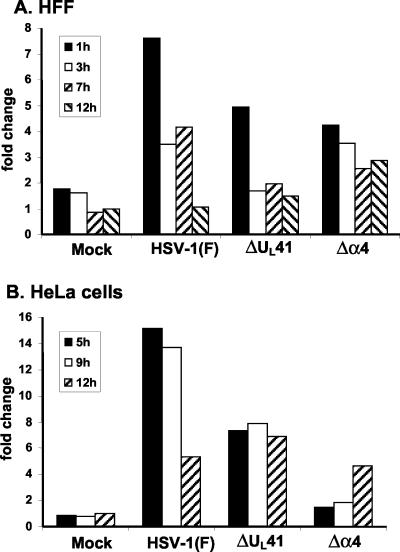
Earlier studies from our laboratory indicated a significant increase in the accumulation of TTP mRNA in HFFs and HeLa cells after infection with wild-type HSV-1 (15, 27, 42). To verify the upregulation of the RNA, we quantified the accumulation of TTP RNA in HFF or HeLa cells infected with wild-type or mutant viruses by using real-time PCR analyses. Quiescent HFFs were mock infected or exposed to 20 PFU of HSV-1(F) or Δα4 or ΔUL41 mutant virus per cell and harvested 1, 3, 7, or 12 h after infection. Total RNAs were extracted, reverse transcribed, and quantified by real-time PCR as previously described (41). The amount of TTP mRNA in cells harvested 12 h after mock infection was used as a calibrator. As shown in Fig. 1A, TTP mRNA was clearly upregulated upon virus infection, even though the levels of induction were different between wild-type and mutant virus-infected cells. For HSV-1(F)-infected cells, the largest increase in TTP mRNA was observed 1 h after infection, decreasing to about half at 3 and 7 h and reaching the levels observed in mock-infected cells by 12 h after infection. The pattern of accumulation of TTP in ΔUL41 mutant-infected cells was similar to that for HSV-1(F)-infected cells, but the amounts of RNA declined more rapidly after the first hour of infection. In cells infected with the Δα4 mutant virus, the amounts of TTP RNA were also increased compared to those in mock-infected cells but were lower than those detected in wild-type virus-infected cells. Furthermore, the amounts recovered 12 h after infection were similar to those detected at earlier time points.
FIG. 1.
Real-time PCR analysis of TTP transcripts in HSV-1-infected cells. Total RNAs were extracted at the indicated times after infection of HFFs (A) or HeLa cells (B) with HSV-1(F) or the Δα4 or ΔUL41 mutant virus and then were analyzed by real-time PCR. The amount of total RNA was normalized with respect to reverse-transcribed 18S rRNA. The amount of TTP RNA that accumulated in infected cells was calculated as the fold change compared to that extracted from cells harvested 12 h after mock infection.
To determine whether the accumulation of TTP transcripts was cell type dependent, we also performed similar tests on total RNAs extracted from infected HeLa cells. These cells were mock infected or exposed to 20 PFU of HSV-1(F) or Δα4 or ΔUL41 mutant virus per cell and then harvested 5, 9, or 12 h after infection. The results are shown in Fig. 1B. TTP mRNA was highly upregulated 5 and 9 h after HSV-1(F) infection but declined thereafter. The level of TTP RNA detected in ΔUL41-infected HeLa cells was lower than that in wild-type virus-infected cells but was still considerably higher than that in mock-infected cells. After infection with the Δα4 mutant virus, the amounts of TTP RNA were slightly modified after 5 and 9 h but were clearly upregulated after 12 h.
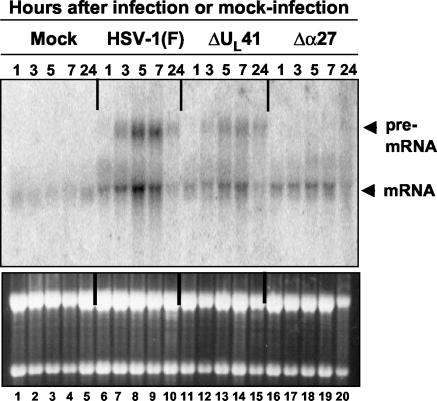
To extend our studies, we performed Northern blot analyses of the TTP RNA with cytoplasmic RNAs extracted from HeLa cells at different times after mock infection or infection with HSV-1(F) or the ΔUL41 or Δα27 mutant virus. The results, shown in Fig. 2, were as follows. (i) A very weak signal corresponding to the size expected for TTP mRNA (∼1.75 kb) was detected in the cytoplasm of cells harvested 1, 3, 5, 7, or 24 h after mock infection (lanes 1 to 5). The intensity of the signal remained stable during the course of the mock infection. (ii) As expected, an increase in the intensity of the signal for TTP RNA was observed for all of the RNA samples purified from HSV-1(F)-infected (lanes 6 to 10), ΔUL41-infected (lanes 11 to 15), or Δα27-infected cells (lanes 16 to 20). The difference in intensities of the signal between wild-type and mutant virus-infected cells reflected what was previously observed by real-time PCR analysis. Earlier reports from our laboratory (41) and by other groups (14) already showed an ICP27-dependent accumulation of unspliced cellular RNA in the cytoplasm of HSV-1-infected cells. Therefore, the slower-migrating band detected in the cytoplasm of the cells infected with wild-type or ΔUL41 mutant virus-infected cells (lanes 6 to 15) represented the unspliced form of TTP RNA. It was indeed absent from cells infected with the Δα27 mutant (lanes 16 to 20). We conclude from these studies that TTP mRNA is upregulated in cells infected with HSV-1(F), and to a lesser extent, in those infected with ΔUL41 and Δα27 mutant viruses.
FIG. 2.
Accumulation of TTP RNA in cytoplasm of HeLa cells infected with HSV-1. HeLa cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUL41 mutant virus per cell. Cytoplasmic RNAs were purified from cells harvested at the indicated times after mock infection (lanes 1 to 5) or infection with HSV-1(F) (lanes 6 to 10), the ΔUL41 mutant (lanes 11 to 15), or the Δα27 mutant (lanes 16 to 20). RNAs (8 μg/lane) were loaded onto a denaturing formaldehyde gel and probed with a 32P-labeled fragment containing the entire coding sequence of TTP. The lower panel represents the ethidium bromide-stained gel showing the relative levels of rRNA. The arrows to the right of the upper panel indicate the positions of the full-length TTP mRNA transcript and the unspliced form of TTP.
TTP protein accumulates in HSV-1(F)-infected cells.
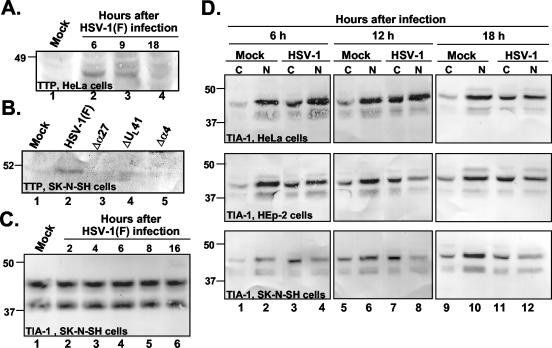
For a determination of whether the accumulation of TTP mRNA in HSV-1-infected cells resulted in a concurrent accumulation of the protein product, HeLa cells harvested 6, 9, and 18 h after exposure to 10 PFU of HSV-1(F) per cell were solubilized, electrophoretically separated in polyacrylamide gels, transferred to a nitrocellulose sheet, and probed with an anti-TTP antibody as described in Materials and Methods. As shown in Fig. 3A, the TTP protein was barely detectable in mock-infected cells (lane 1) but was clearly present 6 and 9 h after HSV-1 infection (lanes 2 and 3) and then disappeared at a later time point (lane 4). To determine whether the increased accumulation of TTP protein was cell type dependent, we performed similar analyses with protein lysates from SK-N-SH cells harvested 17 h after infection with HSV-1(F) or the ΔUL41, Δα27, or Δα4 mutant virus. As shown in Fig. 3B, the TTP protein was readily detected in cells infected with HSV-1(F) at higher levels than those in mock-infected cells. In contrast, the levels of TTP protein detected in lysates of ΔUL41, Δα27, or Δα4 virus-infected cells could not be differentiated from those detected in mock-infected cells. We conclude from these studies that TTP protein levels increase after infection and that, to the extent it was tested, the accumulation of TTP in wild-type virus-infected cells is not cell type dependent. We also determined the level of accumulation of TIA-1, another AU-rich binding protein, in SK-N-SH cells (Fig. 3C). TIA-1 was present in mock-infected cells as a doublet (lane 1), as expected from an alternative splice that was previously described (24). SK-N-SH cells harvested between 2 and 16 h after exposure to 10 PFU of HSV-1(F) per cell (lanes 2 to 6) showed the same level of TIA-1 protein as that present in mock-infected cells. Therefore, the level of TIA-1 showed no significant change after HSV-1 infection.
FIG. 3.
Immunoblots of TTP and TIA-1 proteins after HSV-1 infection. (A and B) Accumulation of TTP after HSV-1 infection. (A) HeLa cells were mock infected (lane 1) or exposed to 10 PFU of HSV-1(F) per cell (lanes 2 to 4) at time zero and were collected at the indicated times after infection. Cell lysates were incubated with an antibody to TTP. (B) SK-N-SH cells were mock infected (lane 1) or exposed to 10 PFU of HSV-1(F) (lane 2) or Δα27 (lane 3), ΔUL41 (lane 4), or Δα4 (lane 5) mutant virus per cell, collected 17 h after infection, and incubated with an antibody to TTP. (C and D) Cytoplasmic accumulation of TIA-1 after HSV-1 infection. (C) Immunoblot of uninfected or HSV-1(F)-infected SK-N-SH whole-cell lysates. The cells were harvested at the indicated times after infection. (D) Immunoblot of cytoplasmic (C) and nuclear (N) fractions of uninfected or HSV-1(F)-infected HeLa, HEp-2, and SK-N-SH cell lysates incubated with a goat polyclonal antibody to TIA-1. The cells were harvested at the indicated times after infection.
TTP localizes in cytoplasm of HSV-1-infected cells.
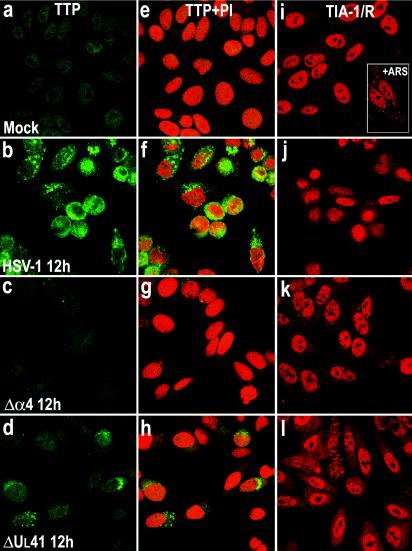
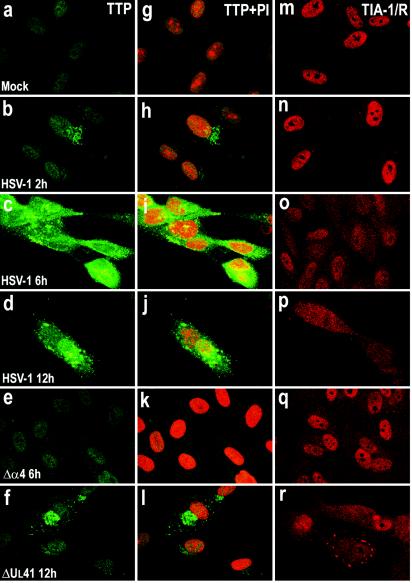
Certain mitogens (serum or phorbol esters) induce the synthesis of TTP and cause it to be rapidly translocated from the nucleus to the cytoplasm by a mechanism that remains to be determined (45). To determine the localization of the TTP protein after viral infection, we examined HFF and HEp-2 cells infected with HSV-1(F) or the Δα4 or ΔUL41 mutant virus by measuring immunofluorescence. HEp-2 cells were fixed 12 h after infection, while HFFs were fixed at various times after infection. Slide cultures containing either HEp-2 cells or HFFs were incubated with an antibody raised against TTP and with propidium iodide to counterstain the nucleus. The results are shown in Fig. 4a to h and 5a to l. For both cell lines, a barely detectable signal corresponding to the TTP protein was observed in the nuclei of mock-infected cells (Fig. 4a and e and 5a and g). Twelve hours after infection with HSV-1(F), the TTP protein was clearly detected in HEp-2 cells throughout the cytoplasm (Fig. 4b and f), while in cells infected with the ΔUL41 mutant virus, the amount of TTP detected was demonstrably lower (Fig. 4d and h). The localization and level of expression of TTP in Δα4 virus-infected cells could not be differentiated from those of mock-infected HEp-2 cells (Fig. 4c and g). Similar results were obtained with infected fibroblasts (Fig. 5). TTP was clearly present in the cytoplasm of cells fixed 2 h after HSV-1(F) infection (Fig. 5b and h). The amount of TTP increased 6 and 12 h after infection and TTP accumulated in granular cytoplasmic structures (Fig. 5c, d, i, and j). In cells infected with the ΔUL41 mutant virus, the TTP protein levels present in the cytoplasm were significantly lower than those detected in wild-type virus-infected cells (Fig. 5f and l). TTP was not modified after Δα4 virus infection, reflecting a similar decrease observed in immunoblots (Fig. 3B). Interestingly, in HSV-1- and ΔUL41 virus-infected cells, TTP appeared to be concentrated in punctate granules of variable size. These studies show that TTP is localized in the cytoplasm in response to HSV-1 infection in both HFF and HEp-2 cells.
FIG. 4.
Localization of TTP and TIA-1 in HEp-2 cells infected with HSV-1. HEp-2 cells were mock infected (a, e, and i) or exposed to 10 PFU of HSV-1(F) (b, f, and j) or the Δα4 (c, g, and k) or ΔUL41 (d, h, and l) mutant virus per cell and fixed by paraformaldehyde and then methanol 12 h after infection. The cells were labeled with an anti-TTP antibody, detected with a FITC-labeled secondary antibody (green), and counterstained with propidium iodide (PI; red), or they were labeled with anti-TIA-1/TIAR antibodies and detected with an Alexa fluor 594-labeled secondary antibody (red). Immunostaining of the monolayers was evaluated by confocal microscopy. Cells were exposed to 0.5 mM arsenite for 30 min.
FIG. 5.
Localization of TTP and TIA-1 in HFFs infected with HSV-1. HFFs were mock infected (a, g, and m) or infected with 10 PFU of HSV-1(F) (b to d, h to j, and n to p) or the Δα4 (e, k, and q) or ΔUL41 (f, l, and r) mutant virus per cell and fixed with paraformaldehyde and then methanol at different times postinfection. The cells were labeled with an anti-TTP antibody, detected with a FITC-labeled secondary antibody (green), and counterstained with propidium iodide (PI; red), or they were labeled with anti-TIA-1/TIAR antibodies and detected with an Alexa fluor 594-labeled secondary antibody (red). Immunostaining of the monolayers was evaluated by confocal microscopy.
TIA-1 and TIAR proteins, markers of SG formation, are translocated from the nucleus to the cytoplasm of infected cells.
We next performed experiments to determine the localization of TIA-1 and TIAR in different cell lines infected with HSV-1. We used two approaches, an analysis by Western blotting of TIA-1 expression in nuclear and cytoplasmic extracts of infected cells and a confocal analysis after immunofluorescence staining using an antibody recognizing both TIA-1 and TIAR.
First, HeLa, HEp-2, and SK-N-SH cells were mock infected or exposed to 10 PFU of wild-type HSV-1 per cell. The cells were collected 6, 12, and 18 h after infection and were fractionated into nuclear and cytoplasmic extracts. Equal amounts of proteins were subjected to electrophoresis in a denaturing gel, transferred to a nitrocellulose sheet, and incubated with antibodies directed against TIA-1. The results, shown in Fig. 3D, were as follows. As expected, TIA-1 was found predominantly in the nuclear fraction of uninfected HeLa, HEp-2, or SK-N-SH cells. While the cytoplasmic fractions of mock-infected HeLa, HEp-2, or SK-N-SH cells harvested 6, 12, or 18 h after mock infection contained only small amounts of TIA-1, the cytoplasmic fraction of cells harvested 6, 12, or 18 h after HSV-1 infection formed two specific TIA-1 bands.
It should be stressed that the total amount of TIA-1 in the whole-cell lysate of mock-infected SK-N-SH cells could not be differentiated from that in infected cells at any time points tested (Fig. 3C). Therefore, the level of TIA-1 seems to be constant during the infection, and the increase in TIA-1 in the cytoplasm observed concomitant with a decrease in the amount of protein in the nucleus fraction reflects a translocation of TIA-1 from the nucleus to the cytoplasm rather than new synthesis of the protein.
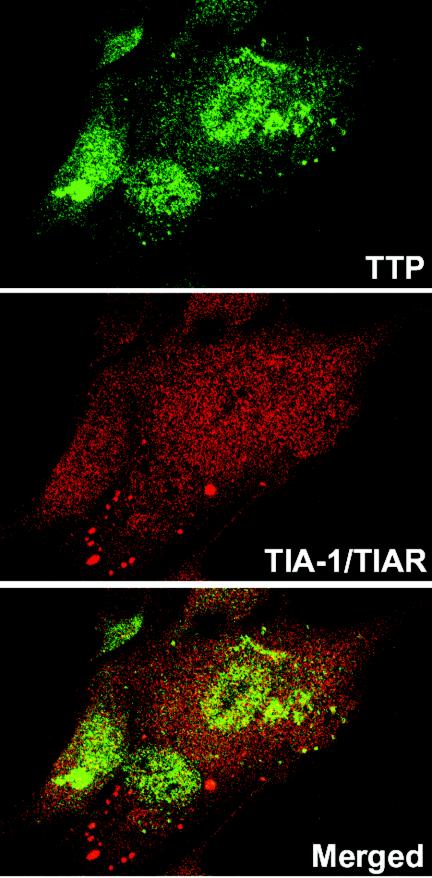
The localization of TIA-1/TIAR was also studied by confocal microscopy. HFF and HEp-2 cells were infected with HSV-1(F) or the Δα4 or ΔUL41 mutant virus as described above and were incubated with an antibody raised against TIA-1/TIAR. The results (Fig. 4i to l and 5m to r) were as follows. In mock-infected HEp-2 cells, TIA-1 and TIAR were predominantly localized in nuclei (Fig. 5i). HEp-2 cells were also treated with arsenite (0.5 mM, 60 min) before being processed for immunofluorescence microscopy. As previously described, arsenite-induced oxidative stress results in the accumulation of TIA-1/TIAR at SGs (Fig. 5i, insert). Twelve hours after infection with HSV-1(F), TIA-1 was partially relocalized in the cytoplasm as a diffuse staining (Fig. 5j). No SGs were visible. The TIA-1 and TIAR proteins were localized essentially in the nuclei of HEp-2 cells infected with the Δα4 mutant virus (Fig. 5k) or the ΔUL41 mutant virus (Fig. 5l). It is noteworthy that a portion of the cells infected with the ΔUL41 mutant virus presented an accumulation of TIA-1/TIAR at granular structures that were reminiscent of SGs. We observed a similar distribution of TIA-1/TIAR in infected fibroblasts. Thus, large amounts of TIA-1/TIAR were concentrated in the nuclei of mock-infected HFFs (Fig. 5m). Again, after HSV-1(F) infection, the TIA-1 protein was present in the cytoplasm as a progressive diffuse staining starting 6 h after infection (Fig. 5n to p). After Δα4 mutant virus infection, the amounts of translocated TIA-1/TIAR were much lower than those in wild-type virus-infected cells (Fig. 5q). The localization of TIA-1 and TIAR proteins detected in HFFs infected with the ΔUL41 mutant virus (Fig. 5r) was different from that observed in cells infected with wild-type HSV-1. A clear portion of the cells showed an accumulation of TIA-1/TIAR in the cytoplasm in granular structures that resembled SGs in size and shape (Fig. 5r). This accumulation was observed as early as 6 h after infection (data not shown). To investigate this distribution further, we incubated ΔUL41 virus-infected cells fixed 12 h after infection with both TTP and TIA-1/TIAR antibodies. An examination of these cultures showed that the TIA-1/TIAR aggregated structures did not colocalized with the granular aggregations of TTP. Extensive studies of these cultures led us to conclude that they were mutually exclusive: cells containing cytoplasmic granular structures of TIA-1/TIAR did not contain cytoplasmic TTP (Fig. 6).
FIG. 6.
Lack of colocalization of induced TTP and aggregated TIA-1/TIAR in ΔUL41 virus-infected cells. HFFs were infected with 10 PFU of ΔUL41 virus per cell, incubated for 12 h, fixed, and then incubated with a rabbit anti-TTP antibody detected with a FITC-labeled secondary antibody (green) and with a mouse anti-TIA-1/TIAR antibody detected with an Alexa fluor 594-labeled secondary antibody (red).
These studies showed that TIA-1 and TIAR accumulate in the cytoplasm of several cell lines infected with HSV-1. It is important to stress that in both HFF and HEp-2 cells infected with wild-type HSV-1, the cytoplasmic TIA-1/TIAR proteins did not form granular structures characteristic of SGs.
Vhs protein physically interacts with TTP.
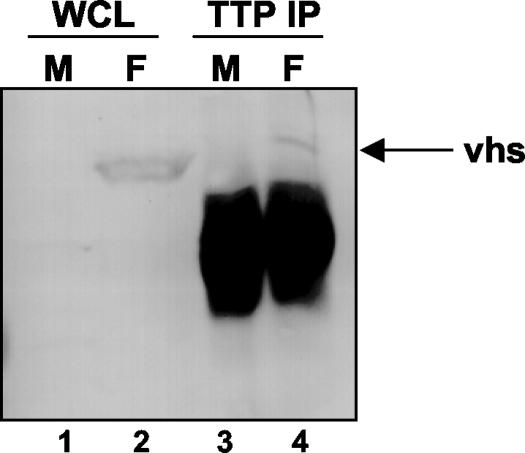
To analyze the potential physical interaction between Vhs and TTP, we performed an immunoprecipitation of TTP from SK-N-SH cell lysates prepared either from mock-infected cells or from cells infected with HSV-1(F). After 16 h of infection at 37°C, the cells were harvested and lysed. The lysate was immunoprecipitated overnight at 4°C with a polyclonal antibody raised against TTP. The precipitate was electrophoretically separated in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and incubated with an anti-Vhs antibody. As shown in Fig. 7, lane 4, the TTP antibody precipitated a fraction of the Vhs present in infected cell lysates. The relatively poor efficiency of the immunoprecipitation of Vhs by the TTP antibody could be related to the small amount of TTP present in the cells at late times after infection, as observed in the immunoblots shown in Fig. 3. These results suggest that Vhs and TTP reside in the same subcellular compartment and indicate a possible mechanism for Vhs-dependent targeting of ARE-containing RNAs.
FIG. 7.
Vhs interacts with TTP in cells infected by HSV-1. SK-N-SH cells were mock infected (lanes 1 and 3) or infected with HSV-1(F) (lanes 2 and 4). Sixteen hours after infection, the cells were harvested, solubilized, immunoprecipitated with a goat polyclonal anti-TTP serum, eluted from protein A-Sepharose, electrophoretically separated in an SDS-10% polyacrylamide gel, transferred to a nitrocellulose sheet, and incubated with a specific antibody directed to the Vhs protein (lanes 3 and 4). The whole-cell lysate was loaded as a control (lanes 1 and 2).
DISCUSSION
In earlier reports, members of our laboratory showed that several upregulated ARE-containing RNAs are degraded in a Vhs-dependent manner by deadenylation and 3′-to-5′ degradation, a process similar to that which turns over ARE-containing RNAs in uninfected cells (15, 42). In the course of a search for stable RNAs, we noted that TTP RNA was upregulated after HSV-1 infection. In light of the role of TTP in the turnover of ARE-containing RNAs, we were interested in examining infected cells with respect to the status of TTP, TIA-1, and TIAR, the last two of which are related proteins involved in the destabilization of ARE-containing RNAs. The salient features of this report are as follows.
(i) We have validated by several methods the upregulation of TTP RNA in at least two cell lines infected with wild-type HSV-1. It is interesting that the amounts of TTP RNA detected in these studies were higher in wild-type virus-infected cells than in cells infected with a ΔUL41 mutant.
(ii) We have shown that TTP is synthesized and accumulates in cytoplasmic granular material during HSV-1 infection. The cytoplasmic accumulation of TTP was detected in HFFs at the earliest time point tested, that is, 2 h after infection. The levels of TTP increased with time after infection and were detected even at late times. As in the case of TTP RNA, the amounts of this protein that accumulated in the cytoplasm were lower in ΔUL41-infected cells that in wild-type virus-infected cells. The localization of TTP in HFFs and HEp-2 cells was not modified by infection with a Δα4 mutant virus.
(iii) An antibody against TTP pulled down the Vhs protein from lysates of SK-N-SH cells harvested 16 h after infection. The amounts of Vhs protein were small The results may reflect the distribution and state of Vhs at late times after infection.
(iv) In mock-infected cells, TIA-1 was present primarily in the nuclear fraction of the cell extract. In infected cells, TIA-1 translocated into the cytoplasm as early as 6 h after infection in the three cell lines tested. The total amount of TIA-1 was constant during the time course of infection, but TIA-1 accumulated progressively in the cytoplasm. These results correlated with confocal microscopy observations. In uninfected cells, TIA-1 and TIAR were readily detected in the nuclei. In infected cells, they were detected 6 h after infection in the form of diffused, cytoplasmic fluorescence. In contrast, TIA-1/TIAR was not detected in the cytoplasm of cells infected with the Δα4 mutant and formed granular aggregates in a significant fraction of ΔUL41-infected cells. TIA-1 is known to accumulate in SGs, and the appearance of the granular material seen in the cytoplasm of ΔUL41 virus-infected cells was similar to that observed after arsenite-induced oxidative stress and to that reported elsewhere (3). In the experiments performed to date, TIA-1 did not physically interact with the Vhs protein.
The following observations are relevant to these studies. (i) The degradation of ARE-containing mRNAs is one of the means by which HSV-1 curtails the ability of the host to respond to viral entry and the expression of viral genes in infected cells. The evolution of a specific HSV pathway for the degradation of ARE-containing RNAs may reflect the fact that these RNAs frequently encode cytokines that signal the presence of an infected cell to the immune system. The mechanisms by which HSV-1 induces the transcription and activation of TTP and the translocation of TIA-1/TIAR remain to be elucidated. It should be noted that TTP is induced by numerous diverse proteins, including insulin and transforming growth factor beta (TGF-β) (33, 45). In cells exposed to cytokines such as TGF-β, TTP activation is rapid and transient within the first 2 h (33), while the upregulation of TTP after HSV-1 infection starts in the first hours of the infection but stays constantly induced for several hours. Microarray analyses suggested that TGF-β mRNA is upregulated in HSV-1-infected cells (41), and in principle we cannot exclude the possibility that a cytokine that is overexpressed in infected cells induces TTP. We should note, however, that the Δα4 mutant virus used in these studies was also made during the course of productive infection in complementing Vero cells and that this mutant did not induce TTP. We exclude the role of virion-associated Vhs in the upregulation of TTP since Vhs would be expected to be present in cells infected with the Δα4 mutant virus.
(ii) While the available data do not support the hypothesis that Vhs induces the synthesis of TTP, the results of our studies indicate that TTP does not accumulate in cells infected with the ΔUL41 mutant. One hypothesis that could explain these observations is that the Vhs protein forms complexes and stabilizes TTP. According to this model, TTP would be transiently induced early in infection but would accumulate only in cells expressing the Vhs protein.
(iii) In HSV-1-infected cells, the degradation of ARE-containing RNAs is Vhs dependent. The observed differences between ΔUL41 mutant and wild-type virus-infected cells are the absence of TTP accumulation and the formation of TIA-1/TIAR-containing SGs in ΔUL41 virus-infected cells. Another important difference is related to the degradation of ARE-containing RNAs in infected and stressed, uninfected cells. As noted in detail elsewhere, partially degraded 5′ domains of ARE-containing RNAs linger in infected cells but not in uninfected cells. We have no data that support a particular role of the Vhs protein in the turnover of cellular RNAs. TTP has been reported to recruit ARE-containing RNAs to the exosome for degradation (10). One model that is consistent with our observations is that the Vhs protein stabilizes TTP but displaces from the exosome a partner of TTP whose normal function is efficient processive RNA degradation.
(iv) The role of TIA-1 and TIAR in the degradation of ARE-containing RNAs remains to be elucidated since at least one, and possibly both, of the proteins is activated in ΔUL41 mutant-infected cells. The observation that the TIA-1 and TIAR proteins form SGs in ΔUL41 mutant-infected cells, but not in wild-type virus-infected cells, suggests that these proteins form different interactions or are in different complexes in these cells. It should be noted that both TIA-1 and TIAR have been found to repress the translation of ARE-containing RNAs (2). This translational silencing can occur even without visible SGs, since both TIA-1 and TIAR repress the translation of TNF-α in the absence of exogenous stress by promoting the assembly of nonpolyribosomal mRNP complexes (20, 25, 34). At this time, no conclusions can be drawn from the differential accumulation of SGs in ΔUL41 mutant-infected cells.
(v) The selective degradation of ARE-containing RNAs mediated by Vhs does not fully explain the global Vhs-dependent shutoff of protein synthesis in infected cells. Consistent with other viral proteins, Vhs may have more than one function, and in this instance, more than one way in which it regulates protein synthesis early in the course of viral infection. One unresolved question, for example, is whether the reported interaction of Vhs with eIF-4H (17) is a component of the RNA degradation reported here or a totally different pathway of RNA degradation.
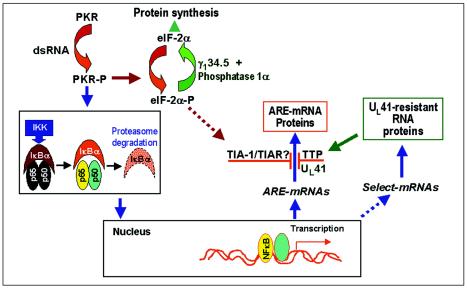
(vi) Finally, we wanted to review the synthesis and degradation of ARE-containing RNAs in the context of the viral gene functions illustrated in Fig. 8. As noted in this figure, HSV-1 replication results in the activation of protein kinase R (PKR) (12). As reported elsewhere, in HSV-1-infected cells the activation of PKR is required for the activation of NF-κB (43). The synthesis of ARE-containing RNAs that have been shown to be upregulated in infected cells (e.g., IEX-1 [4] and IκBα [22]) is NF-κB dependent, and they are degraded in a Vhs-dependent fashion. A correlate of the Vhs-dependent degradation of ARE-containing RNAs is the activation of TTP. Although we do not know the role of TIA-1/TIAR, it has been reported that these proteins are activated by the phosphorylated form of the α subunit of the translation initiation factor 2 (eIF-2α), a product of activated PKR.
FIG. 8.
Schematic representation of a proposed model of NF-κB-dependent RNA synthesis and Vhs-dependent selective degradation in cells infected with HSV-1. Studies reported in this and earlier reports showed that PKR is activated in wild-type virus-infected cells and that in cells infected with HSV-1, NF-κB is translocated into the nucleus by activated PKR through the activation of IKK and the degradation of IκBα. A consequence of activated NF-κB is the upregulation of numerous cellular genes, resulting in the synthesis of both ARE-rich and non-ARE-containing RNAs. ARE-containing RNAs are degraded in a Vhs-dependent manner, and their protein products are either not made or accumulate only transiently in HSV-1-infected cells, whereas RNAs lacking AREs are translated. The degradation of ARE-containing RNAs correlates with the induction and translocation of TTP to the cytoplasm of wild-type virus-infected cells, and to a lesser extent, to that of ΔUL41 virus-infected cells, whereas TIA-1 and TIAR appear to be activated in both wild-type and mutant virus-infected cells. TTP and TIA-1/TIAR represent families of proteins that bind and target ARE-containing mRNAs for degradation. As indicated schematically, it has been reported that the activation and translocation of TIA-1 and TIAR are mediated by the phosphorylation of eIF-2α. Even though eIF-2α-P is efficiently dephosphorylated by the γ1-34.5-phosphatase 1α complex, sufficient eIF-2α-P may be present to activate TIA-1/TIAR.
Numerous studies over the past decade have shown that the fundamental strategy by which HSV-1 takes total control of the host is the binding and subversion of cellular proteins for its own needs. Examples include the recruitment of phosphatase 1α to dephosphorylate eIF-2α and the recruitment of the ubiquitin-conjugating enzyme UbcH5A to degrade the PML protein and forestall exogenous interferon from interfering with viral replication (9, 19, 21). In some instances, the virtues of a specific strategy remain obscure, for example, the choice of dephosphorylating eIF-2α as opposed to blocking the activation of PKR with the US11 protein made early in infection. Since the protein products of the ARE-containing RNAs examined to date do not accumulate, the question arises whether the end products of this complex scheme evolved by HSV-1 during the course of its evolution are designed to produce NF-κB-dependent cellular proteins whose mRNAs lack AREs in their 3′ untranslated domains. The results presented in this and earlier reports (13, 39) suggest that HSV-1 has evolved both the machinery necessary to induce the transcription of a class of cellular genes and the mechanism by which it can block undesirable genes from being expressed.
Acknowledgments
We thank Beatrice Fineschi and the Biological Sciences Collegiate Divisions for making available the real-time PCR equipment, D. W. Wilson for the gift of the Vhs antibody, and P. Anderson for the gift of the 3E6 murine antibody against TIA-1/TIAR.
This study was aided by grants from the National Cancer Institute (CA78766, CA71933, CA83939, CA87661, and CA88860) and the United States Public Health Service. A.E. is a recipient of a fellowship from the Philippe Foundation.
REFERENCES
- 1.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. E2F proteins are posttranslationally modified concomitantly with a reduction in nuclear binding activity in cells infected with herpes simplex virus 1. J. Virol. 74:7842-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, P., and N. Kedersha. 2002. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlt, A., O. Grobe, A. Sieke, M. L. Kruse, U. R. Folsch, W. E. Schmidt, and H. Schafer. 2001. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in HeLa cells. Oncogene 20:69-76. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua, A., M. C. Ceriani, S. Capaccioli, and A. Nicolin. 2003. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J. Cell Physiol. 195:356-372. [DOI] [PubMed] [Google Scholar]
- 6.Blackshear, P. J. 2002. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 30:945-952. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carballo, E., W. S. Lai, and P. J. Blackshear. 1998. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science 281:1001-1005. [DOI] [PubMed] [Google Scholar]
- 9.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, C. Y., R. Gherzi, S. E. Ong, E. L. Chan, R. Raijmakers, G. J. Pruijn, G. Stoecklin, C. Moroni, M. Mann, and M. Karin. 2001. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 107:451-464. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. Y., N. Xu, and A. B. Shyu. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 14.Ellison, K. S., S. A. Rice, R. Verity, and J. R. Smiley. 2000. Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J. Virol. 74:7307-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 101:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairhurst, A. M., J. E. Connolly, K. A. Hintz, N. J. Goulding, A. J. Rassias, M. P. Yeager, W. Rigby, and P. K. Wallace. 2003. Regulation and localization of endogenous human tristetraprolin. Arthritis Res. Ther. 5:R214-R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, M., C. J. Wilusz, S. W. Peltz, and J. Wilusz. 2001. A novel mRNA-decapping activity in HeLa cytoplasmic extracts is regulated by AU-rich elements. EMBO J. 20:1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gueydan, C., L. Droogmans, P. Chalon, G. Huez, D. Caput, and V. Kruys. 1999. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 274:2322-2326. [DOI] [PubMed] [Google Scholar]
- 21.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrdlickova, R., J. Nehyba, A. Roy, E. H. Humphries, and H. R. Bose, Jr. 1995. The relocalization of v-Rel from the nucleus to the cytoplasm coincides with induction of expression of IκBα and NF-κB1 and stabilization of IκBα. J. Virol. 69:403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami, A., Q. Tian, M. Streuli, M. Poe, S. Edelhoff, C. M. Disteche, and P. Anderson. 1994. Intron-exon organization and chromosomal localization of the human TIA-1 gene. J. Immunol. 152:4937-4945. [PubMed] [Google Scholar]
- 25.Kedersha, N., and P. Anderson. 2002. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30:963-969. [DOI] [PubMed] [Google Scholar]
- 26.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khodarev, N. N., S. J. Advani, N. Gupta, B. Roizman, and R. R. Weichselbaum. 1999. Accumulation of specific RNAs encoding transcriptional factors and stress response proteins against a background of severe depletion of cellular RNAs in cells infected with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 96:12062-12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai, W. S., E. A. Kennington, and P. J. Blackshear. 2003. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol. Cell. Biol. 23:3798-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai, W. S., D. J. Stumpo, and P. J. Blackshear. 1990. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J. Biol. Chem. 265:16556-16563. [PubMed] [Google Scholar]
- 32.Lee, G. E., G. A. Church, and D. W. Wilson. 2003. A subpopulation of tegument protein Vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells. J. Virol. 77:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa, K., F. Chen, Y. J. Kim, and Y. Chen. 2003. Transcriptional regulation of tristetraprolin by transforming growth factor-beta in human T cells. J. Biol. Chem. 278:30373-30381. [DOI] [PubMed] [Google Scholar]
- 34.Piecyk, M., S. Wax, A. R. Beck, N. Kedersha, M. Gupta, B. Maritim, S. Chen, C. Gueydan, V. Kruys, M. Streuli, and P. Anderson. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 19:4154-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon, A. P., and B. Roizman. 1997. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant gamma (1)34.5 genes of herpes simplex virus 1. Virology 229:98-105. [DOI] [PubMed] [Google Scholar]
- 36.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawaoka, H., D. A. Dixon, J. A. Oates, and O. Boutaud. 2003. Tristetraprolin binds to the 3′-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J. Biol. Chem. 278:13928-13935. [DOI] [PubMed] [Google Scholar]
- 38.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoecklin, G., B. Gross, X. F. Ming, and C. Moroni. 2003. A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA. Oncogene 22:3554-3561. [DOI] [PubMed] [Google Scholar]
- 41.Taddeo, B., A. Esclatine, and B. Roizman. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc. Natl. Acad. Sci. USA 99:17031-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 77:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taddeo, B., T. R. Luo, W. Zhang, and B. Roizman. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA 100:12408-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taupin, J. L., Q. Tian, N. Kedersha, M. Robertson, and P. Anderson. 1995. The RNA-binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas-mediated apoptotic cell death. Proc. Natl. Acad. Sci. USA 92:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, G. A., M. J. Thompson, W. S. Lai, and P. J. Blackshear. 1996. Mitogens stimulate the rapid nuclear to cytosolic translocation of tristetraprolin, a potential zinc-finger transcription factor. Mol. Endocrinol. 10:140-146. [DOI] [PubMed] [Google Scholar]
- 46.Worthington, M. T., J. W. Pelo, M. A. Sachedina, J. L. Applegate, K. O. Arseneau, and T. T. Pizarro. 2002. RNA binding properties of the AU-rich element-binding recombinant Nup475/TIS11/tristetraprolin protein. J. Biol. Chem. 277:48558-48564. [DOI] [PubMed] [Google Scholar]