Abstract
Mass aggregations and migrations of millipedes despite numerous attempts to find causes for their occurrences are still an enigma. They have been reported from both southern and northern hemisphere countries, from highlands and lowlands of both tropical and temperate regions and they can involve species belonging to the orders Julida and Spirobolida, Polydesmida and Glomerida. According to the main suggestions put forward in the past, mass occurrences in Diplopoda occur: (1) because of a lack of food and a population increase beyond sustainable levels; (2) for the purpose of reproduction and in order to locate suitable oviposition sites; (3) to find overwintering or aestivation sites; (4) because of habitat disruption and changes in the local environment; (5) as a consequence of weather conditions the year (or winter and spring) before. A recent outbreak (November 2014) of a mass migration of the polydesmid Chamberlinius hualienensis Wang 1956 on the Japanese Izu Island of Hachijojima 300 km to the south of Tokyo gave this author an opportunity to review the existing literature on millipede mass migrations and to carry out additional observations on the phenomenon in the field as well as the laboratory. Hitherto unreported heavy infestations with phoretic deutonymphs of the mite Histiostoma sp. as well as dense populations of internal rhabditid nematodes (Oscheius cf. necromena and an unidentified species of the genus Fictor), suggest that infestations of this kind could be necromenic and either have been a contributing factor for the mass migration or been a consequence of so many individuals occurring together at close proximity. It is concluded that mass migrations and aggregations in millipedes do not have one common cause, but represent phenomena that often are seasonally recurring events and appear identical in their outcome, but which have evolved as responses to different causes in different millipede taxa and therefore need to be examined on a case-to-case basis.
Keywords: Myriapoda, Spawning migration, Aggregation behaviour, Diplopod commensals and parasites
INTRODUCTION
Mass aggregations of millipedes are not a recent phenomenon (Hopkin & Read, 1992). They have in fact been reported as far back as 1878 (Tömösváry, 1878 cited in Voigtländer, 2005; Paszlavszky 1879) and possibly even earlier (Verhulst, 1845 cited in Zimmermann, 2013). They are known from countries of the southern hemisphere (Australia: Baker, 1978; Anonymous, 2013; Brazil: Boccardo et al, 1997, 2002; Fontanetti et al, 2010; South Africa: Lawrence, 1952; Robinson, 2005; Madagascar: Wesener & Schütte, 2010) and have been reported from numerous countries in the northern hemisphere, e.g., the USA (Brooks, 1919; Cook, 1924; Morse, 1903; Viosca, 1925), the UK (Chater, 2004; Ormerod, 1890; Scott, 1958a, b), France (Sahli, 1996;Verhoeff, 1900), Germany (reviewed by Voigtländer, 2005), Poland (reviewed by Kania & Tracz, 2005), Austria (Anonymous, 2006; Thaler, 1989; Zimmermann, 2013), Hungary (Korsós, 1998; Paszlavszky, 1879), Romania (Tömösváry, 1878; Ceuca, 1984, cited in Voigtländer, 2005), Northern Yugoslavia (Ćurić & Makarov, 1995), Latvia (Becker, 1929), Norway (Meidell & Simonsen, 1985), Sweden (Lindgren, 1942) and Japan (Esaki, 1934; Niijima, 1998; Niijima & Shinohara, 1988).
Outbreaks have occurred in tropical climates like those of India (Mitra, 1976) and Brazil (Fontanetti et al, 2010), as well as in temperate and even Nordic climes, e.g. Norway (Meidell & Simonsen, 1985) and Sweden (Lindgren, 1942). Millipede mass aggregations are known from low altitude regions (Ehrnsberger, 2002), but equally so from mountainous or hilly areas, e.g., Japan (Hagiwara & Kuwabara, 2008; Niijima & Shinohara, 1988), Switzerland (Anonymous, 2011) and Austria (see first paragraph). Although in most of the above-mentioned cases certain species, e.g., Ommatoiulus sabulosus in Europe and the Parafontaria laminata group in Japan were over-represented, numerous other species were sometimes found to be involved and a list of millipedes in relation to their geographic distribution demonstrates the spread of taxa in connection with the phenomenon of mass migrations.
Juliformia and spirobolid species are the most important and common ones in connection with millipede mass aggregations. Voigtländer (2005) lists Ommatoiulus sabulosus (L), Cylindroiulus caeruleocinctus (Wood), Julus scandinavius (Latzel), J. scanicus (Lohmander) and Ophyiulus pilosus (Newport) as species known from mass occurrences in Germany and in addition Megaphyllum projectum kochi (Verhoeff), M. unilineatum (C.L. Koch), J. terrestris (Porat), Tachypodoiulus niger (Leach), Unciger foetidus (C.L. Koch) and Cylindroiulus londinensis (Leach) as species elsewhere in Europe linked to mass aggregations.
The spirobolid Strongylosoma stigmatosum (Eichwald) has been reported by Ceuca (1984) from Romania, while Parajulus pennsylvanicus was reported from New England by Morse (1903). Streptogonopus phipsoni (Pockcok) and Gymnostreptus pyrrhocephalus (C.L. Koch) are mentioned by Bellairs et al (1967) and Lawrence (1952) in connection with mass aggregations in India and South Africa, respectively. The Iberian species Ommatoiulus moreleti (Lucas) has been known to swarm in South and West Australia (Anonymous, 2013; McKillup et al, 1988), while Urostreptus atrobrunneus (Pierozzi & Fontanetti) is known from Brazilian aggregations (Fontanetti et al, 2010), Spirostreptus sp. from outbreaks in New Mexico (Cook, 1924; Thuringer, 1924) and Spirobolus marginatus (Say) from a mass occurrence in the New Orleans area near Lake Ponchartrain (Viosca, 1925).
Certain species belonging to the polydesmid group of millipedes are also known to swarm and to form mass aggregations, e.g., according to Esaki (1934) Brachydesmus superus (Latzel) in England, Fontaria virginiensis (Gray) and F. brunnea (Bollman) in the USA. Robinson (2005) furthermore mentions Pseudopolydesmus serratus (Say) from Ohio and Bellairs et al (1967) report swarming in Streptogonopus phipsoni (Pocock), while in Central Japan according to Niijima and Shinohara (1988) various subspecies of the Parafontaria laminata group have repeatedly been involved. The Taiwanese polydesmid Chamberlinius hualienensis (Wang, 1956) is not known to swarm in Taiwan, but in some places of Japan, notably the island of Hachijojima (Fujiyama et al, 2012 and this paper) this invasive species does and it then forms huge clusters of several thousands of individuals.
Glomerids are the last group of the Diplopoda known to possess species with a propensity to migrate and to form mass aggregations. From Poland mass occurrences of Glomeris hexasticha (Brandt) and from Madagascar those of Zoosphaerium neptunus (Butler) have been reported by Kania & Tracz (2005) and Wesener & Schütte (2010), respectively. One Australian bristle millipede, the polyxenid Unixenus mjoebergi (Verhoeff) is credited by Robinson (2005), who, however, uses the incorrect name U. nijobergi, to migrate after rainfall according to Koch (1985).
Numerous attempts have been made to find an explanation, i.e., causes and reasons, for the various mass migrations and aggregations amongst millipedes. And the most common suggestions, here briefly summarized on the basis of the works of Brade-Birks (1922), Cloudsley-Thompson (1949) and the publications mentioned above, are (a) population increases that lead to a lack of and consequently search for food; (b) migrations linked to reproduction and attempts to find oviposition sites; (c) the search of animals to locate suitable places to spend the winter or to aestivate during the summer; (d) a need to find wetter or drier habitats, in other words responses to adverse environmental conditions; (e) past weather conditions like mild previous winters in combination with warm springs. Attempts to correlate mass migrations with habitat changes, communal protection against predation, soil conditions and dominating plant communities have also been made.
However, as will be shown point by point in the Discussion, there is not a single suggestion, which is applicable to all of the observed mass migrations and aggregations amongst millipedes. In some instances the phenomenon occurs in predictable cycles and at identical seasons year after year, but in others there is no apparent repetitiveness at all and the phenomenon may occur in areas it has never been seen before and is never seen thereafter again. Given this state of affairs, the recent mass migration of the polydesmid Chamberlinius hualienensis on the island of Hachijojima provided an opportunity to test some of the suggestions that had earlier been offered as likely explanations for millipede mass occurrences.
The polydesmid Chamberlinius hualinenensis is a herbivorous species native to Taiwan (Wang, 1956). In Japan it was first noticed in the autumn of 1983 on the island of Okinawa. From there it reached the major Japanese island of Kyushu in 1999 and soon thereafter Honshu, causing minor outbreaks of mass swarming in the years 2009 and 2012 (Fujiyama et al, 2012). The exact date that the species arrived on the small Izu Island of Hachijojima, 300 km to the south of Tokyo, is not known, but the year 2002 is often mentioned by the locals as the first time they noticed this millipede.
As an invasive and alien species on the subtropical island of Hachijojima with its lack of predators, populations of the species increased rapidly and the locals noticed year after year greater and greater numbers of this millipede and increasingly more substantial mass migrations principally between the months of October to early December. In the autumn of 2014 the largest mass occurrence of Chamberlinius hualienensis to date was seen on the island. It is that event which formed the basis for the investigations reported in this paper.
MATERIAL AND METHODS
On two sites, located at approx. N33°05’ and E139°47’ on the West Coast of the southern half of the Izu island Hachijojima 300 km to the South of Tokyo in the Pacific (Figure 1), mass aggregations of the introduced herbivorous Taiwanese polydesmid millipede Chamberlinius hualienensis Wang 1956 were observed and studied between 15-11-2014 and 24-12-2014.
Figure 1.

Area of mass outbreak (Seen from the west with freeway viaduct in the centre and mountains on the far side (to the east) of the road; the tunnel entrance is to the left)
Facing west to southwest, Site I consisted of a curved approx. 4 m high vertical wall made of concrete with rough surface texture and some parts painted dark brownred and others left cream-colored. The wall marked the entrance to a covered freeway viaduct. Parts of the viaduct support structure facing north and east and painted entirely brownred were also monitored, but not designated as separate sites on account of their close proximities to one another.
Site II, ca. 800 m to the south of Site I and facing north, consisted of an approx. 8 m high vertical cream-coloured concrete, 5.5 m×4.5 m wide support pylon with rough surface texture, on which the motorway rested. The two sites were accessible on foot via an old disused and overgrown narrow road that ran in a north-south direction more or less parallel to the freeway which was opened to traffic in the year 2000. Alongside the small road, there was a steep drop towards the oceanside to the West and a steep rise towards the island’s interior mountains on the East. The slopes on either side of the road were thickly covered by green vegetation that consisted of a rich variety of mixed shrubs, bamboo, native and introduced trees, vines and weeds. Decaying leaf and other plant matter was plentiful and present all over the ground, the latter being basically volcanic in origin.
Daily climatic conditions for the period of observation with information on precipitation, temperatures and humidity were available from the website of the Japanese Meteorological Agency (2014, http: //www.data.jma.go.jp). To check the genders of the individuals in the aggregations or in the migrating swarms, specimens were collected in the field during the day (ca. 1400h) and at night (ca. 2000h) and examined under a binocular microscope at ×20 magnification in the lab. Mites and nematodes, collected from male as well as female specimens, were preserved in 70% ethanol and sent to labs outside Hachijojima (see acknowledgments) for further processing. Observations on biorhythmicity and photoreception were carried out in temperature and humidity controlled climate chambers of the institute set at 20 °C and 75% relative humidity.
RESULTS
Weather conditions immediately prior to and during the outbreak
The weather pattern for the month of November prior to when the mass occurrence of Chamberlinius hualienensis was first noticed on November 15th did not exhibit any unusual or extreme feature except for a very heavy downpour on the 5th with 104 mm recorded for that day alone. However, heavy showers are not rare on Hachijojima, which has an annual precipitation in access of 3 000 mm. On the 11th of November rainfall also exceeded 35 mm, but did not reach the level of November 5th. Nighttime temperature on the two days before the November 5th downpour had dropped from the more usual 18 °C to 15.8 °C on November 3rd and 13.8 °C on November 4th. Thereafter nighttime temperatures averaged again 17.8 °C until November 13th, when they dropped to 13.4 °C and remained around 12-13 °C for the next 10 days after which they climbed again to an average of 15 °C until December 1st. Then, however, day and nighttime temperatures fell rapidly reaching a minimum of 6.2 °C and 4.1 °C on December 18th. Towards December 24th, however, the day all observations ended, temperatures rose again by 3-5 °C. Heavy rainfall in December occurred only on days 1, 4 and 11.
Average relative humidity throughout the 2-week period prior to the start of the recognition of the outbreak on November 15th was always at least 70% and following rainy days even higher than 90%. A sudden drop to 58% occurred during the sunny spell from November 13th to November 16th. The remainder of November exhibited no unusual features with an average relative humidity of 80%, plenty of scattered rain, some sunny periods and gradually lower day and nighttime temperatures, which by early December reached 12 °C and 7 °C, respectively. The weather remained like this until virtually all millipedes, except for a handful of five or six isolated, but still living, stragglers had disappeared from the walls by December 24th.
Prior to November 13th between November 4th - 6th and 11th -13th there had been very strong winds from the north, east and south with gusts in excess of 10 m/sec. The two weeks thereafter winds eased and, averaging 4 m/sec over this period, first changed to blow from the west for a week and thereafter mostly from the southeast and occasionally from the northern but not western directions. Westerly winds occurred again on December 1st and continued to be dominant for the entire month of December.
Migratory activities and densities of Chamberlinius hualienensis individuals
Irrespective as to whether they were exposed to bright sunshine (Site I) or were in the shade almost all day (Site II), dense clusters of individuals were present on all of the walls monitored (Figure 2-4). It made apparently not much of a difference whether the walls were facing the west, southwest, east or north and whether they were lighter or darker in colour. At the height of the mass occurrence between November 15th-18th it was estimated that at Site I, there would be up to approx. 15 000 individuals/m2 (on the basis of counts of 400-600 individuals/20 cm2) on the cream-coloured wall facing west as well as the brownred wall facing east. The area occupied by clusters of millipedes on these walls reached from the ground to more than 3 m above and horizontally to between 3 and 4 m.
Figure 2.

Masses of the polydesmid millipede C. hualienensis on their way to aggregation Site 1
A rough estimate of the total number of millipedes gathered together just on the walls alone was 100 000. An equal or even larger number is thought to have perished, forming a layer on the ground several cm thick (Figure 5) and emitting a smell described by some pedestrians as that of burning plastic. Adding the number of individuals of Site II (see next paragraph) and considering the new daily arrivals at both sites, the total number of millipedes that had come together at both sites could easily have been a million or more.
Figure 5.

Thousands more on the ground are forming many centimetre-thick layers of exhausted and dying individuals
Site II, covered by the bridge overhead and mostly shadowed, also possessed an appreciable number of individuals, but distributed over a larger area they did not quite reach the densities seen at Site I. During the week in which winds from the west were absent (November 17th-25th) densities on the wall of Site I facing north increased significantly at the expense of the areas facing east, west and southwest. At the same time clusters at Site II thinned, but had re-grouped about 6 m above the ground. The snails present on these walls prior to the arrival of the millipedes stayed and apparently neither disturbed the millipedes nor suffered any harm from them.
It was obvious that the millipedes were able to ascend the vertical walls at any place, but that they preferentially used cracks or lines were slabs of concrete had been joined together. Small depressions, holes and crevices in the concrete seemed particularly attractive to them. Although many individuals in these clusters of animals that were moving around and climbed over others that were resting, were frequently paired (Figure 6) or even occurring as triplets with three individuals on top of each other (Figure 7), the majority were seemingly intent on being passive. At night, however, the situation was different. Approximately 15 minutes after sunset almost all of the animals, whether paired or not, became active. The huge aggregations broke up with smaller ones remaining, but the majority of the animals crawling around on the vertical wall did so apparently in no particular direction. At dawn the familiar aggregations of thousands of individuals were present again.
Figure 6.

Paired individuals were a common sight during the aggregation phase
Figure 7.

Sometimes triplet individuals were seen amongst the clustered animals
What was noticeable almost exclusively at night, were individuals that crawled from the surrounding bush along the old disused and overgrown road towards the aggregation Sites I and II. As if pulled by a magnet those as far away as approx. 400 m to the south of Site I approached that latter site and migrated upward in a northerly direction, but those further away and within 400 m of Site II migrated southward and down the sloping road toward their specific aggregation site. At night the road was teeming with crawling millipedes, seemingly single-mindedly heading into one direction only (either north or south) and following a route leading them to their respective aggregation sites.
Mechanically removed from their path by a metre or so, the displaced millipede returned to where their conspecifics were crawling. Using only one side of the road they did not swerve or take a detour when they encountered an obstacle in the form of a shallow puddle left over from a recent shower, but proceeded to crawl across it, even if in the process some drowned. In times of heavy rain, however, no migrations at all occurred. Illumination at night did not stop them, but some individuals did sometimes hesitate before continuing to crawl and some even tried to go around an approx 15 cm wide patch illuminated by the bright white light of a handheld torch.
The path to Site II was particularly intriguing, because in order to reach that site the millipedes first had to crawl downward for about 100 m from the somewhat brighter area into the darkness of the underside of the freeway viaduct and then upward again for the remaining 50 m in order to reach the concrete support pylon which they ascended to form clusters, even at a height of 6 m. Following the road to the pylon, the millipedes first had to head south, then for a few metres turn west and then approach their goal in a southwesterly to southern direction.
Speeds of around 1.6 cm/sec were recorded, which suggests that these millipedes can travel approx. 1 km per night. In case they hadn’t reached their destination by dawn, they sought shelter under stones along the roadside. When there were cockroaches under the stones, the millipedes frequently caused the latter to abandon their shelter or to aggregate in corners the millipedes could not or did not want to reach.
An unexpected “two-way-traffic”, resembling that which is often seen in ants, with slightly more millipedes heading downward and away from the aggregation Site I than towards it and thereby passing the new arrivals, was observed after a small rise in nighttime temperatures from an earlier 12 °C to around 15 °C on the nights of November 23rd - 26th (Figure 8). On which day these “return migrations” ceased was not recorded, but on November 27th only a small number of animals were still arriving, and both Sites I and II were far less populated with few individuals still clinging to the wall and its crevices. None were seen any longer heading in the opposite direction despite a relatively warm nighttime temperature of 14 °C. Given the huge number of animals that over at least 2 weeks had formed the massive aggregations described above, the total number of individuals that had headed back can only have been a trickle. The vast majority of the millipedes that had come together at Sites I and II perished there. And yet, that over several nights some animals returned at all is remarkable and so far has not been reported from any other millipede species.
Figure 8.

Two-way traffic with individuals from the right (arriving) and left (departing) passing each other
From November 29th and during the first week of December with prevailing westerly winds of approx 7 m/sec streams of millipedes, mostly from the northern wall of Site I, where there was still a very substantial cluster, were heading to the opposite side of the freeway. They got there by first crawling up to the ceiling of the covered viaduct and then, upside down, crossing the freeway along 4 m above the traffic with some falling down onto the road, but most reaching the other side. On the other side of the freeway they again formed aggregations, but preferentially on the northern side of a pylon (Figure 9).
Figure 9.

With winds predominantly from the west, dense aggregations began to form on the northern side and on top of the wall
In early December furthermore smaller clusters of around 600-1 000 individuals became established approximately 500 m to the North of Site I along the banister on the opposite side of the freeway above concrete support pylons 14 m off the ground. On December 6th walls of Site I facing west, southwest and east were nearly deserted with just a few isolated clusters of around 50 or so individuals hanging on, but a considerable number still present on the northern side and its flat horizontal top. Wall areas vacated by the millipedes once again became a habitat for geckos that hadn’t been seen there during the times of millipede mass aggregation, but were present there on December 11th and 18th.
Since hardly any millipedes were seen to migrate from Site I to the new clusters that had formed along the freeway’s banister ca. 400 m away and since the new clusters developed only on the banister above support pylons and inside holes and depressions nearby (Figures 10,11), the most likely way the millipedes could have got there was by ascending the 14 m high concrete support pylons on which the freeway rested at that point. A height like this climbed by millipedes would represent a new record.
Sizes and gender distributions
A total of 1, 645 adult C. hualienensis were sexed on 6 separate days in November. When samples taken during the day from Site I aggregations above 1 m were compared with those taken below 1 m the ratios between females and males were 240:205 and 148:137 on November 16th. For Site II the corresponding figures obtained on November 20th were 158:135 and 171:107. Under wet and drizzly conditions on November 22nd the female/male ratio at night of animals approaching Site II was 20: 4 with many individuals (not sexed) lying immobile and presumed dead in puddles.
The ratio between females and males migrating at night on November 26th to Sites I and II were 59:46 and 63:58, respectively. On the same day in bright sunshine at a temperature of 23 °C and moderately strong southerly wind (4.3 m/sec) some millipedes were seen to migrate towards the northern wall of Site I. The female/male ratio in these individuals was 17 : 18.
On November 23rd when millipedes at night were moving away from their aggregation sites passing others that were still arriving, female/male ratios of the departing and arriving animals at Site I were 14:10 and 9:4, respectively. At Site II only individuals still arriving and none leaving were seen and their female/male ratio was 11:11.
Total length measurements were carried out on 20 adult males and females pooled from both sites and killed by freezing. Length measurements on individuals preserved in this way are likely to deviate slightly from measurements of live and crawling individuals, which is why statistical analyses with standard deviations were considered meaningless in connection with the data. Unrolling the thawed and curled up corpses yielded average lengths of males and females of 35.6 mm (range 34-41 mm) and 36 mm (range 35-41 mm). Males were found to be slimmer, often softer and more frequently less opaque due to an empty digestive tube than the females.
Biorhythmicity and responses to light
There were always some individual C. hualienensis that were not at rest, but moving around on the aggregations that had formed on the walls of Sites I and II, even if exposed to bright midday sunshine. However, individuals migrating to or away from the aggregation sites were almost exclusively seen at night. Individuals hiding motionlessly under a stone or log during the day immediately became active as their shelters were taken away, while those that were on the move at night would sometimes stop or even deviate from the direction they were heading into when struck by the beam of some bright white torchlight.
When 40 individuals collected during the day at 1430h were divided into two groups of 20 and at 1500h individuals of one were put into a rectangular transparent plastic terrarium measuring 13 cm×15 cm×20 cm that contained shelters for the millipedes, while those of the other group were put into an identical terrarium at the same time, but in a darkroom with temperature and humidity as in the first group, individuals of the first group immediately found and rested in the hiding places, not appearing again until 15 minutes after sunset. They were then dispersing and freely crawling around the terrarium until dawn.
The animals that were placed into the darkroom, however, were active and moving around when briefly checked at 1600h and 1700h. The next morning at 0900h some 5 or 6 were still out in the open and crawling around, while the remainder were resting. Over the next three days the darkroom group settled to a biorhythm that saw them resting for most of the day, but starting their activity earlier than group one, which waited until sunset and it was getting dark. When they were then illuminated during the night they speedily sought shelters and waited in them motionlessly until the light was switched off again. Ten minutes later they were then crawling around again in the open as before.
Observations on interactions with other species of animals
When live C. hualienensis male as well as female individuals were placed in mild soapy water, hundreds of tiny nematodes, no longer than maximally 0.75 mm long and 0.01 mm thick appeared. Almost certainly residing inside (and not on) the millipedes, the nematodes were identified as members of the Rhabditida group. Light (Figure 12) and scanning electron micrographs (Figure 13) were taken of individuals that were sent in 70% ethanol to Prof. T. Hariyama’s lab of Hamamatsu Medical University and then further processed by Dr. Takaku according to a method recently described (Takaku et al, 2013). Specimens were also sent to Dr. Lynn Carta of the USDA-ARS Nematology Laboratory in the U.S., who confirmed that the nematodes were rhabditids and on the basis of ribosomal DNA markers could be identified as two species, namely Oscheius cf. necromena and a member of the genus Fictor. Their presence suggests that infestations of this kind could be necromenic and either have been an additional cause for the mass migration or been a consequence of so many individuals occurring together at close proximity. When and how they could have colonized individuals of C. hualienensis and to what extent they affected their host’s behaviour is not known, but members of the Rhabditida are known from other arthropods and also occur in decaying matter, where they feed on bacteria and other microorganisms.
Figure 12.
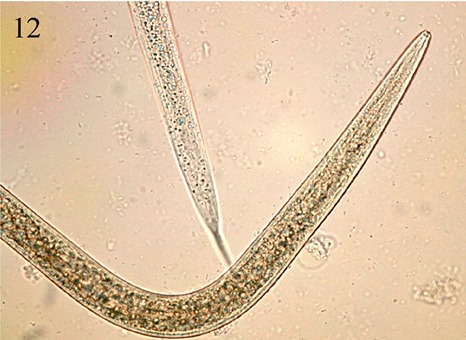
Light micrograph of rhabditid nematode from C. hualienensis (taken by Y. Takaku)
Figure 13.

Scanning electron micrograph of rhabditid nematode from C. hualienensis (taken by Y.Takaku)
Figure 3.

Clusters of C. hualienensis indiviuduals on the north-facing side of a wall, apparently making use of cracks and slight depressions between the blocks of concrete
Figure 4.

Up to approximately 15 000 individuals/m2 were assembled at the aggregation sites
Figure 10.

Cracks and depressions gave the millipedes some protection against the wind and rain (here the word ‘yoko’ in the wall facing east)
Figure 11.

Any hole or depression in the concrete serves the millipedes to hang on (here the word ‘ma’)
On the outer surfaces of C. hualienensis numerous individuals of loosely attached tiny mites, no bigger than 0.3.mm, were present (Figure 14). They were identified by Dr. M. Farfan of the University of Illinois at Chicago as deutonymphs most likely belonging to the genus Histiostoma and almost certainly phoretic rather than parasitic. As with the nematodes when and how the mites ‘boarded’ the millipedes and what effect, if any, they had on their host is still unknown.
Figure 14.

Light micrograph of deutonymph of Histiostoma sp. from the body of migratory C. hualienensis (prepared by H. Schikora)
Macroscopically no animals other than some inactive, presumably hibernating snails were observed amongst the mass aggregations. Once a bright green approximately 5 mm long caterpillar was seen crawling on a cluster of C. hualienensis, but an attempt to capture it failed as it dropped and disappeared amongst multiple layers of dead millipedes on the ground. Nearby on the wall, but obviously not at all interested in the millipedes were occasionally some geckos (Gekko hokouensis Pope) and praying mantises. Under stones in close proximity with the millipedes were cockroaches (Opisthoplatia orientalis Burmeister) of both genders and at various stages in their life cycle. It was obvious, however, that cockroaches and sheltering millipedes avoided each other.
No animal was ever seen to feed on this readily available prey, but millipedes drowned in the rain were approached by large terrestrial snails and partially consumed by them (Figure 15).
Figure 15.

During mild rain unidentified terrestrial snails were feeding on corpses of C. hualienensis.
DISCUSSION
The polydesmid Chamberlinius hualienensis is not the only species of millipede in Japan involved in swarming and forming mass aggregations. Various subspecies of the polydesmid Parafontaria laminata in the mountainous regions of Honshu at altitudes of around 1 000 m have had numerous outbreaks, listed by year, place and dominant local flora by Niijima & Shinohara (1988). Apart from the nuisance that so many animals create by invading dwellings and because of their unpleasant odour, they can also disrupt road and rail traffic as was first reported for Japan by Esaki (1934) after Verhoeff (1900) had already described a similar occurrence for France, albeit not of a migrating polydesmid but a iulid species of millipede. Members of the Julidae, so prominent in European outbreaks, do not feature in Japanese millipede mass aggregations, but apart from the polydesmid taxa mentioned above one other polydesmid family, namely the Strongylosomatidae may contain species in Japan that sometimes swarm (Esaki, 1934).
Possible reasons for swarming and mass aggregations
Comparison between Parafontaria laminata and Chamberlinius hualienensis
Clues as to why and when the two main migratory species groups in Japan swarm could possibly be gleaned from a comparison of the biologies of P. laminata and C. hualienensis, in the local vernacular known as ‘kishayasude and ‘yangbarutosakayasude’, respectively. According to Fujiyama et al (2012) the temperate and sub-Arctic P. laminata has an 8-year cycle with single age groups per year and moults occurring once every 12 months. The tropical C. hualienensis, however, develops much faster and its immature individuals moult monthly. While P. laminata with an optimal temperature range of 10-20 ºC and able to survive subzero temperatures has a narrow food spectrum and occurs predominantly in the forests of hilly or mountainous areas, C. hualienensis dies below 0 °C, is most active between 20-30 °C, has a wide food spectrum and inhabits a range of habitats including forests and cultivated fields of both hilly and lowland areas.
What the two taxa have in common in addition to their rather similar size and appearance are their dietary requirements of decaying plant matter and the number of eggs per cluster. However, while in their lifetime P. laminata females lay their 300-600 eggs within one month, which then enter a period of dormancy, C. hualienensis females mate numerous times over a period of four months and lay their eggs not all at once. Moreover, neither their eggs nor any of their larval stages enter a dormancy period. Higa et al (1992) have counted 70 clusters of 300-600 eggs laid by 100 C. hualienensis pairs over a period of 4 months, but how many eggs one female lays over its lifetime is not known. In P. laminata adults and a small number of sub-adults make up the migratory swarms, which occur mostly in September and October and occasionally even earlier (Niijima & Shinohara 1988), but in C. hualienensis the swarming period lasts mostly from early October to early December and overwhelmingly consists of adult individuals (Fujiyama et al, 2012).
Population pressure and lack of food
The question as to what triggers the migrations, not only of polydesmids in Japan, but millipedes in general, has led to a number of suggestions that will be examined one by one. Lack of food and a population increase beyond sustainable levels have frequently been mentioned and given the often staggeringly large numbers of millipedes involved in the aggregations seems a reasonable suggestion and could perhaps be a contributing factor in some species and situations, e.g., the enormous initial population increases affecting non-native, i.e., invasive species during the first few years after their arrival at a new location as witnessed by the Portuguese O. moreleti in Australia. The species C. hualienensis on Hachijojima island may still be in that initial unchecked population growth phase given that (a) natural predators like toads and some millipede-consuming birds are absent from the island and (b) millipedes generally excrete defensive cyanide derivatives, one of which in C. hualienensis has been identified as the toxic substance mandelonitrite (Noguchi et al, 1997).
However, visual inspection of the areas in which the outbreak of C. hualienensis occurred showed an abundance of rotting and decaying plant matter providing ample food to millipede populations far greater than those seemingly occurring in the area. Although correlations between millipede mass outbreaks have been sought with dominant plant species of the areas in which outbreaks occurred (Niijima & Shinohara 1988; Kania & Tracz, 2005; Voigtländer, 2005), or soil types and habitat characteristics (Barlow, 1957; Helb, 1975; Tajovský, 1993; Voigtländer, 2011; Zimmermann, 2013) or co-inhabiting soil invertebrates (Niijima, 1998), such correlations did not turn out very convincing and certainly do not apply to the euryoecious C. hualienensis and its mass outbreaks on Hachijojima island. Incidentally, a correlation between outbreaks and soil pH changes was rejected by Haacker (1968).
It was suggested by Shintaro Yamashita (pers. comm..) that millipedes choose concrete roads and walls to crawl on, because they could obtain calcium from there. Although the cuticle of millipedes is indeed calcium-rich (Enghoff et al, 2014), it is unlikely that they gather together for the purpose of calcium uptake, for aggregations have also been reported from tree trunks and other surfaces that contain no or little calcium. Moreover, millipedes frequently move along the rails of railway tracks (Verhoeff, 1900; Esaki, 1934), which contain no calcium at all, but allow millipedes to move along unimpededly.
A study of thermal preferences by Boccardo & Penteado (1995a) has led to the speculation that concrete and asphalt roads are preferred as migratory paths, because they retain heat. However, it is unlikely that this could be the main reason for the millipedes’ choice of their migratory route. However, once a route has become established, an olfactory trail may well guide additional individuals along.
Reproduction and search for oviposition sites
Reproduction and a search for suitable oviposition sites by females has frequently been suggested to explain swarming in millipedes, but it does not explain why non-reproductive sub-adults, although in considerably smaller numbers, frequently participate in the mass migrations. Although a high proportion of C. hualienensis individuals were paired at the aggregation sites, pairing and copulations could also be observed at other times of the year and locations far away from the aggregation sites, which suggests that pairing may not be a cause of the mass migration, but the latter can provide individuals with an opportunity to mate, because of the proximity of so many individuals at one place at the same time. As with C. hualienensis, a skewed sex ratio favouring females has also been reported from many other migratory species of millipedes (Kania & Tracz, 2005), but according to Stojałowska (1968, cited in Kania & Tracz, 2005) the ratio between male and females can vary over a period of several months, adding support to the notion of male periodomorphosis (Fairhurst, 1969; Sahli, 1985).
Given the fact that males as well as females of all migratory millipedes appear to have a tendency of climbing upward and ascending walls as well as trees, it does not make much sense if they were in search of suitable oviposition sites, for eggs are usually laid into the soil and other humid habitats (Perttunen, 1953). Therefore, we may have to reject the idea that millipedes primarily swarm in order to mate and that the females then search for places to lay their eggs in.
Weather conditions and climatic factors
The suggestion that the migrations are related to a search for overwintering niches stems from the fact that some species like, for instance, Parafontaria laminata and C. hualienensis migrate in autumn, but in Europe with its more severe winters most of the mass migrations have been reported from late spring and early summer, i.e., many months before the advent of winter. In addition, as with the hypothesized search for oviposition sites, climbing upwards and exposing themselves to the elements can hardly be interpreted as an optimal strategy to find hibernation shelters or, for that matter, to avoid desiccation and enter a state of aestivation during the summer months.
Given that mass migratory outbreaks are not involving all populations of a given species, but are restricted to some, it seems that local weather conditions disrupting the habitat and environment in a particular area may play a role. Temperature extremes and heavy rains, for example, have frequently been noticed to precede an outbreak in both European spring migrations and Japanese autumn migrations. In the case of the Hachijojima outbreak of the C. hualienensis a very heavy downpour with 104 mm of rain in one day occurred on November 6th and could possibly have washed down from the hills huge numbers of millipedes that after cessation of the rains needed to climb to where they had been dislodged from. However, even that cannot be the full explanation, because swarms wandering towards Site II (the concrete support pylon of the viaduct) followed the path of a road that was leading down and not upward. Fleeing excessively wet areas so as not to be drowned and trying to reach drier ground has been suggested and could well be one reason, but seeing at least some C. hualienensis individuals wandering into shallow puddles and getting drowned does not tally with that idea. Neither does the fact that at least some species search for humid areas to lay their eggs in (Perttunen, 1953).
It has also sometimes been suggested that the weather during the previous year and in particular a mild winter and a warm spring before an outbreak year could have been influential in leading to the outbreak. However, for the outbreak of C. hualienensis reported in this paper it is an unlikely explanation, since the weather records of the previous winter and spring did not exhibit any unusual features. Moreover, locals assert that C. hualienensis outbreaks appear to follow roughly a 4-year cycle. Other than the locals’ opinion, however, no hard evidence in support of a 4-year cycle of millipede outbreak on Hachijojima exist and in view of the monthly moults of immature individuals (Fujiyama et al, 2012) actually seems unlikely.
Their extremely well developed clawed tarsi and obviously strong thigmotaxis allows many millipedes generally and C. hualienensis in particular to crawl upside down along rough surfaces even during strong wind. Strong wind, however, is likely to affect the place on the aggregation site where millipedes come to rest, but as the observations on C. hualienensis have shown, while the initially preferred walls were facing west and east at Site 1, the preferred side individuals chose at Site II at the same time was north. The only clear evidence of a preferred side emerged towards late November and early December when winds blew mostly from the west and the remaining aggregation clusters had moved to vertical surfaces of concrete walls all facing north, away from the light, but surprisingly also away from the midday warmth.
Behavioural reactions in particular in relation to light and darkness
All species of millipedes, with few exceptions, avoid bright light and usually occur in dark areas under the bark of trees, in the soil, under stones, amongst leaf litter and rotting vegetation, but there is some evidence that mass aggregations occur preferentially on lighter surfaces. Since aggregations of millipedes were indeed frequently reported to occur on white or at least not very dark surfaces (Voigtländer, 2005) and the fact that some migrations involving O. sabulosus occurred in bright sunlight (Dziadosz, 1966; Fairhurst, 1970; Verhoeff, 1900), has led some researchers to conclude that during their migrations millipedes became positively phototactic. However, contradictory observations, namely that migrating millipedes, including O. sabulosus, sought shelter during the day from the light and became active only at night also exist (Demange, 1960). One question we therefore have to examine is whether millipedes can see at all.
Iulid and spirobolid species, as has been known for over a hundred years, (Graber, 1880; Grenacher, 1880) as well as spirostreptids (Blanke & Wesener, 2014), glomerids (Bedini, 1970) and penicillate millipedes (Müller et al, 2007) possess eyes and undoubtedly can perceive differences in light intensity and perhaps even crude shapes (Nilsson & Kelber, 2007). An endogenous activity rhythm was reported in Gymnostreptus olivaceus (Schubart), but light as an exogenous synchronizer of the millipede’s nocturnal activity seemed insufficient to block the start of the biological clock (Boccardo & Penteado, 1995b). Observations on C. hualienesis gave a different result in that light clearly inhibited the animals (this paper); yet, polydesmids are all eyeless, in fact they are often called “blind” (e.g., Mesibov, 2014). Although that is clearly wrong, as already v. Rath (1890) has shown in Polydesmus complanatus (Latzel) and observations on C. hualienensis (this paper) have confirmed, the question arises how an eyeless polydesmid can detect light and use it as an exogenous synchronizer of its activities.
The fact that individuals active at night almost immediately became immobile and sought shelters when light was shone on them and the fact that individuals continued to crawl around during the day when placed into a totally dark room demonstrates that eyeless polydesmids are not blind. Most likely intracerebral photoreceptors are responsible for the observed dark/light reactions, but candidates for receptors of this kind amongst Myriapoda have to date only been reported from the chilopod Lithobius forficatus L. by Jamault-Navarro (1992), various species of glomerids by Juberthie-Jupeau (1967) and the iulid millipede Cylindroiulus truncorum by Heithier & Melzer (2005). Polydesmid species have apparently not yet been studied with regard to intracerebral photoreceptors, but are expected to have them too, since these internal photoreceptive structures are also present in crustaceans (Bobkova et al, 2003), insects (Hariyama, 2000) and chelicerates (Spreitzer & Melzer, 2003) and, thus, seem an arthropod-wide trait.
Female C. hualienensis outnumbered males by on average 1.24: 1.0 when data were pooled. Under very wet conditions at night, however, females reached an even higher proportion. Since females individuals appear to be stronger than males and are also slightly larger, they are likely to be able to cope with adverse weather conditions better than males. Given the long march that presumably both males and females have behind them when they arrive at the aggregation sites, some weaker males may have perished on the way and this could explain the preponderance of females.
Commensals and parasites
The large numbers of internal nematodes that were collected from males and females of C. hualienensis presumably resided in the posterior intestine as is the case in other diplopodes (e.g., Travassos & Kloss, 1961) or in the haemolymph as with spiders (Lewbart, 2006). The worms most likely belonged to the Diplogastridae (Fictor sp.) and Rhabditidae (Oscheius cf. necromena) line of the Rhabditida. Nematodes are frequent associates as commensals or parasites of arthropods (Kaya & Gaugler, 1993) and have been reported from numerous species of temperate (e.g., Bowie, 1985), tropical (Malysheva & Van Luc, 2012; Malysheva & Spiridonov, 2013) and even desert millipedes (Upton et al, 1983). Some rhabditids like, for example, panagrolaimid nematodes in spiders are considered to be fatal to the infected individuals (Lewbart, 2006). In bark beetles of the family Ipidae it was shown by Yatsenkowsky (1924), cited in Grucmanova & Holusa (2013) that the worms can cause sterility, but an effect, if any, that these worms may have on the health and/or behaviour of C. hualienensis millipedes has yet to be demonstrated. It has, however, been reported by Schulte (1989) that the nematode Rhabditis necromena (=Oscheius necromena) negatively affects the invasive millipede Ommatoiulus moreleti in Australia and Kania & Tracz (2005) have reported 90% and 100% lethality of the millipede O. sabulosus when infected with nematodes of the species Heterorhabditis bacteriophora and Steinernema carpocapsae, respectively.
Equally unknown is the effect another species associated with C. hualienensis might have, namely the deutonymphs of a mite representing a species of the genus Histiostoma. Phoretic mites, collectively known as myriapodophile Acari (Rosenberg, 2009, p357) have been reported from various species of millipedes before (Farfan & Klompen, 2012), including polydesmids, but not from C. hualienensis. The question arises as to whether the nematodes and mites were introduced together with C. hualienensis from Taiwan to Japan or were acquired locally. If the latter is the case, one has to ask if all the millipedes at a time when their swarming phase commenced were already infected by nematodes and mites or whether the close proximity and bodily contacts between individuals in the aggregation clusters caused nematode and mite infestations to spread. A necromenic lifestyle in which an organism ascends a carrier and completes its development on the carrier’s cadavers is a possible scenario and known from some Histiostomatidae (Wirth, 2009).
Parasites or commensals other than those mentioned above were not encountered, although millipedes generally possess a propensity towards internal microorganisms (Hopkin & Read, 1992) and certain microorganisms like bacteria and yeasts actually assist the millipedes in the task of digesting cellulose and also themselves serve as food (Byzov, 2006). However, in another invasive species (the Portuguese Ommatoiulus moreleti), the native Australian nematode Rhabditis necromena Sudhaus and Schulte has been shown to be lethal to the invader because it introduces bacteria to which the millipede is not immune (Schulte, 1989). In Portugal itself, nematomorph worms of the genus Gordius are known to alter the behaviour of their hosts and were shown by Baker (1985) and Sahli (1986) to castrate males and to inhibit maturation of eggs in female O. moreleti individuals.
Since parasites can cause behavioural changes in their hosts we can not totally rule out the possibility that parasite infestations are a factor in millipede outbreaks. According to Dobson (1988) it is in the interest of the parasite to have a high density of host individuals and therefore manipulations by parasites are greatest under conditions of low host population densities and smallest at high host population densities. Moreover, it is known that some parasites that cause infections often alter a natural negative phototaxis “such that infected individuals become indifferent or attracted to light, whereas uninfected ones are strongly repulsed by it” (Cézally et al, 2014).
Reasons and means to control millipede outbreaks
Often described as beneficial on account of their habits to recycle dead and decaying plant matter (Schmidt, 1952; Anonymous, 2010), millipedes can also damage healthy plants and crops (Hopkin & Read, 1992). More importantly they can contain various vectors of plant and animal diseases. For example, in the migratory European species O. sabulosus Kania & Klapeć (2012) found Citrobacter species, known to cause a wide spectrum of infections in humans and being associated with neonatal meningitis and brain abscesses. Other pathogenic bacteria from millipedes were Pantoea agglomerans that “can cause infections in children that involve the bloodstream”, Enterobacter that are “associated with wound, intra-abdominal, respiratory, urinary and blood stream infections representing an increasingly important nosocomial pathogen” and Raoultella planticola as well as Salmonella arizonae, two further species impacting on human health negatively (Kania & Klapeć, 2012). It is therefore important to understand the causes of the outbreaks of millipede mass migrations and to learn how such outbreaks can be controlled. However, what do we know so far?
Millipedes, because of their defensive secretions of benzaldehyde and hydrogen cyanide derivatives, are preyed upon by only very few insect species (e.g., assassin bugs of the subfamily Ectrichodiinae and some firefly larvae: Ohba, 1997) and hardly any mammals, birds, reptiles, and amphibians; control by vertebrate animals is thus not an option. Therefore, a variety of methods (reviewed by, to name but a few, Hopkin & Read, 1992; Kania & Tracz, 2005; Zimmermann, 2013) and categorizable as chemical (e.g., pesticides and poisons), physical (e.g., mechanical barriers, traps, glue-strips and other obstacles) and biological (e.g., parasitoids, parasites and pathogens) have been tried with variable rates of success. Pyrethroid insecticides have been effective, but since these chemicals are toxic not only for millipedes, but also for most other animals, including beneficial species like honeybees, biological control methods have been looked into as alternatives (Kania & Tracz, 2005). Baker (1985) and Sahli (1986) recommended the nematomorph worm Gordius sp. and Baker (1985), additionally, listed the parasitoid fly Egina sp. as agents in the control of the invasive species O. moreleti in Australia. Promising results were also obtained with the native Australian nematode Rhabditis necromena (Schulte, 1989), which carries bacteria to which O. moreleti is not resistant. Kania & Tracz (2005) reported 90% and 100% lethality of the millipede O. sabulosus when infected with the nematodes of the species Heterorhabditis bacteriophora and Steinernema carpocapsae, respectively.
CONCLUSION
While the search for biological control agents seems to result in promising advances, the search for common causes of mass migrations and aggregations in millipedes as well as an answer to the question how these animals orientate and locate their aggregation sites has so far been less successful. For some species in which outbreaks occur at regular intervals related to the species’ life cycle, predictions of outbreak years and seasons are possible and alien, invasive species like O. moreleti in Australia and C. hualienensis on the island of Hachijojima, may exhibit population explosions in their new environment for some years following their arrival until a more balanced situation develops.
Amongst insects numerous species are known, which possess sedentary and migratory morphs that may or may not alternate on a regular basis; the dominance or the prediction of one or the other morph’s occurrence is therefore often not at all possible (Roff & Fairbairn, 2007). What has come under scrutiny recently, however, is the genetic background for this form of polymorphism (Yang et al, 2014). It is thus at least thinkable that also in millipedes we could have two perhaps morphologically hard to distinguish but genetically non-identical morphs, one prone to migrate and the other to remain sedentary. It would certainly seem worthwhile to examine whether those specimens of C. hualienensis that migrate and those that stay behind differ in their gene expression profiles.
There is, however, as this review has shown, not a single suggestion amongst those that have been made to explain the cause or causes of an outbreak and mass migration in the past, which does not have its counter argument. Echoing Morse (1903) more than a hundred years ago, who concluded that each migration seemed to have its own cause, we are left with the possibility that mass migrations and aggregations in millipedes do not have a common cause at all, but represent phenomena that in some locations occur on a seasonal basis and in others not and that are identical in their outcomes of bringing together masses of conspecifics, but which have evolved for different purposes in different millipede taxa and therefore need to be examined not in their entirety (unless the emphasis is on a bio-mathematical treatment or has a modelling perspective, e.g., David, 2012), but on a case-to-case basis.
ACKNOWLEDGMENTS
The author is indebted to Prof. Dr. T. Hariyama, Dr. Y. Takaku and Tsutsui san of Hamamatsu Medical University for preparing light and scanning electron micrographs of the nematodes, Dr. Lynn Carta of the USDA-ARS Nematology Laboratory for identifying the nematodes found in C. hualienensis and Dr. S.V. Malysheva of the A.N. Severtsov’s Institute of Ecology and Evolution in Moscow for information on myriapod nematode taxonomy as well as Drs. C.H.G. Müller and J. Rosenberg for supplying copies or pages of difficult to obtain publications. The author is further grateful to Dr. H.-B. Schikora for preparing the mite light-micrographs and Dr. Monica Farfan of the University of Illinois at Chicago for her information on Histiostomatidae. Thanks are also due to Mr. Isao Nagai of Nakanogo Elementary School and Mr. Kotaro Osawa (Hokkaido University student) for their help with some of the translations of weather tables and Japanese publications. Last but not least the author wishes to acknowledge the role that Mr. S. Yamashita (Hachijojima Councillor) has played in supporting the author in numerous and generous ways and the help that has come from the constructive comments that two anonymous referees had provided.
REFERENCES
- [1].Anonymous. 2006[2006-10-11]. Tausendfüssler überrennen Vorarlberg - News.http://www.20min.ch/print/story/16609580(in German). [Google Scholar]
- [2].Anonymous. 2010[2010-04-26]. Millipedes and centipedes in Atlanta.http://www.callnorthwest.com/2010/04/millipedes-and-centipedes-in-atlanta/ [Google Scholar]
- [3].Anonymous.2011http://www.beobachter.ch/ wohnen/artikel/tausendfuessler-plage/_jede-nacht-krabbelten-tausendfuessler-die-waende-hoch/(in German). [Google Scholar]
- [4].Anonymous.2013Millipedes suspected in Clarkson train crash. Railway Digest (Australia),November 2013: 24. [Google Scholar]
- [5].Baker GH.1978. The distribution and dispersal of the introduced millipede Ommatoiulus moreletii (Diplopoda: Julidae) in Australia.Journal of Zoology (London), 185(1): 1-11. [Google Scholar]
- [6].Baker GH.1985. Parasites of the millipede Ommatoiulus moreletii (Lucas) (Diplopoda: Iulidae) in Portugal, and their potential as biological control agents in Australia.Australian Journal of Zoology, 33(1): 23-32. [Google Scholar]
- [7].Barlow CA.1957. A factorial analysis of distribution in three species of diplopods.Tijdschrift voor Entomologie, 100: 349-426. [Google Scholar]
- [8].Becker R.1929. Beitrag zur Diplopodenfauna Lettlands.Folia Zoologica (Riga), 1: 10-50 (in German). [Google Scholar]
- [9].Bedini C.1970. The fine structure of the eye in Glomeris (Diplopoda).Monitore Zoologico Italiano (N.S.), 4(4): 201-219. [Google Scholar]
- [10].Bellairs V, Bellairs R, Goel S.1967. Studies on an Indian polydesmoid millipede Streptogonopus phipsoni, life cycle and swarming behaviour of the larvae.Journal of Zoology, 199(1): 31-50. [Google Scholar]
- [11].Blanke A, Wesener T.2014. Revival of forgotten characters and modern imaging techniques help to produce a robust phylogeny of the Diplopoda (Arthropoda, Myriapoda). - Arthropod Structure and Development, 43(1): 63-75. [DOI] [PubMed] [Google Scholar]
- [12].Bobkova M, Greve P, Meyer-Rochow VB, Martin G.2003. Description of intracerebral ocelli in two species of North American crayfish: Orconectes limosus and Pacifastus leniusculus (Crustacea, Decapoda, Astacidea).Invertebrate Biology, 122: 158-165. [Google Scholar]
- [13].Boccardo L, Penteado CHS.1995a Preferencias termicas e respostas metabolicas em relacao a temperatura e ao tamanho em Gymnostreptus olivaceus Schubart, 1944 (Diplopoda, Spirostreptidae).Revista brasileira de biologia, 55(3): 445-456 (in Portuguese with English summary). [Google Scholar]
- [14].Boccardo L,Penteado CHS.1995. b. Locomotor and metabolic actoivities of Gymnostreptus olivaceus (Diplopoda, Spirostreptida) at different photoperiod conditions. Comparative Biochemistry and Physiology,Pt A, 112(3-4): 611-617. [Google Scholar]
- [15].Boccardo L, Jucá-Chagas R, Penteado CHS.2002. Migration and population outbreaks of millipedes in the coffee plantations, region Altoparanaíba, MG, Brazil.Holos Environment, 2(2): 220-223. [Google Scholar]
- [16].Boccardo L, Penteado CHS, Jucá-Chagas R.1997. Swarming of millipedes, new case noticed in the district of Patrocinio, MG, Brazil. Journal of Advanced Zoology, 18(1): 62-63. [Google Scholar]
- [17].Bowie JY.1985. New species of rhigonematid and thelostomatid nematodes from indigenous New Zealand millipedes.New Zealand Journal of Zoology, 12(4): 485-503. [Google Scholar]
- [18].Brade-Birks SG.1922. Notes on myriapoda, 27. Wandering millipeds.Annals and Magazine of Natural History (ser. 49), 9(9): 208-212. [Google Scholar]
- [19].Brooks FE.1919. A migratory army of millipeds.Journal of Economic Entomology, 12(6): 462-464. [Google Scholar]
- [20].Byzov BA.2006. Intestinal microbiota of millipedes. In: König H, Varma A. Intestinal Microorganisms of Termites and other Invertebrates. Berlin: Springer, 373-384. [Google Scholar]
- [21].Ceuca T.1984. Migraţiile la diplopode.Nymphaea (Oradea), 10: 237-242 (in Romanian with English summary). [Google Scholar]
- [22].Cézally F, Perrot-Minnot MJ, Rigaud T.2014. Cooperation and conflict in host manipulation: interactions among macro-parasites and micro-organisms.Frontiers in Microbiology, 5: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chater A.2004. A swarm of Cylindroiulus londinensis in Montgomeryshire.Bulletin of the British Myriapod and Isopod Group, 20: 51. [Google Scholar]
- [24].Cloudsley-Thompson JL.1949. The significance of migration in myriapods.Journal of Natural History Series 12, 2(24): 947-962. [Google Scholar]
- [25].Cook OF.1924. Swarming of desert millipeds.Science N.S., 60(1552): 294. [DOI] [PubMed] [Google Scholar]
- [26].Ćurić BPM, Makarov SE.1995. The occurrence of swarming in Megaphyllum unilineatum (C.L. Koch, 1838) (Diplopoda: Julidae), with observations on a case of pedal anomaly.Archives of Biological Sciences (Belgrade), 47(1-2): 67-70. [Google Scholar]
- [27].David JF.2012. First estimate of the intrinsic rate of increase of a millipede: Polydesmus angustus (Polydesmida: Polydesmidae) in a seasonal environment.Annals of the Entomological Society of America, 105(1): 90-96. [Google Scholar]
- [28].Demange JM.1960. Sur un important rassemblement de Schizophyllum sabulosum L. (Myriapode-Diplopode). Cahiers des Naturalistes du Bulletin National de Paris N.S., 16(4): 89-91 (in French). [Google Scholar]
- [29].Dobson AP.1988. The population biology of parasite-induced changes in host behavior.The Quarterly Reviews of Biology, 63(2): 139-165. [DOI] [PubMed] [Google Scholar]
- [30].Dziadosz C.1966. Materiały do znajomości rozmieszczejnia krocinogów (Diplopoda) w Polsce.Fragmenta Faunistica (Warszawa), 13: 1-31 (in Polish). [Google Scholar]
- [31].Ehrnsberger R.2002. Massenauftreten und Wanderung des Diplopoden Ommatoiulus sabulosus in Westniedersachsen.Osnabrücker Naturwissenschaftliche Mitteilungen, 28: 199-203 (in German). [Google Scholar]
- [32].Enghoff H., Manno N., Tchibozo S., List M., Schwarzinger B., Schoefberger W., Schwarzinger & C., Paoletti MG. (2014). Millipedes as food for humans: their nutritional and possible antimalarial value - A first report. Evidence-Based Complementary and Alternative Medicine,ID 651768, doi:10.1155/2014/651768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Esaki T.1934. Diplopoda that disturb the movement of trains.Shokubutsu oyobi Dobutsu, 2: 821-833 (in Japanese). [Google Scholar]
- [34].Fairhurst CP.1970. Activity and wandering in Tachypodoiulus niger (Leach) and Schizophyllum sabulosum (L.).Bulletin du Muséum National d'Histoire Naturelle, 41(S2): 61-66. [Google Scholar]
- [35].Farfan MA, Klompen H.2012. Phoretic mite associations of millipedes (Diplopoda, Julidae) in the northern Atlantic region (North America, Europe).International Journal of Myriapodology, 7: 62-91. [Google Scholar]
- [36].Fontanetti CS, Calligaris IB, Souza TS.2010. A millipede infestation of an urban area of the city of Campinas, Brazil and preliminary toxicity studies of insecticide bendiocarb to the Urostreptus atrobrunneus Pierozzi & Fontanetti, 2006.Arquivos do Instituto Biológico (São Paulo), 77(1): 165-166. [Google Scholar]
- [37].Fujiyama S, Ishida T, Shah SK.2012. Ecology of imigrated diplopoda, Chamberlinius hualienensis with special reference to that of Parafontaria laminata armigera.The Annals of Environmental Science of Shinshu University, 34: 110-116 (in Japanese). [Google Scholar]
- [38].Graber V.1880. Ueber das unicorneale Tracheaten- und speciell das Arachnoiden- und Myriapoden-Auge.Archiv für Mikroskopie und Anatomie, 17: 58-94 (in German). [Google Scholar]
- [39].Grenacher H.1880. Über die Augen einiger Myriapoden. Zugleich eine Entgegnung an V. Graber.Archiv für mikroskopische Anatomie und Entwicklungsmechanik, 18(1): 415-467 (in German). [Google Scholar]
- [40].Grucmanova S, Holusa J.2013. Nematodes associated with bark beetles, with focus on the genus Ips (Coleoptera; Scolytinae) in Central Europe.Acta Zoologica Bulgarica, 65(4): 547-556. [Google Scholar]
- [41].Haacker U.1968. Deskriptive, experimentelle und vergleichende Untersuchungen zur Autökologie rhein-mainischer Diplopoden.Oecologia, 1(1-2): 87-129 (in German with English summary). [DOI] [PubMed] [Google Scholar]
- [42].Hagiwara Y, Kuwabara Y.2008. Outbreak of the train millipede on the northern slope of Mt. Fuji.Fujisan Kenkyu Report, 2: 21-24 (in Japanese). [Google Scholar]
- [43].Hariyama T.2000. The brain as a photoreceptor: intracerebral ocelli in then firefly.Naturwissenschaften, 87(7): 327-330. [DOI] [PubMed] [Google Scholar]
- [44].Heithier N, Melzer RR.2005. The accessory lateral eye of a diplopod, Cylindroiulus truncorum (Silvestri, 1896) (Diplopoda: Julidae).Zoologischer Anzeiger, 244(1): 73-78. [Google Scholar]
- [45].Helb HW.1975. Zum Massenauftreten des Schnurfüssers Schizophyllum sabulosum (Myriapoda: Diplopoda).Entomologia Germanica, 1(3-4): 376-381 (in German). [Google Scholar]
- [46].Higa Y, Kishimoto T, Niijima K.1992. Seasonal abundance of Yanbarutosakayasude in Okinawa.Okinawa Ken Kogai Eisei Kenkyujo Houkoku (Report of the Okinawa Prefectural Pollution Institute), 23: 72-76 (in Japanese). [Google Scholar]
- [47].Hopkin SP, Read HJ.1992. The Biology of Millipedes. Oxford: Oxford University Press. [Google Scholar]
- [48].Jamault-Navarro C.1992. Sur la présence d’une structure rhabdomérique localisée intracérébralement dans le protocérébron de Lithobius forficatus L.Berichte des Naturwissenschaftlich-Medizinischen Vereins Innsbruck Supplement, 10: 81-86 (in French). [Google Scholar]
- [49].Japanese Meteorological Agency.http://www.data.jma.go.jp(in Japanese). [Google Scholar]
- [50].Juberthie-Jupeau L.1967. Existence d’organes neuraux intracérébraux chez les Glomeridia (Diplopodes) épigés et cavernicoles.Comptes Rendus hebdomadaires des Séances de l'Académie des Sciences (Paris), 264: 89-92 (in French). [PubMed] [Google Scholar]
- [51].Kania G, Tracz H.2005. Mass occurrence and migration of Ommatoiulus sabulosus (Linnaeus, 1758) (Diplopoda, Julida: Julidae) in Poland.Peckiana, 4: 57-66. [Google Scholar]
- [52].Kania G, Klapeć T.2012. Seasonal activity of millipedes (Diplopoda) - their economic and medical significance.Annals of Agricultural and Environmental Medicine, 19(4): 649-650. [PubMed] [Google Scholar]
- [53].Kaya HK, Gaugler R.1993. Entomopathogenic nematodes.Annual Review of Entomology, 38: 181-206. [Google Scholar]
- [54].Koch LE.1985. Pincushion millipedes (Diplopoda: Polyxenida): their aggregations and identity in Western Australia.Western Australian Naturalist, 16(2-3): 30-32. [Google Scholar]
- [55].Korsós Z.1998. Ikerszelvényes-invázió magyarországon.Állattani Közlemények, 83: 53-65 (in Hungarian). [Google Scholar]
- [56].Lawrence RF.1952. Variation in the leg numbers of South African millipede Gymnonstreptus pyrrhocephalus C. Koch.Annals and Magazine of Natural History Series, 12: 1044-1051. [Google Scholar]
- [57].Lewbart GA.2006. Invertebrate Medicine.Chichester (U.K.): Wiley-Blackwell, 211. [Google Scholar]
- [58].Lindgren LAH.1942. Ett massuppträdande av en diplopod, Cylindroiulus teutonicus Pocock.Fauna och Flora Uppsala, 2: 79-81 (in Swedish). [Google Scholar]
- [59].Malysheva SV, Van Luc P.2012. Cattiena fansipanis n. sp. (Nematoda: n Rhigonematida: Carnoyidae) from a millipede (Myriapoda: Diplopoda: Spirobolida) in North Vietnam.Systematic Parasitology, 81: 135-146. [DOI] [PubMed] [Google Scholar]
- [60].Malysheva SV, Spiridonov SE.2013. Ichthyocephaloides sumbatus n. sp. (Nematoda: Rhigonematoidea) from Indonesia and additional data on Xystrognathus phrissus Hunt, Pham Van Luc & Spiridonov, 2002.Nematology, 15(5): 575-588. [Google Scholar]
- [61].McKillup SC, Allen PG, Skewes MA.1988. The natural decline of an introduced species following its initial increase in abundance: an explanation for Ommatoiulus moreleti in Australia.Oecologia, 77(3): 339-342. [DOI] [PubMed] [Google Scholar]
- [62].Meidell B, Simonsen A.1985. A mass occurrence of Cylindroiulus londinensis (Leach, 1815) in Norway.Fauna Norvegica B, 32(1): 47-48. [Google Scholar]
- [63].Mesibov R.2014[2015-01-16]. External anatomy of polydesmida.http://www.polydesmida.info/polydesmida. [Google Scholar]
- [64].Mitra TR.1976. Millipedes entering houses.The Entomologist’s Monthly Magazine, 112: 44. [Google Scholar]
- [65].Morse M.1903. Unusual abundance of a myriapod, Parajulus pennsylvanicus (Brandt).Science N.S., 18(445): 59-60. [DOI] [PubMed] [Google Scholar]
- [66].Müller CHG, Sombke A, Rosenberg J.2007. The fine structure of the eyes of some bristly millipedes (Penicillata, Diplopoda): Additional support for the homology of mandibulate ommatidia.Arthropod Structure and Development, 36(4): 463-476. [DOI] [PubMed] [Google Scholar]
- [67].Niijima K.1998. Effects of outbreak of the train millipede Parafontaria laminata armigera Verhoeff (Diplopoda: Xystodesmidae) on litter decomposition in a natural beech forest in Central Japan. 1. Density and biomass of soil invertebrates.Ecological Research, 13(1): 41-53. [Google Scholar]
- [68].Niijima K, Shinohara K.1988. Outbreaks of the Parafontaria laminate group (Diplopoda: Xystodesmidae).Japanese Journal of Ecology, 38: 257-268 (in Japanese with English summary). [Google Scholar]
- [69].Nilsson DE, Kelber A.2007. A functional analysis of compound eye evolution.Arthropod Structure and Development, 36(4): 373-385. [DOI] [PubMed] [Google Scholar]
- [70].Noguchi S, Mori N, Higa H, Kuwahara Y.1997. Identification of mandelonitrite as a major secretary compound of Chamberlinius hualienensis Wang (Polydesmidae, Paradoxosomatidae).Japanese Journal of Environmental Entomology and Zoology, 8: 208-214. [Google Scholar]
- [71].Ohba N.1997. Breeding of the firefly, Rhagophthalmus ohbai (Coleoptera: Rhagophthalmidae).Scientific Reports of Yokosuka City Museum, 45: 51-55. [Google Scholar]
- [72].Ormerod EA.1890. Manual of Injurious Insects with Methods of Prevention and Remedy for Their Attacks to Food Crops, Forest Tress, and Fruit, and with A Short Introduction to Entomology. London: WS Sonnenschein & Allen Publ., 150-151. [Google Scholar]
- [73].Paszlavszky J.1879. Massenhaftes Erscheinen von Tausendfüsslern.Verhandlungen der kaiserlich-königlichen zoologisch-botanischen Gesellschaft in Wien Jahrgang 1878, 28: 545-552 (in German). [Google Scholar]
- [74].Perttunen V.1953. Reactions of Diplopods to the Relative Humidity of the Air: Investigations on Orthomorpha gracilis, Julus terrestris and Schizophyllum sabulosum.Annales Societatis Zoologicae-Botanicæ Fennicæ Vanamo, 16(1): 1-69. [Google Scholar]
- [75].vom Rath O.1890. Ueber die Fortpflanzung der Diplopoden (Chilognathen).Berichte der Naturforschenden Gesellschaft Freiburg, 5(1): 1-28 (in German). [Google Scholar]
- [76].Robinson WH.2005. Urban Insects and Arachnids. Cambridge (U.K.): Cambridge University Press, 424-426. [Google Scholar]
- [77].Roff DA, Fairbairn DJ.2007. The evolution and genetics of migration in insects.Bioscience, 57(2): 155-164. [Google Scholar]
- [78].Rosenberg J.2009. Die Hundertfüsser - Chilopoda: die neue Brehm Bücherei Band 285. Hohenwassersleben (Germnany):Westarp Wissenschafts-Verlagsgesellschaft (in German). [Google Scholar]
- [79].Sahli F.1985. Périodomorphose et males intercalaires des diplopodes Julidae: une nouvelle terminologie.Bulletin Scientifique de Bourgogne, 38(1-2): 23-32 (in French). [Google Scholar]
- [80].Sahli F.1986. Modifications des caractères sexuels secondaires males et stérilisé des femelles sous l’influence d’un Gordius parasite chez le diplopode julide Ommatoiulus omeletti (Lucas) au Portugal.Bulletin Scientifique de Bourgogne, 39(2): 587-598 (in French). [Google Scholar]
- [81].Sahli F.1996. Déplacement en masse dans le sud-est de la France chez Ommatoiulus sabulosus (Myriapoda, Diplopoda, Julidae) avec invasions d’habitations. In: Geoffroy JJ, Mauries JP, Nguyen-Duy-Jacquemin M. Acta Myriapodologica. Paris: Mémoires du Museum National d' Histoire Naturelle, 169: 373-384 (in French). [Google Scholar]
- [82].Schmidt H.1952. Nahrungswahl und Nahrungsverarbeitung bei Diplopoden (Tausendfüβlern).Mitteilungen des Naturwissenschaftlichen Vereines für Steiermark, 81/82: 42-66 (in German). [Google Scholar]
- [83].Schulte F.1989. The association between Rhabditis necromena Sudhaus and Schulte 1989 (Nematoda: Rhabditidae) and native and introduced millipedes in South Australia.Nematologica, 35(1): 82-89. [Google Scholar]
- [84].Scott H.1958a. Migrant millipedes and entering houses 1953-1957.The Entomologist’s Monthly Magazine, 94: 73-77. [Google Scholar]
- [85].Scott H.1958. b. Migrant millipedes entering houses 1958.The Entomologist’s Monthly Magazine, 94: 252-256. [Google Scholar]
- [86].Spreitzer A, Melzer RR.2003. The nymphal eyes of Parabuthus transvaalicus Purcell, 1899 (Buthidae): an accessory lateral eye in a scorpion.Zoologischer Anzeiger, 242(2): 137-143. [Google Scholar]
- [87].Stojałowska W.1968. Materiáły do poznania krocinogów (Diplopoda) [Google Scholar]
- [88].Wyžyny Lubelskiej.Folia Societatis Scientiarum Lublinensis B, 7/8: 83-93 (in Polish). [Google Scholar]
- [89].Tajovský K.1993. Diversity and structure of millipede communities (Diplopoda) in four different iotopes.Ekologia (Bratislava), 12(3): 277-283. [Google Scholar]
- [90].Takaku Y, Suzuki H, Ohta I, Ishii D, Muranaka Y, Shimomura M, Hariyama T.2013. A thin polymer membrane, nano-suit, enhancing survival across the continuum between air and high vacuum.Proceedings of the National Academy of Sciences of the United States of America, 110(19): 7631-7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Thaler K.1989. Kleintiere im Kulturland des Innsbrucker mittelgebirges. In: Köck L, Holaus K. 50 Jahre Landesanstalt für Pflanzenschutz und Samenprüfung. Rinn: Landesanstalt für Pflanzenschutz und Samenprü fung, 59-177 (in German). [Google Scholar]
- [92].Thuringer JM.1924. A note on migration of Myriapoda.Science N.S., 60(1543): 83. [DOI] [PubMed] [Google Scholar]
- [93].Tömösváry Ö.1878. A százlábúak vándorlásához.Természettudományi Közlemények, 10: 365-366 (in Hungarian). [Google Scholar]
- [94].Travassos PL, Kloss GR.1961. Sur un curieux nématode de Robertia leiperi gen. et sp. nov., parasite de l’intestine postérieur de diplopode.Journal of Helminthology, 35(S1): 187-190 (in French). [PubMed] [Google Scholar]
- [95].Upton SJ, Crawford CS, Hoffman RL.1983. A new species of thelastomatid (Nematoda: Thelastomatidae) from the desert millipede, Orthoporus ornatus (Diplopoda, Spirostreptidae).Proceedings of the Helminthological Society (Washington), 50(1): 69-82. [Google Scholar]
- [96].Verhoeff KW.1900. Wandernde Doppelfüβler, Eisenbahnzüge hemmend.Zoologischer Anzeiger, 23: 465-473 (in German). [Google Scholar]
- [97].Verhulst PF.1845. Recherches mathématiques sur la loi d’accroissement de la population.Mémoires de l’Académie Royale des Sciences et Belles Lettres de Bruxelles, 18: 1-42 (in French). [Google Scholar]
- [98].Viosca P Jr.1925. Perambulating millipeds.Science N.S., 61(1566): 19-20. [DOI] [PubMed] [Google Scholar]
- [99].Voigtländer K.2005. Mass occurrences and swarming behaviour of millipedes (Diplopoda: Julidae) in Eastern Germany.Peckiana, 4: 181-187. [Google Scholar]
- [100].Voigtländer K.2011. Preferences of common Central European millipeds for different biotopes (Myriapoda, Diplopoda) in Saxony-Anhalt (Germany).International Journal of Myriapodology, 6: 61-83. [Google Scholar]
- [101].Wang YM.1956. Records of myriapods on Formosa with description of new species (2).Quarterly Journal of the Taiwan Museum, 9(2): 155-159. [Google Scholar]
- [102].Wesener T, Schütte K.2010. Swarming behaviour and mass occurrences in the world’s largest giant pill-millipede species, Zoosphaerium neptunus, on Madagascar and its implication for conservation efforts (Diplopoda: Sphaerotheriida).Madagascar Conservation and Development, 5(2): 89-94. [Google Scholar]
- [103].Wirth S.2009. Necromenic life style of Histiostoma polypori (Acari: Histiostomatidae).Experimental and Applied Acariology, 49(4): 317-327. [DOI] [PubMed] [Google Scholar]
- [104].Yang X, Liu X, Xu X, Li Z, Li Y, Song D, Yu T, Zhu F.2014. Gene expression profiling in winged and wingless cotton aphids, Aphis gossypii (Hemiptera: Aphididae).International Journal of Biological Sciences, 10(3): 257-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yatsenkowsky AV.1924. The castration of Blastophagus of pines by roundworms and their effect of the activity and life phenomena of Ipidae.Publications of the Agriculture Institute of Western White Russia, 3: 1-19. [Google Scholar]
- [106].Zimmermann K.2013. Röns: St. Magnus und die Tausendfüssler. Dornbirn (Austria): Naturmonographie Jagdberggemeinden, 371-386 (in German). [Google Scholar]


