Abstract
Interleukin 1β (IL-1β), the first interleukin to be characterized, plays a key role in regulating the immune response. In this study, we determined the cDNA and genomic DNA sequences of the IL-1β gene from the large yellow croaker, Larimichthys crocea. Phylogenetic analysis indicated that the IL-1β (LcIL-1β) gene was most closely related to that of European seabass (Dicentrarchus labrax), sharing 67.8% amino acid identity. In healthy large yellow croaker, LcIL-1β transcription was detected in all tested tissues, with the highest level found in the head kidney. Upon Vibrio alginolyticus infection, LcIL-1β transcription in all tested tissues was significantly upregulated. Intraperitoneal injection of recombinant LcIL-1β (rLcIL-1β) improved the survival rate and reduced the tissue bacterial load after V. alginolyticus infection. In addition, rLcIL-1β induced monocytes/macrophages (MO/MΦ) chemotaxis and increased phagocytosis and bactericidal activity in vitro. These results suggest that LcIL-1β plays an important role in the large yellow croaker immune response against V. alginolyticus.
Keywords: Interleukin 1β, Large yellow croaker, Survival rate, Vibrio alginolyticus, Monocytes/ macrophages
INTRODUCTION
Large yellow croaker (Larimichthys crocea) is one of the most abundant species in the Northwest Pacific basin, and is also an economically important aquaculture fish species in China. In recent years, farmed production of large yellow croaker has become intensive. However, the farming industry is now threatened with infectious disease outbreaks, with Vibrio alginolyticus regarded as the major bacterial pathogen (Chen et al, 2003; Li et al, 2009). Antibiotics have been used extensively for controlling large yellow croaker diseases, but drug residues in aquatic products and environments have become an increasing threat to human health (Mu et al, 2013). Thus, controlling diseases by understanding immune response modulation in fish species is critical.
The cytokine interleukin-1β (IL-1β) exerts a plethora of systemic and localized biological effects, and is central to the initiation and regulation of immune and inflammatory responses in animals (Hong et al, 2003). Many economically important teleost IL-1β sequences have been studied previously (Fujiki et al, 2000; Lu et al, 2013; Scapigliati et al, 2001; Zou et al, 1999). Some reports have shown that teleost IL-1β is tightly associated with the defense reaction of the host to pathogen infection. For example, IL-1β gene expression increased significantly in ayu (Plecoglossus altivelis) upon Vibrio anguillarum infection (Lu et al, 2013). Recombinant rainbow trout (Oncorhynchus mykiss) IL-1β enhanced their resistance to Aeromonas salmonicida, and increased the migration and phagocytic activity of its head kidney-derived leucocytes in vitro (Hong et al, 2003). An IL-1β derived peptide, P3, which corresponds to fragment 197-206 (YRRNTGVDIS) of the rainbow trout sequence, enhanced the phagocytic and bactericidal activity of rainbow trout head kidney leucocytes (Peddie et al, 2002). Recombinant European seabass (Dicentrarchus labrax) IL-1β stimulated the proliferation of thymocytes (Scapigliati et al, 2001). However, the function of IL-1β in large yellow croaker remains unclear.
In the present study, we determined the cDNA and genomic DNA sequences of the IL-1β gene (LcIL-1β) from the large yellow croaker. LcIL-1β transcription was investigated in healthy fish and in V. alginolyticus-infected fish. The effect of intraperitoneal (i.p.) administration of recombinant LcIL-1β (rLcIL-1β) on the survival rate and tissue bacterial load in large yellow croaker following V. alginolyticus infection was investigated. We also studied the effect of rLcIL-1β on monocytes/macrophages (MO/MΦ) chemotaxis, phagocytosis and bactericidal activity in vitro.
MATERIALS AND METHODS
Fish
Healthy large yellow croaker (15.5±1.3 cm in length, weighing 76.2±5.8 g) were obtained from the Ningbo Hai-Wan Marine Breeding Center, Xiangshan county, Ningbo city, China. Fish without any pathological signs were kept in tanks maintained at 25-27 °C with regular feeding for at least one week prior to experimental use. All experiments were performed according to the Experimental Animal Management Law of China and approved by the Animal Ethics Committee of Ningbo University.
Bacterial challenge
The V. alginolyticus challenge was performed as reported previously (Li et al, 2014). Briefly, overnight cultured V. alginolyticus isolate ATCC 17749 was diluted 1:100 in fresh Tryptic Soy Broth Medium (TSB) and cultured at 28 °C. Cells were harvested in the logarithmic phase of growth, and diluted to the appropriate concentration in PBS. Four groups of fish were infected by i.p. injection of V. alginolyticus (6.5×104 CFU/g in 200 μL PBS), with PBS used in the control group. Each group contained at least three fish. The liver, spleen, heart, head kidney, trunk kidney, brain, intestine and gill were collected at 4, 8, 12, and 24 hours post injection (hpi), frozen in liquid nitrogen and stored at -80 °C until RNA extraction.
Determination of cDNA and genomic DNA sequences of LcIL-1β
The cDNA sequence of LcIL-1β was obtained from transcriptome analysis of large yellow croaker, and the correct sequence was confirmed using PCR amplification combined with sequencing on an ABI 3730 automated sequencer (Invitrogen, Shanghai, China). Genomic DNA of large yellow croaker was isolated from liver tissue using a DNA Extraction Kit (TaKaRa, Dalian, China). Primers gLcIL-1βF: 5'- ATGGAATCTGAGATGAAATGC -3' and gLcIL-1βR: 5'- TCAGGCCTGACCCTCAGT -3' were designed to amplify the genomic sequence. The PCR product was cloned and sequenced. The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used for sequence similarity searching. Protein analysis was performed using online software on the ExPASy Server (http://www.expasy.org/tools/). The ClustalW program (http://clustalw.ddbj.nig.ac.jp/) was used for multiple sequence alignment. MEGA version 5 was used for phylogenetic tree analysis (Tamura et al, 2011). Accession numbers of sequences used are provided in Table 1.
Table 1.
IL-1β sequences used for multiple sequence alignment and phylogenetic tree analysis
| GenBank accession no. | Species | Gene | |
|---|---|---|---|
| Latin name | English name | ||
| NM_001280090 | Takifugu rubripes | Tiger pufferfish | IL-1β |
| AB720983 | Paralichthys olivaceus | Japanese flounder | IL-1β |
| AY257219 | Pagrus major | Red sea bream | IL-1β |
| HF543937 | Plecoglossus altivelis | Ayu | IL-1β |
| AJ535730 | Gadus morhua | Atlantic cod | IL-1β |
| AJ245925 | Oncorhynchus mykiss | Rainbow trout | IL-1β |
| AY617117 | Salmo salar | Atlantic salmon | IL-1β |
| AJ550166 | Melanogrammus aeglefinus | Haddock | IL-1β |
| AJ277166 | Sparus aurata | Gilthead sea bream | IL-1β |
| AJ311925 | Dicentrarchus labrax | European seabass | IL-1β |
| EF513753 | Lateolabrax japonicus | Japanese seabass | IL-1β |
| AJ295836 | Scophthalmus maximus | Turbot | IL-1β |
| EF582837 | Epinephelus coioides | Orange-spotted grouper | IL-1β |
| FJ816103 | Cynoglossus semilaevis | Half smooth tongue sole | IL-1β |
| AJ574910 | Tetraodon nigroviridis | Spotted green pufferfish | IL-1β |
| FM210810 | Danio rerio | Zebrafish | IL-1β |
| BT007213 | Homo sapiens | Human | IL-1β |
Real-time quantitative PCR (qPCR) analysis of LcIL-1β mRNA expression
QPCR was carried out as described previously (Chen et al, 2014; Lu et al, 2013). Briefly, total RNA was extracted from the large yellow croaker liver, spleen, heart, head kidney, trunk kidney, brain, intestine and gill using the RNeasy® Mini Kit (Qiagen, Maryland, USA). First-strand cDNA was synthesized using AMV Reverse Transcriptase (TaKaRa). Primers LcIL-1βF: 5'- TGGGAATGTGCCTGGAGAAC -3' and LcIL-1βR: 5'- CTTCCGTCTTAAGAGGATCA -3' were designed to amplify a 100-base pair (bp) fragment of the LcIL-1β cDNA. As an internal control, primers Lcβ-actinF: 5'-GATGTGGATCAGCA AGCAGG-3' and Lcβ-actinR: 5'-GAGCTGAAGTTGTTGGGT GT-3' were designed to amplify a 120-bp fragment of β-actin cDNA (EU443733). QPCR was performed using SYBR premix Ex Taq (Perfect Real Time) (TaKaRa). The reaction mixture was incubated for 5 min at 95 °C, followed by 35 amplification cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C in an RT-Cycler™ Realtime Fluorescence Quantitative PCR thermocycler (CapitalBio, Beijing, China). The LcIL-1β transcript was normalized relative to β-actin. Amplifications were performed in triplicate for each sample. The relative mRNA expression of LcIL-1β was calculated by the comparative Ct method (2-ΔΔCt method).
Prokaryotic expression, purification and refolding of rLcIL-1β
The ORF sequence of the LcIL-1β gene was amplified from a liver cDNA template with the following primers: LcIL-1βpF: 5'-CCATATGGAATCTGAGATGAAATGC-3' and LcIL-1βpR: 5'-GGGATCCTCAGGCCTGACCCTCAGT-3' (underlined bases are Nde I and BamH I sites, respectively). After restriction enzyme digestion, the amplicon was orientedly inserted into the pET28a vector. The recombinant pET28a-LcIL-1β plasmid was then transformed into Escherichia coli BL21(DE3) pLysS, and its expression was induced with IPTG. The purification and refolding of rLcIL-1β were carried out as described previously (Zhang et al, 2011), with some modifications. Briefly, a His TrapTM FF Crude chelating column (GE Healthcare, Shanghai, China) and 120-mL XK 16/100 column packed with Superdex 75 gel media (GE Healthcare) were used to purify and refold the recombinant protein. The eluted fraction containing refolded rLcIL-1β was then desalted on a Bio-Gel P-6 column (Bio-Rad, Shanghai, China). The size and purity of the peak fractions were monitored by 12% SDS-PAGE followed by Coomassie brilliant blue staining. The purified protein was lyophilized for further study.
Survival rate and bacterial load assays
Four groups of 20 fish were used in the survival rate assay. Thirty minutes after the large yellow croakers were intraperitoneally infected with 6.5×104 CFU/g V. alginolyticus, refolded rLcIL-1β in 200 μL PBS (0, 0.001, 0.01 or 0.1 µg/g body weight) were i.p. injected into the fish. The dose was in line with previous research on rainbow trout (Hong et al, 2003). Morbidity was monitored for nine days, and dead fish were collected daily. The same concentrations of refolded rLcIL-1β were i.p. injected into healthy fish and no impairment was found. Death was considered to be caused by the injected isolate only if the same isolate could be re-isolated as single colonies in pure culture from the head and liver of the moribund or dead fish. The Log-rank test was used to analyze survival rate.
Bacterial load was measured as colony-forming units per mg tissue as per prior study (Li et al, 2014). At 72 h post administration of rLcIL-1β, four groups of six fish were sacrificed, and the tissue samples (liver, spleen, kidney and blood) were collected. The samples from each fish were weighed and homogenized in 1 mL of sterile PBS (pH 7.2). Homogenates and blood were serially diluted in sterile PBS and then plated onto Thiosulfate Citrate Bile Salt (TCBS) agar plates for 12 h at 28 °C. Results were normalized as colonies/weight tissue (0.1 g) or colonies/blood volume (0.1 mL).
Primary culture of large yellow croaker head kidney-derived MO/MΦ
Large yellow croaker MO/MΦ were isolated as perviously described (Lu et al, 2013). Briefly, head kidney leucocyte-enriched fractions were obtained using a Ficoll density gradient (Invitrogen, Shanghai, China). Non-adherent cells were washed off and the attached cells were incubated with RPMI 1640 medium containing 10% FCS and 1% P/S throughout the experiment after overnight incubation at 24 °C. Over 95% of adherent cells were MO/MΦ according to morphological characteristics observed after Giemsa staining.
MO/MΦ chemotaxis, phagocytosis and bactericidal activity assays
The chemotaxis assay was carried out in a Chemotaxicell (Corning, Shanghai, China) following a modified Boyden chamber method (Zhang et al, 2011). Briefly, wells in the lower compartment were loaded with five concentrations (0.001, 0.01, 0.1, 1 and 10 μg/mL) of refolded rLcIL-1β, denatured rLcIL-1β and BSA dissolved in RPMI 1640. A polyvinylpyrrolidone (PVP)-free polycarbonate membrane with a pore size of 5 μm was placed in the lower compartment. Large yellow croaker MO/MΦ were added to the upper compartment. The chamber was sealed and incubated at 24 °C for 4 h. Cells that completely migrated to the lower compartment were counted in five random fields using a light microscope at 400× magnification. Each test was run in triplicate. The chemotactic index was calculated from the number of cells that migrated to the test samples divided by the number of cells that migrated to the medium only.
The phagocytosis assay was carried out following a modified method (Chen et al, 2014). Briefly, E. coli strain DH5α cells were labeled with FITC (FITC-DH5α). Large yellow croaker MO/MΦ were incubated with PBS, 0.001, 0.01 and 0.1 μg/mL rLcIL-1β for 4 h and subsequently incubated with FITC-DH5α at 24 °C for 0.5 h. The uptake of bacteria into cells was captured by a microscope and quantified by measuring fluorescence intensity using ImageJ software (http://rsb.info.nih.gov/ij/).
The bactericidal assay was carried out following a modified method (Chen et al, 2014). Briefly, large yellow croaker MO/MΦ were incubated with PBS, 0.001, 0.01 and 0.1 μg/mL rLcIL-1β at 24 °C for 24 h, and the cells were then washed twice to remove all traces of P/S. The cells were incubated with V. alginolyticus at an MOI of 20 for 0.5 h and subsequently washed in PBS to remove extracellular bacteria. The uptake group cells were lysed with 0.05% Triton X-100 and the killing group wells were incubated for 2 h and then lysed with 0.05% Triton X-100. The cell lysates were plated on TCBS plates, and bacterial counts were enumerated after 12 h. Bacterial survival was determined by dividing the number of colonies in the killing group by those in the uptake group.
Statistical analysis
All data are described as means±SEM. Statistical analysis of the results was conducted by one-way ANOVA with SPSS version 13.0 (SPSS Inc, Chicago, USA), and P<0.05 was considered statistically significant.
RESULTS
Sequence comparison and phylogenetic analysis of the LcIL-1β gene
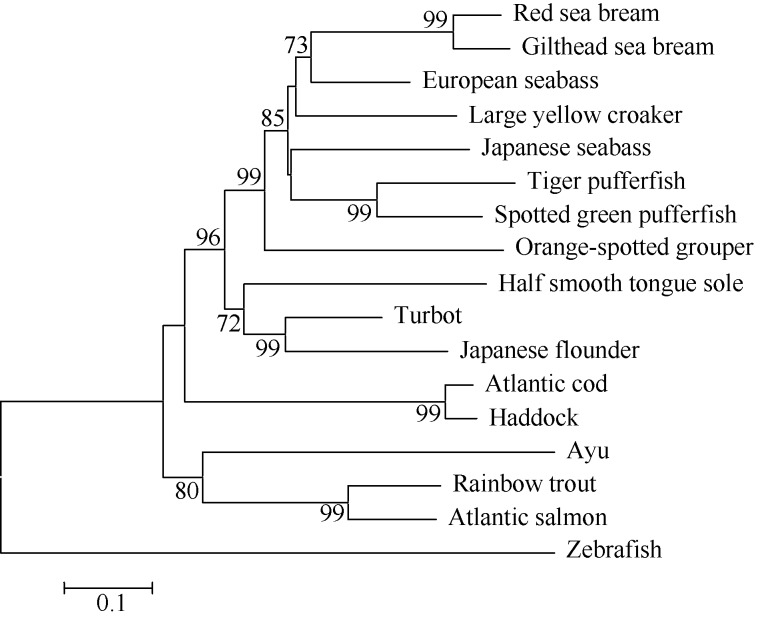
The LcIL-1β cDNA was deposited in GenBank under accession number KJ459927. The sequence consisted of 1274 nucleotides (nt), with a 768-nt ORF that encoded a 255-amino acid (aa) protein with an estimated molecular weight (MW) of 2.86×104 and a theoretical isoelectric point (pI) of 5.6. LcIL-1β contained two conserved cysteine residues (Cys162 and Cys232), which were identified by Husain et al (2012). The IL-1 family signature motif [FCL]-X-S-[ASLV]-X2-[PSR]-X2-[FYLIV]-[LIV]-[SCAT]-T-X7-[LIVMK] was reasonably well conserved in the fish IL-1β, and was identified as L209VSVPYNNWYISTAKENNK PL229 in LcIL-1β (Figure 1). The cleavage site of IL-1β converting enzyme (ICE) (also known as Caspase-1), which is highly conserved in mammalian IL-1β, has not been found in fish IL-1β (Buonocore et al, 2005). Sequence comparisons showed that LcIL-1β shared the highest amino acid identity (67.8%) with that of European seabass. Large yellow croaker IL-1β was most closely related to that of European seabass, gilthead sea bream and red sea bream (Figure 2).
Figure 1.
Multiple alignment of the LcIL-1β amino acid sequence with that of other related animal IL-1β sequences Similar residues are marked with gray shading and identical residues with black shading. The ICE site in humans is indicated with an arrow. Two conserved cysteine residues are marked by “▼”, and the IL-1 family signature is lined above the alignment. Accession numbers of sequences are provided in Table 1.
Figure 2.
Phylogenetic tree analysis of IL-1β amino acid sequences of large yellow croaker and some related fish using the neighbor-joining method The values at the forks indicate the percentage of trees in which this grouping occurred after bootstrapping the data (1 000 replicates; shown only when >60%). Scale bar shows number of substitutions per base. Accession numbers of sequences are provided in Table 1.
The genomic DNA sequence of LcIL-1β was amplified, sequenced and deposited in GenBank under accession number KP057877. The exons and introns of the LcIL-1β gene were identified by comparison with the cDNA sequence. The genomic DNA sequence of LcIL-1β was comprised of three introns and four exons that spanned approximately 1.8 kb, which is the same gene structure as European seabass, Atlantic halibut and spotted green pufferfish.
Alteration of LcIL-1β mRNA expression upon V. alginolyticus infection
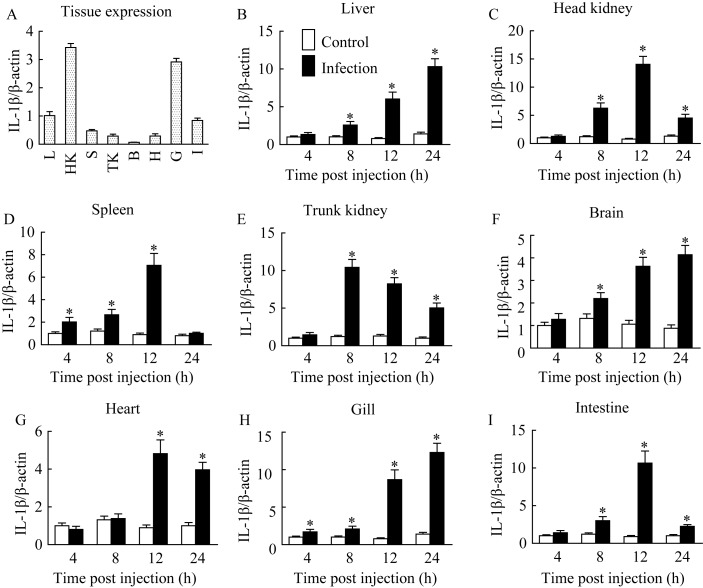
The LcIL-1β transcript exhibited constitutive expression in all tested tissues of healthy large yellow croaker, including the liver, spleen, heart, head kidney, trunk kidney, brain, intestine and gill. The highest LcIL-1β transcription level was in the head kidney, followed by the gill (Figure 3A). When fish were infected with V. alginolyticus, LcIL-1β transcription significantly increased in almost all tested tissues at 4 or 8 hpi compared with that of the control group (Figures 3B-I). The most significant LcIL-1β transcription upregulation was observed in the head kidney (14.1-fold) at 12 hpi, followed by the gill (12.3-fold) at 24 hpi (Figure 3).
Figure 3.
QPCR analysis of the relative mRNA expression of LcIL-1β in different tissues of healthy (A) and V. alginolyticus-challenged large yellow croaker (B-I) A: tissue expression profile of LcIL-1β in healthy fish. L: liver; HK: head kidney; S: spleen; TK: trunk kidney; B: brain; H: heart; G: gill; I: intestine. Results from three fish are expressed as means±SEM. *: P<0.05 versus PBS group.
Prokaryotic expression, purification and refolding of rLcIL-1β
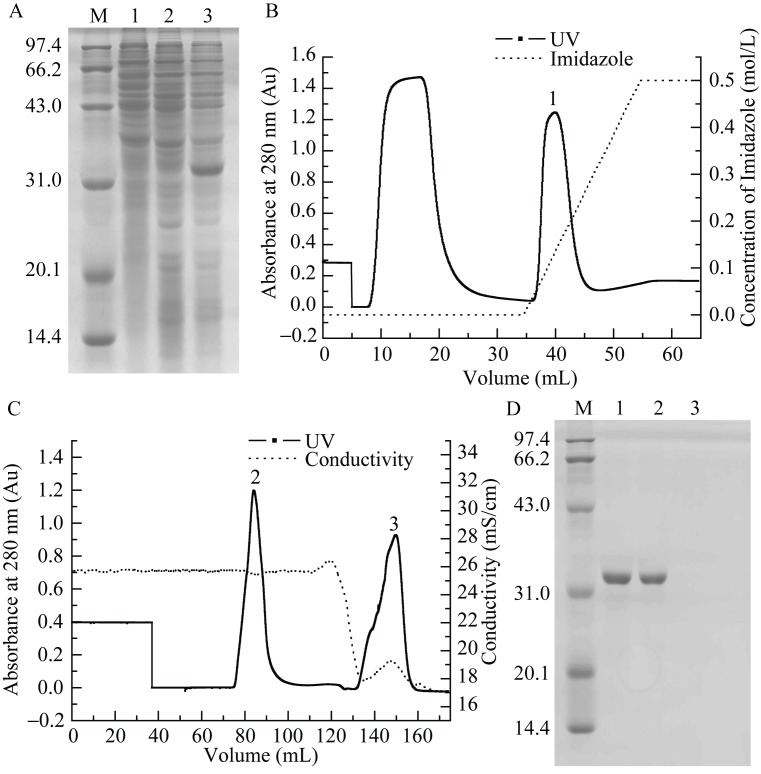
After IPTG induction, a protein band of expected MW (approximately 3.10×104: including intact LcIL-1β and an N-terminal histidine-tag) was observed by SDS-PAGE (Figure 4A). The purifying (Figure 4B) and refolding (Figure 4C) peaks were analyzed by SDS-PAGE (Figure 4D).
Figure 4.
Prokaryotic expression, purification and refolding of rLcIL-1β A: Bacterial lysates were electrophoresed on 12% SDS-PAGE gels. Lane M: protein marker; 1: E. coli BL21 (DE3) transformed with pET28a after IPTG induction; 2: E. coli BL21 (DE3) transformed with pET28a-LcIL-1β before IPTG induction; 3: E. coli BL21 (DE3) transformed with pET28a-LcIL-1β after IPTG induction. B: His affinity chromatography purification of rLcIL-1β using His TrapTM FF Crude column. C: Refolding of rLcIL-1β using urea gradient gel filtration on a Superdex 75 column. D: SDS-PAGE analysis of peaks in B and C. Lane M: protein marker; Lane 1: peak 1; 2: peak 2; 3: peak 3.
Effect of rLcIL-1β on survival rate of V. alginolyticus-infected fish
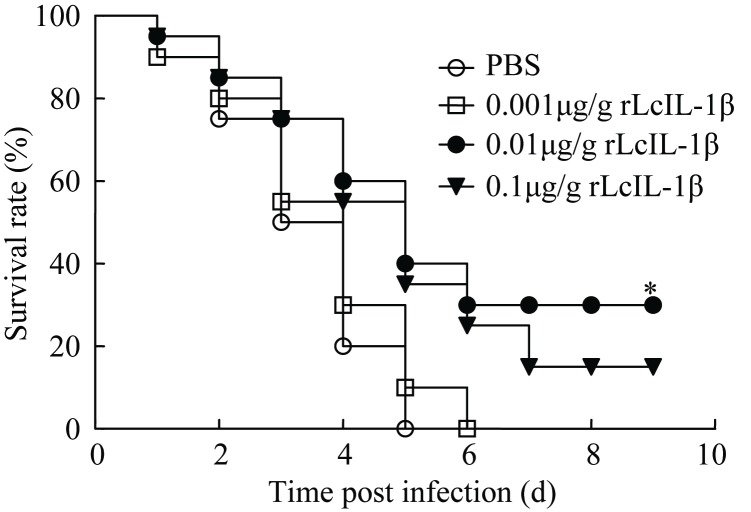
Under pathogen challenge, the 0.01 μg/g rLcIL-1β-treated group showed the highest survival rate out of all treatment groups. The survival rate of rLcIL-1β-treated fish was 30% in the group given 0.01 μg/g rLcIL-1β. However, at 0.1 μg/g rLcIL-1β, the survival rate appeared to be marginally inhibited. This mirrors the pattern previously described in rainbow trout in vitro, where IL-1 receptor saturation and/or receptor sensitization were suggested as possible mechanisms for post-optimal inhibition (Hong et al, 2003; Peddie et al, 2001). The 0.001 μg/g rLcIL-1β-treated group showed no significant increase in survival rate compared with the PBS-treated group, in which all fish were dead by day 6 (Figure 5).
Figure 5.
Effect of different doses of rLcIL-1β on the survival rate of V. alginolyticus-infected large yellow croaker Fish (20 in each group) were i.p.injected with V. alginolyticus (6.5×104 CFU/g). At 0.5 hpi, 0.001, 0.01 or 0.1 µg/g rLcIL-1β was i.p.injected into fish, respectively. The control group received an equal volume of PBS. Fish were monitored for signs of sickness and mortality every 24 h for 9 days. Group survival rates for each treatment were analyzed by the Log-rank test. *: P<0.05 versus PBS-treated group.
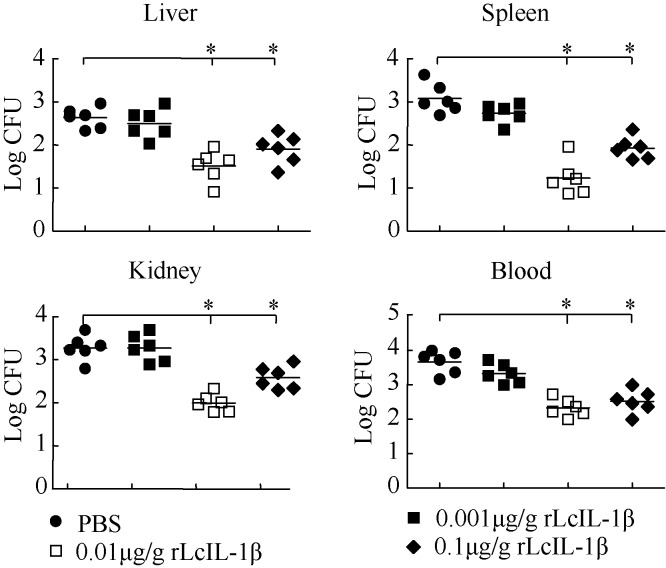
Effect of rLcIL-1β on bacterial load in V. alginolyticus-infected fish
The plate count method was employed to assay bacterial load (Li et al, 2014). Vibrio alginolyticus was undetectable in the liver, spleen, kidney and blood of healthy fish. The number of CFU per 0.1 g of tissue or per 0.1 mL of blood in V. alginolyticus-infected fish is shown in Figure 6. Compared with the PBS-treated group, the 0.01 and 0.1 μg/g rLcIL-1β-treated groups both showed significant reductions in bacterial load in the liver, spleen, kidney and blood, while the 0.001 μg/g rLcIL-1β-treated group showed only a small amount of variation (Figure 6). The 0.01 μg/g rLcIL-1β-treated group achieved the best capacity for bacterial clearance in tissues. The bacterial loads in the liver, spleen, kidney and blood from the 0.01μg/g rLcIL-1β-treated group were 47±7, 17±2 and 126±23 CFU/0.1 g and 214±49 CFU/0.1 mL, respectively. For the control, the bacterial loads in the liver, spleen, kidney and blood from the PBS-treated group were 457±104, 1 202±198 and 1 862±289 CFU/0.1 g and 4 898 ±691 CFU/0.1 mL, respectively.
Figure 6.
Effect of different doses of rLcIL-1β on bacterial loads in immune tissues and blood of large yellow croaker Fish (6 in each group) were i.p.-injected with V. alginolyticus (6.5×104 CFU/g) and received 0.001, 0.01 or 0.1 µg/g of rLcIL-1β at 0.5 hpi, respectively. The control group received an equal volume of PBS. Fish were euthanized at 72 h post treatment of rLcIL-1β. Liver, spleen, kidney, and blood samples were collected. Homogenates and blood were cultured on TCBS agar plates. Colony numbers were normalized to volume (0.1 mL for blood) and tissue weight (0.1 g for liver, spleen and kidney). *: P<0.05 versus PBS-treated group.
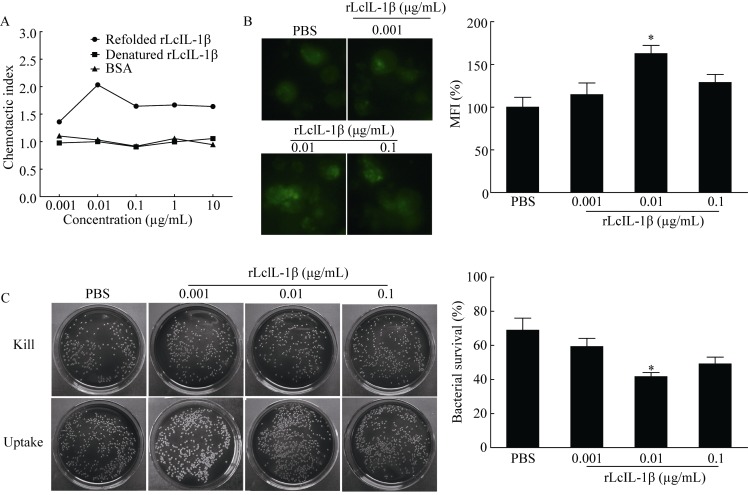
Effect of rLcIL-1β on MO/MΦ chemotaxis, phagocytosis and bactericidal activity
The refolded rLcIL-1β showed a dose-dependent chemotaxis activity to attract large yellow croaker MO/MΦ. The chemotactic index increased with increasing concentrations of refolded rLcIL-1β, showing a chemotactic index peak and reaching a maximum of 2.1 at 0.01 μg/mL (Figure 7A). In contrast, the denatured rLcIL-1β and BSA did not show evident chemotaxis activity. The phagocytosis ability of large yellow croaker MO/MΦ was significantly enhanced to approximately 1.6-fold after treatment with 0.01 μg/mL rLcIL-1β relative to that of treatment with PBS (Figure 7B). Moreover, a 0.001 or 0.1 μg/mL dose of rLcIL-1β also increased phagocytosis ability. Bacterial survival was determined by the CFU counting method to assess the bacterial killing of large yellow croaker MO/MΦ (Figure 7C). Incubation with rLcIL-1β at 0.01 μg/mL significantly decreased bacterial survival to approximately 61% of treatment with PBS.
Figure 7.
Effect of rLcIL-1β on MO/MΦ chemotaxis, phagocytosis and bactericidal activity A: Dose-response relationship of refolded rLcIL-1β to attract large yellow croaker MO/MΦ. Denatured rLcIL-1β and BSA were used as controls. B: Fluorescence images of phagocytosis of FITC-DH5α in MO/MΦ treated with rLcIL-1β. Histogram represents mean fluorescence intensity (MFI) percentage of bacteria engulfed by cells. Magnification ratio: 400X. C: Plates display survival V. alginolyticus from MO/MΦ treated with rLcIL-1β. Histogram demonstrates effects of rLcIL-1β on bacterial killing. Data are representative of at least three independent experiments. *: P<0.05 versus PBS-treated group.
DISCUSSION
IL-1β, which is the first characterized interleukin, plays a key role in regulating the fish immune response. In the present study, we determined the cDNA and genomic DNA sequences of an IL-1β gene from large yellow croaker. LcIL-1β had typical sequence characteristics of the animal IL-1 family (Angosto et al, 2013). Sequence comparisons and phylogenetic tree analysis both confirmed LcIL-1β to be a distinct member of the fish IL-1β family (Husain et al, 2012). In mammals, ICE specifically cleaves IL-1β after residue Asp (Asp116 in humans and Asp117 in mice, respectively), yielding a C-terminal secreted active form (Reis et al, 2012). However, this ICE cleavage site is not found in any known fish IL-1β, suggesting that fish Il-1β is possibly activated by another mechanism.
In teleosts, the tissue expression profile of IL-1β in healthy fish varies greatly in different species. For example, in half smooth tongue sole (Cynoglossus semilaevis) and ayu, high expression of IL-1β was observed in the head kidney, spleen and gill (Yu et al, 2012; Lu et al, 2013), but was not detected at all in haddock (Melanogrammus aeglefinus) (Corripio-Miyar et al, 2007). In this study, strong expression of LcIL-1β was observed in the head kidney and gill, similar to that reported in half smooth tongue sole and ayu. Previous studies have also revealed that IL-1β mRNA expression can be dramatically induced in fish upon bacterial infection (Cai et al, 2004; Lu et al, 2013). For example, Yersinia ruckeri infection significantly increased (thousand-fold) IL-1β transcription in the spleen of rainbow trout (Wang et al, 2009). The present study showed that V. alginolyticus infection induced the mRNA expression of LcIL-1β in all tested tissues of large yellow croaker, consistent with previous reports. This suggests that LcIL-1β was involved in the acute inflammatory response of these fish.
Recently, recombinant IL-1β (rIL-1β) was proven effective in promoting disease resistance in some fish (Hong et al, 2003; Buonocore et al, 2005). In rIL-1β-injected common carp, an increase in resistance to Aeromonas hydrophila infection was found compared with that of the control group (Kono et al, 2002).
In rainbow trout, i.p. injection of rIL-1β (starting at Ala95) prior to infection with A. salmonicida significantly reduced fish mortality (Hong et al, 2003). In this study, V. alginolyticus-infected large yellow croaker i.p. injected with a dose of 0.01 µg/g intact rLcIL-1β at 0.5 h following bacterial infection exhibited a survival rate of 30%, while all control fish died. The tissue bacterial load of rLcIL-1β-treated fish decreased significantly.
Research has shown that rIL-1β can induce cell migration (Ebisawa et al, 1992; Carrero et al, 2012) as well as increase phagocytosis and the bactericidal activity of leucocytes (Hong et al, 2003; Peddie et al, 2002). In humans, rIL-1β treatment induced significant migration of eosinophils (4 h, 5 ng/mL) (Ebisawa et al, 1992) and macrophages (4 h, 25 ng/mL) (Carrero et al, 2012). A rainbow trout IL-1β derived peptide, P3 (0.25 mM), enhanced phagocytosis and bactericidal activity of head kidney leucocytes in vitro (Peddie et al, 2001; Peddie et al, 2002). In the present study, the refolded rLcIL-1β showed chemotaxis activity to attract large yellow croaker MO/MΦ. The higher doses used (0.1, 1 and 10 μg/mL) were significantly less stimulatory, potentially attributable to rLcIL-1β toxicity for cells at this concentration or to receptor saturation (Buonocore et al, 2005). Incubation with 0.01 μg/mL rLcIL-1β demonstrated significant phagocytosis and bactericidal activity. This dose-response is consistent with that noted in humans (Ebisawa et al, 1992), rainbow trout (Hong et al, 2001) and European seabass (Buonocore et al, 2005).
IL-1β can induce the expression of macrophage-derived chemokine (MDC), which is involved in regulating leucocyte migration (Rodenburg et al, 1998) and enhancing phagocytosis and bactericidal activity of peritoneal macrophages (Matsukawa et al, 2000). IL-1β can also reduce pH and lead to acidification in alveolar macrophage endosomes, which is critical for the activation of cysteine proteases involved in bacterial degradation (Bird et al, 2009; Descamps et al, 2012). These results indicate that LcIL-1β could play an important role in phagocytosis and bactericidal activity of large yellow croaker MO/MΦ.
In conclusion, we characterized an IL-1β gene in large yellow croaker, which was tightly involved in the fish innate immune response. Animal experiments showed that even full length rLcIL-1β treatment could increase the survival rate and decrease bacterial load in fish following bacterial infection. It could also induce cell migration and increase phagocytosis and bactericidal activity of MO/MΦ in vitro.
Funding Statement
This project was supported by the National 863 Project (2012AA10A403), the Natural Science Foundation of Ningbo City of China (2014A610187) and the Scientific Research Foundation of Graduate School of Ningbo University (G14041)
REFERENCES
- [1].Angosto D, Montero J, López-Muñoz A, Alcaraz-Pérez F, Bird S, Sarropoulou E, Abellán E, Meseguer J, Sepulcre MP, Mulero V.2013. Identification and functional characterization of a new IL-1 family member, IL-1Fm2, in most evolutionarily advanced fish.Innate Immunity, 20(5): 133-500. [DOI] [PubMed] [Google Scholar]; Angosto D, Montero J, López-Muñoz A, Alcaraz-Pérez F, Bird S, Sarropoulou E, Abellán E, Meseguer J, Sepulcre MP, Mulero V.2013. Identification and functional characterization of a new IL-1 family member, IL-1Fm2, in most evolutionarily advanced fish.Innate Immunity, 20(5): 141-500. [DOI] [PubMed] [Google Scholar]
- [2].Bird PI, Trapani JA, Villadangos JA.2009. Endolysosomal proteases and their inhibitors in immunity.Nature Reviews Immunology, 9(12): 871-882. [DOI] [PubMed] [Google Scholar]
- [3].Buonocore F, Forlenza M, Randelli E, Benedetti S, Bossù P, Meloni S, Secombes CJ, Mazzini M, Scapigliati G.2005. Biological activity of sea bass (Dicentrarchus labrax L.) recombinant interleukin-1β.Marine Biotechnology, 7(6): 609-617. [DOI] [PubMed] [Google Scholar]
- [4].Cai ZH, Song LS, Gao CP, Wu LT, Qiu LH.2004. Molecular cloning and expression of interleukin 1 beta (IL-1β) from red sea bream (Pagrus major).Progress in Natural Science, 14(5): 396-402. [Google Scholar]
- [5].Carrero R, Cerrada I, Lledó E, Dopazo J, García-García F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A, Montero JA, Sepúlveda P.2012. IL1β induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-κB.Stem Cell Reviews and Reports, 8(3): 905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen J, Chen Q, Lu XJ, Li CH.2014. LECT2 improves the outcomes in ayu with Vibrio anguillarum infection via monocytes/macrophages.Fish and Shellfish Immunology, 41(2): 586-592. [DOI] [PubMed] [Google Scholar]
- [7].Chen XH, Lin KB, Wang XW.2003. Outbreaks of an iridovirus disease in maricultured large yellow croaker, Larimichthys crocea (Richardson), in China.Journal of Fish Diseases, 26(10): 615-619. [DOI] [PubMed] [Google Scholar]
- [8].Corripio-Miyar Y, Bird S, Tsamopoulos K, Secombes CJ.2007. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus).Molecular Immunology, 44(6): 1361-1373. [DOI] [PubMed] [Google Scholar]
- [9].Descamps D, Le Gars M, Balloy V, Barbier D, Maschalidi S, Tohme M, Chignard M, Ramphal R, Manoury B, Sallenave JM.2012. Toll-like receptor 5 (TLR5), IL-1β secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing.Proceedings of the National Academy of Sciences of the United States of America, 109(5): 1619-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ebisawa M, Bochner BS, Georas SN, Schleimer RP.1992. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1 beta-induced transendothelial migration.The Journal of Immunology, 149(12): 4021-4028. [PubMed] [Google Scholar]
- [11].Fujiki K, Shin DH, Nakao M, Yano T.2000. Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1β, high affinity immunoglobulin E Fc receptor β subunit and serum amyloid A.Fish and Shellfish Immunology, 10(3): 229-242. [DOI] [PubMed] [Google Scholar]
- [12].Hong S, Peddie S, Campos-Pérez JJ, Zou J, Secombes CJ.2003. The effect of intraperitoneally administered recombinant IL-1β on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss).Developmental and Comparative Immunology, 27(9): 801-812. [DOI] [PubMed] [Google Scholar]
- [13].Hong S, Zou J, Crampe M, Peddie S, Scapigliati G, Bols N, Cunningham C, Secombes CJ.2001. The production and bioactivity of rainbow trout (Oncorhynchus mykiss) recombinant IL-1β.Veterinary Immunology and Immunopathology, 81(1-2): 1-14. [DOI] [PubMed] [Google Scholar]
- [14].Husain M, Bird S, Van Zwieten R, Secombes CJ, Wang T.2012. Cloning of the IL-1β3 gene and IL-1β4 pseudogene in salmonids uncovers a second type of IL-1β gene in teleost fish.Developmental and Comparative Immunology, 38(3): 431-446. [DOI] [PubMed] [Google Scholar]
- [15].Kono T, Fujiki K, Nakao M, Yano T, Endo M, Sakai M.2002. The immune responses of common carp, Cyprinus carpio L., injected with carp interleukin-1β gene.Journal of Interferon & Cytokine Research, 22(4): 413-419. [DOI] [PubMed] [Google Scholar]
- [16].Li HX, Lu XJ, Li CH, Chen J.2014. Molecular characterization and functional analysis of two distinct liver-expressed antimicrobial peptide 2 (LEAP-2) genes in large yellow croaker (Larimichthys crocea).Fish and Shellfish Immunology, 38(2): 330-339. [DOI] [PubMed] [Google Scholar]
- [17].Li SY, Ao JQ, Chen XH.2009. Molecular and functional characterization of a cystatin analogue in large yellow croaker (Pseudosciaena crocea).Molecular Immunology, 46(8-9): 1638-1646. [DOI] [PubMed] [Google Scholar]
- [18].Lu XJ, Chen J, He YQ, Shi YH.2013. Molecular characterization of an IL-1β gene from ayu, Plecoglossus altivelis.Fish and Shellfish Immunology, 34(5): 1253-1259. [DOI] [PubMed] [Google Scholar]
- [19].Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL.2000. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response.The Journal of Immunology, 164(10): 5362-5368. [DOI] [PubMed] [Google Scholar]
- [20].Mu YN, Wan X, Lin KB, Ao JQ, Chen XH.2013. Liver proteomic analysis of the large yellow croaker (Pseudosciaena crocea) following polyriboinosinic: polyribocytidylic acid induction.Fish Physiology and Biochemistry, 39(5): 1267-1276. [DOI] [PubMed] [Google Scholar]
- [21].Peddie S, Zou J, Cunningham C, Secombes CJ.2001. Rainbow trout (Oncorhynchus mykiss) recombinant IL-1β and derived peptides induce migration of head-kidney leucocytes in vitro.Fish and Shellfish Immunology, 11(8): 697-709. [DOI] [PubMed] [Google Scholar]
- [22].Peddie S, Zou J, Secombes CJ.2002. A biologically active IL-1β derived peptide stimulates phagocytosis and bactericidal activity in rainbow trout, Oncorhynchus mykiss (Walbaum), head kidney leucocytes in vitro.Journal of Fish Diseases, 25(6): 351-360. [Google Scholar]
- [23].Reis MIR, Do Vale A, Pereira PJ, Azevedo JE, Dos Santos NM.2012. Caspase-1 and IL-1β processing in a teleost fish.PLoS One, 7(11): e50450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodenburg RJ, Brinkhuis RF, Peek R, Westphal J, Van Den Hoogen FH, Van Venrooij WJ, Van De Putte LB.1998. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1β, tumor necrosis factor alpha, and lipopolysaccharide.Journal of Leukocyte Biology, 63(5): 606-611. [DOI] [PubMed] [Google Scholar]
- [25].Scapigliati G, Buonocore F, Bird S, Zou J, Pelegrin P, Falasca C, Prugnoli D, Secombes CJ.2001. Phylogeny of cytokines: molecular cloning and expression analysis of sea bass Dicentrarchus labrax interleukin-1β.Fish and Shellfish Immunology, 11(8): 711-726. [DOI] [PubMed] [Google Scholar]
- [26].Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S.2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.Molecular Biology and Evolution, 28(10): 2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang TH, Bird S, Koussounadis A, Holland JW, Carrington A, Zou J, Secombes CJ.2009. Identification of a novel IL-1 cytokine family member in teleost fish.The Journal of Immunology, 183(2): 962-974. [DOI] [PubMed] [Google Scholar]
- [28].Yu Y, Zhong QW, Li CM, Jiang LM, Sun YY, Wang XB, Wang ZG, Zhang QQ.2012. Molecular cloning and characterization of interleukin-1β in half-smooth tongue sole Cynoglossus semilaevis.Veterinary Immunology and Immunopathology, 146(3-4): 270-276. [DOI] [PubMed] [Google Scholar]
- [29].Zhang RC, Chen J, Li CH, Lu XJ, Shi YH.2011. Prokaryotic expression, purification, and refolding of leukocyte cell-derived chemotaxin 2 and its effect on gene expression of head kidney-derived macrophages of a teleost fish, ayu (Plecoglossus altivelis).Fish and shellfish Immunology, 31(6): 911-918. [DOI] [PubMed] [Google Scholar]
- [30].Zou J, Grabowski PS, Cunningham C, Secombes CJ.1999. Molecular cloning of interleukin 1β from rainbow trout Oncorhynchus mykiss reveals no evidence of an ICE cut site.Cytokine, 11(8): 552-560. [DOI] [PubMed] [Google Scholar]