Abstract
As a group of intestinal hormones and neurotransmitters, cholecystokinins (CCKs) regulate and affect pancreatic enzyme secretion, gastrointestinal motility, pain hypersensitivity, digestion and satiety, and generally contain a DYMGWMDFG sequence at the C-terminus. Many CCKs have been reported in mammals. However, only a few have been reported in amphibians, such as Hyla nigrovittata, Xenopus laevis, and Rana catesbeiana, with none reported in urodele amphibians like newts and salamanders. Here, a CCK called CCK-TV was identified and characterized from the skin of the salamander Tylototriton verrucosus. This CCK contained an amino acid sequence of DYMGWMDF-NH2 as seen in other CCKs. A cDNA encoding the CCK precursor containing 129 amino acid residues was cloned from the cDNA library of T. verrucosus skin. The CCK-TV had the potential to induce the contraction of smooth muscle strips isolated from porcine gallbladder, eliciting contraction at a concentration of 5.0x10-11 mol/L and inducing maximal contraction at a concentration of 2.0x10-6 mol/L. The EC50 was 13.6 nmol/L. To the best of our knowledge, this is the first report to identify the presence of a CCK in an urodele amphibian.
Keywords: Cholecystokinin, Salamander, Skin, Amphibian
INTRODUCTION
Amphibian skins contain numerous bioactive compounds. Many bioactive peptides exerting defensive and regulatory or hormonal functions have been identified and characterized from amphibian secretions (Lu et al, 2010; Xu & Lai, 2015; Zhang, 2006). Recently, a few neurotoxins acting on ion channels, which also possibly play defensive roles, have been found in amphibian skins (Wu et al, 2011; You et al, 2009). Amphibian peptides with regulatory or hormonal functions are analogs of mammalian hormones and neurotransmitters such as bombesin, gastrin-releasing peptide, bradykinin, caerulein and cholecystokinin (CCK) (Bevins & Zasloff, 1990; Johnsen & Rehfeld, 1992; Lai et al, 2001, 2002; Wakabayashi et al, 1985).
CCKs are important intestinal hormones and neurotransmitters, and play major roles in the physiological regulation of pancreatic enzyme secretion and gastrointestinal (GI) motility (Rourke et al, 1997). Most amphibian CCKs are found in the brain and gastrointestinal tract of animals. Two CCK groups have been identified from the brain and small intestine of the bullfrog Rana catesbeiana using antiserum specific for the common C-terminus of mammalian gastrin and CCK (Rourke et al, 1997). The group of small peptides contains CCK-7 and CCK-8 and another group contains CCK-69 and CCK-70, which are identical to each other and to mammalian CCKs. For example, both CCK-69 and CCK-70 contain the monobasic and dibasic cleavage sites that give rise to CCK-33, CCK-39 and CCK-58 in mammals (Johnsen & Rehfeld, 1992; Johnsen, 1994). Recently, we identified a CCK from the frog skin of Rana nigrovittata (Liu et al, 2007). To date, however, all known amphibian CCKs have been identified in anuran amphibians only, with no CCK yet reported in urodele amphibians. To investigate whether amphibian CCKs are expressed in urodele amphibians, we purified a CCK and cloned cDNA encoding CCK precursors from Tylototriton verrucosus skin.
MATERIALS AND METHODS
Tylototriton verrucosus sample
As per our previous report, adult T. verrucosus (either sex, 20±5 g) were collected from the Yunnan province in China (Mu et al, 2014). They were anesthetized using 2.5% vaporized inhaled isoflurane and the dorsal skin was removed after cleansing with distilled water. The skin was homogenized by a tissue homogenizer with 0.1 mol/L phosphate buffer, pH 6.0 (PBS) (containing 1% (v/v) protease inhibitor cocktail, Sigma, USA, P8340-5). The skin homogenate solutions were quickly centrifuged (10 000 g for 10 min) at 4 ˚C and the supernatants were lyophilized. All experiments were approved by the Kunming Institute of Zoology, Chinese Academy of Sciences.
Peptide purification
An aliquot (1 g) of the lyophilized skin homogenate supernatant was dissolved in 10 mL PBS and centrifuged at 5 000 g for 10 min at 4 ˚C. The supernatant was applied to a Sephadex G-50 (Superfine, Amersham Biosciences, Sweden, 2.6 cm diameter, 100 cm length) gel filtration column equilibrated with 0.1 mol/L PBS for preliminary separation. Elution was performed with the same buffer, collecting fractions of 3.0 mL. The eluted fractions were monitored at 280 nm. The fraction containing smooth muscle contraction-inducing activity was further purified by a C18 reversed-phase high performance liquid chromatography column (RP-HPLC, Gemini C18 column, Phenomenex, USA, 5 μm particle size, 110 Å pore size, 250 mm length, 4.6 mm diameter,). Elution was performed using a linear gradient of 0-80% acetonitrile containing 0.1% (v/v) trifluoroacetic acid in 0.1% (v/v) trifluoroacetic acid/water over 60 min. UV-absorbing peaks were collected and lyophilized. Each purification step was traced by assaying for contraction of smooth muscle strips isolated from porcine gallbladder, as described below.
Primary structure analysis
Peptide sequencing was performed by automated Edman degradation analysis on a pulsed liquid-phase Shimadzu protein sequencer (PPSQ-31A, Shimadzu, Japan) according to the manufacturer’s instructions. Mass spectrometry (MS) was undertaken on an UltraFlex I mass spectrometer (Bruker Daltonics, Germany) by spotting the tested sample (0.5 μL) in 0.1% (v/v) trifluoroacetic acid/water onto a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) plate with a 0.5 μL α-cyano-4-hydroxycinnamic acid matrix (10 mg/mL in 60% acetonitrile). The MS analysis was run in positive ion mode.
Construction and screening of cDNA library
A cDNA library was constructed according to previous research (Mu et al, 2014) using a SMARTTM cDNA Library Construction Kit (Clontech, USA/Canada). The synthesized cDNA was used as a template for PCR to screen the cDNAs encoding the CCK. Two pairs of oligonucleotide primers, (S1: 5’-GA(T/C)TA(C/T)ATGGG(A/T/C/G)TGGATGGA(T/C)TT(T/C)-3’, according to the sequence determined by Edman degradation, in the antisense direction, and primer II A: 5′-AAGCAG TGGTATCAACGCAGAGT-3; S2: 5’-ATGGAGCTATGCCTC ATACTCAC-3′ and primer II A) were used in PCR reactions. The PCR conditions were: 2 min at 95 °C, and 30 cycles of 10 sec at 92 °C, 30 sec at 50 °C, 40 sec at 72 °C followed by a 10 min extension at 72 °C. The PCR products were cloned into a pGEM®-T Easy vector (Promega, Madison, WI, USA). DNA sequencing was performed on a model ABI PRISM 377 DNA sequencer (Applied Biosystems, USA).
Contraction of smooth muscle strips isolated from porcine gallbladder
Preparation of porcine gallbladder muscle strips was according to Nielsen et al (1998). Immediately after removal, the gallbladders were rinsed of bile with Krebs bicarbonate buffer of the following composition, in mmol/L: NaCl, 120.0; KCl, 5.9; MgCl2, 1.2; CaCl2, 2.5; NaH2PO4, 1.4; NaHCO3, 14.9; and glucose, 11.5. The medium was maintained at 37°C and constantly bubbled with a mixture of 95% O2 and 5% CO2, resulting in a pH of 7.4. The gallbladders were dissected free from serosa. Strips (2×10 mm) were cut in the longitudinal direction from the midregion of the gallbladder corpus. They were left for 1 h in Krebs medium, before being attached to strain gauge transducers with a force of 10 mN. After an equilibration period of 45 min, the experiments were performed with cumulative concentrations of CCK-TV peptide.
Synthetic peptide
CCK (DYMGWMDF-NH2, Phe in C-terminus was amidated) was synthesized by GL Biochem Ltd. (Shanghai, China) and analyzed by HPLC and mass spectrometry to confirm purity greater than 98%. The smooth muscle contraction-inducing activity of the synthesized peptide was confirmed to be the same as the natural peptide.
Statistics
All data in this paper are presented as means±SD and were analyzed by Student’s t-tests following parametric one-way analysis of variance.
RESULTS
Purification of CCK-TV
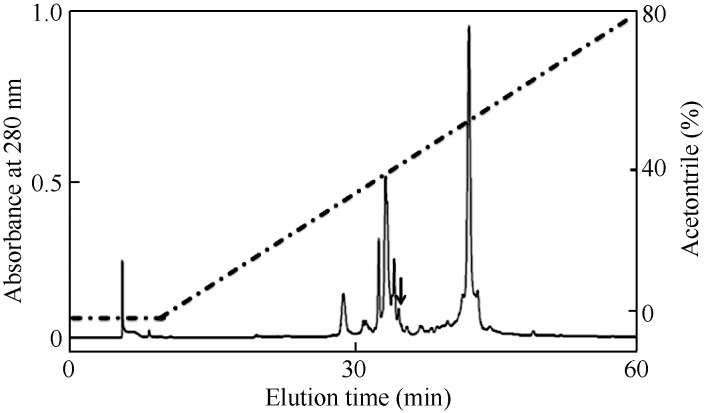
As reported in our previous work (Mu et al, 2014), the skin homogenate supernatant of T. verrucosus was divided into six fractions after Sephadex G-50 gel filtration. The fraction (Fraction VI) containing activity to induce the contraction of smooth muscle strips isolated from porcine gallbladder was pooled and subjected to a C18 RP-HPLC column for further purification (Figure 1). The purified peptide was named CCK-TV (marked by an arrow).
Figure 1.
Purification of CCK-TV from salamander Tylototriton verrucosus by C18 reversed-phase high performance liquid chromatography Peptide purification from the lyophilized skin homogenate supernatant of the salamander was as per our previous method using Sephadex G-50 gel filtration followed by C18 reversed-phase high performance liquid chromatography (RP-HPLC). Each purification step was traced by assaying for contraction of smooth muscle strips isolated from porcine gallbladder. The purified peptide is marked by an arrow.
Structural characterization
The complete amino acid sequence of purified CCK-TV was determined as DYMGWMDF by Edman degradation. CCK-TV was composed of eight amino acid residues. MALDI-TOF-MS gave an observed mass of 1063.1, well-matched to the theoretical molecular weight (1063.2) of CCK-TV containing an amidated C-terminus Phe as seen in other CCKs. The presence of an amidated C-terminus Phe in native CCK-TV was further confirmed by the synthesized peptide, which had the same observed mass and RP-HPLC elution manner as that of the native CCK-TV.
cDNA cloning
The nucleotide sequence encoding the CCK-TV precursor and the encoded amino acid sequence are shown in Figure 2. The sequence contained a coding region of 387 nucleotides and the encoded amino acid sequence corresponded to a polypeptide of 129 amino acids, including mature CCK-TV. A BLAST search indicated that the precursor was a member of the CCK family and contained a conserved DYMGWMDFG domain.
Figure 2.
Nucleotide sequence encoding CCK-TV from salamander skin and the amino acid sequence of the precursor polypeptide The mature CCK-TV peptide sequence is boxed. *: Stop codon.
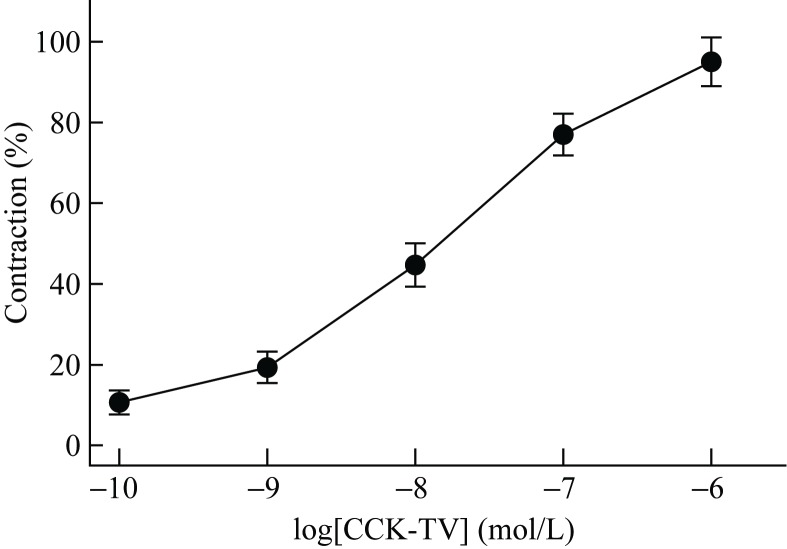
Induction of bladder smooth muscle contraction
Concentration-response experiments were performed with the CCK-TV. The peptide induced contractions in a concentration-dependent manner (Figure 3). CCK-TV elicited a contraction at a concentration of 5.0x10-11 mol/L and induced maximal contraction at a concentration of 2.0x10-6 mol/L. The EC50 value, expressed as -log(mol/L), was 7.87±0.21 (n=5), which corresponded to 13.6 nmol/L.
Figure 3.
Contraction of porcine gallbladder strips by CCK-TV Each gallbladder strip was stimulated with cumulative increases of CCK-TV in alternated order. Responses are expressed as percentages of the maximal contraction induced by CCK-TV. Values represent mean±SE (n=5).
DISCUSSION
CCKs are important intestinal hormones and neurotransmitters that regulate or affect pancreatic enzyme secretion, gastrointestinal motility, pain hypersensitivity, and digestion and satiety. CCKs, which generally contain DYMGWMDFG at the C-terminus, have been extensively reported in mammals and in some anuran amphibians, including H. nigrovittata, X. laevis, and R. catesbeiana (Xu & Lai, 2015). To date, however, no CCKs have been reported in urodele amphibians. In this study, an amphibian CCK was purified from the skin of Tylototriton verrucosus, which is the first report of a CCK from salamander skin. To confirm the presence of CCK in the salamander skin, cDNA clones encoding CCKs were screened from the skin cDNA library of Tylototriton verrucosus. Results indicated that CCKs could be expressed in the skins of urodele amphibians like salamanders, as other amphibian skin-gut-brain triangle peptides (Erspamer et al, 1981).
The diversion of the CCK/gastrin family can be dated early in vertebrate history (Oliver & Vigna, 1996). The CCK/gastrin family may join the increasing number of peptide families that showed early expansion during vertebrate evolution, although the gap between amphibia and the most ‘‘original’’ vertebrates still needs to be filled. However, the existence of CCKs in urodele salamanders may help fill this gap.
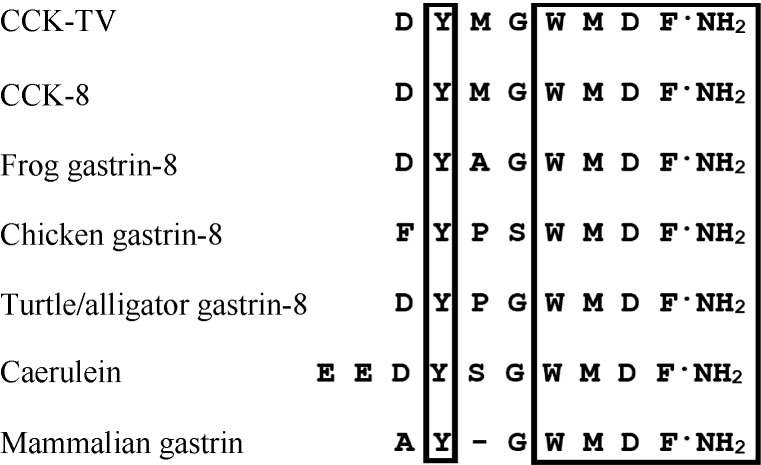
The C-terminal of salamander CCK-TV and frog CCK-8 were identical (Figure 4). As expected for a mammalian system, CCK-TV stimulated porcine gallbladder contraction (Figure 3). The EC50 of CCK-TV on porcine gallbladder contraction was 13.6 nmol/L, which was higher than that of CCK-8 (7.2 nmol/L) (Nielsen et al, 1998). This difference may result from the different modification of the peptides, since the Tyr of the CCK-8 sample was O-sulfated. Amphibian CCKs and gastrin were only distinguished by the substitution of Ala for Met in position six from the C-terminus (Figure 4). Chicken gastrin exhibits clear gastrin properties in both birds and mammals lack of CCK-like properties (Dimaline & Lee, 1990). This was attributed to steric effects of proline residue in position six from the C-terminus (Figure 4). Also, the alligator contains two distinct receptors that discriminate between CCK and gastrin (Oliver & Vigna, 1997). In mammals, the Tyr residue shifted to position six from the C-terminus (Figure 4), which induces a major change in binding to the CCK-A receptor (Oliver & Vigna, 1997). We did not identify gastrin peptide in Tylototriton verrucosus. Further work is needed to clarify whether gastrin peptides exist in salamander and their interactions with the receptors.
Figure 4.
Sequence alignment of CCK/gastrin peptides Conserved amino acid residues in all sequences are boxed. Gaps are added to acquire maximum identity.
Funding Statement
This work was supported by the National Basic Research Program of China (973 Program) (2013CB911300), National Natural Science Foundation of China (U1132601) and the Chinese Academy of Sciences (SAJC201308)
REFERENCES
- [1].Bevins CL, Zasloff M.1990. Peptides from frog skin.Annual Review of Biochemistry, 59: 395-414. [DOI] [PubMed] [Google Scholar]
- [2].Dimaline R, Lee CM.1990. Chicken gastrin: A member of the gastrin/CCK family with novel structure-activity relationships.The American Journal of Physiology, 259(5 Pt 1): G882-G888. [DOI] [PubMed] [Google Scholar]
- [3].Erspamer V, Melchiorri P, Broccardo M, Erspamer GF, Falaschi P, Improta G, Negri L, Renda T.1981. The brain-gut-skin triangle: new peptides.Peptides, 2(Suppl. 2): 7-16. [DOI] [PubMed] [Google Scholar]
- [4].Johnsen AH, Rehfeld JF.1992. Identification of cholecystokinin/gastrin peptides in frog and turtle. Evidence that cholecystokinin is phylogenetically older than gastrin.European Journal of Biochemistry, 207(2): 419-428. [DOI] [PubMed] [Google Scholar]
- [5].Johnsen AH.1994. Identification of cholecystokinin from frog and turtle. Divergence of cholecystokinin and gastrin occurred before the evolution of amphibia.European Journal of Biochemistry, 224(2): 691-702. [DOI] [PubMed] [Google Scholar]
- [6].Lai R, Liu H, Lee W H, Zhang Y.2001. A novel bradykinin related peptide from skin secretions of toad Bombina maxima and its precursor containing six identical copies of the final product.Biochemical and Biophysical Research Communications, 286(2): 259-263. [DOI] [PubMed] [Google Scholar]
- [7].Lai R, Liu H, Lee WH, Zhang Y.2002. A novel proline rich bombesin-related peptide (PR-bombesin) from toad Bombina maxima.Peptides, 23(3): 437-442. [DOI] [PubMed] [Google Scholar]
- [8].Liu X H, Wang Y P, Cheng L H, Song Y Z, Lai R.2007. Isolation and cDNA cloning of cholecystokinin from the skin of Rana nigrovittata.Peptides, 28(8): 1540-1544. [DOI] [PubMed] [Google Scholar]
- [9].Mu L, Tang J, Liu H, Shen C, Rong M, Zhang Z, Lai R.2014A potential wound-healing-promoting peptide from salamander skin.The FASEB Journal, 2014, 28(9): 3919-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lu QM, Lai R, Zhang Y.2010. Animal toxins and human disease: from single component to venomics, from biochemical characterization to disease Mechanisms, from crude venom utilization to rational drug design.Zoological Research, 31(1): 2-16. (in Chinese) [DOI] [PubMed] [Google Scholar]
- [11].Nielsen KG, Bomgren P, Holmgren S, Johnsen AH.1998. Gastrin and cholecystokinin of the bullfrog, Rana catesbeiana, have distinct effects on gallbladder motility and gastric acid secretion in vitro.General and Comparative Endocrinology, 112(2): 247-254. [DOI] [PubMed] [Google Scholar]
- [12].Oliver AS, Vigna SR.1996. CCK-X receptors in the endothermic mako shark (Isurus oxyrinchus).General and Comparative Endocrinology, 102(1): 61-73. [DOI] [PubMed] [Google Scholar]
- [13].Oliver AS, Vigna SR.1997. CCK-A- and CCK-B-like receptors in the gallbladder and stomach of the alligator (Alligator mississippiensis).General and Comparative Endocrinology, 105(1): 91-101. [DOI] [PubMed] [Google Scholar]
- [14].Rourke IJ, Rehfeld JF, Møller M, Johnsen AH.1997. Characterization of the cholecystokinin and gastrin genes from the bullfrog, Rana catesbeiana: evolutionary conservation of primary and secondary sites of gene expression.Endocrinology, 138(4): 1719-1727. [DOI] [PubMed] [Google Scholar]
- [15].Wakabayashi T, Kato H, Tachibana S.1985. Complete nucleotide sequence of mRNA for caerulein precursor from Xenopus skin: the mRNA contains an unusual repetitive structure.Nucleic Acids Research, 13(6): 1817-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu J, Liu H, Yang H L, Yu H N, You D W, Ma Y F, Ye H H, Lai R.2011. Proteomic analysis of skin defensive factors of tree frog Hyla simplex.Journal of Proteome Research, 10(9): 4230-4240. [DOI] [PubMed] [Google Scholar]
- [17].Xu X Q, Lai R.2015. The chemistry and biological activities of peptides from amphibian skin secretions.Chemical Reviews, 115(4): 1760-1846. [DOI] [PubMed] [Google Scholar]
- [18].You D W, Hong J, Rong M Q, Yu H N, Liang S P, Ma Y F, Yang H L, Wu J, Lin D H, Lai R.2009. The first gene-encoded amphibian neurotoxin.The Journal of Biological Chemistry, 284(33): 22079-22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y.2006. Amphibian skin secretions and bio-adaptive significance — Implications from Bombina maxima skin secretion proteome.Zoological Research, 27(1): 101-112. (in Chinese) [Google Scholar]