Abstract
We established small interfering RNA (siRNA) directed against poly(ADP-ribose) polymerase 1 (PARP-1) that effectively reduces the expression of PARP-1 in two human cell lines. Established siRNA against PARP-1 significantly suppressed human immunodeficiency virus type 1 (HIV-1) replication, as well as the activation of the integrated HIV-1 long terminal repeat promoter. These results indicate that PARP-1 is required for efficient HIV-1 replication in human cells. We propose that PARP-1 may serve as a cellular target for RNA interference-mediated gene silencing to inhibit HIV-1 replication.
Poly(ADP-ribose) polymerase 1 (PARP-1) is an abundant nuclear enzyme that catalyzes the successive transfer of the ADP-ribose moiety of NAD+ to a variety of nuclear proteins, including itself (8, 19). Recent studies using cells derived from PARP-1 knockout (PARP-1−/−) mice (17, 29) provide evidence that PARP-1 is required for the activation of NF-κB-dependent target genes, including the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) in reporter constructs (12, 21, 22). In addition, HIV-1 integration is abolished in PARP-1−/− cells (11), suggesting that PARP-1 plays a role in HIV-1 integration. Based on these reports, PARP-1 is probably an important cellular factor involved in HIV-1 replication. Although these studies propose that PARP-1 is involved in HIV-1 replication in murine cells, the function of PARP-1 in HIV-1 replication in human cells remains unclear. To assess the significance of PARP-1 in HIV-1 replication in natural host cells, we developed small interfering RNA (siRNA) directed against PARP-1 and examined the effect of established siRNA on HIV-1 gene expression as well as viral replication in human cells.
Establishment of siRNA directed against PARP-1.
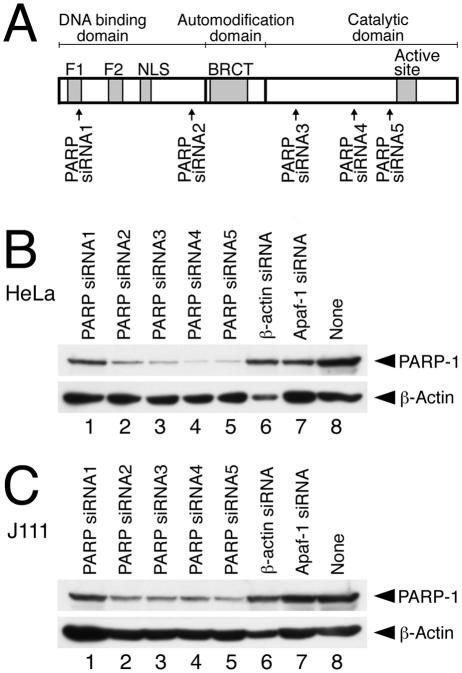
Silencing of gene expression by RNA interference (RNAi) is a new methodology used to assess the role of cellular and viral factors in HIV-1 replication. siRNA, typically comprising a duplex of two 21-mer RNAs with 19 complementary nucleotides and 3′-terminal noncomplementary dimers of uridine, can induce the RNAi-mediated specific suppression of target genes in mammalian cells (9). Five siRNA candidates directed against PARP-1 were designed according to the information found at the siRNA Target Finder website (http://www.ambion.com/techlib/misc/siRNA_finder.html) and were prepared with a Silencer siRNA construction kit (Ambion, Austin, Tex.), according to the manufacturer's instructions. The nucleotide sequences of the siRNA target sites in the PARP-1 gene (Fig. 1A) were as follows: PARP siRNA1, 5′-AAAAGTCCCACACTGGTACCA-3′ (nucleotides [nt] 297 to 317); PARP siRNA2, 5′-AACCCCAAAGGAATTCCGAGA-3′ (nt 1161 to 1181); PARP siRNA3, 5′-AACAAACTGGAACAGATGCCG-3′ (nt 1954 to 1974); PARP siRNA4, 5′-AAGCCTCCGCTCCTGAACAAT-3′ (nt 2401 to 2421); and PARP siRNA5, 5′-AAGATAGAGCGTGAAGGCGAA-3′ (nt 2671 to 2691). All of the position numbers of the PARP-1 nucleotide sequences described here correspond to those of the human PARP-1 cDNA sequence (GenBank accession no. M32721). As controls, siRNAs directed against β-actin and Apaf-1 were purchased from QIAGEN (Tokyo, Japan). RNAiFect transfection reagent (QIAGEN) showed the highest transfection efficiency of the siRNA as well as the lowest cytotoxicity among several kinds of transfection reagents tested and thus was used throughout the experiments. In order to examine the effect of siRNAs for the expression of PARP-1 as well as for HIV-1 replication in various human cell lines, HeLa and J111 (a human acute monocytic leukemia cell line) cells were used in this study. HeLa (Fig. 1B) and J111 (Fig. 1C) cells were transfected with siRNAs against PARP-1 (143 nM), as well as control siRNAs. Forty-eight hours after transfection, cells were lysed in a sample buffer containing sodium dodecyl sulfate (SDS). Samples were separated by SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. Then immunoblot analysis for PARP-1 and β-actin was performed. As shown in Fig. 1B and C, the expression of PARP-1 in cells transfected with PARP siRNA2 (lane 2), PARP siRNA 3 (lane 3), PARP siRNA4 (lane 4), or PARP siRNA5 (lane 5) was significantly reduced compared with that in mock-transfected cells (lane 8). On the other hand, PARP siRNA1 (lane 1) as well as control siRNAs (lanes 6 and 7) had almost no effect on the level of PARP-1 (Fig. 1B and C). In addition, the level of β-actin was moderately reduced in cells transfected with siRNA against β-actin (Fig. 1B and C, lane 6). Four siRNAs against PARP-1 (lanes 2 to 5) reduced the level of PARP-1 more effectively in HeLa cells (Fig. 1B) than in J111 cells (Fig. 1C). This might be due to the higher endogenous level of PARP-1 in J111 cells than in HeLa cells (M. Kameoka, unpublished results) and/or may be due to less efficient transfection of siRNAs into J111 cells. In fact, a lower concentration (72 nM) of PARP siRNA5 still effectively suppressed the expression of PARP-1 in HeLa cells, but not in J111 cells (data not shown). PARP siRNA5 most successfully suppressed the expression of PARP-1 in both cell lines (Fig. 1B and C, lane 5), and PARP siRNA4 also effectively suppressed the expression of PARP-1 in HeLa cells (Fig. 1B, lane 4). Thus, we used PARP siRNA4 and PARP siRNA5 for further studies.
FIG. 1.
(A) Schematic illustration of functional domains of the human PARP-1. F1 and F2, Zn fingers; NLS, nuclear localization signal; BRCT, BRCA1 C-terminal domain. Arrows below the diagram represent the target sites of siRNA designed in this study. (B and C) Immunoblot analysis of cells transfected with siRNA. HeLa (B) and J111 (C) cells were transfected with the indicated siRNAs (143 nM) (lanes 1 to 7) or mock transfected (lane 8). Forty-eight hours after transfection, the cells were harvested. Then samples were immunostained with anti-PARP polyclonal antibody (R&D Systems, Minneapolis, Minn.) (upper panel) or anti-β-actin monoclonal antibody (Sigma-Aldrich) (lower panel). After incubation of the samples with peroxidase-labeled secondary antibodies, the immunocomplex was visualized with ECL Plus enhanced chemiluminescence Western blotting detection reagents (Amersham-Pharmacia Biotech).
siRNA directed against PARP-1 significantly suppresses the single replication cycle of the VSVG-pseudotyped luciferase reporter virus.
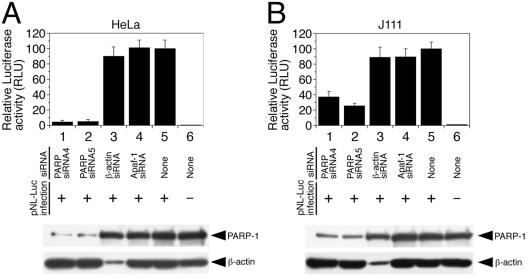
We next examined the efficiency of a single-round cycle of HIV-1 replication in cells transfected with siRNA directed against PARP-1. For this purpose, vesicular stomatitis virus glycoprotein (VSVG)-pseudotyped luciferase reporter virus (pNL-Luc) was prepared by transfecting 293T cells with a pNL4-3-based (1) proviral construct bearing a luciferase reporter gene in place of the viral nef gene, pNL-Luc-E−R+ (5, 7), and VSVG expression vector, pHIT/G (10), using FuGENE 6 transfection reagent (Roche). The concentration of p24Gag antigen released into the culture fluid 48 h after transfection was determined by enzyme-linked immunosorbent assay (ZeptoMetrix Corp., Buffalo, N.Y.). Using this reporter virus, the efficiency of early phases of the HIV-1 replication cycle, including reverse transcription, integration, RNA transcription, and protein translation could be monitored by measuring the luciferase activity in infected cells (5, 7). HeLa (Fig. 2A) and J111 (Fig. 2B) cells were transfected with the indicated siRNAs (143 nM). Then, the cells were infected with pNL-Luc (50 ng of p24). Forty-eight hours after infection, luciferase activity in the infected cells was measured. As shown in Fig. 2A, the luciferase activity in HeLa cells transfected with PARP siRNA4 (lane 1) or PARP siRNA5 (lane 2) was less than 6% of that for cells mock transfected (lane 5) or transfected with control siRNAs (lanes 3 and 4). In addition, the levels of luciferase activity in J111 cells transfected with PARP siRNA4 (lane 1) or PARP siRNA5 (lane 2) were lower by approximately 37 and 24%, respectively, than that of mock-transfected cells (lane 5) (Fig. 2B). These results suggest that siRNAs directed against PARP-1 significantly suppress a single replication cycle of HIV-1 in HeLa (Fig. 2A) and J111 (Fig. 2B) cells. siRNA against PARP-1 suppressed the replication of reporter virus more efficiently in HeLa cells (Fig. 2A) than in J111 cells (Fig. 2B). This might be in part due to PARP siRNA4 and PARP siRNA5 suppressing the levels of PARP-1 more effectively in HeLa cells (Fig. 1B) than in J111 cells (Fig. 1C).
FIG. 2.
siRNA directed against PARP-1 suppresses HIV-1 replication. HeLa (A) and J111 (B) cells were transfected with the indicated siRNAs (143 nM) (lanes 1 to 4) or mock transfected (lanes 5 and 6). Twenty-four hours after siRNA transfection, the cells were mock infected (lane 6) or infected with pNL-Luc (50 ng of p24) (lanes 1 to 5). Forty-eight hours after infection, the cells were lysed in 100 μl of lysis buffer (LCβ-PCG-51; Toyo Ink Mfg., Tokyo, Japan) and the luciferase activity in 20-μl aliquots of lysate was determined with a luciferase assay system (Promega) and TR717 microplate luminometer (Applied Biosystems). Then the relative luciferase activity was calculated by comparing it with the luciferase activity of mock-transfected, pNL-Luc-infected samples (lane 5), which was defined as 100 relative light units (RLU). The data represent the means and standard deviations (error bars) of six independent experiments. The expression levels of PARP-1and β-actin were confirmed by immunoblot analysis (bottom).
In addition, we examined the amount of HIV-1 DNA in siRNA-transfected, pNL-Luc-infected cells. HeLa and J111 cells were transfected with the indicated siRNAs and then were infected with pNL-Luc, as described above. Forty-eight hours after infection, the high-molecular-weight DNA was extracted from the cells. Then 2.5-μl aliquots of extracted DNA were mixed with 22.5 μl of reaction mixture containing 2× TaqMan universal PCR master mix (Applied Biosystems), 300 nM senseprimer (5′-CAGTAGATCCTAGACTAGAGCCCT-3′; nt5837 to 5860 of pNL4-3), 300 nM antisense primer (5′-AGATGCCTAAGGCTTTTGTCAT-3′; nt 5965 to 5944), and 200 nM TaqMan probe (5′-FAM-AGCATCCAGGAAGTCAGCCTAAAACTGCTT-TAMRA-3′; nt 5864 to 5893). The real-time PCR amplification was carried out with ABI PRISM 7900HI (Applied Biosystems), according to the manufacturer's instructions. In addition, β-actin DNA was quantitatively amplified with the TaqMan β-actin control reagents (Applied Biosystems). As shown in Table 1, siRNAs against PARP-1 had almost no effect on the level of HIV-1 DNA in the high-molecular-weight DNA of HeLa cells. In addition, the level of HIV-1 DNA in J111 cells transfected with PARP siRNA4 was rather moderately increased compared with that in mock-transfected cells.
TABLE 1.
Quantification of HIV-1 DNA in siRNA-transfected, pNL-Luc-infected cells
| siRNA | HIV-1 DNA copy no. (copies/104 cells)a
|
|
|---|---|---|
| HeLa | J111 | |
| PARP siRNA4 | 483.5 ± 29.5 | 843.3 ± 32.1 |
| PARP siRNA5 | 551.4 ± 60.0 | 644.2 ± 45.8 |
| β-Actin siRNA | 465.0 ± 15.8 | 595.7 ± 40.0 |
| Apaf-1 siRNA | 397.7 ± 7.0 | 564.0 ± 28.4 |
| None | 499.1 ± 27.9 | 519.7 ± 31.6 |
HIV-1 DNA in the infected cells was quantitatively amplified by real-time PCR. The linear standard curve ranging from 106 to 102 copies of HIV-1 DNA was generated from a series of dilutions with plasmid pNL-Luc-E−R+, and the number of HIV-1 DNA copy was calculated with SDS 2.0 software (Applied Biosystems). In addition, β-actin DNA was quantified in order to determine the input level of cellular DNA in the samples and was used as an endogenous reference to normalize variations due to differences in the cell count or DNA extraction. The normalized value of the HIV-1 DNA was calculated as (HIV-1 copy number/β-actin copy number) × 104 and is expressed as the number of HIV-1 DNA copies per 104 cells. All values represent means ± standard errors for three independent experiments. Note that the numbers of nonspecific amplification products in mock-infected samples were less than 1.4 ± 0.2 copy per 104 cells.
siRNA directed against PARP-1 inhibits the activation of integrated HIV-1 LTR promoter.
MAGIC-5A, CCR5-expressing MAGI (HeLa-CD4-LTR-β-gal) (14, 18), contains a single integrated copy of HIV-1 LTR linked to the β-galactosidase gene. Using this cell line, we next examined whether siRNAs against PARP-1 suppress the activation of integrated HIV-1 LTR promoter. When MAGIC-5A cells were treated with tumor necrosis factor alpha (TNF-α) (Pepro Tech EC, Ltd., London, United Kingdom) or phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich), the level of β-galactosidase activity was elevated in MAGIC-5A cells mock transfected (Fig. 3, lane 5) or transfected with control siRNAs (lanes 3 and 4). In contrast, TNF-α and PMA only slightly enhanced β-galactosidase activity in MAGIC-5A cells transfected with siRNAs against PARP-1 (Fig. 3, lanes 1 and 2). These results suggest that the activation of integrated HIV-1 LTR promoter was suppressed in cells transfected with siRNA directed against PARP-1.
FIG. 3.
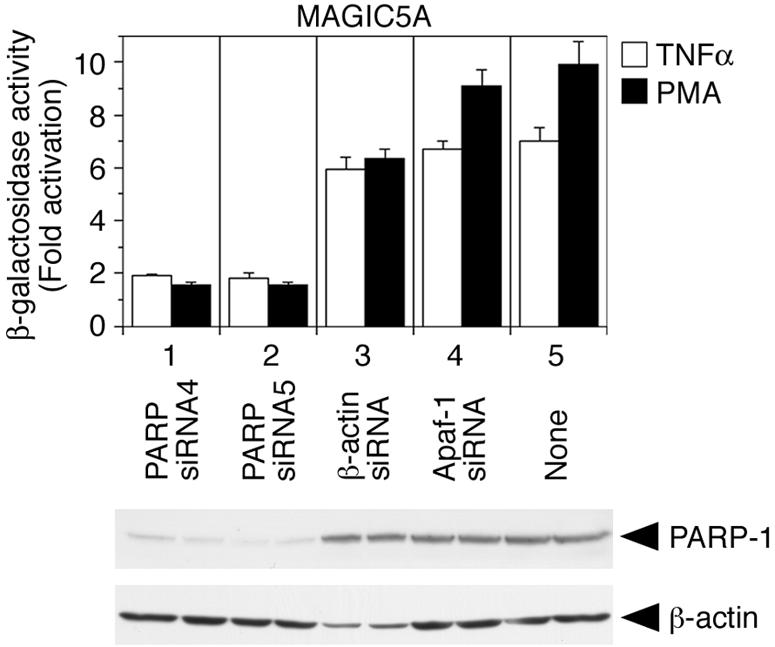
siRNA directed against PARP-1 suppresses the activation of the integrated HIV-1 LTR promoter in MAGIC-5A. MAGIC-5A cells were transfected with the indicated siRNAs (36 nM) (lanes 1 to 4) or mock transfected (lane 5). Forty-two hours after transfection, the cells were mock treated or treated with TNF-α (10 ng/ml) or PMA (100 nM) for 6 h. Then the cells were lysed in 100 μl of lysis buffer (LCβ-PCG-51), and the β-galactosidase activity in 10-μl aliquots of lysate was determined by using a Galacto-Light Plus system (Applied Biosystems) and TR717 microplate luminometer (Applied Biosystems). The β-galactosidase activities are indicated as the relative values of TNF-α-induced (white bar) and PMA-induced (black bar) enhancement (fold activation) over mock-treated samples. The data represent the means and standard deviations (error bars) of four independent experiments. The expression levels of PARP-1 and β-actin were confirmed by immunoblot analysis (bottom).
In this study, we established siRNA that effectively reduces the expression of PARP-1 (Fig. 1). In addition, we showed that siRNAs directed against PARP-1 can significantly suppress HIV-1 replication (Fig. 2), as well as the activation of the HIV-1 LTR promoter (Fig. 3). Previous reports showed that PARP-1 is required for the activation of NF-κB-dependent gene expression in murine cells (12, 21). In addition, although a discrepancy exists between two reports on the efficiency of integration of HIV-1-based lentivirus vectors in PARP-1−/− cells (11, 25), their results indicate that HIV-1 LTR promoter-dependent expression of green fluorescent protein is reduced in PARP-1−/− cells compared with PARP-1+/+ cells. Taken together with the observations made in this report, PARP-1 could be an important cofactor for efficient HIV-1 gene expression in human and murine cells.
We propose here that siRNAs directed against PARP-1 are useful to examine the mechanism by which PARP-1 regulates the HIV-1 replication in human cells. In addition, PARP-1 may be a therapeutic target for the development of anti-HIV-1 drugs. Three independent lines of PARP-1−/− mice were developed (16, 17, 29). These animals are viable and fertile and do not show an overtly abnormal phenotype, except for the spontaneous development of skin hyperplasia observed only in one line (29), indicating that PARP-1 is probably dispensable for normal life. Recent reports show that siRNAs directed against viral and cellular genes, which play important roles in the HIV-1 life cycle, can inhibit HIV-1 replication (2-4, 6, 13, 15, 20, 23, 24, 27) and therefore potentially be applied to the development of therapeutic agents against HIV-1 (26, 28). To prevent the appearance of siRNA-resistant mutants (3), it is necessary to develop a strategy that simultaneously targets multiple host factors, which are required for efficient viral replication (26, 28). We believe that PARP-1 may serve as a cellular target for RNAi-mediated gene silencing to inhibit HIV-1 replication.
Acknowledgments
We thank K. Tokunaga (Department of Pathology, National Institute of Infectious Diseases, Tokyo, Japan) for the gift of pNL-Luc-E−R+ and pHIT/G and M. Tatsumi (Department of Veterinary Science, National Institute of Infectious Diseases, Tokyo, Japan) for the gift of MAGIC-5A. The manuscript was proofread by Medical English Service (Kyoto, Japan).
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J., A. Banerjea, V. Planelles, and R. Akkina. 2003. Potent suppression of HIV type 1 infection by a short hairpin anti-CXCR4 siRNA. AIDS Res. Hum. Retrovir. 19:699-706. [DOI] [PubMed] [Google Scholar]
- 3.Boden, D., O. Pusch, F. Lee, L. Tucker, and B. Ramratnam. 2003. Human immunodeficiency virus type 1 escape from RNA interference. J. Virol. 77:11531-11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capodici, J., K. Karikó, and D. Weissman. 2002. Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J. Immunol. 169:5196-5201. [DOI] [PubMed] [Google Scholar]
- 5.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn, G. A., and B. R. Cullen. 2002. Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference. J. Virol. 76:9225-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 8.D'Amours, D., S. Desnoyers, I. D'Silva, and G. G. Poirier. 1999. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 342:249-268. [PMC free article] [PubMed] [Google Scholar]
- 9.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier, R. A. M., B. E. Meyer, J. H. M. Simon, U. Fischer, and M. H. Malim. 1997. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16:4531-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha, H. C., K. Juluri, Y. Zhou, S. Leung, M. Hermankova, and S. H. Snyder. 2001. Poly(ADP-ribose) polymerase-1 is required for efficient HIV-1 integration. Proc. Natl. Acad. Sci. USA 98:3364-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassa, P. O., and M. O. Hottiger. 1999. A role of poly(ADP-ribose) polymerase in NF-κB transcriptional activation. Biol. Chem. 380:953-959. [DOI] [PubMed] [Google Scholar]
- 13.Jacque, J.-M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, N. S., T. Dohjima, G. Bauer, H. Li, M.-J. Li, A. Ehsani, P. Salvaterra, and J. Rossi. 2001. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20:500-505. [DOI] [PubMed] [Google Scholar]
- 16.Masutani, M., H. Suzuki, N. Kamada, M. Watanabe, O. Ueda, T. Nozaki, K. Jishage, T. Watanabe, T. Sugimoto, H. Nakagama, T. Ochiya, and T. Sugimura. 1999. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA 96:2301-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ménissier de Murcia, J., C. Niedergang, C. Trucco, M. Ricoul, B. Dutrillaux, M. Mark, F. J. Oliver, M. Masson, A. Dierich, M. LeMeur, C. Walztinger, P. Chambon, and G. de Murcia. 1997. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. USA 94:7303-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochizuki, N., N. Otsuka, K. Matsuo, T. Shiino, A. Kojima, T. Kurata, K. Sakai, N. Yamamoto, S. Isomura, T. N. Dhole, Y. Takabe, M. Matsuda, and M. Tatsumi. 1999. An infectious DNA clone of HIV type 1 subtype C. AIDS Res. Hum. Retrovir. 15:1321-1324. [DOI] [PubMed] [Google Scholar]
- 19.Nishizuka, Y., K. Ueda, K. Yoshihara, H. Yamamura, M. Takeda, and O. Hayaishi. 1969. Enzymic adenosine diphosphoribosylation of nuclear proteins. Cold Spring Harb. Symp. Quant. Biol. 34:781-786. [DOI] [PubMed] [Google Scholar]
- 20.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S.-K. Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, F. J., J. Ménissier-de Murcia, C. Nacci, P. Decker, R. Andriantsitohaina, S. Muller, G. de la Rubia, J. C. Stoclet, and G. de Murcia. 1999. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J. 18:4446-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota, K., M. Kameoka, Y. Tanaka, A. Itaya, and K. Yoshihara. 2003. Expression of histone acetyltransferases was down-regulated in poly(ADP-ribose) polymerase-1-deficient murine cells. Biochem. Biophys. Res. Commun. 310:312-317. [DOI] [PubMed] [Google Scholar]
- 23.Park, W.-S., N. Miyano-Kurosaki, M. Hayafune, E. Nakajima, T. Matsuzaki, F. Shimada, and H. Takaku. 2002. Prevention of HIV-1 infection in human peripheral blood mononuclear cells by specific RNA interference. Nucleic Acids Res. 30:4830-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin, X.-F., D. S. An, I. S. Y. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA 100:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siva, A. C., and F. Bushman. 2002. Poly(ADP-ribose) polymerase 1 is not strictly required for infection of murine cells by retroviruses. J. Virol. 76:11904-11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, M. 2003. Dissecting HIV-1 through RNA interference. Nat. Rev. Immunol. 3:851-858. [DOI] [PubMed] [Google Scholar]
- 27.Surabhi, R. M., and R. B. Gaynor. 2002. RNA interference directed against viral and cellular targets inhibits human immunodeficiency virus type 1 replication. J. Virol. 76:12963-12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Q.-C., Q.-H. Nie, and Z.-H. Feng. 2003. RNA interference: antiviral weapon and beyond. World J. Gastroenterol. 9:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Z. Q., B. Auer, L. Stingl, H. Berghammer, D. Haidacher, M. Schweiger, and E. F. Wagner. 1995. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 9:509-520. [DOI] [PubMed] [Google Scholar]