Abstract
The microenvironment is increasingly recognized to play key roles in cancer, and biomaterials provide a means to engineer microenvironments both in vitro and in vivo to study and manipulate cancer. In vitro cancer models using 3D matrices recapitulate key elements of the tumor microenvironment and have revealed new aspects of cancer biology. Cancer vaccines based on some of the same biomaterials have, in parallel, allowed for the engineering of durable prophylactic and therapeutic anticancer activity in preclinical studies, and some of these vaccines have moved to clinical trials. The impact of biomaterials engineering on cancer treatment is expected to further increase in importance in the years to come.
Introduction
The complexity, diversity, and dynamic nature of cancer pose many challenges to both its study and treatment. For example, the tumor microenvironment and stromal cells contribute to tumor progression as well as its escape from host immune surveillance1–3. Cancer cells originated from the same tumor of a patient may also be genetically heterogeneous4–6, solid tumors tend to have leaky vasculature that allow drug access7,8 but also have elevated interstitial fluid pressure (IFP) to impede penetration of therapeutics9,10, and cancer cells can develop drug resistance through multiple mechanisms11,12.
To confront these and additional challenges, many engineering tools and techniques have been created and utilized to both study cancer in vitro, and to develop new anticancer therapeutics. In particular, complex in vitro culturing systems, engineered protein or cell-based diagnostic and therapeutic agents, and sophisticated molecular or cellular delivery devices are in various stages of development. Integration of bioengineering into cancer research and therapy is not only improving the efficacy of traditional cancer treatments such as surgery13,14 and chemotherapy15,16, but is also opening up entirely new modalities of cancer therapy. This Perspective will discuss the current contributions of bioengineering, especially biomaterials engineering, to our understanding of cancer biology and to the development of emerging therapeutic strategies such as cancer immunotherapy. Biomaterial-based delivery systems for chemotherapeutics are now routinely used to treat patients (see Text Box 1), but as there have been many excellent reviews on this topic17–20, it will not be reviewed here.
Text Box 1. Other applications of biomaterials in cancer.
In order to overcome limitations of classic chemotherapy treatment (e.g., toxicity), nanoparticle carriers have been developed to modulate the pharmacokinetics (PK, including absorption, distribution, metabolism and elimination) of chemotherapeutic agents7,17,159–165. To date, several nanoparticle-based anticancer therapeutics have been clinically approved in the United States and the European Union (Doxil, Janssen Products; Lipodox, a generic version of Doxil from Sun Pharma Global; Myocet, Teva UK Limited; DaunoXome, Galen Limited; Marqibo, Spectrum Pharmaceuticals; DepoCyt, Sigma-Tau Pharmaceuticals; Abraxane, Celgene), and many more in various stages of clinical trials. These approved nanodrugs use liposomes, proteins, or synthetic polymers as delivery vehicles, taking advantage of the simple materials design and enhanced permeability and retention (EPR) effect of nanoscale particles (~10–200 nm in diameters) in solid tumors7,159,166. These nanodrugs have clinically demonstrated higher drug accumulation in tumors and reduced side effects compared to the free drugs157,167–169. Besides the early generations of nanodrugs, many exciting new nanomaterials and delivery strategies are being investigated in preclinical studies and clinical trials. For example, a higher patient response rate and overall survival have been shown when using nanoparticles to co-deliver multiple therapeutic agents with precise formulation to tumors compared to conventional administration of drug cocktails170,171. Nanoparticles decorated with ligands that recognize specific receptors of cancer cells172, trigger tumor transport mechanisms173,174, or camouflage as “markers of self”175,176 can exploit cellular pathways to enhance tumor uptake and avoid immune clearance. Inorganic nanomaterials such as silicon, gold, and iron oxide nanoparticles with unique optical or magnetic properties are also being explored for simultaneous drug delivery and tracking177–180. In addition, although not discussed in this Perspective, it is worth mentioning that biomaterials engineering is also impacting cancer diagnostics, offering methods with substantially improved sensitivity and specificity181,182.
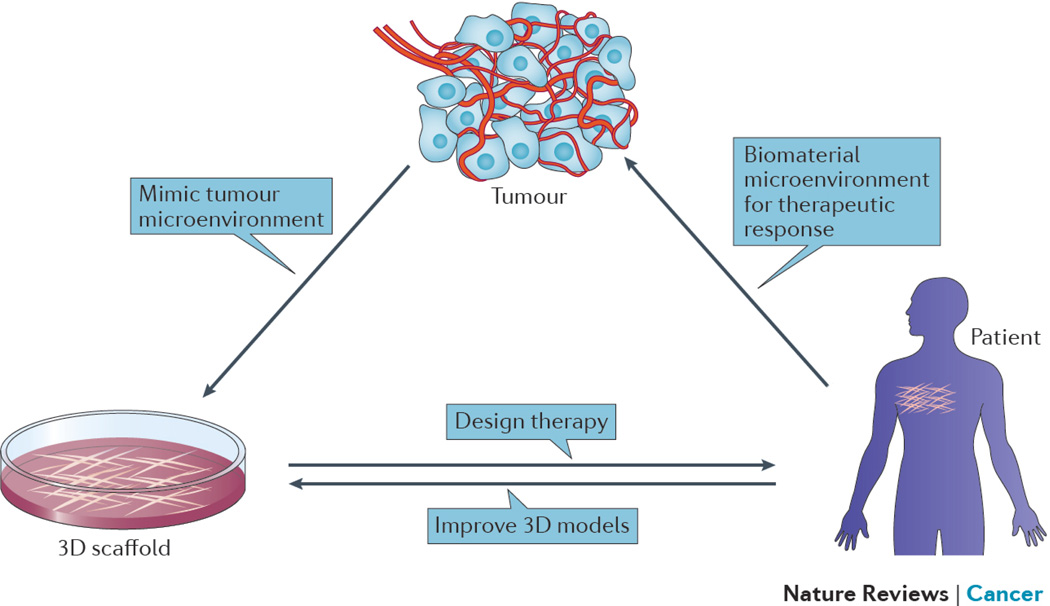
Biomaterials, traditionally defined as materials used in medical devices, provide a highly versatile tool to create defined macro and microenvironments, and manipulate cells and tissues in vitro and in vivo. They have been used since antiquity as simple prosthetics to replace damaged tissues21, but their degree of sophistication has increased rapidly in recent years. New characterization22–24 and synthetic methods25,26, combined with advances in our understanding of biological processes, have greatly extended the variety as well as the size scale at which biomaterials can be designed and synthesized, and how they specifically impact signal transduction pathways. Together, these have transformed biomaterials from being simple structural supports to sophisticated devices that can interact with cells and tissues through well-defined molecular pathways at various size scales to create highly defined microenvironments to direct biological responses. As such, the same materials can now provide both a basis for in vitro mimics of tumors in order to better screen therapeutic approaches and identify new therapeutic targets, and a means to modulate the microenvironment in vivo and direct therapeutic responses against cancerous cells and tumors (Fig. 1).
Figure 1. Creating new microenvironments in vitro and in vivo using biomaterials.
Biomaterials are being used to both create 3D in vitro cancer models that mimic the microenvironmental conditions found in tumors, and to create new microenvironments within the body to allow effective immune cell activation and anti-tumor function. Knowledge gained from studies with 3D in vitro models can inform design of therapeutic biomaterials, while clinical experience resulting from use of biomaterials in vivo can inform the design of new 3D models that more faithfully mimic in vivo biology.
This perspective will focus on two highly interrelated areas where biomaterials engineering may have a substantial impact on the development of new cancer therapies in the future. The use of biomaterials to engineer 3D human tumors in vitro with defined microenvironments will first be reviewed, as these may both further our understanding of cancer and provide new models for drug screening and identification. We will then discuss how biomaterials can be used to manipulate the microenvironment in vivo to alter the immune system, in the context of immunotherapy for cancer.
Biomaterials to create 3D tumor models
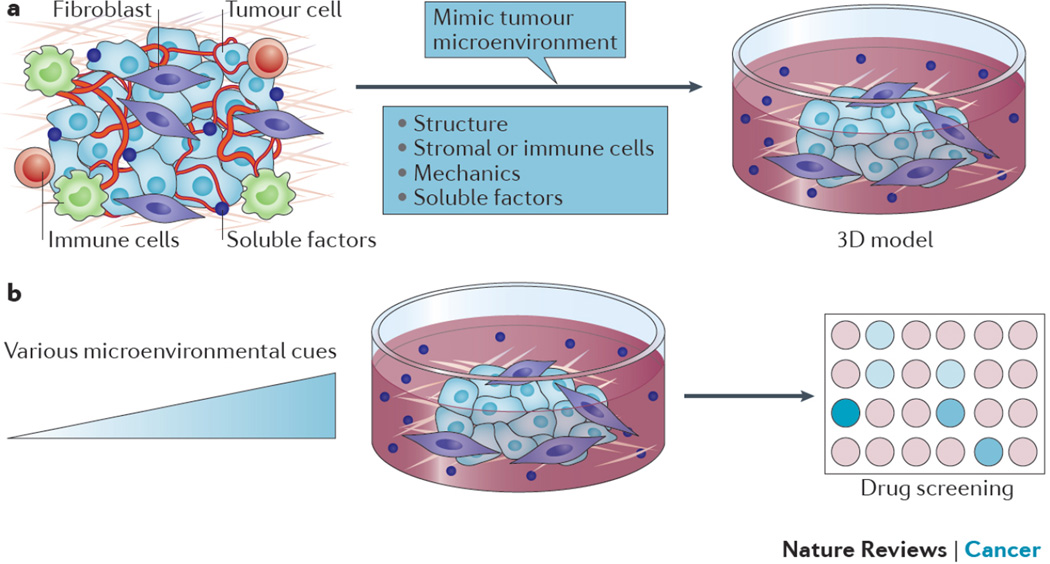
One of the most prevalent challenges in cancer research and therapeutic screening is the limitations of current in vitro models27,28, which relate in part to their inability to accurately reflect microenvironmental cues found in vivo. Conventional two-dimensional (2D) monolayer models of human cancers have been important tools for studying cancer biology and developing anticancer therapeutics, and provide a valuable addition to what can be learned using animal models. However, it is becoming increasingly clear that conventional 2D culture models are insufficient to recapitulate many important characteristics of tumors in vivo and are often poorly predictive of drug response in humans29–31. In contrast to 2D monolayer culture models, tumor cells in vivo are supported by a 3D extracellular matrix (ECM) and non-tumor cells, including endothelial cells, immune cells, and other stromal cells1,32. Early studies have demonstrated that changing the culture of cancer cells from 2D to 3D markedly affects cell behaviors, and that 3D cultures often better resemble tumors than conventional 2D monolayers and can improve our understanding of cancer biology33,34. For example, coupled interactions were found between β-1 integrin and epidermal growth factor receptor (EGFR) signaling in 3D cultures of malignant human mammary epithelial cells but not in 2D culture35. For drug testing, it is important that the cells in the in vitro assay mimic the phenotype within tumors to produce biomedically relevant responses. Although 3D tumor models have not yet had a direct impact on clinical translation at this time point, we expect that more sophisticated 3D models will become important tools for anticancer drug discovery and testing in the future. In order to better mimic different aspects of the microenvironment of human cancers, various 3D culture models have been developed. We here discuss biomaterials engineering approaches to modeling the tumor microenvironment, particularly its mechanical and structural functions, and their potential influence on testing of anticancer drugs (Fig. 2).
Figure 2. Biomaterials to create 3D in vitro human tumor models.
a) Engineered 3D in vitro tumor models recapitulate various microenvironmental cues of human tumors. b) 3D in vitro tumor models can be used to screen anticancer therapeutics as various microenvironmental conditions are modulated, to determine the impact of each condition on efficacy of the therapeutic approach.
Engineered 3D tumors to better model cancer biology
In order to recapitulate the 3D organization and ECM of tumors, various natural and synthetic materials have been developed to provide architectural support to interacting cells26,33. Natural ECM-derived biomaterials such as collagen, laminin, hyaluronic acid, and reconstituted basement membrane (rBM or Matrigel) were the first and are still the most commonly used materials for 3D culture of cancer cells due to their inherent cytocompatibility, intrinsic cell adhesion properties, and ability to be remodeled by cells33,36. However, the batch-to-batch variability, complex molecular composition, and uncontrolled degradation of these materials often make it difficult to study the influence of a particular property of the ECM on tumor cells while maintaining other variables unaltered. Synthetic materials such as poly(ethylene glycol) (PEG) and poly(lactide-co-glycolide) (PLG) can provide more precise experimental control over biochemical and mechanical properties in modeling the tumor ECM. However, as these synthetic materials lack natural cell adhesion sites and are not readily remodeled by cells, cell adhesion ligands and biodegradable crosslinkers are often grafted to the polymers. For example, PEG hydrogels containing the integrin-binding RGD peptide and matrix metalloproteinase (MMP)-degradable peptide crosslinkers promoted 3D epithelial morphogenesis of lung adenocarcinoma cells similarly to Matrigel37. Another class of biomaterials that is increasingly being used in 3D tumor models is naturally-derived polysaccharides such as alginate and chitosan. They are biocompatible and have a broad range of chemical and mechanical properties, but they also lack mammalian cell adhesion sites and often require chemical modification for crosslinking to form gels with desirable physical properties26.
In addition to providing structural support to cancer cells, 3D matrices are also used to create spatially controlled cellular compartments, allowing the interactions between cancer cells and other cells, including immune cells, to be studied in various 3D contexts. For example, collagen and rBM-based 3D systems have been developed to study tumor-induced angiogenesis by co-culturing various types of cancer cells with human umbilical vein endothelial cells or arterial explants38,39. Interactions of cancer cells and stromal fibroblasts in 3D have also been modeled and investigated using collagen gels40,41. In an in vitro bone metastasis model, prostate cancer cells encapsulated in PEG hydrogels were co-cultured with primary human osteoblasts seeded in bone-mimicking polycaprolactone–tricalcium phosphate scaffolds, revealing the paracrine effect of osteoblasts in altering the androgen dependency of prostate cancer cells and supporting their survival42. In the context of tumor-immune cell interactions, it has been shown that melanoma cells cultured in 3D become more resistant to cytotoxic T lymphocytes (CTL) compared to cells grown in 2D monolayers, owing to down regulation of tumor antigens and major histocompatibility complex class I (MHC I), and increased production of lactate43,44. Similarly, 3D culture of lung carcinoma cells diminished their susceptibility to CTL activity. However, this result was attributed to a decrease in heat shock protein 70 (HSP70) expression and associated defective tumor antigen presentation45. Glioma spheroids also appeared to have an increased resistance to natural killer (NK) cell cytotoxicity compared to glioma cells grown in 2D monolayers46. These findings support the importance of using 3D culture for developing in vitro models of tumor-immune cell interactions. While abovementioned 3D models for studying tumor-immune cell interactions primarily use unsupported tumor spheroids, biomaterial matrices mimicking various tumor microenvironments may also be utilized to investigate microenvironmental influences in such interactions. For example, porous scaffolds made from chitosan and alginate have been used to study interactions of human prostate cancer cells and lymphocytes. These scaffolds allow one to track immune cell penetration into tumor spheroids for a longer period of time in a 3D environment due to slower scaffold degradation, as compared to collagen or rBM matrices47. More sophisticated 3D co-culture models, particularly incorporating a model of the vasculature, can be fabricated using 3D printing. Sacrificial templates have been printed using glassy carbohydrate or temperature-responsive hydrogels. The templates are embedded within various cell-laden natural and synthetic hydrogels, and can be subsequently dissolved to generate vascular networks in the hydrogels48,49. Various photolithography50,51 and soft lithography52 methods using photo-crosslinked materials have also been developed to achieve in vitro mimics of 3D vascularized tissues. These sophisticated architectures have not been widely applied yet as 3D tumor models, but are likely to provide important tools for studying various aspects of cancer biology in the future.
As tumors have distinct mechanics compared to normal tissues, biomaterials have also been utilized in 3D culture to model the mechanical properties of the tumor microenvironment, and to study the effects of ECM mechanics on tumor development and progression. Mechanical cues regulate various cell behaviors through mechanotransduction, including proliferation, migration, and differentiation53–55. In the context of cancer, tumor cells remodel the ECM and change its mechanical properties, and the altered mechanical niche in turn is likely to influence tumor progression32. For example, breast cancer stroma is stiffer than normal stroma due to increased collagen deposition and ECM crosslinking. Using 3D in vitro models fabricated from rBM and collagen, it was discovered that high matrix stiffness together with increased collagen concentration or crosslinking induced a malignant phenotype in normal mammary epithelium56,57. To understand the specific role of increased matrix stiffness in this transformation independent of changes in matrix composition and architecture, an interpenetrating polymer network (IPN) of rBM and alginate matrix was developed58. The stiffness of these IPNs can be modulated by simply controlling the ionic crosslinking of alginate without changing the polymer concentration, cell-adhesion-ligand density, or the pore size of the matrix. Using this IPN model, it was found that the increased matrix stiffness was sensed through the β4 integrin, Rac1, and the PI3K pathway, leading to loss of apicobasal polarity, cell invasion into the basement membrane, increased proliferation, and other hallmarks of a malignant phenotype in mammary epithelium58. Other biomaterials have also been used to model the mechanical properties of different tumors. As hyaluronic acid is a major component of brain ECM, 3D glioblastoma models were developed using methacrylate modified hyaluronic acid hydrogels with different mechanical properties. These hydrogels mimic the stiffness encompassing normal and tumorigenic brain tissues, allowing one to study cancer cell invasion into different matrices59. Synthetic hydrogels based on PEG have been used in lung adenocarcinoma models to study the influence of matrix stiffness on the epithelial–mesenchymal transition. The PEG hydrogels were modular and had higher stiffness compared to collagen or rBM matrices37.
Although the importance of ECM mechanics is increasingly recognized, the majority of the studies have focused on the elasticity (stiffness) of ECM, overlooking the potential role of the viscoelasticity (both viscous and elastic characteristics) of the ECM. A recent study showed that viscoelastic substrates could stimulate spreading of osteosarcoma cells to a greater extent than purely elastic substrates with the same initial elastic modulus60. Future 3D models using materials developed to mimic the viscoelasticity of various tumor niches will potentially help to better dissect the function of ECM mechanics on tumor progression.
Engineered 3D matrices have also been developed to recapitulate the spatiotemporal complexity of soluble factors (e.g. oxygen and growth factor gradients) found in tumors, and study their contribution to tumor growth. Conventional 2D culture in reduced oxygen environments has helped to improve our understanding of the role of hypoxia, but does not recapitulate the spatial variation of oxygen in tumors. The use of stacked layers of chromatography paper infused with suspensions of cancer cells in Matrigel was shown to generate defined oxygen gradients in the 3D culture, and necrotic cells as well as overexpression of vascular endothelial growth factor (VEGF) and insulin-like growth factor binding protein 3 (IGFBP3) were observed at the hypoxic core of the 3D culture61. Alginate hydrogels have also been used to modulate oxygen concentration. This 3D system was able to model various uniform or gradient hypoxic conditions by controlling the hydrogel spatial configuration, which allowed studies of the secretion of pro-angiogenic factors (VEGF and interleukin 8 (IL-8)) from cancer cells in response to ECM cues such as integrin-matrix engagement under different hypoxia conditions62,63. To control the spatiotemporal availability of growth factors in a 3D tumor model, controlled drug delivery technologies have been exploited. For example, a hyaluronic acid hydrogel bilayer system was developed to study the function of heparin-binding EGF-like growth factor (HB-EGF) on the growth of prostate cancer spheroids. As HB-EGF is found in prostate stroma and bound to ECM, HB-EGF was encapsulated in microparticles to control its availability. Sustained release of HB-EGF from microparticles embedded in the top-layer hydrogel promoted the growth of tumor spheroids in the bottom layer, and increased VEGF and IL-8 expression in the cancer cells64. Microfluidic65 and photo-patterning66 methods may be useful to achieve more precise spatiotemporal control of soluble factors in 3D culture models through controlling the flow of fluids containing the factors, and photo-induced coupling or cleavage of factors, respectively. These approaches are likely to also be useful to study tumor cell responses to spatiotemporally controlled cytokines in the context of immunotherapy.
Engineered in vitro models for testing anticancer therapeutics
As it is desired to better mimic human biology and dismiss potentially ineffective or unacceptably toxic therapeutic candidates as early as possible, 3D in vitro human tumor models are being increasingly explored for evaluation of new therapies67. While 2D cell culture models have been an invaluable tool to identify potential anticancer agents at the early stages of drug discovery, they provide little information on drug responses influenced by tumor heterogeneity and microenvironment. In contrast, simple tumor spheroid models have shown drug responses more similar to that of tumors in vivo as compared to 2D models, and are increasingly being used to evaluate anticancer agents under 3D conditions68–70. For example, 3D tumor spheroids created through hanging drop methods have been commercialized by companies such as 3D Biomatrix and InSphero, and are being explored for high-throughput drug screening by pharmaceutical companies. To integrate additional influences of the tumor microenvironment, collagen gels containing heterospheroids of liver carcinoma cells and fibroblasts were used to study changes in drug resistance and metabolism associated with culture dimensionality, stromal cells, and the ECM. This study found that suspended 3D heterospheroids had higher drug resistance than 2D monolayers and suspended homospheroids containing only cancer cells, and placing either type of spheroid in a collagen matrix further increased drug resistance71. A 3D model of Ewing sarcoma has also been developed using biodegradable, highly porous polycaprolactone scaffolds fabricated by electrospinning. This 3D culture model showed closer resemblance to xenograft tumors in phenotype and drug resistance as compared to a 2D monolayer model. In addition, cancer cells in the 3D system also had a slower proliferation rate and better mimicked in vivo tumor growth, which allowed investigation of the long-term impact of drug exposure72. While patient-derived xenograft (PDX) cancer models have been established by engrafting and maintaining patient-derived tumor tissues in immunocompromised animals73, the complexity of these models and long latency following engraftment has slowed their broad adoption. To circumvent these and other potential problems (e.g. drift of PDX stromal components from human to host species), patient-derived prostate xenograft tumor cells have been cultured in hyaluronic acid-based hydrogels to mimic the microenvironment of bone metastases in prostate cancer. This 3D system was shown to maintain the cancer cells’ native androgen receptor expression, and a higher docetaxel resistance was observed with patient-derived cells compared to a commonly utilized prostate cancer cell line, although the relevance to patient drug response still needs to be varified74. In vitro 3D models to test immunotherapies are also being developed. For example, a 3D model of mouse mammary carcinoma cells cultured in porous chitosan-alginate scaffolds was used to evaluate CTL function in the presence or absence of tumor-associated fibroblasts. It was found fibroblasts decreased tumor necrosis factor α (TNFα) secretion by CTLs, likely due to elevated production of IL-10 and transforming growth factor beta (TGF-β) in the co-cluture75. In another study, drug inhibition of tumor cell-macrophage paracrine interactions was analyzed using co-culture of breast adenocarcinoma cells and macrophages in alginate hydrogel fibers. Gefitinib, an EGFR inhibitor, and a Rac1 inhibitor were both able to impair macrophage migration to cancer cells, and the 3D cell distribution and cancer cell-macrophage ratio affected drug responses76. Although generally faster than in vivo models, 3D tumor models for drug testing typically require increased processing time relative to 2D cell monolayers. In order to address this problem, automated techniques such as microfluidics have been utilized to achieve high-throughput compound screening in 3D tumor models. For example, a microfluidic-based platform has been developed to fabricate “microtissues” that can encapsulate desired combination of cancer cells, stromal cells, and ECM components. These 3D microtissues allow one to probe cancer cell responses to various microenvironmental cues, as the specific matrices, soluble factors, and stromal cells are changed, and to test drug candidates77.
Engineered 3D tumor models may also potentially help to improve animal models for drug development. Xenograft animal models are typically established by inoculation of cancer cells previously cultured on stiff 2D substrates. However, culture conditions can substantially alter cell gene expression and phenotype78, and certain effects may persist for a long period of time even after altering conditions73,79,80. For example, soft fibrin gels were shown to promote growth and possibly “priming” of stem-cell-like cancer cells in 3D culture, and enhance their ability to form tumors in mice81. Similarly, culture in PLG or chitosan-alginate 3D matrices enhanced the subsequent ability of cancer cells to grow tumors and promote angiogenesis in mice, compared to cells from 2D culture82,83. Although still in a very early stage, further development and evaluation of these approaches may potentially help to create xenograft animal models that can better recapitulate the phenotypes of actual human tumors for drug testing.
Future directions and considerations
Since 3D cancer models are still in the early stages of development, a number of issues still remain to be addressed. First, factors such as specific disease relevance and processing efficiency will need to be evaluated and optimized in large-scale studies in order to fully establish and exploit 3D tumor models for drug discovery and screening. To date, few, if any, of the discoveries made in 3D cancer models have been validated in human cancer patients. This calls for closer collaborations among researchers, physicians, and pharmaceutical industry. Also looking forward, there is a substantial potential for advanced microfluidic 3D cell culture devices that more closely mimic the physiological function of human organs (organs-on-chips) in drug screening than static 3D model systems84. A ‘human-body-on-a-chip’ that combines several different, fluidically linked organs-on-chips85 is a particularly appealing concept for testing new anticancer therapeutics including immunotherapies, as it could provide a human test bed that encompasses the dynamic, multiple cellular interactions required to mount an effective immune response to cancer. This approach may also potentially provide an in vitro platform for personalized therapy evaluation by using cells or tissues directly from patients86. However, the potential benefits of all 3D human cancer models must be balanced with the increased complexity and difficulty of performing large-scale discovery science or drug screening, as compared to the classic 2D systems.
Biomaterials in cancer immunotherapy
Harnessing the immune system to treat cancer has been a goal in medicine for over a century, driven by the potential of providing specific, durable, and adaptive reactivity towards tumors87,88. Recently, the benefit and efficacy of certain cancer immunotherapies, including checkpoint blockade antibodies, were successfully established in large clinical trials89–92, and adoptive cell transfer (ACT) is also extremely promising93,94. Biomaterials may enhance the efficacy of many of these new therapies or other immunotherapies (e.g. cancer vaccines) via control over the microenvironment in which immune cells encounter antigen, stimulatory signals, cancerous cells, or other immunomodulatory cells (Fig. 3). Therapeutic viral particles can also be considered biomaterials, but are not covered here as they are typically not intended to directly alter the microenvironmental conditions of immune cells and have been discussed in previous reviews95.
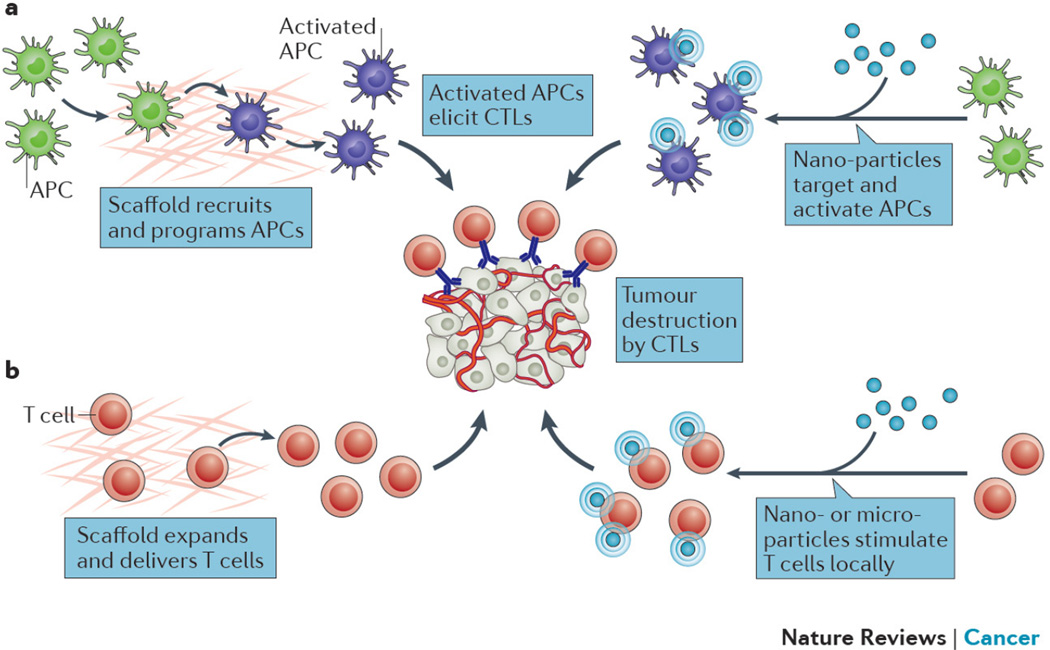
Figure 3. Biomaterials-based cancer immunotherapies.
a) Biomaterial-based cancer vaccines. In one approach (left panel), a scaffold releases recruiting factors (e.g. GM-CSF) from the vaccine into surrounding tissue to direct host DCs to migrate into the device, where the DCs are exposed to tumor antigens and “danger signals” (e.g. CpG) that activate the DCs, and enable their homing to lymph nodes to elicit a cytotoxic T lymphocyte (CTL) anti-tumor response. Alternatively (right panel), nanoparticles are used to target DCs in lymph nodes, and deliver antigens and “danger signals” to again generate a CTL response. b) Biomaterial-based T cell manipulation. Either scaffolds (left panel), or nano- or micro-particles (right panel) are used to provide a stimulatory microenvironment to T cells for ACT in order to expand T cells and maintain their activity against tumors.
Biomaterial-based cancer vaccines
Therapeutic cancer vaccines aim to generate immune reactivity against existing tumors in the host. Through vaccination, antigen presenting cells (APCs) such as dendritic cells (DCs) are activated to elicit tumor specific CTLs87. The activation of APCs can be achieved either ex vivo or in vivo. Provenge, the first cell-based therapeutic cancer vaccine approved by the US Food and Drug Administration (FDA), demonstrated the potential of adaptive immunity in combating cancer, but the complexity of the treatment (e.g. ex-vivo cell manipulation, transportation of living cells between clinic and production facility) and its associated high cost hampered its reach to patients96. In contrast, cancer vaccines based on peptides, viruses, or DNA encoding tumor antigens historically have shown low overall objective response rates in clinical trials97,98, perhaps due to inappropriate temporal or spatial control over antigen presentation and immune cell activation, and a limited ability to overcome the immune inhibitory mechanisms of tumors. To generate tumor specific CTLs, it is critical to activate APCs in a stimulatory microenvironment that induces antigen-specific immunity rather than tolerance99,100. The kinetics of antigen exposure are also important, as too short of a low-dose or too long of a high-dose of antigen exposure, at least in infectious diseases, may either fail to result in a T cell response or cause T cell exhaustion, respectively101–104.
Biomaterial-based vaccines may achieve more precise immune modulation due to their ability to control delivery of antigens and adjuvants in space and time, to regulate immune cell trafficking through their physical and chemical characteristics, and to mimic stimulatory signals of innate immunity. For example, in contrast to the ex-vivo cell manipulation approach of Provenge, implantable cancer vaccines fabricated from porous PLG scaffolds, similar to those used in 3D cancer models in vitro82, have been designed to control immune cell trafficking and activation kinetics in situ. Also different from traditional bolus vaccination, this biomaterial-based vaccine creates a new physical environment in the body that accumulates, activates, and presents antigens and stimulatory signals to DCs over a period of at least 2 weeks105. Specifically, the macroporous PLG vaccine system has a sustained release of granulocyte macrophage-colony stimulating factor (GM-CSF), which promotes DC accumulation at the vaccine scaffold. The vaccine is loaded with tumor lysate as an antigen source and also presents nanoparticles of CpG oligodeoxynucleotides (or CpG for short), which are agonists of toll-like receptor 9 (TLR9) and serve as a “danger signal” for DC activation. The activated and antigen-loaded DCs subsequently migrate to lymph nodes105. This vaccine leads to a strong CTL response against established melanoma (syngeneic B16-F10 model), causing complete regression of tumors in ~47% of mice in preclinical studies106. A human version of this vaccine (called WDVAX) is currently in a Phase I clinical trial for stage IV melanoma (https://clinicaltrials.gov/ct2/show/NCT01753089). Importantly, this type of biomaterial design for a cancer vaccine is modular, and a variety of immunostimulatory agents can be incorporated. For example, inflammatory cytokines such as chemokine (C-C motif) ligand 20 (CCL20) or Fms-related tyrosine kinase 3 ligand (Flt3L) can be used in place of GM-CSF in the vaccine to alter DC subset recruitment and activation, and these also generate anti-tumor responses in a preclinical melanoma model107. Altering the source of tumor lysate used as the antigen allows the PLG vaccine system to demonstrate therapeutic activity in mouse lung carcinoma and rat glioma models108,109. Local immunogenic niches that control immune cell trafficking and activation have also been created using other materials such as gelatin, alginate hydrogels, and mesoporous silica rods110–113. As these materials are either highly deformable or self-organizing, they can be administered by injection, instead of the surgical implantation required for WDVAX. Many of the scaffold vaccine systems use tumor lysates or irradiated tumor cells as the antigen source. This design can create personalized vaccines to address tumor heterogeneity and potentially hit multiple tumor antigens simultaneously. However, this approach may also lead to increased complexity in clinical translation, as compared to using defined antigens. One alternative approach that could be pursued in the future is to use synthetic neoantigens identified by exome sequencing of cancer cells from individual patients114.
Biomaterials, owing to their tunable sizes at the nanoscale, can also be transported to lymph nodes, where they alter the microenvironment of the targeted immune cells. As large numbers of DCs reside in the secondary lymphoid organs, delivery of vaccines to lymph nodes is an attractive alternative to recruiting the migratory DCs to a vaccination site. It has been shown that interstitial particles (e.g. administered subcutaneously or intramuscularly) in the size range of 10–100 nm, depending on the type of material and animal model, are optimal for lymph node targeting through lymphatic drainage102,115,116. Small (25 nm) pluronic copolymer-coated nanoparticles that were intradermally injected were efficiently transported to draining lymph nodes through interstitial flow and retained for at least 120 h. The nanoparticles were readily internalized by DCs and macrophages in the lymph nodes. In comparison, 100 nm nanoparticles appeared only ~10% as efficient. When used to deliver the model antigen ovalbumin (OVA), the 25 nm nanoparticles also induced higher humoral immune responses compared to 100 nm nanoparticles115. A study using another type of nanoparticles (inert polystyrene beads) showed that 40 nm was optimal for targeting DCs in the lymph nodes and generating antitumor immunity in an OVA-expressing mouse lymphoma model (EG7-OVA)117.
Besides exploiting size-dependent targeting to the lymph nodes, biomaterial-based vaccines have also used binding ligands of DC receptors or hitchhiked natural transportation mechanisms in the body to actively target DCs. For example, both inorganic and polymeric nanoparticles have shown enhanced binding and uptake by DCs when coupled with antibodies to CD40 or DEC-205 (also known as CD205 or LY75) receptors118–121. A recent study122 exploited the clinical finding that injected dyes that bind avidly to endogenous albumin were efficiently transported to lymph nodes and filtered by resident APCs123,124. Inspired by this “albumin hitchhiking”, amphiphilic macromolecules were developed to deliver peptide antigens to lymph nodes. The structure comprised a peptide antigen linked to a lipophilic albumin-binding tail, with a hydrophilic PEG polymer chain as the linker to improve solubility. When injected subcutaneously in mice, these amphiphilic macromolecules were efficiently transported with endogenous albumin to lymph nodes, targeting DCs and macrophages. Vaccination with the amphiphilic peptide antigens and CpG adjuvant significantly increased antigen specific CTL expansion and therapeutic efficacy against established tumors (B16F10 melanoma) compared to unmodified peptide and CpG immunizations122. Recent studies have shown that targeting tumor-draining lymph nodes specifically with nanoparticle-based cancer vaccines or adjuvants induced stronger cellular immune responses compared to targeting uninvolved lymph nodes, possibly due to altering the immunosuppressive environment of tumor-draining lymph nodes to a more stimulatory condition and to high exposure of immune cells in these lymph nodes to tumor-associated antigens125,126. An interesting direction for future studies would be to combine aforementioned APC targeting strategies to home in on specific immune cells in the tumordraining lymph nodes127.
Particulate vaccine carriers can also promote a CTL response by controlling the intracellular delivery of antigens and modulating antigen processing. Normally, proteins in the cytosol of APCs (e.g. from invaded viruses) are degraded and loaded on MHC I for CD8+ T cell activation, but soluble antigens which are endocytosed by APCs are typically presented on MHC II to activate CD4+ T cells and induce a humoral response. However, phagocytosis of pathogens or particulate antigens by APCs, in contrast to the endocytosis of soluble antigen, can lead to loading of antigen on MHC I through cross-presentation128. Certain types of nanomaterial vaccine carriers can also actively destabilize endosomes through mechanisms such as altering endosomal osmotic pressure, and release antigen into the cytosol, promoting cross-presentation of antigen129–132. In addition, co-delivery of antigen and TLR agonists simultaneously to APCs can promote CD8+ T cell responses. For example, multilamellar lipid nanoparticles have been loaded with a model antigen together with the TLR4 agonist monophosphoryl lipid A (MPLA). These nanoparticles consist of multiple concentric lipid bilayers that are crosslinked within the vesicle walls to stably retain antigen and MPLA. The nanoparticles elicited a high percentage of antigen specific CD8+ T cell in the peripheral blood of mice after a prime injection and two boosters, and this response was dependent on co-delivery of the stably incorporated TLR agonist133. Similarly, enhanced cellular immunity against melanoma (B16F10) was also observed using other nanomaterial carriers when a melanoma antigen (tyrosinase-related protein 2 peptide) and various TLR agonists were co-delivered134,135. Interestingly, preclinical studies have shown that combined stimulation of multiple TLR or Nod-like receptors (NLRs) may have a synergistic effect and enhance vaccine efficacy136–138. A recent melanoma clinical trial using virus-like particle (VLP) vaccine containing CpG and the melan-A peptide antigen, given together with incomplete Freund's adjuvant (IFA) injection and imiquimod topical cream (a TLR7 agonist), significantly increased memory and effector CD8+ T cell responses as compared to the vaccine alone139. This suggests that incorporating multiple TLR agonists simultaneously into nanoparticle-based cancer vaccines may also potentially improve the efficacy of therapeutic vaccines in humans.
Certain materials have intrinsic adjuvancy or the capacity to create an inflammatory microenvironment, which can be exploited in the design of cancer vaccines. Aluminum salts (alum), whose mechanism is not yet fully understood, were until recently the only type of adjuvant approved for human vaccination140. Silica particles were recently shown to activate the innate immune response and promote cellular immunity, possibly due to inflammasome activation of APCs112,141. ISCOMATRIX adjuvant consists of cage-like nanoparticles comprising saponin, cholesterol, and phospholipid. It does not appear to activate TLRs, but it induces innate immune response by inflammasome-dependent and independent IL-18 production, possibly due to endosomal stress and cell damage142. Vaccination with ISCOMATRIX adjuvant and an HPV16 E6–E7 fusion protein demonstrated both antigen specific humoral and cellular immune responses in a Phase I clinical trial for cervical intraepithelial neoplasia143. Some materials can also generate danger signals of innate immunity by triggering the complement cascade. Studies using polypropylene sulfide nanoparticles coated with Pluronic copolymers showed that the surface terminal hydroxyl groups activated complement, eliciting DC maturation. When the model antigen OVA was coupled to these nanoparticles, effective humoral and cellular immune responses were observed115. It was further shown that the complement activation of these nanoparticles could be controlled by adjusting their core thiolation and surface carboxylation to affect the deposition of complement component C3b144. So far, our understanding of how biomaterials with various chemical and physical properties may interact with the innate immune system is still quite limited, and further knowledge would be invaluable for designing future cancer vaccines.
Biomaterials engineering in adoptive cell transfer
T cell-based ACT, including tumor infiltrating lymphocytes and chimeric antigen receptor (CAR) T cells, has become one of the most successful developments in cancer immunotherapy93,94,145. However, these promising treatments have some notable challenges, including expansion of T cells and maintaining their effector function, both during ex vivo cell production and in vivo after transplantation.
Biomaterials may address many of challenges facing T cell-based ACT by providing a means to locally and specifically provide stimulatory cues. First, these therapies typically require large numbers of autologous tumor specific T cells, but T cells collected directly from patients’ peripheral blood or tissues are normally low in number and can be hyporesponsive146. To activate and expand the cells ex vivo, microparticles coated with anti-CD3 and anti-CD28 antibodies have been developed as “artificial APCs” to provide primary and co-stimulatory signals. These macroparticles also contain superparamagnetic iron oxide for easy separation from cells by a magnetic field. This method has now become a common tool in immunology and is widely used in CAR T cell clinical studies147. More recently, it was reported that biodegradable PLG microparticles containing an additional signal, the T cell growth factor IL-2, further enhanced T cell expansion, especially of CD8+ T cells. The sustained release of IL-2 from microparticles in the vicinity of T-cell contacts, in a paracrine fashion, appeared more effective than simply adding soluble IL-2 to the culture media148. Besides biochemical factors, the physical characteristics, such as the shape and size, of the microparticles also affect their T cell expansion efficiency149. For example, ellipsoidal PLG microparticles showed enhanced CD8+ T cell expansion activity compared to spherical microparticles with the same volume and protein content, possibly due to increased interaction of T cells with the long axis of the ellipsoidal microparticles150.
Biomaterials may also allow one to prevent the decline in viability and function of the transplanted cells in ACT by creating optimal cytokine presentation in the cellular microenvironment. To improve the persistence of the transplanted T cells, systemic administration of cytokines (e.g. IL-2, IL-15) is often used145,151. However, high-dose systemic cytokines can cause severe side effects due to their broad activities152. One can instead enhance transplanted T cell function by conjugating cytokine-loaded nanoparticles to the surface of the transplanted cells153. Stable attachment to the cells for at least 4 days can be achieved by covalent conjugation of liposomal nanoparticles to the surface thiols on T cells. In a B16F10 melanoma model, transplanted CTLs conjugated with nanoparticles that release the cytokines IL-15 superagonist and IL-21 demonstrated a marked improvement in tumor elimination compared to CTLs with systemic co-injection of the free cytokines153. Nanoparticles conjugated to the T cell surface can also be used to deliver small molecular inhibitors (e.g. an inhibitor of the SHP1 and SHP2 phosphatases, which normally down-regulate T-cell receptor activation) into the T-cell synapse, achieving a greater adoptive T cell expansion at the tumor site154. However, one caveat of such approaches is that the cell-bound nanoparticles will be diluted during T cell expansion in the body, limiting the duration of stimulation from the nanoparticles. A possible solution is to target transplanted T cells in vivo using nanoparticles coupled with ligands specific to these cells155. Alternatively, a recent study used a porous alginate scaffold, similar to those used in 3D in vitro cancer models47,63, to expand and deliver CTLs in situ by implanting a CTL-loaded scaffold adjacent to tumors or at resection sites. Microspheres containing IL-15 superagonist and anti-CD3, anti-CD28 and anti-CD137 antibodies were also incorporated into the scaffold to provide a stimulatory microenvironment to the loaded CTLs156.
Future directions and considerations
Biomaterials-based cancer vaccines are expected to complement and synergize with the checkpoint blockade and ACT therapies that are rapidly moving to the frontline of cancer treatment. This combination would address two key factors for effective immunotherapy: generation of robust tumor-specific CTLs and inhibition of tumor-induced immunosuppression. Personalized cancer vaccines are also an important direction due to heterogeneity of tumors between patients, especially from the immunotherapy standpoint. Incorporation of patient-specific neoantigens identified by genetic profiling may enhance the efficacy of biomaterials-based cancer vaccines114.
Clinical adaptation of biomaterial-based immunotherapies will, though, require the pharmaceutical industry to incorporate a much greater emphasis on materials science and engineering than has been typical in the past. This includes the new approaches required to manufacture these types of therapies as contrasted to small molecule drugs and biologics, and altered regulatory and approval pathways relating to the resultant combinations of materials and drugs. The recent manufacturing difficulties for Doxil point to the challenges that can arise from material-drug combinations157.
Conclusions
The ability of biomaterials to create defined microenvironments is expected to have a major impact on both the discovery and clinical translation processes in cancer, and to increase exponentially in the coming years. 3D in vitro models that better recapitulate in vivo tumor biology are beginning to replace 2D models in many areas of cancer research. The first personalized biomaterial-based cancer immunotherapy (WDVAX) has recently moved to a clinical trial158. The frequent use of the same polymers, and even scaffold architectures, in these two developing areas suggest that transfer of knowledge and technologies both within and between the two areas is expected to be rapid and fruitful. This will allow, for example, discoveries on one type of cancer to be quickly applied to another. Also, when the role of specific signals (e.g., local cytokine gradients) in regulating immune cell activation is identified in 3D in vitro systems, these findings could be recapitulated in vivo using the same or a similar material system for therapeutic purposes. Conversely, new understanding of complex immune responses, often involving multiple cell types, that result from therapeutic use of biomaterials will then feedback to the design of new 3D in vitro models. Overall, the addition of biomaterials engineering to cancer research and therapies contributes to an exciting time in the search for better cancer therapies.
Acknowledgments
Relevant work in the authors' laboratory has been supported in part by a grant from the NIH (R01 EB015498).
References
- 1.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Gerlinger M, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelstein B, et al. Cancer Genome Landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 8.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Heldin C-H, Rubin K, Pietras K, Östman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 10.Goel S, et al. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 12.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper Transporters and the Cellular Pharmacology of the Platinum-Containing Cancer Drugs. Mol. Pharmacol. 2010;77:887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation — a new cutting edge. Nat. Rev. Cancer. 2013;13:653–662. doi: 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 16.Zamboni WC, et al. Best Practices in Cancer Nanotechnology: Perspective from NCI Nanotechnology Alliance. Clin. Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 18.Davis ME, Chen Z (Georgia), Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 19.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 20.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binnig G, Rohrer H. Scanning Tunneling Microscopy. IBM J Res Dev. 1986;30:355–369. [Google Scholar]
- 23.Yu G, Yan X, Han C, Huang F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013;42:6697–6722. doi: 10.1039/c3cs60080g. [DOI] [PubMed] [Google Scholar]
- 24.Nel AE, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 25.Whitesides GM, Grzybowski B. Self-Assembly at All Scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 26.Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chem. Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 27.Suggitt M, Bibby MC. 50 Years of Preclinical Anticancer Drug Screening: Empirical to Target-Driven Approaches. Clin. Cancer Res. 2005;11:971–981. [PubMed] [Google Scholar]
- 28.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 29.Gill BJ, West JL. Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology. J. Biomech. 2014;47:1969–1978. doi: 10.1016/j.jbiomech.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Infanger DW, Lynch ME, Fischbach C. Engineered Culture Models for Studies of Tumor-Microenvironment Interactions. Annu. Rev. Biomed. Eng. 2013;15:29–53. doi: 10.1146/annurev-bioeng-071811-150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H-HG, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv. Drug Deliv. Rev. 2014;79–80:19–29. doi: 10.1016/j.addr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauptmann S, et al. Integrin expression on colorectal tumor cells growing as monolayers, as multicellular tumor spheroids, or in nude mice. Int. J. Cancer. 1995;61:819–825. doi: 10.1002/ijc.2910610613. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elsdale T, Bard J. COLLAGEN SUBSTRATA FOR STUDIES ON CELL BEHAVIOR. J. Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill BJ, et al. A Synthetic Matrix with Independently Tunable Biochemistry and Mechanical Properties to Study Epithelial Morphogenesis and EMT in a Lung Adenocarcinoma Model. Cancer Res. 2012;72:6013–6023. doi: 10.1158/0008-5472.CAN-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross VL, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seano G, et al. Modeling human tumor angiogenesis in a three-dimensional culture system. Blood. 2013;121:e129–e137. doi: 10.1182/blood-2012-08-452292. [DOI] [PubMed] [Google Scholar]
- 40.Dolznig H, et al. Modeling Colon Adenocarcinomas in Vitro. Am. J. Pathol. 2011;179:487–501. doi: 10.1016/j.ajpath.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung KE, et al. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PloS One. 2013;8:e76373. doi: 10.1371/journal.pone.0076373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieh S, et al. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone. 2014;63:121–131. doi: 10.1016/j.bone.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Feder-Mengus C, et al. Multiple mechanisms underlie defective recognition of melanoma cells cultured in three-dimensional architectures by antigen-specific cytotoxic T lymphocytes. Br. J. Cancer. 2007;96:1072–1082. doi: 10.1038/sj.bjc.6603664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirt C, et al. ‘In vitro’ 3D models of tumor-immune system interaction. Adv. Drug Deliv. Rev. 2014;79–80:145–154. doi: 10.1016/j.addr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Dangles-Marie V, et al. A Three-Dimensional Tumor Cell Defect in Activating Autologous CTLs Is Associated with Inefficient Antigen Presentation Correlated with Heat Shock Protein-70 Down-Regulation. Cancer Res. 2003;63:3682–3687. [PubMed] [Google Scholar]
- 46.He W, et al. Proteomic Comparison of 3D and 2D Glioma Models Reveals Increased HLA-E Expression in 3D Models is Associated with Resistance to NK Cell-Mediated Cytotoxicity. J. Proteome Res. 2014;13:2272–2281. doi: 10.1021/pr500064m. [DOI] [PubMed] [Google Scholar]
- 47.Florczyk SJ, et al. 3D Porous Chitosan–Alginate Scaffolds: A New Matrix for Studying Prostate Cancer Cell–Lymphocyte Interactions In Vitro. Adv. Healthc. Mater. 2012;1:590–599. doi: 10.1002/adhm.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JS, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolesky DB, et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 50.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichol JW, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens KR, et al. InVERT molding for scalable control of tissue microarchitecture. Nat. Commun. 2013;4:1847. doi: 10.1038/ncomms2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 55.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 56.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Levental KR, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaudhuri O, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 59.Ananthanarayanan B, Kim Y, Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011;32:7913–7923. doi: 10.1016/j.biomaterials.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhuri O, et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015;6:6365. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derda R, et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. Sci. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbridge SS, et al. Oxygen-Controlled Three-Dimensional Cultures to Analyze Tumor Angiogenesis. Tissue Eng. Part A. 2010;16:2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischbach C, et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X, et al. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33:9049–9060. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buchanan CF, et al. Three-Dimensional Microfluidic Collagen Hydrogels for Investigating Flow-Mediated Tumor-Endothelial Signaling and Vascular Organization. Tissue Eng. Part C Methods. 2014;20:64–75. doi: 10.1089/ten.tec.2012.0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeForest CA, Anseth KS. Photoreversible Patterning of Biomolecules within Click-Based Hydrogels. Angew. Chem. Int. Ed. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Controlled Release. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vinci M, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myungjin Lee J, et al. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Invest. 2013;93:528–542. doi: 10.1038/labinvest.2013.41. [DOI] [PubMed] [Google Scholar]
- 71.Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013;433:327–332. doi: 10.1016/j.bbrc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Fong ELS, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc. Natl. Acad. Sci. 2013;110:6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tentler JJ, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fong ELS, et al. Hydrogel-Based 3D Model of Patient-Derived Prostate Xenograft Tumors Suitable for Drug Screening. Mol. Pharm. 2014;11:2040–2050. doi: 10.1021/mp500085p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phan-Lai V, et al. Three-Dimensional Scaffolds to Evaluate Tumor Associated Fibroblast-Mediated Suppression of Breast Tumor Specific T Cells. Biomacromolecules. 2013;14:1330–1337. doi: 10.1021/bm301928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA. Rapid 3D Extrusion of Synthetic Tumor Microenvironments. Adv. Mater. 2015;27:5512–5517. doi: 10.1002/adma.201501729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li CY, Wood DK, Huang JH, Bhatia SN. Flow-based pipeline for systematic modulation and analysis of 3D tumor microenvironments. Lab. Chip. 2013;13:1969–1978. doi: 10.1039/c3lc41300d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenny PA, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilbert PM, et al. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 2012;11:734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischbach C, et al. Engineering tumors with 3D scaffolds. Nat. Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 83.Leung M, et al. Chitosan-Alginate Scaffold Culture System for Hepatocellular Carcinoma Increases Malignancy and Drug Resistance. Pharm. Res. 2010;27:1939–1948. doi: 10.1007/s11095-010-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huh D, et al. A Human Disease Model of Drug Toxicity–Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci. Transl. Med. 2012;4:159ra147–159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 86.Xu Z, et al. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials. 2013;34:4109–4117. doi: 10.1016/j.biomaterials.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 87.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waldmann TA. Immunotherapy: past, present and future. Nat. Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 89.Kantoff PW, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 90.Hodi FS, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamid O, et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Postow MA, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenberg SA, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maude SL, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 96.Dendreon bankrupt. Nat. Biotechnol. 2014;32:1176–1176. [Google Scholar]
- 97.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol. Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawiger D, et al. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo. J. Exp. Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 102.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johansen P, et al. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. 2008;105:5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 105.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8–8. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ali OA, Tayalia P, Shvartsman D, Lewin S, Mooney DJ. Inflammatory Cytokines Presented from Polymer Matrices Differentially Generate and Activate DCs In Situ. Adv. Funct. Mater. 2013;23:4621–4628. doi: 10.1002/adfm.201203859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ali OA, et al. Identification of Immune Factors Regulating Antitumor Immunity Using Polymeric Vaccines with Multiple Adjuvants. Cancer Res. 2014;74:1670–1681. doi: 10.1158/0008-5472.CAN-13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ali OA, et al. The efficacy of intracranial PLG-based vaccines is dependent on direct implantation into brain tissue. J. Controlled Release. 2011;154:249–257. doi: 10.1016/j.jconrel.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 110.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cellcarrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29:3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 111.Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim J, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 2014 doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bencherif SA, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015;6:7556. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 115.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 116.Manolova V, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 117.Fifis T, et al. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 118.Gu L, et al. Multivalent Porous Silicon Nanoparticles Enhance the Immune Activation Potency of Agonistic CD40 Antibody. Adv. Mater. 2012;24:3981–3987. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raghuwanshi D, Mishra V, Suresh MR, Kaur K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine. 2012;30:7292–7299. doi: 10.1016/j.vaccine.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 120.Cruz LJ, et al. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: A comparative study. J. Controlled Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 121.Tacken PJ, et al. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 122.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsopelas C, Sutton R. Why Certain Dyes Are Useful for Localizing the Sentinel Lymph Node. J. Nucl. Med. 2002;43:1377–1382. [PubMed] [Google Scholar]
- 124.Faries MB, et al. Active Macromolecule Uptake by Lymph Node Antigen-Presenting Cells: A Novel Mechanism in Determining Sentinel Lymph Node Status. Ann. Surg. Oncol. 2000;7:98–105. doi: 10.1007/s10434-000-0098-6. [DOI] [PubMed] [Google Scholar]
- 125.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Jeanbart L, et al. Enhancing efficacy of anti-cancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2014 doi: 10.1158/2326-6066.CIR-14-0019-T. canimm.0019.2013. [DOI] [PubMed] [Google Scholar]
- 127.Gerner MY, Torabi-Parizi P, Germain RN. Strategically Localized Dendritic Cells Promote Rapid T Cell Responses to Lymph-Borne Particulate Antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 128.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 129.Murthy N, et al. A macromolecular delivery vehicle for protein-based vaccines: Acid-degradable protein-loaded microgels. Proc. Natl. Acad. Sci. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scott EA, et al. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials. 2012;33:6211–6219. doi: 10.1016/j.biomaterials.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 131.Li WA, Mooney DJ. Materials based tumor immunotherapy vaccines. Curr. Opin. Immunol. 2013;25:238–245. doi: 10.1016/j.coi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J. Controlled Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hamdy S, et al. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 135.Xu Z, et al. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Controlled Release. 2013;172:259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 136.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garaude J, Kent A, Rooijen N van, Blander JM. Simultaneous Targeting of Toll- and Nod-Like Receptors Induces Effective Tumor-Specific Immune Responses. Sci. Transl. Med. 2012;4:120ra16–120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 138.Li AV, et al. Generation of Effector Memory T Cell-Based Mucosal and Systemic Immunity with Pulmonary Nanoparticle Vaccination. Sci. Transl. Med. 2013;5:204ra130–204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goldinger SM, et al. Nano-particle vaccination combined with TLR-7 and-9 ligands triggers memory and effector CD8+T-cell responses in melanoma patients. Eur. J. Immunol. 2012;42:3049–3061. doi: 10.1002/eji.201142361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Flach TL, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 141.Zhang H, et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012;134:15790–15804. doi: 10.1021/ja304907c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilson NS, et al. Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J. Immunol. Baltim. Md 1950. 2014;192:3259–3268. doi: 10.4049/jimmunol.1302011. [DOI] [PubMed] [Google Scholar]
- 143.Frazer IH, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX™ adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23:172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 144.Thomas SN, et al. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32:2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 145.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 147.Milone MC, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Steenblock ER, Fahmy TM. A Comprehensive Platform for Ex Vivo T-cell Expansion Based on Biodegradable Polymeric Artificial Antigen-presenting Cells. Mol. Ther. 2008;16:765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 149.Perica K, Kosmides AK, Schneck JP. Linking form to function: Biophysical aspects of artificial antigen presenting cell design. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2015;1853:781–790. doi: 10.1016/j.bbamcr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sunshine JC, Perica K, Schneck JP, Green JJ. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35:269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncol. Williston Park N. 2002;16:11–20. [PubMed] [Google Scholar]
- 153.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Y, et al. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Controlled Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stephan SB, et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barenholz Y (Chezy) Doxil® — The first FDA-approved nano-drug: Lessons learned. J. Controlled Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 158.Dolgin E. Cancer vaccines: Material breach. Nature. 2013;504:S16–S17. doi: 10.1038/504S16a. [DOI] [PubMed] [Google Scholar]
- 159.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 160.Chu KS, et al. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine Nanotechnol. Biol. Med. 2013;9:686–693. doi: 10.1016/j.nano.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park J-H, et al. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sykes EA, Chen J, Zheng G, Chan WCW. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano. 2014;8:5696–5706. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- 163.Hu C-MJ, Fang RH, Luk BT, Zhang L. Polymeric nanotherapeutics: clinical development and advances in stealth functionalization strategies. Nanoscale. 2014;6:65. doi: 10.1039/c3nr05444f. [DOI] [PubMed] [Google Scholar]
- 164.Arvizo RR, et al. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nanoparticles. PLoS ONE. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Batist G, et al. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared With Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. J. Clin. Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 168.Mross K, et al. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose study. Cancer Chemother. Pharmacol. 2004;54:514–524. doi: 10.1007/s00280-004-0825-y. [DOI] [PubMed] [Google Scholar]
- 169.Gradishar WJ, et al. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. J. Clin. Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 170.Feldman EJ, et al. First-In-Man Study of CPX-351: A Liposomal Carrier Containing Cytarabine and Daunorubicin in a Fixed 5:1 Molar Ratio for the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lancet JE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hrkach J, et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012;4:128ra39–128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 173.Sugahara KN, et al. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Olson ES, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rodriguez PL, et al. Minimal ‘Self’ Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu C-MJ, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gu L, et al. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 2013;4:2326. doi: 10.1038/ncomms3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 179.Schwartz JA, et al. Feasibility Study of Particle-Assisted Laser Ablation of Brain Tumors in Orthotopic Canine Model. Cancer Res. 2009;69:1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 180.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011;63:24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 181.Chi X, et al. Nanoprobes for in vitro diagnostics of cancer and infectious diseases. Biomaterials. 2012;33:189–206. doi: 10.1016/j.biomaterials.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 182.Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]