Abstract
Fusion of rabies virus with membranes is triggered at a low pH and is mediated by a viral glycoprotein (G). Fusion of rabies virus with liposomes was monitored by using a lipid mixing assay based on fluorescence resonance energy transfer. Fusion was detected below pH 6.4, and its extent increased with H+ concentrations to be maximal around pH 6.15. The origin of the partial fusion activity of rabies virus under suboptimal pH conditions (i.e., between pH 6.15 and 6.4) was investigated. We demonstrate unambiguously that fusion at a suboptimal pH is distinct from the phenomenon of low-pH-induced inactivation and that it is not due to heterogeneity of the virus population. We also show that viruses that do not fuse under suboptimal pH conditions are indeed bound to the target liposomes and that the fusion complexes they have formed are blocked at an early stage of the fusion pathway. Our conclusion is that along the fusion reaction, different kinds of fusion machines with different pH thresholds for fusion can be formed. Possible explanations of this difference of pH sensitivity are discussed.
The entry of enveloped viruses into host cells requires fusion of the viral envelope with a cellular membrane. This step is mediated by virally encoded fusogenic glycoproteins. Activation of the fusion capacity involves structural rearrangements of these proteins upon interaction with specific triggers (e.g., low pH in the endosome or specific receptors at the cell surface). One of the consequences of these conformational changes is the exposure of the fusion peptide, which then interacts with and destabilizes one or both of the fusing membranes (32).
Despite extensive study of many enveloped viruses, the actual mechanism of the fusion process is still unknown. However, recent results have revealed striking similarities between different viral families and have suggested that the fusion intermediates along the fusion pathway are similar despite the diversity of the fusogenic proteins (5, 7, 8, 17, 25). All the data obtained so far are consistent with the so-called “stalk-pore” model (6). Furthermore, an increasing number of experiments indicate that the fusion complex is made up of several fusogenic proteins that act in a concerted manner, as shown for influenza virus hemagglutinin (4, 9, 23), for baculovirus Gp64 (24, 29), and for rabies virus glycoprotein G (31). This supramolecular assembly could explain the restriction of lipid diffusion that is observed in many systems (8, 17, 20, 22) during the initial steps of the fusion process.
Rabies virus-induced membrane fusion is mediated by viral transmembrane glycoprotein G which is organized in trimers (3 of 65 kDa each) (13, 34). The fusion of rabies virus with fluorescent liposomes has been studied in detail (14). Fusion is triggered at a low pH, is optimal around pH 6, and is not detected above pH 6.4. The fusion of rabies virus with liposomes is preceded by a lag time, the duration of which increases with lower temperature and higher pH (up to the pH threshold for fusion). Preincubation of the virus in the absence of a target membrane below pH 6.75 leads to the inhibition of viral fusion properties. However, a loss of fusion properties can be reversed by readjusting the pH to above 7. This is the main difference between rhabdoviruses (such as rabies virus) and other viruses that fuse at a low pH, for which low-pH-induced fusion inactivation is irreversible (18).
Low-pH-induced conformational changes of the glycoprotein and their relationships with fusion activity have been studied. Glycoprotein G can assume at least three different states (14). The native state is detected at the viral surface at a pH above 7. The activated hydrophobic state is detected immediately after acidification. It interacts with the target membrane as a first step of the fusion process (11). After prolonged incubation at a low pH, glycoprotein G is in a fusion-inactive conformation that is antigenically distinct from the native state. There is a pH-dependent equilibrium between these states which is shifted toward the fusion-inactive state at a low pH (31).
In this paper, using a liposome assay, we have analyzed the extent of fusion under suboptimal pH conditions. We show that fusion increases with H+ concentrations to be maximal around pH 6.15. We show that this phenomenon of partial fusion activity under suboptimal conditions is distinct from the phenomenon of low-pH-induced inactivation and that it is not due to heterogeneity of the virus population (i.e., that some virions would have a lower pH threshold for fusion). Our results are consistent with the ability for the virions and the liposomes to form different kinds of fusion machines having different pH thresholds for complete membrane fusion. Possible explanations for this difference in pH sensitivity are discussed below.
MATERIALS AND METHODS
Chemicals.
N-(7-nitro-2,1,3-benzoxadiazol-4-yl)-phosphatidylethanolamine (NBD-PE), 2-(4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine (BODIPY500-PC), and 2-(4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphoethanolamine (BODIPY530-PE) were purchased from Molecular Probes. 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (C18LPC) was purchased from Avanti Polar Lipids, Inc. Phosphatidylcholine (PC) (type XVI-E from egg yolk), phosphatidylethanolamine (PE) (type III from egg yolk), and gangliosides (type III from bovine brain) were supplied by Sigma Chemical Co.
Virus purification.
The PV strain of rabies virus was grown in BSR cells (a clone of BHK21, baby hamster kidney, cells) at 37°C in minimum essential medium supplemented with 2% calf serum. Virus particles were purified from the culture supernatant 72 h postinfection. Cell debris was first eliminated by a 30-min centrifugation at 3,500 rpm with a JA14 rotor (Beckman) at 4°C. The virus was then pelleted by centrifugation at 4°C (3 h at 14,000 rpm with a JA14 rotor) and resuspended in TD buffer (137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 25 mM Tris-HCl [pH 7.5]). Viral purification was achieved by another centrifugation of 50 min with 30% glycerol in 10 mM Tris-HCl (pH 7.5), 50 mM NaCl, and 1 mM EDTA at 25,000 rpm with an SW 28 rotor (Beckman) at 4°C. Virus was resuspended in TD buffer and stored at −80°C.
Buffers and pH adjustment.
The buffers used to adjust the pH were phosphate-citrate buffers prepared from a 0.1 M solution of citric acid and 0.2 M dibasic sodium phosphate stock solutions. Viral dilutions in these buffers do not modify the pH.
Preparation of liposomes.
A total of 700 μg of PC, 300 μg of PE, and 100 μg of gangliosides dissolved in organic solvents were mixed either with 2.5 μg of BODIPY500-PC and 2.5 μg of BODIPY530-PE (for fusion experiments) or with 10 μg of NBD-PE (for binding experiments) and dried in vacuo. The lipid film was resuspended in 1 ml of buffer (150 mM NaCl, 5 mM Tris-HCl [pH 8]), and the mixture was bath sonicated for 20 min.
Lipid mixing assay.
Lipid mixing was assayed at 18°C by using a resonance energy transfer method described by Malinin et al. (21). Ten microliters of fluorescent liposomes (about 10 μg of lipids giving a ratio of about 2 liposomes per viral particle) were mixed with 980 μl of phosphate-citrate buffer at the required pH in the cuvette of a thermostated Perkin-Elmer LS50B spectrofluorimeter. Next, 10 μl of virus (about 25 μg of viral proteins) was added, and the increase of BODIPY500-PC fluorescence was monitored continuously. In this range of viral concentrations, the increase of fluorescence was proportional to the viral concentration and thus to the number of fusion events. Excitation was at 503 nm (slit width, 5 nm) and emission was at 518 nm (slit width, 5 nm). The mixture was continuously stirred during the experiments.
In some experiments, when fusion had been triggered under suboptimal pH conditions (pH 6.25), once the plateau was reached, the pH was lowered to 5.9 by the addition of the appropriate volume of a 100 mM citric acid solution. To analyze the sensitivity of this second fusion step to C18LPC, 30 μl of a stock solution (500 μM) of this lipid, freshly prepared in ethanol, was added into the cuvette 1 min before the pH was lowered to 5.9. In all these experiments, the decrease of fluorescence induced by dilution was corrected in the figures presented in this paper.
Experiments performed with the same viral stocks, liposome preparations, and buffers were highly reproducible (fusion extent variations were <3%). However, the extent of fusion under suboptimal pH conditions (pH 6.25) was variable when different buffer stocks were used, due to pH accuracy (pH buffer could vary within ±0.03 pH units).
Separation of unfused liposomes from the virions and second-round fusion experiment.
After a first round of fusion at pH 7.5, 6.25, or 5.9, 200 μl of 1 M Tris-HCl (pH 8) was added in the spectrofluorimeter cuvette, and the mixture was incubated at 37°C for 30 min. This step allowed the detachment of unfused liposomes from the virions. The mixture was then centrifuged at 13, 000 × g for 1 h, and the pellet was resuspended in 10 μl of TD buffer and was mixed with 990 μl of phosphate-citrate buffer at the required pH containing 10 μg of fluorescent liposomes for a second round of fusion.
Liposomes binding to virus.
For binding experiments, 5 μl of virus (about 20 μg of viral proteins) was incubated with 12 μl of fluorescent liposomes (12 μg of lipids giving a ratio of about 3 liposomes per viral particle) containing NBD-PE and with 43 μl of phosphate-citrate buffer at the required pH for various durations at 0 or 18°C. The mixture was then rapidly diluted in 4.7 ml of cold 30% glycerol in phosphate-citrate buffer (pH 6.9) before a 30-min centrifugation at 40,000 rpm in an SW55 rotor (Beckman). The pellet was dissolved in 1 ml of sodium dodecyl sulfate (1%), and the amount of lipids was determined by NBD fluorescence measurements. This first value corresponded to the amount of liposomes, either fused or unfused, associated with the virus. To distinguish between fused and solely bound liposomes, the mixture of viruses and liposomes was incubated for 30 min at pH 8 (by the addition of a concentrated Tris-HCl solution) before dilution in cold glycerol, allowing unfused virions to detach from the liposomes. After dilution in cold glycerol (pH 6.9) and centrifugation, the amount of lipids in the pellet was determined by NBD fluorescence measurements. This second value corresponded to the amount of liposomes that had fused with the virions.
RESULTS
Origin of submaximal responses in low-pH-induced fusion between rabies virus and liposomes.
The resonance energy transfer method described by Malinin et al. (21) that involves the nonexchangeable probes BODIPY500-PC as a fluorescent donor and BODIPY530-PE as a fluorescent acceptor was used to assay for fusion between rabies virus and liposomes containing PC, PE, and gangliosides (7/3/1, wt/wt/wt). It was preferred to the method of Struck et al. (33) which we had previously used (31) because the BODIPY analogues appeared to be more photostable than those of NBD-PE. This stability was important for the long-term kinetic measurements presented in this paper. Nevertheless, similar results were obtained by using the method of Struck et al. (33) (data not shown).
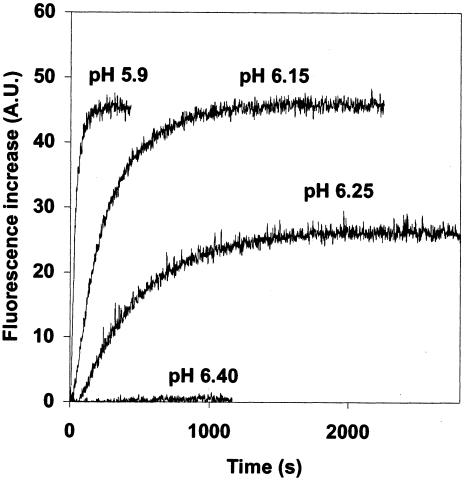
Figure 1 shows typical kinetics of fusions obtained under different pHs at 18°C. Fusion was detected below pH 6.4, and its extent increased with H+ concentrations to be maximal at pH 6.15. Although the rate of fusion was higher at a lower pH, the extent of the reaction was the same at pH 5.9 and 6.15 (Fig. 1 and Table 1).
FIG. 1.
Fusion of rabies virus (PV strain) with gangliosides containing liposomes at indicated pHs and 18°C (see Materials and Methods for details). A.U., arbitrary units.
TABLE 1.
Fusion extent under different pH conditions and at 18°Ca
| pH | Fusion extent (%) |
|---|---|
| 5.9 | 100 |
| 6.15 | 89 ± 11 (n = 2) |
| 6.20 | 73 ± 7 (n = 3) |
| 6.25 | 61 ± 5 (n = 7) |
| 6.3 | 39 ± 2 (n = 4) |
| 7.5 | 4 ± 4 (n = 4) |
Fusion experiments were performed as described in Materials and Methods. Liposomes were made of PC, PE, and gangliosides (weight ratio, 7/3/1). In each set of experiments, the increase of fluorescence at pH 5.9 was defined as 100%, and the other values were calculated as percentages thereof. The mean, the standard deviation, and the number of independent experiments (i.e., performed with different viral stocks and liposome preparations) are given.
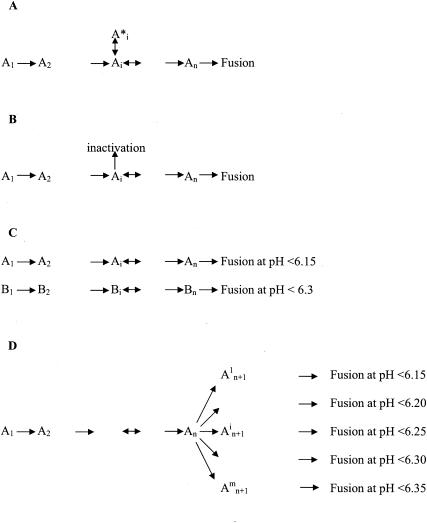
A linear sequence of reactions (Fig. 2A) would have led to the same fusion extent at any pH below the pH threshold in a homogeneous population of virions. Thus, what could explain the difference of the extent of the reaction at pH 6.25 and 5.9?
FIG. 2.
Possible reaction scheme for fusion. (A) Linear reaction scheme with eventual reversible branched reactions. Such a scheme would lead to the same fusion extent at any pH below the pH threshold. Ai (1 ≤ i ≤ n) represents an intermediate stage among n intermediate stages in the fusion pathway. (B) Linear reaction scheme with irreversible inhibitory branches. Such a scheme may result to different fusion extents at different pHs. The extent at a given pH depends on the probability to enter the inhibitory branches. (C) Different subpopulations of viruses (A and B) having different pH thresholds for fusion. (D) Formation of different kinds of fusion intermediates with different pH thresholds for fusion. From the intermediate state An, the virus is able to irreversibly form different kinds of complexes having distinct pH thresholds for complete fusion.
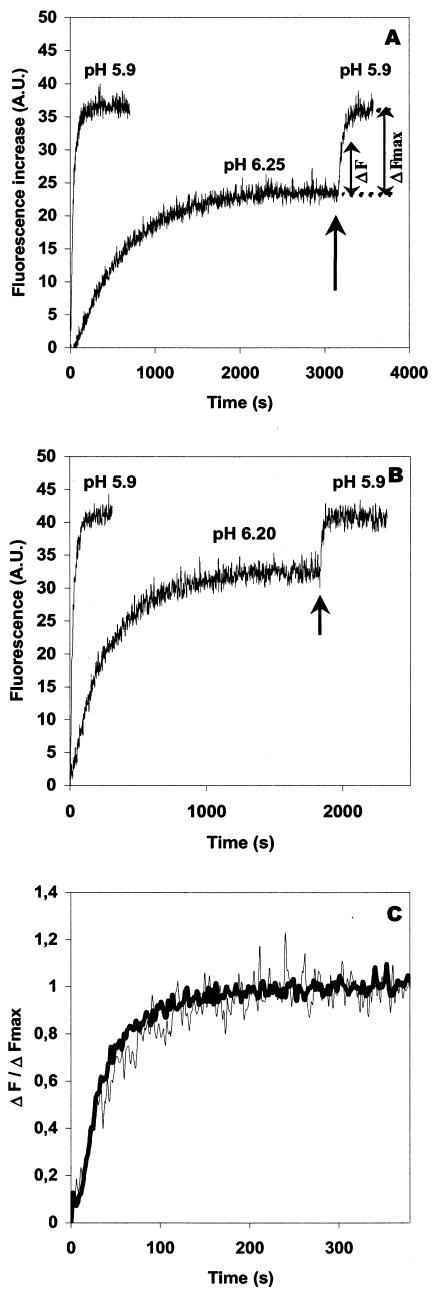
A first explanation could be that, at pH 6.25, inactivation of the fusogenic properties of glycoprotein G competed with fusion (Fig. 2B). This hypothesis can be ruled out because, once the reaction plateau had been reached at pH 6.25, lowering the pH to 5.9 produced new fusion events. In fact, after such a treatment, the total number of fusion events was almost the same as after a single step at pH 5.9 (Fig. 3A). Furthermore, when proteinase K was added in the cuvette after the first round of fusion at pH 6.25, no fluorescence increase was observed when the pH was subsequently lowered to 5.9 (data not shown). This result indicated that the second fusion reaction still required the integrity of the viral glycoproteins.
FIG. 3.
Partial fusion activity of rabies virus at pH 6.25 is distinct from the phenomenon of low-pH-induced inactivation. (A) Rabies virus was added to gangliosides containing fluorescent liposomes at pH 5.9 or at indicated suboptimal pH at 18°C. At a suboptimal pH, once the plateau was reached, the pH was lowered to 5.9 by the addition of the appropriate volume of a 100 mM citric acid solution (indicated by arrows). (B) Same experiment (A) with liposomes devoid of gangliosides. (C) Normalization of fusion curves obtained at pH 5.9 (A) either after a direct incubation at this pH (dark curve) or after a first step of fusion at pH 6.25 (light curve). Normalization was performed by plotting (C) Normalization of fusion curves obtained at pH 5.9 (A) either after a direct incubation at this pH (dark curve) or after a first step of fusion at pH 6.25 (light curve). Normalization was performed by plotting ΔF/ΔFmax (as indicated in panel A) as a function of time. A.U., arbitrary units.
Another explanation for the partial fusion activity observed under suboptimal pH conditions could be that gangliosides, upon the initial interaction between virus and liposomes, might form microdomains that locally modify the pH in the vicinity of a given fusion complex. However, as similar experiments performed on ganglioside-free liposomes gave identical results (Fig. 3B), this explanation was also ruled out.
Finally, the viral population could be heterogeneous and different subpopulations could have different pH thresholds for fusion (Fig. 2C). This heterogeneity might be due to the quasispecies nature of the viral population (1, 19, 27), to transcription errors made by the viral polymerase, or to the presence of damaged viral particles. In this case, viruses that had not fused in the first round at pH 6.25 should be unable to fuse in a second round at the same pH. In order to investigate this hypothesis, fusion between virions and liposomes was triggered at either pH 5.9 or 6.25. After the pH was increased to above 7.5 (allowing the spikes to recover their native structure and thus reset the system), the viral particles (either fused or unfused) were pelleted and resuspended in TD buffer. They were then reincubated with fresh fluorescent liposomes for a second round of fusion.
When the first round of fusion had been triggered at pH 5.9, virtually no fluorescence increase was detected in a second round (Table 2). This result indicated that all the virions have fused in the first round at pH 5.9. The absence of fluorescence increase could have two causes. First, virosomes resulting from the fusion of virions with liposomes could be unable to fuse in the second round at pH 5.9. Second, alternatively, an eventual fusion of these virosomes (that already contain fluorescent probes in their membranes) with liposomes in the second round at pH 5.9 did not result in a fluorescence increase.
TABLE 2.
Relative fluorescence increase after a first round and a second round of fusion at indicated pHsa
| PH
|
Relative fluorescence increase (%)
|
||
|---|---|---|---|
| First round | Second round | First round | Second round |
| 7.5 | 5.9 | 1.5 ± 3.3 | 95.4 ± 4 |
| 5.9 | 5.9 | 100 | 1.8 ± 3 |
| 6.25 | 5.9 | 60.2 ± 5 | 30.0 ± 4.3 |
| 6.25 | 6.25 | 60.9 ± 6.1 | 20.3 ± 5.3 |
Fusion between virions and liposomes was first triggered at the indicated pH. After the pH was increased to above 7.5, the viral particles (either fused or unfused) were pelleted and resuspended in TD buffer. They were then reincubated with fresh fluorescent liposomes for a second round of fusion (see Materials and Methods for details). In each set of experiments, the increase of fluorescence in the first round of fusion at pH 5.9 was defined as 100%, and the other values were calculated as percentages thereof. The mean and the standard deviation of three independent experiments are given.
When fusion was triggered at pH 6.25, about 61% of the virions fused in the first round, and the rest of the population fused at pH 5.9 in the second round (Table 1). When the second round was triggered at pH 6.25, the increase of fluorescence was about 67% (20.3%/30%) of that observed at pH 5.9 (Table 2), indicating that the virions which had not fused in the first round at pH 6.25 were indeed able to fuse at this pH. Thus, the behavior of the virions that had not fused in the first round of fusion at pH 6.25 could not be distinguished from that of the complete population of virions.
All these results led to the hypothesis that along the fusion reaction, different kinds of fusion machines with different pH thresholds for fusion could be formed (Fig. 2D).
Viruses which do not fuse at pH 6.25 are bound to the target liposomes.
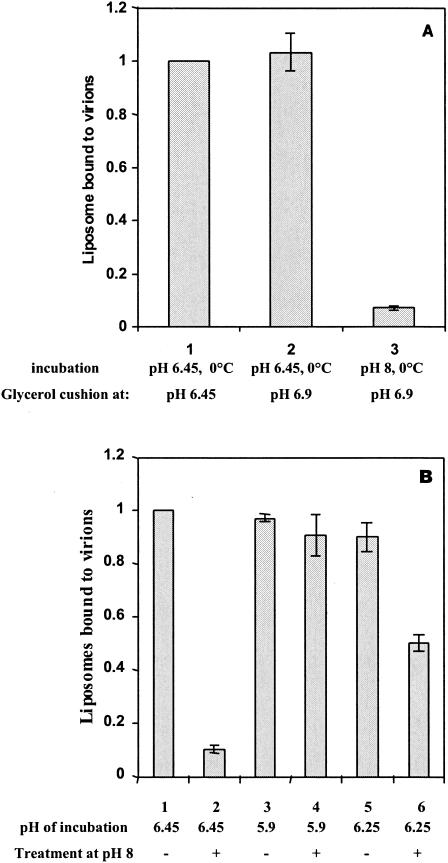
We next investigated at which step fusion was blocked for the virions unable to fuse at pH 6.25. For this, we developed a sedimentation assay that allowed us to differentiate between virions which have fused with the liposomes from those which were bound to only the target membrane in a hydrophobic manner. First, we demonstrated that a rapid dilution of a mixture of viruses and liposomes in 30% glycerol at pH 6.9 and 0°C followed by centrifugation of the complexes neither disrupted virus-liposome complexes preformed at pH 6.45 and 0°C (11, 17, 31) nor allowed their formation (Fig. 4A). Second, we showed that a 10-min incubation at pH 8 and 37°C disrupted any virus-liposome complexes which have not fused (Fig. 4B).
FIG. 4.
Viruses that do not fuse at pH 6.25 are bound to liposomes. (A) Determination of experimental conditions used to study virus-liposome complexes; dilution at pH 6.9 neither disrupted complexes preformed at pH 6.45 at 0°C nor allowed their formation. Rabies virus was incubated for 3 min on ice with fluorescent liposomes containing NBD-PE at pH 6.45 (bars 1 and 2) or pH 8 (bar 3). The mixture was diluted in a cold 30% glycerol solution at pH 6.45 (bar 1) or pH 6.9 (bars 2 and 3) (see Materials and Methods for details) before centrifugation. The amount of liposomes associated with virus in the pellet was determined by fluorescence measurements. In each set of experiments, the value of fluorescence intensity (bar 1) was defined as 100%, and the other values were calculated as percentages thereof. The graph shows the mean of three independent experiments. Error bars indicate standard deviations. (B) Viruses that do not fuse at pH 6.25 are bound to liposomes. Rabies virus was incubated with fluorescent liposomes containing NBD-PE for 3 min on ice at pH 6.45 (bars 1 and 2) or for 10 min at pH 5.9 at 18°C (bars 3 and 4), or for 20 min at pH 6.25 at 18°C (bar 5 and 6). The mixture was diluted in a cold 30% glycerol solution at pH 6.9 either directly (bars 1, 3, and5) or after reincubation at pH 8 at 37°C for 10 min. (bars 2, 4, and 6). The amount of liposomes associated with virus in the pellet was determined by fluorescence measurements. In each set of experiments, the value of fluorescence intensity (bar 1) was defined as 100%, and the other values were calculated as percentages thereof. The graph shows the mean of three independent experiments. Error bars indicate standard deviations.
This assay was then used to test if the virions which have not fused at pH 6.25 were bound to liposomes. The amount of liposomes associated with the virions was the same after an incubation at pH 6.25 or 5.9 and 18°C. A second incubation of the complexes at pH 8 and 37°C resulted in a disruption of about 50% of the complexes made at pH 6.25 but had no effect on the complexes made at pH 5.9. The simplest explanation was that at pH 5.9, all the complexes led to fusion, whereas at pH 6.25, only 50% of the complexes led to fusion (in agreement with the results shown in Fig. 3A and Table 1). This indicated that viruses which did not fuse at pH 6.25 were indeed bound to the liposomes.
Fusion complexes with a low pH threshold for fusion are blocked at an early stage of the fusion pathway at pH 6.25.
We tested if the stage at which the process was blocked at pH 6.25 for the virions which have not fused was located upstream or downstream of stalk formation. Thus, we investigated the sensitivity of the second fusion reaction (triggered at pH 5.9) to C18LPC. The C18LPCs were still able to inhibit the second fusion reaction. Furthermore, after a first step at pH 6.25, the fusion reaction at pH 5.9 does not seem to be kinetically advanced compared with the fusion reaction directly triggered at pH 5.9. Particularly, the normalized kinetics were the same whether the fusion was triggered directly at pH 5.9 or when the plateau had been reached at pH 6.25 (Fig. 3C). These observations are consistent with the fact that the fusion complexes with a low pH threshold for fusion are blocked at an early stage of the fusion pathway at pH 6.25.
DISCUSSION
In this study, we observed a phenomenon of partial fusion activity of rabies virus under suboptimal pH conditions. Our experiments eliminated the possibility that the virus population is heterogeneous and that some virions would have a lower pH threshold for fusion. We also demonstrated that this partial fusion activity is not due to an irreversible inactivation of the fusion process at pH 6.25. The experiments presented here cannot exclude a model of reversible inactivation in which lowering the pH to 5.9 would reverse the inactivation induced at pH 6.25 and would allow fusion to go to completion. Nevertheless, as inactivation of rabies virus fusion properties is more efficient at lower pHs (14), such an explanation is highly unlikely. Thus, our conclusion is that partial fusion activity is due to the existence of different fusion pathways or, to be more precise, to the ability for the virions and the liposomes to form different kinds of fusion machines having different pH thresholds for complete membrane fusion.
These results are reminiscent of results obtained for Sendai virus (28) and influenza virus (30) for which a phenomenon of partial fusion activity has also been described that was not due to low-pH inactivation. It was proposed that partial fusion activity was due to a certain probability of irreversible production of fusion-inactive sites. More recently, kinetic considerations have demonstrated that different and independent fusion machines generate the fusion and hemifusion phenotypes observed in lipid mixing experiments between red blood cells and hemagglutinin-expressing cells. Moreover, the analysis suggested the existence of several different hemifusion machines (26).
A very similar phenomenon has also been described for calcium-triggered exocytosis (2, 3). In this case, it has been demonstrated that cortical vesicles are heterogeneous in their response to calcium due to the formation of fusion complexes with different calcium thresholds.
What could be the molecular origin of this phenomenon? In the case of calcium-triggered exocytosis, one of the proposed explanations was that a local lipidic environment or local ionic conditions might influence the fusion process. Although we have worked with a minimal system (purified virus and liposomes of defined composition), we cannot exclude that some differences of local curvature of the target membranes, due to the size heterogeneity of the liposomes, have an influence on the fusion process. Nevertheless, the phenomenon of partial fusion activity at a given suboptimal pH was quantitatively similar when we used target membranes of different composition, as shown with liposomes containing or not containing gangliosides (Fig. 3A and B). Another explanation for this phenomenon may also reside in the peculiar bullet shape of rabies virus. The curvature of the viral membrane is very different at the round extremity, on the side, or at the blunt extremity of the bullet. The position of the attachment site to the target membrane at the viral surface could affect the pH threshold for fusion. Two experimental observations are consistent with this explanation. First, for vesicular stomatitis virus (another rhabdovirus having the typical bullet shape of this viral family), after incubation at a low pH, virus particles that were fused together at their blunt ends were observed (15). This observation suggests that this viral end is more apt to fuse with a target membrane. Second, the pH threshold to observe syncytium formation in a cell-cell system is lower (about pH 5.9) than the one for virus-liposome fusion (about pH 6.3) (10, 12). In the first experimental system, the bilayers can be considered flat, whereas in the second system, the bilayers are highly bent (particularly at the extremities of the virions). It is thus possible that in the cell-to-cell fusion assay, only the fusion machineries with lower pH thresholds can be formed.
Alternatively, the ability for the virions to form different kinds of fusion complexes having different pH thresholds for complete membrane fusion may be explained by different macromolecular organization of the viral glycoproteins in the fusion complex. For rabies virus, we have previously shown that a large number of glycoproteins are involved in the fusion process (31). We have also obtained results that suggest that rabies virus glycoproteins can self-associate under prefusion conditions (pH 6.6 and 0°C) to form a local hexagonal lattice (16). It is possible that the number of spikes involved in the contact zone and the quality of the lattice (i.e., the presence of penta- or heptagons formed by quasiequivalent contacts between glycoproteins) modify the pKa of some residues and thus have an influence on the pH threshold for fusion. It should be noted that these explanations are not exclusive of one another; the organization of the lattice cannot be the same at the extremities and on the side of the virions and may also depend on the curvature of the target liposome.
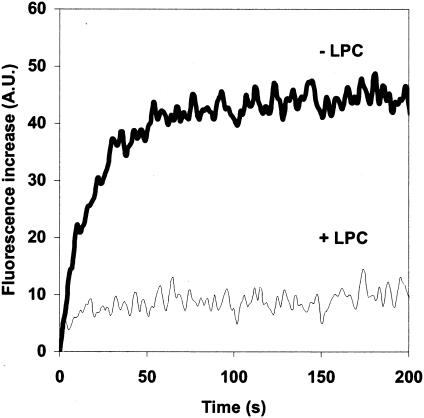
We have previously identified a fusion intermediate (called RVPC for rabies virus prefusion complex) that is trapped when virions and liposomes are incubated between pH 6.4 and 6.7 at 0°C (17). It has been proposed that at this stage, a restricted hemifusion diaphragm is already destabilized although lipid mixing is not detected (17). Indeed, when complete fusion is triggered from the RVPC stage, the reaction appears to be kinetically advanced (14, 17). In this work, we show that viruses that do not fuse under suboptimal pH conditions are bound to the target liposomes. The formation of these complexes certainly involves the insertion of fusion peptides into the liposome membrane because the liposomes do not contain any viral receptor. At this stage, the fusion reaction is still C18LPC sensitive (Fig. 5), suggesting that it is blocked at a stage upstream of stalk formation (7, 8, 17). As it has been shown that lipidic intermediates on the fusion pathway are dynamic, this result has to be interpreted with caution. Indeed, it is difficult to exclude that C18LPC disrupted the lipidic structure of a putative intermediate during the 1-min incubation before the pH was lowered to 5.9. Nevertheless, as the fusion reaction at pH 5.9, after a first round of fusion at pH 6.25, is not kinetically advanced compared with the fusion reaction directly triggered at pH 5.9, these complexes are blocked at a stage that is much less advanced than the RVPC stage. However, even at this early stage, the fusion machinery has already undergone a transition that has engaged it in a pathway that determines its pH threshold for complete fusion of the membranes.
FIG. 5.
The stage at which the fusion process is blocked at pH 6.25 at 18°C is located upstream of stalk formation. Rabies virus was added to gangliosides containing fluorescent liposomes at pH 6.25 and 18°C. After 1,500 s, once the plateau was reached, the pH was lowered to 5.9 by the addition of the appropriate volume of a 100 mM citric acid solution. For the bottom curve (+LPC), C18LPC (final concentration, 15 μM) was added 1 min before the pH was lowered to 5.9. Zero time corresponds to addition of citric acid. A.U., arbitrary units.
Acknowledgments
We thank Rob Ruigrok for helpful discussions and careful reading of the manuscript.
This work was supported by the CNRS (UMR 2472). S.R. is a predoctoral fellow of the Ecole Polytechnique and is supported by a grant of the DGA.
REFERENCES
- 1.Benmansour, A., M. Brahimi, C. Tuffereau, P. Coulon, F. Lafay, and A. Flamand. 1992. Rapid sequence evolution of street rabies glycoprotein is related to the highly heterogeneous nature of the viral population. Virology 187:33-45. [DOI] [PubMed] [Google Scholar]
- 2.Blank, P. S., S. S. Vogel, M. S. Cho, D. Kaplan, D. Bhuva, J. Malley, and J. Zimmerberg. 1998. The calcium sensitivity of individual secretory vesicles is invariant with the rate of calcium delivery. J. Gen. Physiol. 112:569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank, P. S., M. S. Cho, S. S. Vogel, D. Kaplan, A. Kang, J. Malley, and J. Zimmerberg. 1998. Submaximal responses in calcium-triggered exocytosis are explained by differences in the calcium sensitivity of individual secretory vesicles. J. Gen. Physiol. 112:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenthal, R., D. P. Sarkar, S. Durell, D. E. Howard, and S. J. Morris. 1996. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J. Cell Biol. 135:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chernomordik, L., E. Leikina, M. S. Cho, and J. Zimmerberg. 1995. Control of baculovirus gp64-induced syncytium formation by membrane lipid composition. J. Virol. 69:3049-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernomordik, L., M. M. Kozlov, and J. Zimmerberg. 1995. Lipids in biological membrane fusion. J. Membr. Biol. 146:1-14. [DOI] [PubMed] [Google Scholar]
- 7.Chernomordik, L. V., E. Leikina, V. Frolov, P. Bronk, and J. Zimmerberg. 1997. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell Biol. 136:81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., V. A. Frolov, E. Leikina, P. Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danieli, T., S. L. Pelletier, Y. I. Henis, and J. M. White. 1996. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol. 133:559-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmezieres, E., A. P. Maillard, Y. Gaudin, N. Tordo, and P. Perrin. 2003. Differential stability and fusion activity of lyssavirus glycoprotein trimers. Virus Res. 91:181-187. [DOI] [PubMed] [Google Scholar]
- 11.Durrer, P., Y. Gaudin, R. W. Ruigrok, R. Graf, and J. Brunner. 1995. Photolabeling identifies a putative fusion domain in the envelope glycoprotein of rabies and vesicular stomatitis viruses. J. Biol. Chem. 270:17575-17581. [DOI] [PubMed] [Google Scholar]
- 12.Gaudin, Y., C. Tuffereau, D. Segretain, M. Knossow, and A. Flamand. 1991. Reversible conformational changes and fusion activity of rabies virus glycoprotein. J. Virol. 65:4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudin, Y., R. W. Ruigrok, C. Tuffereau, M. Knossow, and A. Flamand. 1992. Rabies virus glycoprotein is a trimer. Virology 187:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudin, Y., R. W. Ruigrok, M. Knossow, and A. Flamand. 1993. Low-pH conformational changes of rabies virus glycoprotein and their role in membrane fusion. J. Virol. 67:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudin, Y., R. W. Ruigrok, and J. Brunner. 1995. Low-pH induced conformational changes in viral fusion proteins: implications for the fusion mechanism. J. Gen. Virol. 76:1541-1556. [DOI] [PubMed] [Google Scholar]
- 16.Gaudin, Y., H. Raux, A. Flamand, and R. W. Ruigrok. 1996. Identification of amino acids controlling the low-pH-induced conformational change of rabies virus glycoprotein. J. Virol. 70:7371-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudin, Y. 2000. Rabies virus-induced membrane fusion pathway. J. Cell Biol. 150:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudin, Y. 2000. Reversibility in fusion protein conformational changes. The intriguing case of rhabdovirus-induced membrane fusion. Subcell. Biochem. 34:379-408. [DOI] [PubMed] [Google Scholar]
- 19.Kissi, B., H. Badrane, L. Audry, A. Lavenu, N. Tordo, M. Brahimi, and H. Bourhy. 1999. Dynamics of rabies virus quasispecies during serial passages in heterologous hosts. J. Gen. Virol. 80:2041-2050. [DOI] [PubMed] [Google Scholar]
- 20.Leikina, E., and L. V. Chernomordik. 2000. Reversible merger of membranes at the early stage of influenza hemagglutinin-mediated fusion. Mol. Biol. Cell 11:2359-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinin, V. S., M. E. Haque, and B. R. Lentz. 2001. The rate of lipid transfer during fusion depends on the structure of fluorescent lipid probes: a new chain-labeled lipid transfer probe pair. Biochemistry 40:8292-8299. [DOI] [PubMed] [Google Scholar]
- 22.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2000. The lipid-anchored ectodomain of influenza virus hemagglutinin (GPI-HA) is capable of inducing nonenlarging fusion pores. Mol. Biol. Cell 11:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markovic, I., E. Leikina, M. Zhukovsky, J. Zimmerberg, and L. V. Chernomordik. 2001. Synchronized activation and refolding of influenza hemagglutinin in multimeric fusion machines. J. Cell Biol. 155:833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markovic, I., H. Pulyaeva, A. Sokoloff, and L. V. Chernomordik. 1998. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J. Cell Biol. 143:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal, A., E. Leikina, L. V. Chernomordik, and J. Bentz. 2003. Kinetically differentiating influenza hemagglutinin fusion and hemifusion machines. Biophys. J. 85:1713-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimoto, K., D. C. Hooper, H. Carbaugh, Z. F. Fu, H. Koprowski, and B. Dietzschold. 1998. Rabies virus quasispecies: implications for pathogenesis. Proc. Natl. Acad. Sci. USA 95:3152-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nir, S., N. Duzgunes, M. C. de Lima, and D. Hoekstra. 1990. Fusion of enveloped viruses with cells and liposomes. Activity and inactivation. Cell. Biophys. 17:181-201. [DOI] [PubMed] [Google Scholar]
- 29.Plonsky, I., and J. Zimmerberg. 1996. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J. Cell Biol. 135:1831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramalho-Santos, J., M. C. Lima, and S. Nir. 1996. Partial fusion activity of influenza virus toward liposomes and erythrocyte ghosts is distinct from viral inactivation. J. Biol. Chem. 271:23902-23906. [DOI] [PubMed] [Google Scholar]
- 31.Roche, S., and Y. Gaudin. 2002. Characterization of the equilibrium between the native and fusion-inactive conformation of rabies virus glycoprotein indicates that the fusion complex is made of several trimers. Virology 297:128-135. [DOI] [PubMed] [Google Scholar]
- 32.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 33.Struck, D. K., D. Hoekstra, and R. E. Pagano. 1981. Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20:4093-4099. [DOI] [PubMed] [Google Scholar]
- 34.Whitt, M. A., L. Buonocore, C. Prehaud, and J. K. Rose. 1991. Membrane fusion activity, oligomerization, and assembly of the rabies virus glycoprotein. Virology 185:681-688. [DOI] [PubMed] [Google Scholar]