Abstract
The immune correlates of protection in human immunodeficiency virus type 1 (HIV-1) infection remain poorly defined, particularly the contribution of CD4+ T cells. Here we explore the effector functions of HIV-1-specific CD4+ T cells. We demonstrate HIV-1 p24-specific CD4+-T-cell cytolytic activity in peripheral blood mononuclear cells directly ex vivo and after enrichment by antigen-specific stimulation. We further show that in a rare long-term nonprogressor, both an HIV-1-specific CD4+-T-cell clone and CD4+ T cells directly ex vivo exert potent suppression of HIV-1 replication. Suppression of viral replication was dependent on cell-cell contact between the effector CD4+ T cells and the target cells. While the antiviral effector activity of CD8+ T cells has been well documented, these results strongly suggest that HIV-1-specific CD4+ T cells are capable of directly contributing to antiviral immunity.
Despite extensive investigation, the immune correlates of protection in human immunodeficiency virus type 1 (HIV-1) infection remain undefined. Control of HIV-1 replication in the absence of antiretroviral therapy is associated with vigorous virus-specific immune responses in both the CD8+- and CD4+-T-cell subsets in persons with undetectable viremia (19, 36). Virus-specific CD8+ cytoxic T lymphocytes (CTL) are typically thought to be the biggest contributors to the control of HIV-1 replication. The fall in viral load after primary HIV-1 infection is temporally correlated with the appearance of major histocompatibility complex class I-restricted CTL (7, 20). Depletion of CD8+ CTL in simian immunodeficiency virus (SIV) infection results in failure to control primary viremia or a rapid increase in virus load in chronic infection (37). However, recent comprehensive analyses of CD8+-T-cell responses reveal no quantitative correlation with viremia (2, 4, 13). Furthermore, while CTL appear to play an important role in HIV-1 infection, they do not afford protection from progression of disease in most individuals (10), and viremia usually persists in spite of strong HIV-1-specific CD8+ CTL responses (reviewed in reference 25). These findings indicate that in most cases CTL alone are not sufficient to control infection.
A growing body of evidence points to a pivotal role for CD4+-T-cell responses in HIV-1 infection. Robust p24-specific proliferative responses are associated with transient control of HIV-1 viremia (36). However, data indicate that high viral load interferes with Th-cell proliferation and that HIV-1-specific proliferative responses prior to interruption of antiretroviral therapy are not predictive of virus control after discontinuation of antiretroviral therapy (26). It is believed that the preferential infection of HIV-1-specific CD4+ T cells (9) contributes to a functional deletion or impairment of helper activity needed to support CTL function, and CD4+-T-cell proliferative responses have been correlated to CTL precursor frequencies (19). Direct mechanistic evidence on the role of virus-specific CD4+ T cells has been lacking.
Effector functions mediated by CD4+ Th cells have not been widely explored in the control of viral infections. In vitro cytolytic activity residing in the CD4+-T-cell subset is seen in a number of viral infections in humans, including those caused by HIV, Epstein-Barr virus, herpes simplex virus, measles virus, influenza virus, varicella-zoster virus, and dengue virus (reviewed in reference 31). CD4+ Th cells have also been shown to secrete antiviral factors, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (42). Most work analyzing effectiveness of CD4+ CTLs has been performed with the murine system. Adoptive transfer of tumor-specific CD4+ CTL into athymic or RAG2−/− mice results in tumor regression after initial growth (29, 45). CD4+ CTL clones can mediate suppression of Friend virus in vitro (17) and influenza in vivo (14). These studies all suggest a potential role for effector functions mediated by Th cells in controlling viral infections.
The present study demonstrates ex vivo-mediated CD4+ cytolytic activity and explores the ability of HIV-1-specific CD4+-T-cell clones to kill T cells expressing the HIV-1 p24 protein. We further demonstrate that CD4+ cytolytic T-cell clones and CD4+ T cells directly ex vivo can suppress HIV-1 replication. These data support the concept that CD4+ T cells not only may provide indirect “help” for CTL but could have a direct role in control of viral replication in vivo.
MATERIALS AND METHODS
Study subjects.
CD4+-T-cell clones were previously derived from 4 individuals (30), and 10 additional subjects were selected, spanning a range of viral loads (Table 1). Long-term nonprogressors are defined as having been HIV-1 infected for more than 10 years and maintaining a virus load of fewer than 2,000 RNA copies/ml of plasma without antiretroviral therapy. Controllers fit the same definition but are infected for less than 10 years. Acutes refer to individuals treated with antiretroviral therapy within six months of HIV-1 seroconversion, and many underwent structured therapy interruption after initial antiretroviral therapy. Seronegative individuals were not HIV-1 infected, determined by HIV-1 antibody testing. All study samples were obtained with informed consent, and the studies were approved by the Massachusetts General Hospital Institutional Review Board.
TABLE 1.
Subject characteristicsa
| Subject | Status | Diagnosis date | CD4 count (cells/ml) | Virus load (RNA copies/ml) | ART |
|---|---|---|---|---|---|
| AC-01 | Acute | 1997 | 875 | <400 | Yes |
| AC-06 | Acute | 1997 | 466 | 109,000 | Off 2 years |
| AC-14 | Acute | 1998 | 598 | 1,530 | Off 3 years |
| AC-18 | Acute | 1998 | 311 | 985,000 | Off 6 months |
| AC-25 | Acute | 1998 | 471 | 150 | Yes |
| AC-42 | Acute | 1999 | 1,074 | <50 | Yes |
| CO-01 | Controller | 2000 | 798 | 601 | Never |
| MGB | Controller | 1998 | 673 | <50 | 1 year, 1998-1999 |
| LT-02 | LTNP | 1991 | 597 | 738 | 2.3 years, 1998-2000 |
| LT-04 | LTNP | 1992 | 729 | <50 | 4 mos, in 1992 |
| LT-06 | LTNP | 1987 | 703 | 384 | 3 mos 1988 |
| LT-10 | LTNP | 1992 | 784 | 1,920 | Never |
| 161J | LTNP | Mid-1980s | 842 | <50 | Never |
| CTS-01 | LTNP | Late 1980s | 571 | 545 | Never |
| SN-01 | HIV− | ||||
| SN-02 | HIV− | ||||
| SN-03 | HIV− | ||||
| SN-04 | HIV− |
CD4 count and viral load are from the time of subject study. LTNP refers to long-term nonprogressor, and HIV− indicates that subjects were not HIV infected. ART, antiretroviral therapy.
Antigens and monoclonal antibodies.
p24 and gp120 proteins and the baculovirus-derived control antigen mgs were from Protein Sciences (Meriden, Conn.), reverse transcriptase protein was from Trinity Biotech (Carlsbad, Calif.), and Nef protein was from ViroGen (Watertown, Mass.). Shorter p24 peptides were generated with an Advanced ChemTech (Texas) 396Ω peptide synthesizer.
Generation of clones and lines.
Clones were generated via limiting dilution and maintained as previously described (32). CD4+-T-cell lines were generated using Rosette-Sep (Stem Cell Technologies, Vancouver, Canada) for depletion of CD8+ cells from blood during Ficoll-hypaque separation, followed by stimulation with p24 protein (5 μg/ml). CD8+- or CD4+-T-cell enrichment was performed using Rosette-Sep to deplete cells bearing CD16, CD19, CD36, or CD56 and CD4+ or CD8+ cells, respectively. CD4+-T-cell enrichment typically resulted in at least 85% CD4+ T cells with up to 7% contamination with CD8+ T cells and 3% CD3− CD4− CD8− cells (data not shown).
Cytotoxicity assays.
Cytolysis was measured as previously described (32). Peripheral blood mononuclear cell (PBMC) cytolytic activity was assessed in an overnight assay, while clones and T-cell lines were assayed over 4 h. Spontaneous lysis was less than 30% unless noted. Specific lysis of greater than 10% was considered significant.
IFN-γ ELISPOT assays.
Enzyme-linked immunosorbent spot (ELISPOT) assays were performed as previously described (32). Target MT-2 cells (5 × 104) and antigen (5 μg/ml) were combined with T-cell clones (100 cells/well) in an overnight assay. Background responses to no antigen ranged from 0.5 to 1.5 spots/well. Responses greater than five spots/well and five times the background level were considered significant.
Flow cytometry.
Intracellular cytokine staining (ICS) was performed using a modification of published techniques (35). Briefly, 0.5 × 106 cells were incubated with 5 μg of p24 protein/ml for 2 h and then treated with 10 μg of brefeldin A (Sigma-Aldrich, St. Louis, Mo.)/ml for 4 h. Cells were surface stained with anti-CD8-PE, anti-CD3-PerCP, and anti-CD4-APC (Becton Dickinson, San Jose, Calif.) for 30 min, washed in phosphate-buffered saline, and then fixed and permeabilized using Caltag reagents (Caltag Laboratories, Burlingame, Calif.). Staining for IFN-γ was performed using anti-IFN-γ-fluorescein isothiocyanate for 30 min. Intracellular p24 staining was performed in a similar fashion according to the manufacturer's instructions (Coulter, Miami, Fla.). In some experiments, clones were first labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at 0.05 μM for 15 min, followed by two washes in phosphate-buffered saline, according to the manufacturer's instructions (Molecular Probes, Inc., Eugene, Ore.). Clones were resuspended in RPMI without serum before CFSE staining, since serum can quench the CFSE signal. Cells were acquired on a FACSCalibur flow cytometer (Becton Dickinson). Control conditions included staining of unstimulated aliquots for IFN-γ and staining with isotype controls for p24.
Virus stocks.
Viruses were obtained as previously detailed (43). HIV-1 IIIB was originally obtained from the laboratory of Robert Gallo. Virus stocks were from the supernatant of freshly infected H9 cells and were stored at −80°C. The titer of virus was determined on C8166 cells as previously described (18).
Virus suppression assays.
MT-2 cells were centrifuged and infected with HIV-1 at a multiplicity of infection (MOI) of 10−2 and incubated for 4 h at 37οC. Target cells were plated in R10 with 50 U of interleukin 2 (IL-2) (R10-50)/ml in 24-well plates at 50,000 cells/well, and effector cells were added at various effector-to-target (E:T) ratios, all in duplicate. In transwell experiments, effector cells were in an upper chamber on Costar plates (0.4-μm-pore-size membrane; Corning, Corning, N.Y.). Supernatants were harvested at 3, 7, and 10 days after infection. For harvesting, 0.7 ml was removed and inactivated with 70 μl of 5% Triton-100, and 0.7 ml R10-50 was replaced. Supernatants were assayed for p24 concentration by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Perkin-Elmer Life Sciences, Boston, Mass.).
Analysis of supernatant from Th clones.
Clones (106/ml) were stimulated with 5 × 105 B-lymphoblastoid cells (B-LCL) and cognate peptide (5 μg/ml). Supernatants were collected at 24, 48, and 72 h and were analyzed by ELISA according to the manufacturer's instructions for RANTES, macrophage inflammatory protein 1α (Mip-1α), Mip-1β, IL-2, IL-4, IL-10, and IFN-γ production (R&D Systems, Minneapolis, Minn.) and α-defensins 1 to 3 (HyCult Biotechnology, Norwood, Mass.). Cytokines were measured before and 24, 48, and 72 h after stimulation. The highest of the four time points was reported.
Statistics.
Two-tailed P values were calculated using GraphPad Prism (GraphPad Software, Inc., San Diego, Calif.). Standard deviations were calculated in Excel (Microsoft Corp., Redmond, Wash.), and the standard error was the standard deviation divided by the square root of the number of replicate assays.
RESULTS
HIV-1-specific CD4+-T-cell cytolytic activity can be measured directly ex vivo.
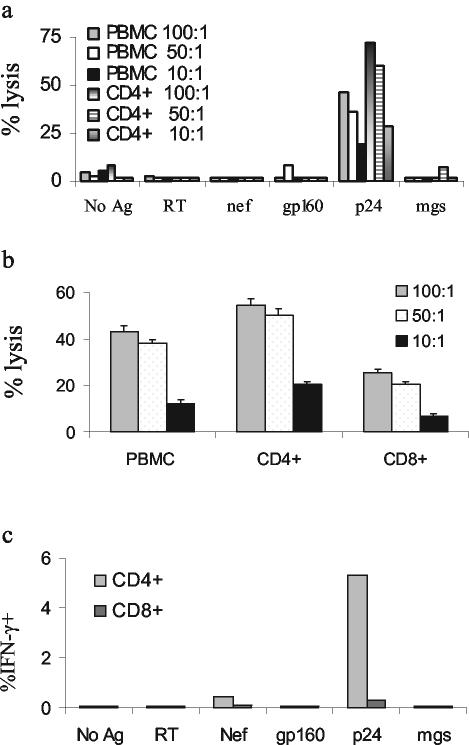
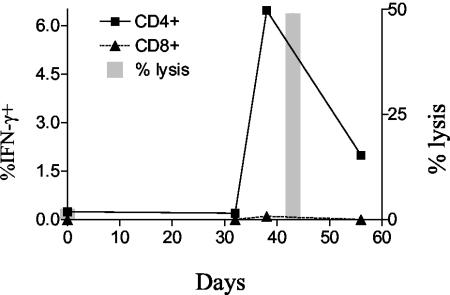
While multiple reports have described HIV-specific CD4+-T-cell-mediated cytolytic activity, relatively few have demonstrated cytolytic activity directly ex vivo (21, 28). Most reports of CD4+-T-cell cytolytic activity have shown killing by cells after weeks of in vitro culture (11), including our previous report on p24-specific CD4+-T-cell clones (32). We therefore assessed p24-specific CD4+-T-cell cytolytic responses ex vivo in 14 HIV-1-infected subjects with virus loads ranging from undetectable to more than 100,000 RNA copies/ml of plasma, including four individuals from whom we originally derived cytolytic clones, AC-01, AC-25, CTS-01, and 161J (Table 2). Four HIV-1-seronegative individuals were included as controls. One subject with long-term nonprogressive infection, 161J, exhibited CD4+-T-cell cytolytic activity ex vivo, directed against a pool of peptides spanning the p24 protein (Fig. 1a). The cytolytic activity was also demonstrable when B-cell targets were incubated with whole, soluble p24 protein and was enhanced with enrichment for CD4+ T cells and diminished with enrichment for CD8+ T cells (Fig. 1b). It is unlikely that natural killer cells played a significant role in the cytotoxic activity, since lysis was increased after CD4 enrichment, a process that depleted CD16- and CD56-expressing cells. Of the subjects studied, 161J possessed the greatest proportion of CD4+ T cells that recognized p24 protein (Fig. 1c and Table 2). We hypothesized that the low frequency of p24-specific Th cells might make ex vivo Th-cell cytolytic activity difficult to detect. To address this issue, we generated T-cell lines by using CD8+-depleted PBMC stimulated with p24 protein from a number of the study subjects and quantitated lytic ability at 2 and 4 weeks after isolation (Table 2, right-hand columns). Data are shown from the latest time point available for each T-cell line; many lines did not survive to the 4-week time point. T-cell lines from subjects AC-25 and LT-04 developed high levels of p24-specific CD4+ cells and possessed lytic ability. The T-cell line from subject LT-02 generated low levels of p24-specific IFN-γ secretion and also possessed a low level of specific lytic ability. CD8+-T-cell responses to p24 were negligible in the T-cell lines (data not shown). After 5 weeks in culture, the AC-25 T-cell line responded to p24 stimulation with 6.47% of CD4+ T cells secreting IFN-γ by ICS (Fig. 2). A cytolytic assay performed at week 6 revealed more than 40% specific lysis at an E:T ratio of 50:1 (Fig. 2). These data demonstrate that cytolytic CD4+ T cells exist in vivo but are rarely detectable, possibly due to the low frequency of p24-specific CD4+ T cells in most of the individuals studied.
TABLE 2.
Ex vivo lysis by CD4+ T cells is seen only rarelya
| Subject | Result
|
||||
|---|---|---|---|---|---|
| PBMC
|
T-cell line
|
||||
| % Lysis | % CD4+ IFN-γ+ | TCL duration (days) | % Lysis | %CD4+ IFN-γ+ | |
| AC-01 | 0 | ND | |||
| AC-06 | 0.9 | ND | |||
| AC-14 | 0 | 0 | |||
| AC-18 | 2.3 | ND | 28 | 0 | 0 |
| AC-25 | 0.6 | 0.24 | 43 | 49 | 6.47 |
| AC-42 | * | 0 | 28 | 1.9 | 0.1 |
| CO-01 | * | 0 | 14 | 0 | 0.06 |
| MGB | 0 | 0 | 30 | 0.7 | ND |
| LT-02 | 4.8 | 0.04 | 15 | 10.1 | 0.09 |
| LT-04 | 1 | 0.05 | 15 | 32.5 | 25.3 |
| LT-06 | 0 | ND | |||
| LT-10 | 0 | ND | 16 | 8.2 | 0.42 |
| 161J | 22.2 | 3.74 | |||
| CTS-01 | 0 | ND | |||
| SN-01 | 0 | ND | |||
| SN-02 | 0 | 0 | 14 | 0 | 0.1 |
| SN-03 | 0 | 0 | |||
| SN-04 | * | 0.05 | 27 | 0 | 0.2 |
The percentage of CD4+ T cells responding to stimulation with 5 μg of p24ml, measured by intracellular cytokine staining, is listed in the third column. The right three columns show the results of T-cell-line (TCL) testing, with significant specific lysis of >10% shown in bold. ND, not done; *, spontaneous lysis of >30%.
FIG. 1.
Ex vivo CD4+-T-cell lysis. (a) The ability of freshly isolated PBMC or CD8+-T-cell-depleted T cells to kill B-cell targets pulsed with HIV-1 or control proteins (reverse transcriptase, Nef, gp160, and mgs) or a peptide pool (p24) was tested in an overnight killing assay. The only HIV-1 antigen to elicit significant lysis was the peptide pool spanning p24, and depletion of CD8+ cells increased lysis at a given E:T ratio. The CD4+-T-cell-enriched population was 89.1% CD3+ CD4+ cells and 6.5% CD3+ CD8+ cells (data not shown). E:T ratios are noted in the legend of each figure. Spontaneous lysis was 33% for the mgs-pulsed B-LCL and 41% for the p24-pulsed B-LCL. Of note, spontaneous lysis of p24-pulsed B-LCL was 17% in the subsequent experiment analyzed in panel b, and there was no change in the outcome of the experiment. We confirmed the ex vivo cytolytic activity in subject 161J on six separate occasions. (b) Enrichment for CD4+ or CD8+ T cells and lysis of targets pulsed with whole p24 protein. CD4+ refers to PBMC enriched for CD4+ T cells during isolation, and CD8+ refers to PBMC enriched for CD8+ T cells. Enrichment for CD4+ T cells led to an increase in lysis, and enrichment for CD8+ T cells led to diminution of lysis. The CD8+-T-cell-enriched population was 88.8% CD3+ CD8+ and 3.0% CD3+ CD4+; the purity of the CD4+-T-cell-enriched population was not tested. (c) Frequency of HIV-1-specific cells ex vivo. Fresh PBMC were stimulated with the indicated whole proteins at a concentration of 5 μg/ml except for p24, where a pool of 18-amino-acid peptides spanning p24 were used as the stimulus. Cells were stained for production of IFN-γ and quantitated by flow cytometry. At least 50,000 live events/condition were recorded. Since whole protein was used as a stimulus for each of the conditions except p24, CD8+-T-cell responses would be expected to be low.
FIG. 2.
Lysis by a T-cell line from patient AC-25. p24-specific lysis was assayed directly ex vivo and at 42 days with a T-cell line. CD4+ and CD8+ T cells producing IFN-γ after stimulation with p24 are shown ex vivo and at three subsequent time points. At least 15,000 and generally more than 40,000 live events were collected/condition. Lysis was demonstrated after the level of p24-specific CD4+ T cells rose above 6%.
Lysis of T-cell targets and recognition of infected CD4+ T cells.
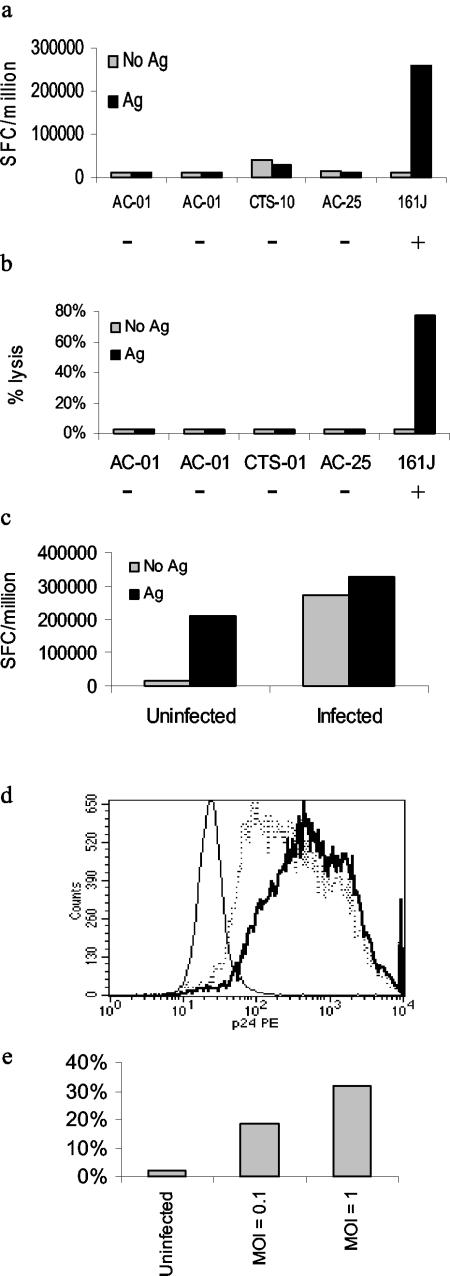
If cytolytic Th cells were to have an impact on control of virus, lysis of CD4+-T-cell targets would be important. As a target we utilized the MT-2 cell line, a type 1 human T-cell leukemia virus-immortalized CD4+-T-cell line capable of replicating CXCR4-tropic (X4) HIV-1 at high levels (15). We demonstrated that an HLA-matched clone (from subject 161J), but not unmatched clones, could recognize cognate peptide presented by MT-2 cells (Fig. 3a). Furthermore, we showed that MT-2 cells were susceptible to lysis by the HLA-matched Th cell clone (Fig. 3b). The degree of lysis seen at an E:T ratio of 10:1 was comparable to that seen in lysis of B-cell targets, implying effective recognition of the CD4+-T-cell target. We demonstrated recognition of infected MT-2 cells by two methods, first by exposing 161J clone cells to infected MT-2 cells overnight and measuring IFN-γ secretion by ELISPOT (Fig. 3c). Infected cells were able to induce IFN-γ secretion, while uninfected cells were not. Exogenous antigen was added to both infected and uninfected cells as a positive control. We also observed IFN-γ production by intracellular cytokine staining, and the 161J clone was activated by the presence of infected but not uninfected MT-2 cells (74% IFN-γ+ cells versus 0.6% IFN-γ+ cells; data not shown). In the second set of experiments, we infected MT-2 cells with HIV-1 IIIB at an MOI of 0.1 or 1 and tested the ability of the 161J clone to lyse the infected targets at day 6 after infection. Intracellular p24 expression was higher in the MT-2 cells infected with the higher dose of virus (Fig. 3d), and cytolytic ability correlated with p24 expression (Fig. 3e). These experiments imply that the 161J clone cells can recognize p24 antigen produced by infected MT-2 cells and can lyse infected cells.
FIG. 3.
Recognition of HIV-1-infected MT-2 cells by an HLA-matched Th-cell clone. (a) MT-2 cells were incubated with a mixture of cognate epitopes for all five of the Th-cell clones. Only the HLA-matched, DR4-restricted clone could recognize antigen presented by the MT-2 cells. The − and + signs below each clone indicate whether or not the clone was HLA matched to the target cell. Positive controls included stimulation with autologous B-LCL pulsed with cognate antigen, confirming each clone's activity (data not shown). (b) 161J clone can lyse MT-2 cells in an antigen-specific fashion. Only the HLA-matched Th-cell clone could lyse MT-2 cells in a 4-h 51Cr release assay. Clonal activity was confirmed in parallel with autologous B-LCL targets pulsed with cognate antigen for each clone (data not shown). (c) Infected MT-2 cells process antigen for recognition by the Th-cell clone 161J. Infected and uninfected cells were used as antigen-presenting cells in an overnight ELISPOT assay for IFN-γ secretion. Cells were infected at an MOI of 1. The uninfected cells induced IFN-γ secretion only if exogenous cognate antigen was added, while the infected cells needed no exogenous antigen to induce IFN-γ secretion. (d) Granular staining of infected MT-2 cells for intracellular p24. The thin histogram represents uninfected cells, the dashed histogram represents cells infected at an MOI of 0.1, and the bold histogram represents cells infected at an MOI of 1. The mean fluorescence intensities for each of the conditions, respectively, were 19.3, 825, and 1032. (e) Lysis of infected MT-2 cells. The 161J clone was able to lyse infected MT-2 cells in a 4-h 51Cr release assay, with greater lysis in targets expressing higher levels of p24 as determined in Fig. 3d.
Inhibition of HIV-1 replication by HIV-1-specific CD4+ T cells.
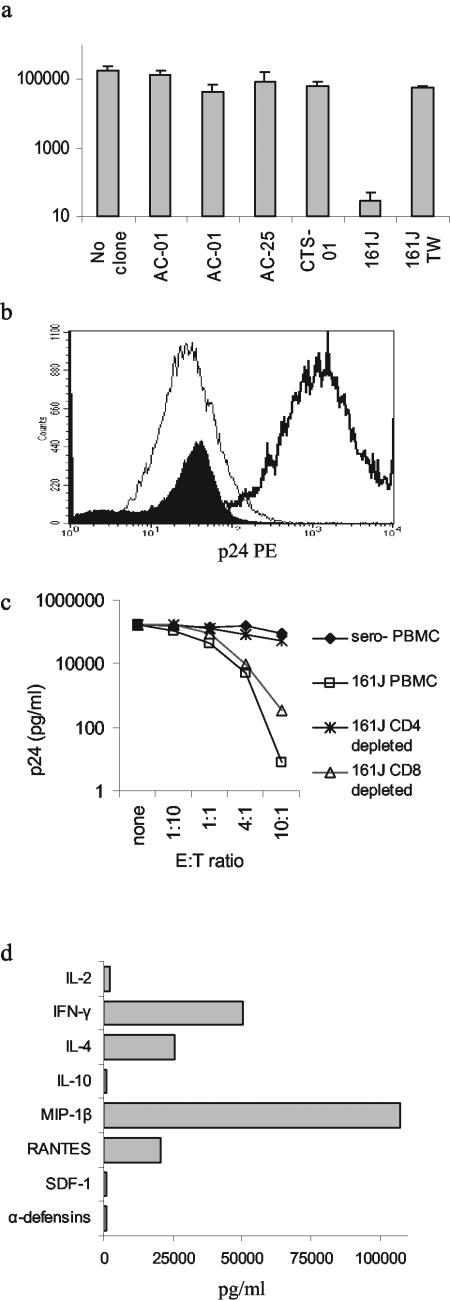
Fratricide committed by gp120-specific CD4+ T cells has been proposed as a possible method for the depletion of CD4+ T cells seen with progression to AIDS (40). We entertained an alternative hypothesis, that CD4+-T-cell HIV-1-specific cytolytic activity might lyse HIV-1-infected CD4+ T cells and suppress viral replication. We tested this hypothesis by infecting MT-2 cells and measuring p24 produced in the supernatant with and without the addition of Th-cell clones (Fig. 4a). Addition of clone 161J resulted in profound suppression of p24 production (P = 0.006). To test whether cell-cell contact was required, we activated the 161J clone with free antigen presented by autologous B-LCL and separated the clone from infected MT-2 cells in a transwell experiment (Fig. 4a). In spite of vigorous IFN-γ secretion by the clone cells in the transwell (data not shown), there was no significant suppression of virus replication (P = 0.32). These results imply that cell-cell contact is required for suppression of virus replication.
FIG. 4.
Suppression of HIV-1 replication and secretion of antiviral factors. (a) MT-2 cells were infected with HIV-1 IIIB, and the supernatant was assayed for p24 production by ELISA at day 7. Each of the clones was added at a 1:1 E:T ratio. Only the HLA-matched 161J clone was able to suppress virus replication. Suppression depended on cell-cell contact, since separation of the clone from the targets with a semipermeable membrane (transwell [TW]) abrogated suppression. Results are averages of at least two independent experiments, and error bars represent the standard error. (b) Intracellular staining of MT-2 cells for p24 at day 11 after infection. MT-2 cells were gated based on their large size and granularity in the forward and side scatter gates. The thin histogram represents uninfected cells, the thick histogram represents infected cells, and the filled histogram represents infected cells incubated with the 161J Th-cell clone at an E:T ratio of 1:1. The mean fluorescence intensities for each of the conditions, respectively, were 27.0, 1670, and 32.3. More than 50,000 events/condition were recorded, except for the infected cells alone, where 22,000 events were acquired due to lower viable cell numbers by day 11. (c) Suppression of HIV-1 replication by 161J PBMC directly ex vivo. Infected MT-2 cells were incubated with various E:T ratios of 161J PBMC or PBMC depleted of CD4+ or CD8+ T cells, and p24 was quantitated by ELISA at day 7. Virus was suppressed by PBMC and the CD4+-T-cell subsets but not PBMC depleted of CD4+ T cells. No virus suppression was demonstrated after coincubation with PBMC from a control HIV-1-seronegative individual. (d) Secretion of cytokines and antiviral factors by 161J Th-cell clone. Clone cells were stimulated with a cognate antigen at 5 μg/ml presented by an autologous B-cell line, and supernatants were harvested at 24, 48, and 72 h. The highest value of the three for each cytokine is listed. IL-4, IFN-γ, and the CCR5 chemokines were secreted at the highest levels.
One explanation for the lack of p24 production in the presence of the Th clone would be the elimination of uninfected MT-2 cells, depriving HIV-1 of a target in which to replicate. We stained MT-2 cells for intracellular p24 before and after HIV-1 infection and in the presence of 161J clone cells (Fig. 4b). Infected MT-2 cells were easily distinguished from uninfected cells by p24 staining. Addition of 161J clone cells to infected MT-2 cells at day zero led to the disappearance of MT-2 cells expressing high levels of p24 and the appearance of MT-2 cells with levels of intracellular p24 comparable to those in uninfected cells at day 11 (mean fluorescence intensity, 27.0 versus 32.3). These data show that lack of p24 production was not due to an absence of MT-2 targets but rather to decreased viral replication in the presence of the clone.
The ability of the 161J Th clone to suppress HIV-1 replication could be ascribed to effector functions obtained by the clone after long-term in vitro culture. To address the issue we isolated fresh PBMC from subject 161J and plated them directly with infected MT-2 cells (Fig. 4c). PBMC from 161J were able to effect a five-log suppression of p24 production at an E:T ratio of 10:1. The effect was abrogated if CD4+ T cells were depleted and was only partially diminished upon depletion of CD8+ T cells. These data do not indicate that CD8+ T cells lack HIV-1-suppressive effects, since the MT-2 cell line is matched to 16 1J at only one class I C allele, while it is matched at all DR and DQ alleles. The data do imply that CD4+-T-cell-mediated suppressive activity can be measured directly ex vivo and is not an artifact of long-term culture of the 161J clone cells.
Examination of antiviral soluble factors secreted by p24-specific CD4+-T-cell clones.
The transwell experiments imply that cell-cell contact is required between the Th clone and the target cell for suppression of virus replication (Fig. 4a). However, it is possible that a soluble factor is still responsible for virus suppression and that the factor can only act at close range. We therefore characterized the secretion of cytokines and antiviral compounds by the 161J clone. After stimulation, the clone produced levels of IFN-γ and IL-4 greater than 1,000 pg/ml (Fig. 4d). We quantitated secretion of β-chemokines, associated with suppression of CCR5-tropic (R5) virus replication, and stromal derived factor 1, shown to inhibit infection with X4 virus (6, 33). We also tested whether or not the clones secreted detectable quantities of α-defensins, possibly contributing to HIV-1 suppression though shown not to be secreted by T cells (47). The only chemokines or defensins secreted in significant quantities were the β-chemokines (Fig. 4d). These chemokines have activity against R5 and not X4 viruses, and IFN-γ has been shown to be inactive against HIV-1 in most systems (24), so it appears the antiviral effect of the Th clone we observed against an X4 virus was mediated through direct cytolysis or an unidentified soluble factor unable to act in transwell assays.
DISCUSSION
HIV-1-specific Th cells are thought to contribute to control of virus by providing help for HIV-1-specific CTL and B cells. Here we demonstrate another role for Th cells, that of direct inhibition of HIV-1 replication. Cytolytic activity mediated by Th cells has long been recognized, though difficult to demonstrate without prior in vitro expansion. We were able to demonstrate such activity directly ex vivo for one subject with high frequencies of HIV-1-specific Th cells. T-cell lines from other subjects possessed HIV-1-specific cytolytic activity after short-term in vitro culture (Fig. 2 and Table 2). CD4+-T-cell clones not only were able to lyse B-cell targets but also could kill other Th cells infected with HIV-1. While this could lead to depletion of the Th subset in vivo, we also showed that a Th clone possessed HIV-1-suppressive activity that was dependent on cell-cell contact. The suppression was not likely an artifact of prolonged in vitro culture, since CD4+ T cells ex vivo also possessed suppressive activity.
The presence of cytolytic CD4+ T cells has been previously demonstrated in HIV-1 infection in vitro. HIV-1-specific cytolytic CD4+-T-cell clones can be isolated from the cerebrospinal fluid (38), blood (22, 32), and cervical cells (27) of HIV-1-infected individuals. Cytolytic activity mediated by CD4+ T cells directly ex vivo directed against Gag, Pol, or Env proteins has only rarely been witnessed in HIV-1-infected persons (16, 21, 28), though characterization of HIV-1-specific Th-cell frequency has not been simultaneously quantified. The only study to examine the mechanism of action of ex vivo cytolytic CD4+ T cells found them to act using non-perforin-dependent pathways (16), in contrast to our findings (32; also data not shown). CD4+ T cells with a cytolytic phenotype have recently been identified ex vivo in an HIV-1-infected long-term nonprogressor and cytomegalovirus-infected healthy adults, implying that cytolytic CD4+ T cells may occur more frequently than previously appreciated (46). It has also been noted that about 20% of CD4+ T cells from HIV-1-infected patients express perforin, compared to 2% in seronegative controls, and that CD4+ T cells from HIV-1-infected individuals can lyse targets in a redirected killing assay (3). We found only one HIV-1-infected individual of 14 with detectable ex vivo cytolytic Th cells. Of note, the frequency of HIV-1-specific CD4+ T cells in this subject was more than one order of magnitude higher than that in the other subjects surveyed. Supporting the notion that Th-cell effector frequency limited the ability to detect ex vivo CD4+ CTL was the fact that T-cell lines expanded in vitro could mediate HIV-1-specific cytolysis, presumably through expansion of antigen-specific Th cells. While we were able to demonstrate ex vivo CD4+-T-cell lysis with one individual, the experiments with T-cell lines from other subjects suggest but do not prove the existence of in vivo cytolytic activity in these individuals, since Th cells in even relatively short-term in vitro culture have been shown to develop cytolytic activity (11). Most HIV-1-infected individuals have relatively low frequencies of HIV-1-specific Th cells (4), so we would expect that ex vivo Th-cell cytolytic activity would be difficult to detect for most subjects. Detecting significant proportions of antigen-specific cytolytic Th cells ex vivo may require study of viruses with higher frequencies of antigen-specific Th cells, such as cytomegalovirus (5), or use of more-sensitive techniques to detect cytolysis at the single-cell level (23, 39). The correlation between frequency of antigen-specific Th cells and lytic activity is likely not absolute, and we saw a low level of lysis in the T-cell line from subject LT-02 with undetectable IFN-γ secretion (Table 2). It is also possible that individuals with high viral load and detectable p24-specific IFN-γ secretion would lack cytolytic activity, a question not answered by the present study.
The ability of T cells to kill B-cell targets is interesting, but to envision a role for Th-cell cytolytic activity in the direct control of HIV-1, CD4+ T cells themselves would have to be targets of lysis as well. Th cells can present endogenously synthesized HIV-1 gp160 to CD4+ CTL (34). Our studies demonstrated that the MT-2 T-cell line can effectively process and present an epitope within p24 Gag and sensitize itself for lysis by an HLA-matched Th-cell clone. While HIV-1-specific Th-cell lysis appears to be mediated via perforin in a fashion similar to that of CD8+-CTL lysis (32), it is unlikely that CD4+ and CD8+ CTL would have the same in vivo effects. CD4+ CTL would target cells that express major histocompatibility complex class II molecules, primarily antigen-presenting cells and other T cells. These cells can present peptides through the exogenous pathway after endocytosis, without being productively infected (40). If these cells are preferential targets for cytolytic CD4+ T cells, elimination of bystander Th cells could contribute to CD4+-T-cell depletion in HIV-1. It appears unlikely that bystander lysis was the primary function of the Th cells we studied, since only MT-2 cells with high levels of p24 were completely eliminated (Fig. 4b). Alternatively, CD4+ CTL could target the reservoirs of HIV-1 replication, potentially contributing to control of the virus.
Mechanistically, CTL can inhibit HIV-1 replication in vitro via cytolytic and noncytolytic pathways (8, 44). Suppression of HIV-1 replication mediated by a Th clone appeared to rely on cell-cell contact, since separation of the clone from the target cells by a semipermeable membrane abolished the suppressive effect. Modest reductions in HIV-1 replication have been noted upon addition of p24-specific Th-cell clones to infected human T lymphoblastoid CEM cells, and in those experiments Th1 clones appeared to be more effective than Th0 clones (41). The cytokine profile of the clone we studied was Th0, with both IL-4 and IFN-γ secretion. Additional recent work has documented the suppression of R5 and X4 viruses by naïve, nonspecifically activated CD4+-T-cell supernatants, and X4 suppression was mediated by chemokine ligand 22 (1). We did not examine CCL22, but the clone did not appear to secrete any other soluble factors associated with suppression of X4 viruses, including stromal derived factor 1 and α-defensins. Prior work has demonstrated secretion of R5-blocking chemokines by Th cells (12), consistent with the soluble factors we found in the present study.
In summary, we propose that the Th-cell effector activity demonstrated above may contribute to control of HIV-1 replication. Part of the reason Th cell effector functions have not been typically documented is likely the relatively low frequency of HIV-1-specific Th cells in natural infection. While Th cell effector responses alone would be unlikely to eliminate the virus, they could function as one more defense employed by the immune system in its struggle to control HIV-1.
Acknowledgments
This work was supported by the National Institutes of Health (grants AI-01698-01 and AI-040873). D.E.K. was supported by the Swiss Foundation for Grants in Biology and Medicine, die Schweizerische Stiftung für medizinisch-biologische Stipendien (SSMBS.). E.S.R. and P.J.N. were supported by the Doris Duke Charitable Foundation. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2, catalog no. 136 from Hoffman-La Roche, Inc.
We are grateful to Bruce D. Walker for helpful comments and thoughtful review of the manuscript and to Walter Moretto and John Heitman for expert technical assistance.
REFERENCES
- 1.Abdelwahab, S. F., F. Cocchi, K. C. Bagley, R. Kamin-Lewis, R. C. Gallo, A. DeVico, and G. K. Lewis. 2003. HIV-1-suppressive factors are secreted by CD4+ T cells during primary immune responses. Proc. Natl. Acad. Sci. USA 100:15006-15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay, V., J. J. Zaunders, L. Papagno, J. Sutton, A. Jaramillo, A. Waters, P. Easterbrook, P. Grey, D. Smith, A. J. McMichael, D. A. Cooper, S. L. Rowland-Jones, and A. D. Kelleher. 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168:5954-5958. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV-1)-specific CD4(+) and CD8(+) t-cell responses: relationship to viral load in untreated HIV-1 infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitmansour, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzas, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 167:1151-1163. [DOI] [PubMed] [Google Scholar]
- 6.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-1-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D., J. Brenchley, M. Betts, D. Ambrozak, B. Hill, Y. Okamoto, J. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. Price, M. Connors, and R. Koup. 2002. HIV-1 preferentially infects HIV-1-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 10.Draenert, R., C. L. Verrill, Y. Tang, T. M. Allen, A. G. Wurcel, M. Boczanowski, A. Lechner, A. Y. Kim, T. Suscovich, N. V. Brown, M. M. Addo, and B. D. Walker. 2004. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J. Virol. 78:630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischer, B. 1984. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature 308:365-367. [DOI] [PubMed] [Google Scholar]
- 12.Furci, L., G. Scarlatti, S. Burastero, G. Tambussi, C. Colognesi, C. Quillent, R. Longhi, P. Loverro, B. Borgonovo, D. Gaffi, E. Carrow, M. Malnati, P. Lusso, A. G. Siccardi, A. Lazzarin, and A. Beretta. 1997. Antigen-driven C-C chemokine-mediated HIV-1 suppression by CD4(+) T cells from exposed uninfected individuals expressing the wild-type CCR-5 allele. J. Exp. Med. 186:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. deq Uiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-1-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 14.Graham, M. B., V. L. Braciale, and T. J. Braciale. 1994. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 180:1273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada, S., Y. Koyanagi, and N. Yamamoto. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563-566. [DOI] [PubMed] [Google Scholar]
- 16.Heinkelein, M., I. Euler-Konig, H. Klinker, H. Ruckle-Lanz, and C. Jassoy. 1996. Lysis of human immunodeficiency virus type 1 antigen-expressing cells by CD4 and CD8 T cells ex vivo. J. Infect. Dis. 174:209-213. [DOI] [PubMed] [Google Scholar]
- 17.Iwashiro, M., K. Peterson, R. J. Messer, I. M. Stromnes, and K. J. Hasenkrug. 2001. CD4(+) T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J. Virol. 75:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, V. A., and B. D. Walker. 1990. HIV-1-infected cell fusion assay, p. 92-94. In A. Aldovani and B. D. Walker (ed.), Techniques in HIV-1 research. Stockton Press, New York, N.Y.
- 19.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kundu, S. K., and T. C. Merigan. 1992. Equivalent recognition of HIV-1 proteins, Env, Gag and Pol, by CD4+ and CD8+ cytotoxic T-lymphocytes. AIDS 6:643-649. (Erratum, AIDS 6:following 1051.) [PubMed] [Google Scholar]
- 22.Littaua, R. A., M. B. Oldstone, A. Takeda, and F. A. Ennis. 1992. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J. Virol. 66:608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, L., A. Chahroudi, G. Silvestri, M. E. Wernett, W. J. Kaiser, J. T. Safrit, A. Komoriya, J. D. Altman, B. Z. Packard, and M. B. Feinberg. 2002. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat. Med. 8:185-189. [DOI] [PubMed] [Google Scholar]
- 24.Mackewicz, C. E., H. Ortega, and J. A. Levy. 1994. Effect of cytokines on HIV-1 replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 153:329-343. [DOI] [PubMed] [Google Scholar]
- 25.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV-1. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 26.McNeil, A. C., W. L. Shupert, C. A. Iyasere, C. W. Hallahan, J. Mican, R. T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 98:13878-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musey, L., Y. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura, T., K. Iwakabe, M. Sekimoto, Y. Ohmi, T. Yahata, M. Nakui, T. Sato, S. Habu, H. Tashiro, M. Sato, and A. Ohta. 1999. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J. Exp. Med. 190:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris, P. J., H. F. Moffett, C. Brander, T. M. Allen, K. M. O'Sullivan, L. A. Cosimi, D. E. Kaufmann, B. D. Walker, and E. S. Rosenberg. 2004. Fine specificity and cross-clade reactivity of HIV-1 type 1 Gag-specific CD4+ T cells. AIDS Res. Hum. Retrovir. 20:315-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris, P. J., and E. S. Rosenberg. 2002. CD4(+) T helper cells and the role they play in viral control. J. Mol. Med. 80:397-405. [DOI] [PubMed] [Google Scholar]
- 32.Norris, P. J., M. Sumaroka, C. Brander, H. F. Moffett, S. L. Boswell, T. Nguyen, Y. Sykulev, B. D. Walker, and E. S. Rosenberg. 2001. Multiple effector functions mediated by human immunodeficiency virus-specific CD4+ T-cell clones. J. Virol. 75:9771-9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberlin, E., A. Amara, F. Bachelerie, C. Bessia, J. L. Virelizier, F. Arenzana-Seisdedos, O. Schwartz, J. M. Heard, I. Clark-Lewis, D. F. Legler, M. Loetscher, M. Baggiolini, and B. Moser. 1996. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833-835. [DOI] [PubMed] [Google Scholar]
- 34.Orentas, R. J., J. E. Hildreth, E. Obah, M. Polydefkis, G. E. Smith, M. L. Clements, and R. F. Siliciano. 1990. Induction of CD4+ human cytolytic T cells specific for HIV-1-infected cells by a gp160 subunit vaccine. Science 248:1234-1237. [DOI] [PubMed] [Google Scholar]
- 35.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 38.Sethi, K. K., H. Naher, and I. Stroehmann. 1988. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-1-specific and HLA-restricted cytotoxic T-cell clones. Nature 335:178-181. [DOI] [PubMed] [Google Scholar]
- 39.Sheehy, M. E., A. B. McDermott, S. N. Furlan, P. Klenerman, and D. F. Nixon. 2001. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J. Immunol. Methods 249:99-110. [DOI] [PubMed] [Google Scholar]
- 40.Siliciano, R. F., T. Lawton, C. Knall, R. W. Karr, P. Berman, T. Gregory, and E. L. Reinherz. 1988. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV-1 sequence variation and a mechanism for CD4+ cell depletion. Cell 54:561-575. [DOI] [PubMed] [Google Scholar]
- 41.Vyakarnam, A., P. M. Matear, S. J. Martin, and M. Wagstaff. 1995. Th1 cells specific for HIV-1 gag p24 are less efficient than Th0 cells in supporting HIV-1 replication, and inhibit virus replication in Th0 cells. Immunology 86:85-96. [PMC free article] [PubMed] [Google Scholar]
- 42.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-1-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimura, A., H. Shiku, and E. Nakayama. 1993. Rejection of an IA+ variant line of FBL-3 leukemia by cytotoxic T lymphocytes with CD4+ and CD4-CD8- T cell receptor-alpha beta phenotypes generated in CD8-depleted C57BL/6 mice. J. Immunol. 150:4900-4910. [PubMed] [Google Scholar]
- 46.Zaunders, J. J., W. B. Dyer, B. Wang, M. L. Munier, M. Miranda-Saksena, R. Newton, J. Moore, C. R. Mackay, D. A. Cooper, N. K. Saksena, and A. D. Kelleher. 2004. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood 103:2238-2247. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]