Abstract
Aims
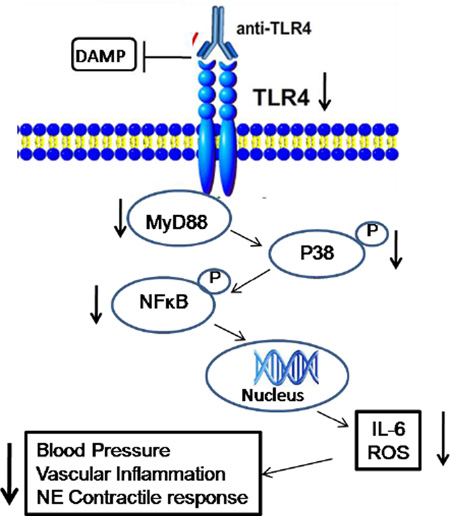
Hypertension is associated with increased levels of circulating cytokines and recent studies have shown that innate immunity contributes to hypertension. The mechanisms which hypertension stimulates immune response remain unclear, but may involve formation of neoantigens that activate the immune system. Toll like receptor 4 (TLR4) is an innate immune receptor that binds a wide spectrum of exogenous (lipopolysaccharide) and endogenous ligands. TLR4 signaling leads to activation of nuclear factor kappa B (NFκB) and transcription of genes involved in inflammatory response. We previously demonstrated that TLR4 blockade reduces blood pressure and the augmented vascular contractility in spontaneously hypertensive rats (SHR). Here we hypothesized that inhibition of TLR4 ameliorates the vascular inflammatory process by a NFκB signaling pathway.
Main methods
SHR and Wistar rats were treated with anti-TLR4 antibody (1µg/day) or unspecific IgG for 15 days (i.p.).
Key findings
Anti-TLR4 treatment decreased production of reactive oxygen species and expression of IL-6 cytokine in mesenteric resistance arteries from SHR, when compared with IgG-treated SHR. Anti-TLR4 treatment also abolished the increased vascular reactivity to noradrenaline observed in IgG-treated SHR, as described before, and inhibition of NFκB decreased noradrenaline responses only in IgG-treated SHR. Mesenteric arteries from SHR treated with anti-TLR4 displayed decreased expression of MyD88, but not TRIF, key molecules in TLR4 signaling. Phosphorylation of p38 and NF-κB p65 were decreased in arteries from anti-TLR4-treated SHR versus IgG-treated SHR.
Significance
Together, these results suggest that TLR4 is a key player in hypertension and vascular inflammatory process by a NFκB signaling pathway.
Keywords: hypertension, toll-like receptor, NF-κB, innate immune response
Graphical abstract
1. INTRODUCTION
Hypertension is considered a chronic inflammatory disease, with elevated levels of vascular and circulatory pro-inflammatory cytokines and augmented activation of the transcription factor NFκB [1, 2]. Inflammation of the vascular wall is associated with blood vessels dysfunction [3]. Experimental and clinical evidence supports the concept that the innate and the adaptive immune responses contribute to the pathophysiology of cardiovascular diseases, including arterial hypertension [4]. Cells from the adaptive immune response, mainly T lymphocytes, play critical roles in the development of angiotensin II and deoxycorticosterone-salt-induced hypertension, and in the progression of vascular remodeling, which is a hallmark of hypertension [4, 5]. However the participation of the innate immune system in hypertension is still scarcely investigated.
The innate immune response is the first line of defense of the organism against pathogens. Innate immune cells recognize molecules from pathogens through activation of pattern recognition receptors (PRR), such as the toll-like receptors (TLRs) [6]. We recently demonstrated that mesenteric resistance arteries from spontaneously hypertensive rats (SHR) exhibit TLR4 overexpression compared to Wistar arteries, and that inhibition of TLR4 activation by chronic treatment with anti-TLR4 antibody decreases blood pressure and restores vascular hypercontractility, by a cyclooxygenase (COX)-dependent mechanism [7].
TLR4 is expressed in many cell types, including endothelial cells, smooth muscle cells and macrophages. Recent studies demonstrated that TLR4 is activated not only by pathogens, but also by endogenous ligands from injury and damaged tissues. Accordingly, TLR4 has been well studied in sterile inflammatory conditions [8]. TLR4 activation culminates in the recruitment of adaptor proteins and activation of two major intracellular signaling pathways depending on the adaptors recruited to the TLR. The MyD88-dependent pathway involves activation of IRAK-1 and 4 (IL-1R-associated kinases), TRAF-6 (TNF receptor-associated factor-6), MAPK (mitogen-activated kinases), resulting in the activation of NFκB, which mediates the transcription of COX-2, several pro-inflammatory cytokine genes and generation of reactive oxygen species. The TRIF-dependent pathway (TIR-domain-containing adapter-inducing interferon-β) leads to activation of the interferon regulated factors (IRF) family of transcription factors, resulting in the synthesis of interferon (IFN) [9].
In view that TLR4 is upregulated in mesenteric resistance arteries from SHR and that several pro-inflammatory factors contributes to the pathogenesis of hypertension, we hypothesized that in addition to decreasing blood pressure, TLR4 blockade in SHR would ameliorate the inflammatory process via an NFκB -dependent pathway. Therefore, the aim of this study was to investigate whether the inhibition of TLR4 by a treatment with anti-TLR4 antibody, reduces arterial expression of pro-inflammatory molecules, such as IL-6, TNF-α, and oxidative stress in SHR. In addition, considering that TLR4 activates the nuclear factor NF-κB, we determined whether inhibition of NFκB activity contributes to the beneficial effects of anti-TLR4 treatment in SHR.
2. MATERIALS AND METHODS
2.1 Animals
Male SHR and Wistar rats (15 weeks-old; Harlan Laboratories, Indianapolis, IN, USA) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Georgia Regents University Committee on the Use of Animals in Research and Education and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The animals were housed on a 12 h light/dark cycle and fed a standard chow diet with free water intake. Mean blood pressure was measured by the tail-cuff method (model DT-100; Utah Medical Products, Midvale, Utah, USA) before the beginning and at the end of the treatment. At the end of the treatments, in all experiments, rats were anesthetized using isoflurane and euthanized.
2.2 Treatment with anti-TLR4 antibody
Treatment using anti-TLR4 was performed as described by Milanski et al (2009) and Bomfim et al (2012) [7, 10]. Fifteen weeks-old SHR and Wistar rats were treated by a daily intraperitoneal (i.p.) injection of 200 µl solution containing 1.0 µg of anti-TLR4 IgG antibody (Santa Cruz Biotechnology sc-13591) diluted in saline, for 15 days, in order to molecularly block TLR4 [7, 10]. Control Wistar and SHR groups received daily i.p. injections containing 1.0 µg of unspecific IgG antibody (Santa Cruz Biotechnology) to rule out unspecific effects of antibody.
2.3 Western Blotting
Proteins (50 µg) from mesenteric arteries were extracted using a lysis buffer and separated by electrophoresis on a 10 % polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5 % milk in Tris-buffered solution (10 mM Tris,100 mM NaCl, and 0.1 % Tween 20) for 1 h at room temperature. Membranes were then incubated with primary antibodies overnight at 4°C. Primary antibodies, with their respective target molecular weight and dilutions, were: anti-MyD88 (33 kDa, 1:750), anti-phospho-p38 (43 kDa, 1:1000), anti-p38 (43 kDa, 1:1000), anti-phospho-ERK (42, 44 kDa, 1:2000), anti-ERK (42, 44 kDa, 1:2000), anti-phospho-NFκB p65 (65 kDa, 1:500), anti-NFκB p65 (65 kDa, 1:500) (all antibodies from Cell Signaling Technology, Beverly, MA, USA) and anti-TNF-α (23 kDa, 2µg/mL), anti-IL-6 (21, 28 kDa, 1:500) and anti-TRIF (66 kDa, 1:1000) (Cayman, USA). Membranes were washed with Tris-buffered solution and incubated for 1 hour at room temperature with the appropriate secondary antibody. After incubation membranes were washed with Tris-buffered solution and signals were revealed with chemiluminescence and quantified using Alpha Imager software (Alpha Innotech, San Leandro, CA). The same membrane was stripped and used to determine β-actin protein expression using a monoclonal antibody against β-actin (1:15000), and its content was used to normalize protein expression in each sample. All representative Western blot images shown in each figure were obtained from samples run on the same membrane.
2.4 Vascular function studies
After euthanasia, second-order mesenteric arteries were removed and dissected in a modified Krebs solution (in mM: 130 NaCl, 14.9 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 1.56 CaCl2·2H2O, 0.026 EDTA and 5.5 glucose). Segments (2 mm in length) from the mesenteric arteries were cleaned of connective tissues and perivascular fat and mounted on 40 µm wires in a small vessel myograph for isometric tension recording. The segments were equilibrated for about 30 min in Krebs solution gassed with 5 % CO2 in O2, maintaining pH equal 7.4. The relationship between resting wall tension and internal circumference was determined, and the internal circumference, L100, corresponding to a transmural pressure of 100 mmHg for a relaxed vessel in situ, was calculated. The vessels were set to the internal circumference L1, given by L1 = 0.9×L100. The effective internal lumen diameter was determined as L1 = L1/π. After stabilization, arterial integrity was assessed by stimulation of vessels twice with 120 mM KCl, to assess maximal contractility. Endothelial integrity was assessed by testing the relaxant effect of acetylcholine (1 µM) on vessels pre-contracted with noradrenaline (3 µM). Only segments with intact endothelium (>?% ACh-induced relaxation) were used in this study.
Cumulative concentration-response curves to noradrenaline (10 nM to 100 µM) were performed in mesenteric arteries incubated with vehicle or pyrrolidine (NFκB inhibitor), which was added 30 min before generating the concentration–response curves.
2.5 Reactive oxygen species measurement in small mesenteric arteries
In situ ROS production was evaluated by the oxidative fluorescent dye dihydroethidine (Invitrogen, USA), as described before [11]. Small mesenteric arteries incubated with vehicle or noradrenaline (0.1µM) for 30 min were embedded in freezing medium and transverse sections (20 µm) were collected on glass slides and equilibrated for 10 min in phosphate buffer in a humidified chamber at 37 C. Phosphate buffer containing dihydroethidine (2.5 µM) was applied to each tissue section. The slides were incubated in a light-protected humidified chamber at 37 C for 30 min. Immediately after this time, digital images were collected on an epifluorescence microscope (Carl Zeiss, Germany) coupled to a camera (DS-U3; Nikon, Japan). The collected images were analyzed by measuring the mean optical density of the fluorescence of the vessel, using Image J software (National Institute of Health, USA).
2.6 Data Analysis
Results are shown as mean ± standard error of the mean (SEM) and “n” represents the number of animals used in the experiments. Contractile responses are expressed in mN as maximal force to each agonist concentration. Concentration–response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software Inc.) and two pharmacological parameters were obtained: the maximal effect generated by the agonist (Emax) and −log EC50 (pD2). Statistical analyses were performed using one-way ANOVA followed by Tukey post-hoc test or Student t test, when appropriate. Values of P<0.05 were considered statistically significant.
3. RESULTS
3.1 Effect of anti-TLR4 treatment on blood pressure and body weight
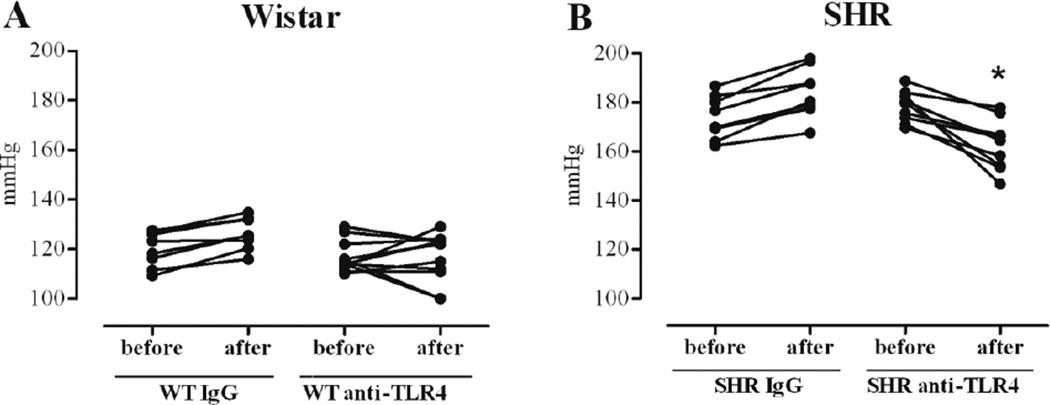
Anti-TLR4 treatment decreased mean blood pressure in SHR, compared with values before treatment. However, no changes in blood pressure were observed in SHR after treatment with unspecific IgG or in Wistar groups after treatments with anti-TLR4 or IgG antibodies (Figure 1 and Table 1). Treatment with anti-TLR4 did not change body weight (g) in the groups. These results reproduce our previous findings (7) and suggest that increased activation of TLR4 contributes to augmented blood pressure observed in SHR.
Figure 1. Anti-TLR4 treatment decreases SHR blood pressure.
Mean arterial pressure (MAP, mmHg) was evaluated in Wistar (A) and SHR (B) treated with IgG or anti-TLR4. Each point refers to one animal and the trace to other point indicates the MAP of the same animal before and after treatment. n= 6. *P< 0.05 vs.anti-TLR4-SHR group before the treatment. Statistical test: Student’s t test
Table 1.
Blood pressure values before and after treatment with IgG or anti-TLR4; and maximum response (Emax) and sensitivity (pD2) to noradrenaline with or without pyrrolidin in mesenteric resistance arteries from Wistar and SHR treated with IgG or anti-TLR4
| Wistar IgG | Wistar anti-TLR4 | SHR IgG | SHR anti-TLR4 | |||||
|---|---|---|---|---|---|---|---|---|
| before | after | before | after | before | after | before | after | |
| Mean blood pressure (mmHg) | 119.9±2.5 | 126.2±2.2 | 116.9±1.9 | 116.5±2.9 | 174±3.1‡ | 184.3±3.6 | 178.4±2.1 | 162.6±3.4# |
| pD2 | Emax | pD2 | Emax | pD2 | Emax | pD2 | Emax | |
| Nor (mN) | 6.32±0.05 | 20.0±0.71 | 6.15±0.07 | 17.44±0.95 | 5.99±0.04 | 26.3±0.97 | 5.92±0.08 | 19.4±1.2$ |
| Nor + pyrrolidin (mN) | 6.09±0.05 | 21.21±0.82 | 6.18±0.08 | 17.44±0.95 | 5.79±0.07 | 16.3±0.66$ | 5.61±0.04 | 18.05±0.77 |
Values are mean ±SEM.
P≤0.05 vs Wistar IgG before;
vs SHR IgG before;
P < 0.05 vs SHR IgG Nor.
Before: before treatment with IgG or anti-TLR4; after: after treatment with IgG or anti-TLR4; pD2: −logEC50 or sensitivity; Emax: maximal response; Nor: noradrenaline.
3.2 Effect of anti-TLR4 treatment on vascular contractility
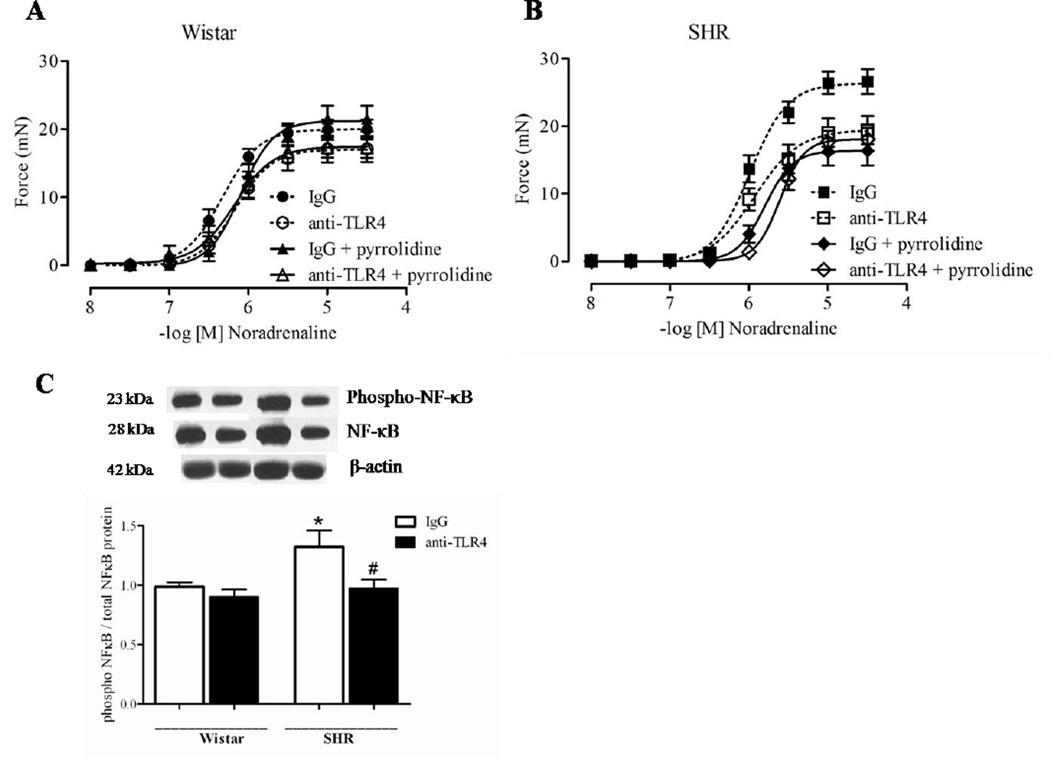
Vascular reactivity (Emax and pD2 values) to noradrenaline was significantly decreased in endothelium-intact mesenteric resistance arteries from anti-TLR4-treated SHR, compared to arteries from unspecific IgG-treated SHR (Figure 2B and Table 1), as described before [7]. No differences were observed in noradrenaline responses between the Wistar groups (Figure 2A and Table 1). Pre-incubation of mesenteric resistance arteries with pyrrolidine (NFκB inhibitor) decreased noradrenaline Emax value in arteries from IgG-treated SHR, but not in arteries from anti-TLR4-treated SHR or arteries from the Wistar groups (Figure 2A–B and Table 1). Together, these data demonstrate that anti-TLR4 treatment decreases augmented vascular contractile responses observed in SHR, through a NFκB-dependent mechanism.
Figure 2. Anti-TLR4 treatment decreases NFκB p65 phosphorylation and attenuates noradrenaline-induced constriction in mesenteric resistance arteries from SHR.
Cumulative concentration-response curves to noradrenaline in the presence of vehicle or pyrrolidine (NFκB inhibitor), in mesenteric resistance arteries from (A) Wistar and (B) SHR treated with IgG or anti-TLR4. (C) Protein expression of phosphorylated and total NFκB p65 in mesenteric resistance arteries from Wistar and SHR treated with IgG or anti-TLR4. Each curve point represents the mean ± SEM of maximal response to each concentration of noradrenaline. Bar graphs show the relative expression of proteins (mean ± SEM) after normalization to β-actin. On the top are representative Western blotting images of protein expression. n = 5–6. Statistical test: One-way ANOVA.
3.3 Effect of anti-TLR4 treatment on cytokines and TLR4 signaling pathway
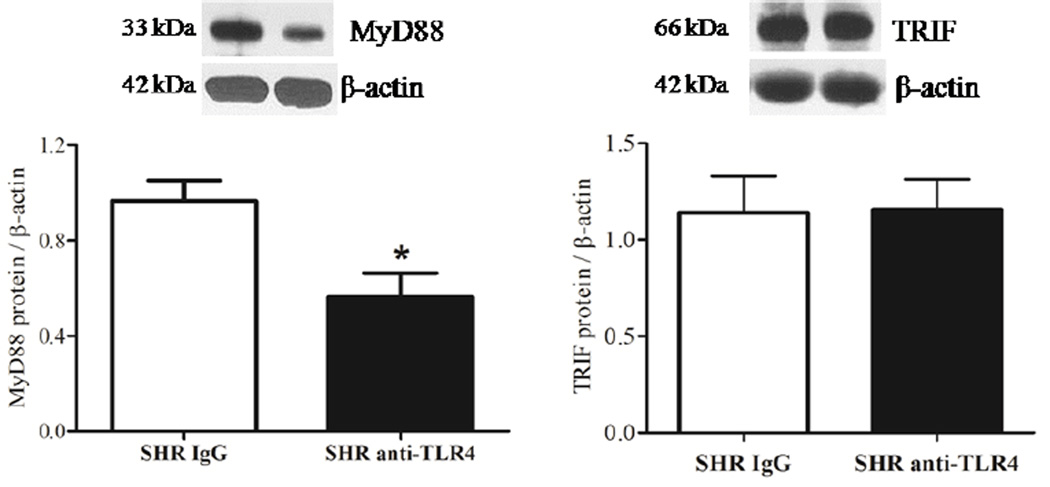
We determined activation of molecules involved in TLR4 signaling pathway in mesenteric arteries from SHR. MyD88 protein expression was decreased in arteries from anti-TLR4-treated SHR when compared to arteries from IgG-treated SHR. No differences in TRIF expression in arteries from SHR after anti-TLR4 treatment were observed (Figure 3). The phosphorylation of NFκB p65 in small mesenteric arteries from IgG-treated SHR was increased compared to arteries from IgG-treated Wistar rats. The anti-TLR4 treatment decreased vascular NFκB p65 phosphorylation in SHR, but not in Wistar rats (Figure 2C).
Figure 3. MyD88 signaling pathway is decreased in SHR after anti-TLR4 treatment.
(A) MyD88 and (B) TRIF protein expression in mesenteric resistance arteries from IgG- (white bars) and anti-TLR4-treated (black bars) SHR, On top, representative western blot images of MyD88 (A) and TRIF (B) protein expression. Bar graphs show the relative expression of proteins after normalization to β-actin. Each bar represents the mean ± SEM, n = 5–6. *P < 0.05. Statistical test: Student’s t test.
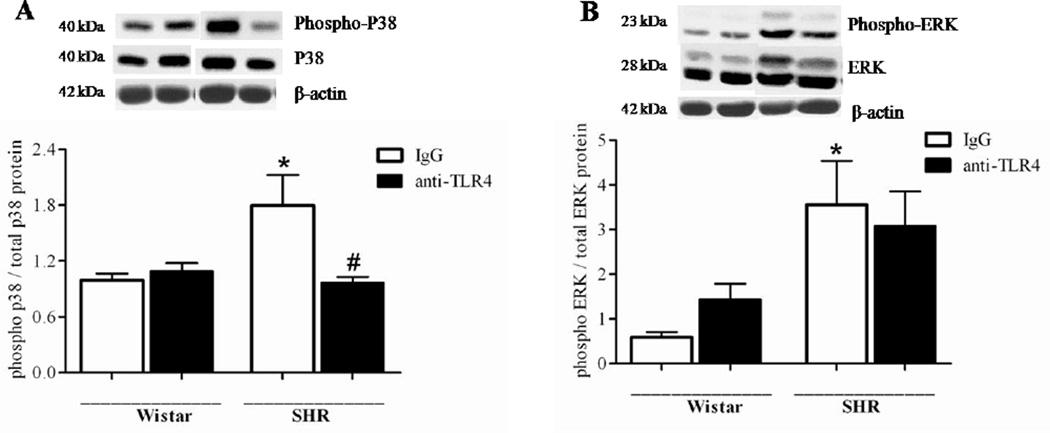
Vascular expression and activity of MAPK were also determined. Accordingly, p38 and ERK phosphorylation was increased in small mesenteric arteries from IgG-treated SHR, compared to arteries from IgG-treated Wistar rats. The anti-TLR4 treatment decreased p38, but not ERK, phosphorylation in SHR (Figure 4).
Figure 4. Anti-TLR4 treatment decreases vascular p38 phosphorylation in SHR.
Phosphorylated and total p38 (A) and ERK1/2 (B) protein expression in mesenteric resistance arteries from Wistar and SHR treated with IgG or anti-TLR4. On the top, representative Western blotting images of (A) p38 and (B) ERK1/2. On the bottom, corresponding bar graphs showing the relative expression of p38 and ERK1/2, after normalization to β-actin expression. n=6 in each experimental group. *P < 0.05 compared with Wistar IgG; # P < 0.05 compared with SHR IgG. Statistical test: One-way ANOVA.
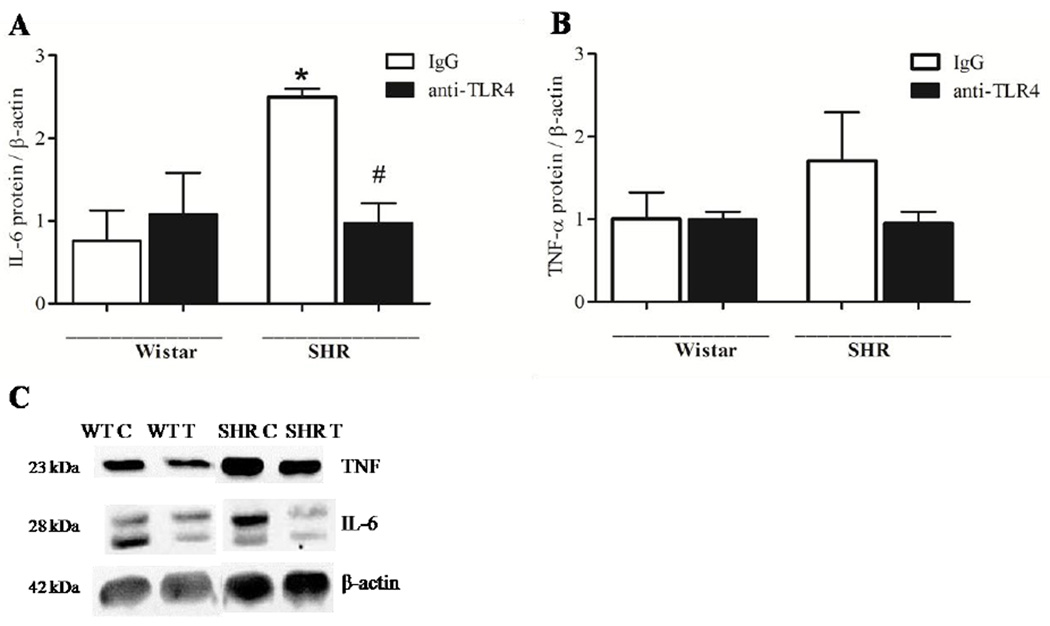
Protein expressions of pro-inflammatory cytokines IL-6 and TNF-α were determined in small mesenteric arteries. IL-6, but not TNF-α expression was increased in IgG-treated SHR versus IgG-treated Wistar, and the treatment with anti-TLR4 decreased IL-6 levels in SHR. (Figure 5).
Figure 5. TLR4 blockade reduces vascular inflammation in SHR.
Protein expression of IL-6 (A) and TNF-α (B) cytokines were analyzed in mesenteric resistance arteries from Wistar and SHR treated with IgG (white bar) or anti-TLR4 (black bar). Representative western blot images of IL-6, TNF-α and β–actin protein expression are shown on C. Bar graphs show the relative protein expression after normalization to β-actin. Values are means ± SEM, n= 4. *P < 0.05 compared with Wistar IgG; # P < 0.05 compared with SHR IgG. Statistical test: One-way ANOVA.
These data demonstrate that TLR4 inhibition attenuated the activation of inflammatory markers and TLR4 signaling proteins, as demonstrated by decreased expression of MyD88 and IL-6 and reduced p38 and NFκB phosphorylation.
3.4 Effect of anti-TLR4 treatment on ROS production
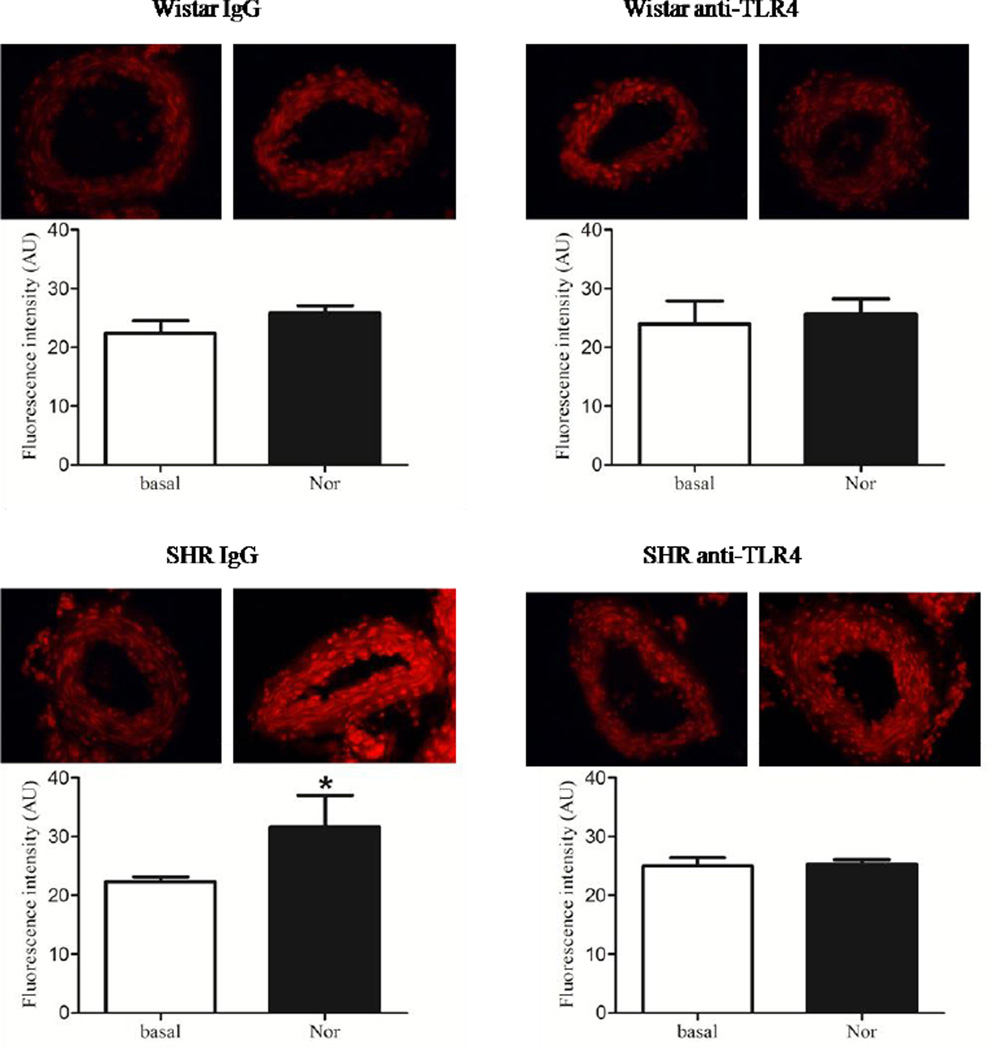
Vascular generation of reactive oxygen species was determined in basal conditions and after stimulation with noradrenaline in samples from Wistar and SHR rats treated with IgG or anti-TLR4. Increased ROS production was found only in noradrenaline-stimulated arteries from IgG-treated SHR compared to basal conditions. No differences in ROS generation were observed in arteries from anti-TLR4-treated SHR suggesting that treatment with anti-TLR4 abolished augmented ROS production in small mesenteric arteries from SHR.
4. DISCUSSION
In this study, we show for the first time that inhibition of TLR4 activation, by treatment with an anti-TLR4 antibody, in addition to decreasing blood pressure, decreases p38 and NFκB phosphorylation as well as ROS generation in mesenteric resistance arteries from SHR. The involvement of the innate immune system in hypertension pathogenesis and in the development of hypertension-related vascular dysfunction is a novel concept that has recently been under intense investigation. Our aim in this work was to identify the signaling pathways involved in TLR4 activation in resistance vessels in hypertension. A decrease in NFκB activity and expression of pro-inflammatory cytokines represents a potential mechanism by which the anti-TLR4 treatment decreases vascular reactivity and blood pressure in SHR.
Recognition of host-derived molecules by TLRs and subsequent TLR activation may be an important link between activation of the immune system and hypertension. Many studies have clearly shown that TLR4 is responsible for inflammation induced by endogenous ligands, such as C-reactive protein and heat shock proteins (HSP60 and HSP70), in smooth muscle cells and many other cell types [12–14]. Circulating levels of these molecules are increased in hypertension. They may act as long-term TLR4 activators, resulting in augmented expression of several pro-inflammatory cytokines in cardiovascular tissues, contributing to blood pressure elevation. We demonstrated that treatment with anti-TLR4 decreases blood pressure in SHR, but not in normotensive controls, showing that TLR4 activation contributes to spontaneous hypertension.
An augmented expression of TLR4 during hypertension has been demonstrated in many tissues and in different hypertensive models. Eisler et al, demonstrated an increased cardiac TLR4 expression in SHR and L-NAME-induced hypertension. De Batista et al, showed an increased TLR4 expression in SHR aorta and that anti-TLR4 treatment ameliorates inflammatory parameters such as oxidative stress in this vessel. Previous studies from our laboratory demonstrated an increase in TLR4 expression in mesenteric arteries from SHR. Additionally, we observed that anti-TLR4 treatment reduced this receptor expression as well as IL-6 and TNF-α serum levels. suggesting that TLR4 blockade in hypertension has significant systemic anti-inflammatory effects. We now investigate the NFκB-dependent mechanism involved in the TLR4-mediated vascular inflammatory process.
Activation of TLR signaling pathways by endogenous molecules have been poorly investigated in different cell types, but recent studies report recruitment of distinct adaptor molecules and induction of distinct signaling pathways downstream of TLRs after stimulation with exogenous or endogenous molecules. Activation of TLR4 by bacteria-derived LPS can induce both TRIF and MyD88-dependent pathways [8]. In contrast, it has been shown that endogenous TLR4 agonists, such as heat shock proteins [15], fibrinogen [16] and uric acid [17], signal via MyD88. All these endogenous ligands are found in high levels in hypertensive subjects, suggesting that they can activate TLR4 by a MyD88-dependent signaling pathway in mesenteric resistance arteries of SHR.
An important signaling pathway recruited by TLR4 activation via MyD88-dependent pathway is the MAPK cascade [18]. To confirm that TLR4 activates p38 and ERK in mesenteric resistance arteries from SHR, we determined phosphorylation levels of these proteins. Western blotting analysis showed that anti-TLR4 treatment decreased p38, but not ERK, phosphorylation only in SHR. In fact, C-reactive protein triggers an inflammatory response in rat vascular smooth muscle cells by inducing TNF-α secretion, which is mediated by p38 MAPK–TLR4 signaling pathway [19]. These data suggest an involvement of p38 MAPK in TLR4 signaling pathway in hypertension.
The present study also demonstrates that IL-6, but not TNF-α, protein expression is increased in mesenteric resistance arteries from IgG-treated SHR and that anti-TLR4 treatment normalizes expression of these pro-inflammatory cytokines. In our previous study, we showed reduced IL-6 in serum from TLR4-treated SHR versus IgG-treated SHR [7]. Protein expression measurements of this cytokine in vascular tissues showed a local effect action of TLR4 blockade in mesenteric arteries. The inflammatory response is associated with cytokine release and ROS production, which play an important role in renal damage and increased vascular resistance associated with hypertension. Inflammation has also been clinically associated with increased arterial stiffness, which independently predicts cardiovascular events in hypertensive patients [20, 21]. TNF-α and IL-6 are pro-inflammatory cytokines positively associated with hypertension [22, 23]. These cytokines regulate the expression and function of several proteins, including adhesion molecules, MAPK, extracellular matrix components and growth factors, which are important in vessel hypertrophy and vascular dysfunction described in hypertension [24]. IL-6 also seems to influence the balance between vasoconstrictor and vasodilator factors, as well as regional differences in the release and responsiveness to these factors, contributing to the increased responsiveness within a specific vascular bed in hypertension [25]. Activation of TLR4 culminates in NFκB phosphorylation and IL-6 and TNF-α expression in different cell types and tissues [12, 26]. However some works have showed that TLR4 activation can stimulate specific cytokines release. Lukic L., (2014) showed that diabetes type 2 patients with hypertension have high levels of IL-6 but not TNF-α compared with patients without hypertension [27].
The IkB/NFkB system is considered a major intracellular inflammatory pathway, mediating most of the vascular inflammatory responses [28]. NFκB is a transcription factor containing DNA binding and dimerization domains, nuclear translocation signal, and binding site for the inhibitor of κB, which is involved in regulating genes of the inflammatory cascade [29]. Upon activation by extracellular stimuli, NFκB translocates from the cytoplasm into the nucleus and induces gene expression. for cytokines such as IL-6, COX-2, chemokines and adhesion molecules involved in recruitment of monocytes/macrophages to sites of inflammation in the vascular wall [28]. It was previously shown that, in SHR, renal NFκB activation and immune cell infiltration occur very early in life, being observed in 3 weeks-old SHR, when compared to Wistar-Kyoto rats (WKY) [30]. However, vascular NFκB activation has not been demonstrated in resistance arteries from experimental models of hypertension. In this study, we not only observed increased vascular NFκB phosphorylation, but also that increased NFκB activity is, in part, dependent on TLR4 signaling.
In addition, we previously showed that vascular reactivity, assessed through the constrictor responses to the α-adrenergic agonist noradrenaline in isolated mesenteric resistance arteries, is modulated by anti-TLR4 treatment. Accordingly, anti-TLR4 treatment decreases contractile responses to noradrenaline in SHR [7]. Here we demonstrated that increased vasoconstriction is normalized by NFκB inhibition with pyrrolidine. Considering that 1) cytokines, such as IL-6, activate several signaling pathways and mediators of vascular contraction; 2) cytokines expression is often regulated by NFκB, which, in turn, produces mediators (as the COX enzyme) that amplify the vascular inflammatory response, our results suggest that the inflammatory mediators regulated by NFκB potentiate the increased vascular contractile response to noradrenaline.
5. CONCLUSION
Our findings demonstrate that TLR4 signaling is activated in mesenteric resistance arteries from SHR, leading to activation of MyD88/p38/NFκB pathway. In addition, we showed that anti-TLR4 treatment decreases blood pressure, as well as restores hyperreactivity to noradrenaline via NFκB inhibition, lowering of IL-6 and reactive oxygen species generation. This study reinforces the concept that hypertension is a pro-inflammatory disease and strengthens the idea that TLR4 activation contributes to the pathogenesis of hypertension.
Figure 6. Anti-TLR4 treatment abolishes noradrenaline-induced augmented ROS production in SHR.
The bar graphs represent a quantitative analysis of ROS production from mesenteric arteries of Wistar and SHR treated with IgG or anti-TLR4. On top of each graphs representative images of transverse DHE-treated sections of small mesenteric arteries stimulated with vehicle or noradrenaline. The results show densitometric analysis of fluorescence intensity of mesenteric arteries from Wistar and SHR treated with IgG or anti-TLR4 incubated with vehicle or noradrenaline and are expressed as mean ± SEM, (n= 4–5/group). *P < 0.05 vs respective group stimulated with noradrenaline. Statistical test: Student’s t test.
Acknowledgments
We are grateful to Ana Rita Gonçalves Araujo, Antonio Garcia Soares Junior, Marta Rodrigues da Silva and Sonia Maria Rodrigues Leite for their excellent technical support.
Sources of Funding
This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brazil, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G.F. Bomfim, Email: gifacholi@yahoo.com.br.
C. Echem, Email: cinthya.echem@gmail.com.
C.B. Martins, Email: cla_cbm@hotmail.com.
T.J. Costa, Email: tiago.januario@uol.com.br.
S.M. Sartoretto, Email: simone.sartoretto@gmail.com.
R.A. Dos Santos, Email: reichlerusp@gmail.com.
M.A. Oliveira, Email: cidora@yahoo.com.
E.H. Akamine, Email: eliakamine@gmail.com.
Z.B. Fortes, Email: zbfortes@icb.usp.br.
R.C. Tostes, Email: rtostes@usp.br.
R.C. Webb, Email: cwebb@gru.edu.
M.H.C. Carvalho, Email: mhcarval@icb.usp.br.
REFERENCES
- 1.Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens. 2003;12(2):181–187. doi: 10.1097/00041552-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Guzik TJ, Harrison DG. Endothelial NF-kappaB as a mediator of kidney damage: the missing link between systemic vascular and renal disease? Circ Res. 2007;101(3):227–229. doi: 10.1161/CIRCRESAHA.107.158295. [DOI] [PubMed] [Google Scholar]
- 3.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17(4):223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DG, et al. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10(2):203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzik TJ, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Bomfim GF, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122(11):535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 10.Milanski M, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akamine EH, et al. Correction of endothelial dysfunction in diabetic female rats by tetrahydrobiopterin and chronic insulin. J Vasc Res. 2006;43(4):309–320. doi: 10.1159/000093196. [DOI] [PubMed] [Google Scholar]
- 12.Liu N, et al. C-reactive protein triggers inflammatory responses partly via TLR4/IRF3/NF-kappaB signaling pathway in rat vascular smooth muscle cells. Life Sci. 2010;87(11–12):367–374. doi: 10.1016/j.lfs.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Wallin RP, et al. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23(3):130–135. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 14.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, et al. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105(12):1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, et al. MyD88-dependent nuclear factor-kappaB activation is involved in fibrinogen-induced hypertrophic response of cardiomyocytes. J Hypertens. 2009;27(5):1084–1093. doi: 10.1097/HJH.0b013e3283293c93. [DOI] [PubMed] [Google Scholar]
- 17.Liu-Bryan R, et al. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52(9):2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 19.Liu N, et al. C-reactive protein induces TNF-alpha secretion by p38 MAPK-TLR4 signal pathway in rat vascular smooth muscle cells. Inflammation. 2011;34(4):283–290. doi: 10.1007/s10753-010-9234-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118–1122. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 22.Navarro-Gonzalez JF, et al. Association of tumor necrosis factor-alpha with early target organ damage in newly diagnosed patients with essential hypertension. J Hypertens. 2008;26(11):2168–2175. doi: 10.1097/HJH.0b013e32830e2545. [DOI] [PubMed] [Google Scholar]
- 23.Bautista LE, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19(2):149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 24.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290(3):H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 25.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putra AB, et al. Jellyfish collagen stimulates production of TNF-alpha and IL-6 by J774.1 cells through activation of NF-kappaB and JNK via TLR4 signaling pathway. Mol Immunol. 2014;58(1):32–37. doi: 10.1016/j.molimm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Lukic L, et al. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health. 2014;11(4):3586–3598. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Martin R, et al. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20(11):E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 29.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107(3):255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Iturbe B, et al. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315(1):51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]