Ischemic heart disease remains the leading cause of death in the developed world, and mechanisms to reduce cardiac ischemic damage are being actively investigated. A potent protective mechanism for the heart is ischemic preconditioning (IPC), a phenomenon in which brief ischemic episodes protect the heart from tissue damage and cell death resulting from subsequent periods of ischemia and reperfusion1. The precise molecular mechanisms at play during IPC are not known, but the opening of a mitochondrial ATP-sensitive potassium channel, mitoKATP, is believed to be necessary for IPC-induced activation of several pro-survival pathways and processes2.
Although the discovery of a putative mitoKATP occurred over twenty years ago3, progress on identifying its molecular composition has been limited. The mitoKATP was determined to be both functionally and molecularly distinct from sarcolemmal KATP channels, which have a relatively minimal role in IPC protection and are insensitive to several drugs that affect mitoKATP4, 5. Initial studies using immunoreactivity identified the inward rectifying K+-channel subunit Kir6.1 as localizing to mitochondria6, 7, and thus Kir6.1 became an attractive candidate as a possible subunit of the mitoKATP. However, further research by mass spectrometry into the specificity of the Kir6.1 antibody revealed that the antibody does not recognize Kir6.1, and that Kir6.1 is not isolated from more thorough proteomic screens of mitochondria8. Another investigation led to purification of an inward-rectifying K+-channel component of the mitochondria, but did not identify any specific protein9. In a separate study, sulfonylurea receptor (SUR)2 was presented as a possible candidate for a mitoKATP channel component10. The long form of SUR2 is needed for the diazoxide-sensitive K+ current. The short form of SUR2 localizes to mitochondria, and is able to form a glibenclamide- and ATP-sensitive K+ current on the cell surface with Kir6.1. The protective effects of SUR2 have been shown directly only in E. coli, and not in cardiac cells. Whether SUR2 is truly necessary for the ATP-sensitive K+ current in the mitochondria, and whether it plays a role in IPC, remains to be seen. The mitoKATP has also been proposed to be a multiprotein complex, and succinate dehydrogenase was found to play a regulatory role in mitoKATP activity11. However, the identity of the protein that is responsible for the K+-channel activity in this complex is not known.
The search for mitoKATP subunits has been complicated by the lack of specificity of pharmacological agents that target the mitoKATP. Diazoxide, a commonly used drug for mitoKATP activation, has been shown to activate the sarcolemmal mitoKATP as well12. This drug, along with the mitoKATP inhibitor 5-HD13, also affect metabolism and therefore may have a confounding contribution to the protective effects of IPC that is not directly related to the mitoKATP. Thus, pharmacological manipulation alone has proven to be insufficient in identifying mitoKATP components.
In this issue of Circulation Research, Foster et al. combine a high-throughput proteomic screen with pharmacological and genetic manipulation to provide evidence that renal outer medullary potassium channel (ROMK) is a component of the K+ channel of the cardiac mitoKATP14. The authors employ mass spectrometry analysis of a scaled-up preparation of bovine heart mitochondria to identify ROMK as an inner membrane component, and verify its localization to the mitochondria. They also determine that the ROMK inhibitor Tertiapin Q decreases mitoKATP activity, and that ROMK knockdown inhibits ATP-sensitive and diazoxide-activated mitochondrial uptake of the K+ surrogate thallium. Finally, they find that ROMK overexpression protects against cell death in H9c2 cardiomyoblasts, while ROMK knockdown increases cell death.
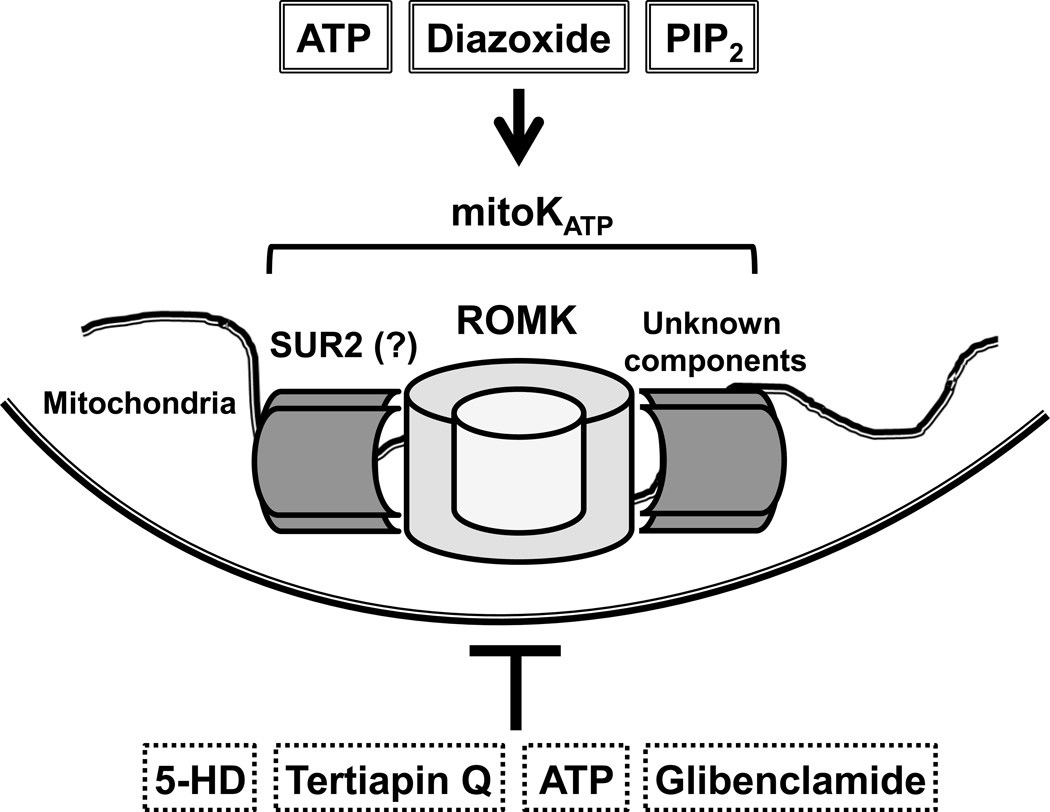
The findings by Foster et al. are novel and compelling in identifying ROMK as a subunit of the mitoKATP channel. This study also brings up new questions about the mitoKATP. First, while the relatively low abundance of ROMK isoforms in the mitochondria presents particular technical difficulties in studying them, it is now pertinent to investigate what other proteins bind to endogenous levels of ROMK. Specifically, given the previous data on SUR2, it would be of interest to see whether endogenous cardiac SUR2 and ROMK can co-precipitate and form functional K+ channels in the mitochondria (Figure). Additionally, it is unclear whether ROMK2 is the particular ROMK isoform present in the mitoKATP. More detailed studies on the function of each isoform are needed to determine if there are isoform-specific differences in ROMK localization and KATP activity. It is also unknown whether ROMK transports K+ selectively in the mitochondria, or if it is involved in the transport of other ions as well. Moreover, ROMK was chosen as a candidate for further study from the proteomics screen based on its known K+-transporter properties. However, additional targets from the screen may also be part of the mitoKATP channel, even if they are not known to be K+ transporters. A thorough second look at the results of this screen may reveal other potential candidates for mitoKATP components.
Figure. Proposed MitoKATP structure.
A proteomic screen of inner mitochondrial membrane components identified ROMK as a protein possessing mitoKATP channel activity which responds to known regulators of the mitoKATP channel, such as the pharmacologic activator diazoxide and the inhibitor 5-hydroxydecanoate (5-HD). While not established, ROMK may interact with another proposed component of the mitoKATP channel, the short splice variant of sulfonylurea receptor 2 (SUR2).
As many pharmacological agents have been proven to lack specificity for mitoKATP, research on the mitoKATP must take care to utilize genetic manipulation in addition to pharmacological treatment. Foster et al. combined both methods by separately using ROMK knockdown and Tertiapin Q to show inhibition of mitoKATP. However, in addition to its known inhibition of ROMK, Tertiapin Q may also have nonspecific ROMK-independent effects that contribute to its reduction of mitoKATP activity. If there is any ROMK-independent mitoKATP inhibition by Tertiapin Q, identification of the potential nonspecific effects would be needed for future studies utilizing this toxin.
A logical next step to follow up on the results from Foster et al. is to investigate whether ROMK confers mitoKATP activity in vivo as well. ROMK knockout or transgenic animals should now be studied for mitoKATP activity as well as IPC protection. These experiments would be important in determining whether ROMK is a physiologically relevant factor of mitoKATP and IPC in vivo.
In summary, the components of the mitoKATP have remained elusive since its initial discovery, as investigation into its composition has been hindered by the low abundance of K+ transporters in the mitochondria as well as a lack of pharmacological specificity for the mitoKATP. Foster et al. now demonstrate that ROMK is a novel component of the mitoKATP through the combination of a relatively large and specific proteomic screen with pharmacological and genetic techniques. Future studies will reveal what other proteins bind to ROMK, whether there are ROMK isoform-specific differences in mitoKATP function, and whether this protein is a part of the in vivo mitoKATP.
Acknowledgments
Sources of Funding
H.A. is supported by National Institutes of Health grants K02 HL107448, R01 HL087149, R01 HL104181, and 1P01 HL108795, and the American Heart Association.
Non-standard abbreviations
- IPC
Ischemic preconditioning
- SUR
sulfonylurea receptor
- ROMK
renal outer medullary potassium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Ardehali H, O'Rourke B. Mitochondrial k(atp) channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, atp-dependent k+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- 4.Sato T, Sasaki N, Seharaseyon J, O'Rourke B, Marbán E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal k(atp) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O'Rourke B, Marbán E. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal katp channels and native cardiac mitochondrial katp channels. Mol Pharmacol. 1999;55:1000–1005. [PubMed] [Google Scholar]
- 6.Lacza Z, Snipes JA, Miller AW, Szabó C, Grover G, Busija DW. Heart mitochondria contain functional atp-dependent k+ channels. J Mol Cell Cardiol. 2003;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of kir6.0 and sur2 atp-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 8.Foster DB, Rucker JJ, Marbán E. Is kir6.1 a subunit of mitok(atp)? Biochem Biophys Res Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mironova GD, Negoda AE, Marinov BS, Paucek P, Costa AD, Grigoriev SM, Skarga YY, Garlid KD. Functional distinctions between the mitochondrial atp-dependent k+ channel (mitokatp) and its inward rectifier subunit (mitokir) J Biol Chem. 2004;279:32562–32568. doi: 10.1074/jbc.M401115200. [DOI] [PubMed] [Google Scholar]
- 10.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardehali H, Chen Z, Ko Y, Mejía-Alvarez R, Marbán E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial atp-sensitive k+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'hahan N, Moreau C, Prost AL, Jacquet H, Alekseev AE, Terzic A, Vivaudou M. Pharmacological plasticity of cardiac atp-sensitive potassium channels toward diazoxide revealed by adp. Proc Natl Acad Sci U S A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J. K(atp) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O'Rourke B. The mitocondrial romk channel is a molecular component of mitokatp. Circ Res. 2012;111 doi: 10.1161/CIRCRESAHA.112.266445. xxx-xxx. [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]