Abstract
We are fortunate to live in an age in which biomedical technology has provided us with unprecedented ability to supplant the functions of organs and support the physiologic processes of the human body. Ingenious doctors, physiologists, and engineers helped create these advances with new and innovative ideas. One of these pioneers was Dr. Theodor Kolobow. He is best known for one of his earliest inventions, the spiral coil membrane lung. His contributions to medical innovation, however, are diverse, as he also contributed to advances in hemodialysis, improvements in extracorporeal life support (ECLS) technology/circuit components, and through his laboratory experiments helped shape our current understanding of cardiopulmonary pathophysiology. In retrospect, much of Kolobow’s work was unified by the theme of preventing iatrogenic lung injury due to mechanical ventilation. This tenet became more obvious as his later studies progressed to developing techniques and devices intended to limit ventilator pressures, and prevent bacterial colonization of the lungs. Although he formally retired from his research endeavors in 2009, the impact of his contributions remains prominent in our everyday use of techniques and equipment that he either originated or helped to develop. (Picture 1)
Introduction
Theodor (Ted) Kolobow was born in the small island village of Kardla, Estonia on July 20, 1931. The youngest of three children, early life for Ted was idyllic as he enjoyed the boyhood freedom of life on a rural island. Ted’s father was a Russian Orthodox priest and lawyer who was promoted to judge in the Estonian Supreme Court in Tallinn in the late 1930’s. As such, education and religion were both important in the daily life of the Kolobow family. Ted’s mother would often tell her young son that there were three professions that people always need: doctors, lawyers, and shoemakers. Ted took this to heart, but as a young boy growing up on an island he could not have possibly foreseen the impact he would have on critical care medicine.
The events of WWII forced Ted’s family to flee Estonia to a deported person’s camp in Augsburg, Germany in 1940. Nine-year old Ted used education and learning as a means of clinging to normalcy during this dark time in the world’s history. In after-hours lessons held after German children had finished at school, Ted learned to speak Russian, German and English.
As a teenager, Ted was able to turn his language skills into a job at World Church Services in Munich, Germany, where he assisted fellow Estonians in completing their applications to immigrate to America. Ted soon decided that America could provide him with the educational opportunities he sought, and was awarded a scholarship to Heidelberg College (now University) in Tiffin, Ohio. One month before his 19th birthday, with only $20 in his pocket and his father’s crucifix, Ted left his family and boarded the US troop transport ship Harry S. Taylor to New York City where he was met by Heidelberg’s dean of students.
While at Heidelberg College, Ted was in the work-study program and washed dishes in the mess hall. He also participated in track and field, and enjoyed shot put and discus events. Academically his passion was mechanical engineering, and it was in the machine shop where he became interested in applying his ideas to the field of medicine. In 1954 he graduated second in his class with a degree in mathematics and physics, and immediately enrolled in the school of medicine at Case Western Reserve University. As a first year medical student he was required to choose a research project, and fortunately, he chose to work with Dr. George H.A. Clowes, a cardiothoracic surgeon who sought to develop new methods for oxygenating blood during cardiopulmonary bypass. Dr. Clowes had begun research to identify which plastic films (membranes) were suitable for exchanging respiratory gas. 1 Dr. Kolobow’s contributions as a student to testing these membranes led him to authorship on a paper that was presented at the founding meeting of the American Society for Artificial Internal Organs (ASAIO) in 1955. 2 This was the first of many publications that distinguished Kolobow’s long career. He continued to work in the Clowes laboratory until completing his medical education at Case Western Reserve University in 1958.
Kolobow continued his medical training as a house officer in internal medicine and pulmonology at Cleveland Metropolitan General Hospital. During his time there he was occasionally solicited to babysit for the infant child of one of his co-residents. It was during one of these sittings that he met a young woman named Danielle, the infant’s aunt. The babysitting sessions became more and more frequent, likely due to Ted and Danielle’s fondness for each other. The couple was married in 1963, and went on to have 4 children and 9 grandchildren.
After completing his training in Cleveland in 1962, Kolobow entered the Public Health Service (PHS) and prepared to leave for Fort Defiance in Arizona. Prior to traveling across the country, he had a chance meeting and informal conversation with Dr. Robert L. Bowman, the distinguished director of the Laboratory of Technical Development at the National Heart Institute (NHI) (a division of the National Institute of Health [NIH] that would later become the National Heart Lung and Blood Institute [NHLBI]). Dr. Bowman was a remarkable man; he developed the fluorescence spectrophotometer, was an editor of “SCIENCE”, and had been at NHI since it’s founding. Kolobow idolized immigrant physician scientists such as Drs. Willem Kolff and Pierre Galletti, and realized that under Dr. Bowman’s mentorship at the NHI he could follow in their footsteps and perhaps have a similar broad impact on medical progress. He accepted a PHS position as a staff associate at the National Heart Institute, and remained at the NIH for his entire career.
At the NIH Kolobow had the opportunity to pursue his multifaceted interests. While he continued work on membrane oxygenators, his other early projects were aimed at temporary mechanical hemodynamic support and included a cardiac cup assistor and leg counterpulsation. Starting in the 1960s and through 2010, he trained numerous physician fellows in his laboratory (approximately 50 from Italy alone), many of whom would become leaders in the fields of biotechnology and critical care. (Picture 2) He earned a reputation for being a kind and patient teacher, “He never raised his voice in all the years I knew him” (personal communication-W. Zapol, NIH fellow 1967-1970).
Picture 2.
Dr. Kolobow (top row, fourth from left) in Milan 9/19/2008 reunited with Dr. Robert Bartlett (top row second from left) and Dr. Warren Zapol (top row third from left) and many of his Italian Fellows of whom he referred affectionately as the “Italian Mafia,” including Dr. Luciano Gattinoni (top row fifth from left), Dr. Antonio Pesenti, Dr. Gianluigi Li Bassi, Dr. Massimiliano Cressoni, Dr. Maurio Panigada and Dr. Alberto Zanella - Photo courtesy of Dr. Lorenzo Berra
Dr. Luciano Gattinoni (NIH fellow 1975-1977) said of Kolobow; “He is one of the few persons I met in my life with the capability of looking to reality with different and innocent eyes. Where everybody saw grey he could discriminate different mixtures of white and black” (personal communication). Kolobow’s talents were recognized early, and he was promoted to section chief of Pulmonary and Cardiac Assist Devices in 1970 with the approval of Donald Fredrickson MD, then director of the NHI. Dr. Kolobow’s scholarly work is a clear example of effective translational research, wherein laboratory developments are rapidly tested in animals and subsequently implemented in clinical trials. Despite his many successes, Kolobow remained very humble. “Ted was painfully shy, never spoke at meetings unless invited to speak, and told me often that it is far more important to publish than to speak” (personal communication- W. Zapol). Fortunately, Dr. Kolobow published many of his scientific ideas extensively. What follows is a brief review of his most important contributions; many are overlapping and interrelated. According to his wife, when asked what his greatest achievement had been, he would say, “I survived the war.” If asked what his greatest contribution to medicine was, he would proudly say, “ECMO” (extracorporeal membrane oxygenation) (personal communication-D. Kolobow).
ECLS Components
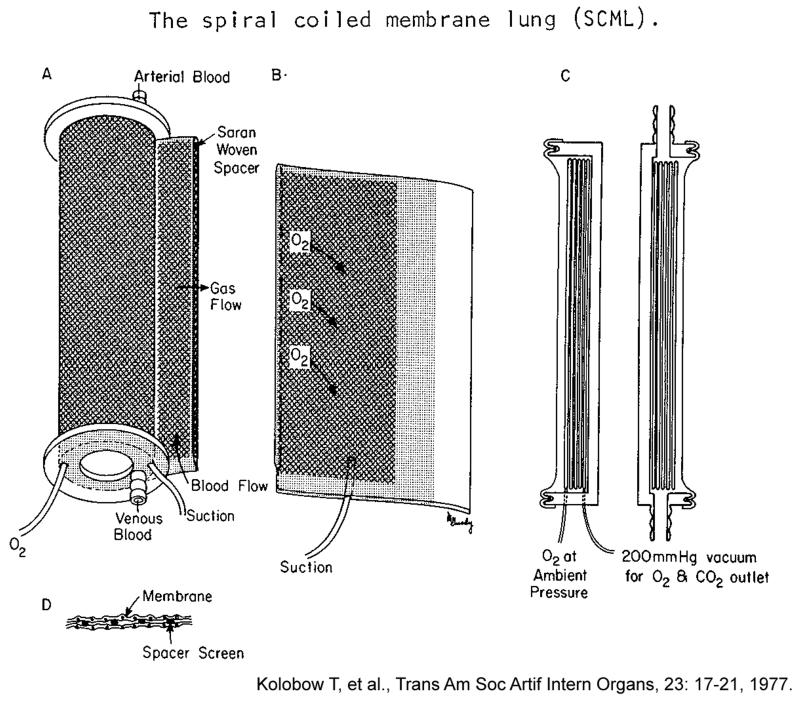
Kolobow’s work in biomedical engineering began with what is recognized as one of his greatest contributions, the disposable membrane lung. He recalled the process in a personal retrospective published in 2004. 1 When he entered the field in 1955, membrane lungs were simply composed of large flat sheets of thin silicone rubber separating blood and gas. It was recognized that membrane lungs needed to be more compact and ideally disposable for easy use. Initial attempts included stacking the membranes, however these configurations were bulky and took many hours to construct. Dr. Willem Kolff constructed a coiled lung design, 3 however the device had a high resistance and large priming volume making it impractical. Kolobow was inspired by Kolff’s coiled polyethylene artificial lung and decided that assembling a silicone membrane in a spiral configuration would be even more compact, and provide the large surface area (several square meters) needed for gas exchange, yet have a small priming volume to allow use in children. While in the Clowes laboratory, he was able to test a coiled configuration using polyethylene membrane tubing, which contained a vinyl fiberglass screen inside the tube to allow gas to easily flow. Instead of blowing oxygen at positive pressure through the device, as done in Kolff’s design, Kolobow applied slight suction which allowed formation of a tight seal. If membrane leaks occurred (and in that era they often did) the hypobaric gas would not embolize into the blood stream. These ideas ultimately led him to the silicone rubber spiral coil membrane lung, for which the NIH was issued a patent in 1970. (Picture 3) Over the next two decades, as the inherent advantages of membrane lungs over bubble oxygenators (less blood damage) became widely recognized, disposable membrane lungs completely replaced bubble oxygenators for cardiopulmonary bypass in cardiac operating rooms.
Picture 3.
Design of the spiral coil membrane lung
Kolobow first attempted canine studies of the membrane lung at the NHI with the assistance of his fellow Dr. Warren Zapol. When dogs proved to be too active as experimental animals, the investigators shifted to studying long-term (days to weeks) bypass in awake, 5-10kg lambs. The NIH veterinarian Dr. Joseph Pierce was remarkably helpful in obtaining and maintaining lambs for these studies, and in helping to formulate the animal study designs. Not being certain of the safety of pumping blood through the apparatus, the initial experiments utilized arteriovenous (AV) perfusion (much as a fetus supplies the placenta). The investigators then progressed to employing a blood pump, allowing venovenous (VV) perfusion, and eventually venoarterial (VA) perfusion.
To assure adequate blood flow during bypass, Kolobow first tackled the problem of high resistance and kinking in vascular access cannulae. Kolobow designed and produced thin walled flexible polyurethane cannulas with steel wire reinforcement to allow cannulation of the fetal umbilical vessels with low blood flow resistance. 4 “Ted was truly inventive- since the manufacturer would not give us polyurethane for medical use (they were afraid of legal suits), Ted simply dissolved a Lycra brassiere in dimethyl sulfoxide and recast the material as a polymeric vascular catheter!” (personal communication-W. Zapol). In 1967 Kolobow and his team designed a complete system consisting of the thin walled, wire reinforced cannulae, a non-occlusive roller pump with Kolobow’s novel polyurethane pump-head, and a silicone rubber reservoir and tubing. With this new system they reported total arteriovenous perfusion of fetal lambs for 48-72 hours, and published their results in “SCIENCE.” 5 Subsequently In 1970 they demonstrated that up to 1 week of support was possible in lambs with minimal blood damage in both the venovenous and venoarterial (VA) configurations; by 1971 Kolobow’s group was able to achieve venovenous perfusions lasting up to 16 days. 6 (Picture 4) Their membrane lung’s ability to support prolonged gas exchange without protein denaturation or hemolysis allowed for eventual safe transition of prolonged cardiopulmonary bypass out of the operating room to the intensive care unit (ICU), helping to set the stage for the implementation of clinical ECMO.
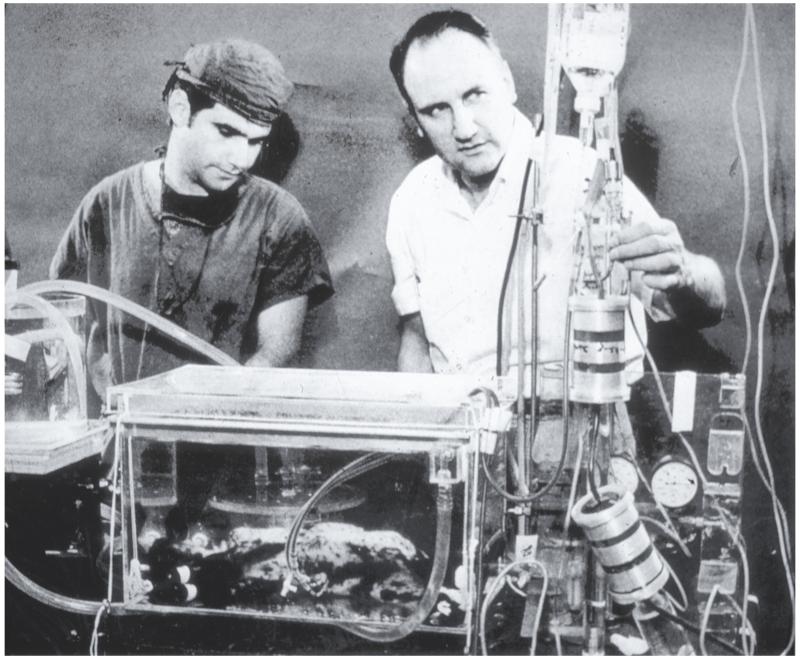
Picture 4.
Drs. Kolobow (right) and Dr. Zapol perfusing a newborn lamb, 1969- Courtesy of Warren Zapol
In building his own membrane lungs in the 1970s, Kolobow was plagued by pinholes in commercially available silicone membranes. To address this problem, he developed a novel dispersion casting technique to create superior silicone rubber membrane for circuit components. Since he also recognized the importance of blood-surface interactions with circuit components, Kolobow pioneered techniques for the manufacture of silicone rubber membrane with superior blood compatibility, and conducted studies regarding the thrombogenicity of silicone rubber. He also examined the platelet response to using membrane lungs without heparin, and studied the relationship between heparin dosing and platelet concentration. 7 A summary of his work regarding membrane lungs and blood surface interactions entitled “The Promise of the Membrane Artificial Lung” was published in 1978. 8
Dr. Kolobow’s ideas in this field of research led to biotechnological advances useful in other areas of translational research. Over the course of his career Kolobow was granted over 20 patents. He helped develop new dialysis machines, cuff-less endotracheal tubes, and additional devices to prop open right-sided heart valves to avoid left heart distention during percutaneous cardiopulmonary bypass. The products and offspring of many of his innovative ideas have entered clinical practice, and his laboratory studies sparked the practical and widespread application of clinical ECLS.
Clinical ECLS
While never again clinically active himself, Dr. Kolobow was a frequent advisor to clinicians in the Critical Care Service at NIH in Bethesda; His expertise was invaluable in promoting ECLS technology. As the circuit components reached a safe and an improved level in animal studies, Kolobow’s team attempted to apply the technique in patients. In September 1968, Kolobow and Zapol performed venovenous ECMO in a young woman with respiratory failure at the NHI. Although she survived ECMO, she subsequently died before discharge from the clinical center. Given their successful experience supporting newborn lambs, the group attempted to support infants with infant respiratory distress syndrome. In 1969 Kolobow and Zapol traveled to San Juan, PR and performed ECMO perfusion via umbilical cannulation in five premature newborns with hypoxia. Hemorrhage plagued these attempts at life support, and none of the infants survived. In a second attempt in 1969, the NIH group combined with Dr. Gordon Avery at Children’s Hospital of DC to perform AV ECMO (via umbilical cannulation with Kolobow’s polyurethane cannulae) in hypoxic newborns. Several children were perfused but unfortunately again none survived. Although dissatisfied, Dr. Kolobow was not discouraged; as one of his former fellows said “Of course the early unsuccessful clinical trials were not published, Ted had no interest in discouraging the world from trying ECMO” (personal communication-W. Zapol). The Department of Defense (DOD) became interested in ECMO technology for treatment of “Danang Lung” or what we would today call Acute Respiratory Distress Syndrome (ARDS). Kolobow had larger spiral coil lungs built by Sci-Med Corporation with DOD funding and in March 1970 sent Zapol off to Danang, Vietnam, to perfuse soldiers with “Danang lung”. Luckily the war had slowed significantly prior to his arrival and there were no victims of blast injuries to serve as candidates for the study.
In the early 1970s others succeeded with ECMO where Kolobow and Zapol had not; Dr. J.D. Hill successfully supported an adult male with venoarterial bypass after traumatic lung injury, Dr. Robert H. Bartlett supported a two year old boy with VA ECMO after cardiac surgery, and Dr. John J. White was making progress using venovenous ECMO in infants with respiratory distress syndrome. 9-11 Kolobow was confident in the physiologic principles of extracorporeal support, and he continued to attempt perfusions at the NHI. In 1974 his group published a case report detailing the use of a membrane lung via venovenous perfusion as therapy for an 11-year-old boy with respiratory failure due to bilateral pneumonia. After being treated for several days at fraction of inspired oxygen (FiO2) of 1.0 and a positive end expiratory pressure (PEEP) of 15 cm H2O, the patient’s arterial partial pressure of oxygen (PaO2) fell below 40 mm Hg. He was cannulated (internal jugular infusion, femoral drainage) and maintained on bypass for 10 days, which at the time was the longest successful clinical effort to provide extracorporeal respiratory support. 12 The boy was discharged 7 weeks later, and 4 months later was participating in athletic events at school. Two years later the boy remained in excellent health, and Kolobow’s group published a follow up report describing his spirometric results, confirming that a patient whose lungs are severely damaged by ARDS may recover complete function if given time to heal via ECLS support. 13 This remarkable finding was in contrast to popular opinion at that time, which deemed that ARDS survivors would have decreased lung function and become respiratory cripples. Dr. Kolobow used this success to help promote the use of ECLS for respiratory assistance as a viable and useful treatment option.
The Dangerous Ventilator
A disappointment to many clinicians who had great hopes for ECLS technology was an NIH funded randomized clinical trial in 1979, which failed to show any differences in patient survival when management of pulmonary failure using conventional ventilation was compared to conventional ventilation plus venoarterial ECMO. 14 Kolobow ’s explanation for these disappointing findings focused on the extreme criteria used for patient selection for ECMO; he noted that it was often moribund patients with irreversible lung damage who were selected for ECMO and thus predisposed the study to failure. He also emphasized that the patients in these studies had previously, and continued to be, exposed to high ventilator pressures while on ECMO support. He hypothesized that the high FiO2 and ventilator pressures provided prior to initiation of ECLS and during ECMO surely contributed to ongoing lung injury in these patients. He argued that the use of lower ventilator pressures while on ECMO was what allowed Dr. Robert Bartlett at University of California, Irvine, to achieve higher success rates in neonates. 15 This idea led to future recommendations that ECMO should be used to augment gas exchange, in order to allow lungs to be managed at lower respiratory rates, tidal volumes, and peak inspiratory pressures during ECMO. 16 In addition Kolobow advocated for earlier implementation of ECLS therapy to avoid ventilator associated lung damage, an argument still resonating to this day.
To provide evidence for his arguments, the Kolobow laboratory investigated the phenomenon of ventilator associated lung damage in sheep. 15 They showed that ventilator peak airway pressures of 50cm H2O or greater consistently induced ARDS and multisystem organ failure in lambs, who subsequently died within two days of ventilation at these ventilator settings. Deterioration of lung function, whilst delayed, was also observed at peak airway pressures as low as 30 cm H2O. Hyperventilation, even at normal pressures, was also damaging to the sheep’s healthy lung, and resulted in ARDS. 16 Future studies would show that the histological injury caused by high airway pressures was indistinguishable from ARDS. 17
These experimental studies suggested that high airway pressures propagate lung injury in the ARDS patients and contributed to the failure of the NIH clinical trial. It became clear to Kolobow that it was necessary to provide adequate alveolar ventilation of the healthy regions of the lung while maintaining peak pressures within the normal range in order to protect the diseased lung regions from over-distension. On the basis of his experimental work, Kolobow recommended limiting peak inspiratory pressures to 20 mmHg; similar settings would later be recommended by the ARDS Network two decades later. These seminal experiments started discussions and laid the foundation for future studies of barotrauma, which helped spark movement towards lung protective ventilation for ARDS.
While oxygenation could be maintained, one result of lower ventilator pressures was hypercapnia. In order to support ARDS patients without using high-pressure ventilator strategies, Kolobow in conjunction with his fellow Luciano Gattinoni of Milan, developed the technique of extracorporeal CO2 removal. Later, with other collaborators, he also developed a technique for intra-tracheal pulmonary ventilation.
Limiting Barotrauma
Techniques for respiratory support and carbon dioxide removal were maturing in the Laboratory for Technical Development in lamb models for several years and would become a major project of Kolobow’s distinguished career. Working with his fellow Dr. Luciano Gattinoni, Kolobow theorized that employing extracorporeal arteriovenous perfusion to remove CO2 would reduce the requirement for pulmonary ventilation, thereby limiting ventilator pressures and significantly reducing ventilator induced lung injury. Oxygenation of venous blood could be provided via the expanded native lung at low pressure. The first demonstration of the feasibility of using low flow ECLS to remove sufficient CO2 to reduce ventilatory requirements was provided by Kolobow and Gattinoni in 1977. 18 They performed a series of experiments in lambs using air as the ECMO sweep gas and relied on the native lungs to provide continuous oxygenation assisted only by continuous positive airway pressure (CPAP); they discovered that lambs could be supported for several days using this technique despite remaining totally apneic. For extended use, the addition of low frequency ventilator breaths was effective in maintaining the functional residual capacity, compliance, and preventing atelectasis. Other large animal studies followed in subsequent years demonstrating the effectiveness of this technique in eliminating CO2 and reducing barotrauma. Moreover, lambs treated in this manner had an increased cardiac output and urine output, and a decreased pulmonary artery pressure as compared to animals treated with conventional ventilation.
In 1979 Gattinoni and co-workers in Milan published the first clinical case report of treatment with Low Frequency Positive Pressure Ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R). 19 The report described a 25 year old woman with ARDS who, after beginning ECCO2R, was able to decrease her minute ventilation from 14L/min to 1.5L/min while maintaining arterial oxygen tensions constant; this allowed her lung function to recover. Unfortunately she eventually succumbed to unrelated complications of a laparotomy. Gattinoni continued his clinical studies in Milan and reported more successful cases in subsequent years. An excellent summary entitled “Motionless Lungs”, published in 1983, reported the theory, laboratory experiments, and clinical application of the LFPPV-ECCO2R technique. 20 The authors reviewed 20 patients treated with LFPPV-ECCO2R and reported an overall survival of 60%, a great improvement over the 10% survival reported in the 1979 NIH-ECMO trial. They concluded that the improvement in survival was due to a reduction in barotrauma, and that the laboratory studies and the clinical results provided evidence for the detrimental effect of high ventilator settings in ARDS patients treated by traditional ECLS. The Milan group continued to demonstrate their success with LFPPV-ECCO2R, and published another report in 1988 showing 47% survival of patients with ARDS treated with up to 32 days of support. 21
Unfortunately LFPPV-ECCO2R did not become a standard treatment as Kolobow had hoped; It was expensive, labor intensive and required skilled and dedicated physicians. Kolobow’s group therefore sought other less invasive methods to limit ventilator induced iatrogenic lung injury. Working with researchers and clinicians in Boston and Miami, their next endeavor was to provide Intra-tracheal pulmonary ventilation. The technique involves using a small catheter to provide continuous flow of oxygen at the level of the trachea, as well as a reverse thrust catheter, to allow gas to continuously escape and remove CO2. This technique helps to limit the peak inspiratory pressure, and ensures that all parts of the lung, whether diseased or not, are ventilated at equal pressures. This technique could be coupled with frequent, small volume breaths, and later evolved into an advanced form of high frequency oscillatory ventilation (HFOV). Intra-tracheal pulmonary ventilation was first reported in infants with congenital diaphragmatic hernia in 1992, and was eventually expanded with success to other pediatric patients and adults. 22
Improving Respiratory Care Devices and the Prevention of Ventilator Associated Pneumonia (VAP)
Kolobow’s investigations continued to focus on improving the management of the severely injured lung. With the goal of reducing endotracheal tube resistance and dead space, he designed and fabricated an ultrathin-wall, two-stage, twin endotracheal tube. This elaborate endotracheal tube design mimicked the low resistance and airflow characteristics of the un-intubated human airway, allowing further reductions of ventilator pressures. This design was of special interest to neonatologists, since when used with a specially designed “Y” piece at the lips it was shown to reduce the dead space ventilation by almost three to seven fold. 23
Since it was well known that ventilator-associated pneumonia often follows prolonged ventilator management, Kolobow focused on the prevention of this complication while maintaining optimal respiratory care. To reduce aspiration, Kolobow designed a cuff-less endotracheal (ET) tube. The cuff-less tube utilized “gills” to maintain an airway seal and was shown to be effective even at high inflation pressures. 24 This design had the added benefits of avoiding tracheal injury caused by high-pressure balloon inflation, as well as promoting physiologic mucociliary clearance.
Many of Kolobow’s more recent interventions and recommendations were logical and progressive advances, but remain at this time unexplored in clinical trials. For example, he postulated that keeping intubated patients semi-recumbent at an angle of 30-45 degrees might promote micro aspiration, and facilitate the development of pneumonia. In sheep, he demonstrated that if ET tubes were oriented horizontally, mucus drained spontaneously and there was little or no bacterial colonization of the lungs. In contrast, when sheep were maintained at a 30-45 degree semi-recumbent angle there was a high degree of bacterial colonization of the lungs. 1, 25
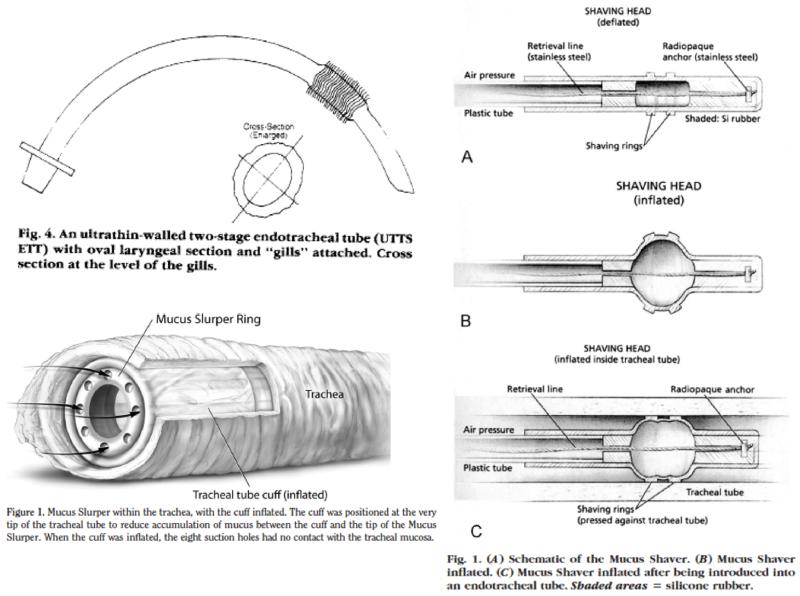
Since positioning ET tubes in a horizontal manner could pose inherent difficulties in nursing critically ill patients, Kolobow developed more than 50 different anti-adhesive, bactericidal and antistatic coatings that could limit pathogen colonization and biofilm formation on the ET tube. Other novel Kolobow devices sought to help clear mucous from the trachea, either by using a suction device to aspirate secretions, or by having a sliding balloon fitted with “shaving rings” to help scrape the mucous out of the ET tube (Picture 5). 26, 27 Several publications focused on experimental animal studies of these devices and demonstrated that these ET tube and respiratory device designs reduced bacterial colonization of both the ET tube and of the lungs of healthy sheep. 28 Variations and extrapolations of these ingenious and straightforward designs are beginning to become commercially available.
Picture 5.
Design of cuff-less ET tube,24 Mucous Shaver,27 and Mucous Slurper26
In Summary
We have described Dr. Theodor Kolobow’s professional journey in learning to “treat lungs, rather than numbers” (T. Kolobow). 1 “The membrane artificial lung is, was, and will remain a most powerful tool in our armamentarium, both surgical and medical. Yet, we should apply it carefully, recognizing that ancillary factors such as injuries from mechanical ventilation, or intubation/ventilator associated nosocomial pneumonia, may well have been the primary cause for lack of survival/recovery of many of our patients” (T. Kolobow). 1 This statement captures the essence of Kolobow’s perspectives. “First do not harm the lung!” he would exclaim to his research fellows. Driven by a pioneering spirit and an insatiable desire to learn, he first noted that when peak ventilator pressures were reduced in patients supported by VV ECMO, the native lungs recovered. This prompted other laboratory experiments, which demonstrated the pathological effects of barotrauma in large animals, and led to the development of ingenious clinical strategies to reduce mechanical ventilator requirements, and prevent VAP.
As Gattinoni, one of his fellows and collaborators, notes, “Ted’s (thinking) was at least 20 years advanced over current thinking. I would not be surprised to find many more of his ideas as routine practice in ICU patients 10 years from now” (personal communication). Unfortunately, Dr. Kolobow’s dream of artificial lung assistance becoming as commonplace, and easy to manage, as hemodialysis has not yet been realized. Perhaps such routine application awaits us in the future. Despite this, the medical and surgical community is eternally grateful for Kolobow’s inspired endeavors, as he propelled us into the modern era of successful extracorporeal support and care of the critically ill patient.
Picture 1.
Dr. Kolobow relaxing in the Laboratory, 1967- Courtesy of Warren Zapol
Acknowledgments
We thank Danielle “Gigi” Kolobow, who provided valuable information regarding the early life of her husband, and described her husband’s dedication to his remarkable work and to his fellows. Also, special thanks to Dr. Luciano Gattinoni for illuminating comments regarding his mentor.
Funding: This study was in part supported by NIH grant T32HL007854-20 (MAH); and NIH Grant 2R01 HD015434-29 (RHB)
Footnotes
Disclaimer: None
Disclosures: The authors declare no conflicts of interest.
Works Cited
- 1.Kolobow T. The artificial lung: The past. A personal retrospective. ASAIO Journal. 2004;50:xliii–xlviii. doi: 10.1097/01.mat.0000147960.14376.d5. [DOI] [PubMed] [Google Scholar]
- 2.Clowes G, Jr, Hopkins A, KOLOBOW T. Oxygen diffusion through plastic films. ASAIO Journal. 1955;1:23. hyhen. [Google Scholar]
- 3.Kolff W, Balzer R, CLEVELAND M. The artificial coil lung. ASAIO Journal. 1955;1:39. hyhen. [Google Scholar]
- 4.Kolobow T, Zapol W. A new thin-walled nonkinking catheter for peripheral vascular cannulation. Surgery. 1970;68:625–629. [PubMed] [Google Scholar]
- 5.Zapol WM, Kolobow T, Pierce JG, Bowman RL. Artificial placenta: Two days of total extrauterine support of the isolated premature lamb fetus. Science. 1969;166:617–618. doi: 10.1126/science.166.3905.617. [DOI] [PubMed] [Google Scholar]
- 6.Kolobow T, Spragg RG, Pierce JE, Zapol WM. Extended term (to 16 days) partial extracorporeal blood gas exchange with the spiral membrane lung in unanesthetized lambs. ASAIO Journal. 1971;17:350–354. [PubMed] [Google Scholar]
- 7.Uziel L, Agostoni A, Pirovano E, et al. Hematologic survey during low frequency positive pressure ventilation with extracorporeal co2 removal. ASAIO Journal. 1982;28:359–364. [PubMed] [Google Scholar]
- 8.Kolobow T. The promise of the membrane artificial lung. The International journal of artificial organs. 1978;1:15–20. [PubMed] [Google Scholar]
- 9.White JJ, Andrews HG, Risemberg H, Mazur D, Haller JA. Prolonged respiratory support in newborn infants with a membrane oxygenator. Surgery. 1971;70:288–296. [PubMed] [Google Scholar]
- 10.Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the bramson membrane lung. The New England journal of medicine. 1972;286:629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett R, Gazzaniga A, Jefferies M, Huxtable R, Haiduc N, Fong S. Extracorporeal membrane oxygenation (ecmo) cardiopulmonary support in infancy. ASAIO Journal. 1976;22:80–92. [PubMed] [Google Scholar]
- 12.Kolobow T, Stool EW, Sacks KL, Vurek GG. Acute respiratory failure. Survival following ten days’ support with a membrane lung. The Journal of thoracic and cardiovascular surgery. 1975;69:947–953. [PubMed] [Google Scholar]
- 13.Newball HH, Stool EW, Kolobow T. Follow-up respiratory function of a patient treated with a membrane lung 1–3. American Review of Respiratory Disease. 1975;112:725–731. doi: 10.1164/arrd.1975.112.5.725. [DOI] [PubMed] [Google Scholar]
- 14.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. Jama. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 15.Kolobow T, Borelli M, Spatola R. Artificial lung (oxygenators) Artificial organs. 1986;10:370–377. doi: 10.1111/j.1525-1594.1986.tb02583.x. [DOI] [PubMed] [Google Scholar]
- 16.Kolobow T. Acute respiratory failure. On how to injure healthy lungs (and prevent sick lungs from recovering) ASAIO transactions / American Society for Artificial Internal Organs. 1988;34:31–34. [PubMed] [Google Scholar]
- 17.Tsuno K, Miura K, Takeya M, Kolobow T, Morioka T. Histopathologic pulmonary changes from mechanical ventilation at high peak airway pressures. The American review of respiratory disease. 1991;143:1115–1120. doi: 10.1164/ajrccm/143.5_Pt_1.1115. [DOI] [PubMed] [Google Scholar]
- 18.Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J. The carbon dioxide membrane lung (cdml): A new concept. ASAIO Journal. 1977;23:17–21. doi: 10.1097/00002480-197700230-00005. [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal co2 removal (lfppv-ecco2r) in treatment of adult respiratory distress syndrome (ards) The International journal of artificial organs. 1979;2:282–283. [PubMed] [Google Scholar]
- 20.Gattinoni L, Pesenti A, Kolobow T, Damia G. A new look at therapy of the adult respiratory distress syndrome: Motionless lungs. International anesthesiology clinics. 1983;21:97–117. doi: 10.1097/00004311-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Pesenti A, Gattinoni L, Kolobow T, Damia G. Extracorporeal circulation in adult respiratory failure. ASAIO Journal. 1988;34:43–47. [PubMed] [Google Scholar]
- 22.Kolobow T, Giacomini M, Reali-Forster C, Trawoger R. The current status of intratracheal-pulmonary ventilation (itpv) The International journal of artificial organs. 1995;18:670–673. [PubMed] [Google Scholar]
- 23.Kolobow T, Berra L, DeMarchi L, Aly H. Ultrathin-wall, two-stage, twin endotracheal tube: A tracheal tube with minimal resistance and minimal dead space for use in newborn and infant patients*. Pediatric Critical Care Medicine. 2004;5:379–383. doi: 10.1097/01.pcc.0000128602.22489.2d. [DOI] [PubMed] [Google Scholar]
- 24.Kolobow T, Tsuno K, Rossi N, Aprigliano M. Design and development of ultrathin-walled, nonkinking endotracheal tubes of a new“ no-pressure” laryngeal seal design. A preliminary report. Anesthesiology. 1994;81:1061–1067. doi: 10.1097/00000542-199410000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Bassi GL, Zanella A, Cressoni M, Stylianou M, Kolobow T. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: Possible role in the pathogenesis of ventilator–associated pneumonia. Critical care medicine. 2008;36:518–525. doi: 10.1097/01.CCM.0000299741.32078.E9. [DOI] [PubMed] [Google Scholar]
- 26.Bassi GL, Curto F, Zanella A, Stylianou M, Kolobow T. A 72-hour study to test the efficacy and safety of the “mucus slurper” in mechanically ventilated sheep. Critical care medicine. 2007;35:906–911. doi: 10.1097/01.CCM.0000257332.62358.0E. [DOI] [PubMed] [Google Scholar]
- 27.Kolobow T, Berra L, Bassi GL, Curto F. Novel system for complete removal of secretions within the endotracheal tubethe mucus shaver. The Journal of the American Society of Anesthesiologists. 2005;102:1063–1065. doi: 10.1097/00000542-200505000-00028. 1063-1065. [DOI] [PubMed] [Google Scholar]
- 28.Berra L, Panigada M, De Marchi L, et al. New approaches for the prevention of airway infection in ventilated patients. Lessons learned from laboratory animal studies at the national institutes of health. Minerva anestesiologica. 2003;69:342–347. [PubMed] [Google Scholar]