Abstract
Herpes simplex virus (HSV) normally undergoes productive infection in culture, causing cell destruction and plaque formation. Here we characterize an unusual pattern of HSV type 1 (HSV-1) infection in MDBK cells which surprisingly results in suppression of replication, cell recovery, and maintenance of virus. Compared to Vero cells, MDBK cells supported a normal productive infection at a high multiplicity with complete cell destruction. At low multiplicity, HSV also showed an identical initial specific infectivity in the two cell types. Thereafter, the progression of infection was radically different. In contrast to the rapid plaque expansion and eventual destruction in Vero monolayers, in MDBK cells, after initial plaque formation, plaque size actually decreased and, with time, monolayers recovered. Using a green fluorescent protein (GFP)-VP16-expressing virus, we monitored infection in live individual plaques. After early stages of intense GFP-VP16 expression, expression regressed to a thin boundary at the edge of the plaques and was completely suppressed by 10 days. Cells lacking expression then began to grow into the plaque boundaries. Furthermore, following media replacement, individual cells expressing GFP-VP16 could be observed reinitiating infection. The results indicated the production of a potent inhibitory component during infection in MDBK cells, and we show the continued and prolonged presence of interferon in the medium, at times when there was no longer evidence of ongoing productive infection. We exploited the ability of V protein of simian virus 5 to degrade Stat1 and prevent interferon signaling. We established MDBK cells constitutively expressing the V protein with the resultant loss of Stat1. In comparison to the parental cells, infection in these cells now progressed at a rapid rate with expanding plaque formation. We believe the conclusions have significant implications for the study of HSV-1 and interferon signaling both in culture and in animal models.
Herpes simplex virus (HSV) normally undergoes a productive cycle of replication in culture which results in cell destruction within 18 to 24 h and virus production (15). The replication cycle in culture is studied in a wide range of cells, including not only human cells but also cells of primate, murine, canine, or bovine origin. In vivo, in its natural host, after acute-stage productive infection in epithelial cells at mucocutaneous boundaries, the virus is transported to neuronal cells innervating the primary sites, where it undergoes a nonproductive infection resulting in latency. Periodic reactivation occurs whereby HSV undergoes a productive infection and is transported back to surface sites where it may undergo further rounds of productive replication (22, 46). Various systems have been established in culture to recapitulate this cyclical pathway of productive infection, repression, and reactivation, usually involving the use of virus mutants defective in several functions and/or the use of inhibitors to suppress virus replication (20, 34, 35, 38, 41, 44, 45, 47).
Cells have evolved many diverse mechanisms of innate and acquired antiviral responses to combat virus infection (15). One of the most important innate antiviral response mechanisms is the production and secretion of interferon (IFN) and the subsequent paracrine activation of signaling via IFN receptors (16). IFN-α (encompassing 24 related isotypes) and IFN-β are secreted by most cells in response to infection while IFN-γ production is largely restricted to T cells and NK cells. IFN-α/β bind to and activate a common single receptor while IFN-γ recognizes a separate receptor (42). IFN binding results in the activation of the JAK/Stat pathway and the ensuing induction of expression of antiviral components, such as double-stranded RNA-dependent protein kinase R (PKR), 2′ 5′ oligoadenylate synthase, and RNase L (25, 39, 40, 42). These factors are induced in uninfected cells as inactive primed precursors and are then rapidly activated at early stages of infection to suppress replication or even induce cell apoptosis. In turn, viruses have developed countermeasures in the attempt to facilitate productive infection and overcome the host immune responses (23). For example, the HSV protein ICP34.5 was reported to recruit a cellular phosphatase to dephosphorylate the α subunit of eukaryotic initiation factor 2 and thus counteract the activity of PKR (21), which phosphorylates the α subunit of eukaryotic initiation factor 2 in an inhibitory response to infection. Another HSV gene product, US11, appears to prevent PKR activation (3, 32), and virus mutants which are defective in ICP34.5 exhibit increased sensitivity to IFN (29). More recently, results from several laboratories indicate that the immediate-early protein ICP0 may also act to counteract aspects of the IFN pathway. ICP0 is required and sufficient to repress the induction of IFN-stimulated genes (ISGs) (8, 19), and viruses lacking functional ICP0 were shown to be hypersensitive to IFN in culture (28, 29). By analysis of the IFN pathway after infection in the presence of protein synthesis inhibitors (31) or infection with UV-inactivated or defective viruses (8, 27), it has been demonstrated that virus binding or the entry of virion components is sufficient to trigger expression of ISGs. Correspondingly, it has been shown that HSV products synthesized de novo during normal infection with wild-type (wt) viruses are very efficient blockers of such triggering (8, 19, 27). For example, the induction of ISG54, one of the most responsive genes observed during infection in the presence of cycloheximide, is undetectable during wt infection (31). These very effective viral countermeasures therefore account for the general observation that wt HSV replication is comparatively resistant to IFN signaling and IFN-mediated responses in culture (8, 28, 31, 37).
Here we characterize a virus-host cell interaction, using wt HSV in culture, where a progressive repressive effect is established in a paracrine fashion. In MDBK cells, which are of bovine origin and frequently used to characterize aspects of HSV replication and protein targeting, HSV induced the expression and secretion of IFN and subsequent Stat1 phosphorylation in uninfected cells. Even with infection at multiplicities of between 0.01 and 0.1 in a population of cells, the result of the progressive resistance to infection was the apparent clearance of active replication and cell recovery. Active IFN was maintained in infected cultures for a prolonged period, and its removal by medium replacement resulted in the reinitiation of virus gene expression in isolated individual cells. By using the V protein of the paramyxovirus simian virus 5 (SV5) to target Stat1 for degradation (6), we show that the repressive effect and establishment of a form of persistent infection is due to IFN- and Stat1-mediated signaling. The results are discussed with respect to the mechanisms involved in IFN induction and its suppression, the issue of HSV persistence, and the general nature of low-multiplicity-dependent phenotypes of certain virus mutants. We also discuss whether lack of function of candidate virus proteins in this system is due to species specificity in identified signaling pathways and possible implications for studies of pathogenesis in animal models.
MATERIALS AND METHODS
Cells and viruses.
MDBK, HeLa, and Vero cells were grown in Dulbecco's modified minimal essential medium supplemented with 10% newborn calf serum. Virus stocks used were wt HSV-1 [17] and the green fluorescent protein (GFP)-VP16-expressing derivative of HSV-1 [17], HSV-1 [44] (22a).
Plaque assays.
Cells were seeded in six-well cluster dishes at a density of 2 × 106 cells per dish such that monolayers were confluent the following day. Monolayers were infected with serial dilutions of virus in serum-free Dulbecco's modified minimal essential medium for 1 h, the inoculum was removed, and cells were then incubated in medium containing 2% newborn calf serum with or without 1% neutralizing human serum as indicated. For assays of IFN and conditioned medium, cells were seeded at a density of 106 cells per well. Universal IFN-α (uIFN) (IFNαA/D; Sigma) or conditioned medium from infected cultures was serially diluted as indicated and added to the monolayers for 12 h. The medium was then removed, and cells were infected with a standard amount of virus (1,000 PFU) of HSV-1 as described above. Plaques were counted after 3 days.
Western blot analysis of Stat phosphorylation.
To examine the phosphorylation of Stat1 by uIFN or conditioned medium, cells were seeded in six-well dishes at a density of 106 cells per dish and treated the day after with different dilutions of uIFN for 1 h. Conditioned medium from MDBK cells previously infected with different amounts of HSV-1 was harvested at the times indicated, and any virus was removed by filtration using polyvinylidene difluoride syringe filters with a pore size of 0.1 μm. Cells treated with uIFN or conditioned medium were harvested after 1 h, washed in phosphate-buffered saline, and lysed in sodium dodecyl sulfate (SDS) lysis buffer, and proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). After transfer to nitrocellulose membranes, the membranes were blocked with Tris-buffered saline containing 0.1% Tween 20 and 1% bovine serum albumin. The membranes were incubated overnight at 4°C with primary antibody in the same buffer, washed three times, and incubated for a further 1 h in buffer containing the appropriate horseradish peroxidase-conjugated secondary antibody. After further washing in Tris-buffered saline containing 0.1% Tween 20, membranes were processed for detection by chemiluminescence with standard reagents (Pierce). Primary antibodies used for probing membranes were anti-Stat1 for total Stat1 (1:1000; Upstate) and anti-pStat1 specifically for Y701 phosphorylated species (1:1,000; Cell Signaling).
Analysis of MDBK plaque progression with GFP-VP16-expressing HSV.
MDBK cells were plated in six-well dishes and infected when confluent with 2,000 PFU of HSV-1 [V44] (which expresses GFP fused to the tegument protein VP16) per well (22a). GFP-VP16 expression was monitored by confocal microscopy with an inverted microscope using a 20 × objective lens. To monitor the fate of single plaques, areas of the dish corresponding to initial foci were visualized with a normal objective at approximately 24 h after infection and then labeled by moving a marker objective (which contains an inked end piece in place of a lens) into position. The marker objective was raised to contact the dish, ringing an area which could then be relocated and repeatedly imaged. The same plaque was thus monitored every 24 h thereafter for up to 10 days.
Selection of a V5-expressing MDBK cell line.
MDBK cells were seeded in 60-mm-diameter dishes at a cell density of 106 cells per dish and transfected the day after by the calcium phosphate precipitation procedure modified as described previously (17). Plasmid DNA used for transfection was pIRES/V, kindly provided by R. Randall, which expresses the V protein together with a gene for neomycin resistance. After 16 h, the medium was replaced with fresh medium containing 400 μg of Geneticin/ml. Fresh Geneticin was replaced three times a week, and colonies were isolated approximately 4 weeks after transfection. Several individual colonies were isolated as cell lines, and one of several lines expressing V protein was used for further studies. The monoclonal antibody 336 anti-pK against V protein was used (1:10,000) to confirm expression by Western blot analysis and immunofluorescence.
RESULTS
Unusual outcome during HSV-1 infection on MDBK cells.
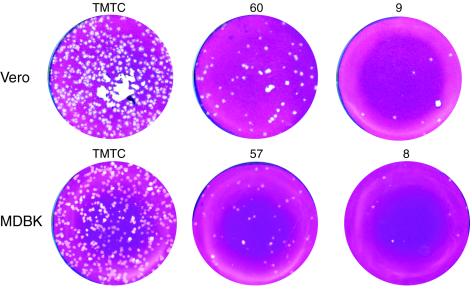
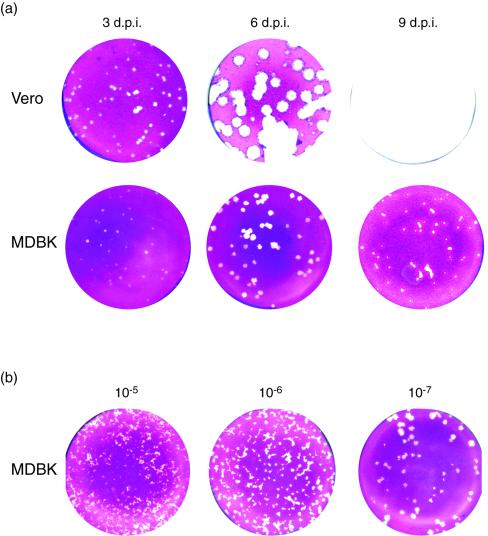
As part of our analysis of HSV-1 replication in MDBK cells, we initially compared plaque efficiency in these cells with that in the commonly used Vero cell line. The results, monitoring plaque formation at 3 days postinfection in each cell type, demonstrated virtually identical plaquing efficiency, with equal numbers of plaques and a modest difference in plaque size (Fig. 1). However, a surprising difference was observed when we extended the time over which we compared plaque formation (Fig. 2). In Vero cells there was a normal progression of infection with visible plaque formation by 3 days, progressively increasing plaque size with large plaques by 6 days, and eventually complete monolayer destruction by 9 days. In contrast, in MDBK cells, while as indicated above, the same numbers of plaques were visible at 3 days, only a moderate increase was observed by 6 days (Fig. 2a). This accentuated the difference in plaque size between MDBK and Vero cells. Strikingly, from about 6 days onward, plaque sizes in MDBK cells not only did not develop but actually decreased. This resulted in a dramatically different outcome of infection by 9 days, with complete monolayer destruction in Vero cells compared to cell recovery in MDBK cells (Fig. 2a). Two other features of plaque progression at late times in MDBK cells were notable. Plaques started to show an altered morphology and, instead of a more regular round appearance, began to become irregular in shape with flat cells at the periphery appearing to invade the plaque centers caused by earlier cell destruction. It was also notable at later times during infection that plaque size depended on plaque number, being smaller with higher amounts of initial virus inoculum (Fig. 2b). Plaques from the highest dilution formed distinctly larger plaques, whereas plaques at the lower dilutions were smaller and had the altered morphology with more irregular shapes described above.
FIG. 1.
Plaque assay in Vero (upper panel) and MDBK (lower panel) cells with HSV-1 [17]. Numbers of plaques (indicated above the plates) were assayed at 3 days postinfection. Both cell types have equal numbers of plaques and a moderate difference in plaque size. TMTC, too many to count.
FIG. 2.
Progression of plaque formation with time in Vero and MDBK cells. (a) Plaque formation was assayed after inoculation with 50 PFU of HSV-1 [17] in Vero and MDBK cells as indicated. Monolayers were fixed and stained at 3, 6, and 9 days after infection. (b) Plaque formation was assayed in MDBK cells after inoculation with different dilutions of HSV-1 [17], representing 5,000, 500, and 50 plaques. Monolayers were fixed and stained at 6 days after infection. d.p.i., days postinfection.
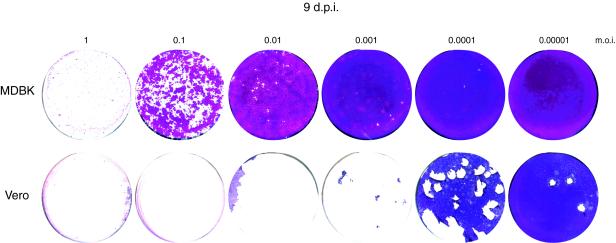
It is important to note that the profound difference in outcome of infection in the two cell types was manifest under conditions where not all cells were initially infected. In single-step growth curves at high multiplicities of infection (MOI) where all cells would be infected from the outset, MDBK cells were as susceptible as Vero cells, with complete cell destruction over the same time course (18 to 24 h) and similar yields (data not shown) (Fig. 3). However, when not all cells were initially infected, this apparent progressive suppression of infection in MDBK cells meant that even after infection with 0.01 to 0.1 PFU/cell, the monolayers could eventually recover (Fig. 3).
FIG. 3.
Cell recovery versus multiplicity. MDBK and Vero cells were infected in parallel with a dilution series of HSV-1 corresponding to the MOI indicated. Monolayers were stained 9 days after infection. In MDBK cells, even with infection with an MOI of 0.1, suppression of infection was evident by 9 days and cell growth had begun to reestablish the monolayer. d.p.i., days postinfection.
MDBK cell recovery during HSV-1 infection.
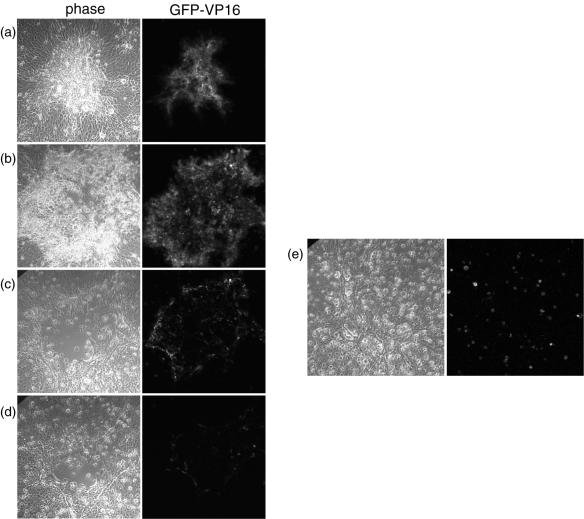
To monitor virus infection in live cells tracking the progression of individual plaques over time we used HSV-1 [V44], which expresses GFP fused to the tegument protein VP16. After infection with approximately 2,000 PFU, isolated individual cells expressing VP16-GFP could be detected by approximately 12 h, expanding into foci of 6 to 12 VP16-GFP-expressing cells by 18 h. By 24 to 36 h, small autofluorescent plaques of rounded cells had developed. To monitor the fate of single plaques, individual cells or small foci were identified at approximately 12 h, labeled by using a marker objective, and then monitored for up to 12 days. The results of a typical plaque progression are shown in Fig. 4. The focus containing numerous VP16-GFP-expressing cells had developed by 24 to 36 h (Fig. 4a). By 4 days (Fig. 4b), an increase in plaque size was observed, with more intense green cells more towards the periphery and rounded green cells beginning to thin out in the middle of the plaque. Two days later (6 days) (Fig. 4c), a noticeable change was observed. While there was significant cell loss in the center of the plaque, there was no increase in plaque size. Moreover, the expression of GFP-VP16 was much less intense and restricted to a ring that was 1 or 2 cells deep at the outside edge. By 10 days, a clear decrease in plaque size could be now seen (Fig. 4d). Expression of GFP-VP16 had by this time virtually disappeared, with cells now growing inward from the previous green fluorescent boundary. Thus, using VP16-GFP as a marker, virus gene expression appears to have been suppressed by between 4 and 6 days, with apparently healthy cells dividing or growing back to fill the central area resulting from earlier cell loss. This new cell growth likely explains the change in plaque morphology mentioned above. With time, a relatively healthy monolayer grew back to near confluence and GFP-VP16 was not detectable.
FIG. 4.
Plaque progression in MDBK cells infected with HSV-1 [V44]. MDBK cells grown in a six-well dish were infected with 2,000 PFU of HSV-1 [44]. Single plaques were ringed at 24 h postinfection with a marker objective, and plaque progression in individual plaques was monitored every day for 12 days by confocal microscopy on live cells. Typical results are shown. Images were taken at 2 (a), 4 (b), 6 (c), and 10 (d) days after infection, with the same microscope settings for imaging by phase contrast and by GFP fluorescence. After 10 days, the medium in the dish was replaced with fresh medium, and the cells were imaged 5 days later (e).
We conclude that while the specific infectivity of HSV in MDBK cells was identical to that in Vero cells and the early stages of infection and cell destruction (plaque formation) were similar, a progressive suppression of infection in MDBK cells ensued. The decrease in plaque size equates to cell growth into the original plaque, with complete recovery of the monolayer frequently observed.
One explanation for this behavior and outcome of virus-host interaction would be the production of an inhibitory substance into the medium of infected cultures. We reasoned that the greater the initial inoculum, the greater would be the initial production of this substance. Hence, plaque size would be inversely proportional to plaque number. We further reasoned that while infection could progress for an initial period, the ensuing production of this inhibitor from additional infected cells would eventually establish a profound refractory condition on uninfected cells, such that infection would die out. To provide evidence for this and also investigate whether virus remained in the infected monolayer in some recoverable form, we replaced the media at 10 days after infection with HSV [V44], which is when the VP16-GFP expression was no longer detectable and the monolayer had started the recovery phase. After medium replacement, the monolayer was monitored for GFP-VP16 expression. Approximately 5 days after the medium was replaced, individual green spots, indicative of GFP expression from single cells, were now detectable in the monolayer (Fig. 4e).
IFN production in response to HSV-1 infection in MDBK cells.
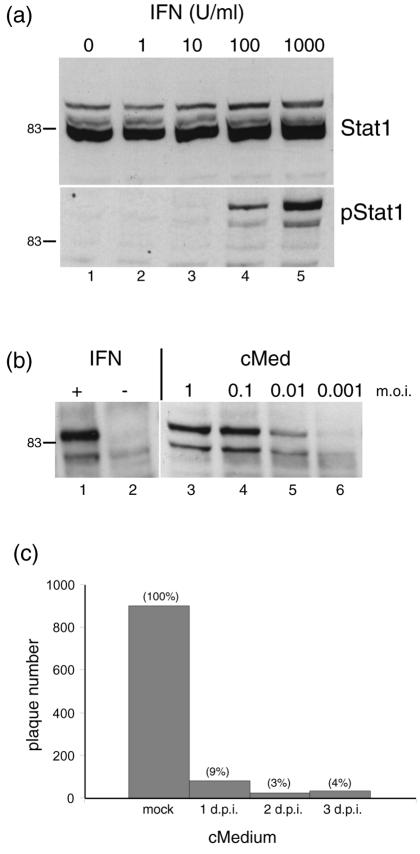
These results were consistent with the proposal that infection was suppressed by a constituent present within the media, and one obvious candidate for this component was IFN-α/β. We wished to examine the presence of physiological levels of functional IFN in the medium of infected cultures (as opposed to measuring expression by transcript analysis, see Discussion). No characterized antibodies to bovine IFN were readily available, and therefore, we used the phosphorylation of Stat1 at Tyr701 as a readily accepted surrogate marker for the presence of IFN (42). Conditioned medium from infected cells was applied to uninfected cells, and phosphorylation of Stat1 was assayed by Western blot analysis with a phosphospecific antibody. We first verified whether MDBK cells could be stimulated by treatment with commercial IFN-α. MDBK cells were treated for 1 h with increasing concentrations of IFN-α (1 to 1,000 U/ml), the cells were lysed, and extracts were probed for total Stat1 and phospho-Stat1 (pStat1). The total amount of Stat1 did not alter significantly (Fig. 5a, upper panel), whereas treatment with between 10 and 100 U/ml resulted in detectable phosphorylation of Stat1, visible as a doublet in Western blot analysis (Fig. 5a, lower panel).
FIG. 5.
Production of IFN in MDBK cells. (a) MDBK cells were treated with medium containing increasing doses of standard uIFN-α (as indicated) and harvested 1 h later. Cell aliquots were then separated by SDS-PAGE and probed by Western blotting for total Stat1 (upper panel) and pStat1 (lower panel). (b) MDBK cells were infected with HSV-1 [17] at the dilutions indicated, medium was harvested 24 h later, and virus was removed by using sterile polyvinylidene difluoride syringe filters and the media were then applied to a fresh uninfected MDBK monolayer. These cells were harvested after 1 h and probed for pStat1 (lanes 3 to 6). Lanes 1 and 2, treatment with uIFN as a control. +, present; −, absent. (c) MDBK cells were mock infected or infected with 7,000 PFU of HSV-1, and conditioned medium was harvested at 1, 2, or 3 days after infection, filtered, and applied to a fresh MDBK monolayer. Twelve hours later, the conditioned medium was removed and the cells were infected with 1,000 PFU of HSV-1 [17]. Plaques were counted 3 days later. Plaque formation after application of the conditioned medium (cMed, cMedium) is indicated as a percentage relative to that of the control medium (taken as 100%). d.p.i., days postinfection.
We next sampled medium at approximately 22 h after infection of cells with a range of MOI of approximately 1, 0.1, 0.01, and 0.001 corresponding to 2 × 106, 2 × 105, 2 × 104, and 2,000 PFU, respectively, in each dish. We then used the conditioned medium to treat fresh, uninfected MDBK monolayers. Note that the medium was filtered to remove any virus that may have been carried over in the medium. Complete removal of virus was confirmed. The medium (or control medium from mock-infected cells) was applied for 1 h, after which time the target cells were harvested and assayed for pStat1 (Fig. 5b). Medium from cells infected at MOI of 1, 0.1, and 0.01 induced the phosphorylation of Stat1 (lanes 2 to 5), showing the typical doublet observed in the positive control treated with uIFN (lane 1). No detectable induction was observed in this experiment with medium from cells infected with 2,000 PFU (MOI, 0.001) and harvested at 22 h (see below).
To further investigate the production of IFN during MDBK infection and relate this to the suppression of HSV replication, conditioned medium was applied to uninfected cells prior to infection to perform a plaque reduction assay. Cells were mock infected or infected with 7,000 PFU (MOI, 0.04), and medium from these cells was harvested at 1, 2, or 3 days and applied to fresh MDBK uninfected monolayers for 12 h. The treatment was performed in parallel with medium from mock-infected cells or, as a positive control, with serial dilutions of uIFN. The test monolayers were then inoculated with 1,000 PFU of HSV-1, and plaques were counted at 3 days postinfection. Conditioned medium taken at 2 or 3 days after infection showed a pronounced inhibition of plaque formation (Fig. 5c), with residual plaque numbers at 3 to 4% of the controls. No effect was observed in the control with conditioned medium from mock-infected cells. These results reinforce the observation of IFN production as assessed by pStat1 induction. While other factors may be contributory (see below), the simplest explanation is that infection in MDBK cells causes the production of bovine IFN (or a pStat1 signaling component), which has a profound inhibitory effect on HSV infection. For the sake of clarity, we use the term IFN for the pStat1-inducing activity while accepting that other cytokines could be responsible for, or contribute to, the stimulation. However, we have demonstrated that bovine IFN-β is induced during HSV infection of MDBK cells by reverse transcription (RT)-PCR and that conditioned medium inhibits encephalomyocarditis virus replication by a standard assay (data not shown).
IFN produced from MDBK cells is species specific.
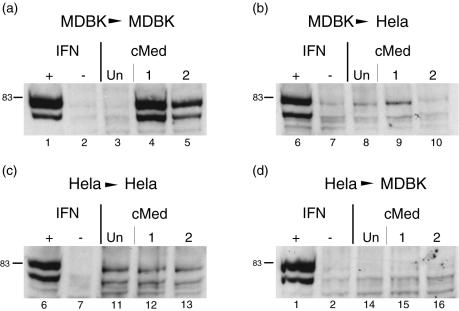
We next wished to address whether the induction of IFN observed in MDBK cells could also be observed in other cell types and examined in parallel whether conditioned media from MDBK cells could activate an antiviral response in heterologous cell types. We infected HeLa and MDBK cells with 7,000 or 700 PFU of HSV-1 per well and harvested the media after 2 days. After removal of any virus by filtration, medium was applied to fresh homologous MDBK cells or to HeLa cells. In parallel, both cell types were treated with uIFN as a positive control and with medium from mock-infected cells as a negative control. Medium was applied for 1 h as before, and the cells were then analyzed for the induction of pStat1 (Fig. 6). As shown above, medium from HSV-infected MDBK cells induced Stat1 phosphorylation in MDBK cells (Fig. 6a, lanes 4 and 5). However, when the same medium was used to treat HeLa cells, little significant induction of Stat1 phosphorylation could be detected (Fig. 6b, lanes 8 to 10), even though these cells were responsive to IFN signaling with standard uIFN (Fig. 6b, lanes 6 and 7). Surprisingly, in parallel, when medium from HSV-infected HeLa cells was used on homologous HeLa cells, no induction of pStat1 could be observed (Fig. 6c, lanes 11 to 13). Again in positive controls, HeLa cells were responsive to IFN signaling with standard uIFN. As expected from the lack of response in HeLa cells, conditioned media from HeLa cells had no effect on MDBK cells (Fig. 6d). From these results, we conclude first that the IFN induced from infected MDBK cells is unable to induce the phosphorylation of Stat1 in human HeLa cells (despite their competence to respond to human IFN). Second, accounting for the difference in MDBK and HeLa cells, it appears that HSV either does not induce IFN in HeLa cells or is able to effectively block (at any of a number of possible levels) IFN production and secretion into the medium. In MDBK cells, IFN is obviously induced and secreted.
FIG. 6.
Analysis of IFN signaling from conditioned medium (cMed) in homologous and heterologous cells. HeLa and MDBK cells were mock infected (Un) or infected and with 7,000 and 700 PFU of HSV-1 per well (1 and 2 in each set of assays). Medium from these cells was harvested 2 days after infection and applied to uninfected homologous and heterologous cells as indicated. Cells were harvested 1 h later and lysed, and cell aliquots were probed for the induction of pStat1. (a) MDBK > MDBK. Conditioned medium samples from uninfected MDBK cells (lane 3) or HSV-infected MDBK cells (lanes 4 and 5) were applied to fresh uninfected MDBK cells, and these cells were assayed 1 h later for pStat1. In each case, cells were also treated or untreated with 1,000 U of uIFN/ml as positive and negative controls (lanes 1 and 2, respectively). (b) MDBK > HeLa. Medium samples from uninfected MDBK cells (lane 8) or HSV-infected MDBK cells (lanes 9 and 10) were applied to fresh uninfected HeLa cells in parallel with positive and negative controls with uIFN (lanes 6 and 7). (c) HeLa > HeLa. Medium samples from uninfected (lane 11) or HSV-infected HeLa cells (lanes 12 and 13) were applied to fresh HeLa monolayers. (d) HeLa > MDBK. Medium samples from uninfected (lane 14) or HSV-infected HeLa cells (lanes 15 and 16) were applied to fresh MDBK monolayers. +, present; −, absent.
Temporal analysis of IFN production.
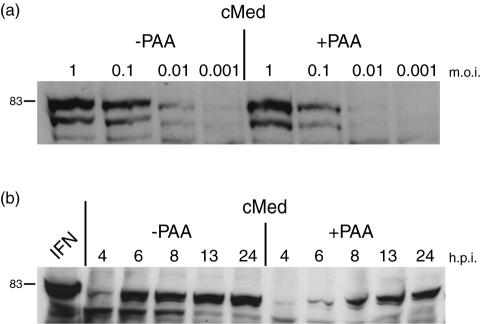
To further characterize aspects of the induction of IFN in MDBK cells, we analyzed the time course of accumulation of IFN in medium after infection in the presence or absence of phosphonoacetic acid (PAA, 400 μg/ml) to inhibit DNA replication and the production of progeny virus (Fig. 7a). First, we tested the effect of PAA on the production of IFN 24 h after infection by using a series of virus doses in the inoculum. Conditioned medium was harvested and applied to uninfected cells to test the induction of pStat1. In the absence of PAA, the induction of pStat1 was observed as before with an MOI of 0.01, decreasing to barely detectable levels at 0.001 (2,000 PFU). In the presence of PAA, the induction of pStat1 was diminished but was observed readily at an MOI of 0.1 and, while quite low, was above the background level at 0.01. This result indicated that some amplification of virus was required to induce IFN to levels detectable by this assay, i.e., assaying actual IFN in the medium by its ability to stimulate Stat1 phosphorylation in naive cells. Nevertheless, IFN induction could be observed with between 1 in 10 to 1 in 100 of the cells initially infected.
FIG. 7.
IFN production in MDBK cells in the absence of DNA replication. (a) MDBK cells were infected with HSV-1 [17] at the MOI indicated in the presence (+) or absence (−) of 400 μg of PAA/ml. Conditioned medium (cMed) was harvested 24 h later, filtered to remove any residual virus, and used to treat uninfected MDBK cells. These cells were harvested 1 h later, and the induction of pStat was assayed as before. (b) MDBK cells were infected with HSV-1 at a high MOI (10 PFU/cell) in the presence or absence of PAA, and medium was harvested at the times indicated. Conditioned medium was filtered and applied to uninfected cells, and the induction of pStat1 was assayed as before. Maximum levels of IFN were observed by 6 h after infection in the absence of PAA. In the presence of PAA, IFN production was stimulated but was delayed significantly.
We next examined IFN production under conditions where every cell was infected. Cells were infected at an MOI of 10, in the presence or absence of PAA, and conditioned medium was harvested at 4, 6, 8, 13, and 24 h after infection and tested for the induction of pStat1 in target cells (Fig. 7b). In the absence of PAA, IFN was detected in the medium between 4 and 6 h after infection, with comparatively little increase thereafter. In the presence of PAA, there was a pronounced delay in IFN production, with only a modest amount seen at 6 h, increasing continuously to maximal levels at 24 h. This result indicates that, while DNA replication and late virus gene expression are not absolutely required for IFN stimulation, late events augment IFN production even within the first 6 h of infection. Correspondingly, the results in the presence of PAA also indicate that even when all cells were infected, with infection progressing to immediate-early and early expression, it was not until 6 to 8 h that significant levels of functional IFN capable of inducing Stat1 phosphorylation were detected in the medium.
Suppression of Stat1 in MDBK cells alters the progression of infection.
IFN production is one of the main innate antiviral responses mounted by infected cells, and viruses have in turn evolved diverse mechanisms to overcome IFN-mediated pathways. It has been demonstrated that the V protein from the paramyxovirus SV5 eliminates the IFN cascade by targeting Stat1 for proteasome-mediated degradation (1, 6). This activity has been exploited with the establishment of cell lines constitutively expressing the V protein, resulting in the reduction or absence of Stat1, lack of IFN-induced Stat1 phosphorylation, and alteration of the outcome of SV5 infection in that cell type (6).
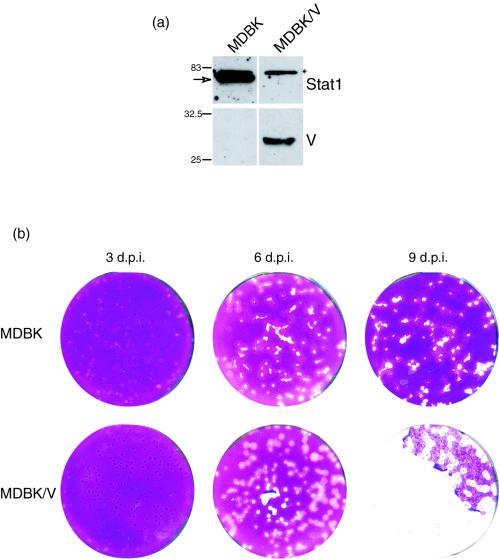
To pursue our proposal that the IFN pathway was a main factor in determining the nature of the outcome of HSV-1 infection in MDBK cells, we therefore established MDBK cells expressing the SV5 V protein and compared the progression of infection with that in the parental cells. MDBK cells were transfected with an expression plasmid for SV5 V protein together with a selectable marker. Independent lines were established, and expression of the V protein was confirmed by Western blotting and by immunofluorescence. In the line (MDBK/V) expressing the V protein, Stat1 was reduced to below detectable levels (Fig. 8a). Our results are consistent with expectation from the previous data showing a virtual quantitative loss of Stat1 in the presence of V protein in human cells (6). As expected, no induction of Stat1 phosphorylation was detected in MDBK/V cells treated with IFN (data not shown).
FIG. 8.
Establishment of MDBK cells expressing the V protein and lacking Stat1. MDBK cells expressing the SV5 V protein were established as described in Materials and Methods. (a) Samples of the parental MDBK line and the V expressing line, MDBK/V, were separated by SDS-PAGE and probed for expression of Stat1 and the V protein as indicated. Constitutive expression of the 27-kDa V protein was observed in the MDBK/V cells which correspondingly lacked Stat1. Note that the band migrating at ca. 83 kDa (indicated by an asterisk) in both lines is a cross-reacting band which migrates just above Stat1. (b) Comparison of plaque progression in MDBK and MDBK/V cells. Monolayers were infected with approximately 200 PFU of HSV-1 [17] per dish, fixed, and stained 3, 6, and 9 days later. The upper panel corresponds to MDBK cells, and the lower panel corresponds to MDBK/V cells. A significant difference in plaque formation and the outcome of infection was observed, with progression and monolayer destruction in MDBK/V cells, as opposed to regression in the parental MDBK cells. d.p.i., days postinfection.
We next compared plaque formation in these cells in parallel with that in the parental MDBK cells (Fig. 8b). By 3 days after infection, little difference in plaque number or size was observed between MDBK and MDBK/V cells, and if anything, plaques in the MDBK/V cells were slightly smaller. However, by 6 days, a clear difference was observed. Plaques in infected MDBK/V cells were more rounded and had increased significantly in size while those in the MDBK cells were now smaller than those in the MDBK/V cells and more irregular in size. By 9 days, a profound difference in the outcome of infection was apparent. Little progression was evident in MDBK cells, with smaller plaques and cell growth of the monolayer. In contrast in MDBK/V cells, plaques had continued to increase in size and there was now extensive destruction of the monolayer. These results provide strong evidence that the progressive repression of infection and cell recovery in MDBK cells is mediated by IFN and the Stat signaling pathway.
Prolonged IFN production during suppressed infection in MDBK cells.
As shown above (Fig. 3 to 4), HSV-1 expression and replication was suppressed from approximately 4 to 6 days onward, resulting in the absence of GFP-VP16 expression between 8 and 12 days. However, upon replenishment with fresh media, individual cells expressing VP16 began to appear.
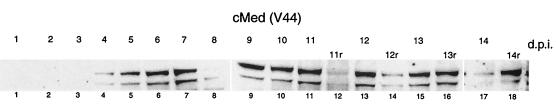
Therefore, we wished to address the question of whether IFN was still present in the medium at times when VP16 expression was no longer detectable. We monitored IFN levels in conditioned medium for up to 14 days after infection (2,000 PFU) and also examined the effect of medium replacement (Fig. 9). In this experiment, the production of IFN was slightly delayed, with levels sufficient to induce Stat1 phosphorylation in naive cells appearing by 4 days. Thereafter, there was a progressive increase to a maximum at about 9 days. In parallel, GFP-VP16 expression had been reduced to undetectable levels, yet IFN levels were maintained at least until 13 days. In a parallel set of infected cells, the medium was replaced with fresh medium at 11 days and harvested at various time intervals thereafter (2 h and 1, 2, and 3 days). These samples correspond to 11r, 12r, 13r, and 14r, respectively (Fig. 9). As expected, IFN levels dropped immediately upon medium reversal (11r), but production resumed and accumulated over the next 2 to 3 days to levels seen before the reversal. These results indicate first that, at times after strong suppression of virus infection, IFN levels remained high in the medium and second that, upon medium reversal, active IFN production resumed, possibly due to the accompanying early reinitiation of virus gene expression (see Discussion).
FIG. 9.
Long-term time course of IFN production in infected MDBK cells. MDBK cells were infected with 2,000 PFU of HSV-1 [V44] per 60-mm-diameter dish. The medium was harvested every day for 14 days and filtered. In parallel, in a set of infected cells, medium was removed at day 11 and replaced with fresh medium, which was then harvested at 11, 12, 13, and 14 days (11r, 12r, 13r, and14r). The conditioned medium (cMed) samples harvested each day without any replacement (lanes 1 to 11 and 13, 15, and 17) and conditioned medium samples replaced at day 11 and harvested at 11, 12, 13, and 14 days (lanes 12, 14, 16, and 18) were then applied to fresh uninfected MDBK cells for 1 h and assayed for Stat1 phosphorylation as before. d.p.i., days postinfection.
DISCUSSION
Much of our understanding of the replication of HSV comes from studies with tissue culture where the virus normally undergoes a productive cycle of replication resulting in cell destruction and virus production. In vivo, after acute-stage productive infection, HSV undergoes a nonproductive infection in neurons resulting in latency. Periodic reactivation occurs whereby HSV undergoes a productive infection and is transported back to surface sites where it may undergo further rounds of productive replication (15). Normally, wt HSV is cytocidal in culture. However, early reports on HSV replication in culture described certain situations, including T cells and MDBK cells, wherein a population of cells survives infection, allowing the maintenance of the virus in culture (4, 7, 18, 26). Furthermore, various systems, usually involving the use of virus mutants defective in several functions and/or the use of inhibitors, e.g., acycloguanosine or cycloheximide, have been pursued to recapitulate aspects of the cyclical pathway of productive infection, repression, and reactivation (20, 34, 35, 38, 41, 44, 45, 47). Here we expand on the characterization of an unusual pattern of infection with wt HSV-1 in MDBK cells, which results in the progressive suppression of replication, cell recovery, and regrowth of infected monolayers and in the maintenance of virus. We demonstrate that HSV does not block IFN expression and secretion in these cells and that the outcome is controlled by the IFN signaling system (or a secreted component which functions through Stat signaling). The results have relevance for understanding the nature of the events inducing and blocking IFN production and intercellular signaling. They also have relevance for understanding the basis of multiplicity-dependent phenotypes of viral mutants and have implications in other areas, including latency, persistence, and the appropriateness of animal models of pathogenesis.
Induction of IFN by HSV.
Work from several laboratories examining the induction of IFN and ISGs has indicated that HSV infection normally triggers a cellular response but then effectively suppresses it. While IFN signaling certainly influences the outcome of infection in murine models of pathogenesis (24), in culture, HSV is normally thought of as being relatively resistant to IFN and the IFN-mediated antiviral state. During wt infection in the absence of inhibitors of virus gene expression, little induction of IFN or ISGs was observed in human or primate cells, such as Vero or human embryonic lung cells (8, 19, 28, 31). Induction of IFN and ISGs is observed during infection in the presence of cycloheximide (31), with UV-inactivated virus (29), or with multiply defective viruses which express no immediate genes (8). Thus, the process of entry and/or components of the virion trigger IFN production and ISG expression. However, we show that wt HSV efficiently stimulates IFN production in MDBK cells (despite normal virus replication if most cells were initially infected) and that this production dictates a profound and progressive suppression of replication, even of wt HSV.
Two not mutually exclusive explanations are likely to be involved. It may be that HSV triggers IFN production in MDBK cells much more potently than in other cell types or that the initial triggering is similar but HSV products do not subsequently function to suppress IFN production as infection progresses, or it may be a combination of both aspects. We note here an important point in our determination of IFN production. Our measurements of IFN are based upon conditioned medium inducing the phosphorylation of Stat1 at Y701. The lack of available antibodies to bovine IFN prevented direct assay of IFN production in the medium. However, Stat1 phosphorylation is a known and accepted surrogate for measurements of IFN, which we compared in initial bioassays. We were interested, in any case, in measuring the production of IFN at physiologically relevant levels able to induce signaling in naive cells in a paracrine manner. This requires the stimulation of IFN expression, its synthesis, and its secretion. Previous studies have examined the transcription of IFN and/or ISGs by transcript analysis (Northern, RT-PCR, or microarray) and have clearly demonstrated that the very early aspects of the infection process and/or virion components induce IFN transcription. However, these studies are frequently performed at high MOI, and in one analysis using mutant HSV defective in immediate-early gene expression, high MOI of more that 10 PFU/cell were required to observe a response (8). Here we show that MDBK cells appear to be very sensitive to infection, detecting IFN secreted into the medium after infection with between 0.01 and 0.1 PFU/cell, under conditions where second-round amplification was blocked. On the other hand, under conditions where every cell was infected, inhibition of DNA synthesis delayed and reduced IFN production. Therefore, it may be that while the induction of IFN by very early events does occur, it is by itself relatively weak in mediating the physiologically relevant event of secretion at levels which could trigger signaling in naive cells. It appears that later events are important triggers and that IFN induction and secretion in response to these events are not suppressed in MDBK cells. Indeed, from preliminary results with UV-inactivated virus, we were unable to detect IFN in conditioned medium of MDBK cells even when using high MOI. It will be interesting to examine whether IFN induction can be detected under these conditions by RT-PCR, in which case this would not appear to lead to sufficient IFN in the medium to induce detectable pStat1. If IFN induction is not detectable by RT-PCR, then some additional stimulus is likely to be involved in these cells. This system, where potent IFN production is the outcome, may help identify later key triggering events or components.
Blocking induction of IFN.
The other not mutually exclusive explanation for HSV induction of IFN in MDBK cells is, rather than triggering being exceptionally efficient, that HSV fails to block induction after the initial stimulus. One of the key virus-encoded components which has been shown to be involved in the suppression of IFN and ISG induction is ICP0 (8, 19, 28, 29). Indeed, using viruses expressing only ICP0 at the immediate-early stage, it appears that ICP0 is sufficient to block IFN induction in the right cell type (8). In addition, viruses lacking functional ICP0 are known to be hypersensitive to IFN (28). ICP0 is a ubiquitin E3 ligase (2, 10), reverses the SUMO modification of several key target proteins, and induces their degradation in a proteosome-dependent fashion (12, 13), but the link between these activities and the precise mechanism of IFN suppression remains unclear. It may therefore be that some aspect of ICP0 function is defective in MDBK cells. Recent data (not shown) indicates that ICP0 is made at comparable levels and with kinetics in MDBK cells similar to those of other cell types. Future analysis of specific differences in the localization, biochemical activities, interactions, and host cell modifications of ICP0 in MDBK cells may help identify which aspects of ICP0 are directly related to its role in subverting IFN induction.
However, particularly since later events in infection in MDBK cells may also be involved in triggering IFN production and secretion, it may also be that other virus functions, including for example ICP34.5, are defective in blocking these processes in MDBK cells.
MOI and kinetics of progression and suppression.
While we show recovery of MDBK monolayers infected with MOI of up to 0.01 to 0.1 clearly, if enough cells were infected initially, infection progressed and the cells were completely destroyed. When fewer cells were initially infected, the production of IFN from these cells induced a refractory state in uninfected cells. We note that infections at the boundaries of plaques are likely to be at high multiplicities. While such infection was progressively suppressed, a high-MOI infection in which all cells were infected from the outset resulted in replication and cell destruction. Presumably, the suppression in the plaque setting is because sufficient time has elapsed to allow the establishment of a potent antiviral response, whereas by definition, in an initial high-MOI infection, all cells are immediately infected. Thus, the greater the initial number of infected cells, the greater the IFN response from those initial infected cells and, likely, the greater the refractory response or increased numbers of refractory cells. The outcome therefore becomes a kinetic consideration, with the variables being the initial number of single infected cells (and thus the initial production of IFN), the rate of virus production from those cells, and the rate of establishment of the induced refractory state. We believe this consideration is important for our understanding of the multiplicity dependence of certain viral mutants. The multiplicity dependence of a mutation reflects the observation that a defect is not pronounced when all cells are infected but becomes apparent only when a subpopulation is infected, usually with a single initiating particle. The basis for multiplicity dependence has not been clarified. However, most explanations usually involve proposals of the candidate locus having a role in specific infectivity within a cell or the chances of success when a single particle infects, including, for example, the suggestion that multiple copies of infecting genomes titrate out repressors and overcome the lack of the function. We propose that multiplicity dependence of a particular genetic mutation may frequently reflect a role of the corresponding locus in suppressing a paracrine inhibitory pathway. Take an example in which a virus factor had no role in augmenting intracellular infectivity but had a role exclusively in suppressing extracellular signaling to uninfected cells. This role would only be evident under circumstances where not all cells were infected and multistep progression was being assayed. The role would not be necessary or evident under conditions where all cells were infected. The defect seen under low-multiplicity conditions then reflects not a role in specific infectivity per se but in suppressing communication of a repressive state to uninfected cells. By definition, to be evident, this role requires a low-multiplicity assay. It also requires a cell system in which the repressive effect can be mounted. Thus, if the cells were no longer able to mount a repressive response, then the role of the viral factor becomes dispensable and its mutation or loss has little effect, even at low multiplicity. Conversely, if the low-multiplicity-dependent phenotype of a mutation remains after paracrine effects can be discounted, then the locus would indeed have a role in intracellular specific infectivity. This model for multiplicity-dependent phenotypes accounts for several observations and also makes certain predictions. For example, ICP0 mutant viruses exhibit multiplicity dependence (14, 36, 43). Such mutants are also IFN hypersensitive (28). But the low-multiplicity-dependent phenotype of ICP0 mutants also varies considerably with cell type. The model predicts that this would correlate with variations in paracrine signaling. Vero cells, where loss of ICP0 function has relatively little effect, are known to be defective in IFN production (5, 9). On the other hand, ICP0 loss of function has a profound effect in human embryonic lung cells, and the prediction would be that these cells mount a potent paracrine response. Moreover, in other cells, e.g., U2OS cells, ICP0 is thought to be completely dispensable, although this is usually attributed to an ICP0-like cellular function complementing the defect (48). If these cells mounted an IFN response, such a conclusion would be warranted and ICP0 would have a role in intracellular specific infectivity. However, a defect in key paracrine pathways in U2OS cells may also account for ICP0 independence. On the other hand, previous work has indicated that induction of IFN-responsive gene expression does not strictly correlate with permissiveness for ICP0 mutant virus replication (33). Also, although IFN knocks down all immediate-early gene expression (30), ICP0-null mutants are not defective for immediate-early gene expression, even in low-MOI infections of restrictive cell lines (11). Further work is required to clarify these effects and to test the several predictions from our proposals for multiplicity dependence.
HSV persistence.
Finally, one interesting feature of the outcome of HSV infection in MDBK cells is maintenance of virus in some form which can be reactivated by the removal of IFN. Our present results in this system indicate that HSV can persist in a suppressed form for months, with the limiting factor likely being technical aspects of the culture conditions and health of the monolayer. Although we show progressive suppression of VP16-GFP expression, we presently do not know the extent, if any, of virus gene expression during this persistent state and, in preliminary experiments, we have not detected expression. This raises an interesting question about the nature of the maintenance of IFN, whether its expression is continuously and chronically stimulated, and what the nature of the stimulus is. Nor do we know the population status of cells which harbor virus that can be reactivated, but the use of GFP viruses for detailed single-cell analysis of live populations combined with infectious center or in situ analysis will help describe the dynamic nature of the virus-cell interaction on a quantitative basis. It will be interesting to examine how the virus is suppressed, whether the LAT locus is expressed (which seems unlikely), in what form the genome is maintained, and whether other routes, e.g., histone deacetylase inhibitors, will overcome suppression. We note that IFN signaling can be species specific in that IFN from one species may be unable to trigger the appropriate responses in cells from another species. Indeed, we show this for the substance produced in MDBK cells, which could clearly stimulate Stat1 phosphorylation in homologous cells but failed to do so in HeLa cells. Correspondingly, it is possible that the countermeasures which HSV has developed may also operate in a species-specific manner and that, for example, IFN induction and secretion is not blocked precisely because these are nonprimate cells. If this were the case, it would be a singular example highlighting the need for caution in interpreting the role of specific genes and the effect (or not) of their deletion in different animal models.
In conclusion, this study demonstrates that wt HSV can be suppressed in culture and maintained in some type of persistent form by a paracrine mechanism. Considering that the IFN system has been shown through the use of knockout mice to have a significant influence on the outcome of infection in vivo (24), this present system, particularly combined with live-cell analysis of GFP viruses, is likely to add an additional dimension to studies of suppressed HSV-cell interactions in culture.
Acknowledgments
We thank R. Randall for kindly providing plasmids and antibodies to us and Dan Bailey, Rick Randall, Steve Goodbourne, and John MacCauley for helpful discussions.
This work was supported by Marie Curie Cancer Care.
REFERENCES
- 1.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings, P. G., and R. J. Lakomy. 1981. Characterization of herpes simplex virus persistence in a human T lymphoblastoid cell line. Infect. Immun. 34:817-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desmyter, J., J. L. Melnick, and W. E. Rawls. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 2:955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn, J. E., W. J. Meinke, and J. Spizizewn. 1979. Further characterisation of herpes virus persistence. J. Gen. Virol. 43:467-472. [DOI] [PubMed] [Google Scholar]
- 8.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247-252. [DOI] [PubMed] [Google Scholar]
- 10.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett, R. D., C. M. Preston, and N. D. Stow. 1991. Functional and genetic analysis of the role of Vmw110 in herpes simplex virus replication, p. 49-76. In E. K. Wagner (ed.), Herpesvirus transcription and its regulation. CRC Press, Boca Raton, Fla.
- 15.Flint, S. J., L. W. Enquist, R. M. Krug, V. R. Racaniello, and A. M. Skalka. 2000. Principles of virology. ASM Press, Washington, D.C.
- 16.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 17.Greaves, R., and P. O'Hare. 1989. Separation of requirements for protein-DNA complex assembly from those for functional activity in the herpes simplex virus regulatory protein Vmw65. J. Virol. 63:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampar, B., and M. A. K. Burroughs. 1969. Mechanisms of persistent herpes simplex virus infection in vitro. J. Natl. Cancer Inst. 43:621-631. [PubMed] [Google Scholar]
- 19.Harle, P., B. Sainz, Jr., D. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology 293:295-304. [DOI] [PubMed] [Google Scholar]
- 20.Harris, R. A., and C. M. Preston. 1991. Establishment of latency in vitro by the herpes simplex virus type 1 mutant in1814. J. Gen. Virol. 72:907-913. [DOI] [PubMed] [Google Scholar]
- 21.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, T. J. 1985. Herpes simplex virus latency, p. 175-240. In B. Roizman (ed.), The herpesviruses. Plenum Press, New York, N.Y.
- 22a.La Boissière, S., A. Izeta, S. Malcomber, and P. O’Hare. 2004. Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J. Virol. 78:8002-8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leib, D. A. 2002. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269:171-185. [DOI] [PubMed] [Google Scholar]
- 24.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 26.Mannini-Palenzona, A., A. M. Bartoletti, L. Foa'-Tomasi, M. Baserga, M. Tognon, and R. Manservigi. 1985. Establishment and characterization of a persistent infection of MDBK cells with herpes simplex virus. Microbiologica 8:165-180. [PubMed] [Google Scholar]
- 27.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholl, M. J., and C. M. Preston. 1996. Inhibition of herpes simplex virus type 1 immediate-early gene expression by alpha interferon is not VP16 specific. J. Virol. 70:6336-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 32.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston, C. M., and J. Russell. 1991. Retention of nonlinear viral DNA during herpes simplex virus latency in vitro. Intervirology 32:69-75. [DOI] [PubMed] [Google Scholar]
- 35.Russell, J., N. D. Stow, E. C. Stow, and C. M. Preston. 1987. Herpes simplex virus genes involved in latency in vitro. J. Gen. Virol. 68:3009-3018. [DOI] [PubMed] [Google Scholar]
- 36.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sainz, B., Jr., and W. P. Halford. 2002. Alpha/beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76:11541-11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 72:3307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-581. [DOI] [PubMed] [Google Scholar]
- 41.Shiraki, K., and F. Rapp. 1989. Protein analysis of herpes simplex virus latency in vitro established with cycloheximide. Virology 172:346-349. [DOI] [PubMed] [Google Scholar]
- 42.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 43.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 44.Stuart-Jamieson, D. R., L. H. Robinson, J. I. Daksis, M. Nicholl, and C. M. Preston. 1995. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus type 1 Vmw65 mutants. J. Gen. Virol. 76:1417-1431. [DOI] [PubMed] [Google Scholar]
- 45.Wigdahl, B., A. C. Scheck, R. J. Ziegler, C. E. De, and F. Rapp. 1984. Analysis of the herpes simplex virus genome during in vitro latency in human diploid fibroblasts and rat sensory neurons. J. Virol. 49:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wildy, P., H. J. Field, and A. A. Nash. 1982. Classical herpes latency revisited, p. 133-167. In B. W. J. Mahy, A. C. Minson, and G. K. Darby (ed.), Synposium 33, Society for General Microbiology. Cambridge University Press, Cambridge, United Kingdom.
- 47.Wu, N., S. C. Watkins, P. A. Schaffer, and N. A. DeLuca. 1996. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J. Virol. 70:6358-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]