Loss of UDP-glucuronate 4-epimerases GAE1 and GAE6 in Arabidopsis thaliana causes a dramatic reduction of pectin in the cell wall and compromised immunity to Pseudomonas syringae and Botrytis cinerea.
Abstract
Plant cell walls are important barriers against microbial pathogens. Cell walls of Arabidopsis thaliana leaves contain three major types of polysaccharides: cellulose, various hemicelluloses, and pectins. UDP-d-galacturonic acid, the key building block of pectins, is produced from the precursor UDP-d-glucuronic acid by the action of glucuronate 4-epimerases (GAEs). Pseudomonas syringae pv maculicola ES4326 (Pma ES4326) repressed expression of GAE1 and GAE6 in Arabidopsis, and immunity to Pma ES4326 was compromised in gae6 and gae1 gae6 mutant plants. These plants had brittle leaves and cell walls of leaves had less galacturonic acid. Resistance to specific Botrytis cinerea isolates was also compromised in gae1 gae6 double mutant plants. Although oligogalacturonide (OG)-induced immune signaling was unaltered in gae1 gae6 mutant plants, immune signaling induced by a commercial pectinase, macerozyme, was reduced. Macerozyme treatment or infection with B. cinerea released less soluble uronic acid, likely reflecting fewer OGs, from gae1 gae6 cell walls than from wild-type Col-0. Although both OGs and macerozyme-induced immunity to B. cinerea in Col-0, only OGs also induced immunity in gae1 gae6. Pectin is thus an important contributor to plant immunity, and this is due at least in part to the induction of immune responses by soluble pectin, likely OGs, that are released during plant-pathogen interactions.
INTRODUCTION
The cell walls in Arabidopsis thaliana leaves are mainly primary cell walls consisting of three major types of polysaccharides—cellulose, various hemicelluloses, and various pectic polysaccharides—as well as some structural proteins (Liepman et al., 2010). Arabidopsis leaf walls contain ∼14% cellulose, a homopolymer of (1,4)-β linked d-glucose subunits (Zablackis et al., 1995; Carpita, 2011). Hemicelluloses are a diverse class of polysaccharides that includes xylans, xyloglucans, mannans, glucomannans, and mixed-linkage β-glucans (Scheller and Ulvskov, 2010). In Arabidopsis leaves, the major hemicellulose is xyloglucan, which constitutes ∼20% of the wall polysaccharides. Xyloglucan contains a (1,4)-β-linked glucan backbone substituted with (1,6)-α-linked xylosyl residues or side chains of xylosyl, galactosyl, and fucosyl residues (Zablackis et al., 1995; Liepman et al., 2010). Glucuronoarabinoxylan (4% of the wall) is also found in Arabidopsis leaves (Zablackis et al., 1995). Primary walls of dicotyledonous plants generally also contain 3 to 5% of the hemicelluloses mannan and glucomannan (Scheller and Ulvskov, 2010). Hence, hemicelluloses in primary walls of dicotyledonous plants are mainly composed of Glc, Xyl, Ara, Gal, and Man.
Pectins are a diverse group of polysaccharides that all contain galacturonic acid (GalA) and make up ∼50% of Arabidopsis leaf walls (Zablackis et al., 1995; Harholt et al., 2010). Homogalacturonan (HG) is a linear homopolymer of (1,4)-α-linked GalA residues and it makes up ∼65% of all pectin in Arabidopsis leaf walls (Zablackis et al., 1995; Mohnen, 2008). A linear (1,4)-α-linked GalA backbone substituted with single xylose residues is called xylogalacturonan, and a polymer with complex side chains containing borate ions and sugars such as Ara, Rha, Gal, Xyl, or Fuc is referred to as rhamnogalacturonan II (Mohnen, 2008; Harholt et al., 2010). Xylogalacturonan and rhamnogalacturonan II make up less than 10% of leaf cell wall pectin (Zandleven et al., 2007; Mohnen, 2008). In contrast, rhamnogalacturonan I backbones consist of a repeating α-1,4-d-GalA-α-1,2-l-Rha disaccharide and are substituted with β-(1,4)-galactan, branched arabinan, or arabinogalactan side chains (Mohnen, 2008; Harholt et al., 2010). RGI constitutes ∼20 to 25% of pectin in primary walls (Mohnen, 2008). Hence, pectin in Arabidopsis leaf cell walls consists mostly of GalA, Rha, and smaller amounts of other sugars, including Ara, Gal, Xyl, and Fuc.
In general, carbohydrate biosynthesis requires nucleotide sugars provided by nucleotide sugar interconversion pathways (Seifert, 2004). Most nucleotide sugars are synthesized from UDP-Glc. UDP-glucuronic acid is made from UDP-Glc by UDP-glucose dehydrogenase activity or via an alternative pathway requiring inositol oxygenase activity (Tenhaken and Thulke, 1996; Loewus and Murthy, 2000). UDP-d-glucuronate 4-epimerases (GAEs) interconvert UDP-d-GlcA and UDP-d-GalA, the monomeric precursor of pectin. There are six GAE genes encoded by the Arabidopsis genome. When heterologously expressed in Escherichia coli or Pichia pastori, GAE1, GAE4, and GAE6 showed activity in interconverting UDP-d-GlcA and UDP-d-GalA (Gu and Bar-Peled, 2004; Mølhøj et al., 2004; Usadel et al., 2004). GAE1 and GAE6 were found to be Golgi localized and strongly expressed in various plant tissues (Mølhøj et al., 2004; Usadel et al., 2004; Parsons et al., 2012). GAE1 and GAE6 have been hypothesized to be evolutionarily older than the other GAE family members (Usadel et al., 2004) and might have overlapping functions that are distinct from the other family members.
Plant cell wall composition and architecture affects wall strength and flexibility, and cell walls present a physical barrier to potential plant pathogens. Besides preformed physical barriers, such as a cell wall, plants have a sophisticated immune system to defend themselves against harmful microbial pathogens (Chisholm et al., 2006; Jones and Dangl, 2006). Immune signaling involves changes in phytohormone levels, the most important for plant immunity being salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) (Grant and Jones, 2009; Pieterse et al., 2012). Other major regulators of plant immunity include PHYTOALEXIN DEFICIENT4 (PAD4) and ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) (Wiermer et al., 2005). Immune signaling generally involves changes in the transcriptome that, in turn, lead to altered cellular responses (Tao et al., 2003). For instance, induced expression of the cytochrome P450 PAD3 is thought to increase production of the antimicrobial secondary metabolite camalexin (Zhou et al., 1999).
Because plant cell walls are important barriers against pathogenic microbes, alterations in wall structural properties can lead to changes in plant immunity. To date, only a few examples of cell wall-related mutants with altered pathogen phenotypes have been studied in detail. Mutants impaired in callose biosynthesis, powdery mildew resistant4 (pmr4), display enhanced resistance to the powdery mildew pathogens Golovinomyces orontii and Golovinomyces cichoracearum (Nishimura et al., 2003). These plants have constitutively high SA levels, and SA signaling is required for the resistance phenotype (Nishimura et al., 2003). Other plants with enhanced resistance to these powdery mildew pathogens include pmr5, a mutant enriched in pectin, and pmr6, a mutant with a defect in a pectate lyase-like gene (Vogel et al., 2002, 2004). Plants with mutations in cellulose synthases required for primary and secondary wall formation also show increased resistance to pathogens (Ellis and Turner, 2001; Hernández-Blanco et al., 2007). Enhanced resistance to powdery mildew and Pseudomonas syringae pv maculicola ES4326 (Pma ES4326) in constitutive expression of VSP 1 (cev1), which is mutated in the primary wall cellulose synthase CesA3, was linked to constitutive high JA and ET levels in these plants (Ellis and Turner, 2001; Ellis et al., 2002a). Interestingly, irregular xylem1 (irx1), irx3, and irx5 plants, which carry mutations in the secondary wall-associated cellulose synthases CesA8, CesA7, and CesA4, showed enhanced resistance to the necrotrophic pathogens Plectosphaerella cucumerina, Ralstonia solanacearum, and Botrytis cinerea as well as the biotrophic powdery mildew G. cichoracearum, whereas growth of P. cucumerina and R. solanacearum pathogens was unaltered in pmr5, pmr6, and cev1 plants (Hernández-Blanco et al., 2007). This enhanced resistance of the irx mutants was independent of SA, JA, or ET signaling, but instead involved abscisic acid signaling (Hernández-Blanco et al., 2007). Changes in wall structure are likely monitored by the plants and effects may be counteracted through initiation of defense responses that can lead to altered phytohormone levels.
Although plant cell walls serve as barriers against pathogen entry, many pathogens secrete cell wall-degrading enzymes and thus are able to successfully infect their host plants (Albersheim et al., 1969). For example, the B. cinerea polygalacturonase Bc-PG1 is required for full virulence of this pathogen, and the gene encoding it shows evidence for diversifying selection, as would be expected for a virulence gene (ten Have et al., 1998; Rowe and Kliebenstein, 2007). Secretion of cell wall-degrading enzymes can trigger the release of cell wall fragments, which can act as damage-associated molecular patterns that activate plant immune responses (Hahn et al., 1981; Ferrari et al., 2013). Similarly, pectin fragments known as oligogalacturonides (OGs) can trigger plant immune responses (Côté and Hahn, 1994; Ferrari et al., 2013). Such responses include the production of reactive oxygen species (ROS), activation of mitogen-activated protein kinase (MAPK) activation and enhanced expression of defense-related genes such as PAD3 (Ferrari et al., 2007; Denoux et al., 2008; Galletti et al., 2008; Galletti et al., 2011). Some of these responses require SA, JA and ET, whereas others are independent of these phytohormones (Ferrari et al., 2007).
Here, we describe two GAE family members, GAE1 and GAE6, whose expression is repressed upon pathogen challenge. A gae1 gae6 double mutant has brittle leaves and its walls are reduced in pectin, specifically HG and likely RGI. Resistance to the bacterial pathogen Pma ES4326 and the fungal necrotroph B. cinerea is compromised in gae1 gae6. Callose deposition is reduced in gae1 gae6 plants after treatment with B. cinerea isolate Gallo 1. Furthermore, these plants show altered activation of immune signaling in response to treatment with macerozyme, a polygalacturonase that degrades pectin, and they also are hyperresponsive to JA signaling. Macerozyme treatment released less soluble, likely low molecular weight pectin from isolated gae1 gae6 cell walls than from Col-0 cell walls. B. cinerea-induced accumulation of soluble, likely low molecular weight pectin was also decreased in gae1 gae6 plants. Additionally, gae1 gae6 plants are impaired in macerozyme-induced immunity to B. cinerea, possibly due to decreased release of OGs from their cell walls.
RESULTS
GAE1 and GAE6 Expression Is Repressed by Pma ES4326-Induced Immune Signaling
Our previous research suggested that the status of pectin, specifically pectin modification by methylesterification, is important for plant immunity against Pma ES4326 (Bethke et al., 2014). Here, we investigate whether pectin abundance, in addition to its specific structure, is also important for plant immunity by taking a closer look at the formation of the pectin precursor UDP-GalA by GAEs.
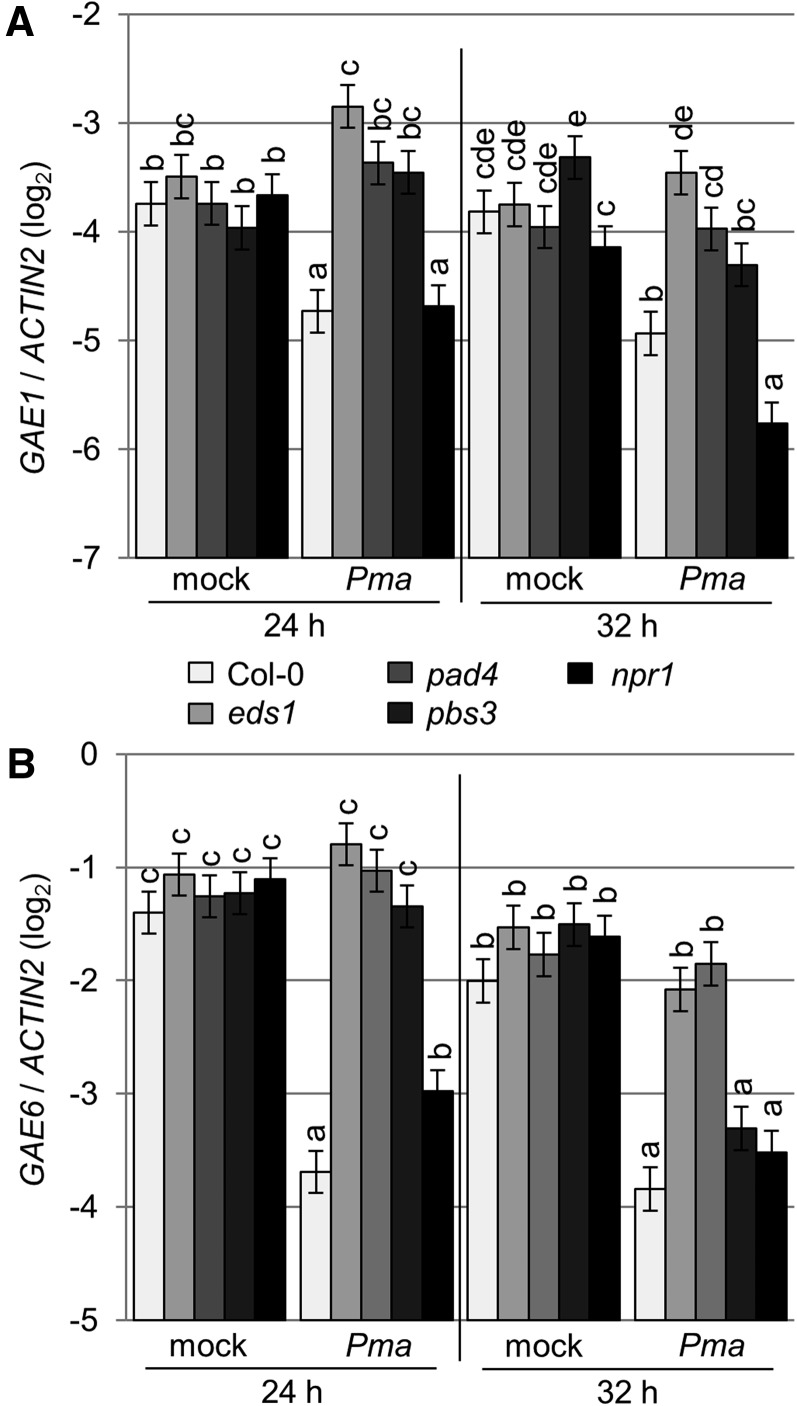
Microarray experiments showed that expression of GAE1 and GAE6 was repressed in Arabidopsis plants treated with Pma ES4326 (Wang et al., 2008). Repression of GAE1 and GAE6 in wild-type Arabidopsis Col-0 plants treated with Pma ES4326 was confirmed by qRT-PCR (Figure 1). To test if this repression is mediated by immune signaling, qRT-PCR was also performed in mutants with blocked immune signaling (Figure 1; Supplemental Figure 1). Generally, immunity to Pma ES4326 requires intact SA signaling. Repression of both GAE1 and GAE6 required the presence of the major immune regulators EDS1 and PAD4, required for SA signaling as well as other immune responses (Jirage et al., 1999; Wiermer et al., 2005; Bartsch et al., 2006; Wang et al., 2008) at both early and late time points. The presence of AVRPPHB SUSCEPTIBLE3 (PBS3), a regulator of many immune responses including SA signaling, (Nobuta et al., 2007), was also required for repression of GAE1 and GAE6 at an early time point (Figure 1). CALMODULIN BINDING PROTEIN 60-LIKE a (CBP60a) (a negative regulator of SA synthesis and immunity; Truman et al., 2013), CBP60g, SYSTEMIC ACQUIRED RESISTANCE DEFICIENT1 (SARD1) (both promote SA biosynthesis and regulation of other immune responses; Wang et al., 2011), SALICYLIC ACID INDUCTION DEFICIENT2 (SID2) encodes a biosynthetic enzyme for SA; Wildermuth et al., 2001), and NONEXPRESSER OF PR GENES1 (a regulator of the majority of SA-induced immune signaling; Cao et al., 1997; Yan and Dong, 2014) were not required for this repression (Figure 1; Supplemental Figure 1). Thus, repression of GAE1 and GAE6 requires a regulatory function that is dependent on EDS1 and PAD4, partially dependent on PBS3, but independent of SA.
Figure 1.
Pma ES4326-Induced Repression of GAE1 and GAE6 Expression Requires PAD4, EDS1, and PBS3.
(A) Expression of GAE1 in leaves of wild-type Col-0 and mutant plants. Expression was measured 24 or 32 h after either inoculation with Pma ES4326 (OD600 = 0.002) or mock inoculation with 5 mM MgSO4 and was then normalized to the level of ACTIN2. Data from three biological replicates were combined using a mixed linear model. Bars represent mean log2 ratios to ACTIN2 ± se. Letters indicate significantly different groups with q < 0.05, where 0.05 represents the maximum false discovery rate at which the test may be called significant.
(B) Expression of GAE6 measured as described in (A).
There are six GAE family members in Arabidopsis. GAE2 expression was induced by Pma ES4326 in the absence of PAD4 or EDS1, GAE3 expression was unaltered, GAE4 was repressed early, and GAE5 expression was induced in all genotypes (Supplemental Figure 2). The similarity of the immune-regulated expression of GAE1 and GAE6 suggested a redundant role of these genes in Arabidopsis immunity to Pma ES4326.
Resistance to Pma ES4326 Is Compromised in gae1 gae6 and gae6 Plants
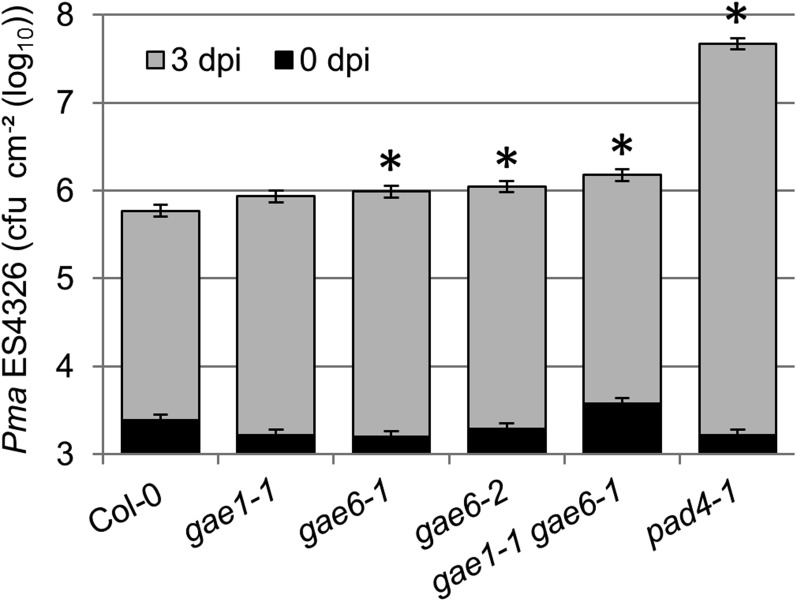
To study the roles of GAE1 and GAE6 in plant immunity in more detail, plants with T-DNA insertion mutations in GAE1 and GAE6 were obtained from the SALK collection as described in the Accession Numbers section in Methods. We identified two GAE6 knockout alleles, gae6-1 and gae6-2, and a T-DNA insertion in GAE1, gae1-1, that caused reduced expression of this gene (Supplemental Figure 3). A double mutant was created by crossing gae1-1 and gae6-1. Both gae6 and gae1 gae6 plants were more susceptible to Pma ES4326 than wild-type Col-0 and gae1 plants, supporting a role of GAE6 in plant immunity to Pma ES4326 (Figure 2).
Figure 2.
Susceptibility of gae6 and gae1 gae6 Plants to Pma ES4326 Is Increased Relative to Wild-Type Col-0.
Leaves of plants of the indicated genotypes were inoculated with Pma ES4326 (OD600 = 0.0002) as described in Methods. Bacterial titers in leaves were determined either immediately (0 dpi) or after 3 d (3 dpi). Bars represent the mean ± se of four independent experiments, each with 4 or 12 biological replicates at 0 and 3 dpi, respectively. Data were combined using a mixed linear model. Asterisks indicate significant differences from Col-0 wild type (q < 0.05). Susceptible pad4 plants were included as a positive control. cfu, colony-forming units.
gae6 and gae1 gae6 Plants Have Brittle Leaves
While inoculating leaves of gae1 gae6 plants, we noticed that the petioles and midribs of these plants tended to break easily when leaves were bent against the proximal-distal axis of the leaves (Figure 3; Supplemental Figures 4D to 4F). They tended to break less often when twisted around the proximal-distal axis. To quantify this phenotype, leaves were bent by 180° (Figure 3A) and breaking of midribs or petioles was recorded. Most gae1 gae6 leaves broke, whereas fewer leaves of gae6 plants and very few leaves of wild-type Col-0 and gae1 plants did (Figure 3B; Supplemental Figure 4C). Although gae1 gae6 plants had slightly shorter leaves and petioles than wild-type plants (Supplemental Figures 4A and 4B), no correlation between leaf size or petiole length and breaking frequency was detected. We also investigated tissue integrity by monitoring ion leakage. Interestingly, when leaves were inoculated with deionized water, leaf discs of gae1 gae6 leaked more ions than wild-type Col-0 discs (Figure 3C), suggesting that the increased brittleness is correlated with a structural defect that promotes increased ion leakage. Taken together, these results showed that loss of GAE6 causes brittle leaves and additional loss of GAE1 strongly enhances this phenotype. We conclude that GAE1 and GAE6 contribute to normal leaf flexibility.
Figure 3.
Mutant gae6 and gae1 gae6 Plants Have Brittle Leaves.
(A) Depiction of the assay for brittleness of gae mutant leaves. Fully expanded leaves of 4- to 5-week-old plants were bent by 180° so that the adaxial surface of the leaf was parallel with the soil. Broken leaf petioles or midribs were considered brittle.
(B) Quantification of broken midribs or petioles. For each genotype, 66 to 82 leaves were analyzed for brittleness as described in (A). Bars represent the ratio of broken to total number of leaves tested. Results were analyzed using Fisher’s exact test. Letters indicate significantly different groups at P < 0.05.
(C) Conductivity measurements. Leaves of 4-week-old plants were inoculated with deionized water and then leaf discs were cut out and placed on deionized water. Conductivity was measured at the indicated time points. After 30 min, the water was replaced with fresh water (30 min fresh weight) to account for wounding-related ion leakage from the edges of the leaf discs. Three independent experiments with six biological replicates each were performed and data were analyzed using a t test for each time point. P values were corrected using the Bonferroni method. Data show means ± se; asterisks indicate significant differences from wild-type Col-0 at q < 0.01.
Cell Walls of gae6 and gae1 gae6 Plants Have Lower Levels of Pectin
GalA is a major component of pectin. We analyzed wall monosaccharide composition in gae mutants to investigate whether the increased brittleness was associated with an altered wall composition of gae leaves. To approximate pectin content of gae mutant walls, the total uronic acid concentration was measured using a simple colorimetric assay (Filisetti-Cozzi and Carpita, 1991; van den Hoogen et al., 1998). Total uronic acid was reduced in gae6-2 and strongly reduced in gae1 gae6 plants but was indistinguishable from wild-type Col-0 in gae6-1 and gae1 (Supplemental Figure 5). This suggested that gae1 gae6 and possibly gae6 plants have reduced pectin.
To investigate the cell wall composition in leaves of gae plants in more detail, we measured the levels of seven neutral monosaccharides, the acidic sugars GalA (the monomeric subunit of the pectin backbone; Harholt et al., 2010) and GlcA (Figure 4). We also estimated the crystalline cellulose content (Updegraff, 1969). Cell walls of both gae6 mutants and gae1 gae6 were reduced in GalA (Figure 4A). In gae1 gae6, GalA content in cell walls was reduced by more than 40% compared with the wild-type level. GlcA levels were indistinguishable between mutants and the wild type (Figure 4B). Cellulose content was higher in gae6-2 and gae1 gae6 walls, likely due to the loss of pectins (Figure 4C). Cell walls of gae1 gae6 plants were also enriched in all neutral sugars measured, presumably due to the substantial proportional loss of GalA (Figure 4D). The levels of most wall monosaccharides were even higher in the gae1 gae6 double mutant with the exception of Rha, which did not exhibit a significant increase compared with the gae6 single mutants. The absence of a compensational increase in Rha suggested that RGI, the dominant Rha contributor to the wall, may be considered reduced in gae1 gae6 mutants compared with the wild type albeit not to such an extent as HG.
Figure 4.
Cell Walls of gae6 and gae1 gae6 Plants Are Reduced in GalA.
Levels of GalA (A), GlcA (B), cellulose (C), and neutral sugars (D) in AIR extracted from leaves of 4-week-old plants of the indicated genotypes. Bars represent means ± se of four biological replicates combined using a mixed linear model. Letters indicate significantly different groups (P < 0.05).
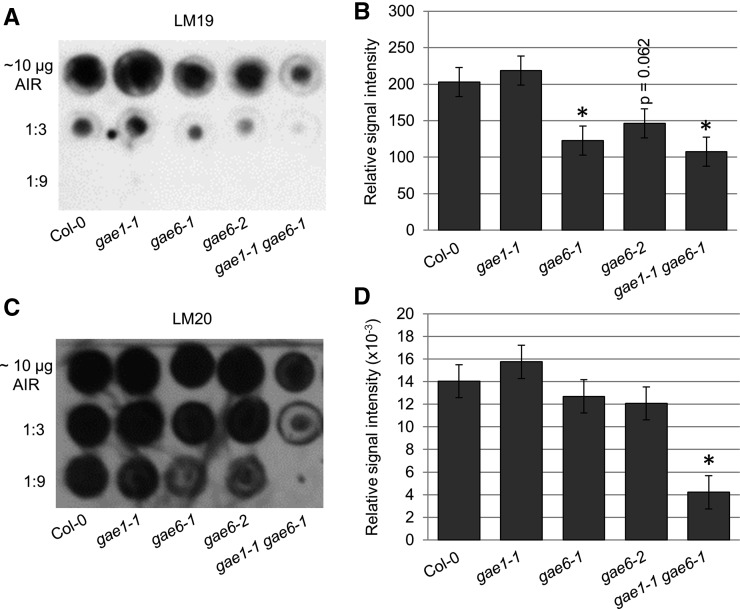
To further investigate which pectic components were altered in gae1 gae6, dot blot experiments using pectin isolated from the various genotypes were performed. Both LM19 and LM20 antibodies recognize HG (Verhertbruggen et al., 2009a). As expected, LM19 binding was reduced in gae6-1 and gae1 gae6 (Figures 5A and 5B) and LM20 binding was reduced in gae1 gae6 (Figures 5C and 5D). This result confirmed that gae1 gae6 cell walls are reduced in HG. Using the same samples, we detected no difference from the wild type in binding of LM5, which recognizes a tetramer of (1-4)-β-d-galactans found in RGI (Jones et al., 1997), LM6, which recognizes a linear pentasaccharide in (1-5)-α-l-arabinans found in RGI (Willats et al., 1998; Verhertbruggen et al., 2009b), LM8, which recognizes xylogalacturonan (Willats et al., 2004), or CCRC-M7, which recognizes an arabinosylated (1-6)-β-d-galactan epitope occurring on RGI (Puhlmann et al., 1994; Steffan et al., 1995; Pattathil et al., 2010) (Supplemental Figure 6). Collectively, these cell wall composition data show that gae1 gae6 plants are reduced in HG. Due to the strong proportional reduction of HG in the walls, levels of all other components should be higher. However, Rha indicative of RGI was not higher in gae1 gae6, suggesting that RGI may also be reduced in gae1 gae6 walls.
Figure 5.
Cell Walls of gae1 gae6 Plants Are Reduced in Homogalacturonan.
Dot blot analyses with anti-LM19 and anti-LM20 antibodies. Pectin was extracted from AIR prepared from the indicated genotypes using 1 μL of extraction buffer per 10 µg of AIR. Pectin was serially diluted and 1 μL of undiluted, 1:3 diluted, and 1:9 diluted samples were spotted on a nitrocellulose membrane. Three biological replicates were spotted twice to obtain two technical replicates each. All replicates produced similar results. Relative signal intensities were measured using Image J software. Measurements from the three biological replicates were combined using a linear model after Box-Cox power transformation as described in Methods. Bars represent means ± se. Asterisks indicate a difference from wild-type Col-0 at P < 0.05.
(A) Representative dot blot using anti-LM19 antibody.
(B) Relative signal intensities of the spots in the dot blot in (A).
(C) Representative dot blot using anti-LM20 antibody.
(D) Relative signal intensities of the LM20 dot blot shown in (C).
GAE1 and GAE6 Are Required for Immunity to Specific B. cinerea Isolates
We hypothesized that plant immunity to B. cinerea might be affected by pectin content in the host cell wall because this pathogen requires polygalacturonases and pectin methylesterases for full virulence (ten Have et al., 1998; Valette-Collet et al., 2003; Kars et al., 2005b; Espino et al., 2010). Because B. cinerea is genetically diverse and has a high diversity of polygalacturonases (Rowe and Kliebenstein, 2007), we measured relative fungal growth at both 2 and 3 d postinoculation (dpi) using 10 different B. cinerea isolates (Figure 6). The B. cinerea isolates Fresa 525 and Gallo 1 showed enhanced virulence on gae1 gae6 plants at both time points, whereas KT and UK Razz showed enhanced virulence in gae1 gae6 plants at 3 but not 2 dpi (Figure 6A). We did not detect any significant changes in relative fungal growth between wild-type Col-0 and gae1 gae6 plants when using Acacia, Apple 517, DN, Grape, Pepper, or Rasp isolates of B. cinerea. Therefore, we continued our study using Gallo 1 and Fresa 525. We also included Pepper for comparison, as this isolate has been used in a large-scale analysis of plant gene expression in response to B. cinerea infection (Windram et al., 2012).
Figure 6.
Growth of B. cinerea on gae1 gae6 Plants and the Effect of B. cinerea on Expression of GAE1 and GAE6.
(A) Growth of B. cinerea isolates. Leaves of Col-0 and gae1 gae6 plants were inoculated with 2.5 × 105 spores mL−1 of the B. cinerea isolates indicated in the figure. Samples were collected two (2 dpi) and three (3 dpi) days later. Bars represent means ± se of six biological replicates for each fungus, combined using a mixed linear model. Asterisks indicate significant differences from wild-type Col-0 for each isolate (P < 0.05).
(B) and (C) Expression of GAE1 (B) and GAE6 (C) 48 h after inoculation with the B. cinerea isolates indicated. Expression levels were measured by qRT-PCR. Bars represent mean log2 ratios to ACTIN2 ± se of three biological replicates, combined using a mixed linear model. Letters indicate significantly different groups (q < 0.05).
We next investigated whether expression of GAE1 and GAE6 was affected by challenge with B. cinerea. Inoculation of wild-type Col-0 plants with any isolate repressed GAE1 and GAE6 expression (Figures 6B and 6C), and this repression was generally independent of PAD4 (Figures 6B and 6C). Expression of GAE2 and GAE3 was unaltered by B. cinerea treatment. GAE4 was repressed only by Fresa 525 treatment, while GAE5 expression was enhanced with all three isolates tested (Supplemental Figure 7). As seen for infection with Pma ES4326, the expression of GAE1 and GAE6 was repressed upon B. cinerea treatment. This indicates a role for GAE1 and GAE6 in the immune response to B. cinerea.
Pectinase-Induced Immune Signaling Is Altered in gae1 gae6 Plants
Because the amount of pectin substrate for pathogen polygalacturonases is reduced, we hypothesized that immunity phenotypes of gae1 gae6 plants may result from reduced generation of OGs by these enzymes. In turn, the reduced OGs could lead to reduced immune responses. To test this, we used macerozyme, a commercial pectinase, as a proxy for pathogen pectinases. We isolated cell walls as a subcellular fraction known as alcohol-insoluble residue (AIR; Gille et al., 2009). This was treated with macerozyme and tested for release of soluble, likely low molecular weight, pectin fragments (Figure 7). Treatment with 0.25% macerozyme released significantly more uronic acid from wild-type Col-0 AIR relative to gae1 gae6 AIR (Figure 7B). To test activity of these pectin-containing solutions, we infiltrated them into wild-type Col-0 plants and measured PAD3 expression. PAD3 is required for synthesis of the phytoalexin camalexin (Zhou et al., 1999) and its expression is known to be induced by OG treatment (Ferrari et al., 2007). Fractions from macerozyme-treated Col-0 walls induced significantly more PAD3 expression than fractions derived from gae1 gae6 walls, suggesting that macerozyme treatment did indeed release more active soluble uronic acid, possibly OGs, from wild-type Col-0 walls than from gae1 gae6 walls (Figure 7C).
Figure 7.
Levels of Soluble Pectin and Expression of PAD3 in Fractions Produced Following Macerozyme Treatment of gae1 gae6 Cell Walls.
(A) Schematic overview of the experimental procedure for (B) and (C).
(B) Uronic acid measurements. AIR from leave tissue of 4-week-old wild-type Col-0 or gae1 gae6 plants was treated with water or 0.25% macerozyme and assayed for uronic acid levels. Data from 11 biological replicates were combined using a mixed linear model. Bars represent means ± se. Letters indicate significant differences (P < 0.05).
(C) Expression of PAD3 in Col-0 plants treated with the fractions from (B). Extracts from (B) were heat inactivated, pooled, and diluted 5-fold with water. Pooled extracts were inoculated into 4-week-old Col-0 plants. PAD3 expression was measured 3 h after inoculation and normalized to the level of ACTIN2 expression. Data from four biological replicates were combined using a mixed linear model. Bars represent mean log2 ratios to ACTIN2 ± se. Letters indicate significant differences (P < 0.05).
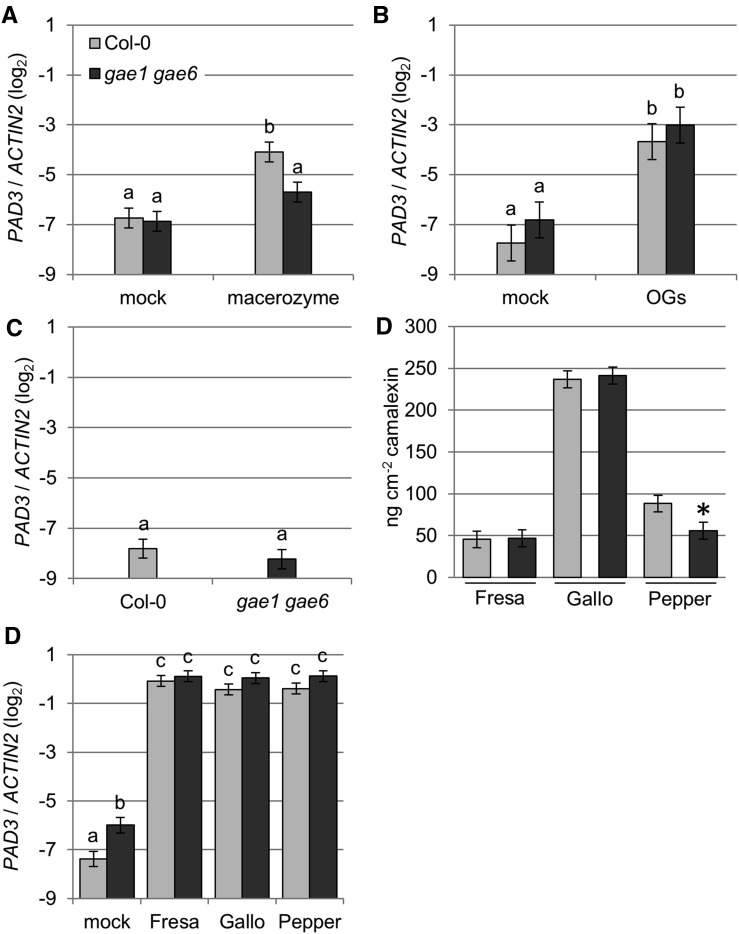
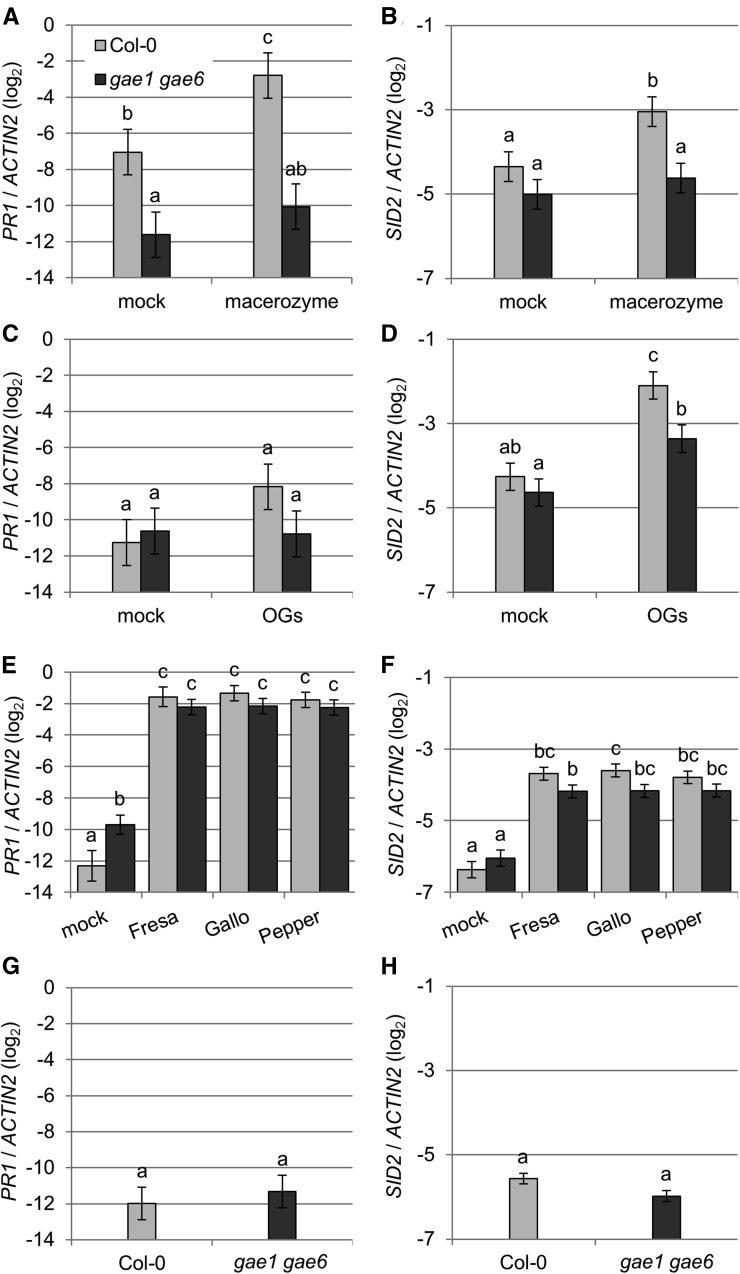
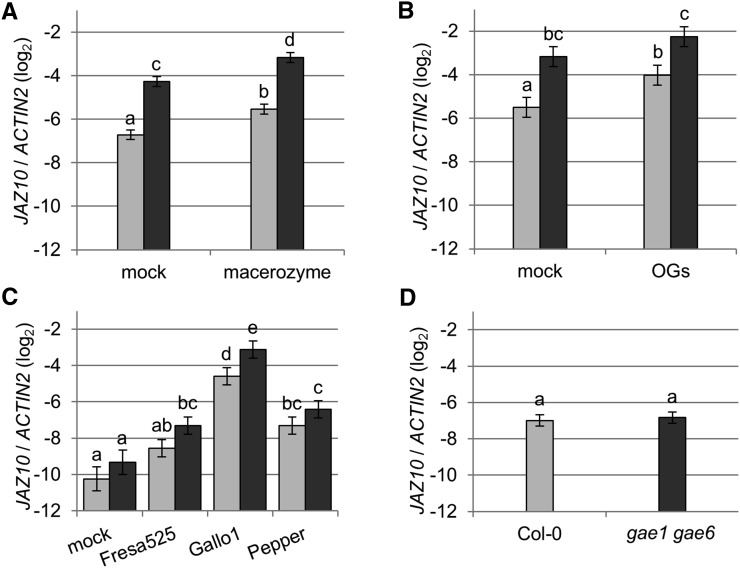
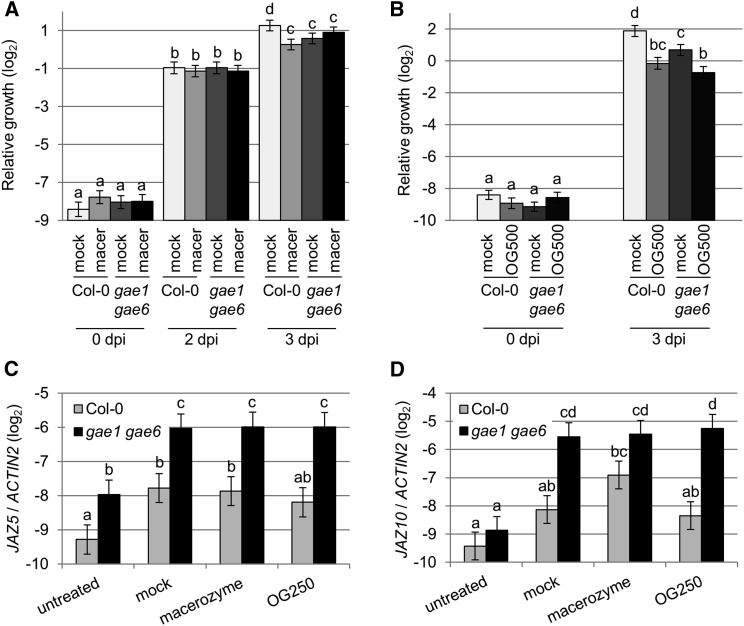
Next, we studied the effect of macerozyme treatment on expression of PAD3 in intact plants (Figure 8). This treatment did not lead to any visible leaf damage (Supplemental Figure 8). In wild-type Col-0, PAD3 expression was induced three h after treatment with 0.01% macerozyme (Figure 8A). By contrast, no macerozyme-induced induction of PAD3 expression was detected in gae1 gae6 (Figure 8A). However, OG treatment induced the expression of PAD3 in both genotypes, indicating that gae1 gae6 plants do respond to OGs (Figure 8B). Macerozyme treatment also induced expression of the SA marker genes PATHOGENESIS RELATED1 (PR1) and SID2 in Col-0, but not in gae1 gae6 (Figures 9A and 9B). PR1 was not significantly induced by purified OGs (Figure 9C) at the time and concentration tested, but SID2 was induced by OG treatment in both genotypes (Figure 9D). However, the induction was stronger in wild-type Col-0. In addition, the JA markers JASMONATE-ZIM-DOMAIN PROTEIN5 (JAZ5) and JAZ10 were induced by macerozyme and OG treatment in both Col-0 and gae1 gae6 (Figure 10A; Supplemental Figure 11). Interestingly, expression of JAZ5 and JAZ10 was already strongly induced in gae1 gae6 after mock inoculation (Figure 10; Supplemental Figure 11). We observed no hyperresponsive induction by mock treatment of PLANT DEFENSIN 1.2 (PDF1.2), a necrotrophy and late JA signaling marker gene (Supplemental Figures 12A to 12D). PDF1.2 was also not induced by macerozyme or OG treatments (Supplemental Figures 12A to 12D). This suggests that early JA signaling is induced by mock treatment in gae1 gae6. Because it is known that JA and SA signaling act antagonistically (Pieterse et al., 2012), the strong upregulation of JA-induced immune signaling in gae1 gae6 might explain the lower OG-induced SID2 expression in this genotype.
Figure 8.
Macerozyme-Induced PAD3 Expression Is Absent in gae1 gae6.
(A) Expression of PAD3 after macerozyme treatment. Four-week-old plants were either inoculated with 0.01% macerozyme or mock inoculated with water, and PAD3 expression was measured 3 h later by qRT-PCR. Data from four biological replicates were combined using a mixed linear model. Mean log2 ratios to ACTIN2 ± se were plotted. Letters indicate significantly different groups (P < 0.05).
(B) Expression of PAD3 after OG treatment. PAD3 expression was measured as described in (A) 3 h after inoculation with 100 µg mL−1 OGs or mock inoculation with water. Data are from six biological replicates.
(C) PAD3 expression in untreated plants. Three biological replicates were performed and data analyzed as described in (A).
(D) Camalexin accumulation after B. cinerea treatment. Camalexin accumulation was measured in 4-week-old plants inoculated with the B. cinerea strains shown in the figure. Eight biological replicates each were combined using a mixed linear model. Asterisks indicate significant differences from wild-type Col-0 for each isolate (P < 0.05).
(E) PAD3 expression 28 h after treatment with the indicated B. cinerea strains. Data from six biological replicates were collected and analyzed as described in (A). Data in all figure parts represent means ± se. Letters indicate significantly different groups (q < 0.05).
Figure 9.
Macerozyme-Induced Expression of the SA Marker Genes SID2 and PR1 Is Abolished in gae1 gae6 Plants.
(A) to (H) Expression of PR1 ([A], [C], [E], and [G]) or SID2 ([B], [D], [F], and [H]) in 4-week-old Col-0 and gae1 gae6 plants. Data were combined using a mixed linear model. Bars represent mean log2 ratios of expression versus ACTIN2 ± se for all figure parts. Letters indicate significantly different groups at P < 0.05 for (A) to (D), (G), and (H) and q < 0.05 for (E) and (F).
(A) and (B) Plants were treated with 0.01% macerozyme or mock treated with water and samples were collected 3 h later. Data from four biological replicates are shown.
(C) and (D) Plants were inoculated with 100 µg mL−1 OGs or mock treated with water and samples collected 3 h later. Data from six biological replicates are shown.
(E) and (F) Plants were inoculated with the indicated B. cinerea isolates or mock inoculated with B. cinerea inoculation medium and samples collected 48 h later. Data from eight biological replicates are shown.
(G) and (H) Expression in untreated plants. Three biological replicates were performed.
Figure 10.
The Expression of the JA Marker Gene JAZ10 is Highly Responsive in gae1 gae6 Plants.
(A) to (D) JAZ10 expression in 4-week-old plants. Data were combined using a mixed linear model. Mean log2 ratios to ACTIN2 ± se are shown. Letters indicate significantly different groups at P < 0.05 for (A), (B), and (D) and q < 0.05 for (C).
(A) Plants were inoculated with 0.01% macerozyme or mock inoculated using water and samples were collected 3 h later. Data from four biological replicates are shown.
(B) Plants were inoculated with 100 µg mL−1 OGs or mock inoculated using water and samples collected 3 h later. Data are from six biological replicates.
(C) JAZ10 expression 48 h after treatment with the indicated B. cinerea strains. Data are from six biological replicates.
(D) JAZ10 expression in untreated plants. Data are from three biological replicates.
To test whether other immune responses are altered in gae1 gae6 plants, we analyzed mitogen-activated protein kinase (MAPK) activity following macerozyme and OG treatments. OGs are known to activate the MAPKs MPK3 and MPK6 in Arabidopsis (Galletti et al., 2011). Macerozyme treatment activated MPK3 and MPK6 in wild-type Col-0, whereas MAPK activation in gae1 gae6 was slightly reduced in four out of five experiments performed (Supplemental Figure 13). By contrast, OG-induced MAPK activity was similar or increased in gae1 gae6 in four out of five experiments (Supplemental Figure 13). Additionally, mock treatment induced MAPK activity in gae1 gae6 more strongly than it did in wild-type Col-0 in four out of five experiments performed (Supplemental Figure 13). Collectively, these results illustrate that activation of several immune signaling components—camalexin synthesis, SA and JA signaling, and possibly MAPK activation—by macerozyme is substantially altered in gae1 gae6. This suggests that release of soluble pectin fragments, possibly OGs, by pectinolytic enzymes might be reduced in gae1 gae6 plants while recognition of OGs is intact.
Following B. cinerea Infection, Production of Soluble Pectin and Deposition of Callose Are Reduced in gae1 gae6 Plants
Although pad3 plants were more susceptible to the B. cinerea isolates Fresa 525 and Gallo 1, which also grew better on gae1 gae6, growth of the isolate Pepper was similar on Col-0 and pad3 (Supplemental Figure 9A). However, we did not detect any significant changes in camalexin production after treatment with B. cinerea isolate Gallo 1 and Fresa 525 in gae1 gae6 (Figure 8D). Neither did we detect changes in PAD3 expression after treatment with any B. cinerea isolate in gae1 gae6 (Figure 8E). Additionally, SID2 and PR1 were similarly induced by all B. cinerea isolates in both Col-0 and gae1 gae6 (Figure 9E and F) and sid2 plants, which are deficient for pathogen-induced SA biosynthesis, did not show any differences in relative B. cinerea growth (Supplemental Figure 9B). Expression of JAZ10 was induced more strongly by Gallo 1 treatment in gae1 gae6 than in wild-type Col-0 but not by Fresa 525 or Pepper (Figure 10C), while dde2 plants, deficient for JA biosynthesis, showed enhanced susceptibility to all B. cinerea isolates tested (Supplemental Figure 9B). Curiously, exogenous application of methyl jasmonate (MeJA) did not lead to altered relative fungal growth of B. cinerea (Supplemental Figure 9C), and cev1/isoxaben resistant1 (ixr1) plants, which have constitutively high JA levels (Ellis and Turner, 2001), did not show any changes in susceptibility to the B. cinerea isolates tested (Supplemental Figure 9D). Hence, neither differences in B. cinerea isolate susceptibility to camalexin nor altered SA or JA signaling by themselves explained the enhanced susceptibility of gae1 gae6 to these B. cinerea isolates.
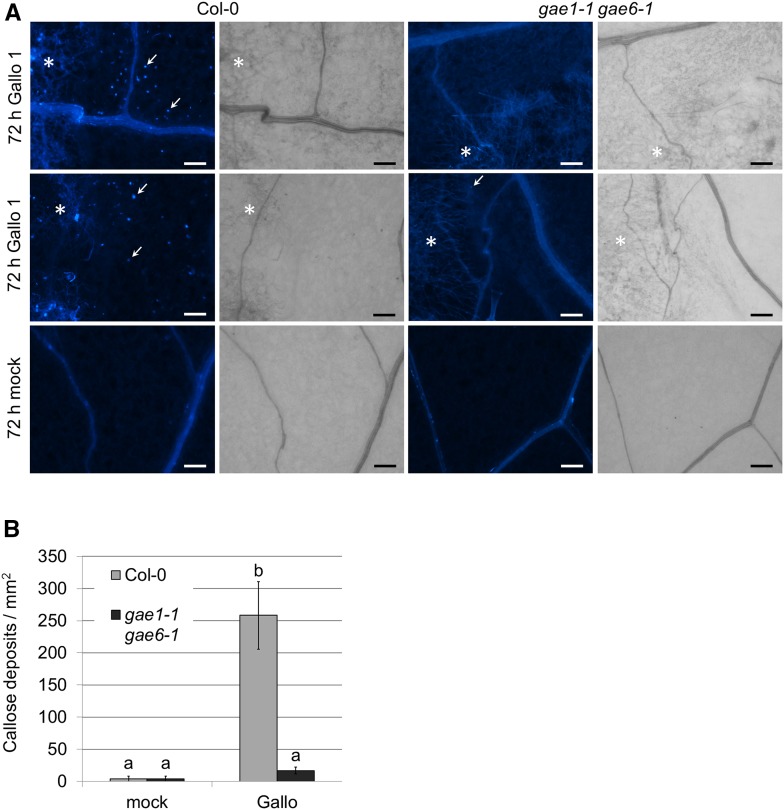
Hydrogen peroxide accumulation in response to B. cinerea isolates Fresa 525, Gallo 1, and Pepper was not obviously altered in gae1 gae6 plants (Supplemental Figure 14). However, callose deposition upon treatment with B. cinerea isolate Gallo 1 was strongly reduced in gae1 gae6 (Figure 11). It was previously shown that OG treatment induces callose deposition (Denoux et al., 2008), suggesting that the reduction in callose deposition might be due to reduced OG release in gae1 gae6. It is possible that this reduction in callose deposition contributes to the reduced immunity of gae1 gae6 plants to B. cinerea isolate Gallo 1.
Figure 11.
Gallo 1-Induced Callose Deposition Is Reduced in gae1 gae6 Plants.
(A) Callose deposition in 4-week-old Col-0 and gae1 gae6 plants. Plants were inoculated with B. cinerea isolate Gallo 1 (2.5 × 105 spores mL−1) or mock inoculated with B. cinerea inoculation medium, and samples were collected after 72 h. Callose was stained with aniline blue and visualized using a Nikon Eclipse Ni-U microscope with a 4′,6-diamidino-2-phenylindole filter. Three leaves were stained per treatment. Asterisks indicate fungal hyphae, and arrows indicate representative callose spots. Bars = 100 µm.
(B) Quantification of callose deposition. Callose deposits were counted in 200 × 200-µm squares laid out across the images shown here. Bars represent means ± se from six squares each. Letters indicate significantly different groups (P < 0.05) according to a t test analysis.
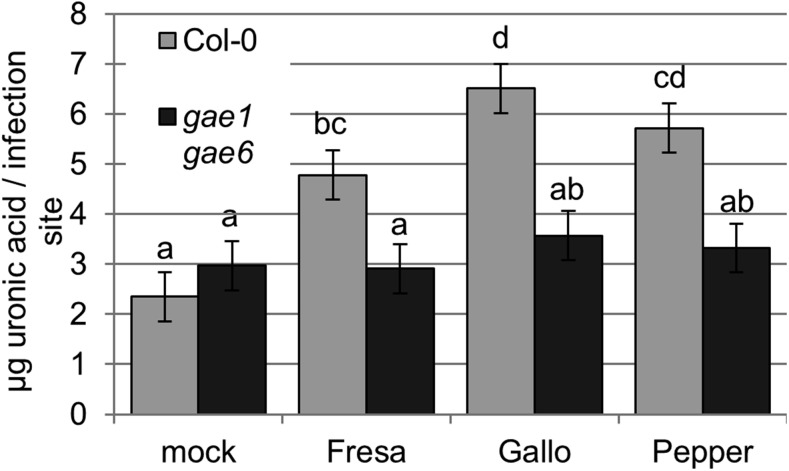
Because macerozyme could be used to release soluble, likely low molecular weight pectin from isolated cell walls, we next investigated whether infection with B. cinerea was accompanied by accumulation of soluble pectin as well. We prepared cell walls (AIR fraction) from leaves infected with B. cinerea, incubated these cell walls with water, and measured uronic acid content of the extracts. B. cinerea treatment did in fact lead to increased accumulation of soluble pectin in wild-type Col-0 plants when compared with mock-treated samples (Figure 12). No such increased accumulation of water-soluble pectin fractions was observed in gae1 gae6 (Figure 12).
Figure 12.
Less Soluble Pectin Accumulates in B. cinerea-Infected gae1 gae6 Leaves.
Four-week-old Col-0 and gae1 gae6 plants were inoculated with 10-μL droplets of the indicated B. cinerea isolate (2.5 × 105 spores mL−1) or mock inoculated with B. cinerea inoculation medium. Leaves were collected 48 h later. AIR was extracted from leaf samples and water added (20 μL/infection site) to extract soluble pectin fragments. Samples were vortexed for 7 h and centrifuged at 12,000g, and the uronic acid concentration of the supernatant was measured. Data from nine biological replicates were combined using a mixed linear model. Means ± se are shown. Letters indicate significant differences (q < 0.05).
Macerozyme-Induced Pattern-Triggered Immunity against B. cinerea Is Abolished in gae1 gae6
It was previously shown that OG treatment can induce pattern-triggered immunity (PTI) to B. cinerea (Ferrari et al., 2007). We tested macerozyme treatment to determine whether it could also induce PTI to the B. cinerea isolate Gallo 1 (Figure 13). Indeed, pretreatment with 0.01% macerozyme for 24 h led to reduced B. cinerea growth in wild-type Col-0 but not gae1 gae6 plants (Figure 13A). Interestingly, mock and macerozyme pretreated gae1 gae6 plants exhibited similar growth of B. cinerea Gallo 1 as did macerozyme-treated Col-0 plants. This result is likely due to activation of JA signaling in gae1 gae6 during the pretreatment procedure (Figures 13C and 13D). OG-induced PTI was observed in both genotypes when using a high dose of OGs (Figure 13B), whereas a lower dose of OGs only induced PTI in Col-0 (Supplemental Figure 15). This suggests that OG perception in gae1 gae6, at least above a certain threshold, is unaltered, while release of immunity-activating pectin fragments, possibly OGs, is reduced. We conclude that immune responses induced by macerozyme treatment, which do not occur in gae1 gae6 plants, are important for plant immunity to the B. cinerea isolate Gallo 1.
Figure 13.
Macerozyme-Induced PTI Is Lost in gae1 gae6 While OG-Induced PTI Is Unaltered.
(A) Macerozyme-induced PTI in Col-0 and gae1 gae6 plants. Four-week-old plants were inoculated with 0.01% macerozyme or mock inoculated with boiled macerozyme and were infected with B. cinerea isolate Gallo 1 (2.5 × 105 spores mL−1) 24 h later. Samples were collected immediately (0 dpi) and after two (2 dpi) and three (3 dpi) days. Data show mean ± se of six to 14 biological replicates each combined using a linear model. Letters indicate significantly different groups (q < 0.05).
(B) OG-induced PTI in Col-0 and gae1 gae6 plants. Four-week-old plants were inoculated with 500 µg mL−1 OGs and infected with B. cinerea as described in (A). Six to eight biological experiments were performed.
(C) and (D) Expression of JAZ5 (C) and JAZ10 (D) was measured in untreated plants or 24 h after inoculation with water (mock), 0.01% macerozyme, or 250 µg mL−1 OGs. Data show mean log2 ratios to ACTIN2 ± se from three biological replicates combined using a mixed linear model. Letters indicate significantly different groups (q < 0.05). The q-value for comparison of JAZ10 expression in the wild type and mutant after macerozyme treatment is 0.056.
DISCUSSION
Here, we showed that loss of GAE1 and GAE6 leads to a dramatic reduction in pectin content in the cell wall (Figure 4), likely causing the brittleness of leaves that we observed (Figure 3). Petioles and midribs of gae1 gae6 plants broke easily when bent, and inoculation of leaves with deionized water resulted in increased ion leakage, possibly due either to microscopic fractures of the cell wall induced by inoculation stress or to a reduced capability of these walls to withstand increased turgor pressure. The increased brittleness was associated with altered cell wall composition. In our analysis, the GalA content of gae1 gae6 leaf walls was reduced by more than 40% and the total uronic acids by over 60% (Figure 4; Supplemental Figure 5). This is a major change in the overall cell wall composition, considering that pectin makes up ∼50% of the plant cell walls found in Arabidopsis leaves (Zablackis et al., 1995; Nobuta et al., 2007; Harholt et al., 2010). Interestingly, quasimodo1 (qua1) plants, which have a mutation in a glycosyltransferase family 8 gene, are reduced in GalA by 25% and show reduced binding of HG-specific antibodies (Bouton et al., 2002).
Furthermore, qua2 plants, which have a mutation in a putative methyltransferase, have ∼13% less total GalA and lack 50% of cell wall HG (Mouille et al., 2007). Likewise, cgr2 cgr3 plants, carrying mutations in another class of Golgi-localized putative methyl-transferases, have leaf cell walls that are reduced by ∼40% in uronic acid (Kim et al., 2015). All three mutants are severely dwarfed (Bouton et al., 2002; Mouille et al., 2007; Kim et al., 2015). Additionally, qua1 and qua2 mutants show a distorted overall shape, likely due to reduced cell cohesion, and are extremely fragile (Bouton et al., 2002; Mouille et al., 2007). By contrast, gae1 gae6 plants with a similar strong reduction in GalA and total uronic acids have an overall normal shape and are only slightly smaller than wild-type Col-0 (Supplemental Figure 4). In the wall analysis performed here, we did not detect changes in other wall polysaccharides or structures that might compensate for the reduction of pectin in gae1 gae6. It is possible the severe morphological alterations in qua2 and cgr2 cgr3 plants are due to reduction in pectin methylesterification, which is unaffected in gae1 gae6. It was speculated that quality control mechanisms in the Golgi prevent secretion of inefficiently esterified pectin in cgr2 cgr3 (Kim et al., 2015), suggesting that the Golgi of these plants might overaccumulate pectin and that this may contribute to the severe dwarf phenotype of these plants. The gae1 gae6 plants described here provide an opportunity to study the effects of reduction of pectin in plants that do not show severe morphological phenotypes.
We found that gae1 gae6 plants are also compromised in resistance to B. cinerea and Pma ES4326, indicating that loss of pectin reduces immunity to these pathogens (Figures 2 and 6). We found that gae1 gae6 plants were slightly more susceptible to Pma ES4326 but showed a strong increase in susceptibility to specific B. cinerea isolates. This was not unexpected because B. cinerea is known to interact with pectin as part of its infection strategy. It secretes various pectin-degrading enzymes early during infection and requires some of these enzymes, including PG1, PG2, and PME1, for full virulence (ten Have et al., 1998; Valette-Collet et al., 2003; Kars et al., 2005b; Espino et al., 2010). Additionally, Arabidopsis plants expressing an antisense construct of the polygalacturonase inhibitor PGIP1 are significantly more susceptible to B. cinerea (Ferrari et al., 2006). On the other hand, Pma ES4326 is not known to require cell wall degrading enzymes for full virulence even though it encodes two pectate lyase genes in its genome (PMA4326_07094 and PMA4326_04621; www.bacteria.ensembl.org). B. cinerea isolates are also known to be genetically quite diverse in their polygalacturonase loci, and genetic variability in PG2 is associated with varying growth on pectin containing media (Rowe and Kliebenstein, 2007). Furthermore, the contribution of PG1 and PG2 to B. cinerea virulence seems to depend on the specific B. cinerea isolate and the host species (Kars et al., 2005a; Zhang and van Kan, 2013). B. cinerea isolates are also known to be differently sensitive to the antimicrobial secondary metabolite camalexin (Kliebenstein et al., 2005). In line with these observations, the susceptibility of gae1 gae6 plants differed depending on the specific B. cinerea isolate. Whereas Fresa 525, Gallo 1, UK Razz, and KT grew better in gae1 gae6, we detected no difference in Acacia, Apple 517, DN, Grape, Pepper, or Rasp growth (Figure 6).
B. cinerea was shown to metabolize pectin and to possibly utilize GalA as a carbon source during in planta growth (Zhang et al., 2011; Zhang and van Kan, 2013). Our data show that gae1 gae6 plants, which are reduced in GalA content, did allow enhanced growth of specific B. cinerea isolates, but no reduction in growth of any B. cinerea isolates tested was found. This suggests that utilization of pectin as a carbon source was not a contributing factor to alterations in B. cinerea susceptibility. It is possible that changes in the cell wall composition affect the structural integrity of the wall and affect hyphal penetration of specific B. cinerea isolates. However, OGs (small HG fragments) are likely released during the infection process (Ferrari et al., 2013). OGs function as damage-associated molecular patterns and initiate immune responses in Arabidopsis (Ferrari et al., 2013). Treatment of Arabidopsis with OGs having a degree of polymerization of 10 to 15 sugar subunits was previously shown to enhance resistance to B. cinerea (Ferrari et al., 2007). We showed that pretreatment with OGs or macerozyme-induced enhanced immunity to B. cinerea isolate Gallo 1 in wild-type Col-0, but only OGs, and not macerozyme, induced immunity in gae1 gae6 (Figure 13). Our data suggest gae1 gae6 plants, which have reduced pectin content, may release less OGs and that this reduction leads to the increased susceptibility to B. cinerea.
Consistently, we found that macerozyme treatment released less soluble pectin from gae1 gae6 cell walls than from wild-type walls. Fractions containing soluble pectin released from gae1 gae6 were also less active in inducing PAD3 gene expression than the fractions released from wild-type walls (Figure 7). We also showed that less soluble pectin accumulated in gae1 gae6 than in wild-type plants 48 h after treatment with B. cinerea (Figure 12).
We observed that loss of pectin leads to alterations in Arabidopsis immune signaling. Macerozyme treatment induced expression of PAD3 and the SA marker genes PR1 and SID2 in wild-type Col-0 but not in gae1 gae6, whereas OG treatment led to increased PAD3 and SID2 expression in both the wild type and gae1 gae6 (Figures 8 and 9). This suggests that although release of active soluble pectin in these plants might be altered, OG recognition is intact. In fact, cell walls isolated from qua1, another mutant known to be reduced in HG, released less OGs (degree of polymerization 1 to 6) than did wild-type cell walls upon endopolygalacturonase treatment (Bouton et al., 2002). This was also true for gae1 gae6 cell walls, as macerozyme treatment of isolated gae1 gae6 cell walls released less soluble pectin than treatment of Col-0 cell walls. Because the fraction from gae1 gae6 plants was also less active in inducing PAD3 expression (Figure 7), it seems possible that biologically active OGs were the active component inducing PAD3 expression. Interestingly, expression of the JA marker genes JAZ5 and JAZ10 was higher in gae1 gae6 than in wild-type mock-inoculated plants but was comparable to the wild type in untreated plants, suggesting that JA signaling in gae1 gae6 is hyperresponsive (Figure 10; Supplemental Figure 11). This induction of JA signaling might be due to wounding stress incurred during the inoculation process. JA and SA signaling generally act antagonistically (Pieterse et al., 2012). Increased JA signaling activity might hence explain why mock- and macerozyme-treated gae1 gae6 plants exhibited reduced expression of the SA marker PR1 and why OG treatment induced expression of SID2, another SA marker, to a lower extent in gae1 gae6 than in wild-type plants (Figure 9).
No single immune signaling component was found to be the sole contributor to the compromised immunity of gae1 gae6 to specific B. cinerea isolates, such as Gallo 1 and Fresa 525. These isolates grew better on pad3, while growth of Pepper, another B. cinerea isolate that did not show altered virulence on gae1 gae6, was unaltered (Supplemental Figure 9). This suggested that differences in camalexin sensitivity of these B. cinerea isolates might account for the differences in gae1 gae6 susceptibility. However, camalexin accumulation and PAD3 expression were the same for wild-type Col-0 and gae1 gae6 for the B. cinerea isolates Fresa 525 and Gallo 1 (Figure 8), which showed enhanced growth on gae1 gae6 plants. It should be noted that B. cinerea virulence is often estimated by comparing lesion sizes. Lesion size and measurements of relative fungal growth by qPCR do not always show the same results. For example, although Denby et al. (2004) showed that Pepper causes larger lesions on pad3 than on the wild type, this study showed that relative fungal growth of Pepper on pad3 was unaltered.
We observed that callose deposition was strongly reduced in gae1 gae6 plants 72 h after treatment with B. cinerea isolate Gallo 1 (Figure 11), possibly due to reduced release of active soluble pectin. Interestingly, OG-induced PTI to an unspecified isolate of B. cinerea was intact in the pmr4 mutant, which does not produce callose (Galletti et al., 2008). Due to the high SA levels in pmr4 plants (Nishimura et al., 2003) and the fact that different B. cinerea isolates vary both in induction of and reaction to defense responses (Kliebenstein et al., 2005; Rowe et al., 2010), we could not determine whether reduced callose deposition causes the increased susceptibility of gae1 gae6 to B. cinerea isolate Gallo 1.
We did not observe any changes in B. cinerea growth either on cev1/ixr1 plants, which are known to have constitutively high JA and OPDA levels (Ellis et al., 2002b) or on plants exogenously treated with MeJA (Supplemental Figure 9). Because plants were kept uncovered for 3 h after MeJA treatment, it is unclear how much MeJA was bioavailable in the plant. However, JAZ10 expression was induced 3 h after spraying with MeJA (Supplemental Figure 10). Because pathogen growth generally depends on the combination of a multitude of immune signaling events, we conclude that multiple changes to the immune signaling in gae1 gae6 together account for the altered B. cinerea growth phenotypes detected.
We showed that expression of GAE1 and GAE6 is repressed by the bacterial hemibiotroph Pma ES4326. This repression did not occur in an eds1 or pad4 background or in pbs3 at the 24-h time point (Figure 1; Supplemental Figure 1). During the Arabidopsis-Pma ES4326 interaction, the expression of many defense related genes is altered in a PAD4/EDS1-dependent fashion (Wang et al., 2008). Some of these expression changes require SA. CBP60g and SARD1 are thought to act downstream of PAD4 and EDS1 but upstream of SA-dependent responses (Wang et al., 2011). PBS3 is also thought to act downstream of PAD4 and EDS1 and upstream of SA, but the set of genes whose expression it affects is only partially overlapping with those affected by CBP60g/SARD1 (Wang et al., 2008, 2011). Thus, GAE1 and GAE6 repression is regulated by the part of the immune signaling network that requires PAD4/EDS1 and PBS3 but is independent of CBP60g/SARD1 and SA. Because EDS1, PAD4, and PBS3 are important components of the plant immune signaling network (Wiermer et al., 2005; Nobuta et al., 2007), one might conclude that repression of GAE1 and GAE6 may be part of the plant immune response to Pma ES4326. B. cinerea treatment also reduced GAE1 and GAE6 expression but this was mostly independent of PAD4 (Figure 6); while both Pma ES4326 and B. cinerea repress GAE1 and GAE6 expression, the host signaling mechanisms responsible likely differ.
In summary, our data show that Arabidopsis GAE1 and GAE6 are required for pectin biosynthesis, leaf flexibility, and immunity to Pma ES4326 and to specific B. cinerea isolates. We used macerozyme, a commercial pectinase, to mimic the effect of B. cinerea pectinases. Macerozyme treatment released less pectin from gae1 gae6 cell walls and induced less immune signaling in gae1 gae6 plants. Less water-soluble pectin also accumulated in B. cinerea-infected gae1 gae6 plants, and macerozyme-induced immunity to B. cinerea isolate Gallo 1 was reduced in these plants. We conclude that the reduced pectin content in these mutants may lead to reduced release of soluble pectin including active OGs during pathogen attack and that this compromises immunity.
METHODS
Plant Materials and Growth Conditions
Wild-type Columbia (Col-0) and all mutant plants (in Col-0 background) were grown on sterilized BM2 germinating mix (Berger) in a controlled environment chamber (Conviron) with a 12-h photoperiod under 100 µmol m−2 s−1 fluorescent illumination at 22°C and 75% relative humidity. Germplasm used is described in the Accession Numbers section at the end of Methods.
Data Analysis and Replicates
For biological replicates, samples were obtained from separate plant tissues and, in many cases, from plants grown in different flats. In some experiments, multiple technical replicates were measured per biological replicate. For statistical analysis, all technical replicates were averaged and the averages were considered one biological replicate. Independent experiments were performed at different times and often consisted of multiple biological replicates per experiment.
Data analysis was performed using mixed linear models in the R programming environment (Bates et al., 2015), unless otherwise indicated. Generally, genotypes, treatments, and time points were used as fixed effects, and replicate-specific effects, like different pots, flats, etc., were considered random effects. We reported P values, obtained from the mixed linear model, when less than 10 comparisons were performed and q-values for more than 10 comparisons to correct for multiple comparison testing. Q-values were calculated using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995) unless specified otherwise.
Pathogen Strains, Growth Conditions, and Pathogen Growth Assays
Growth of Pseudomonas syringae pv maculicola ES4326 (Pma ES4326) and bacterial growth assays were performed as previously described (Bethke et al., 2014). For qRT-PCR assays, 4-week-old plants were inoculated with bacteria (OD600 = 0.002) or mock (5 mM MgSO4). At least four leaves from two plants were collected at the indicated time points per biological replicate.
Prior to each experiment, Botrytis cinerea isolates were grown on 1× PDA medium (Difco) for 10 d at room temperature. All isolates used have been described previously (Rowe and Kliebenstein, 2007). The spores were washed from the surface of the plate using an inoculation medium (1× Gamborg’s B-5 basal salt mixture [Sigma-Aldrich; G5768], 2% [w/v] glucose, and 10 mM phosphate buffer, pH 6.4), and fungal hyphae were removed from the suspension by filtering through four layers of cheesecloth. The concentration of spores was determined using a hemocytometer and adjusted to 2.5 × 105 spores mL−1. Four-week-old Arabidopsis thaliana plants were inoculated by placing one 10-μL droplet of the B. cinerea spore solution or inoculation medium (mock) on the adaxial leaf surface of fully expanded leaves. Inoculated plants were kept at 100% relative humidity. Infection sites were collected at various time points using a cork borer. For qRT-PCR assays, at least four leaf discs from one plant were combined for each biological sample. For B. cinerea growth assays, at least 12 leaf discs from three to six plants were pooled per biological sample. Relative fungal growth was determined by measuring the abundance of a fungal gene relative to a plant gene as described below. In brief, DNA from infected tissue was extracted and equal amounts of total DNA were used to perform qPCR reactions using the B. cinerea cutinase A and the Arabidopsis SK11 gene, respectively. Primers were described by Gachon and Saindrenan (2004) and are listed in Supplemental Table 1. Two technical replicates were combined for each biological replicate. B. cinerea is a necrotrophic fungal pathogen and may destroy plant DNA as disease progresses. However, decreases in the amount of plant DNA over the course of our experiments were only observed at late time points with very susceptible plants, so the qPCR assay is generally a reasonable estimate of fungal biomass (Supplemental Figure 16).
Treatment with Macerozyme or OGs
For PTI assays, 4-week-old plants either were inoculated with 0.01% (w/v) macerozyme R-10 (Yakult), a Rhizopus polygalacturonase (EC 3.2.1.15) that possesses high pectinase and hemicellulase activity, or were mock inoculated using heat-inactivated macerozyme. Other similar plants were either inoculated with 250 or 500 µg mL−1 OGs or mock inoculated with water. One day later, plants were infected with B. cinerea and samples were collected immediately (0 dpi) and 2 or 3 d later. To investigate the role of MeJA on B. cinerea growth, plants were sprayed with 1 mM MeJA in water with 0.01% (v/v) Tween 20. Covers were removed so that leaves could dry off before plants were inoculated with B. cinerea 3 h later. Samples were harvested at the indicated time points. For expression analysis and MAPK activation assays, plants were either inoculated with 100 µg mL−1 OGs or mock inoculated with water. OGs used for experiments described in Figures 8 to 10 and Supplemental Figure 12 were a kind gift from Simone Ferrari. OGs used in experiments described in Figure 13 and Supplemental Figures 11, 13, and 15 were prepared as previously described (Kohorn et al., 2014).
Expression Analysis
Leaf tissue from inoculated plants was harvested at the indicated time points, flash frozen, pulverized, and RNA extracted using Trizol (Invitrogen). Quantitative RT-PCR was performed using the SuperScript III Platinum SYBR Green One-Step quantitative RT-PCR kit (Invitrogen) and a Lightcycler 480 Real-Time PCR system (Roche) as previously described (Truman and Glazebrook, 2012). In brief, equal amounts of total RNA and a gene-specific primer were used for each reaction. The crossing point (Cp) was calculated using the second derivative max method provided with the Lightcycler software for each amplification curve. Each reaction was run with two technical replicates and the Cp values for these replicates were averaged. ACTIN2 was used as a stably expressed reference gene. At least three biological replicates each were performed and analyzed using a mixed-linear model. RT-PCR was performed to verify T-DNA insertion lines using the One-Step RT-PCR kit (Qiagen) according to the manufacturer’s instructions. The primers used can be found in Supplemental Table 1.
Electrolyte Leakage Assay
Fully expanded leaves of 4-week-old plants were inoculated with water (Milli-Q grade) using a needleless syringe. Leaf discs were collected using a cork borer and three leaf discs from one leaf were placed with the adaxial surface down onto 2 mL water in a 12-well cell culture plate. Conductivity was measured using a Horiba B-173 conductivity meter after 30 min and then the water was replaced. Conductivity was measured again 30 min later (1 h total) as indicated in Figure 3C. Six biological replicates each were performed in three independent experiments and means and standard errors were calculated. Two-sided t tests were performed for each time point.
3,3′-Diaminobenzidine and Aniline Blue Staining
Leaves from 4-week-old plants were either mock inoculated with B. cinerea inoculation medium or inoculated with 10-μL droplets of a B. cinerea spore solution (2.5 × 105 spores mL−1) of the indicated isolates. Samples were collected 72 h later. For 3,3′-diaminobenzidine staining, two to five inoculated leaves were vacuum infiltrated with 3,3′-diaminobenzidine (1 mg mL−1 in water, pH 3.8) solution and stained overnight in the dark. Leaves were destained using 100% ethanol at 65°C and then transferred to 50% (v/v) glycerol and photographed.
Callose was visualized by staining leaves with aniline blue as previously described (Adam and Somerville, 1996). Briefly, leaves were vacuum infiltrated with alcoholic lactophenol (1 volume of phenol:glycerol:lactic acid:water [1:1:1:1], mixed with two volumes of ethanol) and destained at 65°C. Next, leaves were moved through an alcohol gradient (50, 20, and 10% [v/v] ethanol) into water. The destained leaves were stained for 30 min in 150 mM K2HPO4 (pH 9.5) with 0.01% (w/v) aniline blue. Samples were mounted in water and examined using a Nikon Eclipse Ni-U microscope at 10× magnification using a 4′,6-diamidino-2-phenylindole filter (excitation at 325 to 375 nm; emission at 435 to 485 nm). For callose quantification, six 200 × 200-µm squares were counted for each sample type. Callose deposits per mm2 were calculated and data analyzed using a t test. Note that there is an inherent uncertainty associated with such counts because callose deposits may not all be in focus at the same time.
MAPK Activity Assay
Fully expanded leaves of 4-week-old plants were inoculated with 0.01% macerozyme, 100 µg mL−1 OGs, 100 nM flg22, or water (mock). flg22, a peptide from bacterial flagella that induces immune responses, was used as a positive control for MAPK activation (Asai et al., 2002) and was purchased from EZBiolab. Samples were harvested 10 min later and immediately frozen in liquid nitrogen. Protein was extracted using an extraction buffer containing 25 mM Tris, pH 7.8, 75 mM NaCl, 1 mM DTT, 1 mM NaF, 0.5 mM Na3VO4, 0.1% (v/v) Tween 20, one cOmplete Mini protease inhibitor cocktail tablet (Roche), and one PhosSTOP phosphatase inhibitor cocktail tablet (Roche) per 10 mL buffer. Fifteen micrograms of total protein was electrophoresed on a 10% SDS-polyacrylamide gel and then blotted onto a PVDF membrane (Bio-Rad; 162-0177). Activated MAPKs were detected using p44/42 MAPK (Erk1/2) antibody (9102S Cell Signal, 1:2500 in TBST [20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% (v/v) Tween 20]), anti-Rabbit IgG-HRP (1:15,000 in TBST and Sigma-Aldrich A6154), and ECL Plus substrate (Pierce; 32132). MPK3 and MPK6 were detected with an anti-MPK3 antibody (Sigma-Aldrich M8318, 1:2000 in TBST and 3% [w/v] milk) or an anti-MPK6 antibody (Sigma-Aldrich A7104, 1:6000 in TBST and 3% [w/v] milk), respectively. The secondary antibody and detection were the same as for the p44/42 antibody. Five independent experiments were performed.
Preparation of AIR, Pectin Extraction, and Uronic Acid Measurements
Fully expanded leaves from 4-week-old plants that were incubated for 48 h in the dark to reduce starch were harvested, flash frozen, and pulverized. AIR was extracted by washing ground material twice in 70% (v/v) ethanol, three times in a mixture of chloroform and methanol (1:1 [v/v]), and once in acetone (Gille et al., 2009) and was then air dried. Cell wall pectin was extracted from AIR with cell wall extraction buffer (50 mM Trizma and 50 mM CDTA, pH 7.2) at 95°C for 15 min. Samples were homogenized using a paint shaker (Harbil 5G-HD) and glass beads (3-mm diameter). For dot blot experiments, 500 μL of cell wall extraction buffer was used per 10 mg of AIR. Debris was precipitated by centrifugation for 10 min at 10,000g.
Total uronic acid content of pectin, extracted as described above using 500 μL of cell wall extraction buffer per 1 mg of AIR, was measured as described previously (Filisetti-Cozzi and Carpita, 1991; van den Hoogen et al., 1998). First, 36 μL of pectin extract were mixed with 4 μL of 4 M sulfamic acid. Then, 200 μL of sulfuric acid containing 120 mM sodium tetraborate was added and samples were incubated at 80°C for 1 h. Following cooling on ice, the optical density of samples was measured at 490 nm. Next, 40 μL of m-hydroxydiphenyl reagent (100 μL of m-hydroxydiphenyl in DMSO at 100 mg/mL mixed with 4.9 mL 80% [v/v] sulfuric acid just before use) was added and the samples were mixed. The optical density at 490 nm was measured again. The optical density before m-hydroxydiphenyl reagent addition was subtracted from the optical density measured after addition of the dye. The concentration of uronic acid was calculated using known amounts of GalA as a standard.
Release of Pectin from Arabidopsis Cell Walls Using Macerozyme
AIR preparations from leaves of 4-week-old plants were treated with 0.25% (w/v) macerozyme R-10 (Yakult) or water (mock) at a concentration of 100 μL per mg AIR. Samples were incubated for 3 h at room temperature on a vortex shaker and centrifuged for 10 min at 12,000g. Total uronic acid concentration of 5-fold diluted supernatants was determined for two technical replicates each. The concentration of uronic acid was calculated using known amounts of GalA as a standard. For subsequent qRT-PCR experiments, supernatants from multiple extractions were combined, boiled for 20 min to inactivate macerozyme, diluted 10-fold with water, and inoculated into 4-week-old Col-0 plants. Heat-inactivated, 10-fold diluted macerozyme solutions were used as mock treatment. Samples were collected 3 h later and PAD3 expression was determined.
Release of Pectin from Arabidopsis Leaves Infected with B. cinerea
Fully expanded leaves of 4-week-old plants were either inoculated with up to five 10-μL droplets of B. cinerea spore solution (2.5 × 105 spores mL−1) or mock inoculated with B. cinerea inoculation medium. For each biological replicate, leaves with a combined count of at least 20 infection sites were collected 48 h later. AIR was extracted as described above but plants were not dark-treated or freeze-dried and the first ethanol step was performed using 85% (v/v) ethanol. To extract soluble pectin fragments, AIR samples were mixed with water at a concentration of 20 μL per infection site, incubated for 7 h at room temperature on a vortex shaker, and centrifuged for 10 min at 12,000g. Total uronic acid concentration of supernatants was determined for two technical replicates each. The concentration of uronic acid was calculated using known amounts of GalA as a standard.
Measurement of Uronic Acids, Neutral Monosaccharides, and Cellulose Content in Cell Walls
Neutral sugars and uronic acids from noncellulosic polysaccharides in cell walls (AIR) were released by trifluoroacetic acid (TFA) hydrolysis. Approximately 1 mg of AIR was hydrolyzed with 2 M TFA at 121°C for 90 min. The TFA-soluble material was split into two equal parts, one for uronic acid and one for neutral sugar determination. The uronic acid content was determined by analyzing the hydrolyzate using a CarboPac PA200 anion-exchange column with an ICS-3000 Dionex chromatography system. The elution profile consisted of a linear gradient of 50 to 200 mM sodium acetate in 0.1 M NaOH in 10 min at 0.4 mL per min. Alditol acetate derivatives of neutral sugars were produced as described (York et al., 1985). In brief, monosaccharides in the hydrolyzate were reduced with NaBH4 and peracetylated with acetic anhydride and pyridine at 121°C for 20 min. The generated alditol acetates were analyzed by gas chromatography-mass spectrometry. Crystalline cellulose was measured according to Updegraff (1969). After hydrolysis of noncellulosic polysaccharides from AIR with the Updegraff reagent (acetic acid:nitric acids:water, 8:1:2 [v/v]), the remaining pellet was hydrolyzed in 72% sulfuric acid. The resulting glucose quantity was determined by the anthrone method (Scott and Melvin 1953).
Dot Blot Experiments
Pectin solutions, extracted using 50 μL of cell wall extraction buffer per mg AIR, were serially diluted and nitrocellulose membranes were spotted with 1 μL of the diluted pectin solutions. Membranes were dried overnight, blocked with 5% milk (w/v) in 1× PBS (8 g L−1 NaCl, 0.2 g L−1 KCl, 1.44 g L−1 Na2HPO4, and 0.24 g L−1 KH2PO4, pH 7.4), and probed with LM5 (Jones et al., 1997), LM6 (Willats et al., 1998), LM8 (Willats et al., 2004), LM19, and LM20 (Verhertbruggen et al., 2009a) or CCRC-M7 (Steffan et al., 1995) antibodies. LM series antibodies were diluted 1:250 and CCRC-M7 was diluted 1:500 in 5% milk powder (Nestle) in 1× PBS. For LM series antibodies, a goat anti-Rat HRP conjugated antibody (Bethyl A110-105P, 1:5000 diluted) and for CCRC-M7 an anti-Mouse IgG HRP conjugate (Promega W402B, 1:2500 diluted) was used as secondary antibody in 5% milk powder in 1× PBS. LM series antibodies were obtained from PlantProbes and CCRC-M7 from CarboSource. Membranes were washed after incubation with the primary and secondary antibodies with 1× PBS. Dot blots were developed using the ECL system (GE Healthcare; Amersham ECL prime). For quantification of signals, dot blot results on x-ray films were photographed using a CCD camera and intensities were measured using Image J’s integrated density function. Locally adjusted background was subtracted. As the dynamic range of the signal on an x-ray film was narrow, nonlinearity in the measured values was corrected for each genotype in each biological replicate using the values from different dilutions in two technical replicates. We noticed that the residuals were not normally distributed and performed Box-Cox power transformation (Box and Cox, 1964) to obtain normally distributed values for the subsequent statistical analysis of the data. For LM19, Box-Cox selected power transformation was done to the power of 0.38th and for LM20 to the power 0.75th. After correction of the transformed measured values with the effect of the biological replicates, the values were fit to a linear model with the Arabidopsis genotype as the fixed effect. This linear model was used both to generate the plots in Figures 5B and 5D and to test significant differences.
Camalexin Measurements
Four-week-old plants were infected with B. cinerea and samples (three leaf disks from one plant each) were harvested at the indicated time points, flash-frozen, and pulverized. Each sample was extracted with 300 μL of 90% methanol (v/v). Fifty microliters of the extract was run on an Agilent Lichrocart 250-4 RP18e 5-µm column using an Agilent 1100 series HPLC. Camalexin was detected using a diode array at 330 nm and with a fluorescence detector at emission 318 nm/excitation 385 nm (Agilent). Separation was achieved using the following program with aqueous acetonitrile: 5-min gradient from 63 to 69% acetonitrile, 30-s gradient from 69 to 99% acetonitrile, 2 min at 99% acetonitrile, and a post-run equilibration of 3.5 min at 63% acetonitrile (Denby et al., 2004; Kliebenstein et al., 2005). Purified camalexin was used to produce a standard curve to identify and quantitate camalexin.
Accession Numbers
Microarray data used can be accessed at the NCBI Gene Expression Omnnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE18978. Sequence information for all Arabidopsis genes described in this article can be found in TAIR (www.arabidopsis.org) using the following accession numbers: PAD3 (CYP71B15, At3g26830), SID2 (ICS1, At1g74710), GAE1 (At4g30440), GAE2 (At1g02000), GAE3 (At4g00110), GAE4 (At2g45310), GAE5 (At4g12250), GAE6 (At3g23820), PR1 (At2g14610), JAZ5 (At1g17380), JAZ10 (At5g13220), ACTIN2 (At3g18780), PDF1.2a (At5g44420), PDF1.2b (At2g26020), and AtSK11 (At5g26751). The sequence of the B. cinerea cutinase Bc-CutA (Z69264) can be found in the DNA Data Bank of Japan (http://getentry.ddbj.nig.ac.jp). Germplasm used included dde2-2 (At5g42650; von Malek et al., 2002), pad3-1 (At3g26830; Zhou et al., 1999), pad4-1 (At3g52430; Jirage et al., 1999), sid2-2 (At1g74710; Wildermuth et al., 2001), pbs3-2 (At5g13320, SALK_018225; Nobuta et al., 2007), cbp60a-1 (At5g62570, SALK_124410; Truman et al., 2013), cbp60g-1 sard1-2 (At5g26920, SALK_023199; At1g73805, SALK_052422; Wang et al., 2011), cbp60a-1 cbp60g-1 sard1-2 (Truman et al., 2013), gae1-1 (At4g30440, SALK_085554), gae6-1 (At3g23280, SALK_104454), gae6-2 (At3g23280, SALK_017191), ixr1-1/cev1 (At5g05170; Ellis and Turner, 2001; Scheible et al., 2001). eds1 plants were derived by introgression of the Landsberg erecta eds1-2 allele into Col-0 that contains two copies of EDS1 (At3g48090 and At3g48080; Bartsch et al., 2006), mpk6-2 (At2g43790, SALK_073907; Liu and Zhang, 2004), mpk3-DG (At3g45640, a fast neutron deletion mutant; Miles et al., 2005), and npr1-1 (At1g64280; Cao et al., 1997). T-DNA insertion lines were part of the SALK collection (Alonso et al., 2003) and were obtained from the ABRC.
Supplemental Data
Supplemental Figure 1. Pma ES4326-Induced Repression of GAE1 and GAE6 Expression Does Not Require CBP60s or SID2.
Supplemental Figure 2. Expression of GAE Family Members upon Treatment with Pma ES4326.
Supplemental Figure 3. Characterization of T-DNA Insertions in GAE1 and GAE6.
Supplemental Figure 4. Mutant gae6 and gae1 gae6 Plants Have Brittle Leaves and Are Slightly Smaller Than Wild-Type Col-0 Plants.
Supplemental Figure 5. Cell Walls of gae1 gae6 Plants Contain Less Uronic Acid Than Wild-Type Col-0 Cell Walls.
Supplemental Figure 6. Antibodies Raised against the RGI or XG Components of Pectin Show No Difference in Binding to gae Mutant and Col-0 Cell Walls.
Supplemental Figure 7. The Effect of B. cinerea Treatment on the Expression of GAE Family Genes.
Supplemental Figure 8. Treatment with 0.01% Macerozyme Does Not Cause Any Visible Damage to Col-0 Plants.
Supplemental Figure 9. B. cinerea Growth on Plants with Defects in Immune Signaling.
Supplemental Figure 10. MeJA-Induced Expression of the JA Marker Gene JAZ10.
Supplemental Figure 11. The Expression of the JA Marker Gene JAZ5 Is Highly Responsive in gae1 gae6 Plants.
Supplemental Figure 12. The Expression of PDF1.2 Is Unaltered in gae1 gae6 Plants.
Supplemental Figure 13. Macerozyme Treatment Activates MPK3 and MPK6.
Supplemental Figure 14. B. cinerea-Induced ROS Production in Wild-Type Col-0 and gae1 gae6 Plants.
Supplemental Figure 15. OG-Induced PTI Using 250 µg mL−1 OGs.
Supplemental Figure 16. Abundance of Plant DNA in B. cinerea-Treated Plants.
Supplemental Table 1. Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank William Truman for helpful discussions, Simone Ferrari (Sapienza Universita di Roma) for the generous gift of OGs, Bruna Bucciarelli (USDA-ARS) for help with fluorescence microscopy, the ABRC for T-DNA insertion lines, Paul Knox’s lab (University of Leeds) for LM5, LM6, LM8, LM19, and LM20 antibodies, and the Complex Carbohydrate Resource Center (University of Georgia) for the CCRC-M7 antibody. Most of this work was funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-FG02-05ER15670 to J.G. This work was also supported by National Science Foundation Awards IOS 1339125 and IOS 1021861 to D.J.K., National Science Foundation Award IOS 1121425 to F.K., and the USDA National Institute of Food and Agriculture, Hatch project number CA-D-PLS-7033-H to D.J.K.
AUTHOR CONTRIBUTIONS
G.B. and J.G. designed the research. G.B., G.X., A.T., B.L., N.E.S., and N.H. performed research. D.J.K. provided tools. G.B., G.X., M.P., A.T., B.L., R.A.H., and F.K. analyzed data. G.B. and J.G. wrote the article with input from all authors.
Glossary
- GalA
galacturonic acid
- HG
homogalacturonan
- SA
salicylic acid
- JA
jasmonic acid
- ET
ethylene
- dpi
days postinoculation
- AIR
alcohol-insoluble residue
- MAPK
mitogen-activated protein kinase
- MeJA
methyl jasmonate
- PTI
pattern-triggered immunity
- TFA
trifluoroacetic acid
References
- Adam L., Somerville S.C. (1996). Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 9: 341–356. [DOI] [PubMed] [Google Scholar]
- Albersheim P., Jones T.M., English P.D. (1969). Biochemistry of the cell wall in relation to infective processes. Annu. Rev. Phytopathol. 7: 171–194. [DOI] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983. [DOI] [PubMed] [Google Scholar]
- Bartsch M., Gobbato E., Bednarek P., Debey S., Schultze J.L., Bautor J., Parker J.E. (2006). Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Machler M., Bolker B.M., Walker S.C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B Met. 57: 289–300. [Google Scholar]
- Bethke G., Grundman R.E., Sreekanta S., Truman W., Katagiri F., Glazebrook J. (2014). Arabidopsis PECTIN METHYLESTERASEs contribute to immunity against Pseudomonas syringae. Plant Physiol. 164: 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton S., Leboeuf E., Mouille G., Leydecker M.T., Talbotec J., Granier F., Lahaye M., Höfte H., Truong H.N. (2002). QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14: 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box G.E.P., Cox D.R. (1964). An analysis of transformations. J. R. Stat. Soc. B 26: 211–252. [Google Scholar]
- Cao H., Glazebrook J., Clarke J.D., Volko S., Dong X. (1997). The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63. [DOI] [PubMed] [Google Scholar]
- Carpita N.C. (2011). Update on mechanisms of plant cell wall biosynthesis: how plants make cellulose and other (1→4)-β-D-glycans. Plant Physiol. 155: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm S.T., Coaker G., Day B., Staskawicz B.J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814. [DOI] [PubMed] [Google Scholar]
- Côté F., Hahn M.G. (1994). Oligosaccharins: structures and signal transduction. Plant Mol. Biol. 26: 1379–1411. [DOI] [PubMed] [Google Scholar]
- Denby K.J., Kumar P., Kliebenstein D.J. (2004). Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. Plant J. 38: 473–486. [DOI] [PubMed] [Google Scholar]
- Denoux C., Galletti R., Mammarella N., Gopalan S., Werck D., De Lorenzo G., Ferrari S., Ausubel F.M., Dewdney J. (2008). Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 1: 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Turner J.G. (2002a). Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant Microbe Interact. 15: 1025–1030. [DOI] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Wasternack C., Turner J.G. (2002b). The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Turner J.G. (2001). The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino J.J., Gutiérrez-Sánchez G., Brito N., Shah P., Orlando R., González C. (2010). The Botrytis cinerea early secretome. Proteomics 10: 3020–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Denoux C., De Lorenzo G., Ausubel F.M., Dewdney J. (2007). Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 144: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Vairo D., Cervone F., De Lorenzo G. (2006). Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol. Plant Microbe Interact. 19: 931–936. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Savatin D.V., Sicilia F., Gramegna G., Cervone F., Lorenzo G.D. (2013). Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filisetti-Cozzi T.M., Carpita N.C. (1991). Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 197: 157–162. [DOI] [PubMed] [Google Scholar]
- Gachon C., Saindrenan P. (2004). Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiol. Biochem. 42: 367–371. [DOI] [PubMed] [Google Scholar]
- Galletti R., Denoux C., Gambetta S., Dewdney J., Ausubel F.M., De Lorenzo G., Ferrari S. (2008). The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 148: 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R., Ferrari S., De Lorenzo G. (2011). Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellin-induced resistance against Botrytis cinerea. Plant Physiol. 157: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S., Hänsel U., Ziemann M., Pauly M. (2009). Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc. Natl. Acad. Sci. USA 106: 14699–14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.R., Jones J.D.G. (2009). Hormone (dis)harmony moulds plant health and disease. Science 324: 750–752. [DOI] [PubMed] [Google Scholar]
- Gu X., Bar-Peled M. (2004). The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of Arabidopsis UDP-D-glucuronic acid 4-epimerase. Plant Physiol. 136: 4256–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M.G., Darvill A.G., Albersheim P. (1981). Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 68: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Suttangkakul A., Vibe Scheller H. (2010). Biosynthesis of pectin. Plant Physiol. 153: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Blanco C., et al. (2007). Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19: 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D., Tootle T.L., Reuber T.L., Frost L.N., Feys B.J., Parker J.E., Ausubel F.M., Glazebrook J. (1999). Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA 96: 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D.G., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Jones L., Seymour G.B., Knox J.P. (1997). Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1->4)-beta-D-galactan. Plant Physiol. 113: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars I., Krooshof G.H., Wagemakers L., Joosten R., Benen J.A.E., van Kan J.A.L. (2005b). Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 43: 213–225. [DOI] [PubMed] [Google Scholar]
- Kars I., McCalman M., Wagemakers L., VAN Kan J.A.L. (2005a). Functional analysis of Botrytis cinerea pectin methylesterase genes by PCR-based targeted mutagenesis: Bcpme1 and Bcpme2 are dispensable for virulence of strain B05.10. Mol. Plant Pathol. 6: 641–652. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Zemelis S., Keegstra K., Brandizzi F. (2015). The cytoplasmic localization of the catalytic site of CSLF6 supports a channeling model for the biosynthesis of mixed-linkage glucan. Plant J. 81: 537–547. [DOI] [PubMed] [Google Scholar]
- Kliebenstein D.J., Rowe H.C., Denby K.J. (2005). Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J. 44: 25–36. [DOI] [PubMed] [Google Scholar]
- Kohorn B.D., Kohorn S.L., Saba N.J., Martinez V.M. (2014). Requirement for pectin methyl esterase and preference for fragmented over native pectins for wall-associated kinase-activated, EDS1/PAD4-dependent stress response in Arabidopsis. J. Biol. Chem. 289: 18978–18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman A.H., Wightman R., Geshi N., Turner S.R., Scheller H.V. (2010). Arabidopsis - a powerful model system for plant cell wall research. Plant J. 61: 1107–1121. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus F.A., Murthy P.P.N. (2000). Myo-inositol metabolism in plants. Plant Sci. 150: 1–19. [Google Scholar]
- Miles G.P., Samuel M.A., Zhang Y., Ellis B.E. (2005). RNA interference-based (RNAi) suppression of AtMPK6, an Arabidopsis mitogen-activated protein kinase, results in hypersensitivity to ozone and misregulation of AtMPK3. Environ. Pollut. 138: 230–237. [DOI] [PubMed] [Google Scholar]
- Mohnen D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11: 266–277. [DOI] [PubMed] [Google Scholar]
- Mølhøj M., Verma R., Reiter W.D. (2004). The biosynthesis of D-Galacturonate in plants. functional cloning and characterization of a membrane-anchored UDP-D-Glucuronate 4-epimerase from Arabidopsis. Plant Physiol. 135: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G., Ralet M.C., Cavelier C., Eland C., Effroy D., Hématy K., McCartney L., Truong H.N., Gaudon V., Thibault J.F., Marchant A., Höfte H. (2007). Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J. 50: 605–614. [DOI] [PubMed] [Google Scholar]
- Nishimura M.T., Stein M., Hou B.H., Vogel J.P., Edwards H., Somerville S.C. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301: 969–972. [DOI] [PubMed] [Google Scholar]
- Nobuta K., Okrent R.A., Stoutemyer M., Rodibaugh N., Kempema L., Wildermuth M.C., Innes R.W. (2007). The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 144: 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons H.T., et al. (2012). Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 159: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C.M.J., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S.C.M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28: 489–521. [DOI] [PubMed] [Google Scholar]
- Puhlmann J., Bucheli E., Swain M.J., Dunning N., Albersheim P., Darvill A.G., Hahn M.G. (1994). Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1-->2)-linked fucosyl-containing epitope. Plant Physiol. 104: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H.C., Kliebenstein D.J. (2007). Elevated genetic variation within virulence-associated Botrytis cinerea polygalacturonase loci. Mol. Plant Microbe Interact. 20: 1126–1137. [DOI] [PubMed] [Google Scholar]
- Rowe H.C., Walley J.W., Corwin J., Chan E.K.F., Dehesh K., Kliebenstein D.J. (2010). Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 6: e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible W.R., Eshed R., Richmond T., Delmer D., Somerville C. (2001). Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc. Natl. Acad. Sci. USA 98: 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H.V., Ulvskov P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61: 263–289. [DOI] [PubMed] [Google Scholar]
- Scott T.A., Melvin E.H. (1953). Determination of dextran with anthrone. Anal. Chem. 25: 1656–1661. [Google Scholar]
- Seifert G.J. (2004). Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Curr. Opin. Plant Biol. 7: 277–284. [DOI] [PubMed] [Google Scholar]
- Steffan W., Kovác P., Albersheim P., Darvill A.G., Hahn M.G. (1995). Characterization of a monoclonal antibody that recognizes an arabinosylated (1-->6)-beta-D-galactan epitope in plant complex carbohydrates. Carbohydr. Res. 275: 295–307. [DOI] [PubMed] [Google Scholar]
- Tao Y., Xie Z., Chen W., Glazebrook J., Chang H.S., Han B., Zhu T., Zou G., Katagiri F. (2003). Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R., Thulke O. (1996). Cloning of an enzyme that synthesizes a key nucleotide-sugar precursor of hemicellulose biosynthesis from soybean: UDP-glucose dehydrogenase. Plant Physiol. 112: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Have A., Mulder W., Visser J., van Kan J.A.L. (1998). The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant Microbe Interact. 11: 1009–1016. [DOI] [PubMed] [Google Scholar]
- Truman W., Glazebrook J. (2012). Co-expression analysis identifies putative targets for CBP60g and SARD1 regulation. BMC Plant Biol. 12: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman W., Sreekanta S., Lu Y., Bethke G., Tsuda K., Katagiri F., Glazebrook J. (2013). The CALMODULIN-BINDING PROTEIN60 family includes both negative and positive regulators of plant immunity. Plant Physiol. 163: 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D.M. (1969). Semimicro determination of cellulose in biological materials. Anal. Biochem. 32: 420–424. [DOI] [PubMed] [Google Scholar]
- Usadel B., Schlüter U., Mølhøj M., Gipmans M., Verma R., Kossmann J., Reiter W.D., Pauly M. (2004). Identification and characterization of a UDP-D-glucuronate 4-epimerase in Arabidopsis. FEBS Lett. 569: 327–331. [DOI] [PubMed] [Google Scholar]
- Valette-Collet O., Cimerman A., Reignault P., Levis C., Boccara M. (2003). Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces virulence on several host plants. Mol. Plant Microbe Interact. 16: 360–367. [DOI] [PubMed] [Google Scholar]
- van den Hoogen B.M., van Weeren P.R., Lopes-Cardozo M., van Golde L.M.G., Barneveld A., van de Lest C.H.A. (1998). A microtiter plate assay for the determination of uronic acids. Anal. Biochem. 257: 107–111. [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y., Marcus S.E., Haeger A., Ordaz-Ortiz J.J., Knox J.P. (2009a). An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 344: 1858–1862. [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y., Marcus S.E., Haeger A., Verhoef R., Schols H.A., McCleary B.V., McKee L., Gilbert H.J., Knox J.P. (2009b). Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 59: 413–425. [DOI] [PubMed] [Google Scholar]
- Vogel J.P., Raab T.K., Schiff C., Somerville S.C. (2002). PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14: 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Raab T.K., Somerville C.R., Somerville S.C. (2004). Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 40: 968–978. [DOI] [PubMed] [Google Scholar]
- von Malek B., van der Graaff E., Schneitz K., Keller B. (2002). The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216: 187–192. [DOI] [PubMed] [Google Scholar]
- Wang L., Mitra R.M., Hasselmann K.D., Sato M., Lenarz-Wyatt L., Cohen J.D., Katagiri F., Glazebrook J. (2008). The genetic network controlling the Arabidopsis transcriptional response to Pseudomonas syringae pv. maculicola: roles of major regulators and the phytotoxin coronatine. Mol. Plant Microbe Interact. 21: 1408–1420. [DOI] [PubMed] [Google Scholar]
- Wang L., Tsuda K., Truman W., Sato M., Nguyen V., Katagiri F., Glazebrook J. (2011). CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant J. 67: 1029–1041. [DOI] [PubMed] [Google Scholar]
- Wiermer M., Feys B.J., Parker J.E. (2005). Plant immunity: the EDS1 regulatory node. Curr. Opin. Plant Biol. 8: 383–389. [DOI] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- Willats W.G.T., Marcus S.E., Knox J.P. (1998). Generation of monoclonal antibody specific to (1-->5)-alpha-L-arabinan. Carbohydr. Res. 308: 149–152. [DOI] [PubMed] [Google Scholar]
- Willats W.G.T., et al. (2004). A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 218: 673–681. [DOI] [PubMed] [Google Scholar]
- Windram O., et al. (2012). Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 24: 3530–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Dong X. (2014). Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 20: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York W.S., Darvill A.G., Mcneil M., Albersheim P. (1985). Structure of plant cell walls. 16. 3-Deoxy-D-manno-2-octulosonic acid (Kdo) is a component of rhamnogalacturonan-II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 138: 109–126. [Google Scholar]
- Zablackis E., Huang J., Müller B., Darvill A.G., Albersheim P. (1995). Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandleven J., Sørensen S.O., Harholt J., Beldman G., Schols H.A., Scheller H.V., Voragen A.J. (2007). Xylogalacturonan exists in cell walls from various tissues of Arabidopsis thaliana. Phytochemistry 68: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Zhang L., Thiewes H., van Kan J.A.L. (2011). The D-galacturonic acid catabolic pathway in Botrytis cinerea. Fungal Genet. Biol. 48: 990–997. [DOI] [PubMed] [Google Scholar]
- Zhang L., van Kan J.A.L. (2013). Botrytis cinerea mutants deficient in D-galacturonic acid catabolism have a perturbed virulence on Nicotiana benthamiana and Arabidopsis, but not on tomato. Mol. Plant Pathol. 14: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Tootle T.L., Glazebrook J. (1999). Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11: 2419–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.