A transmembrane region of the guard cell SLAC1 anion channel contains sites of CO2 signal perception, which could be different from the sites of ABA signal perception.
Abstract
The guard cell S-type anion channel, SLOW ANION CHANNEL1 (SLAC1), a key component in the control of stomatal movements, is activated in response to CO2 and abscisic acid (ABA). Several amino acids existing in the N-terminal region of SLAC1 are involved in regulating its activity via phosphorylation in the ABA response. However, little is known about sites involved in CO2 signal perception. To dissect sites that are necessary for the stomatal CO2 response, we performed slac1 complementation experiments using transgenic plants expressing truncated SLAC1 proteins. Measurements of gas exchange and stomatal apertures in the truncated transgenic lines in response to CO2 and ABA revealed that sites involved in the stomatal CO2 response exist in the transmembrane region and do not require the SLAC1 N and C termini. CO2 and ABA regulation of S-type anion channel activity in guard cells of the transgenic lines confirmed these results. In vivo site-directed mutagenesis experiments targeted to amino acids within the transmembrane region of SLAC1 raise the possibility that two tyrosine residues exposed on the membrane are involved in the stomatal CO2 response.
INTRODUCTION
Stomata are pores formed by pairs of guard cells that mediate gas exchange between plants and the atmosphere. Adjustment of stomatal apertures is achieved by controlled transport of osmoregulatory ions through several types of ion channels (Hetherington and Woodward, 2003; Kim et al., 2010). Blue light activates a H+-ATPase that induces plasma membrane hyperpolarization to drive K+ uptake through inward K+ channels, causing stomatal opening (Kinoshita and Hayashi, 2011). By contrast, when guard cells perceive a high CO2 concentration, ozone, and the plant hormone abscisic acid (ABA), anion channels are activated and the efflux of anions induces plasma membrane depolarization that activates outward K+ channels, causing stomatal closure (Kim et al., 2010; Kollist et al., 2011; Negi et al., 2014).
SLAC1, an S-type anion channel that was genetically isolated from mutant screenings, plays a key role in stomatal closing (Negi et al., 2008; Vahisalu et al., 2008). Most studies of SLAC1 regulation have been concerned with the phosphorylation of the SLAC1 N-terminal region in the ABA signaling pathway. OPEN STOMATA1 (OST1), an important kinase in stomatal ABA signaling (Mustilli et al., 2002; Yoshida et al., 2002), activates the SLAC1 by phosphorylating Ser-120 (S120) in the N-terminal region (Geiger et al., 2009; Lee et al., 2009; Vahisalu et al., 2010). The PP2C-type phosphatase ABA INSENSITIVE1 (ABI1) inhibits this phosphorylation by interacting with OST1. CALCIUM-DEPENDENT PROTEIN KINASE6 (CPK6) is involved in ABA-induced stomatal closure (Mori et al., 2006). Oocyte electrophysiology experiments demonstrated that CPK6 interacts with SLAC1, leading to its activation via phosphorylation of S59 in the N-terminal region of SLAC1. ABI1 directly dephosphorylates S59 and other N-terminal residues (Brandt et al., 2012). Replacement of both S59 and S120 with alanine severely impairs ABA activation of S-type anion channels and stomatal closing in vivo (Brandt et al., 2015). This mechanism for SLAC1 activation by ABA was confirmed by reconstitution experiments in Xenopus laevis oocytes using CPK6 or OST1, ABI1, and the ABA receptor PYRABACTIN RESISTANCE1 (Brandt et al., 2012). CPK23, GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1), and CBL INTERACTING PROTEIN KINASE23 (CIPK23) also activate SLAC1 via phosphorylation of its N-terminal region in X. laevis oocytes (Geiger et al., 2010; Hua et al., 2012; Maierhofer et al., 2014). The target amino acid residues of these kinases remain to be directly determined.
In addition to the N-terminal region of SLAC1, the C-terminal region is also phosphorylated by OST1 (Lee et al., 2009). Site-directed mutagenesis experiments showed that the phosphorylation-mimic mutant T513D, in which T513 of SLAC1 is replaced with aspartic acid, exhibits kinase-independent S-type anion channel activity, suggesting the possibility that T513 is involved in the activation of SLAC1 (Maierhofer et al., 2014). When T513 is replaced with alanine (T513A), SLAC1 channel activity is still observed upon coexpression with OST1 or CPK6 (Maierhofer et al., 2014).
Although several studies have provided detailed information about ABA-induced SLAC1 activation, the mechanism for CO2-induced SLAC1 activation remains unclear. The carbonic anhydrases BETA CARBONIC ANHYDRASE1 (βCA1) and βCA4 catalyze fast, reversible hydration of CO2 to bicarbonate in guard cells and accelerate CO2-induced stomatal closing (Hu et al., 2010). HIGH LEAF TEMPERATURE1 (HT1) was identified as a negative regulator of the CO2 response pathway (Hashimoto et al., 2006). These studies suggest the possible existence of a CO2-specific pathway. RESISTANT TO CO2 (RHC1) was also recently characterized as a component of the CO2-specific signaling pathway (Tian et al., 2015). Oocyte electrophysiology experiments and kinase assays suggested that RHC1 mediates SLAC1 activation by inhibiting HT1. By contrast, ost1 mutants were reported to be insensitive to both CO2 and ABA, suggesting that the convergence point of both signal transduction pathways occurs at the level of OST1 or earlier (Xue et al., 2011).
In addition, F450 is located in the pore domain of the SLAC1 channel and is a key amino acid residue for gating the channel (Chen et al., 2010). Very large SLAC1 currents are observed when a mutant SLAC1 protein in which F450 is replaced by alanine (F450A) is expressed in oocytes, suggesting that the side chain of F450 occludes the SLAC1 channel pore (Chen et al., 2010).
These previous studies demonstrated mechanisms for SLAC1 activation in vitro. However, little research has been performed from the viewpoint of the stomatal responses to CO2. Here, we searched for sites in SLAC1 that are necessary for the stomatal response to CO2 using transgenic plants. Using several independent methods, we found that transgenic plants expressing SLAC1 proteins truncated at both the C- and N-terminal regions restored the slac1 CO2-insensitive phenotype, whereas these truncated forms of SLAC1 did not restore the ABA-insensitive phenotype. These observations suggested that SLAC1 perceives CO2 signals at a transmembrane region via an ABA-independent pathway.
RESULTS
Transgenic Lines Expressing SLAC1 Proteins Truncated at Both the C- and N-Terminal Regions Restore the slac1 CO2-Insensitive Phenotype
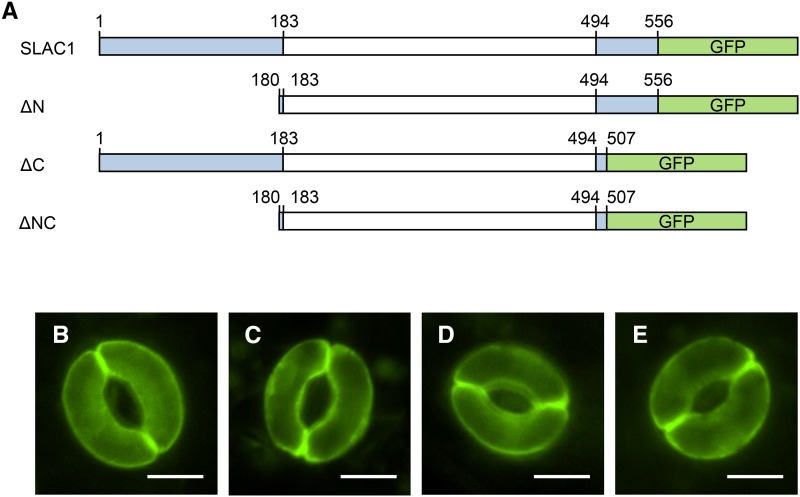
To dissect the sites of SLAC1 necessary for stomatal CO2 response in vivo, transgenic plants expressing several truncated variants of SLAC1 protein were analyzed for their ability to respond to CO2. SLAC1 truncated constructs in the N-terminal, C-terminal, or both terminal regions (ΔN, ΔC, and ΔNC, amino acids 180 to 556, 1 to 507, and 180 to 507, respectively) and a full-length SLAC1 construct were introduced into slac1-4, a T-DNA insertion null mutant, under the control of the native SLAC1 promoter (Figure 1A). GFP was fused to the C terminus of all truncated and full-length SLAC1 proteins in order to confirm their localization to stomatal plasma membranes by fluorescence microscopic imaging (Figures 1B to 1E).
Figure 1.
Truncated Variants of SLAC1 Used in This Study.
(A) Illustration of the full-length SLAC1 protein and its truncated variants. GFP was fused to a series of truncated SLAC1 proteins at the C-terminal region. The amino acid residue numbers are as indicated. A transmembrane region (amino acids 183 to 494) predicted using the ARAMEMNON database (http://aramemnon.uni-koeln.de/) is shown in white.
(B) to (E) Subcellular localization of truncated SLAC1 proteins in the slac1-4 transgenic plants. Fluorescence microscopy images of GFP fused to SLAC1 and its truncated variant proteins, full-length SLAC1 (B), ΔN (C), ΔC (D), and ΔNC (E), in guard cells. Bars = 5 µm.
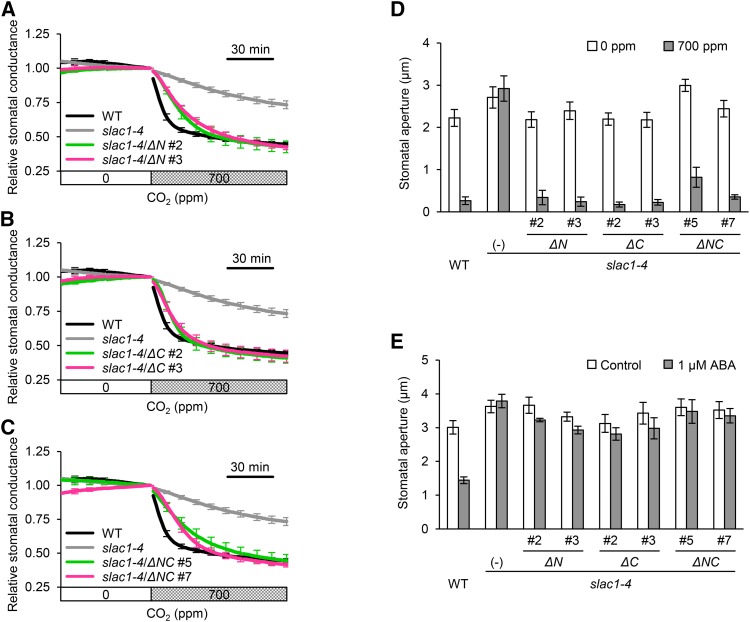
Using gas-exchange measurements, we investigated whether ∆N, ∆C, and ∆NC transgenic lines restored the slac1 CO2-insensitive phenotype. Increases in CO2 concentration from 0 to 700 ppm reduced stomatal conductance in wild-type plants, whereas the conductance was hardly reduced in the slac1-4 mutant (Figures 2A to 2C). Unexpectedly, the ΔN, ΔC, and ΔNC transgenic lines were still able to respond to CO2 (Figures 2A to 2C), even though the responses in the ΔN and ΔNC transgenic lines were slightly slower. We also examined the stomatal apertures of transgenic lines exposed to low or high CO2 levels. When plants were exposed to high CO2 concentrations, the stomata of the transgenic lines closed in a manner similar to those of the wild type (Figure 2D), consistent with the stomatal conductance measurements. These results suggested that the sites involved in stomatal CO2 response exist in the transmembrane region of SLAC1.
Figure 2.
SLAC1 Truncated Proteins in the N-, C-, or N- and C-Terminal Regions Are Functional in the Stomatal Response to CO2, Whereas the Truncated Forms of SLAC1 Do Not Function in the ABA Response.
(A) to (C) Time-resolved relative stomatal conductance in response to [CO2] changes in the wild type, slac1-4 mutant, ΔN transgenic slac1-4 (slac1-4/ΔN) (A), ΔC transgenic slac1-4 (slac1-4/ΔC) (B), and ΔNC transgenic slac1-4 (slac1-4/ΔNC) (C). Wild-type (black) and slac1-4 mutant (light gray) control traces are the same in (A) to (C). Two independent transgenic lines per construct (magenta or green) were analyzed. Plants were kept under constant white light of 150 µmol m−2 s−1 at 22°C in 45% relative humidity. Conductance was normalized to the average conductance at the last 0 ppm data point. Error bars indicate ± se; n = 5 to 7.
(D) CO2 regulation of stomatal aperture in the wild type, slac1-4 mutant, and transgenic lines. Stomata in the transgenic plants closed in response to high [CO2]. White bars indicate low CO2 (0 ppm), and gray bars indicate high CO2 (700 ppm) conditions. Plants were kept under constant white light of 42 µmol m−2 s−1 at 22°C in 45% relative air humidity. Error bars indicate ±se; data from four independent experiments were averaged.
(E) ABA regulation of stomatal aperture in the wild type, the slac1-4 mutant, and transgenic lines. White and gray bars indicate stomatal apertures in the absence (control) and presence of 1 µM ABA. All of the transgenic lines, ΔN, ΔC, and ΔNC, failed to respond to ABA. Error bars indicate ± se; data from four to five independent experiments were averaged.
ABA also activates the SLAC1 channel and induces stomatal closure. We investigated the stomatal ABA response in the ΔN, ΔC, and ΔNC transgenic lines. Measurements of stomatal aperture showed that the stomatal ABA response was not restored in any of the transgenic lines (Figure 2E). Thus, not only the N-terminal region but also the C-terminal region of SLAC1 is involved in the response to ABA in vivo.
Replacement of S120 by Alanine Has No Effect on the Stomatal CO2 Response in Vivo
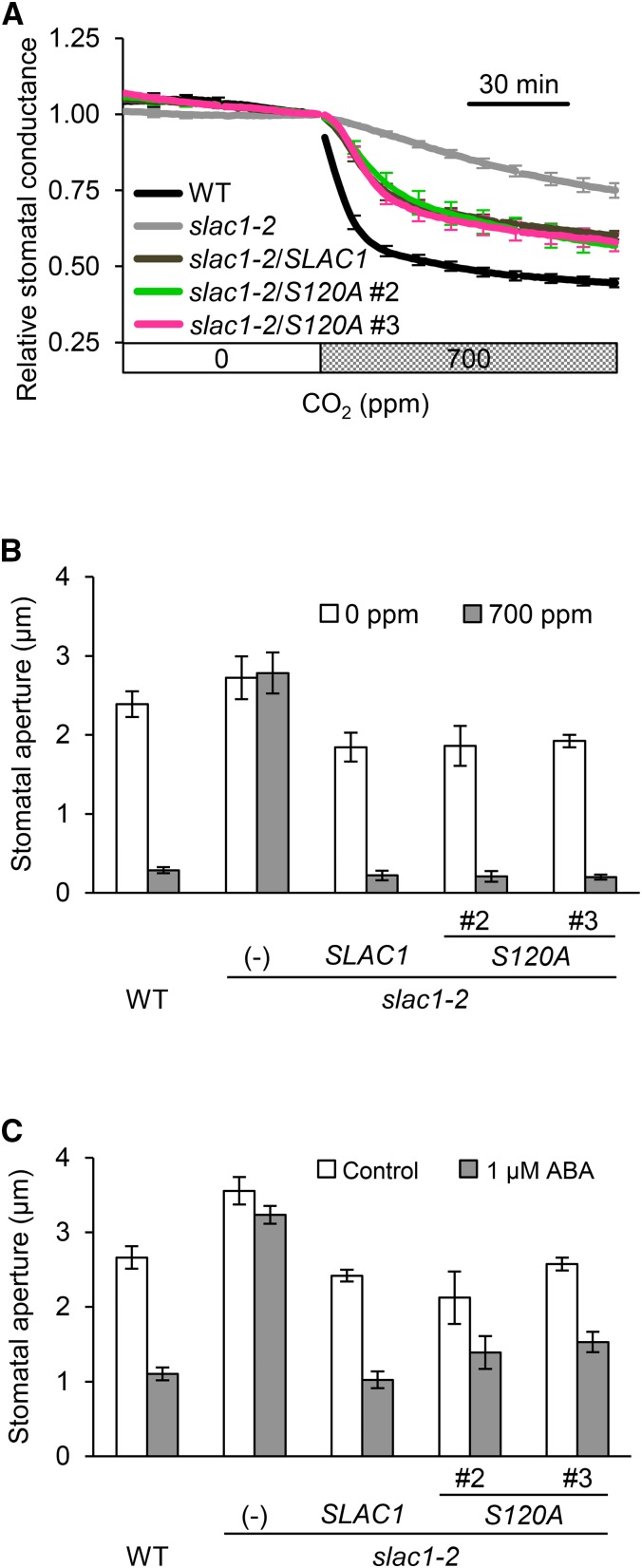
The N-terminal region of SLAC1 harbors multiple phosphorylation sites, including S120, which is reported to be phosphorylated by OST1 (Geiger et al., 2009; Lee et al., 2009; Vahisalu et al., 2010). OST1, an important kinase in stomatal ABA signaling, also participates in CO2 signaling (Xue et al., 2011; Merilo et al., 2013; Tian et al., 2015). To assess the effect of phosphorylating S120 on the stomatal CO2 response in vivo, a complementation line expressing a mutant SLAC1 protein in which S120 was replaced with alanine (S120A) was analyzed. The SLAC1(S120A)-GFP and SLAC1(WT)-GFP fusion constructs were introduced into a slac1 mutant, slac1-2, under the control of the native SLAC1 promoter. Gas-exchange measurements demonstrated that the S120A lines and the wild-type SLAC1 complementation line had similar declining patterns of stomatal conductance in the presence of elevated CO2 levels (Figure 3A). Similar results were obtained from stomatal aperture measurements (Figure 3B). The degree of response to CO2 examined by stomatal aperture seems to be larger than those for stomatal conductance. This inconsistency may be due to differences in the measurement method. Either way, the stomata of these transgenic plants were irrefutably able to close in response to CO2. In the ABA response, stomata of the S120A lines also closed (Figure 3C), a result in agreement with a previous report (Brandt et al., 2015). Our data provide evidence that phosphorylation of S120 contributes relatively little to in vivo stomatal CO2 responses.
Figure 3.
The Transgenic Line Expressing SLAC1 S120A Restores the slac1-2 CO2-Insensitive Phenotype.
(A) Time-resolved relative stomatal conductance response to [CO2] in the wild type (black), the slac1-2 mutant (light gray), wild-type SLAC1 complementation line (dark gray), and transgenic lines expressing SLAC1 S120A (magenta or green). Two independent transgenic lines were analyzed. Plants were kept under constant white light of 150 µmol m−2 s−1 at 22°C in 45% relative humidity. Conductance was normalized to the average conductance at the last 0 ppm data point. Error bars indicate ± se; n = 5 to 7.
(B) CO2 regulation of stomatal aperture in the wild type, slac1-2 mutant, and transgenic lines expressing SLAC1 S120A. White bars indicate low CO2 (0 ppm), and gray bars indicate high CO2 (700 ppm) conditions. Stomata in the transgenic plants closed in response to high [CO2]. Plants were kept under constant white light of 42 µmol m−2 s−1 at 22°C in 45% relative humidity. Error bars indicate ± se; data from four independent experiments were averaged.
(C) ABA regulation of stomatal aperture in the wild type, the slac1-2 mutant, and transgenic lines. White and gray bars indicate stomatal apertures in the absence (control) and presence of 1 µM ABA. Error bars indicate ± se; data from four to five independent experiments were averaged.
The Sites Involved in CO2-Induced S-Type Anion Channel Activation Could Be Different from the Sites of ABA Activation
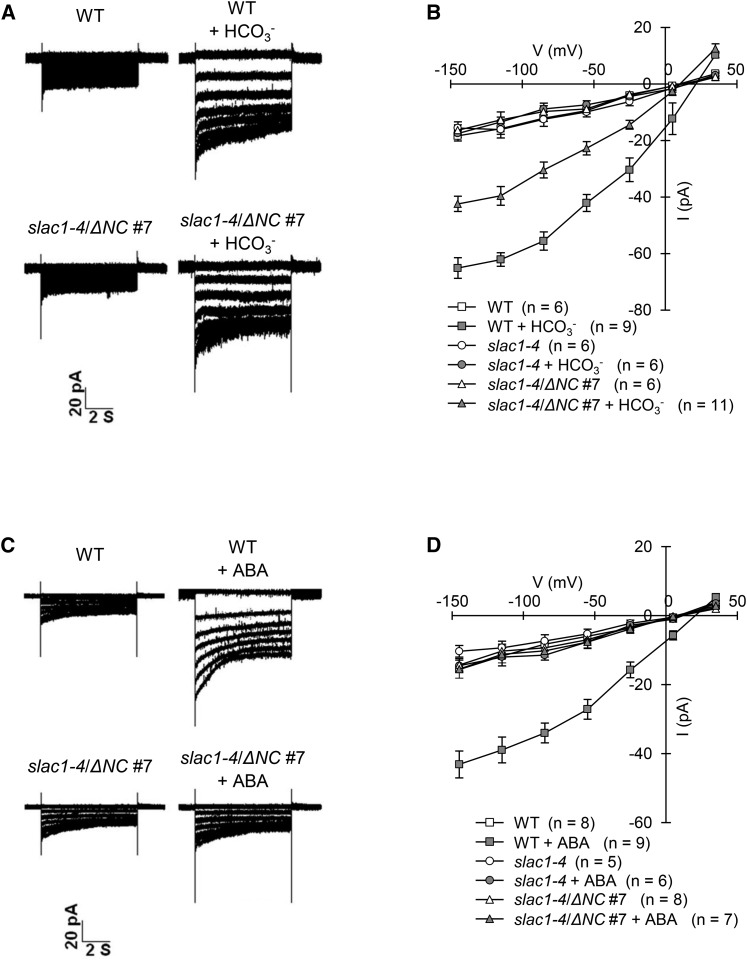
We examined CO2- or ABA-induced S-type anion channel activity in guard cells of the ΔNC transgenic lines using whole-cell patch clamp experiments. Plants respond to elevated CO2, and 13.5 mM intracellular bicarbonate (11.5 mM free [HCO3−]/2 mM free [CO2]) activates S-type anion channels in guard cells (Hu et al., 2010; Xue et al., 2011). Accordingly, we used 13.5 mM bicarbonate as a CO2 stimulus for S-type anion channel activation. In addition, ABA activation of S-type anion channels was investigated. In these analyses, guard cell protoplasts (GCPs) were prepared from wild-type plants, the slac1-4 mutant, and ΔNC transgenic slac1-4 lines. Whole-cell patch clamp experiments showed that bicarbonate activation of S-type anion currents was restored in the slac1-4 guard cells expressing the ΔNC protein, although S-type anion currents were partially impaired compared with the wild type (Figures 4A and 4B; Supplemental Figures 1A and 1B). Since SLAC1 is impermeable to bicarbonate (Geiger et al., 2009; Xue et al., 2011), this result suggested that SLAC1 was activated by CO2 signal perception in the transmembrane region. By contrast, ABA activation of S-type anion currents in the ΔNC transgenic slac1-4 guard cells was completely disrupted, similar to the slac1-4 mutant (Figures 4C and 4D; Supplemental Figures 1C and 1D). Therefore, the mechanism for CO2-induced SLAC1 activation differs from that for ABA.
Figure 4.
Bicarbonate Activation of the S-Type Anion Currents Is Restored in Guard Cells of the ΔNC Transgenic Line, Whereas ABA Activation of the S-Type Anion Currents Is Impaired.
(A) Whole-cell currents in guard cell protoplasts of the wild type and the ΔNC transgenic line (slac1-4/ΔNC #7) with or without bicarbonate.
(B) Steady state current-voltage relationships of the whole-cell currents recorded in the wild type (squares), slac1-4 mutant (circles), and the ΔNC transgenic line (triangles) with (closed symbols) or without (open symbols) bicarbonate. Error bars indicate ± se; n = 6 to 11 guard cells.
(C) Whole-cell currents in guard cell protoplasts of the wild type and the ΔNC transgenic line (slac1-4/ΔNC #7) with or without ABA.
(D) Steady state current-voltage relationships of the whole-cell currents recorded in the wild type (squares), slac1-4 mutant (circles), and the ΔNC transgenic line (triangles) with (closed symbols) or without (open symbols) ABA. Error bars indicate ± se; n = 5 to 9 guard cells.
Stomatal Response of the Single Amino Acid Substitution Lines
To investigate the possibility that SLAC1 is phosphorylated in the CO2-signaling pathway in a manner similar to the ABA-signaling pathway, we performed immunoblot analysis using SDS-PAGE gels containing Phos-tag, a ligand that shifts the mobility of phosphorylated proteins (Kinoshita et al., 2006). For this analysis, proteins were extracted from GCPs that were prepared from the transgenic plants producing SLAC1-GFP after treatment with 13.5 mM bicarbonate or 10 µM ABA. SLAC1-GFP from GCPs treated with bicarbonate had a broad migration pattern that was similar to samples obtained from GCPs treated with ABA, reflecting the phosphorylated forms of SLAC1-GFP (Supplemental Figure 2). This result raises the possibility that SLAC1 is phosphorylated by CO2 stimuli in vivo.
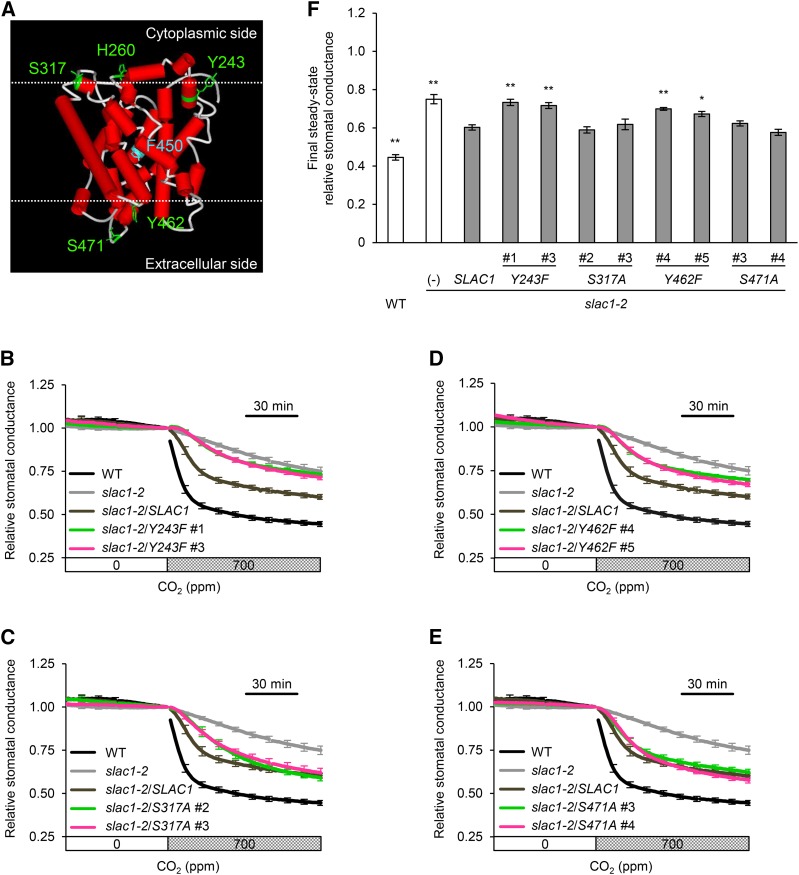
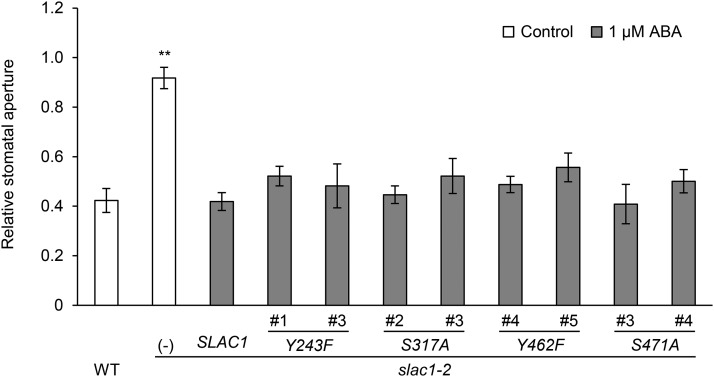
We evaluated the influence of replacing some potentially phosphorylatable amino acids in the stomatal CO2 response using site-directed mutagenesis experiments. Serine, threonine (T), tyrosine (Y), and histidine (H) residues, potential sites for activation by phosphorylation in plant proteins, were selected as candidates for these experiments from the amino acid residues that are exposed on the membrane of the SLAC1 ΔNC protein based on a model of the protein structure (Figure 5A). Next, because the SLAH1 and SLAH3 genes were reported to complement the slac1-2 CO2-insensitive phenotype (Negi et al., 2008), we narrowed down the candidates to Y243, H260, S317, Y462, and S471, five amino acid residues that are conserved among the SLAC1, SLAH1, and SLAH3 proteins. Transgenic lines in which each one of these five amino acids was individually substituted were constructed using the same method as for the construction of S120A. In addition, we constructed the slac1-2 mutant producing the mutant SLAC1 protein F450A. F450 acts as a gate that regulates the SLAC1 anion channel (Chen et al., 2010). F450A exhibited constitutively low stomatal conductance regardless of the CO2 concentration (Supplemental Figure 3), showing that this measurement works well for evaluating the influence of replacing amino acids. GFP signals were observed in the Y243F, S317A, Y462F, and S471A lines (Supplemental Figure 4), but not in the H260F lines. These observations suggest that H260F protein is not stable in guard cells; thus, H260F lines were not used. Gas-exchange measurements of the slac1 complementation lines showed that elevated CO2 concentrations reduced stomatal conductance in the S317A and S471A lines at levels similar to that of the wild-type SLAC1 complementation line, whereas Y243F and Y462F lines were impaired in the CO2 response (Figures 5B to 5F). These results were in agreement with stomatal movement analyses (Supplemental Figure 5). We also tested several slac1 complementation lines for their ability to respond to ABA. All of the lines restored the ABA regulation of stomatal movements (Figure 6; Supplemental Figure 6), indicating that these amino acid substitutions do not cause structural defects in the SLAC1 channel. From these findings, we conclude that it is likely that phosphorylation of some amino acids is involved in CO2-induced SLAC1 activation.
Figure 5.
Stomatal Response of the slac1-2 Mutant Expressing SLAC1 Proteins Containing a Single Amino Acid Substitution.
(A) Structural model of the transmembrane region of SLAC1 (amino acids 180 to 507) generated by homology modeling using Modeler (Fiser and Sali, 2003). The crystal structure of HiTehA, a bacterial homolog (Haemophilus influenza) of SLAC1 (PDB ID 3M72), was used as the template for the modeling according to Chen et al. (2010). The substituted amino acids are shown in green. Serine (S) was substituted with alanine (A). Tyrosine (Y) and histidine (H) were substituted with phenylalanine (F). Phe-450 (F450) that has been reported to gate a central five-helix transmembrane pore of SLAC1 is shown in blue. The cytoplasmic and extracellular membrane surfaces were predicted according to Spassov et al. (2002) and are depicted in upper and lower dotted lines, respectively.
(B) to (E) Time-resolved relative stomatal conductance response to [CO2] in the transgenic lines expressing mutant SLAC1 protein Y243F (B), S317A (C), Y462F (D), and S471A (E). The wild type (black), slac1-2 mutant (light gray), and wild-type SLAC1 complementation line (dark gray) control traces are the same in (B) to (E). Two independent transgenic lines per construct (magenta or green) were analyzed. Plants were kept under constant white light of 150 µmol m−2 s−1 at 22°C in 45% relative humidity. Conductance was normalized to the average conductance at the last 0 ppm data point. Error bars indicate ± se; n = 5 to 7.
(F) Final steady state relative stomatal conductance of the transgenic lines. Asterisks denote comparison with wild-type SLAC1 complementation line: **P < 0.05 and *P < 0.1 by one-way ANOVA with Dunnett's post hoc test.
Figure 6.
Amino Acid Substitutions Did Not Cause Structural Defects.
ABA regulation of relative stomatal aperture in the wild type, the slac1-2 mutant, and transgenic lines expressing mutant SLAC1 protein. Two independent transgenic lines per construct were analyzed. White and gray bars indicate relative stomatal apertures in the absence (control) and presence of 1 µM ABA. Error bars indicate ± se; data from four to five independent experiments were averaged. Asterisks denote comparison with wild-type SLAC1 complementation line: **P < 0.05 by one-way ANOVA with Dunnett's post hoc test.
DISCUSSION
In this study, we demonstrated that sites involved in the stomatal CO2 response in vivo exist in the transmembrane region of SLAC1 and that in contrast to ABA activation, the N and C termini of SLAC1 are not completely essential for CO2-induced stomatal closing. We showed that all the slac1 mutant transgenic lines producing SLAC1 proteins truncated in the N, C, or N and C termini, ΔN, ΔC, and ΔNC, responded to CO2, whereas the same lines did not respond to ABA (Figure 2). Whole-cell patch clamp experiments revealed that lines expressing the ΔNC protein had CO2-induced S-type anion channel activity in vivo, whereas these lines had no activity in response to ABA (Figure 4; Supplemental Figure 1). These results are in accordance with stomatal movement analyses of the ΔNC transgenic lines. In ΔNC guard cells, CO2-induced S-type anion channel activity was partially impaired compared with wild-type guard cells. Thus, our data do not exclude the possible existence of sites involved in the CO2 response in the N- or C-terminal regions. However, CO2-induced S-type anion currents were significantly different from those resulting from ABA treatment in ΔNC guard cells. Taken together, the transmembrane region of the SLAC1 channel appears to be sufficient for CO2-induced S-type anion channel activity in stomata.
Phosphorylation of S59 and S120 in the N-terminal region of SLAC1 by CPK6 and OST1 are crucial for SLAC1 activation in X. laevis oocytes (Geiger et al., 2009; Lee et al., 2009; Brandt et al., 2012, 2015). CPK23, GHR1, and CIPK23 also activate SLAC1 by phosphorylating the N-terminal region (Geiger et al., 2010; Hua et al., 2012; Maierhofer et al., 2014). The fact that the ΔN transgenic lines did not respond to ABA (Figure 2E) is in line with results from analyses with X. laevis oocytes and in vitro kinase assays. Whereas the N-terminal region of SLAC1 is known to be crucial for the ABA response, the significance of the C-terminal region of SLAC1 in response to ABA remains to be established in plants. Although replacement of T513 in the C-terminal region of SLAC1 by aspartate, which mimics phosphorylation, renders the SLAC1 constitutively active in the absence of any kinases in vitro (Maierhofer et al., 2014), the physiological role of the C-terminal region of SLAC1 for stomatal responses is unknown. We showed that stomata of the ΔC truncated lines were impaired in their response to ABA in planta (Figure 2E). Our results support the findings of the previous study (Maierhofer et al., 2014) and demonstrate that the C-terminal region of SLAC1 is essential for ABA-induced stomatal closure.
S120 is phosphorylated by OST1 in vitro. OST1 is a protein kinase that is involved in not only ABA signaling but also CO2 signaling (Xue et al., 2011; Merilo et al., 2013; Tian et al., 2015). To evaluate the effect of replacing S120 with alanine, we compared the S120A transgenic lines and a wild-type SLAC1 complementation line. These transgenic lines had a similar response to high CO2 concentrations and ABA (Figure 3), suggesting that phosphorylation of S120 is not strictly required for the stomatal response in vivo. In the ost1-3 mutant, bicarbonate activation of S-type anion channels is not completely impaired (Xue et al., 2011), providing evidence for the possible existence of some OST1-independent pathways. Since SLAC1 S120A cannot be activated by OST1 in X. laevis oocytes (Geiger et al., 2009; Brandt et al., 2015), S120 is an absolute requirement for OST1-mediated SLAC1 activation. Thus, ΔN, ΔNC, and S120A used in this study were not regulated though direct activation by OST1. Since these transgenic lines responded to CO2 (Figures 2 and 3), the following possibilities are suggested: (1) The transmembrane region of SLAC1 could perceive CO2 signals via an OST1-independent pathway, or (2) some downstream factors of OST1 interact with the transmembrane region of SLAC1. A tilling line, slac1-7, with the S120F mutation in SLAC1 is impaired in ozone-, drought-, and CO2-induced stomatal closing, suggesting the importance of S120 in regulating SLAC1 in vivo (Vahisalu et al., 2010; Merilo et al., 2013). However, since the slac1-7 is a tilling mutant, this line might contain additional mutations in other genes in addition to SLAC1. We cannot rule out the possibility that such mutations cause impairments in the stomatal movements of slac1-7.
Some previous studies demonstrated that phosphorylation regulates SLAC1 activity in response to ABA as described above, but the mechanism for the CO2 response remains unknown. The experiments using Phos-tag raise the possibility that SLAC1 is phosphorylated in response to CO2 in vivo (Supplemental Figure 2). In addition, several reports provide evidence that phosphorylation of some amino acids exposed on the membrane in the transmembrane region of other channel proteins is crucial for phosphorylation-dependent regulation of channel activity (Surti et al., 2005; Baek et al., 2014; Verdoucq et al., 2014). Based on these results, we hypothesized that phosphorylation of amino acids in the transmembrane domain causes SLAC1 activation in the CO2 response and performed site-directed mutagenesis experiments with transgenic plants. We analyzed stomatal responses of slac1 complementation lines expressing mutant SLAC1 proteins in which potentially phosphorylatable amino acids that are exposed on the membrane in the transmembrane region were substituted. We found that two single mutants, Y243F and Y462F, of SLAC1 could not complement the CO2 response in the slac1 transgenic plant, whereas these mutant proteins could restore the ABA response in the plant (Figures 5B to 5F and 6; Supplemental Figure 5). These results suggest that Y243 and Y462 in wild-type SLAC1 might be promising candidates for phosphorylation that would trigger activation of the channel. These residues are located at exposed sites within the α-helical transmembrane domain in the 3D model (Figure 5A). Y243 is located on the cytoplasmic side of the membrane. Thus, cytosolic kinases may catalyze the phosphorylation of Y243. By contrast, Y462 is located on the extracellular side. A secreted protein kinase was reported to phosphorylate extracellular domains of transmembrane proteins (Bordoli et al., 2014). We cannot presently exclude the notion that Y462 is phosphorylated by such secreted proteins. We also used Phos-tag SDS-PAGE experiments to detect mutant SLAC1 proteins. The mutant SLAC1 proteins treated with bicarbonate or ABA had broad migration patterns, and no differences were observed between mutant and wild-type SLAC1. We postulate that SLAC1 has multiple phosphorylation sites including those in the N- or C-terminal regions that are involved in the CO2 response. Based on this hypothesis, one possible explanation for the results of the Phos-tag SDS-PAGE experiments is that the broad migration pattern of SLAC1 may result from the accumulation of multiply phosphorylated SLAC1 protein, and differences in phosphorylation of a single amino acid are undetectable in the case of SLAC1 protein. Thus, Phos-tag SDS-PAGE experiments have provided no convincing evidence that Y243 and Y462 are phosphorylated. To identify the phosphorylation sites, mass spectroscopy (nano-liquid chromatography-tandem mass spectrometry) analysis is an effective technique (Sugiyama et al., 2008) that we attempted to use for analyzing SLAC1. However, because of the low expression of SLAC1 protein in planta, sufficient quantities of peptides necessary for this analysis were not obtained. Although it remains unknown which amino acids in SLAC1 are phosphorylated in the CO2 response, our key observation that two tyrosine residues exposed on the membrane are involved in the stomatal CO2 response offers new opportunities to investigate this phenomenon.
The point of convergence of CO2 and ABA signaling remains to be determined (Murata et al., 2015). Previous studies have shown that ABA enhances CO2-induced stomatal closing (Raschke, 1975; Merilo et al., 2015). Recently, Chater et al. (2015) suggested the following possibilities: (1) CO2-induced stomatal responses rapidly trigger ABA signaling, and (2) ABA increases the sensitivity of CO2-induced stomatal responses. Our research provides evidence for the second model. In the CO2-specific signaling pathway, a recent study has shown that the CO2-permeable PIP2;1 aquaporin protein is a key interactor of the β-carbonic anhydrase 4 that catalyzes a reaction converting CO2 into bicarbonate and protons, and mediates downstream CO2 signaling (Wang et al., 2016). The participation of protein kinases in bicarbonate activation of S-type anion channels has been shown in guard cells (Hashimoto et al., 2006; Xue et al., 2011; Tian et al., 2015), and a new study also shows that SLAC1 is a bicarbonate sensor in CO2 regulation of SLAC1 activation (Wang et al., 2016). Taken together, these results suggest that multiple pathways could be involved in the mediation of CO2 responses in vivo, consistent with a recent study on carbonic anhydrase functions (Hu et al., 2015). Reconstitution experiments using X. laevis oocytes are effective for understanding the individual pathways of CO2 signaling. For the application of reconstitution experiments to ΔNC SLAC1 protein, identification of the upstream kinases that interact with the transmembrane region of SLAC1 in CO2 signaling is required. Our next step would be to identify the upstream kinases using the yeast two-hybrid system and elucidate the distinct role(s) of the two tyrosines that we identified in this research by reconstitution experiments.
METHODS
Plant Materials and Growth Conditions
All lines of Arabidopsis thaliana used were derived from the Columbia ecotype. The mutants used in this study were slac1-2 (Negi et al., 2008) and slac1-4 (Salk_137265). Transgenic plants were generated by Agrobacterium tumefaciens-mediated transformation as previously described (Clough and Bent, 1998). Plants were grown on solid Murashige and Skoog medium for 19 d in a growth chamber (constant white light of 35 µmol m−2 s−1 at 22°C, 70% RH) and then transplanted into vermiculite pots supplemented with mineral nutrients. Plants 22 to 24 d old were used for the whole-plant gas-exchange experiments and microscopy analysis of stomatal responses.
Transgene Construction
For truncation constructs, pHGW (Karimi et al., 2002) digested with EcoRV and self-ligated was used as a binary vector. A NOS terminator was inserted between the SacI and HindIII sites of this vector. All sequences, including the SLAC1 promoter (−1568 to +1) and GFP, were isolated by PCR using the previously described pBI101-SLAC1-GFP construct (Negi et al., 2008) and inserted between the SalI and SacI sites of the binary vector. For ΔN, ΔC, and ΔNC, genomic sequences corresponding to the coding regions for amino acids 180 to 556, 1 to 507, and 180 to 507 of the SLAC1 protein were used, respectively. All primers used for plasmid construction are listed in Supplemental Table 1. For single amino acid substitution constructs, a fragment including the single amino acid substitution was prepared by recombinant PCR and inserted into the corresponding sites of pBI101-SLAC1-GFP (Negi et al., 2008). The DNA sequences harboring mutations causing the amino acid substitutions are listed in Supplemental Table 2.
Transgene Expression Analysis
Leaf epidermal peels prepared from stable transgenic plants harboring the ΔN, ΔC, or ΔNC constructs were analyzed for GFP fluorescence using a fluorescence microscope (IX73; Olympus). The filter set used was U-FGFP (exciter, band-pass 460 to 480 nm; emitter, band-pass 495 to 540 nm).
Whole-Plant Stomatal Conductance Measurements and Microscopy Analysis of Stomatal Responses
The whole-plant stomatal conductance to water vapor (gs) was measured with a portable gas-exchange fluorescence system (GFS-3000; Heinz Walz) equipped with a 3010-A Arabidopsis chamber. The gs response to CO2 was measured by increasing the [CO2] from 0 to 700 ppm at a constant light intensity (150 µmol m−2 s−1), 22°C, and 45% RH. The flow rate in the system was kept constant (750 µmol s−1) throughout the gas-exchange experiments. All measurements were taken every minute. For the microscopy analysis of stomatal responses, abaxial epidermal peels of the plants were used immediately to measure stomatal apertures. In the CO2 treatment, plants were equipped with an automatic CO2 control unit (FR-SP; Koito). After 90 min of adaptation to low [CO2] (0 ppm) or high [CO2] (700 ppm), epidermal tissues were peeled. In the ABA treatment, epidermal peels were floated on a test medium containing 30 mM KCl, 10 mM MES-KOH (pH 6.15), and 0.1 mM CaCl2 and were incubated in the growth chamber. ABA was added to the solution after 1 h of illumination and stomatal apertures were measured 2 h later. The stomatal apertures were observed in epidermal peels with a fluorescence microscope (IX71; Olympus). Results of stomatal apertures in response to CO2 and ABA were confirmed by double-blind analysis.
Isolation of Guard Cell Protoplasts
GCPs were isolated as described elsewhere with some modifications (Negi et al., 2008). Detached leaves were blended for 1 min and filtered through a 100-µm nylon mesh. Harvested epidermal peels were transferred to digestion solution 1 (0.5% [w/v] cellulase R-10 [Yakult Pharmaceutical Industry Co.], 0.002% [w/v] pectolyase Y-23 [Seishin Pharmaceutical], 0.1% [w/v] polyvinylpyrrolidone K-30, 0.2% [w/v] BSA, 0.25 M mannitol, 0.5 mM CaCl2, 0.5 mM MgCl2, and 10 mM MES-KOH, pH 5.5) and incubated at 24°C for 1 h in a vigorously shaking water bath. Subsequently, the peels were transferred to digestion solution 2 (1.3% [w/v] cellulase RS [Yakult Pharmaceutical Industry Co.], 0.0075% [w/v] pectolyase Y-23, 0.2% [w/v] BSA, 0.4 M mannitol, 0.5 mM CaCl2, and 0.5 mM MgCl2, pH 5.5) and incubated at 24°C for 1 h. Protoplasts were filtered through four layers of 10-µm nylon mesh and collected by centrifugation at 1000g for 10 min. To remove mesophyll cell fragments and other contaminants, the protoplasts were subjected to density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich) according to the method described previously (Negi et al., 2008).
Patch Clamp Analyses
Guard cell protoplasts were washed twice with washing solution containing 1 mM MgCl2, 1 mM CaCl2, 5 mM MES, and 500 mM d-sorbitol (pH 5.6 with Tris) by centrifugation for 10 min at 200g. During patch clamp recordings of S-type anion currents, the membrane voltage was adjusted to potentials starting at +35 to −145 mV for 7 s with −30-mV decrements and holding potential of +30 mV. The bath solutions contained 30 mM CsCl, 2 mM MgCl2, 10 mM MES-Tris (pH 5.6), and 1 mM CaCl2, with an osmolality of 485 mmol/kg. The pipette solutions contained 3.35 mM CaCl2, 6.7 mM EGTA, 2 mM MgCl2, 10 mM HEPES-Tris (pH 7.1), and 150 mM CsCl, with an osmolality of 500 mmol/kg. The final osmolalities of the both bath and pipette solutions were adjusted with d-sorbitol. Fresh Mg-ATP (5 mM) was added to the pipette solution before use. For analysis of the ABA activation of S-type anion channels, the guard cell protoplasts were preincubated with 50 μM ABA, which does not override activation of ABA signaling mutants, for 20 min before patch clamping, and patch clamp experiments were performed in the presence of 50 μM ABA in the bath and pipette solutions. For analysis of the bicarbonate activation of S-type anion channels, 13.5 mM bicarbonate (11.5 mM free [HCO3−]/2 mM free [CO2]) was freshly dissolved in the pipette solution before patch clamp experiments, and the free calcium concentration was 2 µM (5.86 mM CaCl2 in the pipette solution). Note that control solutions in which no bicarbonate was added also contained 2 µM Ca2+ in the pipette solution. The concentrations of free bicarbonate and CO2 were calculated using the Henderson-Hasselbalch equation (pH = pK1+log [HCO3−]/[CO2]), where [HCO3−] represents the free bicarbonate concentration and [CO2] represents the free CO2 concentration; pK1 = 6.352 was used for the calculation (Speight, 2005).
Phos-Tag SDS-PAGE and Immunological Analysis
GCPs were treated with 13.5 mM CsHCO3 or 10 µM ABA in a buffer containing 30 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM MES-Tris (pH 5.6), and 0.4 M mannitol for 30 min. Total proteins were extracted from the GCPs in a lysis buffer containing 150 mM NaCl, 10% glycerol, 50 mM Tris-HCl (pH 7.5), 0.5% (v/v) Triton X-100, and 1 mM EDTA. Subsequently, an equal quantity of sample buffer containing 4% (w/v) SDS, 20% (v/v) glycerol, 125 mM Tris-HCl (pH 6.8), and 200 mM DTT was added to the protein lysate. The separation of phosphorylated proteins with Phos-tag SDS-PAGE was described previously (Kinoshita et al., 2006). We added 35 μM Phos-tag reagent (code No. 304-93521; Wako) and 70 μM MnCl2 to the separation gel mixture of a standard 7% SDS polyacrylamide gel prior to polymerization. After electrophoresis, proteins were transferred onto PVDF membrane. Immunodetection of SLAC1-GFP was performed with anti-GFP polyclonal antibody (code No. 598; MBL).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession number At1g12480 (SLAC1).
Supplemental Data
Supplemental Figure 1. Bicarbonate Activation of the S-Type Anion Currents Is Restored in Guard Cells of an Independent ΔNC Transgenic slac1-4 Line, Whereas ABA Activation of S-Type Anion Currents Is Impaired.
Supplemental Figure 2. The Mobility Shift of SLAC1 Protein Treated with Bicarbonate or ABA in SDS-PAGE Gels Containing Phos-tag.
Supplemental Figure 3. Stomatal Response of Transgenic Plants Expressing SLAC1 F450A.
Supplemental Figure 4. Subcellular Localization of Mutant SLAC1 Proteins in the slac1 Transgenic Plants.
Supplemental Figure 5. Stomatal Aperture of the Transgenic Lines in Response to CO2.
Supplemental Figure 6. Stomatal Aperture of the Transgenic Lines in Response to ABA.
Supplemental Table 1. Oligonucleotide Primers Used in This Study.
Supplemental Table 2. DNA Sequences of the Amino Acid Substitutions.
Supplementary Material
Acknowledgments
We thank Y. Ishihama for nano-liquid chromatography-tandem mass spectrometry analysis and E. Kasuya for helpful discussion. We also thank N. Kawahara and Y. Johno for their technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (Nos. 21114002, 26221103 to K.I. and No. 15K18556 to J.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; by CREST, JST; by a grant from the National Science Foundation (MCB1414339 to J.I.S.); and by a grant from the National Institutes of Health (GM060396-ES010337 to J.I.S.).
AUTHOR CONTRIBUTIONS
Y.Y. performed the experiments on constructing and analyzing transgenic plants and the immunoblot analysis. C.W. performed whole-cell patch clamp experiments assays. Y.I. created the structural model. K.I., J.I.S., J.N., and Y.Y. designed the experiments and wrote the article.
Glossary
- ABA
abscisic acid
- GCP
guard cell protoplast
References
- Baek J.H., Rubinstein M., Scheuer T., Trimmer J.S. (2014). Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures. J. Biol. Chem. 289: 15363–15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoli M.R., et al. (2014). A secreted tyrosine kinase acts in the extracellular environment. Cell 158: 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 109: 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Munemasa S., Wang C., Nguyen D., Yong T., Yang P.G., Poretsky E., Belknap T.F., Waadt R., Aleman F., Schroeder J.I. (2015). Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. Elife 4: 03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C., et al. (2015). Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 25: 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Hu L., Punta M., Bruni R., Hillerich B., Kloss B., Rost B., Love J., Siegelbaum S.A., Hendrickson W.A. (2010). Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature 467: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Fiser A., Sali A. (2003). Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 374: 461–491. [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 107: 8023–8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A., Romeis T., Hedrich R. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Negi J., Young J., Israelsson M., Schroeder J.I., Iba K. (2006). Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 8: 391–397. [DOI] [PubMed] [Google Scholar]
- Hetherington A.M., Woodward F.I. (2003). The role of stomata in sensing and driving environmental change. Nature 424: 901–908. [DOI] [PubMed] [Google Scholar]
- Hu H., Boisson-Dernier A., Israelsson-Nordström M., Böhmer M., Xue S., Ries A., Godoski J., Kuhn J.M., Schroeder J.I. (2010). Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12: 87–93, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Rappel W.J., Occhipinti R., Ries A., Böhmer M., You L., Xiao C., Engineer C.B., Boron W.F., Schroeder J.I. (2015). Distinct cellular locations of carbonic anhydrases mediate carbon dioxide control of stomatal movements. Plant Physiol. 169: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193–195. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Hayashi Y. (2011). New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. Int. Rev. Cell Mol. Biol. 289: 89–115. [DOI] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006). Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5: 749–757. [DOI] [PubMed] [Google Scholar]
- Kollist H., Jossier M., Laanemets K., Thomine S. (2011). Anion channels in plant cells. FEBS J. 278: 4277–4292. [DOI] [PubMed] [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maierhofer T., Diekmann M., Offenborn J.N., Lind C., Bauer H., Hashimoto K., S Al-Rasheid K.A., Luan S., Kudla J., Geiger D., Hedrich R. (2014). Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 7: ra86. [DOI] [PubMed] [Google Scholar]
- Merilo E., Jalakas P., Kollist H., Brosché M. (2015). The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol. Plant 8: 657–659. [DOI] [PubMed] [Google Scholar]
- Merilo E., Laanemets K., Hu H., Xue S., Jakobson L., Tulva I., Gonzalez-Guzman M., Rodriguez P.L., Schroeder J.I., Broschè M., Kollist H. (2013). PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I.C., Murata Y., Yang Y., Munemasa S., Wang Y.F., Andreoli S., Tiriac H., Alonso J.M., Harper J.F., Ecker J.R., Kwak J.M., Schroeder J.I. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Mori I.C., Munemasa S. (2015). Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66: 369–392. [DOI] [PubMed] [Google Scholar]
- Mustilli A.C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Hashimoto-Sugimoto M., Kusumi K., Iba K. (2014). New approaches to the biology of stomatal guard cells. Plant Cell Physiol. 55: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Raschke K. (1975). Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta 125: 243–259. [DOI] [PubMed] [Google Scholar]
- Spassov V.Z., Yan L., Szalma S. (2002). Introducing an implicit membrane in generalized Born/solvent accessibility continuum solvent models. J. Phys. Chem. B 106: 8726–8738. [Google Scholar]
- Speight J.G. (2005). Lange's Handbook of Chemistry, 16th ed. (New York: McGraw-Hill Press; ). [Google Scholar]
- Sugiyama N., Nakagami H., Mochida K., Daudi A., Tomita M., Shirasu K., Ishihama Y. (2008). Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surti T.S., Huang L., Jan Y.N., Jan L.Y., Cooper E.C. (2005). Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc. Natl. Acad. Sci. USA 102: 17828–17833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., et al. (2015). A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6: 6057. [DOI] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.F., Nishimura N., Chan W.Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., et al. (2010). Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 62: 442–453. [DOI] [PubMed] [Google Scholar]
- Verdoucq L., Rodrigues O., Martinière A., Luu D.T., Maurel C. (2014). Plant aquaporins on the move: reversible phosphorylation, lateral motion and cycling. Curr. Opin. Plant Biol. 22: 101–107. [DOI] [PubMed] [Google Scholar]
- Wang C., Hu H., Qin X., Zeise B., Xu D., Rappel W.-J., Boron W.F., Schroeder J.I. (2016). Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as carbonic anhydrase 4 interactor. Plant Cell 28: 10.1105/tpc.15.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Hu H., Ries A., Merilo E., Kollist H., Schroeder J.I. (2011). Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 30: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Hobo T., Ichimura K., Mizoguchi T., Takahashi F., Aronso J., Ecker J.R., Shinozaki K. (2002). ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 43: 1473–1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.