Abstract
Interleukin-10 (IL-10) suppresses the maturation and cytokine production of dendritic cells (DCs), key regulators of adaptive immunity, and prevents the activation and polarization of naïve T cells towards protective gamma interferon-producing effectors. We hypothesized that human cytomegalovirus (HCMV) utilizes its viral IL-10 homolog (cmvIL-10) to attenuate DC functionality, thereby subverting the efficient induction of antiviral immune responses. RNA and protein analyses demonstrated that the cmvIL-10 gene was expressed with late gene kinetics. Treatment of immature DCs (iDCs) with supernatant from HCMV-infected cultures inhibited both the lipopolysaccharide-induced DC maturation and proinflammatory cytokine production. These inhibitory effects were specifically mediated through the IL-10 receptor and were not observed when DCs were treated with supernatant of cells infected with a cmvIL-10-knockout mutant. Incubation of iDCs with recombinant cmvIL-10 recapitulated the inhibition of maturation. Furthermore, cmvIL-10 had pronounced long-term effects on those DCs that could overcome this inhibition of maturation. It enhanced the migration of mature DCs (mDCs) towards the lymph node homing chemokine but greatly reduced their cytokine production. The inability of mDCs to secrete IL-12 was maintained, even when they were restimulated by the activated T-cell signal CD40 ligand in the absence of cmvIL-10. Importantly, cmvIL-10 potentiates these anti-inflammatory effects, at least partially, by inducing endogenous cellular IL-10 expression in DCs. Collectively, we show that cmvIL-10 causes long-term functional alterations at all stages of DC activation.
Human cytomegalovirus (HCMV) can establish and maintain a persistent subclinical infection in fully immunocompetent individuals. Efficient induction of immune responses to HCMV appears to be important in preventing overwhelming virus infection since serious complications ensue in hosts with an immature (fetuses and newborns) or a compromised (e.g., transplant recipients and AIDS patients) immune system (6). CMV-specific cytotoxic T lymphocytes (CTLs) potently control viral replication, but they cannot completely eliminate infected cell reservoirs (57, 72). This can be explained, in part, by the fact that HCMV has developed several ingenious pathways to disrupt potential interactions between infected cells, on the one hand, and effector T cells and natural killer (NK) cells on the other. For example, the open reading frame (ORF) UL18 encodes a major histocompatibility complex (MHC) class I homolog. Another HCMV gene product, gpUL40, induces surface expression of HLA-E. Both can prevent NK cell recognition and lysis (13, 15, 67, 71). Moreover, HCMV evades CTL detection by interfering with the processing and presentation of viral antigens by MHC class I molecules through a group of glycoproteins: gpUS2, gpUS3, gpUS6, and gpUS11 (54, 69).
Dendritic cells (DCs) exhibit great functional plasticity and can discriminate between different classes of microorganisms (30, 56). DCs are the only antigen-presenting cells thought to stimulate primary immune responses and are the key cells that regulate the magnitude and quality of the ensuing immune responses (2). Therefore, inactivation or attenuation of DCs through different strategies would likely compromise virus-specific immune responses. Multiple viruses, including measles virus, vaccinia virus, and murine cytomegalovirus (MCMV), directly infect DCs and disrupt DC functionality (1, 22, 24). Recent studies have provided in vitro evidence that high titers of endothelial cell-adapted HCMV strains can infect DCs, thereby paralyzing DC-mediated stimulation of adaptive immune responses (46, 53). However, the primary targets of HCMV in vivo are likely cell types other than DCs (48), raising the question whether HCMV could utilize mechanisms other than direct infection to delay and/or skew DC-mediated immune responses.
Recently, a viral interleukin-10 (vIL-10) homolog, ORF UL111A, was identified in the genomes of HCMV and other primate CMVs (36, 37). UL111A is not essential for HCMV replication in vitro (73) but may be crucial for viral immune evasion in vivo. HCMV-encoded IL-10 (cmvIL-10) forms homodimers that bind to the ligand-binding subunit of the IL-10 receptor (IL-10R1) with essentially the same affinity as that of cellular IL-10 (cIL-10) (33) and transduce through the signaling subunit IL-10R2 (36). The function(s) of this viral gene has not been fully characterized (65). However, it appears critical for the life cycle of HCMV in vivo since its sequence is highly conserved in multiple tissue culture-adapted strains and clinical isolates (R. Hector and A. Davison, Abstr. 9th Int. Cytomegalovirus Workshop, abstr. C.02, 2003). Although a number of viruses, including Epstein-Barr virus and Orf poxvirus, encode vIL-10 homologs, cmvIL-10 is the most divergent vIL-10 discovered thus far (23, 29, 36, 37). Its protein sequence shares only 25 to 27% identity with its cIL-10 counterpart, suggesting that it may not merely copy the cellular function but have different or additional properties. It is, thus, important to directly compare the cIL-10 and cmvIL-10 functions.
IL-10 strongly inhibits cell-mediated immunity by regulating DC functions (45). DCs coordinate immune responses through their ability to sense and respond to changes in the microenvironment (52). Upon DC activation, immature DCs (iDCs) differentiate and migrate to lymphoid tissues for priming naïve T cells (19). IL-10 inhibits DC maturation, a prerequisite for T-cell priming (39, 43), thus accounting for the inhibitory effects of IL-10 on DC-induced T-cell alloreactivity (10, 47). Furthermore, IL-10 blocks the ability of lipopolysaccharide (LPS)-stimulated DCs to migrate (16). Since migration of mature DCs (mDCs) to secondary lymphoid tissues is essential for the subsequent activation of naïve T cells, these data indicate that cIL-10 suppresses the induction of strong T-cell responses. This study was undertaken to elucidate the functions of cmvIL-10, in comparison with those of cIL-10. In particular, we focused on determining the effects of cmvIL-10 on multiple stages of DC activation. The results of our study support the hypothesis that secretion of cmvIL-10 by virus-infected cells is an indirect immune evasion mechanism that causes long-term inhibition of DC function.
MATERIALS AND METHODS
Virus and cells.
HCMV strains AD169 (kindly provided by W. J. Britt, University of Alabama, Birmingham) and Toledo (kindly provided by J. A. Wiedeman, University of California, Davis) and an AD169-derived mutant virus, TS359 (73), were used in this study. MRC-5 cells (human embryonic lung fibroblasts; ATCC CCL-171) were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal calf serum and 2 mM l-glutamine-100 U of penicillin/ml-100 μg of streptomycin/ml. Virus stocks used for fibroblast infection were prepared from 0.45-μm-pore-size-filtered supernatant in a 1:1 mixture with autoclaved 9% nonfat milk and stored at −70°C. Purified HCMV virions used for DC stimulation were prepared according to procedures described previously (12). Virus titers were determined by standard plaque assay, as described previously (11). Replication kinetics of HCMV were determined by single-step or multiple-step growth curve analyses. Briefly, MRC-5 cells at 3 × 105 cells/well of six-well plates were infected at an indicated multiplicity of infection (MOI). Supernatants from triplicate infected cultures were harvested daily, centrifuged at 600 × g for 5 min to remove cell debris, and stored at −70°C.
RNA extraction and cDNA synthesis.
Total RNA from HCMV-infected cells was purified with the RNeasy minikit (Qiagen, Valencia, Calif.) and treated with DNase (TURBO DNA-free kit; Ambion Woodward, Austin, Tex.) according to the manufacturer's instructions. For first-strand cDNA synthesis, an annealing reaction was first performed with 5 μg of RNA and 0.5 μg of oligo(dT)12-18 primer (Invitrogen) at 70°C for 5 min and then 4°C for 3 min. Reverse transcription was carried out in a 40-μl reaction mixture with Superscript II reverse transcriptase (200 U; Invitrogen) at 42°C for 90 min in the presence of RNaseOUT RNase inhibitor (60 U; Invitrogen), followed by inactivation of reverse transcriptase at 70°C for 15 min. cDNA samples were stored at −20°C and later used as templates for quantitative real-time PCR analyses.
Quantitative PCR analysis.
Real-time PCR was performed from reverse-transcribed cDNA samples for relative quantification of HCMV UL111A transcript copy numbers. Primer and probe sequences were designed specific to the second exon of the UL111A ORF with Primer Express software (Applied Biosystems, Foster City, Calif.). The sequences of the forward and reverse primers were 5′-TGT TGA GGC GGT ATC TGG AGA-3′ and 5′-CCG TCT TGA GTC CGG GAT AG-3′, respectively. Probe sequence CGT GTT TCC CGC AGG CGA CC contained 5′-tetrachloro-6-carboxyfluorescein (TET) as the reporter dye and 3′-6-carboxytetramethylrhodamine (TAMRA) as the quencher dye (Applied Biosystems). Standard curves were generated by using 10-fold serial dilutions of a plasmid (from 107 copies to 1 copy/μl) containing the genomic sequence of UL111A ORF (pWC154). Quantitative real-time PCR for UL111A was performed with 1× TaqMan universal PCR master mixture (Applied Biosystems), 200 nM (each) primer, 100 nM probe, and template cDNA (2 μl of each synthesized cDNA sample or diluted plasmid) in a 20-μl reaction volume. Ready-for-use glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe and primers were obtained from Applied Biosystems (TaqMan GAPDH control reagents). PCR was performed with the ABI/Prism 7900HT sequence detection system (Applied Biosystems) (1 cycle of 50°C for 2 min and 95°C for 10 min and then 40 cycles of 95°C for 15 s and 60°C for 1 min). Quantification of the PCR signals was performed by comparing the cycle threshold value of each sample in triplicate with the cycle threshold values of the GAPDH reference gene. The detection limit of reverse-transcribed UL111A transcripts was 10 copies/μl.
Immunoblotting.
HCMV-infected cells were collected, washed with cold phosphate-buffered saline (PBS), and then lysed with Novex Tris-glycine-sodium dodecyl sulfate sample buffer (Invitrogen). The protein extracts were size-separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and then probed with the following antibodies: anti-HCMV glycoprotein B (gB) (monoclonal antibody [MAb] clone 27-180; kindly provided by W. J. Britt), anti-β-actin (MAb clone AC-74; Sigma, St. Louis, Mo.), and biotinylated anti-cmvIL-10 polyclonal immunoglobulin G (IgG; R&D Systems, Minneapolis, Minn.). After removal of the primary antibody and multiple washes with PBS-0.5% Tween 20, the membranes were then incubated with peroxidase-conjugated anti-mouse IgG (Sigma) or streptavidin (Vector Laboratories, Burlingame, Calif.). Immunoreactive proteins were detected with the ECL Plus Western blotting detection reagents and scanning with a Typhoon 9410 variable mode imager (Amersham Biosciences, Piscataway, N.J.).
Generation of monocyte-derived DCs.
Human peripheral blood mononuclear cells were isolated from buffy coats of healthy individuals (obtained from the Sacramento Blood Center) by Ficoll-Paque gradient centrifugation (Amersham Biosciences). CD14+ monocytes from human peripheral blood mononuclear cells were then positively selected using anti-CD14 MAb-conjugated magnetic microbeads (Miltenyi Biotec, Auburn, Calif.). Purified monocytes (>95% purity, as determined by flow cytometry with staining for CD3, CD4, CD8, and CD20) (data not shown) were cultured at a density of 1.5 × 106 to 2 × 106 cells/ml in 12-well plates (1 ml per well) or 24-well plates (0.5 ml per well). DC medium consisted of RPMI 1640 (Invitrogen) containing 2 mM l-glutamine-100 U of penicillin/ml-100 μg of streptomycin/ml, 50 μM 2-mercaptoethanol (Sigma), 10 mM HEPES (Invitrogen), 10% endotoxin-free fetal calf serum (Invitrogen), and recombinant human granulocyte-macrophage colony-stimulating factor and IL-4 (1,000 U/ml each; R&D Systems). Cytokines were replenished every other day by adding 20% fresh DC medium to each well.
DC stimulation and treatment.
For DC activation, LPS from Escherichia coli O127:B8 (Sigma) or purified recombinant human soluble CD40 ligand (sCD40L) (Research Diagnostics, Flanders, N.J.) was added 6 days after culture onset. For IL-10 treatment, recombinant human IL-10 (hIL-10) (Sf21 cell-expressed) or cmvIL-10 (ORF UL111A of HCMV Towne strain expressed in E. coli) (both from R&D Systems) was added to the cultures concomitantly with the appropriate activation signals. At various times after onset, DCs were collected by gentle resuspension. The numbers of viable DCs were determined by trypan blue exclusion. Binding of cmvIL-10 to its cognate receptor on DCs was inhibited with anti-IL-10R1 MAb (5 μg/ml; R&D Systems). Purified mouse IgG1 (R&D Systems) was used as an isotype control antibody for the assay. MAbs were added to the DC cultures 30 min before the addition of recombinant IL-10 proteins and LPS. Endotoxin levels of all reagents (except LPS) were tested by Limulus amebocyte lysate assays (<1 EU/μg of cytokine-sCD40L; <0.1 EU/μg of antibody).
ELISA.
Levels of cmvIL-10 in culture supernatants were measured by an antigen-capture enzyme-linked immunosorbent assay (ELISA). cmvIL-10-specific IgG (100 ng/well; affinity purified, polyclonal; R&D Systems) and biotinylated cmvIL-10-specific IgG (10 ng/well) were used for coating and detection, respectively. The relative units of cmvIL-10 within the supernatant from HCMV-infected cultures were calculated from standard curves obtained using recombinant cmvIL-10 (1 U = 1 pg/ml). The levels of tumor necrosis factor-α (TNF-α), IL-6, IL-12 (p40 plus p70), and endogenous cIL-10 secreted by DCs were quantified using ELISA kits purchased from U-CyTech (Utrecht, The Netherlands). Protein concentrations were calculated from a standard curve obtained with recombinant protein standards. The limits of detection for the ELISA are as indicated: cmvIL-10, 10 pg/ml or 10 U; cIL-10, 5 pg/ml; IL-6, 5 pg/ml; IL-12 (p40 plus p70), 5 pg/ml; and TNF-α, 5 pg/ml.
Flow cytometry.
Four-color flow cytometry was performed using a FACSCalibur (BD Biosciences, San Jose, Calif.) cell sorter. Directly conjugated antibodies were purchased from BD Biosciences, Beckman Coulter (Fullerton, Calif.), and R&D Systems: anti-HLA-ABC-fluorescein isothiocyanate (FITC)-allophycocyanin (APC) (G46-2.6), anti-HLA-DR-peridinin chlorophyll protein (PerCP) (L243), anti-CD1a-APC (HI149), anti-CD3-FITC (SP34), anti-CD4-phycoerythrin (PE) (M-T477), anti-CD8-PerCP (SK1), anti-CD20-APC (L27), anti-CD40-PE (5C3), anti-CD80-FITC (MAb 104), anti-CD83-FITC (HB15e), anti-CD83-PE (HB15a), anti-CD86-APC (2331), anti-CCR1-PE (53504.111), anti-CCR2-APC (48607), anti-CCR5-APC (3A9), and anti-CCR7-PE (3D12). Appropriate isotype-matched antibodies were used as controls. Data were analyzed and illustrated using FlowJo software (Tree Star Inc., San Carlos, Calif.).
Endocytosis and phagocytosis assays.
To assess the endocytic capacity of DCs, untreated DCs or DCs exposed to hIL-10 or cmvIL-10 (50 ng/ml) for 30 min were incubated with FITC-conjugated dextran (molecular weight, 70,000; Molecular Probes, Eugene, Oreg.) for 2 h at 37°C at a final concentration of 10 μg/ml. To study the effects of IL-10 exposure on the phagocytic capacity of DCs, cells were incubated with FITC-conjugated carboxylate-modified polystyrene beads (0.5-μm diameter; Polysciences, Warrington, Pa.) at 500 beads per cell in the presence or absence of hIL-10 or cmvIL-10 (50 ng/ml) for 24 h. After incubation, cells were washed three times with ice-cold PBS, immunostained, and analyzed by fluorescence-activated cell sorting (FACS).
Chemotaxis assay.
In vitro transwell migration assays were performed by adding 5 × 105 DCs in 100 μl of medium to the upper chambers (5-μm pore size) of a 6.5-mm-diameter transwell plate (Costar, Corning, N.Y.). Recombinant macrophage inflammatory protein 1α (MIP-1α) and MIP-3β (R&D Systems) were diluted in assay medium (plain RPMI plus 0.5% bovine serum albumin) at 100 ng/ml, and 600-μl aliquots were placed in the lower wells. DC migration was evaluated by FACS analysis after incubation at 37°C for 2 h. Absolute numbers of mDCs that had migrated were determined using TruCOUNT tubes (BD Biosciences) and assessed on a FACSCalibur cell sorter.
Statistical analysis.
Student's t tests (paired, one-tailed) were performed to evaluate the differences among experimental groups.
RESULTS
UL111A exhibits late expression kinetics during viral replication.
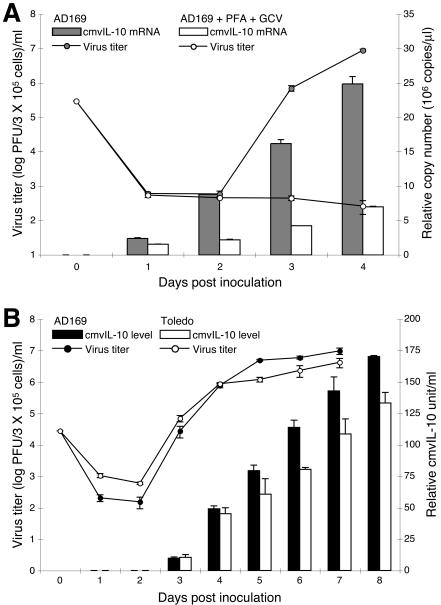
HCMV gene expression is regulated by events at the transcriptional, posttranscriptional, translational, and posttranslational levels (44). Although cmvIL-10 transcripts have previously been identified in HCMV-infected cells (36), cmvIL-10 expression kinetics have not been evaluated at either the RNA or the protein level. To define the temporal expression of cmvIL-10 in infected fibroblasts, a single-step growth curve analysis was performed in the presence and absence of a combination of two replication inhibitors, foscarnet and ganciclovir (Fig. 1A, shown as lines). To measure the levels of cmvIL-10 mRNA, a real-time PCR assay was developed to quantify UL111A transcripts from reverse-transcribed cDNA samples. The relative copy number of UL111A cDNA was determined by normalizing the cycle threshold values to those of GAPDH. Transcription of UL111A exhibited late gene kinetics. Both UL111A transcript copy number and the production of infectious virions were profoundly reduced in the presence of the two replication inhibitors (Fig. 1A, gray and open bars, respectively).
FIG. 1.
Expression kinetics of HCMV ORF UL111A during viral replication. (A) UL111A is transcribed with late gene kinetics. MRC-5 cells were infected in triplicate with HCMV AD169 at an MOI of 1 and cultured in the presence (open symbols) or absence (filled symbols) of foscarnet (PFA; 167 μM) and ganciclovir (GCV; 4 μM). Supernatants were collected daily to quantify progeny virus titers by standard plaque assays (shown as lines), and total RNA was extracted for cDNA synthesis and quantitative real-time PCR analyses. The relative copy numbers of UL111A cDNA after normalization with GAPDH copy numbers are shown as bars. (B) Accumulation of cmvIL-10 protein in the supernatants of infected cultures. Cells were infected with HCMV strain AD169 (filled symbols) or Toledo (open symbols) at an MOI of 0.1. Supernatants were collected at indicated time points to measure virus titers (shown as lines) and cmvIL-10 protein concentrations (shown as bars). Shown are mean values ± standard deviations for triplicate cultures.
The expression kinetics of ORF UL111A were also evaluated at the protein level. MRC-5 cultures were infected with two HCMV strains (AD169 and Toledo), and the supernatants were collected daily to determine virus titers and cmvIL-10 levels. Although the mRNA level of UL111A increased prior to the first burst of progeny virus (Fig. 1A), cmvIL-10 protein became detectable in the supernatants coincidently with the presence of extracellular virus (Fig. 1B and 2B). Accumulation of virally encoded cmvIL-10 protein in the supernatants of infected cultures continued even though the production of progeny virus peaked around day 5 postinoculation (Fig. 1B). Whether cmvIL-10 is actively secreted by infected cells or released from dead cells could not be distinguished in this analysis. Previous studies have demonstrated that transfection of cells with a full-length clone of UL111A results in the steady accumulation of extracellular cmvIL-10 (65), consistent with the presence of a predicted leader peptide (36, 37). Since most of the HCMV structural proteins are encoded at the late stage of the viral replication cycle (44), our data indicate that cmvIL-10 accumulates with other abundantly expressed viral antigens in the microenvironment of the infected tissues.
FIG. 2.
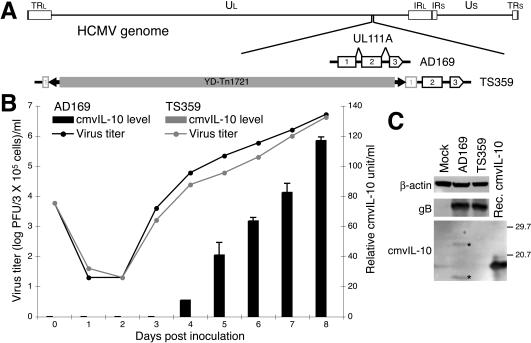
Genome structure and in vitro phenotype of HCMV AD169 mutant TS359. (A) Schematic diagram of HCMV genome structure with expansion of the UL111A ORF (exons and introns shown) of AD169 and the UL111A-mutated variant TS359. The UL111A ORF of TS359 is disrupted by the 3.66-kb transposon YD-Tn1721 insertion within its first exon (73). TRl, IRL, IRS, and TRS, terminal or internal inverted repeats flanking the Ul and Us components. (B) Multiple-step growth curves of AD169 (black symbols) and TS359 (gray symbols). Cells were infected with AD169 or TS359 at an MOI of 0.02, and supernatants were collected daily for measurement of progeny virus titers (shown as lines) and cmvIL-10 protein concentrations (shown as bars). ELISA data are shown as mean values ± standard deviations for triplicate cultures. (C) Immunoblot analysis for cmvIL-10 expression within HCMV-infected cells. Cell lysates were prepared after infection with AD169 or TS359 (MOI of 1) for 96 h. Immunoblots were probed for β-actin, HCMV gB, and cmvIL-10. The immunoblot of recombinant cmvIL-10 probed with anti-cmvIL-10 antibodies is also shown. Numbers at right are molecular masses in kilodaltons.
HCMV-encoded IL-10 possesses a biological function.
To evaluate the biological activity of cmvIL-10 expressed by HCMV-infected cells, DC functions were analyzed in the presence and absence of supernatant derived from cells infected with HCMV. Supernatant from cells transfected with a UL111A-deletion variant of HCMV, TS359 (73), was used in parallel to determine the relative contribution of cmvIL-10 to alterations of DC functions. TS359 is derived from the lab-adapted AD169 strain of HCMV and contains a disruption of the first exon of UL111A due to the insertion of a 3.66-kb transposon (Fig. 2A). This variant virus exhibited normal replication kinetics in fibroblasts (Fig. 2B) in the absence of cmvIL-10 protein expression. cmvIL10 was never detected by ELISA or immunoblotting in cells infected with TS359, as opposed to the robust expression observed in cells infected with AD169 (Fig. 2B and C). Two sizes of cmvIL-10 were observed in the immunoblots of extracts from cells infected with AD169 (Fig. 2C, asterisks). The larger band was slightly greater than the predicted size of full-length cmvIL-10 protein (175 amino acids) and may be the result of posttranslational glycosylation. Recently, a novel UL111A transcript expressed during latent infection but not during productive infection within permissive cells has been identified (32). This processed transcript consists of a single splicing event between the first and second exons of UL111A and is predicted to encode a 139-amino-acid protein. The size of the smaller protein detected by immunoblotting (Fig. 2C) is consistent with this form of cmvIL-10. However, Kotenko et al. have previously demonstrated that this truncated variant protein does not transduce through the IL-10R complex (36), probably because the COOH-terminal part encoded by the third exon is important for the formation of cmvIL-10 homodimers.
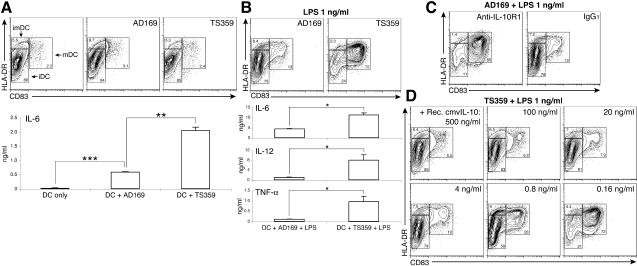
Conditioned media from AD169- and TS359-infected fibroblast cultures (Fig. 2B, collected on day 7 postinoculation) were used to assess the effects of cmvIL-10 on DC function. Since activation of DCs leads to a burst of synthesis and surface translocation of MHC class II (49) and up-regulation of CD83 molecules, we used those markers, CD83 and HLA-DR, to identify DC populations. Analysis of CD83 and HLA-DR surface expression following DC activation enabled the subdivision of the DC population into iDCs (CD83− HLA-DRlow), intermediate DCs (imDCs; CD83− HLA-DRhi), and mDCs (CD83+ HLA-DRhi) (Fig. 3). The effects of IL-10 on stimulus-induced DC maturation were monitored by changes in these subpopulations. Treatment of DCs with either AD169- or TS359-conditioned medium did not significantly promote DC maturation (Fig. 3A) despite the presence of viral antigens in the conditioned media of both HCMV strains (Fig. 2B). Similar results were observed when iDCs were incubated with purified HCMV AD169 virions (data not shown). It should be noted that DCs cannot be infected by the fibroblast-adapted HCMV (27). Although DCs cocultured with conditioned media did not phenotypically mature, IL-6 production was significantly induced compared to that with untreated DCs (Fig. 3A). It has been demonstrated that HCMV triggers IL-6 production in lung fibroblasts through binding of gB to its cellular receptor and subsequent NF-κB signaling (9). gB-mediated IL-6 induction appeared to be sensitive to cmvIL-10 inhibition because TS359-conditioned medium induced a significantly higher level of IL-6 than that of AD169 (Fig. 3A).
FIG. 3.
Inhibitory effects of HCMV-encoded IL-10 on DCs. iDC cultures were treated as indicated for 24 h, and their phenotypes were assessed by FACS and ELISA. Supernatants from AD169- and TS359-infected cultures were collected on day 7 postinoculation (Fig. 2B). Untreated DCs and DC cultures were incubated with conditioned medium from AD169- or TS359-infected cells (A), conditioned medium plus 1 ng of LPS/ml (B), conditioned medium from AD169-infected culture with 5 μg of anti-IL-10R1 MAb or IgG1 control/ml (C), or conditioned medium from TS359-infected culture with 1 ng of LPS/ml and indicated concentrations of recombinant cmvIL-10 (D). Surface expression of CD83 and HLA-DR on DCs was assessed by FACS to define immature (iDC; CD83− HLA-DRlow), intermediate (imDC; CD83− HLA-DRhigh), and mature (mDC; CD83+ HLA-DRhigh) DC populations, as indicated by boxes. Numbers represent frequencies of gated cells among the total DC population. Shown are 5% contour plots with outliers of cells after gating by forward scatter-side scatter to identify DCs by size. Cytokine data are expressed as the mean concentrations ± standard deviations of triplicate cultures. Results are representative of DCs from three different donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To demonstrate the effects of cmvIL-10 on DC functionality, the potent DC stimulus LPS was used to initiate the maturation of DCs in the presence of conditioned media. In the presence of a low dose of LPS (1 ng/ml) alone, the majority of DCs progressed to the mDC phenotype (Fig. 4A, top row). When DCs were coincubated in LPS plus TS359-conditioned medium, a significant number of cells became mature (Fig. 3B). This stood in marked contrast to cells coincubated with LPS plus AD169-conditioned medium. Moreover, AD169 supernatant significantly inhibited proinflammatory cytokine production by LPS-activated DCs, in contrast to DCs coincubated with TS359-conditioned medium and LPS (Fig. 3B). The effects of AD169 supernatant on DCs were specifically mediated through the cIL-10R. When DC cultures were incubated with AD169-conditioned medium and LPS in the presence of anti-IL-10R1 MAb, normal levels of maturation were observed (Fig. 3C). DCs were refractory to maturation when an isotype control antibody was substituted for the anti-IL-10R antibody. Taken together, these data demonstrate that virally encoded cmvIL-10 profoundly alters DC maturation and cytokine production.
FIG. 4.
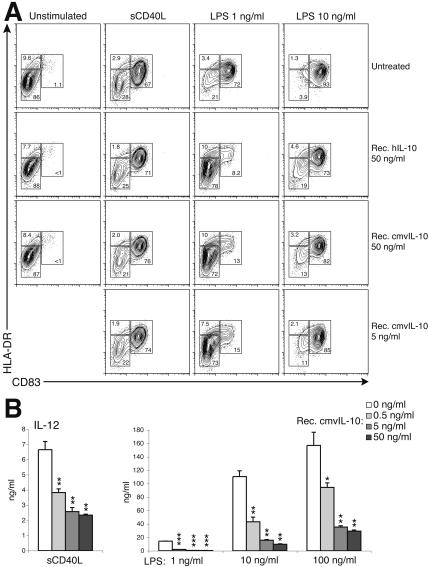
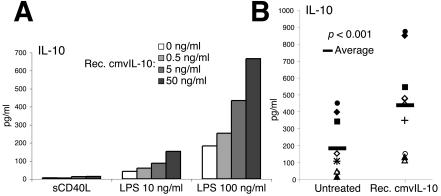
Recombinant cmvIL-10 inhibits maturation of DCs and/or alters their cytokine expression profiles. (A) IL-10 inhibits LPS-stimulated but not CD40L-stimulated phenotypic maturation of DCs. Cells were unstimulated or stimulated with sCD40L (1 μg/ml) or LPS (at the indicated concentration) for 24 h in the absence (top panel) or presence of recombinant hIL-10 (second panel) or cmvIL-10 (bottom two panels). Results are representative of 10 separate experiments. (B) cmvIL-10 alters the IL-12 expression profiles of activated DCs. Cells were stimulated with 1 μg of sCD40L/ml or the indicated concentration of LPS and concomitantly treated with recombinant cmvIL-10 (0, 0.5, 5, and 50 ng/ml). Data are expressed as the mean concentrations ± standard deviations of triplicate cultures and are representative of five separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to result with no cmvIL-10 treatment.
Quantification of biological activity of HCMV-encoded IL-10.
A critical question regarding the biological function of cmvIL-10 is whether its concentration in the extracellular medium is sufficient to regulate DCs during the normal course of viral replication. To quantify the biological activity of cmvIL-10 in conditioned medium, the levels of DC maturation were evaluated following treatment with serial dilutions of purified recombinant cmvIL-10. It should be noted that the standard assay for quantifying hIL-10 activity, stimulation of murine mast cell proliferation (66), was not feasible for quantifying cmvIL-10 since we did not see any functional activity of cmvIL-10 on mouse cells (W. L. W. Chang and N. Baumgarth, unpublished data). Nonstimulated DCs were cocultured with LPS, TS359-conditioned medium, and fivefold serial dilutions of recombinant cmvIL-10 (Fig. 3D). The inhibition of DC maturation produced with AD169-conditioned medium collected on day 7 postinoculation (Fig. 3B) was comparable to 0.8 to 4 ng of recombinant cmvIL-10/ml (Fig. 3D). Thus, the biological activity of conditioned medium on DC maturation was the result of low concentrations of cmvIL-10, suggesting that infected cells can produce physiologically relevant concentrations of cmvIL-10.
The biological activity of recombinant cmvIL-10 was comparable to that of cIL-10. Essentially equal levels of inhibition of DC maturation were observed when DCs were incubated in LPS (1 ng/ml) and either hIL-10 (50 ng/ml) or cmvIL-10 (5 and 50 ng/ml) (Fig. 4A). However, neither source of IL-10 significantly inhibited DC maturation when a higher concentration (10 ng/ml) of LPS was used. The sensitivity of DCs to LPS activation varied from donor to donor (data not shown). Therefore, multiple doses of LPS were used in most of our experiments. For most donor samples, 10 ng of LPS/ml activated >90% DCs in culture (Fig. 4A). At this concentration of LPS, the majority of DCs exhibited the mDC phenotype in the presence of recombinant hIL-10 and cmvIL-10, respectively (Fig. 4A, right column). CD40L is a well-characterized DC activation signal provided by activated T cells. CD40-CD40L ligation is unlikely to occur before antigen-specific naïve T cells are primed by activated DCs during HCMV infection. Both hIL-10 and cmvIL-10 failed to inhibit DC maturation when cells were stimulated with the sCD40L cognate maturation signal (1 μg/ml) (Fig. 4A, second column), consistent with results from other studies (8, 50).
Despite the inability of cmvIL-10 to block DC phenotypic maturation in the presence of either sCD40L or high concentrations of LPS, recombinant cmvIL-10 inhibited proinflammatory cytokine production for all stimulation conditions. Treatment of activated cmvIL-10 resulted in a dose-dependent inhibition of IL-12, IL-6, and TNF-α production by DCs following stimulation (Fig. 4B; only the IL-12 data are shown). This occurred even when most DCs had fully matured following sCD40L and LPS (10 and 100 ng/ml) stimulation. Inhibition of cytokine production saturated at a concentration of 5 ng of cmvIL-10/ml.
cmvIL-10-IL-10R engagement enhances cIL-10 production.
Results of previous studies have led to the conclusion that cIL-10 modulates its own expression, since neutralization of endogenous cIL-10 diminishes IL-10 mRNA accumulation in activated DCs (14). Based on this precedent for cIL-10, cmvIL-10-treated DCs were analyzed for changes in the levels of cIL-10 expression. In contrast to the effects of cmvIL-10 on proinflammatory cytokines in DCs, cIL-10 production was increased in a dose-dependent manner by cmvIL-10 treatment (Fig. 5A). Increased cIL-10 production was also observed in sCD40L-stimulated DC cultures, despite the fact that sCD40L is not a potent IL-10 stimulus, compared with LPS. The absolute level of cIL-10 produced by DCs varied from donor to donor, but the trend of cIL-10 induction by cmvIL-10 treatment was consistently observed in all tested donors (Fig. 5B). These data suggested that cIL-10 production by DCs could be up-regulated through a positive feedback mechanism in vivo, independent of whether IL-10 is encoded by the cells or by HCMV. Since activated DCs have a lower surface expression of IL-10R1 than do iDCs (14, 34), iDCs accumulating at the site of primary HCMV infection probably represent a prominent in vivo target of cmvIL-10.
FIG. 5.
cmvIL-10 induces endogenous cIL-10 production. (A) cmvIL-10 enhances IL-10 secretion by activated DCs in a dose-dependent fashion. DCs treated with indicated concentrations of cmvIL-10 were stimulated for 24 h with sCD40L or indicated doses of LPS. Shown are mean concentrations from duplicate cultures representative of three separate experiments. (B) Shown are levels of cIL-10 produced by DCs from various donors (n = 9). Cells were stimulated with 10 ng of LPS/ml in the presence or absence of recombinant cmvIL-10 (50 ng/ml). Each symbol represents one individual donor. In all cases, cmvIL-10 stimulated the expression of cIL-10. The P value between untreated and cmvIL-10-treated groups is shown.
Functional analogy of cmvIL-10 and cIL-10.
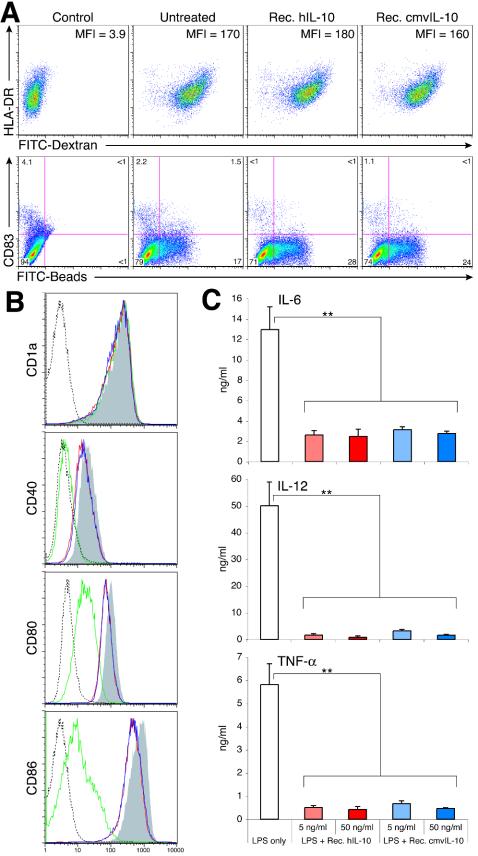
Although the cmvIL-10 homodimer can bind to IL-10R1, it has evolved a different interdomain angle that may alter the orientation of IL-10R1 on the cell surface (33). This structural change may interrupt IL-10R2 recruitment, and consequently, its biological effects might be distinct from those of cIL-10. A central question is whether cmvIL-10 precisely mimics the function of cIL-10 or whether there are functional differences commensurate with the sequence difference. Therefore, cIL-10 and cmvIL-10 were functionally compared to evaluate the effects of both IL-10 molecules on multiple stages of the DC life cycle. iDCs are very efficient in antigen capture through several pathways, including macropinocytosis, receptor-mediated endocytosis, and phagocytosis (2). Although both forms of IL-10 strongly inhibited maturation of iDCs (Fig. 4A), both recombinant hIL-10- and cmvIL-10-treated iDCs efficiently captured antigens via endocytosis or phagocytosis. Endocytic activity following IL-10 treatment was essentially equal to that observed in untreated iDCs (Fig. 6A, top panel). Both IL-10 molecules slightly enhanced the phagocytic capacity of iDCs compared to that of untreated cells (Fig. 6A, bottom panel).
FIG. 6.
cmvIL-10 and hIL-10 share comparable potency in modulating the functionality of DCs. (A) IL-10 enhances phagocytic capacity of iDCs. DC cultures left untreated or treated with recombinant hIL-10 or vIL-10 (50 ng/ml) were analyzed by FACS for their ability to take up FITC-conjugated dextran (top panels) or FITC-conjugated beads (bottom panels) for assessment of endocytic and phagocytic capacity, respectively. Control panels on the left show untreated DCs with no FITC-dextran or FITC-conjugated beads added to the cultures. MFI, mean fluorescence intensity (FITC). Results are representative of two separate experiments. (B) IL-10 suppresses the induction of surface expression of costimulatory molecules on activated DCs. Cells were stimulated with 10 ng of LPS/ml in the presence (hIL-10, red open histogram; cmvIL-10, blue open histogram) or absence (gray shaded histogram) of recombinant IL-10 (5 ng/ml) for 24 h. Surface expression of indicated molecules on DCs was analyzed by FACS and presented as histograms after gating by forward scatter-side scatter-CD83-HLA-DR to identify mDC subsets. Expression levels of indicated molecules on the surface of iDCs are also shown (green open histogram). Isotype control staining is shown by dashed lines. (C) IL-10 alters cytokine secretion profiles of activated DCs. Levels of indicated proinflammatory cytokines in supernatants of DC cultures 24 h after LPS stimulation (10 ng/ml) and IL-10 treatment were determined by ELISA. Data are expressed as the mean cytokine concentrations ± standard deviations of triplicate cultures. Results are representative of eight separate experiments. **, P < 0.01.
Following LPS-mediated maturation of iDCs to mDCs, in addition to CD83 and MHC class II, there was a concomitant increase in surface expression of MHC class I (data not shown) and various costimulatory molecules involved in T-cell activation, including CD40, CD80, and CD86 (Fig. 6B, compare iDC [green open histogram] versus mDC [gray shaded histogram]). Since IL-10 potently inhibited maturation of iDCs, only the CD83+ HLA-DRhi mDC subpopulation was gated to evaluate the effects of both IL-10 molecules on the surface expression of these costimulatory molecules. Treatment of mDCs with either cIL-10 or cmvIL-10 resulted in slightly reduced levels of CD40, CD80, and CD86, compared to those of mDCs that did not receive IL-10-mediated signals (Fig. 6B, red and blue open histograms). In contrast, IL-10 treatment did not alter the surface expression of CD1a (Fig. 6B), CD83, and HLA-DR (Fig. 4A) on the activated DC population. The effects of cellular and viral forms of IL-10 on alteration of the mDC phenotype were indistinguishable from each other.
A direct comparison of both IL-10 molecules on proinflammatory cytokine production by activated DCs was also performed (Fig. 6C). Quantification of secreted cytokines by ELISA demonstrated that cIL-10 and cmvIL-10 had equivalent inhibitory activity for IL-6, IL-12, and TNF-α expression. Thus, despite the considerable sequence evolution of cmvIL-10 and cIL-10, no functional distinctions were observed between the two in these assays.
Dual effects of IL-10 on DC migration.
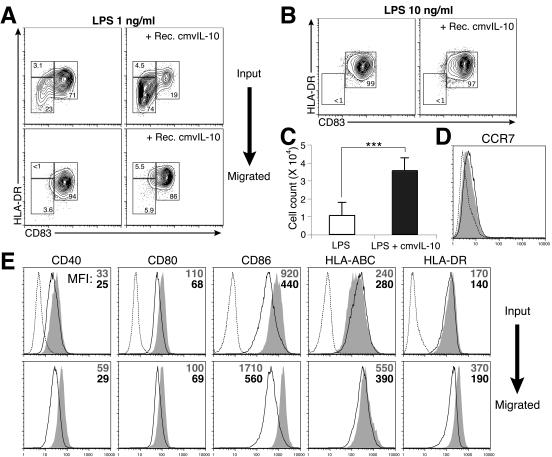
The migratory capacity of DCs is tightly correlated with their maturation status and is one of the most important features of DC physiology (21). DC trafficking is associated with the differential expression of inflammatory chemokine receptors or the lymph node homing chemokine receptor CCR7 at distinct stages of the DC life span. The maturation process of DCs coordinates the down-regulation of CCR1, CCR2, and CCR5 and up-regulation of CCR7 and therefore leads mDCs to migrate towards lymphoid tissues (21, 59, 63). To assess the effects of cmvIL-10 on the migration capacity of the mDC population, chemotaxis of cmvIL-10-treated and untreated LPS-stimulated DCs to MIP-3β was assayed in a transwell system. FACS analysis of input and migrated cell populations revealed that only mDCs (CD83+ HLA-DRhi) were able to migrate towards MIP-3β (Fig. 7A). Thus, because cmvIL-10 inhibits DC maturation, it reduced the extent of overall DC migration. Importantly, the DC subpopulation that overcame the restraint of cmvIL-10 and differentiated into the mature phenotype of CD83+ HLA-DRhi (Fig. 7A) and CCR1− CCR2− CCR5− CCR7+ (data not shown) remained capable of migration towards MIP-3β.
FIG. 7.
Dual effects of cmvIL-10 on DC migration. (A) cmvIL-10 inhibits maturation of DCs and, thereby, inhibits their ability to migrate towards MIP-3β. Migration in response to MIP-3β (100 ng/ml) was assessed for DCs stimulated for 24 h with 1 ng of LPS/ml in the absence or presence of recombinant cmvIL-10 (50 ng/ml). Shown are 5% contour plots of input cells (top panels) and cells present in the bottom chamber 2 h after assay setup (bottom panels). Note that the only cells capable of migration were CD83+ HLA-DRhigh mDCs. Cells were gated by forward scatter-side scatter (FSC-SSC) to identify DCs by size. Results are representative of two separate experiments. (B to E) cmvIL-10 modulates the phenotype of migrating DCs. Cells stimulated with 10 ng of LPS/ml in the presence or absence of recombinant cmvIL-10 (50 ng/ml) for 24 h were used for chemotaxis assays. One representative experiment of three separate experiments is shown. (B) Input DCs were analyzed for surface expression of CD83-HLA-DR prior to chemotaxis. (C) Absolute numbers of CD83+ HLA-DRhigh cells in each well that migrated. Data are expressed as the mean numbers ± standard deviations of cells that migrated from triplicate assays. ***, P < 0.001. (D) Shown are the overlaid histograms for CCR7 expression on cmvIL-10-treated (open histogram) and untreated (gray shaded histogram) mDCs and the isotype control staining (dashed line). (E) The phenotypes of mDCs before (top panels) and after (bottom panels) cell migration towards MIP-3β were assessed by FACS. Surface expression of indicated molecules was analyzed by flow cytometry after gating by FSC-SSC-CD83-HLA-DR to identify mDC subsets. Results are presented as histograms, and numbers indicate mean fluorescence intensities (MFI) for the respective fluorochromes (gray number and shaded histogram, untreated; black number and open histogram, cmvIL-10 treated).
Somewhat surprisingly, when migration capacity was compared between LPS-matured mDCs treated with cmvIL-10 and those not treated with cmvIL-10 (Fig. 7B), cmvIL-10 consistently enhanced the frequency of mDCs that migrated (Fig. 7C). Similar data were obtained when cells were treated with recombinant hIL-10 (data not shown). cmvIL-10 treatment did not appreciably affect the surface levels of CCR7 on mDCs (Fig. 7D). The observed increase in chemotaxis of IL-10-treated mDCs was, therefore, not due to an IL-10-induced up-regulation of the MIP-3β receptor CCR7. In addition to MIP-3β, the inflammatory chemokine MIP-1α was used for transwell migration assays. LPS-stimulated mDCs, either treated or untreated with recombinant IL-10, did not respond to MIP-1α, consistent with the surface chemokine receptor expression pattern on those DCs (data not shown).
cmvIL-10 has long-term effects on activated DCs.
It has been suggested that terminal maturation of DCs occurs during their migration towards secondary lymphoid tissues and upon receiving signals provided by activated T cells (2). This is supported by our FACS analyses of those DCs that had migrated towards MIP-3β. The cells that migrated in our transwell studies showed higher expression levels of CD40, CD86, HLA-ABC, and HLA-DR than those of the input population (Fig. 7E, gray-shaded histograms). Our data demonstrated that this step of DC maturation was also disrupted by the regulatory events triggered by early cmvIL-10-IL-10R engagement during DC activation. The up-regulation of these surface molecules following cell migration was less pronounced in the cmvIL-10-treated DCs (Fig. 7E, open histograms). Whether the difference in surface expression levels of these molecules between the DCs that had migrated and those that had not was due to preferential migration of highly expressive cells or differences in induction of surface markers could not be determined. However, in some donors, CD86 and HLA-DR expression by cells that had migrated surpassed the levels of the input cell population (data not shown), suggesting that further up-regulation of these surface markers indeed occurred during DC migration.
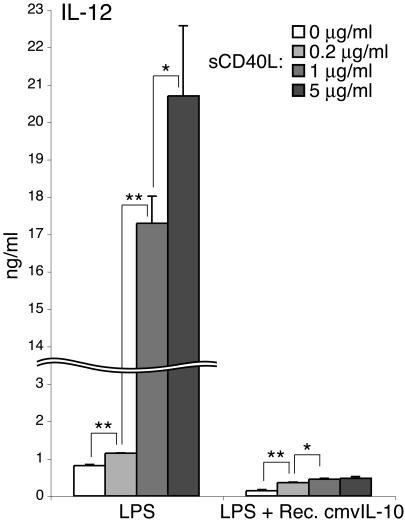
The long-term effects of cmvIL-10 on the functionality of DCs were further shown by its continuing suppression of cytokine production by repeat-stimulated mDCs. A recent in vivo study has demonstrated that CD40 ligation (of activated DCs) and CD40L ligation (of activated T cells) facilitate production of IL-12 through preferential induction of p35 transcription in DCs primed with microbial signals (61). To evaluate the impact of cmvIL-10 on the CD40-dependent regulation of DC maturation, LPS-stimulated cells were harvested and restimulated with sCD40L to mimic physiological interactions between DCs and T cells in vivo. After culture for 24 h, supernatants and cells were collected for IL-12 ELISA and FACS analysis, respectively. Secondary stimulation of LPS-primed mDCs with sCD40L significantly increased their IL-12 production in a stimulation-dose-dependent manner (Fig. 8) but did not further up-regulate the expression of MHC and costimulatory molecules (data not shown). In contrast, DCs exposed to cmvIL-10 during primary LPS stimulation showed a strong reduction in IL-12 secretion both prior to and following stimulation with sCD40L (Fig. 8). These data indicate that cmvIL-10-triggered inhibitory events continuously suppress their maturation and alter their cytokine expression profiles after activated DCs migrate from the peripheral tissues toward the draining lymphoid tissues. The affected DC population is, therefore, likely to be less capable of stimulating strong antiviral adaptive immune responses, even in the lymph node, a site where cmvIL-10 is unlikely to be present in vivo.
FIG. 8.
Activated mDCs treated with cmvIL-10 do not respond to further stimulation via CD40L with increased IL-12 production. DCs were stimulated with LPS (10 ng/ml) in the absence or presence of recombinant cmvIL-10 (50 ng/ml) for 12 h. Cells were collected, washed with PBS, and then stimulated with indicated concentrations of sCD40L for a further 24 h. Supernatants were harvested for measurement of IL-12 levels by ELISA. Results are expressed as the mean IL-12 concentrations ± standard deviations of triplicate cultures. *, P < 0.05; **, P < 0.01.
DISCUSSION
HCMV establishes a persistent infection in the face of an immune system fully capable of initiating and maintaining antiviral responses. How this virus can evade immune surveillance remains to be fully elucidated. It is known that HCMV can interrupt interactions between virus-infected cells and effector cells, such as NK cells and T cells, by encoding multiple viral gene products (13, 54, 67). Our study shows that the HCMV-encoded IL-10 homolog is expressed with late kinetics at a level that can inhibit DC functions. The temporal secretion of cmvIL-10 is contemporaneous with the release of progeny virions from infected cells. Exposure of DCs to cmvIL-10 demonstrated that this virokine strongly inhibits the maturation of DCs and irreversibly alters their functions. Since DCs are key regulators of cell-mediated immunity, alterations in the extent of their activation and the quality of their responses likely blunt the specific antiviral immunity induced against HCMV in vivo.
An important implication of our study is that it does not require direct infection of DCs by HCMV to alter the host's immune responses to virus infection. Rather, the results suggest that there is a spatial separation between the sites of virus infection and perturbation of adaptive immune responses. Our data indicated that iDCs exposed to cmvIL-10 in vitro remain capable of taking up antigens through endocytosis or phagocytosis. Although cmvIL-10 reduces the levels of multiple costimulatory molecules on mDCs, their levels are still higher than those on iDCs. Accordingly, activated DCs exposed to cmvIL-10 in vivo should be capable of priming naïve T cells and stimulating de novo antiviral immune responses. This notion is further supported by the in vitro chemotaxis assay showing that DCs that overcome the IL-10 restraint can migrate towards MIP-3β. It should be noted that this result is in contrast to the finding of D'Amico et al., who showed that concomitant exposure of DCs to LPS and IL-10 blocks DC migration due to the lack of chemokine receptor switch associated with DC maturation (16). Our results demonstrate that cmvIL-10 engenders a DC-directed cytokine milieu that would, seemingly, favor the establishment of a persistent infection. Early exposure of DCs to cmvIL-10 during their activation induced a distinct IL-10high IL-12low cytokine production profile. The production of IL-12 by mDCs during T-cell priming is critical for polarizing CD4+ T cells toward gamma interferon production (20, 40, 41). IL-12 is a key cytokine that also induces proliferation and enhances cytotoxic activity of NK cells, one of the crucial effector cells for the elimination of intracellular pathogens (70) and for controlling the spread of CMV (4). Thus, cmvIL-10-induced reduction of IL-12 production by DCs, at the site of viral infection or in secondary lymphoid tissues, might directly and indirectly reduce the activation of crucial antiviral defenses by CD4+ T cells and CD8+ CTLs as well as NK cells.
A salient finding of our study is that cmvIL-10 coopts normal cellular processes to exert its effects. In addition to binding to the IL-10Rs, cmvIL-10 significantly up-regulates the production of endogenous cIL-10 by LPS-activated DCs. Thus, HCMV may subvert the degree and quality of DCs activated by local virus replication though expression of the vIL-10 homolog and the induction of endogenous cIL-10 production from cells that are not directly infected by the virus. In contrast to other cytokines expressed by DCs, the promoter activity of IL-10 is independent of NF-κB activation. Instead, it is strongly dependent on two constitutively expressed transcription factors, Sp1 and Sp3 (5, 68). IL-10 transcription would, therefore, be unaffected by an IL-10-induced NF-κB inhibition that affects many other DC cytokines (60). Sp1 and Sp3 are present in many cell types and can be induced by LPS stimulation (38). Our data support and extend these findings by showing that cIL-10 production by DCs is LPS dose dependent (Fig. 5A). Another checkpoint of cIL-10 expression is the control of its own mRNA stability (51). Ligation of IL-10R triggers various mechanisms that regulate cytokine or chemokine expression posttranscriptionally (7, 35, 64). The mechanism that underlies the positive feedback of IL-10-IL-10R ligation on IL-10 production in DCs is unknown but is likely to involve a number of posttranscriptional mechanisms.
Somewhat surprisingly, given the high degree of divergence at the sequence level, our results indicate that cmvIL-10 and cIL-10 share comparable potency in modulating DC activities. The local concentration of cmvIL-10 at the HCMV-infected cell-DC interface would not need to be especially high to achieve its modulatory function, primarily because cmvIL-10 binds to cIL-10R1 with an affinity essentially equal to that of cIL-10 (33). As a consequence, secretion of cmvIL-10 from infected cells in concentrations comparable to those of cIL-10 should produce biologically relevant downstream effects. Although physiologically relevant levels of recombinant IL-10 (0.5 to 5 ng/ml) were sufficient to inhibit DC maturation and to skew the cytokine expression schemes of activated DCs in vitro, recombinant IL-10 was administered at a higher level (50 ng/ml) for some experiments to ensure that IL-10-mediated regulatory events were triggered in all cells in culture. One important question is whether cmvIL-10 secreted by virally infected cells can, indeed, inhibit DC maturation and alter its functions in vivo. Although in vivo studies will be required to answer this question fully, the biological activity of cmvIL-10 expressed by HCMV-infected cells (Fig. 3) was higher than that of cIL-10 secreted by an equivalent number of DCs activated by high doses of LPS (Fig. 5). LPS treatment is known to potently induce endogenous cIL-10 production that can inhibit DC activation in an autocrine fashion (14, 18). These data lead to the hypothesis that cmvIL-10 plays an important immunomodulatory role during HCMV infection.
Support for a crucial role of cmvIL-10 in viral natural history comes from a recent report on the remarkable degree to which the coding region and sequence for cmvIL-10 (ORF UL111A) are well conserved among all HCMV isolates examined thus far (Hector and Davison, Abstr. 9th Int. Cytomegalovirus Workshop). The stability of the coding region in different clinical isolates implies that cmvIL-10 confers a selective advantage in vivo. Counterparts of HCMV UL111A have also been found in the genomes of other primate CMVs, including those isolated from rhesus macaques, African green monkeys, and baboons (37). Intriguingly, the HCMV UL111A counterpart is not present within the genome of chimpanzee CMV (CCMV), the closest known relative of HCMV, although the remainder of the genome is highly conserved (17). The reason for this is unknown, but it is possible that CCMV utilizes other pathways to achieve the same ends as those of the UL111A encoded by HCMV. Precedence for this idea can be found with MCMV, which also does not encode a vIL-10 homolog. Nevertheless, MCMV induces cIL-10 production within infected macrophages (55), consistent with the idea that modulation of cIL-10 is instrumental in CMV natural history. It is also interesting that MCMV efficiently infects DCs and paralyzes their functions, thereby temporarily impairing the generation of antiviral immunity (1). Since MCMV does not encode a vIL-10 homolog, MCMV infection of DCs may be a necessary requisite for efficient dissemination of the virus. The UL111A locus of primate CMV appears to be in a state of genetic flux (3). One interpretation of the presence and absence of cmvIL-10 in the primate and nonprimate CMV genomes is that both branches of the CMV lineage evolved convergent mechanisms targeting the same cell type. It is likely that CCMV, for reasons that remain to be determined, has lost (or deleted) the cmvIL-10 coding region after branching from its common ancestor with HCMV to replicate better in chimpanzees.
HCMV may have evolved distinct pathways for disrupting DC function that involve both indirect mechanisms (via cmvIL-10) and direct infection. HCMV establishes latency within a small percentage of myeloid progenitors (28) and productively infects differentiated macrophages (31, 42). Although disruption of DC functions might be a key pathogenic mechanism among many, if not all, CMV strains, the mechanism underlying the inhibition of DCs might vary among different CMV strains. Previous studies have characterized infection of DCs by HCMV in vitro, although requiring very high MOIs of endothelial cell-adapted strains (e.g., TB40/E) (46, 53, 58). In contrast, DCs appear to be highly resistant to infection by fibroblast-adapted strains of HCMV (58, 62). The reason for the differences in infectivity between viruses derived after passage in different cell types is undoubtedly genetically based, but the precise mechanisms remain to be determined. The 15-kb UL131-128 region, absent in the genome of a fibroblast-adapted HCMV strain, AD169, has been identified as essential to viral tropism for both endothelial cells and leukocytes (G. Hahn, F. Baldanti, A. Gallina, G. Campanini, M. Wagner, M. Patrone, E. Percivalle, A. Sarasini, M. G. Revelo, and G. Gerna, Abstr. 28th Int. Herpesvirus Workshop, abstr. 1.02, 2003). However, Gerna et al. recently reported that both Towne and AD169 strains, lacking both endothelial cell tropism and leukotropism, reacquire both properties after a high number of sequential passages in endothelial cells (25, 26). In contrast to the in vitro studies, in vivo data indicate that DCs do not constitute a favored target of infection. A recent report indicated that HCMV mainly infects cell types other than DCs and macrophages in vivo (48). Further studies are required to address the importance of DC infection during HCMV natural history, particularly during the earliest stages of primary infection.
Taken together, our in vitro data suggest that DCs are clearly an important, if indirect, target of HCMV. Since DCs represent the link between innate and adaptive immunity, our results also suggest that HCMV modulates host immune responses at the earliest stage of primary infection. Inhibition or neutralization of cmvIL-10 through therapeutic or vaccination approaches might result in the generation of more vigorous and effective DC responses that can facilitate the induction of fully protective cellular immunity to this virus.
Acknowledgments
We thank T. Shenk, W. Britt, and J. Wiedeman for generously providing the HCMV strains and the anti-gB hybridoma clone. We also acknowledge the invaluable assistance of A. Spinner with flow cytometry and M. Eberhardt with immunoblotting.
This work was supported by NIH grant AI49342 and California National Primate Research Center grant RR00169 to P.A.B.
REFERENCES
- 1.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Barry, P. A., and W. L. Chang. Primate betaherpesviruses. In A. M. Arvin, G. Campadelli-Fumi, E. S. Mocarski, P. S. Moore, B. Roizman, R. Whitley, and K. Yamanishi (ed.), Human herpesviruses: biology, therapy and immunoprophylaxis, in press. Cambridge University Press, Cambridge, United Kingdom. [PubMed]
- 4.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 5.Bondeson, J., K. A. Browne, F. M. Brennan, B. M. Foxwell, and M. Feldmann. 1999. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J. Immunol. 162:2939-2945. [PubMed] [Google Scholar]
- 6.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2522. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 7.Brown, C. Y., C. A. Lagnado, M. A. Vadas, and G. J. Goodall. 1996. Differential regulation of the stability of cytokine mRNAs in lipopolysaccharide-activated blood monocytes in response to interleukin-10. J. Biol. Chem. 271:20108-20112. [DOI] [PubMed] [Google Scholar]
- 8.Buelens, C., V. Verhasselt, D. De Groote, K. Thielemans, M. Goldman, and F. Willems. 1997. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur. J. Immunol. 27:1848-1852. [DOI] [PubMed] [Google Scholar]
- 9.Carlquist, J. F., L. Edelman, D. W. Bennion, and J. L. Anderson. 1999. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-κB. J. Infect. Dis. 179:1094-1100. [DOI] [PubMed] [Google Scholar]
- 10.Caux, C., C. Massacrier, B. Vanbervliet, C. Barthelemy, Y. J. Liu, and J. Banchereau. 1994. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int. Immunol. 6:1177-1185. [DOI] [PubMed] [Google Scholar]
- 11.Chang, W. L., V. Kirchoff, G. S. Pari, and P. A. Barry. 2002. Replication of rhesus cytomegalovirus in life-expanded rhesus fibroblasts expressing human telomerase. J. Virol. Methods 104:135-146. [DOI] [PubMed] [Google Scholar]
- 12.Chang, W. L., A. F. Tarantal, S. S. Zhou, A. D. Borowsky, and P. A. Barry. 2002. A recombinant rhesus cytomegalovirus expressing enhanced green fluorescent protein retains the wild-type phenotype and pathogenicity in fetal macaques. J. Virol. 76:9493-9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman, T. L., A. P. Heikeman, and P. J. Bjorkman. 1999. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity 11:603-613. [DOI] [PubMed] [Google Scholar]
- 14.Corinti, S., C. Albanesi, A. la Sala, S. Pastore, and G. Girolomoni. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166:4312-4318. [DOI] [PubMed] [Google Scholar]
- 15.Cosman, D., N. Fanger, and L. Borges. 1999. Human cytomegalovirus, MHC class I and inhibitory signalling receptors: more questions than answers. Immunol. Rev. 168:177-185. [DOI] [PubMed] [Google Scholar]
- 16.D'Amico, G., G. Frascaroli, G. Bianchi, P. Transidico, A. Doni, A. Vecchi, S. Sozzani, P. Allavena, and A. Mantovani. 2000. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat. Immunol. 1:387-391. [DOI] [PubMed] [Google Scholar]
- 17.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 18.Demangel, C., P. Bertolino, and W. J. Britton. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32:994-1002. [DOI] [PubMed] [Google Scholar]
- 19.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Smedt, T., M. Van Mechelen, G. De Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229-1235. [DOI] [PubMed] [Google Scholar]
- 21.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 23.Fleming, S. B., C. A. McCaughan, A. E. Andrews, A. D. Nash, and A. A. Mercer. 1997. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerna, G., E. Percivalle, A. Sarasini, F. Baldanti, G. Campanini, and M. G. Revello. 2003. Rescue of human cytomegalovirus strain AD169 tropism for both leukocytes and human endothelial cells. J. Gen. Virol. 84:1431-1436. [DOI] [PubMed] [Google Scholar]
- 26.Gerna, G., E. Percivalle, A. Sarasini, F. Baldanti, and M. G. Revello. 2002. The attenuated Towne strain of human cytomegalovirus may revert to both endothelial cell tropism and leuko- (neutrophil- and monocyte-) tropism in vitro. J. Gen. Virol. 83:1993-2000. [DOI] [PubMed] [Google Scholar]
- 27.Grigoleit, U., S. Riegler, H. Einsele, K. Laib Sampaio, G. Jahn, H. Hebart, P. Brossart, F. Frank, and C. Sinzger. 2002. Human cytomegalovirus induces a direct inhibitory effect on antigen presentation by monocyte-derived immature dendritic cells. Br. J. Haematol. 119:189-198. [DOI] [PubMed] [Google Scholar]
- 28.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu, D. H., R. de Waal Malefyt, D. F. Fiorentino, M. N. Dang, P. Vieira, J. de Vries, H. Spits, T. R. Mosmann, and K. W. Moore. 1990. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science 250:830-832. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 31.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins, C., A. Abendroth, and B. Slobedman. 2004. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J. Virol. 78:1440-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, B. C., N. J. Logsdon, K. Josephson, J. Cook, P. A. Barry, and M. R. Walter. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. USA 99:9404-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalinski, P., J. H. Schuitemaker, C. M. Hilkens, and M. L. Kapsenberg. 1998. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161:2804-2809. [PubMed] [Google Scholar]
- 35.Kontoyiannis, D., A. Kotlyarov, E. Carballo, L. Alexopoulou, P. J. Blackshear, M. Gaestel, R. Davis, R. Flavell, and G. Kollias. 2001. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20:3760-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockridge, K. M., S. S. Zhou, R. H. Kravitz, J. L. Johnson, E. T. Sawai, E. L. Blewett, and P. A. Barry. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272-280. [DOI] [PubMed] [Google Scholar]
- 38.Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276:13664-13674. [DOI] [PubMed] [Google Scholar]
- 39.Macatonia, S. E., T. M. Doherty, S. C. Knight, and A. O'Garra. 1993. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J. Immunol. 150:3755-3765. [PubMed] [Google Scholar]
- 40.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071-5079. [PubMed] [Google Scholar]
- 41.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minton, E. J., C. Tysoe, J. H. Sinclair, and J. G. Sissons. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra, R. S., T. A. Judge, F. O. Nestle, L. A. Turka, and B. J. Nickoloff. 1995. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J. Immunol. 154:2668-2677. [PubMed] [Google Scholar]
- 44.Mocarski, E. S. 1996. Cytomegaloviruses and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 45.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 46.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 47.Peguet-Navarro, J., C. Moulon, C. Caux, C. Dalbiez-Gauthier, J. Banchereau, and D. Schmitt. 1994. Interleukin-10 inhibits the primary allogeneic T cell response to human epidermal Langerhans cells. Eur. J. Immunol. 24:884-891. [DOI] [PubMed] [Google Scholar]
- 48.Pereira, L., E. Maidji, S. McDonagh, O. Genbacev, and S. Fisher. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77:13301-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierre, P., S. J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R. M. Steinman, and I. Mellman. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388:787-792. [DOI] [PubMed] [Google Scholar]
- 50.Poe, J. C., D. H. Wagner, Jr., R. W. Miller, R. D. Stout, and J. Suttles. 1997. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1β synthesis and rescue from apoptosis. J. Immunol. 159:846-852. [PubMed] [Google Scholar]
- 51.Powell, M. J., S. A. Thompson, Y. Tone, H. Waldmann, and M. Tone. 2000. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region. J. Immunol. 165:292-296. [DOI] [PubMed] [Google Scholar]
- 52.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 53.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 54.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831-844. [DOI] [PubMed] [Google Scholar]
- 55.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 56.Ricciardi-Castagnoli, P., and F. Granucci. 2002. Opinion: interpretation of the complexity of innate immune responses by functional genomics. Nat. Rev. Immunol. 2:881-889. [DOI] [PubMed] [Google Scholar]
- 57.Riddell, S. R., K. S. Watanabe, J. M. Goodrich, C. R. Li, M. E. Agha, and P. D. Greenberg. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257:238-241. [DOI] [PubMed] [Google Scholar]
- 58.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 59.Sallusto, F., P. Schaerli, P. Loetscher, C. Schaniel, D. Lenig, C. R. Mackay, S. Qin, and A. Lanzavecchia. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28:2760-2769. [DOI] [PubMed] [Google Scholar]
- 60.Schottelius, A. J., M. W. Mayo, R. B. Sartor, and A. S. Baldwin, Jr. 1999. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J. Biol. Chem. 274:31868-31874. [DOI] [PubMed] [Google Scholar]
- 61.Schulz, O., A. D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453-462. [DOI] [PubMed] [Google Scholar]
- 62.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1998. Growth of human cytomegalovirus in primary macrophages. Methods 16:126-138. [DOI] [PubMed] [Google Scholar]
- 63.Sozzani, S., P. Allavena, G. D'Amico, W. Luini, G. Bianchi, M. Kataura, T. Imai, O. Yoshie, R. Bonecchi, and A. Mantovani. 1998. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161:1083-1086. [PubMed] [Google Scholar]
- 64.Sozzani, S., S. Ghezzi, G. Iannolo, W. Luini, A. Borsatti, N. Polentarutti, A. Sica, M. Locati, C. Mackay, T. N. Wells, P. Biswas, E. Vicenzi, G. Poli, and A. Mantovani. 1998. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J. Exp. Med. 187:439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson-Snipes, L., V. Dhar, M. W. Bond, T. R. Mosmann, K. W. Moore, and D. M. Rennick. 1991. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J. Exp. Med. 173:507-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, B. P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. J. McMichael, and G. W. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287:1031. [DOI] [PubMed] [Google Scholar]
- 68.Tone, M., M. J. Powell, Y. Tone, S. A. Thompson, and H. Waldmann. 2000. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J. Immunol. 165:286-291. [DOI] [PubMed] [Google Scholar]
- 69.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 70.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 71.Ulbrecht, M., S. Martinozzi, M. Grzeschik, H. Hengel, J. W. Ellwart, M. Pla, and E. H. Weiss. 2000. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J. Immunol. 164:5019-5022. [DOI] [PubMed] [Google Scholar]
- 72.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 73.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]