Abstract
SER virus, a paramyxovirus that is closely related to simian virus 5 (SV5), is unusual in that it fails to induce syncytium formation. The SER virus F protein has an unusually long cytoplasmic tail (CT), and it was previously observed that truncations or specific mutations of this domain result in enhanced syncytium formation. In addition to the long CT, the SER F protein has nine amino acid differences from the F protein of SV5. We previously observed only a partial suppression of fusion in a chimeric SV5 F protein with a CT derived from SER virus, indicating that these other amino acid differences between the SER and SV5 F proteins also play a role in regulating the fusion phenotype. To examine the effects of individual amino acid differences, we mutated the nine SER residues individually to the respective residues of the SV5 F protein. We found that most of the mutants were expressed well and were transported to the cell surface at levels comparable to that of the wild-type SER F protein. Many of the mutants showed enhanced lipid mixing, calcein transfer, and syncytium formation even in the presence of the long SER F protein CT. Some mutants, such as the I310 M, T438S, M489I, T516V, and N529K mutants, also showed fusion at lower temperatures of 32, 25, and 18°C. The residue Asn529 plays a critical role in the suppression of fusion activity, as the mutation of this residue to lysine caused a marked enhancement of fusion. The effect of the N529K mutation on the enhancement of fusion by a previously described mutant, L539,548A, as well as by chimeric SV5/SER F proteins was also dramatic. These results indicate that activation to a fusogenic conformation is dependent on the interplay of residues in the ectodomain, the transmembrane domain, and the CT domain of paramyxovirus F proteins.
Fusion of the paramyxovirus envelope and the host cell membrane is brought about by the interaction of two surface glycoproteins, the hemagglutinin-neuraminidase protein (HN) and the fusion (F) protein. The F protein is synthesized as an inactive precursor, F0, that is posttranslationally cleaved by a host protease into two disulfide-linked subunits called F1 and F2. The cleavage of F is essential for virus-cell and cell-cell membrane fusion and also for virus infectivity (12, 21, 28). A well-conserved hydrophobic domain (fusion peptide) at the amino terminus of F1 is exposed by the cleavage (13, 18) and is considered to be involved in the fusion event (8, 22). Three heptad repeat (HR) domains are found in the F1 ectodomain (4, 9). HR1 is immediately adjacent to the carboxyl terminus of the fusion peptide, while HR2 is close to the transmembrane (TM) domain; HR3 is located between the HR1 and HR2 domains (9). The importance of these domains for fusion was shown by mutational analysis (26, 31) and also by the use of peptide analogues which inhibit fusion, presumably because they interfere with the conformational changes in the molecule that are necessary for fusion (10). Crystallographic analyses have shown that polypeptide fragments representing the HR1 domain of the F protein of simian virus 5 (SV5), Newcastle disease virus, or human respiratory syncytial virus form a trimeric coiled-coil structure to which three antiparallel helices of the polypeptide fragments representing the HR2 domain can bind (3, 5, 39). Similar six-helix bundle structures are also found in other viral fusion proteins and represent the final postfusion form of the protein (7, 14).
The cytoplasmic tail (CT) domain of some viral fusion proteins has also been shown to play a regulatory role in membrane fusion. Among the paramyxoviruses, F-protein CT truncations in Newcastle disease virus show highly reduced syncytium formation (30). Truncations in the CTs of the parainfluenza virus type 3 and SV5 F proteins abolished fusion activity (2, 37), whereas truncation of the CT of the human parainfluenza virus type 2 F protein did not affect its fusion activity (37). In SV5, deletion of the CT of the fusion protein inhibited fusion pore enlargement (6). The region of the C-terminal 16 amino acids of the envelope protein of murine leukemia virus, called the R peptide, is also known to be inhibitory to membrane fusion (11, 17, 29, 36). SER virus is a recently identified paramyxovirus, which is very closely related to SV5 but replicates without syncytium formation (34). Comparison of the CT sequences between the two viruses revealed the presence of a stop codon at amino acid 530 in the SV5 F CT, whereas an additional 22-amino-acid-extended CT is found in the SER F protein. Truncations or mutations in the CT domain of the SER virus F protein were found to enhance syncytium formation, indicating that the elongated CT interferes with membrane fusion in a sequence-dependent manner (33, 34). Besides the long CT domain, SER virus F differs from SV5 F by nine residues, six of which are in the ectodomain, one of which is in the TM domain, and two of which are in the CT domain.
Previous studies have demonstrated that SV5 strains differ in their requirements for coexpression of the HN protein to induce membrane fusion and that these differences can be mapped to specific amino acid residues in the external domain of the F protein (15, 25). The WR strain F protein requires the coexpression of HN for fusion activity, whereas the W3a strain F protein exhibits HN-independent fusion; however, even for the W3a F protein, HN coexpression enhances F-mediated fusion (27). A proline residue at position 22 was demonstrated to be important for conferring HN-independent fusion as well as faster kinetics and a lower temperature for the fusion activity of the W3a F protein, and conversion of a serine at position 443 to proline further enhanced its fusion activity in the presence or absence of HN (25). The SER virus F protein contains proline residues at positions 22 and 443 in the external domain, thus corresponding to a highly fusogenic S443P mutant of SV5 described by Paterson et al. (25). However, we observed a complete lack of fusion by SER virus F but only a partial suppression of fusion activity in a chimeric SV5 F carrying the SER virus F CT (33). These results indicate a role of other amino acid differences between the SER virus and SV5 F proteins in conferring a more active fusion phenotype.
For the present study, we investigated the effects of the nine amino acids which differ between the SER virus and SV5 F proteins on lipid mixing, content mixing, and syncytium formation. We also studied the effect of these amino acid differences in the context of previously described mutants (33) which were found to enhance F-mediated membrane fusion.
MATERIALS AND METHODS
Cells, viruses, and vectors.
HeLa T4 cells were maintained in Dulbecco's modified minimal essential medium (DMEM) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, Utah). The recombinant vaccinia virus vTF7-3 and the wild-type (wt) vaccinia virus strain IHD-J were kindly provided by Bernard Moss (National Institutes of Health, Bethesda, Md.). The vaccinia virus stocks were propagated and titrated on CV-1 cells. Plasmid pGINT7 β-Gal was provided by Edward Berger (National Institutes of Health). The previously characterized pGEM-3-SER F and pGEM-3-SER HN plasmids (34) were expressed in HeLa T4 cells. The pGEM-3-SV5 F and pGEM-3-SV5 HN plasmids were described previously (34). A rabbit anti-SV5 antibody was a kind gift from R. A. Lamb (Northwestern University, Chicago, Ill.). SER virus was propagated in MDBK cells and virus titers were determined by a hemagglutination assay using guinea pig or chicken erythrocytes.
Generation of mutant F proteins.
Mutations in the SER virus F gene were generated by use of a Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The pGEM-SER F plasmid was used as the template and the two synthetic oligonucleotide primers designed to carry the mutation at the desired site were complementary to each other (Sigma Genosys, The Woodlands, Tex.). A schematic representation of the mutations in the ectodomain region is shown in Fig. 1B. All constructs were sequenced to confirm the presence of the desired mutations and the absence of additional mutations.
FIG. 1.
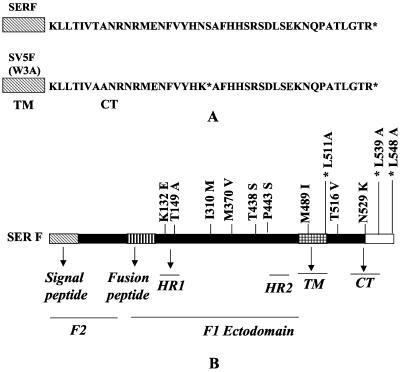
Schematic diagram of SER virus and SV5 F proteins and mutants. (A) Differences in CT domains and presence of a stop codon at amino acid position 530 in the SV5 F CT followed by a 21-amino-acid coding sequence identical to that of the extended CT of the SER virus F protein. (B) SER virus F protein and nine amino acid differences between the SER virus and SV5 F proteins. The amino acid preceding the residue number denotes the wt SER virus F residue and the amino acid after the residue number denotes the SV5 F residue. L511A, a mutation of a conserved residue which served as a negative control, and mutants L539A and L548A, which were described earlier, are also shown (indicated with asterisks).
Transfection, radiolabeling, and immunoprecipitation.
The mutant and wt SER virus F proteins were expressed by using the vaccinia virus-bacteriophage T7 RNA polymerase transient expression system. Briefly, 35-mm-diameter dishes of subconfluent cells were infected with the vTF7-3 virus at a multiplicity of infection (MOI) of 10 for 1 h and were later transfected with 3 μg of plasmid DNA by the use of Lipofectin (Invitrogen, Carlsbad, Calif.). At 18 h posttransfection, the transfected cells were starved in DMEM lacking methionine and cysteine for 45 min, pulse labeled with 100 μCi of [35S]methionine-cysteine/ml for 30 min at 37°C, and then chased with DMEM containing 10% fetal calf serum for 2 h. The cells were washed thrice and then lysed with cell dissociation buffer (10 mM Tris-HCl [pH 8.0], 250 mM NaCl, 0.5% Triton X-100, and 0.5% sodium deoxycholate). The cell lysate was incubated with an SV5 hyperimmune serum for 2 h at 4°C, followed by precipitation with protein-A agarose (Immunopure; Pierce Chemical, Rockford, Ill.) for 2 h at 4°C. The proteins were characterized by sodium dodecyl sulfate (SDS)-8% polyacrylamide gel electrophoresis and subsequently by autoradiography.
Cell surface biotinylation assay.
The expression of proteins at the cell surface was detected by a biotinylation assay, as described previously (19). At 18 h posttransfection, the transfected cells were starved in DMEM lacking methionine and cysteine for 45 min, pulse labeled with 100 μCi of [35S]methionine-cysteine/ml for 30 min at 37°C, and then chased with DMEM containing 10% fetal calf serum for 2 h. The cells were washed thrice with ice-cold phosphate-buffered saline (PBS) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-CM) and then incubated with 1 ml of a 0.5-mg/ml solution of sulfosuccinimidyl-2-(biotin-amido)ethyl-1,3-dithiopropionate (NHS-SS-biotin; Pierce) in PBS-CM at 4°C for 30 min. Free biotin was removed by a brief incubation with DMEM containing 10% fetal calf serum, and the cells were washed thrice with PBS-CM. The biotinylated cell surface proteins were lysed and immunoprecipitated with an anti-SV5 antibody (which also recognizes SER virus-encoded proteins) and protein A-agarose beads (Pierce). The cell lysate-carrying beads were washed thrice and divided into two aliquots. One aliquot was used for immunoprecipitation and the other aliquot was boiled in 10 μl of 10% SDS and diluted with 1 ml of lysis buffer. The supernatant from the protein A-agarose beads was incubated with streptavidin-agarose beads for 2 h at 4°C. The proteins were characterized by SDS-8% polyacrylamide gel electrophoresis and autoradiography. Quantitation of the cell surface expression of the mutant proteins was performed by fluorescence-activated cell sorting (FACS) analysis. The results are presented as percentages of the cell surface expression levels obtained for the corresponding F proteins compared to that of the wt SER virus or SV5 F protein.
Pulse-chase analysis.
Dishes (35-mm diameter) of subconfluent HeLa T4 cells were infected for 1 h with the vTF7-3 virus at an MOI of 10 and then transfected with wt and mutant SER virus F plasmids. At 18 h posttransfection, the transfected cells were starved in DMEM lacking methionine and cysteine for 45 min, pulse labeled with 100 μCi of [35S]methionine-cysteine/ml for 30 min at 37°C, and then chased with DMEM containing 10% fetal calf serum for 0, 15, 30, 45, 60, or 120 min. The cells were washed thrice with ice-cold PBS-CM at the indicated time points and incubated with 1 ml of a 0.5-mg/ml solution of NHS-SS-biotin (Pierce) in PBS-CM at 4°C for 30 min. The cells were washed and then lysed with cell dissociation buffer (10 mM Tris-HCl [pH 8.0], 250 mM NaCl, 0.5% Triton X-100, and 0.5% sodium deoxycholate) at the indicated time points. The surface expression of the SER virus wt and mutant F proteins was monitored by surface biotinylation studies as described above.
Cell fusion assays.
Dishes (35-mm diameter) of subconfluent HeLa T4 cells were infected for 1 h with the vTF7-3 virus at an MOI of 10 and then transfected with 3 μg of a wt or mutant SER virus F construct with or without cotransfection of the wt SER HN plasmid by the use of Lipofectin. At 16 to 20 h posttransfection, the cells were monitored for syncytium formation under a light microscope.
Quantitative cell fusion assays were performed according to the fusion-dependent reporter gene activation method described by Nussbaum et al. (23). In brief, one population of HeLa T4 cells was infected with the recombinant vTF7-3 vaccinia virus, transfected with plasmids encoding the wt SER HN and a wt or mutant SER F protein, and then treated with neuraminidase before the two cell types were mixed. Cytosine arabinoside (40 μg/ml) was added to each plate. A second population of HeLa T4 cells was infected with wt vaccinia virus strain IHD-J and transfected with the pGINT7 β-Gal plasmid, which contains the β-galactosidase (β-Gal) gene under the control of the T7 promoter. At 16 h posttransfection, the two cell populations were collected and mixed by the addition of 100 μl of each cell population (106 cells/ml) to a flat-bottomed 96-well tissue culture plate. The plate was incubated at 37°C for 5 h, and cell fusion was measured by a colorimetric lysate assay as described previously. The quantity of β-galactosidase was calculated by comparing the hydrolysis rate for each sample with that of a standard commercial preparation of Escherichia coli β-galactosidase (Roche). The data were analyzed with the Delta Soft II Microplate analysis program. The fusion activity of the mutants was determined as a percentage of the β-galactosidase production observed in cells coexpressing wt SV5 F and HN.
Temperature-dependent lipid mixing and calcein transfer assay.
Guinea pig erythrocytes (RBCs) were labeled with the hydrophobic fluorescent dye R18 (Molecular Probes, Eugene, Oreg.) as described by Bagai and Lamb (1). In brief, 15 μl of a 1-mg/ml solution of R18 in ethanol was added to 2 ml of 50% hematocrit fresh guinea pig RBC suspension in 10 ml of PBS that was being mixed on a vortex machine. After incubation at room temperature in the dark for 30 min, 35 ml of DMEM with 10% fetal calf serum was added to the suspension and further incubated for 20 min to adsorb the unbound probe. After being labeled, the RBC suspension was washed five times with 50 ml of PBS each time to remove the unincorporated R18. The last washing step was done with PBS containing an additional 1 mM CaCl2 and 1 mM MgCl2 (PBS++), and the cells were resuspended to 50% hematocrit. For the preparation of doubly labeled RBCs, R18-labeled RBCs were loaded with calcein-AM (Molecular Probes, Leiden, Netherlands) as described by Melikyan et al. (20). R18-labeled RBCs (0.3 ml) were washed in swelling buffer (7.125 ml of PBS++ and 4.275 ml of H2O). Calcein-AM was added to a 50% hematocrit RBC suspension to a final concentration of 5 μM, and the cells were washed and resuspended in 15 ml of PBS++ (1% hematocrit). For determinations of lipid mixing and calcein transfer, BHK21 or HeLa T4 cell monolayers expressing SER virus F and HN or SER virus mutant F and HN and incubated with 50 mU of neuraminidase/ml in DMEM for 16 to 18 h posttransfection at 31°C were washed twice with PBS and first incubated with 1 ml of R18- and calcein-labeled RBCs (0.2% hematocrit) in PBS++ at 4°C for 1 h in the dark with intermittent gentle agitation and then incubated at various temperatures for 15 min. Fusion was monitored and photographed by use of an inverted fluorescence microscope. Vaccinia virus-infected and mock-transfected cells were used as negative controls.
RESULTS
Construction and expression of mutant proteins.
It was previously observed that the elongated cytoplasmic domain of the SER virus F protein is inhibitory to the process of membrane fusion (34). Serial truncations in the CT indicated the importance of an eight-amino-acid stretch between amino acids 535 and 542 in suppressing membrane fusion activity. Alanine scanning mutagenesis studies confirmed the importance of this amino acid sequence as well as a leucine residue at position 548 in the regulation of SER virus F-induced membrane fusion (33). We also recently obtained evidence that the presence of the long CT in the SER virus F protein stabilizes the metastable prefusion conformation of the F protein so that the conversion to the fusion active conformation does not occur efficiently at a neutral pH or physiological temperature but may be triggered by exposure to a reduced pH or increased temperature. We observed that the lipid mixing, cytoplasmic content mixing, and syncytium formation ability of the SER virus F protein coexpressed with the SER virus HN protein were enhanced, both qualitatively and quantitatively, at elevated temperatures or under reduced pH conditions ranging between 4.8 and 6.2 (32). We also found that the fusion inhibition effect of the long CT of the SER virus F protein could be partially transferred to the SV5 F protein (33), but a residual level of fusion was observed, indicating that other amino acid differences also play a role in the observed differences in fusion activity of the SER virus and SV5 F proteins.
A comparison of the nucleotide sequences of the F genes of SER virus (EMBL nucleotide sequence database; accession no. AJ278916) and the W3 strain of SV5 (24) revealed the presence of nine amino acid substitutions spanning the SER virus F protein sequence (as shown in Fig. 1A) in addition to the presence of the long SER virus F CT. To determine the effects of the six ectodomains, one TM domain, and two CT differences on the lack of fusion by the SER virus F protein, we introduced these changes individually into the SER virus F sequence. The transport and stability of the mutant SER virus F proteins were examined by performing pulse-chase experiments. All of the mutants were found to be expressed at the cell surface and to have stability comparable to that of the wt SER virus F protein (Fig. 2A). The cell surface expression levels of the mutant proteins were estimated by FACS analysis, as shown in Fig. 2B. Except for the M370V mutant, which showed a surface expression level of 72% that of the wt SER virus F protein, all of the other SER virus F protein CT substitution mutants had surface expression levels of 88 to 96% that of wt F.
FIG. 2.
Surface expression of mutant SER virus F proteins. (A) Pulse-chase analysis of wt and mutant SER virus F proteins. HeLa T4 cells were infected with vTF7-3 at an MOI of 10 for 1 h at 37°C and then transfected with plasmids expressing the K132E, T149A, I310M, M370V, T438S, P443S, M489I, T516V, N529K, and L511A mutants and the SER virus F protein. At 18 h posttransfection, the cells were metabolically labeled with methionine-cysteine for 30 min and chased with cold methionine-cysteine for 0, 15, 30, 45, 60, or 120 min. At the end of the chase period, cell surface proteins were biotinylated and immunoprecipitated with an anti-SV5 antibody. F proteins on the cell surface were detected by precipitating the cell immunoprecipitate with streptavidin-agarose beads as described in Materials and Methods. (B) FACS analysis. For the quantitation of cell surface proteins, HeLa T4 cells were infected with a vTF7-3 (MOI = 10)-expressing wt or mutant SER virus F protein or vTF7-3-expressing T7 polymerase as a negative control. At 18 h posttransfection, the cells were detached with 5 mM EDTA in PBS, washed in PBS, incubated with an anti-SV5 antibody (1:100) followed by a FITC-conjugated secondary antibody, and suspended in PBS. HeLa T4 cells (105) were then analyzed with a FACSCalibur instrument (Becton Dickinson) and WINMDI 2.8 software (Scripps Research Institute Cytometry Software). By comparison to a negative control, only positive cells (20%) were gated to evaluate the geometric mean fluorescence intensity (MFI).
Effect of mutations on lipid mixing and content mixing.
To investigate the effects of these mutations on different stages of membrane fusion, we transiently expressed the SER virus F and HN proteins in HeLa T4 cells in the presence of neuraminidase to prevent cell-cell fusion, and at 16 h posttransfection, we incubated the cells with RBCs that were doubly labeled with R18 and calcein for 1 h at 4°C. After unadsorbed RBCs were removed, the cells were incubated at temperatures ranging from 37 to 18°C for 15 min. The extents of lipid mixing and calcein transfer activities were assayed by counting the number of labeled cells, averaged for six fields, and fusion was expressed as the number of lipid-mixing or content-mixing events per microscopic field. Dye transfer of both R18 and calcein to large syncytia was seen in cells coexpressing most of the mutant F proteins with SER virus HN and incubated at 37°C (Fig. 3). The extent of lipid mixing and the number of content-mixing events were found to be fairly coincident in most of the mutants studied. The M370V mutant showed very low levels of R18 dye transfer and little or no calcein transfer at 37°C, suggesting that this mutant F protein has a higher energy requirement for undergoing complete fusion. However, no enhancement of fusion was observed at higher temperatures (45 and 55°C; data not shown).
FIG. 3.
Lipid mixing and calcein transfer assays at different temperatures. Guinea pig RBCs were doubly labeled with the hydrophobic fluorescent dye R18 and with calcein (Molecular Probes) as described in Materials and Methods. For the determination of lipid mixing and calcein transfer, HeLa T4 cell monolayers expressing SER virus F and HN or SER virus F mutants and HN were treated with 50 mU of neuraminidase/ml in DMEM for 16 to 18 h posttransfection at 31°C, washed twice with PBS, and first incubated with 1 ml of R18- and calcein-labeled RBCs (0.2% hematocrit) in PBS++ at 4°C for 1 h in the dark with intermittent gentle agitation and then incubated at various temperatures for 15 min. The extents of lipid mixing and calcein transfer activities were assayed by counting the number of labeled cells, averaged over six fields, and fusion was expressed as the number of lipid-mixing or content-mixing events per microscopic field. Vaccinia virus-infected and mock-transfected cells were used as negative controls. The lipid mixing and calcein transfer assays were performed independently. (A) Lipid mixing assay; (B) calcein transfer assay.
Dye transfer at a lower temperature of 25 or 18°C was also observed (to a much lesser extent) with the I310 M, T438S, M489I, T516V (TM domain mutant), and N529K mutants. As shown in Fig. 4, dye transfer to smaller syncytia was observed at 25°C and was restricted to single cells at 18°C. Maximum dye transfer, both qualitatively and quantitatively, was observed with the CT mutant N529K. Vaccinia virus-infected, mock-transfected cells served as negative controls and showed no dye transfer. For comparison, we also mutated a conserved leucine 511 residue to alanine as a negative control and found no effect on lipid mixing or calcein transfer at any of the temperatures studied. These results show that mutations in the external domain as well as the TM domain and the CT can overcome the fusion inhibition effect of the long CT of the SER virus F protein and can lower the activation energy for its conversion to a fusogenic conformation.
FIG. 4.
Calcein transfer of I310M, N529K, and L511A mutants at various temperatures. Guinea pig RBCs were labeled with the fluorescent dye calcein (Molecular Probes) as described in Materials and Methods. For the determination of calcein transfer, HeLa T4 cell monolayers expressing SER virus F mutants and HN were treated with 50 mU of neuraminidase/ml in DMEM for 16 to 18 h posttransfection at 31°C, washed twice with PBS, and first incubated with 1 ml of calcein-labeled RBCs (0.2% hematocrit) in PBS++ at 4°C for 1 h in the dark with intermittent gentle agitation and then incubated at various temperatures for 15 min. Calcein transfer was monitored and photographed by using an inverted fluorescence microscope. Magnification, ×200.
Cell fusion activity of mutant F proteins.
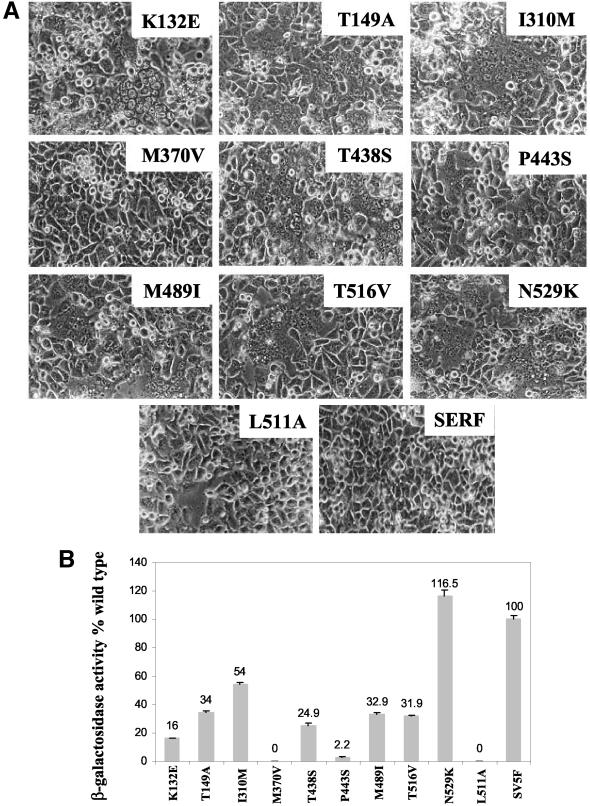
To investigate the effects of the mutations on the induction of syncytium formation, we coexpressed the mutant F proteins with the SER virus HN protein. As shown in Fig. 5, most of the ectodomain mutants induced extensive syncytium formation. The extent of syncytium formation was quantitated by a reporter gene activation assay (23), and the results were expressed as the percentages of β-Gal activity of the mutant F proteins relative to the β-Gal activity of wt SV5 F-HN. As shown in Fig. 5B, mutants K132E and P443S showed β-Gal activities ranging from 2 to 16% that of the wt SV5 F protein, whereas the T149A, I310M, T438S, M489I, and T516V mutants showed β-Gal activities of 25 to 54% that of the wt. The M370V mutant showed no β-Gal activity. The N529K mutant showed a fusion activity of 116% compared to the wt SV5 F protein coexpressed with the SV5 HN protein. The L511A mutant served as a negative control, as it showed no β-Gal activity. These results indicate that the N529K and I310M ectodomain mutants can substantially overcome the suppressive effect of the long SER virus F protein CT, whereas other ectodomain mutants, such as T149M, M489I, and T516V, also enhance fusion by the SER virus F protein, but to a lesser extent.
FIG. 5.
Effect of mutations on cell fusion activity. (A) Cell fusion assays. Dishes (35-mm diameter) of subconfluent HeLa T4 cells were infected for 1 h with the vTF7-3 virus at an MOI of 10 and then cotransfected with 3 μg of a wt or mutant SER virus F construct and a wt SER virus HN plasmid by the use of Lipofectin. At 16 to 20 h posttransfection, the cells were monitored for syncytium formation under a phase-contrast microscope. (B) Reporter gene activation assay. One population of HeLa T4 cells was infected with a recombinant vaccinia virus and transfected with wt SER virus HN and a series of F mutants. The other cell population was infected with the wt vaccinia virus and transfected with a reporter gene construct. At 16 h posttransfection at 31°C, the two cell populations were mixed at a density of 106 cells/ml in a 96-well tissue culture plate and incubated for 5 h at 37°C, after which cell fusion was quantitated by a colorimetric lysate assay. The data are percentages of the β-Gal activity observed in wt SV5 F-HN-expressing cells. The results are averages of three independent experiments.
Effect of N529K mutation on another SER virus mutant F protein and on mutant chimeric SV5/SER virus F proteins.
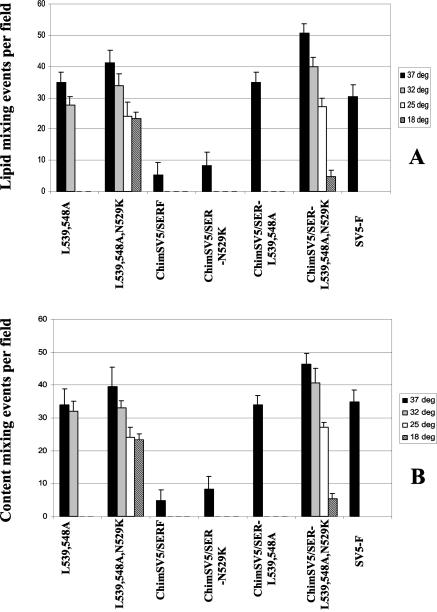
Since the effect of the CT mutation N529K on lipid mixing and content mixing was the most dramatic, we further studied the effect of this mutation in the context of a previously described SER virus F mutant, L539,548A, as well as a chimeric SV5/SER virus F protein carrying the CT from SER virus F or a chimeric SV5/SER-L539,548A mutant (33). All of the N529K mutants were expressed well and were transported to the cell surface at levels comparable to those of the respective original proteins (Fig. 6). The N529K mutants were found be less stable with a 120-min chase period than the original mutant proteins. As shown in Fig. 7, both lipid mixing and calcein transfer were enhanced to a large extent in the L539,548A,N529K and chimeric SV5/SER-L539,548A,N529K mutants; however, the fusion activity of a chimeric SV5/SER virus F mutant harboring the N529K mutation was enhanced to a lesser extent. The effect of the N529K mutation on lipid mixing and content mixing was also studied at low temperatures, and it was found that the L539,548A,N529K and chimeric SV5/SER-L539,548A,N529K mutants were able to induce dye transfer at temperatures as low as 18°C, whereas neither the original SER virus F mutant L539,548A nor the chimeric SV5/SER virus F protein showed dye transfer at reduced temperatures. The L539,548A,N529K, chimeric SV5/SER-N529K, and chimeric SV5/SER-L539,548A,N529K mutants also showed enhanced syncytium formation compared to the original mutant proteins. The chimeric SV5 F mutant L539,548A,N529K showed syncytia at 10 to 12 h posttransfection and was found to be highly fusogenic, with large multinucleate cells seen in all fields. In comparison, the SER virus F mutant L539,548A,N529K showed no syncytium formation until 16 h posttransfection (Fig. 8). The introduction of the N529K mutation also resulted in an increase in fusion, as assessed by a reporter gene activation assay (data not shown). This chimeric SV5/SER-L539,548A,N529K mutant showed the highest fusion activity of any of the constructs studied, suggesting that Asn 529 is one of the key residues involved in modulating fusion by the SER virus CT.
FIG. 6.
Pulse-chase analysis of mutant SER virus F and chimeric SV5/SER virus F proteins. HeLa T4 cells were infected with vTF7-3 at an MOI of 10 for 1 h at 37°C and then transfected with plasmids expressing the following mutants: SER virus F-L539,548A; SER virus F-L539,548A,N529K; chimeric SV5/SER virus F; chimeric SV5/SER-N529K; chimeric SV5/SER-L539,548A; and chimeric SV5/SER-L539,548A,N529K. At 18 h posttransfection, the cells were metabolically labeled with methionine-cysteine for 30 min and chased with cold methionine-cysteine for 0, 15, 30, 45, 60, or 120 min. At the end of the chase period, cell surface proteins were biotinylated and immunoprecipitated with an anti-SV5 antibody. F proteins on the cell surface were detected by precipitating the cell immunoprecipitate with streptavidin-agarose beads as described in Materials and Methods.
FIG. 7.
Temperature-dependent lipid mixing and calcein transfer assay. Guinea pig RBCs were doubly labeled with the hydrophobic fluorescent dye R18 and with calcein (Molecular Probes) as described in Materials and Methods. For the determination of lipid mixing and calcein transfer, HeLa T4 cell monolayers expressing SER virus F mutants and SER virus HN or chimeric SV5/SER virus F mutants and SV5 HN with 50 mU of neuraminidase/ml in DMEM for 16 to 18 h posttransfection at 31°C were washed twice with PBS and first incubated with 1 ml of R18-labeled RBCs (0.2% hematocrit) in PBS++ at 4°C for 1 h in the dark with intermittent gentle agitation and then incubated at various temperatures for 15 min. Fusion was monitored and photographed by using an inverted fluorescence microscope. Vaccinia virus-infected and mock-transfected cells were used as negative controls. (A) Lipid mixing assay; (B) calcein transfer assay. The following mutants were studied: L539,548A; L539,548A,N529K; chimeric SV5/SER virus F; chimeric SV5/SER-N529K; chimeric SV5/SER-L539,548A; and chimeric SV5/SER-L539,548A,N529K.
FIG. 8.
Effect of N529K mutation on fusion activity. Dishes (35-mm diameter) of subconfluent HeLa T4 cells were infected for 1 h with vTF7-3 at an MOI of 10 and then cotransfected with the mutant chimeric SV5/SER-L539,548A,N529K and SER-L539,548A,N529K F constructs and a wt SV5 HN or SER virus HN plasmid by the use of Lipofectin. The cells were monitored for syncytium formation under a phase-contrast microscope at different times posttransfection. Magnification, ×200.
DISCUSSION
SER virus, a paramyxovirus, exhibits minimal fusion activity, and previous studies demonstrated that an extended CT domain in its F protein plays an important role in fusion suppression (34). We also previously demonstrated that various site-specific mutations in the CT of the SER virus F protein can overcome the fusion-suppressive effect of the long CT domain (33, 34). SER virus is very closely related to SV5 serologically and in its protein profile; however, besides the presence of the additional 22 amino acids in the CT, there are 9 amino acid differences spanning the ectodomain, the TM domain, and the CT domain between the SER virus and SV5 F proteins. Since the long CT of the SER virus F protein could only partially suppress fusion in a chimeric SV5 F protein carrying the CT of the SER virus F protein (33), we further studied the possible effects of these amino acid differences on fusion activity by mutating individual SER virus F residues to the corresponding SV5 residues.
We observed that the ectodomain mutants showed lipid mixing, calcein transfer, and syncytium formation to various extents. We performed our assays at a physiological pH to determine if these mutations could induce fusion under conditions in which the wt SER virus F protein shows no fusion. The SER virus F protein, when expressed without HN, does not show fusion at low pHs (32). Many of the mutant proteins also showed dye transfer at a less-than-optimal temperature for fusion (32, 25, and 18°C), indicating that these mutations lower the energy requirement for the SER virus F protein to induce fusion. It was shown previously that the presence of prolines at residues 22 and 443 destabilizes the SV5 F protein and decreases the energy requirement for triggering the conformational change to the fusion active state (25, 35). In contrast, in the wt SER virus F protein, prolines are present at both position 22 and position 443, but the protein is nonfusogenic. The P443S mutant showed some lipid mixing and calcein transfer at 37 and 32°C, although limited syncytium formation was observed at these temperatures. Taken together, these results indicate that the effect of the proline-to-serine change at residue 443 depends on the sequence context of the F protein.
We observed extensive dye transfer and syncytium formation when the Asn residue at position 529 was mutated to lysine, and fusion was found to be very dramatic, with extensive multinucleate cells. It is interesting that this single residue, also in the CT domain, can overcome the fusion-suppressive effect of the long CT. We also found that this mutation further enhanced the fusion activity of fusogenic mutants that we described earlier, such as the SER virus F L539,548A and chimeric SV5/SER virus F or chimeric SV5/SER-L539,548A F proteins (33). Surprisingly, the fusion observed with the SER-N529K mutant was more profound than that with the chimeric SV5/SER-N529K mutant. This indicates an effect of differences in the overall context of the SER virus F ectodomain compared with that of SV5. A highly fusogenic state was observed with the chimeric SV5/SER-L539,548A,N529K mutant, which had enhanced fusion kinetics (with syncytium formation observed as early as 12 h posttransfection) and showed fusion to a greater extent, suggesting a lower activation energy requirement for fusion. However, these mutants showed no fusion in the absence of the SER virus HN protein, in contrast to the SER virus L539,548A mutant, which eliminated the requirement for HN to induce fusion (33). These results indicate that the HN dependence of fusion is not necessarily correlated with differences in the overall fusogenicity of the F proteins.
Whereas the WR and W3a isolates of SV5 are highly fusogenic and have short CTs in their F proteins, several closely related SV5 isolates from other species have long CTs and are much less fusogenic (16, 38). We previously suggested (34) that the SV5 viruses with truncated F proteins may have arisen as cytopathic variants, as they were identified based on their cytopathic effects in monkey kidney cell cultures. The F proteins of these viruses possess a stop codon at position 530, after which they exhibit an open reading frame precisely matching that of the SER virus F protein. The present results further suggest that the external domain of the SER virus F protein has other structural features that have evolved precisely to minimize its fusogenic potential. The lack of fusion activity may provide an advantage to the virus in minimizing the viral cytopathic effect, which may enable a persistent virus infection and long-term virus production at high levels (38). It will therefore be interesting to compare these closely related viruses in terms of their disease potential in animal models.
In conclusion, we found that some mutations in the ectodomain, the TM domain, and the CT domain can confer fusion activity upon the SER virus F protein. Asn529 plays a critical role in the regulation of fusion activity, as the mutation of this residue to lysine causes a state of hyperfusion. This mutation might destabilize the association between monomers in the CT of the SER virus F protein trimer, and the altered conformation may result in a lower energy barrier for transition to the fusion active state. These results strongly suggest that the activation to a fusogenic conformation is brought about by the interplay of residues in the ectodomains, the TM domains, and the CT domains of paramyxovirus F proteins. They also indicate that structural features in multiple domains are involved in determining the nonfusogenic phenotype of the SER virus F protein.
Acknowledgments
This study was supported by grant AI 52542 from the National Institutes of Health.
We thank Tanya Cassingham for assistance in preparing the manuscript.
REFERENCES
- 1.Bagai, S., and R. A. Lamb. 1995. Quantitative measurement of paramyxovirus fusion: differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J. Virol. 69:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagai, S., and R. A. Lamb. 1996. Truncation of the COOH-terminal region of the paramyxovirus SV5 fusion protein leads to hemifusion but not complete fusion. J. Cell Biol. 135:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, P., C. R. Pringle, and A. J. Easton. 1992. Sequence analysis of the gene encoding the fusion glycoprotein of pneumonia virus of mice suggests possible conserved secondary structure elements in paramyxovirus fusion glycoproteins. J. Gen. Virol. 73:1717-1724. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., J. J. Gorman, J. McKimm-Breschkin, L. J. Lawrence, P. A. Tulloch, B. J. Smith, P. M. Colman, and M. C. Lawrence. 2001. The structure of the fusion glycoprotein of Newcastle disease virus suggests a novel paradigm for the molecular mechanism of membrane fusion. Structure (Cambridge) 9:255-266. [DOI] [PubMed] [Google Scholar]
- 6.Dutch, R. E., and R. A. Lamb. 2001. Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement. J. Virol. 75:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 8.Gething, M. J., J. M. White, and M. D. Waterfield. 1978. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc. Natl. Acad. Sci. USA 75:2737-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh, J. K., M. Ovadia, and Y. Shai. 1997. A leucine zipper motif in the ectodomain of Sendai virus fusion protein assembles in solution and in membranes and specifically binds biologically-active peptides and the virus. Biochemistry 36:15451-15462. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh, J. K., and Y. Shai. 1998. A peptide derived from a conserved domain of Sendai virus fusion protein inhibits virus-cell fusion. A plausible mode of action. J. Biol. Chem. 273:7252-7259. [DOI] [PubMed] [Google Scholar]
- 11.Gray, K. D., and M. J. Roth. 1993. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J. Virol. 67:3489-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homma, M., and M. Ouchi. 1973. Trypsin action on the growth of Sendai virus in tissue culture cells. III. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J. Virol. 12:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu, M., A. Scheid, and P. W. Choppin. 1981. Activation of the Sendai virus fusion protein (f) involves a conformational change with exposure of a new hydrophobic region. J. Biol. Chem. 256:3557-3563. [PubMed] [Google Scholar]
- 14.Hughson, F. M. 1997. Enveloped viruses: a common mode of membrane fusion? Curr. Biol. 7:R565-R569. [DOI] [PubMed] [Google Scholar]
- 15.Ito, M., M. Nishio, M. Kawano, S. Kusagawa, H. Komada, Y. Ito, and M. Tsurudome. 1997. Role of a single amino acid at the amino terminus of the simian virus 5 F2 subunit in syncytium formation. J. Virol. 71:9855-9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito, M., M. Nishio, H. Komada, Y. Ito, and M. Tsurudome. 2000. An amino acid in the heptad repeat 1 domain is important for the haemagglutinin-neuraminidase-independent fusing activity of simian virus 5 fusion protein. J. Gen. Virol. 81:719-727. [DOI] [PubMed] [Google Scholar]
- 17.Januszeski, M. M., P. M. Cannon, D. Chen, Y. Rozenberg, and W. F. Anderson. 1997. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J. Virol. 71:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohama, M. T., J. M. Cardenas, and J. T. Seto. 1981. Immunoelectron microscopic study of the detection of the glycoproteins of influenza and Sendai viruses in infected cells by the immunoperoxidase method. J. Virol. Methods 3:293-301. [DOI] [PubMed] [Google Scholar]
- 19.Le Bivic, A., A. Quaroni, B. Nichols, and E. Rodriguez-Boulan. 1990. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J. Cell Biol. 111:1351-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melikyan, G. B., J. M. White, and F. S. Cohen. 1995. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J. Cell Biol. 131:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai, Y., H. D. Klenk, and R. Rott. 1976. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology 72:494-508. [DOI] [PubMed] [Google Scholar]
- 22.Novick, S. L., and D. Hoekstra. 1988. Membrane penetration of Sendai virus glycoproteins during the early stages of fusion with liposomes as determined by hydrophobic photoaffinity labeling. Proc. Natl. Acad. Sci. USA 85:7433-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterson, R. G., T. J. Harris, and R. A. Lamb. 1984. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc. Natl. Acad. Sci. USA 81:6706-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paterson, R. G., C. J. Russell, and R. A. Lamb. 2000. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology 270:17-30. [DOI] [PubMed] [Google Scholar]
- 26.Reitter, J. N., T. Sergel, and T. G. Morrison. 1995. Mutational analysis of the leucine zipper motif in the Newcastle disease virus fusion protein. J. Virol. 69:5995-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheid, A., and P. W. Choppin. 1974. The hemagglutinating and neuraminidase protein of a paramyxovirus: interaction with neuraminic acid in affinity chromatography. Virology 62:125-133. [DOI] [PubMed] [Google Scholar]
- 29.Schultz, A., and A. Rein. 1985. Maturation of murine leukemia virus Env proteins in the absence of other viral proteins. Virology 145:335-339. [DOI] [PubMed] [Google Scholar]
- 30.Sergel, T., and T. G. Morrison. 1995. Mutations in the cytoplasmic domain of the fusion glycoprotein of Newcastle disease virus depress syncytia formation. Virology 210:264-272. [DOI] [PubMed] [Google Scholar]
- 31.Sergel-Germano, T., C. McQuain, and T. Morrison. 1994. Mutations in the fusion peptide and heptad repeat regions of the Newcastle disease virus fusion protein block fusion. J. Virol. 68:7654-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth, S., A. Vincent, and R. W. Compans. 2003. Activation of fusion by the SER virus F protein: a low-pH-dependent paramyxovirus entry process. J. Virol. 77:6520-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seth, S., A. Vincent, and R. W. Compans. 2003. Mutations in the cytoplasmic domain of a paramyxovirus fusion glycoprotein rescue syncytium formation and eliminate the hemagglutinin-neuraminidase protein requirement for membrane fusion. J. Virol. 77:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong, S., M. Li, A. Vincent, R. W. Compans, E. Fritsch, R. Beier, C. Klenk, M. Ohuchi, and H.-D. Klenk. 2002. Regulation of fusion activity by the cytoplasmic domain of a paramyxovirus F protein. Virology 301:322-333. [DOI] [PubMed] [Google Scholar]
- 35.Tsurudome, M., M. Ito, M. Nishio, M. Kawano, H. Komada, and Y. Ito. 2001. Hemagglutinin-neuraminidase-independent fusion activity of simian virus 5 fusion (F) protein: difference in conformation between fusogenic and nonfusogenic F proteins on the cell surface. J. Virol. 75:8999-9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, C., and R. W. Compans. 1997. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J. Virol. 71:8490-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao, Q., and R. W. Compans. 1995. Differences in the role of the cytoplasmic domain of human parainfluenza virus fusion proteins. J. Virol. 69:7045-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, D. F., L. Didcock, and R. E. Randall. 1997. Isolation of highly9 fusogenic variants of simian virus 5 from persistently infected cells that produce and respond to interferon. J. Virol. 71:9333-9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao, X., M. Singh, V. N. Malashkevich, and P. S. Kim. 2000. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. USA 97:14172-14177. [DOI] [PMC free article] [PubMed] [Google Scholar]