Abstract
To characterize the induction of antigen-specific immune response mediated by baculovirus, vectors expressing the E2 glycoprotein of hepatitis C virus or the carcinoembryonic antigen (CEA) under the control of the cytomegalovirus immediate-early promoter-enhancer were constructed. Additionally, a baculovirus vector encoding the E2 glycoprotein (Bac-G-E2) and expressing vesicular stomatitis virus glycoprotein (VSV-G) in the viral envelope was generated by inserting the VSV-G coding sequence downstream of the polyhedrin promoter. Mice were subjected to intramuscular, intranasal, or subcutaneous inoculations with Bac-E2 and the cellular immune response was monitored by ELISPOT and intracellular staining. Additionally, humoral response was monitored by titrating anti-E2 antibodies. Induction of a measurable anti-E2 T-cell response was observed only after intramuscular injection and was predominantly CD8+ specific. The immunogenic properties of baculovirus as vaccine vector were not restricted to E2 because a CEA-specific CD4+ T-cell response was observed upon intramuscular injection of Bac-CEA. Interestingly, the Bac-G-E2 vector was shown to be a more efficient immunogen than Bac-E2, in view of the 10-fold difference in the minimal dose required to elicit a measurable T-cell response upon intramuscular injection. Induction of inflammatory cytokines such as gamma interferon, tumor necrosis factor alpha, and interleukin-6 was detected as early as 6 h postinjection of Bac-G-E2. Most importantly, both vectors elicited CD8+ T cells with effector function capable of lysing target cells loaded with a hepatitis C virus-specific epitope. Additionally, enhanced NK cytolytic activity was detected in immunized mice. Thus, these results further demonstrate that baculovirus may be considered a useful vector for gene therapy.
Recent advances in immunology and molecular biology have stimulated the development of gene-based vaccines. These vaccines are designed to generate both cellular and humoral responses that can be used to prevent infectious diseases and to treat cancer, allergies, and autoimmune diseases (38). Two general classes of gene-based vaccines are being developed. The first is based on the use of plasmid DNA encoding the target antigen. However, the use of plasmid DNA as a gene transfer vehicle for immunization is somewhat limited by the low transduction efficiency that hampers its immunogenic potential. To overcome this problem, numerous strategies are being developed that use electrical stimulation, microparticles, adjuvants, and costimulatory molecules to enhance both the gene transfer capabilities and the intrinsic immunogenicity of plasmid DNA (24).
Viruses have highly evolved structures that enable them to bind to cells and deliver genes into the cells that they infect. Thus, it is reasonable to consider using live virus vaccines to induce cytolytic T lymphocytes to treat a disease in which cellular immunity is an absolute requirement. To this end, a variety of viral vectors, primarily based on poxvirus and adenovirus, have been tested, with encouraging results (21, 36). However, these vectors are themselves antigenic and can cause inflammation, which, depending on the site of injection and on the recipient's state of health, may constitute a liability for their use as vehicles for vaccination. An additional drawback is that existing immunity due to previous infection (as in the case of adenovirus) or to vaccination (smallpox) may limit the potency of the vaccine by clearing the viral vector before it can infect cells and deliver its payload of antigen genes (8, 13). Thus, there is a need to identify unrelated viral vectors that function efficiently as gene transfer vehicles for immunization whose efficacy is not limited by problems connected with toxicity or existing immunity.
In recent years, baculovirus has emerged as a vector with great potential for gene transfer in mammalian cells (31). Baculoviruses are a group of insect viruses possessing a rod-shaped capsid in which is packaged a condensed DNA genome, a double-stranded, covalently closed circular molecule ranging between 80 and 200 kbp in length (30). The baculovirus Autographa californica multiple nucleopolyhedrovirus has long been used as a biopesticide and as a tool for efficient recombinant protein production in insect cells (26, 28). Although its host specificity was originally thought to be restricted to cells derived from arthropods, baculovirus was shown to infect a number of mammalian cells and animal models (2, 6, 10, 12, 18, 23, 32, 33, 34, 37, 39, 40, 42). Gene transfer mediated by baculovirus carrying the reporter gene under the control of a strong mammalian promoter such as the immediate-early promoter of cytomegalovirus (CMV) or the chimeric CMV early enhancer (CAG) promoter was demonstrated in a number of human primary cell lines and liver tissue (6, 42). This vector was also shown to be capable of carrying large inserts and efficiently infecting a variety of cell lines without any apparent viral replication or cytopathic effect, even at a high multiplicity of infection (MOI) (12, 37). Furthermore, enhanced gene transfer efficiency was observed in a variety of cell lines with recombinant baculovirus vectors expressing surface glycoprotein G of vesicular stomatitis virus (VSV-G). This protein was demonstrated to enhance the escape of baculovirus vectors from intracellular endosomes, increasing the transduction efficiency of the virus (4).
The use of baculovirus as a vector for vaccination was initially described by Aoki and coworkers, who demonstrated that injecting mice with a recombinant vector expressing pseudorabies virus glycoprotein B elicited a measurable humoral response directed against this viral glycoprotein (3). More recently, an immune response to the hemagglutinin glycoprotein of influenza virus was elicited upon vaccination with a baculovirus vector expressing the virus structural component. Additionally, induction of a strong innate immune response was also detected upon injection of wild-type baculovirus (1).
We have further examined the efficiency of baculovirus as a vector for gene therapy. To this end, we have determined the induction of humoral and cell-mediated immune responses to the E2 glycoprotein of hepatitis C virus (HCV) and to carcinoembryonic antigen (CEA) in mice vaccinated with recombinant baculovirus vectors expressing the putative structural component of the HCV virions or the epithelial tumor antigen. The results obtained further demonstrate that significant cell-mediated immunity to target antigens can be elicited upon injection of recombinant baculovirus which express them.
MATERIALS AND METHODS
Virus construction.
Viral constructs were prepared with the pFastBac1 plasmid and the Bac-To-Bac baculovirus expression system (Life Technologies). To construct Bac-E2, the pV1J/TPA-E2 vector encoding the chimeric version of the HCV E2 glycoprotein (43) was digested with ApaLI and BstXI to isolate the CMV-tpaE2-bovine growth hormone polyadenylation signal expression cassette. The purified fragment was ligated into the pFastBac vector, which was previously digested with HindIII. To construct Bac-G-E2, pV1J/TPA-E2-F78 was digested with ApalI and BstXI as well and cloned into the pFastBac/G vector (32) as described for Bac-E2.
To construct the Bac-CEA vector, plasmid pVIJ-hCEAopt, which carries the codon-optimized cDNA for human CEA (Mennuni et al., unpublished data), was digested with BstXI and DraIII and treated with T4 DNA polymerase. The DNA fragment corresponding to the CEA coding sequence flanked by the CMV promoter and intron A and by the bovine growth hormone polyadenylation signal was inserted into the StuI site of plasmid pFastBac.
The recombinant FastBac constructs were individually transformed into Escherichia coli DH10Bac cells (Life Technologies) to generate the corresponding recombinant bacmids. Sf9 insect cells (American Type Culture Collection, Rockville, Md.) were transfected with the recombinant bacmid DNA with Cellfectin (Life Technologies), and the recombinant baculovirus vectors were amplified by repeated passages.
Insect cell culture and virus amplification and purification.
Sf9 cells were cultured at 28°C. Cells were grown and maintained in SF900 II medium supplemented with 10% fetal bovine serum (insect cell tested), 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 U of streptomycin per ml. Cells were infected at an MOI of 1. To purify the virus, cell debris was first removed by centrifugation for 10 min at 2000 rpm Infected cell supernatant was then layered over 27% sucrose and centrifuged at 24000 rpm for 75 min in SW28 tubes. The virus pellet was resuspended in phosphate-buffered saline (PBS, pH 7.5) and centrifuged in SW28 tubes at 27,000 rpm for 150 min. The final pellet was resuspended in PBS. Purified virus was titrated in Sf9 cells with the BD BacPAK baculovirus rapid titer kit (BD Biosciences, Pharmingen) and stored at 4°C in the dark. Viral titers were quantified as focus forming units (FFU).
Detection of CEA and E2 proteins in virus-infected cultured cells.
The human hepatoma cell line Huh7 was maintained in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum and infected at an MOI of 100 with Bac-CEA and MOIs 10, 100, and 1,000 with Bac-E2 and Bac-G-E2 in the presence of 10 μM dexamethasone (12). Forty-eight hours after infection, the cells were lysed with lysis buffer (50 mM Tris-HCl, pH 6.8, 0.1 M dithiothreitol, 2% sodium dodecyl sulfate, 10% glycerol). Cell extracts were resolved by sodium dodecyl sulfate-7% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. Western blotting was carried out as previously described (32, 43) with an anti-E2 mouse polyclonal antiserum. Expression of the VSV-G protein was monitored with purified virus samples with a mouse monoclonal antibody specific for VSV-G (PD54; Sigma). Expression of CEA was detected with a rabbit polyclonal antiserum (Fitzgerald).
Animals and treatment.
All animal studies described in this report were approved by the Istituto di Ricerche di Biologia Moleculare institutional animal care and use committee. Eight-week-old BALB/c female mice were purchased from Charles River Italy (Lecco, Italy). Groups of 6 to 10 mice were immunized with different doses of baculovirus vectors ranging from 108 to 1010 FFU in a 50-μl volume. Prime-boost regimens were implemented with injections 3 weeks apart. Where indicated, intramuscular, intranasal, and subcutaneous injections were performed. Blood samples were obtained by retroorbital puncture. Two weeks after the last injection, mice were euthanized, and splenocytes were harvested for immunological assays.
ELISPOT assay.
Mouse splenocytes secreting gamma interferon (IFN-γ) in an antigen-specific manner were detected with a standard enzyme-linked immunosorbent assay (ELISPOT) (43). Spleen cells were prepared from immunized mice and resuspended in R10 medium (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin per ml, 10 mM HEPES, 50 μM 2-mercaptoethanol). Multiscreen 96-well filtration plates (Millipore, Bedford, Mass.) were coated with affinity-purified rat anti-mouse IFN-γ (immunoglobulin G1, clone R4-6A2; Pharmingen). After overnight incubation, plates were washed with sterile PBS and incubated with R10 medium for 2 h at 37°C to block nonspecific binding. Splenocytes from immunized mice were resuspended in R10 medium and incubated for 20 h at a density of 2 × 105 and 5 × 105 cells/well in the presence of the indicated peptide pool at a concentration of 1 μg/ml. After washing with PBST (0.05% Tween in PBS), plates were incubated for 12 h at 4°C with 50 μl/well of biotin-conjugated rat anti-mouse IFN-γ (rat immunoglobulin G1, clone XMG 1.2; PharMingen) diluted 1:250 in assay buffer (PBS with 5% fetal bovine serum and 0.05% Tween 20). Plates were then washed and incubated for 2 h at room temperature with streptavidin-alkaline phosphatase conjugate (Pharmingen) diluted 1:2,500 in assay buffer. After extensive washing, plates were developed by adding 50 μl/well of nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) (Pierce) until development of spots was observed at the microscope. The reaction was stopped by washing plates thoroughly with distilled water. Dimethyl sulfoxide (DMSO) and concanavalin A (5 μg/ml) were used as negative and positive internal controls for each sample. Plates were allowed to air dry, and spots were counted with an automated ELISPOT reader (AID ELR02 coupled with AID ELISPOT 2.6 software). Lyophilized human CEA and E2 peptides were purchased from Bio-Synthesis and resuspended in DMSO at 40 mg/ml.
Flow cytometry analysis.
Intracellular IFN-γ was measured according to the BD Pharmingen standard protocol. Briefly, 2 × 106 splenocytes from immunized mice were incubated overnight in R10 medium with or without 2 μg/ml of peptide 1664 (EATYSKCGSGPWLTPRCMVD) or peptide pool D and brefeldin A as a protein transport inhibitor (Cytofix/Cytomer Plus kit; BD Pharmingen). DMSO was tested with the splenocytes as a background control. Cells were stained with allophycocyanin-conjugated anti-mouse CD3e, phycoerythrin-conjugated anti-mouse CD4, and peridinin chlorophyll protein-anti-mouse CD8α (Pharmingen). The splenocytes were then washed, fixed, permeabilized, and stained for intracellular IFN-γ by fluorescein isothiocyanate-conjugated anti-IFN-γ monoclonal antibody. All samples were acquired within 24 h with a FACSCalibur flow cytometer and CellQuest software (BD Immunocytometry Systems). T-lymphocyte IFN-γ was calculated as 100 × [(IFN-γ+, CD3+ and CD4+ or CD8+)/(CD3+ and CD4+ or CD8+)].
Cytotoxicity assay.
The cytotoxic T-lymphocyte assay was performed as described (43). Briefly, p815 cells (ATCC TIB64) expressing the H-2d class I molecule were used as target cells. A total of 4 × 106 spleen cells were resuspended in R10 supplemented with 10 μg of CD8+-specific peptide (peptide 1664) and 10 U of recombinant human interleukin-2 (Boehringer Mannheim GmbH, Mannheim, Germany) per ml and plated in quadruplicate in 24-well flat-bottomed plates (multiwell-tissue culture plate; Falcon catalog number 3047). After 7 days of in vitro restimulation, cultures were assayed for cytotoxic activity in a standard 51Cr release assay. Briefly, target cells were labeled with Na51CrO4 (Amersham Pharmacia Biotech, Buckinghamshire, England) and pulsed with either peptide 1664 or medium alone for 2 h at 37°C and 5% CO2. After extensive washing with R10 medium, target cells were mixed with cytotoxic T lymphocytes at the designated effector-to-target-cell ratios in 96-well round-bottomed plates (Cell Wells; Corning Glass Works, Corning, N.Y.) and incubated for 4 h at 37°C and 5% CO2; 30-μl samples of supernatants were transferred to a LumaPlate-96 (Packard Instrument Company, Meridien, Conn.), allowed to dry overnight, and then counted (TopCount microplate scintillation counter; Packard Instrument Company) to determine the amount of 51Cr released in each well. The percent specific lysis was calculated with the formula 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)], where the spontaneous and maximum release refer to the number of counts in DMSO or 1% Triton X-100 target cell lysate, respectively.
NK assay.
The cytolytic activity of NK cells was monitored as described (20). Splenocytes from immunized and control mice were resuspended in R10 medium at a density of 107 cells/ml and plated as indicated into Costar V-bottomed microtiter plates (100 μl/well). Target Yac-1 murine lymphoma cells were labeled by incubation for 90 min at 37°C with 150 μCi sodium 51Cr and resuspended at a density of 105 cells/ml. One hundred microliters of target cell suspension was added to the microtiter plates containing the effector spleen cells to obtain the designated effector-target ratio. Plates were incubated at 37°C for 4 h. After the incubation, 30 μl of the supernatant from each well was harvested and analyzed in a beta counter to determine the amount of 51Cr released in each well. The percent specific lysis was calculated with the same formula applied to the cytotoxicity assay.
ELISA.
Induction of anti-E2 antibodies was monitored by enzyme-linked immunosorbent assay (ELISA) as described (43). Ninety-six-well Nunc ELISA plates were coated overnight at 4°C with leptin from Galanthus nivalis (GNA) (Sigma), washed with washing buffer (PBS, 0.05% Tween 20), and incubated with blocking buffer (2% bovine serum albumin, 1× PBS, 0.05% Tween 20) to remove nonspecific binding. Saturating amounts of E2 protein produced by transient transfection of 293 cells were incubated in blocking buffer for 3 h at room temperature. A fixed amount (100 μl/well) or threefold dilutions (from 1:30 to 1:21,870) of sera were used in seroconversion and titration assays, respectively. Bound antibodies were detected with anti-mouse immunoglobulin G Fc-specific alkaline phosphatase-conjugated antibody (catalog number A-7434; Sigma) diluted 1:2,000 in blocking buffer. Serum dilution was plotted versus optical density values and fitted with a Michaelis-Menten curve (Abelbeck Software KaleidaGraph, version 3.0.1).
RNase protection assays.
Induction of cytokines in injected mice was monitored by RNase protection analysis. Quadriceps from three mice injected with 1010 FFU were removed at the indicated time and snap frozen in liquid nitrogen. Total RNA was extracted with Ultraspec II RNA (Biotecx) according to the manufacturer's instructions. Probes were transcribed from a multitemplate mCK3b set (Riboquant; BD PharMingen) with [32P]UTP (DuPont NEN, Boston, Mass.). The freshly labeled radioactive probe set was hybridized overnight with the indicated RNA samples, digested with RNase A and T1, followed by proteinase K according to the manufacturer's instructions. Digested RNA samples were extracted, precipitated, and resolved on 6% polyacrylamide-urea gels. Gels were dried, exposed to film, and quantified with the use of a Phosphorimager.
RESULTS
Construction of recombinant vectors expressing the HCV E2 or CEA glycoprotein.
To characterize the efficiency of baculovirus as a vehicle for gene therapy, vectors expressing the E2 glycoprotein of HCV or the human tumor antigen CEA were constructed. These proteins were selected as target antigens in view of their association with important diseases such as viral hepatitis and various carcinomas affecting colon, breast, lung, and pancreas (16, 22). Additionally, immune responses to these antigens have been induced in animal models and in humans by means of genetic vaccination protocols (5, 27, 35, 43).
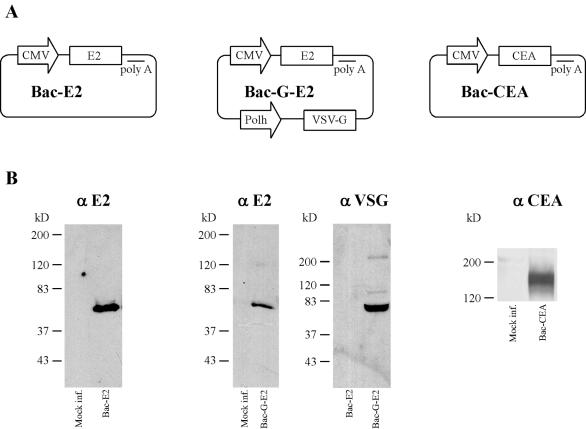
To express the E2 protein of HCV, a cDNA encoding a chimeric version of the glycoprotein ectodomain fused to a highly cross-reactive variable region was selected. This particular construct has been shown to be immunogenic in mice upon electroinjection of plasmid DNA (43). Figure 1A shows the vectors used in this study. Vectors Bac-E2 and Bac-G-E2 carry HCV E2 under the control of the CMV immediate-early promoter. Furthermore, the latter virus also carries the VSV-G coding sequence inserted downstream of the polyhedrin promoter (pPolh), and vectors expressing the VSV-G glycoprotein have been shown to infect cells more efficiently and to transduce mouse skeletal muscle (4, 32). Lastly, Bac-CEA, carrying the CMV-CEA expression cassette, was also constructed.
FIG. 1.
(A) Schematic representation of the recombinant baculovirus vectors used in this study. Baculovirus transfer plasmids were derived from pFastBAc1 as described in Materials and Methods. The HCV E2 glycoprotein, CEA, and VSV-G coding sequences are indicated. The name of each vector is also indicated. Expression of E2 and CEA is driven by the CMV immediate-early promoter. The baculovirus polyhedrin promoter (pPolh) drives the expression of VSV-G. The bovine growth hormone polyadenylation signal (polyA) flanks the E2 and CEA coding sequences. (B) Expression of E2 and CEA in baculovirus-infected cells. Huh-7 cells were infected with Bac-E2, Bac-G-E2, or Bac-CEA and processed for Western blot analysis as described in Materials and Methods. To determine the association of VSV-G with viral particles, Bac-G-E2 and Bac-E2 preparations were purified by centrifugation and processed for Western blot analysis with a monoclonal antibody specific for VSV-G. The specificity of the antibody used for the Western blot is indicated. The positions of molecular size standards (in kilodaltons) are also shown.
Viruses were produced at high titers, ranging from 5 × 108 to 1 × 1012 FFU/ml, and the structure of the baculovirus genomic DNA was confirmed by Southern blot analysis (data not shown). Expression of the CEA and E2 glycoproteins due to infection with recombinant baculoviruses was examined by Western blot analysis with E2- and CEA-specific antibodies. A protein of approximately 64 kDa was detected in Bac-E2- and Bac-G-E2-infected Huh-7 cell lysates, whereas it was not detected in the mock-infected sample. Similarly, a 180-kDa protein band was detected with anti-CEA antiserum in Bac-CEA-infected cell lysates, whereas expression of this target antigen was not observed in the mock-infected control sample. Finally, to determine whether the recombinant virus Bac-G-E2 actually expresses and contains the VSV-G protein, purified virus was analyzed by Western blot with the VSV-G-specific monoclonal antibody P5D4. Expression of the VSV-G protein was detected in the Bac-G-E2 preparation, whereas no VSV-G protein was present in the Bac-E2 viral preparation (Fig. 1B).
In addition, to confirm that Bac-G-E2 has greater transduction efficiency than Bac-E2, Huh-7 cells were infected at different MOIs with each of the two vectors, and the expression of E2 glycoprotein was compared by Western blot analysis. As shown in Fig. 2, the protein band corresponding to the E2 glycoprotein was detected in Huh-7 cells transduced with Bac-G-E2 at an MOI of 10, whereas the HCV protein was detected upon transduction of Huh-7 cells with an MOI of at least 100 with Bac-E2. Thus, in agreement with previous reports (6), these data demonstrate that recombinant baculovirus can be grown to high titer and are efficient vehicles for gene expression in mammalian cells. In addition, the presence of VSV-G protein on the viral envelope significantly increased its transduction efficiency (4).
FIG. 2.
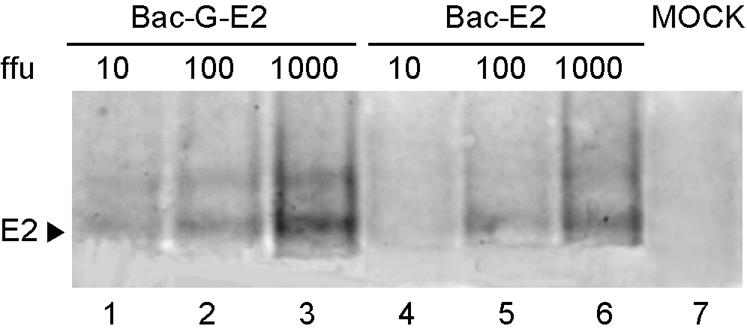
Comparison of transduction efficiency of the Bac-G-E2 and Bac-E2 vectors. Huh-7 cells were infected with Bac-G-E2 or Bac-E2 vectors at MOIs of 10, 100, and 1,000 and processed for Western blot analysis as described in Materials and Methods. Ten micrograms of total cell lysate was loaded on the gel for each sample. The position of the E2 glycoprotein is shown.
Characterization of immunogenic efficiency of baculovirus vectors.
The immunization efficacy of Bac-E2 was initially evaluated by measuring the amplitude of the anti-E2 immune response elicited in mice upon vaccination through different inoculation routes. The efficiency of intranasal, intramuscular, and subcutaneous inoculation was compared by immunizing BALB/c mice at 0 and 3 weeks with 1010 FFU of Bac-E2. The cellular immunity elicited by the different immunization protocols was measured by ELISPOT assay 2 weeks after the last injection. Antigen-specific IFN-γ secretion from stimulated splenocytes was measured with a pool of 15-mer peptides overlapping by 11 amino acids and encompassing the entire E2 glycoprotein. As a negative control, cytokine production was also measured upon stimulation of the splenocytes with DMSO at the same concentration used to solubilize the E2 peptides.
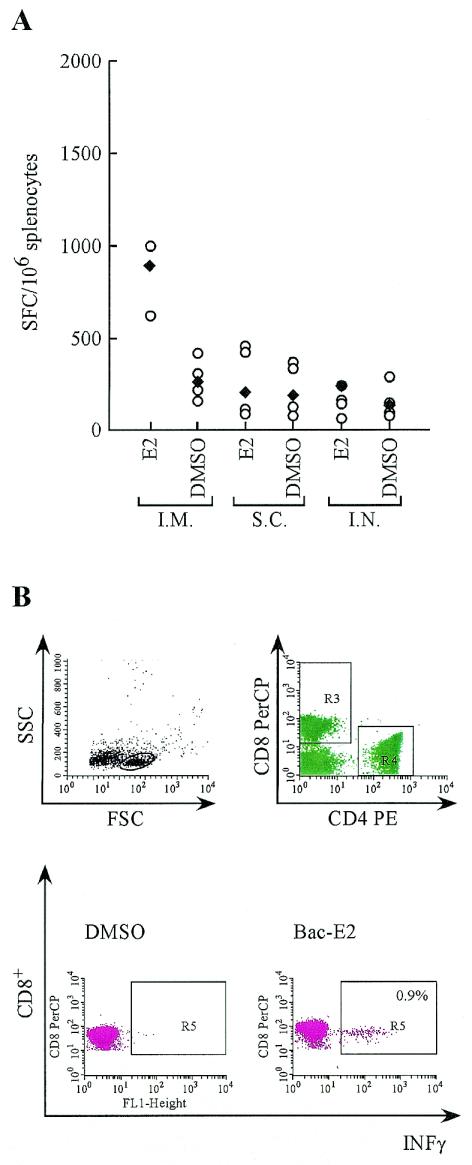
As shown in Fig. 3A, significant cell-mediated immunity was detected upon intramuscular inoculation of mice with Bac-E2 (geometric mean value, 888 spot-forming cells (SFC)/106 splenocytes), whereas intranasal and subcutaneous inoculation of this vector yielded much lower values that did not differ from their corresponding control samples. Interestingly, splenocytes harvested from baculovirus-injected mice routinely produced a high background of nonspecific IFN-γ production in the ELISPOT assay. The background level differed significantly from that of BALB/c mice immunized with unrelated vectors (data not shown), suggesting that a generalized activation of lymphocytes had occurred upon baculovirus injection.
FIG. 3.
Comparison of different inoculation routes for baculovirus immunization. BALB/c mice were injected intramuscularly, subcutaneously, or intranasally with 1010 FFU of Bac-E2 vector at 0 and 3 weeks. Two weeks after the last injection, the cellular immune response was examined as described in Materials and Methods. (A) The IFN-γ ELISPOT assay was performed with a peptide pool encompassing the entire E2 protein sequence. Antigen-specific (E2) and nonspecific (DMSO) IFN-γ production is shown for each experimental group. The SFC values for individual mice (open circles) and geometric means (solid diamonds) are shown. (B) IFN-γ intracellular staining of pooled splenocytes from mice immunized intramuscularly was performed with peptide 1664. Whole lymphocyte gating and gating for CD8+ and CD4+ T cells are shown.
To define the T-cell specificity elicited upon intramuscular inoculation of Bac-E2, intracellular IFN-γ staining was carried out on mouse splenocytes from injected mice with peptides containing CD4+ and CD8+ epitopes that had been previously mapped within the E2 protein sequence (43). As shown in Fig. 3B, a significant CD8+-specific response was detected with peptide 1664 in mice inoculated with Bac-E2 (0.9%). Additionally, no antigen-specific T-cell response could be detected in mice inoculated intranasally or subcutaneously, in agreement with the ELISPOT analysis. Similarly, no measurable IFN-γ production indicative of a CD4+ Th1 response could be detected with this assay in any of the immunized animals (data not shown).
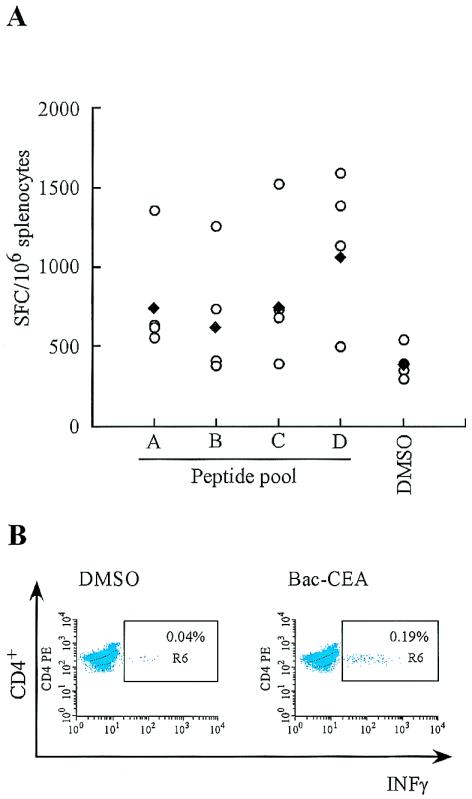
To determine whether the immunogenic efficacy of recombinant baculovirus vectors was not restricted to the E2 glycoprotein of HCV, a baculovirus vector expressing human CEA was injected intramuscularly at a dose of 1010 FFU. The CEA-specific response was assessed by stimulating splenocytes from immunized BALB/c mice with four pools of 15-mer peptides overlapping by 11 amino acids that collectively encompass the entire protein sequence. Pool A covers amino acids 1 to 147, pool B covers amino acids 137 to 237, pool C covers amino acids 315 to 507, and pool D covers amino acids 497 to 702. As shown in Fig. 4A, the immune response elicited by Bac-CEA was primarily directed towards the C-terminal end of the protein, as indicated by the SFC value measured with peptide pool D (1,052 SFC/106 splenocytes, P < 0.05). The other peptide pools yielded lower SFC values that were not significantly higher than that of the negative control (380 SFC/106 splenocytes). The antigen-specific T-cell response directed to the C terminus of CEA was further characterized by intracellular IFN-γ staining with peptide pool D. Interestingly, a predominant CD4+ response was identified (Fig. 4B), while no specific CD8+ response could be detected (data not shown). Thus, these data confirm the observation that baculovirus can elicit an antigen-specific immune response to different target antigens (1). The type of immune response is antigen dependent and is elicited mainly upon intramuscular injection.
FIG. 4.
Analysis of cell-mediated immune response elicited by Bac-CEA. BALB/c mice were injected intramuscularly with 1010 FFU at 0 and 3 weeks. (A) At two weeks postboost, the number of IFN-γ-secreting T cells specific for CEA was determined by ELISPOT assay on splenocytes from individual mice (open circles) with peptide pools that encompass the entire protein. Geometric mean values (solid diamonds) are also indicated. Nonspecific IFN-γ production (DMSO) is shown for each mouse. (B) IFN-γ intracellular staining of pooled splenocytes from mice immunized intramuscularly was performed with peptide pool D.
Expression of VSV-G increases the immunogenic efficiency of baculovirus vectors.
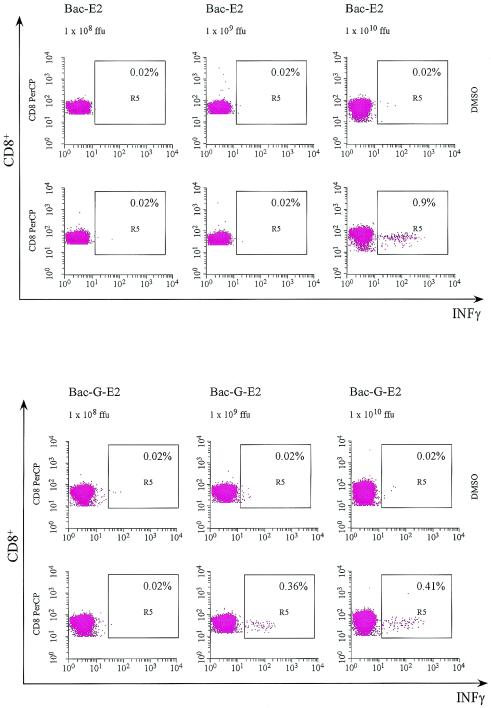
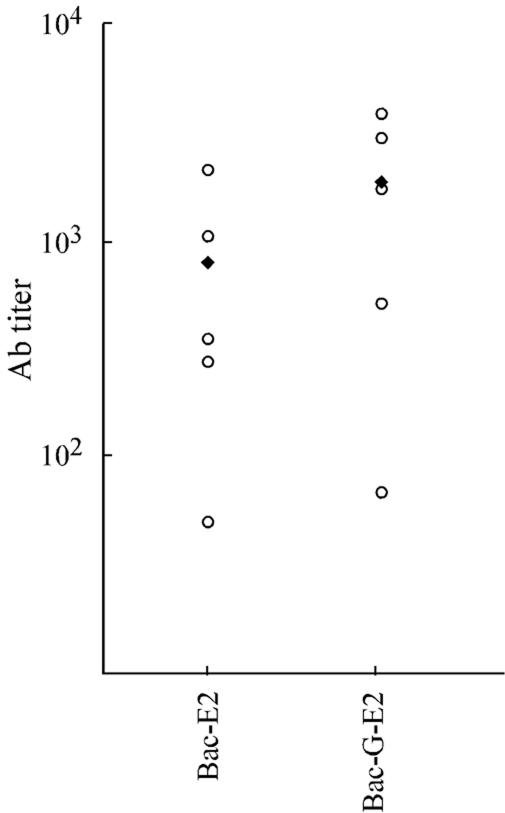
Previous studies have demonstrated that baculovirus vectors that express the VSV-G glycoprotein and incorporate it into the viral particle transduce a variety of mammalian cells more efficiently (4). Additionally, gene transfer in mouse skeletal muscle has been demonstrated with this vector (32). To assess whether the enhanced transduction efficiency of the modified baculovirus vector would also lead to greater immunogenicity, the immune responses elicited by Bac-E2 and Bac-G-E2 were compared. BALB/c mice were inoculated intramuscularly at 0 and 3 weeks with 1010, 109, and 108 FFU of both vectors. The CD8+ E2-specific immune response was measured by intracellular IFN-γ staining with the CD8+-specific epitope. As shown in Fig. 5, a significant CD8+ response was detected upon inoculation of 1010 FFU of Bac-E2 and Bac-G-E2 (0.96 and 0.41%, respectively). The E2-specific CD8+ response could still be detected upon injection of 109 FFU of Bac-G-E2 (0.36%), whereas it was no longer detectable upon injection of 108 FFU. In contrast, E2-specific CD8+ T-cell response was not observed upon injection of 109 and 108 FFU of Bac-E2 vector. The induction of humoral response to E2 was examined by measuring antigen-specific antibodies. Both 1010 FFU of Bac-E2 and Bac-G-E2 elicited comparable antibody titers specific for E2. (Fig. 6).
FIG. 5.
Analysis of E2-specific CD8+ T cells elicited by recombinant baculovirus vectors. BALB/c mice were injected intramuscularly with Bac-E2 and Bac-G-E2 vectors at 1010, 109, and 108 FFU. Two weeks postboost, pooled splenocytes from immunized mice of each experimental group were tested by intracellular staining for IFN-γ production with peptide 1664.
FIG. 6.
Antibody titers from mice immunized with baculovirus vectors. Individual titers against recombinant E2 were measured by ELISA on each serum from mice immunized with the Bac-E2 and Bac-G-E2 vectors. Geometric mean values are also shown (solid diamonds).
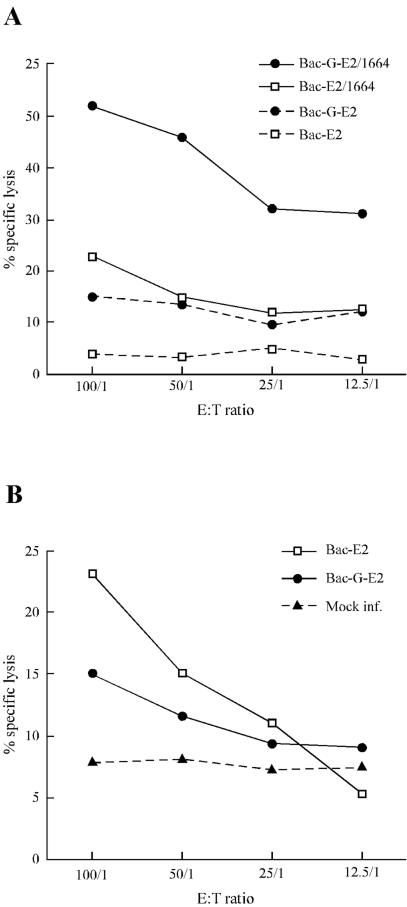
E2-specific CD8+ T cells elicited upon inoculation of Bac-E2 and Bac-G-E2 were tested for effector function, and cytotoxic activity was detected with both vectors. Interestingly, a greater percentage of cell lysis was noted upon stimulation of splenocytes derived from Bac-G-E2-injected mice (Fig. 7A). Additionally, the induction of innate immune response was measured by examining NK cytolytic activity. As shown in Fig. 7B, comparable NK cytolytic activity was noted in splenocytes from mice injected with both vectors. Thus, these results demonstrate that expression of VSV-G glycoprotein increases by 10-fold the immunogenic efficiency of recombinant vectors, leading to a greater induction of antigen-specific CD8+ T cells.
FIG. 7.
Analysis of the T- and NK cell-mediated cytolytic activity of immunized mice. (A) Two weeks postboost, splenocytes from mice immunized intramuscularly with 1010 FFU of Bac-E2 and Bac-G-E2 were tested for E2-specific cytotoxic T-lymphocyte response. Splenocytes were restimulated in vitro and tested for cytotoxic activity against p815 cells pulsed with HCV peptide 1664 or DMSO. (B) NK activity was assessed by measuring lysis of target Yac-1 cells mediated by splenocytes from immunized and control mice. Effector cell-target cell ratios are indicated on the abscissa. The percent specific killing is reported on the vertical axis.
Bac-G-E2 induces inflammatory cytokines in vivo.
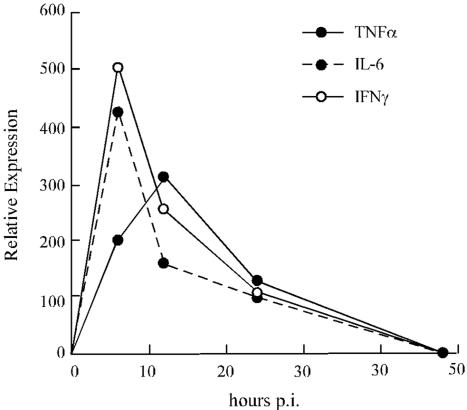
To better characterize the mechanism associated with induction of immune response upon Bac-G-E2 inoculation, the expression of inflammatory cytokines was monitored in a time course experiment. To this end, BALB/c mice were injected intramuscularly with 1010 FFU, and RNAs from injected quadriceps were collected at 0, 6, 12, 24, and 48 h postinjection. The expression of inflammatory cytokines was first monitored by RNase protection assay on RNA samples from immunized mice. As shown in Fig. 8, peak induction of IFN-γ and interleukin-6 mRNAs was detected as early as 6 h postinjection. In contrast, tumor necrosis factor alpha expression reached maximum levels at 12 h postinjection. Expression of all three cytokines declined progressively thereafter and returned to background levels by 48 h postinjection. Thus, these data indicate that a complex pattern of inflammatory cytokines that may contribute to the intrinsic immunogenic properties of this vector is induced upon Bac-G-E2 inoculation.
FIG. 8.
Analysis of cytokine production following intramuscular administration of Bac-G-E2. Quadriceps from three mice injected with 1010 FFU were removed at 0, 6, 12, 24, and 48 h postinjection, and total RNA was extracted. The induction of cytokines was monitored by the RNase protection assay as described in Materials and Methods.
DISCUSSION
We have characterized the immunogenic properties of recombinant baculovirus vectors. This study was prompted by the consideration that the low cytotoxicity of baculovirus and inherent inability to replicate in mammalian cells make baculovirus a suitable gene delivery vehicle for genetic vaccination. The results of this study provide further evidence in support of using baculovirus as a vector for genetic immunization.
Comparison of various routes of inoculation showed that greater antigen-specific immune response is elicited upon intramuscular injection, whereas intranasal and subcutaneous inoculation did not lead to significant cell-mediated immunity (Fig. 3). Interestingly, recombinant baculovirus vectors expressing the hemagglutinin of influenza virus induced higher levels of antibody upon intramuscular injection, in agreement with the results observed in this study. However, protection from influenza virus challenge was achieved only upon intranasal immunization (1). Although we have not examined the antiviral activity of the anti-E2 immune response, simply because there is no mouse model available for HCV replication, the E2-specific CD8+ T cells have effector function, as demonstrated by the cytotoxic assay, and thus should be able to lyse HCV-infected cells (Fig. 7). The therapeutic efficacy of the anti-E2 immune response would probably depend on the viral load and immunocompetence of the recipient as well as on the accessibility of the HCV-infected cells to the activated CD8+ lymphocytes.
It is not clear why diverse routes of baculovirus inoculation lead to differences in the amplitude or extent of immune response. These differences could be due, at least in part, to the ability of baculovirus to transduce the different cell types ultimately involved in the induction of the immune response. The exposure of baculovirus to complement could also contribute to the immunogenic efficiency of this vector, in view of its sensitivity to complement-mediated inactivation (17). Nonetheless, differences in the amplitude of the immune response as a result of the immunization route employed have also been observed with different genetic vaccines, such as DNA immunization with a gene gun (19). This observation suggests that ultimately, the choice of route for inoculation must be based on the intrinsic transduction properties of the vector chosen and on the type of immune response that is desired.
Abe and coworkers reported that wild-type baculovirus imparts a nonspecific antiviral activity that can protect the immunized mice from influenza virus challenge. This property is linked to the ability of baculovirus to stimulate an innate immune response, possibly through transduction and/or activation of macrophages, and is perhaps due to the presence of glycans not normally found in mammalian cells that are linked to the envelope protein gp64 (1). This conclusion is partially supported by the observation that recombinant baculovirus vectors stimulate NK cytolytic activity in immunized mice (Fig. 7). The observation that this activity is detected with both Bac-E2 and Bac-G-E2 suggests that stimulation of the innate immune response cannot be directly ascribed to a specific glycoprotein expressed on the surface of the viral particle but rather may be associated with the type of posttranslational modifications of the surface components of the virus that take place in insect cells and may be highly immunogenic in mammals.
Interestingly, induction of a measurable immune response through baculovirus vaccination was also observed with the Bac-CEA vector, demonstrating that this virus can function as a vehicle for vaccination in an antigen-independent manner (Fig. 4). However, it is worth noticing that, in these experimental conditions, a prevalent CD4+ T-cell response was elicited against CEA in the immunized mice. In contrast, E2-specific T cells activated by Bac-E2 and Bac-G-E2 were predominantly CD8+. The difference in the character of the immune response elicited by these two antigens is probably attributable to the intrinsic immunogenic properties of E2 and CEA, since immune analyses were performed with an inbred mouse strain, with the same expression vector used for vaccination, and comparable levels of protein synthesis (data not shown). The mechanism that determines the type of T-cell response elicited against a given antigen is neither fully understood nor unprecedented (29) and may be influenced by factors such as folding, oligomerization, secretion or localization in different subcellular compartments, and affinity for major histocompatibility complex-encoded molecules.
On the other hand, the observed priming of different arms of the immune response detected upon injection of the E2- and CEA-expressing baculovirus vectors could also be, at least in part, a specific phenomenon associated with the use of BALB/c mice as the animal model for these studies. The CD8+ and CD4+ T-cell responses elicited by these vectors should also be examined in additional mouse strains to ensure that the same immunogenic profile is detected. If so, the combination of a particular vector with the general immunological characteristics of the target antigen may significantly influence the type of immune response that is elicited upon vaccination.
The results obtained also demonstrate that the Bac-G-E2 vector is more immunogenic than Bac-E2, as indicated by the minimal dose required to elicit a measurable immune response to HCV E2 (Fig. 5) and by the differences in cytotoxic activity elicited by these two vectors (Fig. 7). Although expression of the VSV-G glycoprotein could have influenced the type of cells that are transduced in vivo by baculovirus, thereby affecting the amplitude of the immune response, it is highly probable that the differences in immunogenic efficiency between these two vectors reflect the enhanced transduction potential of baculovirus vectors that express VSV-G (4).
Intramuscular injection of Bac-G-E2 stimulates the production of inflammatory cytokines, including interleukin-6, tumor necrosis factor alpha, and IFN-γ within 6 h. The pattern of inflammatory cytokine production observed in baculovirus-injected mice does not differ from that detected upon administration of adenovirus (25), suggesting that the mechanism of cytokine production is probably similar and depends on direct interaction between dendritic cells and macrophages with the baculovirus. The induction of IFN-α and -β from mammalian cells, including mouse embryo fibroblast cells, has been reported (15). Additionally, transduction of macrophages in vitro by baculovirus has been shown by Abe and coworkers, who demonstrated the induction of significant levels of interleukin-6 and tumor necrosis factor alpha (1). These results confirm the observation that dendritic cells and macrophages exert a central role in acute inflammatory responses to baculovirus injection in mice by secreting high levels of inflammatory cytokines that are essential for both innate and adaptive immunities.
Although we have not examined the induction of an immune response to baculovirus in the injected mice, intramuscular injection of the baculovirus-VSV-G vector expressing the mouse erythropoietin protein elicits a significant antibody response to the virus (32). Therefore, it is highly probable that neutralizing antibodies have also been induced for the E2- and CEA-expressing vectors. How this response influences the ability to repeatedly administer the baculovirus as a vehicle for vaccination remains to be determined, but as observed for adenovirus, induction of neutralizing antibodies is likely to hamper the efficacy of multiple injections (13, 8). Thus, protocols based on heterologous prime-boosts should be explored in which injection of baculovirus vectors is coupled with alternative gene delivery vehicles. A similar immunization regimen has been tested for plasmid DNA coupled with adenovirus or poxvirus vectors in mice and nonhuman primates (7, 9, 11, 14, 41). The rationale behind this strategy is that by using different vectors as boosters, it should be possible to bypass the immune response elicited against the primer and also strengthen the immune response against the target antigen.
This study confirms published reports indicating that baculovirus vectors can elicit an immune response against a target antigen. In view of the ease with which baculovirus can be modified, it is highly probable that the transduction efficiency and expression capacity of this virus can be further improved by specific modifications of the virus structure. These changes could enhance the immunogenic efficiency of baculovirus. Therefore, the intrinsic biological characteristics of this virus highlight the great potential of baculovirus as a vector for genetic vaccination.
Acknowledgments
We thank L. Tomei, M. Arcuri, C. Paolini, and A. Nicosia for helpful suggestions and useful reagents. We also thank J. Clench for editorial assistance, M. Emili for graphics, and LAR personnel for technical support in conducting animal experiments. We are also grateful to E. Scarselli, P. Monaci, and G. Ciliberto for critical reviews.
REFERENCES
- 1.Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133-1139. [DOI] [PubMed] [Google Scholar]
- 2.Airenne, K. J., M. O. Hiltunen, M. P. Turunen, O. H. Laitinen, M. S. Kulomaa, and Y. S. Herttuala. 2000. Baculovirus mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 7:1499-1504. [DOI] [PubMed] [Google Scholar]
- 3.Aoki, H., Y. Sakoda, K. Jukuroki, A. Takada, H. Kida, and A. Fukusho. 1999. Induction of antibodies in mice by a recombinant baculovirus expressing pseudorabies virus glycoprotein B in mammalian cells. Vet. Microbiol. 68:197-207. [DOI] [PubMed] [Google Scholar]
- 4.Barsoum, J. R. Brown, M. Mckee, and F. M. Boyce. 1997. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum. Gene Ther. 8:2011-2018. [DOI] [PubMed] [Google Scholar]
- 5.Berinstein, N. L. 2002. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J. Clin. Oncol. 20:2197-2207. [DOI] [PubMed] [Google Scholar]
- 6.Boyce, F. M., and N. L. R. Bucher. 1996. Baculovirus mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA 93:2348-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruna-Romero, O., G. Gonzàlez-Aseguinolaza, J. C. R. Hafalla, M. Tsuji, and R. S. Nussenweig. 2001. Complete, long lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98:11491-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Aulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid recombinant vaccinia virus and replication deficient adenovirus expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casimiro, D., A. Tang, L. Chen, T. M. Fu, R. K. Evans, M. E. Davies, D. C. Freed, W. Hurni, J. M. Aste-Amezaga, L. Guam, R. Long, L. Huang, W. Harris, D. K. Nawrocki, H. Mach, R. T. Troutman, L. A. Isopi, K. K. Murthy, K. Rice, K. A. Wilson, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Vaccine induced immunity in baboons using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type I gag gene. J. Virol. 77:7663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condreay, J. P., S. M. Witherspoon, W. C. Clay, and T. A. Kost. 1999. Transient and stable gene expression in mammalian cells transduced by a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA 96:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estcourt, M. J., A. J. Ramsay, A. Brooks, S. A. Thomson, C. J. Medveckzy, and I. A. Ramshaw. 2002. Prime-boost immunization generates a high frequency, high-avidity, CD8+ cytotoxic T lymphocyte population. Int. Immunol. 14:31-37. [DOI] [PubMed] [Google Scholar]
- 12.Fipaldini, C., B. Bellei, and N. La Monica. 1999. Expression of Hepatitis C virus cDNA in human hepatoma cell line mediated by a hybrid baculovirus-AAV vector. Virology 255:302-311. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. J. Ertl. 2003. A simian replication defective adenoviral recombinant vaccine to HIV gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalo, R. M., G. Del Real, J. R. Rodriguez, D. Rodriguez, R. Heljasvaara, P. Lucas, V. Arraga, and M. Esteban. 2002. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 20:1226-1231. [DOI] [PubMed] [Google Scholar]
- 15.Gronowski, A. M., D. M. Hilbert, K. C. F. Sheehan, G. Garotta, and R. D. Schreiber. 1999. Baculovirus stimulates antiviral effect in mammalian cells. J. Virol. 73:9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge, J. W. 1996. Carcinoembryonic antigen as a target for cancer vaccines. Cancer Immunol. Immunother. 43:127-134. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann, C., and M. Strauss. 1998. Baculovirus mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 5:531-536. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann, C., V. Sandig, G. Jennings, M. Rudolph, P. Schlag, and M. Strauss. 1995. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA 92:10099-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, K., K. Ito, Shinhara, N., and S. Kato. 2003. DNA immunization via intramuscular and intradermal routes using gene gun provides different magnitudes and duration son immune response. Mol. Immunol. 39:847-854. [DOI] [PubMed] [Google Scholar]
- 20.Kaneno, R., A. J. S. Duarte, and A. Borelli. 2002. Natural Killer activity in the experimental privational rickets. Immunol. Lett. 81:183-189. [DOI] [PubMed] [Google Scholar]
- 21.Kwak, H., H. Horing, and H. L. Kaufman. 2003. Poxviruses as vectors for cancer immunotherapy. Curr. Opin. Drug Discov. Dev. 6:161-168. [PubMed] [Google Scholar]
- 22.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 23.Lehtolainen, P., K. Tyynela, J. Kannasto, K. J. Airenne, and S. Yla-Herttuala. 2002. Baculovirus exhibits restricted cell type specificity in rat brain: a comparison of baculovirus-and adenovirus-mediated intracerebral gene transfer in vivo. Gene Ther. 9:1693-1699. [DOI] [PubMed] [Google Scholar]
- 24.Lemiex, P. 2002. Technological advances to increase immunogenicity of DNA vaccines. Exp. Rev. Vaccines 1:85-93. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Q., and D. A. Muruve. 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10:935-940. [DOI] [PubMed] [Google Scholar]
- 26.Luckow, V. A., and M. D. Summers. 1988. Signals important for high-level expression of foreing genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology 167:56. [DOI] [PubMed] [Google Scholar]
- 27.Matsui, M., O. Moriya, and T. Akatsuka. 2003. Enhanced induction of hepatitis C virus specific cytotoxic T lymphocytes and protective efficacy in mice by vaccination followed by Adenovirus boosting in combination with the interleukin 12 expression plasmid. Vaccine 21:1629-1639. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura, Y., R. D. Possee, H. A. Overton, and D. H. L. Bishop. 1987. Baculovirus expression vector: the requirement for high level expression of proteins. Including glycoproteins. J. Gen. Virol. 68:1233. [DOI] [PubMed] [Google Scholar]
- 29.Moore, A. C., W. P. Kong, B. K. Chakrabarti, and G. J. Nabel. 2002. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 76:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual. Oxford University Press, Oxford, United Kingdom.
- 31.Pieroni, L., and N. La Monica. 2001. Towards the use of baculovirus as gene therapy vector. Curr. Opin. Mol. Ther. 3:465-467. [PubMed] [Google Scholar]
- 32.Pieroni, L., D. Maione, and N. La Monica. 2001. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum. Gene Ther. 12:871-881. [DOI] [PubMed] [Google Scholar]
- 33.Sandig, V., C. Hofmann, S. Steinert, G. Jennings, P. Schlag, and M. Strauss. 1996. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum. Gene Ther. 7:1937-1945. [DOI] [PubMed] [Google Scholar]
- 34.Sarkis, C., C. Serguera, S. Petres, D. Buichet, J. L. Rider, L. Edelman, and J. Mallet. 2000. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc. Natl. Acad. Sci. USA 97:14638-14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seong, Y. R., S. Choi, J.-S. Lim, C.-H. Lee, C.-K. Lee, and D. S. Im. 2001. Immunogenicity of E1E2 proteins of hepatitis C virus expressed by a recombinant adenovirus. Vaccine 19:2955-2964. [DOI] [PubMed] [Google Scholar]
- 36.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K., Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. dubey, L. Huang, W. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schelif, Zhu, D. C. Freed, N. V. Persaud, L Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Colling, G. J. Heldecker, W. R. Fernadez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R., Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication incompetent adenoviral vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 37.Shoji, I., H. Aizaki, H. Tani, K. Ishihi, T. Chiba. I. Saito, T. Myamura, and Y. Matsuura. 1997. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J. Gen. Virol. 78:2657-2664. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava, I. K., and M. A. Liu. 2003. Gene vaccines. Ann. Intern. Med. 138:550-559. [DOI] [PubMed] [Google Scholar]
- 39.Tani, H., C. K. Limn, C. C. Yap, M. Onishi, M. Nozaki, Y. Nishimune, N. Okahashi, Y. Kitagawa, R. Watanabe, R. Mochizuki, K. Moriishi, and Y. Matsuura. 2003. In vitro and in vivo gene delivery by recombinant baculoviruses. J. Virol. 77:9799-9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tani, H., M. Mishijima H,. Ushijima, T. Miyamura, and Y. Matsuura. 2001. Characterization of cell-surface determinants important for baculovirus infection. Virology 279:343-353. [DOI] [PubMed] [Google Scholar]
- 41.Wierzibicki, A., I. Kiszka, H. Kaneko, D. Kmieciak, T. J. Wasik, J. Gzyl, Y. Kaneko, and D. Kozbor. 2002. Immunization strategies to augment oral vaccination with DNA and viral vectors expressing HIV envelope glycoprotein. Vaccine 20:1295-1307. [DOI] [PubMed] [Google Scholar]
- 42.Yap, C. C., K. Ishii, Y. Aoki, H. Aizaki, H. Tani, H. Shimuzu, Y Ueno, T. Miyamura, and Y. Matsuura. 1997. A hybrid baculovirus-T7 RNA polymerase system for recovery of infectious virus from cDNA. Virology 231:192-200. [DOI] [PubMed] [Google Scholar]
- 43.Zucchelli, S., S. Capone, E. Fattori, A. Folgori, A. Di Marco, D. Casimiro, A. J. Simon, R. Alufer, N. La Monica, R. Cortese, and A. Nicosia. 2000. Enhancing B- and T-cell immune response to hepatitis C virus E2 DNA vaccine intramuscular electrical gene transfer. J. Virol. 74:11598-11607. [DOI] [PMC free article] [PubMed] [Google Scholar]