Significance
Rehabilitation is often prescribed after brain injury, but the basis for how training can influence brain plasticity and recovery is unclear. In this study, we show that intense rehabilitation training after focal brain injury drives significant structural changes in brain cells located adjacent to the injury. Importantly, a key brain modulatory system, the basal forebrain cholinergic system, is required for enabling rehabilitation to impact brain structure. Damage to the cholinergic system, which can occur naturally during aging, completely blocks brain plasticity mediated by rehabilitation and significantly attenuates functional recovery. These results provide new insights into how rehabilitation may promote recovery and suggest that brain cholinergic systems may be a possible therapeutic target for influencing recovery.
Keywords: cell filling, corticospinal neurons, cholinergic, morphology, plasticity
Abstract
We determined whether rehabilitation after cortical injury also drives dynamic dendritic and spine changes in functionally distinct subsets of neurons, resulting in functional recovery. Moreover, given known requirements for cholinergic systems in mediating complex forms of cortical plasticity, including skilled motor learning, we hypothesized that cholinergic systems are essential mediators of neuronal structural and functional plasticity associated with motor rehabilitation. Adult rats learned a skilled forelimb grasping task and then, underwent destructive lesions of the caudal forelimb region of the motor cortex, resulting in nearly complete loss of grasping ability. Subsequent intensive rehabilitation significantly enhanced both dendritic architecture and spine number in the adjoining rostral forelimb area compared with that in the lesioned animals that were not rehabilitated. Cholinergic ablation markedly attenuated rehabilitation-induced recovery in both neuronal structure and motor function. Thus, rehabilitation focused on an affected limb robustly drives structural compensation in perilesion cortex, enabling functional recovery.
Studies over the past decade have indicated that the adult brain is structurally dynamic (1–3). Indeed, dendritic spines dynamically turn over in the adult brain (3, 4), and learning of novel tasks is associated with further increases in spine turnover (4). Moreover, total and stable increases in spine number together with enhanced dendritic complexity can be detected when analyses are focused specifically on neuronal subpopulations that are functionally related to a newly learned motor skill (5). For example, we recently reported that cortical layer V pyramidal neurons, which project to spinal segment C8 and are specifically engaged when learning a skilled forelimb grasping task, elaborate a 22% increase in apical dendritic spines and exhibit significant increases in dendritic branching and total dendritic length (5); an adjoining control population of cortical layer V pyramidal neurons that project to C4, which are not specifically shaped by the skilled motor task, exhibits no change in spines or dendritic complexity when the same task is learned (5). The detection of stable structural increases in neurons engaged by skilled motor learning in contrast to a lack of change in adjacent neurons that are not engaged by learning advances our understanding of mechanisms underlying experience-dependent cortical plasticity.
Damage to the adult CNS also generates adaptive brain plasticity. For example, focal cortical lesions evoke cortical map plasticity (6, 7), extension of new axonal connections (7, 8), and neurogenesis (9). A very important and unresolved question in the neural plasticity and injury fields is whether rehabilitation—that is, specific retraining of injured neural circuits—can drive, alter, or enhance neural plasticity subsequent to brain lesions. Whereas extensive literature has shown that rehabilitation can increase the numbers of dendritic spines and dendritic complexity in the cortical hemisphere opposite a brain lesion (10–13) and is associated with improved skill in the limb unaffected by the lesion, effects of rehabilitation on neuronal structure in perilesioned cortex have not been described. Indeed, some studies suggest either stability or early loss of dendritic structure in perilesion cortex (14–16). However, knowing whether rehabilitation can drive adaptive brain plasticity could be essential in improving outcomes of numerous CNS disorders acquired in adulthood, including stroke, traumatic brain injury, and spinal cord injury.
Prior studies that have sought to interrogate neuronal structure after injury have been limited by their use of nonspecific cellular sampling methods, such as Golgi–Cox staining or EM; these approaches lack the ability to specifically sample structural changes in neurons associated with specific tasks that are practiced in rehabilitation. Sampling from subpopulations of neurons mediating specific behaviors, such as skilled grasping in the motor cortex, may yield far more sensitive measures of changes in dendritic structure and spine number as a function of rehabilitation, fundamentally advancing our understanding of the role of experience and rehabilitation on structural neuronal plasticity.
Another consideration in understanding cortical mechanisms underlying plasticity after CNS injury is the contribution of subcortical systems that modulate cortical activity, including cholinergic inputs. Studies have identified an essential role for cholinergic activation in modulating cortical plasticity associated with learning (17–19) and motor map plasticity that is evoked after lesions of the caudal forelimb region of the motor cortex (6, 20). These observations raise the possibility that cholinergic inputs to the motor cortex are also essential for generating neuronal structural adaptations in response to rehabilitation training after injury.
In this study, we hypothesized that rehabilitation after injury to the adult brain drives adaptive plasticity, rebuilding spines and enhancing dendritic architecture in neurons surrounding the lesion site. We further hypothesize that these changes are cholinergic-dependent. We examined specific subpopulations of layer V cortical neurons directly related to the learning, loss, and subsequent relearning of skilled forelimb grasping, allowing detailed and specific sampling of structural parameters among subpopulations of neurons specifically engaged in the skilled grasping task.
Results
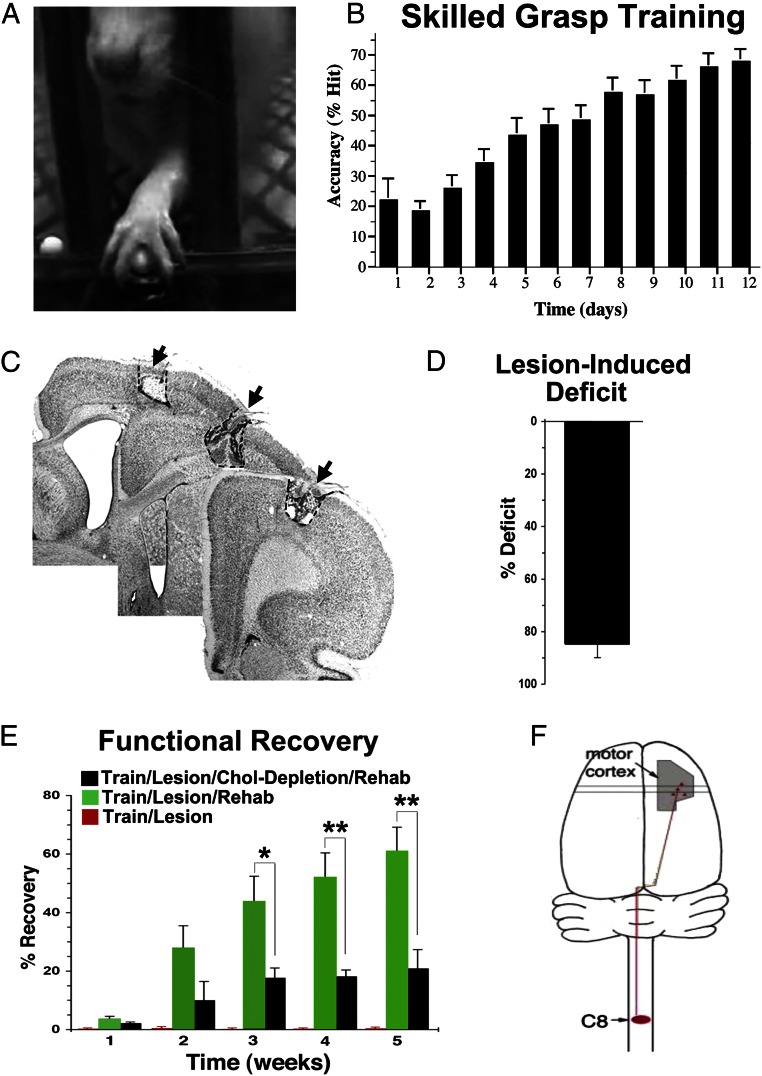
Sixteen adult Fischer 344 rats underwent skilled grasp training over 2 wk (Figs. 1 and 2) as previously described (19, 21). Starting from a pellet retrieval success rate of ∼20% during initial testing, animals improved to a mean proficiency of 70% over 12 d of intensive training (Fig. 2B) [repeated measures ANOVA F(11,165) = 92.7; P < 0.001]. Previously, it has been shown that skilled grasp training is associated with plasticity of evoked cortical motor maps within the caudal forelimb region (19, 22).
Fig. 1.
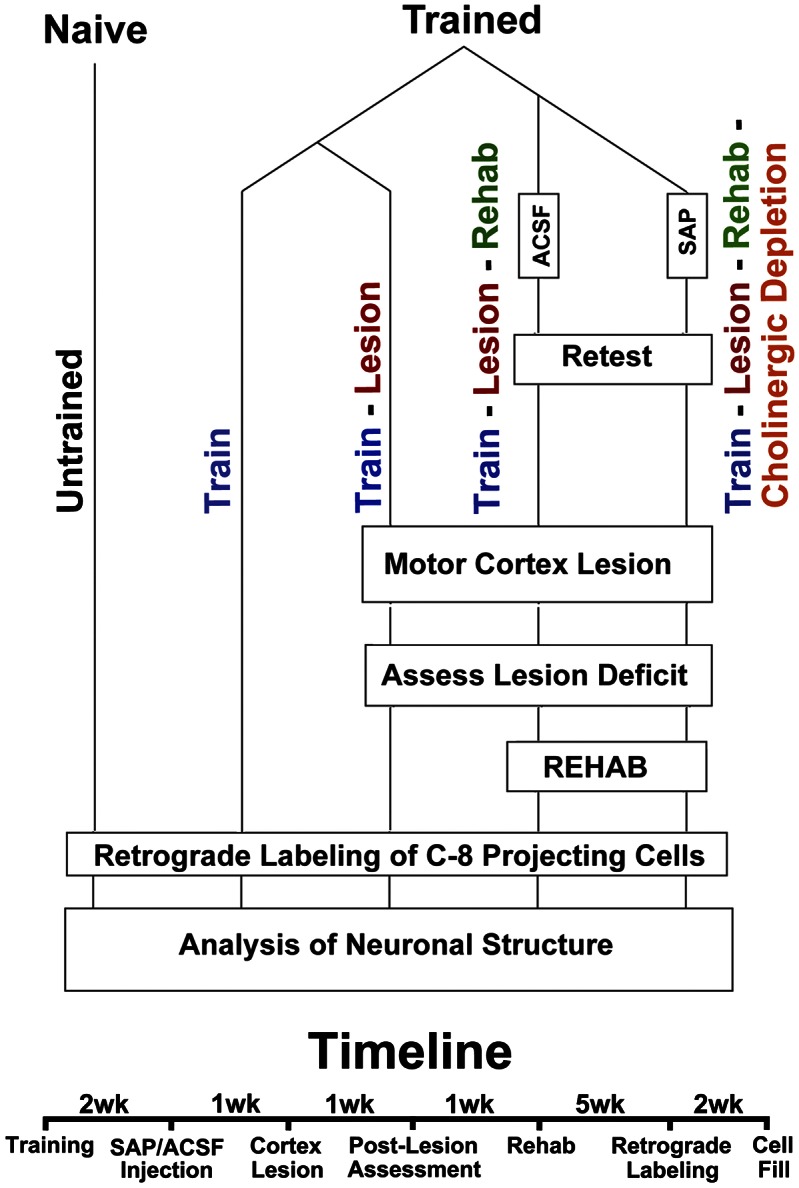
Experimental design and timeline.
Fig. 2.
Skilled motor training, lesions, and rehabilitation. (A) Animals underwent 2 wk of skilled forelimb reach and grasp training. (B) Motor training resulted in gradual increases in pellet retrieval accuracy [repeated measures ANOVA F(11,165) = 92.7; P < 0.001]. (C) Electrolytic lesions were then placed in the motor cortex controlling movement of the caudal forelimb (6). (D) Lesions resulted in an 85% reduction in the ability of rats to performed the skilled forelimb reach and grasp task compared with their prelesion levels of performance [n = 4 rats per group (12 total); t test t(1) = 0.03; P < 0.001]. Animals with intact or lesioned cholinergic systems exhibited the same degree of initial deficit postlesion [t test t(7) = 0.03; P = 0.97]. (E) Over 5 wk of rehabilitation training, rats with intact cholinergic systems recovered 60.9% ± 8.3% of their prelesion grasping ability (n = 4 animals). Animals with cholinergic lesions recovered only 20.7% ± 6.6% of their prelesion grasping performance (n = 4 animals), representing a significant impairment relative to animals with functioning cholinergic system [two-way mixed ANOVA (group × week) F(4,24) = 6.90; P < 0.001]. Asterisks indicate comparisons on individual weeks (Fisher’s posthoc test). Error bars ± SEM. *P < 0.05; **P < 0.01. (F) All animals then underwent injections of retrograde tracers in the C8 spinal cord segment, which controls muscles related to the skilled forelimb reach and grasp task (5). Chol, cholinergic.
One week after completion of skilled grasp training, four rats underwent cholinergic ablations by injecting 192-IgG saporin (SAP) into the nucleus basalis as previously described (19). Four additional trained rats underwent injections of artificial cerebrospinal fluid (ACSF) into the same sites to control for the injection procedure. One week later, these eight rats plus four additional forelimb grasp-trained animals underwent electrolytic lesions of the caudal forelimb motor cortex (6) (Experimental Procedures and Fig. 2C). Postlesion retesting 1 wk later showed a significant 85% loss in skilled grasping ability compared with prelesion level of performance among all lesioned animal groups [repeated t test t(11) = 18.54; P < 0.001] (Fig. 2D). Two groups then underwent intensive rehabilitation training on the same skilled grasping task over 5 additional wk, including a train/lesion/rehabilitation group (n = 4) and a train/lesion/rehabilitation/cholinergic ablation group (n = 4), whereas the third group did not undergo rehabilitation (a train/lesion group; n = 4).
Rehabilitation resulted in a gradual progression in recovery to 60% of the original prelesion level of performance in animals with intact cholinergic systems (train/lesion/rehabilitation group) (Fig. 2E), whereas animals without rehabilitation showed no functional recovery at all (train/lesion group) (Fig. 2E). Rehabilitated animals with cholinergic ablation (train/lesion/rehabilitation/cholinergic ablation group) exhibited a slight degree of functional recovery, although this recovery was only one-third that observed in rehabilitated animals with intact cholinergic systems by the fifth week of rehabilitation [two-way mixed ANOVA (group × week): F(4,24) = 6.90; P < 0.001; posthoc t test between groups: week 3 t(7) = −2.78; P < 0.05; week 4 t(7) = −3.87; P < 0.01; week 5 t(7) = 3.78; P < 0.01]. Thus, motor rehabilitation is associated with a significant improvement of skilled grasping, and most of this improvement requires a functioning cholinergic system.
Skilled Forelimb Training Enhances Neuronal Complexity and Spine Number in the Rostral Forelimb Area.
To determine whether rehabilitation-induced recovery of skilled forelimb function is associated with alterations in neuronal structure, we used a combination of fluorescent retrograde tracing and single-cell filling with Lucifer Yellow (LY) as previously described (5) (Figs. 2A and 3). Using this technique, we specifically examined structural plasticity within the subset of layer V cortical pyramidal neurons that project to the C8 segment of the spinal cord, which is required to execute a skilled forelimb grasp (5). Our analysis focused on layer V pyramidal neurons located in the rostral forelimb region, which is adjacent to the lesioned caudal forelimb region. Previously, we have shown using intracortical microstimulation that focal lesions of the caudal forelimb region followed by intensive rehabilitation results in compensatory motor map plasticity in the rostral forelimb region together with recovery of skilled reaching (6); subsequent ablation of the rostral forelimb region entirely abolishes rehabilitation-induced recovery, indicating the essential nature of the rostral forelimb region for rehabilitation-induced recovery on the skilled grasping task.
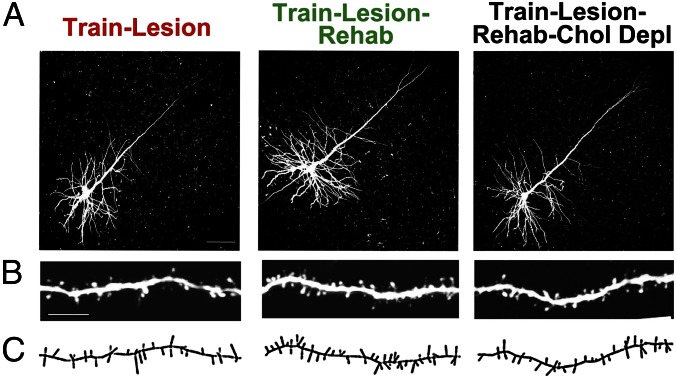
Fig. 3.
Layer V C8-projecting corticospinal motor neurons in the rostral forelimb area. Corticospinal neurons in the rostral forelimb area were retrogradely labeled by injections of fluorescent dextran beads into the C8 spinal segment; on completion of the experiment, brain slices were prepared, and retrogradely labeled neurons were filled with LY to enable analysis of dendritic architecture and spines of cortical neurons associated with skilled grasp to reach training. (A) LY-filled neurons in animals that underwent training and caudal forelimb lesions. Animals that were not rehabilitated (train/lesion), were rehabilitated (train/lesion/rehabilitation), or were rehabilitated after cholinergic ablation (train/lesion/rehabilitation/cholinergic depletion) are shown. Chol Depl, cholinergic depletion. (B) Higher magnification shows distal apical dendrites from roughly the same region among different animals. (Scale bar: 20 µm.) (C) Neurolucida reconstruction reflecting dendritic spines of the same images in B. Qualitatively, spines appear to be more numerous in the trained and rehabilitated group than in the nonrehabilitated group. Quantification is shown in Fig. 4.
We first determined whether there is plasticity of neuronal structure in the rostral forelimb area simply as a function of skilled grasp learning before placement of a caudal forelimb lesion. We quantified the density of distal apical spines in layer V corticospinal neurons that project to C8 in the rostral forelimb area, because distal apical spines of layer V neurons that project to C8 in the caudal forelimb area exhibit the most robust spine changes in association with this behavior (5). Indeed, we observed, in the rostral forelimb area, a significant increase in distal apical spine density from 11.2 ± 0.3 spines per 10-µm length in untrained animals (n = 16 neurons in four animals) to 12.2 ± 0.4 spines per 10-µm length in animals that underwent skilled grasp training (n = 16 neurons in four animals), a 9.2% increase [two tail t test t(30) = 1.99; P < 0.05]. Thus, as in the caudal forelimb region, layer V C8-projecting corticospinal neurons of the rostral forelimb area exhibit structural plasticity in association with learning the skilled grasp task.
Subsequent analyses focused on the effects of rehabilitation training on neuronal dendritic structure, comprehensively examining changes in apical and basilar dendrite architecture and spines in the rostral forelimb area after caudal forelimb area lesions.
Skilled Motor Rehabilitation Increases Dendritic Spine Density and Complexity, and Cholinergic Lesions Block Rehabilitation-Associated Enhancements in Neuronal Structure.
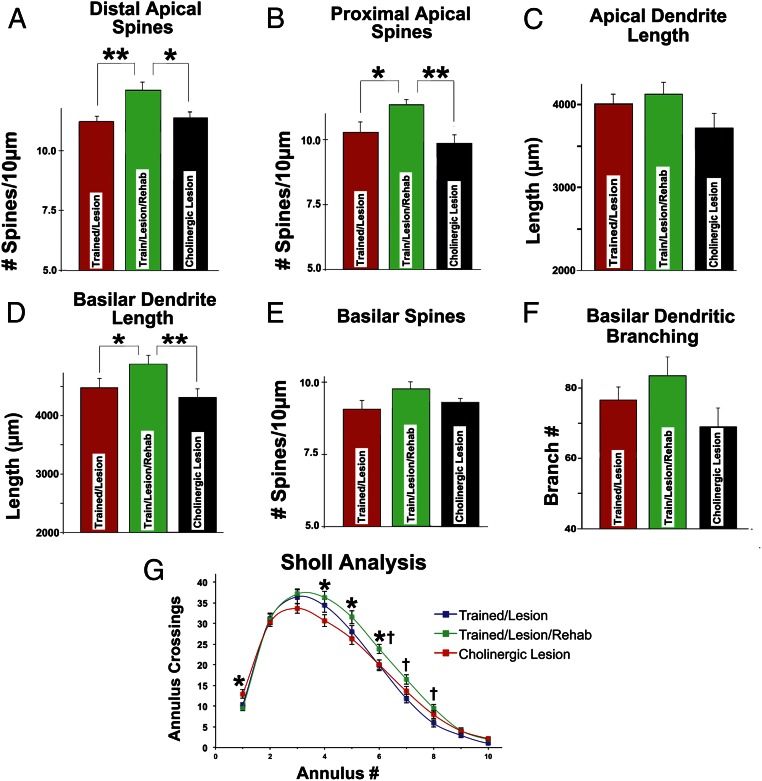
All assessments focused on layer V corticospinal neurons in the rostral forelimb area that project to C8 and influence inputs to muscles of the forelimb involved in skilled grasping. Notably, animals that underwent 5 wk of motor rehabilitation training on the skilled forelimb grasp task after caudal forelimb area lesions exhibited significant expansions in neuronal structure that were detected on measures of both spine density and elaboration of dendritic architecture (Figs. 3 and 4). Overall, there were significant differences among groups of animals in the density of distal apical spines [ANOVA F(2,45) = 4.94; P = 0.01], with significant increases in spine density among lesioned animals that underwent rehabilitation compared with animals that did not undergo rehabilitation (P < 0.01; Fisher’s posthoc test) (Fig. 4A). Cholinergic ablation entirely eliminated the formation of additional spines in animals that underwent rehabilitation (P < 0.05; Fisher’s posthoc test) (Fig. 4A), paralleling the marked reduction of functional recovery in these subjects (Fig. 2F).
Fig. 4.
Rehabilitation restores neuronal structure and is dependent on cholinergic systems. (A) Rehabilitation resulted in significant increases in distal apical dendritic spines in corticospinal neurons projecting to C8 spinal segments. Cholinergic depletion entirely blocked rehabilitation-induced recovery of spines. *P < 0.05; **P < 0.01. (B) Rehabilitation also resulted in significant increases in proximal apical dendritic spines, and cholinergic ablation eliminated these compensatory changes. *P < 0.05; **P < 0.01. (C) Apical dendritic length did not increase significantly with rehabilitation (ANOVA P = 0.15), although there was a trend toward reduced length in animals with cholinergic lesions. (D) Basilar dendritic length did, however, significantly increase with rehabilitation, and cholinergic ablation blocked this increase. *P < 0.05; **P < 0.01. (E) Basilar dendritic spine density also increased in rehabilitated animals, although this effect was not significant (ANOVA P = 0.27); cholinergic ablation attenuated these changes. (F) Basilar dendritic branching exhibited similar effects (ANOVA P = 0.28). (G) Sholl (23) analysis also showed significant effects of rehabilitation that were blocked by cholinergic lesions. *P < 0.05 (comparing the train/lesion/rehabilitation group with the train/lesion/rehabilitation/cholinergic depletion group); †P < 0.05 (comparing the train/lesion/rehabilitation group with the train/lesion group).
Similarly, there were overall group differences in the number of proximal apical spines among groups of lesioned animals [ANOVA F(2,45) = 5.20; P < 0.01]; once again, rehabilitation was associated with a significant increase in proximal apical spine density compared with in animals that were not rehabilitated (P < 0.05; Fisher’s posthoc test) (Fig. 4B). Moreover, cholinergic ablation entirely eliminated rehabilitation-associated increases in spine plasticity (P < 0.01; Fisher’s posthoc test) (Fig. 4B).
Measurements of total apical dendritic length did not detect overall group differences, although the data trended in this direction [ANOVA F(2,57) = 1.75; P = 0.15] (Fig. 4C), with cholinergic depletion tending to reduce the total length of apical dendrites compared with animals that were actively rehabilitated. Rehabilitation did, however, result in significant group differences in total basilar dendritic length [ANOVA F(2,57) = 4.32; P < 0.01] (Fig. 4D); again, rehabilitation was associated with a significant increase in total basilar dendritic length compared with in animals that were not rehabilitated (P < 0.05; Fisher’s posthoc test) (Fig. 4D), and cholinergic lesions prevented these rehabilitation-induced increases (P < 0.01; Fisher’s posthoc test) (Fig. 4D).
Although not statistically significant, measures of basilar dendritic spine numbers and total basilar dendritic branching increased with rehabilitation training [ANOVA F(2,45) = 1.36; P = 0.27 and ANOVA F(2,57) = 1.30; P = 0.28, respectively], and cholinergic ablation tended to counter these increases (Figs. 4 E and F).
Overall changes in dendritic complexity were further assessed using a Sholl analysis (23), wherein concentric rings are placed around a neuronal soma, and the number of crossings of these rings is quantified to reflect dendritic complexity (Fig. 4G). A greater number of crossings of more distant annuli from the soma suggests greater dendritic complexity. Repeated measures mixed ANOVA indicated a significant interaction effect [ANOVA (group × annulus) F(18,513) = 2.70; P < 0.01 (P < 0.03)], with significant expansions of dendritic architecture in animals that underwent rehabilitation training, particularly annuli that were more distant from the cell soma [posthoc ANOVA (group) P < 0.05 for annuli 4–8; Fisher posthoc test P < 0.05 at annuli 6–8 for train/lesion vs. train/lesion/rehabilitation]. Once again, cholinergic ablation significantly reduced rehabilitation-related expansions of dendritic architecture (Fisher posthoc test P < 0.05 at annuli 4–6 for train/lesion/rehabilitation vs. cholinergic lesion) (Fig. 4G).
Assessment of Cholinergic Depletion.
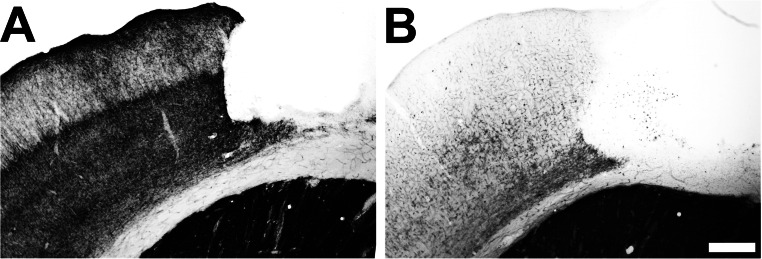
Acetylcholinesterase histochemistry confirmed that cholinergic depletion of cortical innervation was greater than 98% in SAP-injected animals (Fig. S1), which is in agreement with previous studies from our group using this technique (6). Thus, no animals were excluded from analysis based on incomplete cholinergic lesions.
Fig. S1.
Cholinergic ablation. Acetylcholinesterase staining of intact animals that received (A) infusions of ACSF into the nucleus basalis/substantia innominate and (B) infusions of SAP. Animals with toxin injections exhibited >95% depletion of cholinergic fibers. (Scale bar: 250 μm.)
Discussion
The adult CNS is understood today to exhibit far more plasticity at several levels of structure and function than previously known. In the intact CNS, existing spines are dynamically produced and eliminated, but total numbers do not ordinarily change (3, 4); however, when learning occurs, spine turnover is increased, and the building of stable new spines can be detected when analyses focus on specific, functionally relevant neuronal subsets (5). Recent progress has also identified structural alterations that occur as a result of stroke or focal lesions placed in the CNS, with changes in axonal branching and growth (7, 8). This work shows that rehabilitation drives adaptive structural plasticity in the dendritic compartment of neurons that is directly related to functional recovery of the lesioned cortex, a hypothesis that has been frequently cited but has little supporting evidence. Previous studies showed plasticity of spines in the cortex contralateral to a cortical lesion (10–13), but in contrast to our findings, these observations were of unclear relevance to behavioral recovery of the limb affected by a lesion. Using analysis of neuronal networks related to specific motor functions, we now show clear rehabilitation-driven plasticity of dendritic complexity and spine number and a specific requirement for subcortical cholinergic systems to facilitate neuronal remodeling and functional recovery. Relating structure to function, we find that elimination of structural plasticity by cholinergic ablation is associated with significantly attenuated functional recovery. Thus, neural plasticity occurs in the dendritic compartment after CNS injury and notably, can be driven by rehabilitation. This plasticity requires both local circuitry and remote inputs. Taken together with our previous finding that rehabilitation drives plasticity of evoked motor maps in the rostral forelimb region after lesions of the caudal forelimb region (6), the same region examined structurally in this experiment, we conclude that rehabilitation is a potent and effective driver of adaptive plasticity in the brain, subsequently leading to significant functional improvement.
We hypothesized that cholinergic systems would exert an essential role in adaptive structural plasticity and functional recovery, because other studies have proven the role of cholinergic modulation in complex cortical plasticity. This hypothesis was confirmed: cholinergic ablation both eliminated the structural changes associated with rehabilitation and significantly attenuated approximately two-thirds of the functional recovery observed in animals with intact cholinergic systems. Indeed, had we, instead, found that cholinergic ablation abolished functional recovery without changing dendritic complexity or spine number, one could call into question the relevance of neuronal structural changes to functional recovery. The critical role that cholinergic systems have in rehabilitation-linked plasticity raises the possibility that agents augmenting cholinergic functioning, such as cholinesterase inhibitors, may also enhance functional recovery in the setting of rehabilitation after cortical damage resulting from stroke or traumatic brain injury. A few small studies have addressed this possibility, although none have been sufficiently powered to draw clear conclusions (24–27). These findings support the rationale for proceeding with appropriately designed and powered studies to determine whether augmentation of cholinergic function will improve clinical outcomes after cortical injury; cholinesterase inhibitors are clinically approved to enhance function in patients with Alzheimer’s disease (28) and well-tolerated in patients with cortical damage (24).
Our findings are relevant to the current treatment of humans after stroke and CNS trauma. We show that rehabilitation drives structural enhancement of neurons adjacent to an injury site and that this is associated with significant functional recovery; lack of structural enhancement is associated with a failure of functional recovery. Rehabilitation required several weeks of intensive training and practice of the impaired function, with rats typically undergoing 300–500 individual rehabilitation trials in an effort to regain ∼60% of prelesion skilled grasping ability. Human rehabilitation is rarely this prolonged and intense, and it is rarely focused narrowly on recovery of highly specific functions (29); instead, clinical rehabilitation sessions tend to focus on a variety of tasks, including compensation for lost function with an unaffected limb. Because it had not been previously established that rehabilitation drives enhancement of brain structure immediately surrounding a lesion site, the value and importance of intense rehabilitation of lost functions may not have been fully appreciated. Although studies generally support the concept that more frequent rehabilitation is associated with improved outcomes after stroke (30–34), few clinical trials have specifically tested whether highly repetitive, narrowly focused rehabilitation improves motor outcomes (29). Prospective, controlled, and adequately powered studies addressing this specific point have not been performed. Currently, many medical care plans limit the duration and extent of rehabilitation, but our findings suggest that more prolonged, focused, and intensive rehabilitation may improve function. Intensive rehabilitation, in turn, could improve quality of life and independence and reduce the costs of chronic medical care. Our findings strongly support the need for proper clinical trials of intense, focused rehabilitation.
Conclusions
Findings of this study provide clear evidence that rehabilitation drives compensatory structural adaptations in functionally relevant subsets of neurons to enhance behavioral recovery after brain injury. Cholinergic ablation is associated with an elimination of structural adaptation and functional recovery, identifying a key role for acetylcholine in rehabilitation-mediated recovery from brain lesions.
Experimental Procedures
All procedures and animal care adhered strictly to Association for Assessment and Accreditation of Laboratory Animals, Society for Neuroscience, and Veterans Administration Medical Center guidelines for experimental animal health, safety, and comfort.
Subjects.
Experimental subjects consisted of male Fischer 344 rats weighing 150–200 g. Initially, 30 rats were randomly assigned to five groups; group 1 naïve animals (n = 4) underwent no skilled forelimb learning or surgical manipulation, group 2 animals (n = 4) underwent skilled forelimb training and no additional manipulation, group 3 animals (n = 7) underwent skilled forelimb training followed by focal motor cortex lesions in the caudal forelimb area, group 4 animals (n = 8) underwent skilled grasp training followed by focal motor cortex lesions and motor rehabilitation training over several weeks, and group 5 animals (n = 7) underwent skilled grasp training followed by injections of SAP to remove cholinergic innervation to the cortex, focal motor cortex lesions, and motor rehabilitation over several weeks. Six rats were removed from the study because of incomplete motor cortex lesions as evidenced by grasping deficits of less than 50% (n = 2 from group 3, n = 3 from group 4, and n = 1 from group 5). Four additional rats were omitted from overall analysis because of retrograde tracer dye leakage beyond the intended dorsal gray matter seen when examined in serial spinal cord sections under fluorescent microscopy (n = 1 from group 3, n = 1 from group 4, and n = 2 from group 5). Results presents data only from animals that completed both functional and anatomical analyses, with a final n = 4 per group.
Skilled Motor Forelimb Grasp Training and Rehabilitation.
Motor training was carried out using single-pellet retrieval boxes as described below (6, 19). During acquisition training in groups 2–5, rats were initially food-restricted to increase motivation (weight maintained at >85% of the free-feeding baseline) and then, trained to grasp through a slot and retrieve sugar pellets from a tray outside the test chamber for 60 trials per day for 2 wk. Rehabilitative training in groups 4 and 5 consisted of 60 trials per day for 5 d per week for 5 wk. All rehabilitated animals received the same number of reaching trials per day: 60.
Surgery.
All surgical procedures were carried out under ketamine/xylazine/acepromazine anesthesia. Intraparenchymal injections of SAP (Advanced Targeting Systems) in group 5, which was diluted to a concentration of 0.375 mg/mL in ACSF, were made using a 0.5-μL Hamilton syringe into two sites within the nucleus basalis (6, 19). This paradigm selectively destroys cholinergic cell bodies within the nucleus basalis and substantia innominata and reduces cholinergic afferent input to the cortex but does not induce nonspecific damage to noncholinergic cell populations within the basal forebrain (19, 35). Group 4 received identical injections of ACSF. Histological assessment using previously described methods (19) confirmed a nearly complete loss of basal forebrain cholinergic innervation to the sensorimotor cortex. Cholinergic innervation to the cortex was not significantly affected by either motor cortex lesions or ACSF injections.
Motor Cortex Lesion.
Focal motor cortex lesions were performed as previously described (6). Briefly, electrolytic lesions were placed in two sites (site 1: anterior/posterior = 0, medial/lateral = ±3.75 mm; site 2: anterior/posterior = +1.5 mm, medial/lateral = ±3.75 mm from Bregma) specifically targeting the distal forelimb representation of the caudal forelimb area of the motor cortex. A 100-μm stainless steel electrode was initially lowered to a depth of 1.7 mm, and a 1-mA dc (Grass Model DCLM5A) was passed for 20 s. The electrode was then raised 1 mm, and the current was applied for another 20s.
Retrograde Labeling.
To label corticospinal neurons projecting to the C8 cervical spinal segment, which contains lower motor neurons that activate muscles controlling distal forelimb movements that are required for grasping (36), the dura between C7 and T1 was visualized, a small puncture in the dura was made using a 25-gauge needle, and a glass micropipette (tip <50 μm) containing red fluorescent latex microspheres (Lumafluor) was inserted into the dorsal horn of the spinal cord (depth of 0.25 mm and 0.3 mm lateral to midline). Using a Picospritzer II (General Valve, Inc.), 100 nL fluorescent latex microspheres were injected into each side of the spinal cord. In all cases, the extent of tracer diffusion was assessed postmortem in a coronal series of spinal cord sections collected at 50-µm intervals. Animals with tracer diffusion into the dorsal columns, dorsolateral fasciculus, or >500 µm beyond the targeted dorsal gray matter were excluded from additional analysis (n = 1 from group 3, n = 1 from group 4, and n = 2 from group 5). Tracers in all animals were injected within 3 d of completion of all behavioral training, and 2 wk were allowed for retrograde transport to the motor cortex.
Intracellular Neuronal Filling with LY.
Intracellular injection of neurons in lightly fixed slices was performed as previously described with modifications (37, 38). All animals underwent intracellular injections of LY. Animals were perfused with 9.25% (wt/vol) sucrose at 37 °C for 1 min at 300 mmHg perfusion pressure followed by 4% paraformaldehyde in PBS (pH 7.4, 37 °C) for 1 min at 300 mmHg and then, 50 mmHg for 4 min using a Perfusion One Apparatus (Myneurolab). The brains were postfixed in the same fixative for 30 min, and coronal slices (200-μm thick) containing motor cortex were sectioned with a vibratome at 30° anterior to the midcoronal plane; this angle matched the projection orientation of layer V pyramidal neurons in an effort to capture the entire apical and basilar processes in a single plane. Slices were kept in Tris-buffered saline at 4 °C.
Slices were placed under a 40× water immersion objective (N.A. 1.4) and observed with an Olympus BX50WI Microscope using infrared differential interference contrast optics (Olympus). Neurons with red fluorescent microspheres in the hemispheres contralateral to the preferred forelimbs were then filled. Cells toward the middle of the slice were sought to capture entire dendritic trees. A glass micropipette (o.d. = 1.00 mm, i.d. = 0.58 mm) pulled on a vertical puller to a tip diameter <1 μm (David Kopf Instruments) and backfilled with 5% aqueous LY (Sigma) was positioned in the microscopic field with a 4D micromanipulator (Siskiyou, Inc.). The soma of the targeted neuron was impaled and iontophoretically injected with 8-nA positive constant current for 3 min through an iontophoresis pump (Union-200; Kation Scientific). Successful injection was characterized by complete filling of the dendritic arbor, including small-caliber (<1 μm) dendrites and spines. After several cells were filled, the slices were placed in ice-cold 4% paraformaldehyde overnight and then, coverslipped using Fluoromount G (Southern Biotechnology).
Image Acquisition and Analysis.
Specimens were examined using an Olympus FV300/Spectra Physics Mai Tai IR Laser-Scanning Two-Photon Microscope (Olympus). A 20× water-immersion objective (N.A. 0.5) was used to image LY-filled neurons. A 60× oil immersion (N.A. 1.4) objective was used to image spines of LY-filled neurons.
Filled corticospinal motor neurons were then visualized and reconstructed using Neurolucida (MBF Bioscience) and analyzed using NeuroExplorer (MBF Bioscience). To be included in the data analysis, individual filled neurons had to fulfill the following criteria. (i) The apical and basilar arborization of the pyramidal neuron had to be intact and visible in the plane of section and not obscured by branches from other cells. (ii) The tip of the apical dendrites had to be in layers I and II. Extent of dendritic branching was analyzed using the concentric ring method by Sholl (23): the number of intersections of dendrites with a series of concentric circles spaced at 20-μm intervals from the center of the cell body was counted for each cell.
Spine density was calculated by quantifying the number of spines per measured length of the parent dendrite and expressed as the number of spines per 10-µm length of dendrite. The length of each dendritic segment used for spine densitometry was at least 20 μm but not greater than 50 µm in length. For each filled neuron, at least two segments of second-order or greater apical dendrite branches were quantified in the distal apical tuft located in layers I–III of motor cortex. We also quantified at least two segments of second-order or greater apical dendrite branches in proximal apical dendrites close to the cell body and at least three segments of third-order or greater basilar dendritic branches. Branch order was determined for apical dendrites, such that branches originating from the primary apical dendrite were first order; after the next bifurcation, the branches were second order and so on. The mean value of each dendritic compartment from each neuron per animal was calculated. In intact, untrained animals and intact, trained animals, we analyzed 5 neurons in each of four animals per group (20 neurons total per group). In the following three groups, we measured 5 neurons in each of four animals per group (20 neurons total per group): lesioned, nonrehabilitated; lesioned, rehabilitated; and lesioned, rehabilitated, SAP lesion. In total, 800 dendritic segments (apical/distal: −200; apical/proximal: 300; and basilar: 300) from neurons projecting to C8 were analyzed, including group 1, 160 dendritic segments (apical/distal: 40; apical/proximal: 60; and basilar: 60); group 2, 160 dendritic segments (apical/distal: 40; apical/proximal: 60; and basilar: 60); group 3, 160 dendritic segments (apical/distal: 40; apical/proximal: 60; and basilar: 60); group 4, 160 dendritic segments (apical/distal: 40; apical/proximal: 60; and basilar: 60); and group 5, 160 dendritic segments (apical/distal: 40; apical/proximal: 60; and basilar: 60).
Statistical Analysis.
For morphological measures, multiple group comparisons were made using ANOVA, and posthoc differences were tested by Fisher’s probable least square difference. For behavioral performance and Sholl analysis (23), repeated measures ANOVA was used. A significance level of P < 0.05 was used for all analyses. Data are presented as mean values ± SEMs. All cell quantification and behavioral training were performed with the examiner blinded to group identity.
Acknowledgments
These studies were funded by NIH Grant AG10435, the Veterans Administration, and grants from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. The microscopy core facility at the University of California, San Diego is supported by NIH Grants P30NS047101 and NS047101.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514682113/-/DCSupplemental.
References
- 1.Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23(2):353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 3.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Conner JM, Rickert J, Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci USA. 2011;108(6):2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46(2):173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Dancause N, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25(44):10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13(12):1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohab JJ, Carmichael ST. Poststroke neurogenesis: Emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14(4):369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 10.Chu CJ, Jones TA. Experience-dependent structural plasticity in cortex heterotopic to focal sensorimotor cortical damage. Exp Neurol. 2000;166(2):403–414. doi: 10.1006/exnr.2000.7509. [DOI] [PubMed] [Google Scholar]
- 11.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14(4):2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: A quantitative electron microscopic examination. Brain Res. 1996;733(1):142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- 13.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581(1):156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 14.Jones TA, Adkins DL. Behavioral influences on neuronal events after stroke. In: Cramer SCNR, editor. Brain Repair After Stroke. Cambridge Univ Press; Cambridge, United Kingdom: 2010. pp. 23–33. [Google Scholar]
- 15.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39(4):1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- 16.Brown CE, Boyd JD, Murphy TH. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J Cereb Blood Flow Metab. 2010;30(4):783–791. doi: 10.1038/jcbfm.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 18.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 20.Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci. 2009;29(18):5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whishaw IQ, Kolb B. Sparing of skilled forelimb reaching and corticospinal projections after neonatal motor cortex removal or hemidecortication in the rat: Support for the Kennard doctrine. Brain Res. 1988;451(1-2):97–114. doi: 10.1016/0006-8993(88)90753-6. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 23.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett KM, et al. Mayo Acute Stroke Trial for Enhancing Recovery (MASTER) Study Group Enhancing recovery after acute ischemic stroke with donepezil as an adjuvant therapy to standard medical care: Results of a phase IIA clinical trial. J Stroke Cerebrovasc Dis. 2011;20(3):177–182. doi: 10.1016/j.jstrokecerebrovasdis.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Nadeau SE, et al. Donepezil as an adjuvant to constraint-induced therapy for upper-limb dysfunction after stroke: An exploratory randomized clinical trial. J Rehabil Res Dev. 2004;41(4):525–534. doi: 10.1682/jrrd.2003.07.0108. [DOI] [PubMed] [Google Scholar]
- 26.Walker W, et al. The effects of Donepezil on traumatic brain injury acute rehabilitation outcomes. Brain Inj. 2004;18(8):739–750. doi: 10.1080/02699050310001646224. [DOI] [PubMed] [Google Scholar]
- 27.Cramer SC. Drugs to enhance motor recovery after stroke. Stroke. 2015;46(10):2998–3005. doi: 10.1161/STROKEAHA.115.007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KL, et al. The Tacrine Collaborative Study Group A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N Engl J Med. 1992;327(18):1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- 29.Thielbar KO, et al. Training finger individuation with a mechatronic-virtual reality system leads to improved fine motor control post-stroke. J Neuroeng Rehabil. 2014;11:171. doi: 10.1186/1743-0003-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 32.Veerbeek JM, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock A, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;4:CD001920. doi: 10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayward KS, Brauer SG. Dose of arm activity training during acute and subacute rehabilitation post stroke: A systematic review of the literature. Clin Rehabil. 2015;29(12):1234–1243. doi: 10.1177/0269215514565395. [DOI] [PubMed] [Google Scholar]
- 35.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221(2):555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 36.McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: A retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419(3):286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Gao WJ, Zheng ZH. Target-specific differences in somatodendritic morphology of layer V pyramidal neurons in rat motor cortex. J Comp Neurol. 2004;476(2):174–185. doi: 10.1002/cne.20224. [DOI] [PubMed] [Google Scholar]
- 38.Buhl EH, Lübke J. Intracellular lucifer yellow injection in fixed brain slices combined with retrograde tracing, light and electron microscopy. Neuroscience. 1989;28(1):3–16. doi: 10.1016/0306-4522(89)90227-3. [DOI] [PubMed] [Google Scholar]