Significance
Leydig cells, derived from stem cells, are the primary source of testosterone in males. Testosterone deficiency has been linked to sexual dysfunction and decreased bone density, muscle mass, and cognition. Thus, the formation and maintenance of the Leydig cell population is of fundamental importance. Through the use of a unique tubule culture system, we have identified critical niche factors that control the proliferation and differentiation of the Leydig stem cells. Additionally, we report on the isolation and purification of these cells through a specific cell surface marker protein.
Keywords: Leydig cell, stem cell, DHH, CD90, testosterone
Abstract
Testicular Leydig cells are the primary source of testosterone in males. Adult Leydig cells have been shown to arise from stem cells present in the neonatal testis. Once established, adult Leydig cells turn over only slowly during adult life, but when these cells are eliminated experimentally from the adult testis, new Leydig cells rapidly reappear. As in the neonatal testis, stem cells in the adult testis are presumed to be the source of the new Leydig cells. As yet, the mechanisms involved in regulating the proliferation and differentiation of these stem cells remain unknown. We developed a unique in vitro system of cultured seminiferous tubules to assess the ability of factors from the seminiferous tubules to regulate the proliferation of the tubule-associated stem cells, and their subsequent entry into the Leydig cell lineage. The proliferation of the stem Leydig cells was stimulated by paracrine factors including Desert hedgehog (DHH), basic fibroblast growth factor (FGF2), platelet-derived growth factor (PDGF), and activin. Suppression of proliferation occurred with transforming growth factor β (TGF-β). The differentiation of the stem cells was regulated positively by DHH, lithium- induced signaling, and activin, and negatively by TGF-β, PDGFBB, and FGF2. DHH functioned as a commitment factor, inducing the transition of stem cells to the progenitor stage and thus into the Leydig cell lineage. Additionally, CD90 (Thy1) was found to be a unique stem cell surface marker that was used to obtain purified stem cells by flow cytometry.
Testicular Leydig cells are the primary source of testosterone (T) in males. T is essential for the development of the male reproductive system and the maintenance of male reproductive functions (1, 2). In addition to defects in reproductive system, its deficiency in the adult contributes to other symptoms that include increased body fat, decreased muscle mass, increased fatigue, depressed mood, decreased cognitive function (3, 4), and reduced immune response (5, 6). In aged men, low T has been reported to contribute to mortality (7). Thus, the formation and maintenance of a functional Leydig cell population throughout adult life is of fundamental importance.
In rodents and humans, T production gradually increases from the peripubertal period through the adult, coincident with the development of adult Leydig cells. There now is compelling evidence that most, if not all, of the adult Leydig cells arise from stem cells, not from the transdifferentiation of fetal Leydig cells (8, 9). In previous studies, we and others isolated cells from neonatal testes that expressed platelet-derived growth factor receptor-α (PDGFα) or nestin (9–11). Depending on culture conditions, these cells were shown to be capable of proliferating indefinitely or of differentiating into T-producing Leydig cells and, thus, were identified as stem Leydig cells (9–11). During their differentiation, these cells proceeded through two intermediate stages, progenitor Leydig cells and immature Leydig cells, before becoming adult Leydig cells (12). The gene expression pattern by the stem Leydig cells was similar to that of bone marrow stem cells and quite different from the patterns of the cells in the Leydig cell lineage (13).
The adult Leydig cells, once established, turn over slowly during adult life. However, when these cells are eliminated from adult testes by treating the rats with ethane dimethanesolfonate (EDS), new, fully functional adult Leydig cells reappear (14, 15). In initial efforts to localize the precursor cells, seminiferous tubules were isolated from EDS-treated testes and cultured with luteinizing hormone (LH) (16). Cells on the tubule surfaces first underwent division, and then differentiated and produced T (17). These results suggested that in addition to reported perivascular locations in the interstitial compartment (18), stem cells also were located on the surfaces of the seminiferous tubules (16, 19, 20).
Studies of a number of tissues have shown that stem cell self-renewal and differentiation are regulated by the interactions between cues that are intrinsic to the cells and extracellular signaling from the local environment, the latter referred to as the niche. In many tissues, including the testis, anatomic complexity combined with the inability to specifically mark stem cells make it difficult to identify these cells, characterize the niche, or determine the extrinsic factors involved in stem cell functions. Thus, as yet the extent to which the testicular environment influences the ability of the stem cells to proliferate and/or differentiate remains unknown. In the present study, we used a unique in vitro system of cultured seminiferous tubule to identify stem Leydig cells on the surface of seminiferous tubules and to assess the ability of factors associated with the tubules to regulate their proliferation and entry into the Leydig cell lineage (i.e., their differentiation). We provide evidence that the proliferation and subsequent differentiation of the stem Leydig cells are regulated by multiple niche factors from the seminiferous tubules, including PDGF, basic fibroblast growth factor (FGF2), transforming growth factor β (TGF-β), activin, Notch, Wnt, and most importantly, Desert hedgehog (DHH). Additionally, we report on the isolation of the stem cells by flow cytometric sorting through a specific cell surface marker protein, CD90.
Results
Differentiation of Stem Leydig Cells Associated with Seminiferous Tubules.
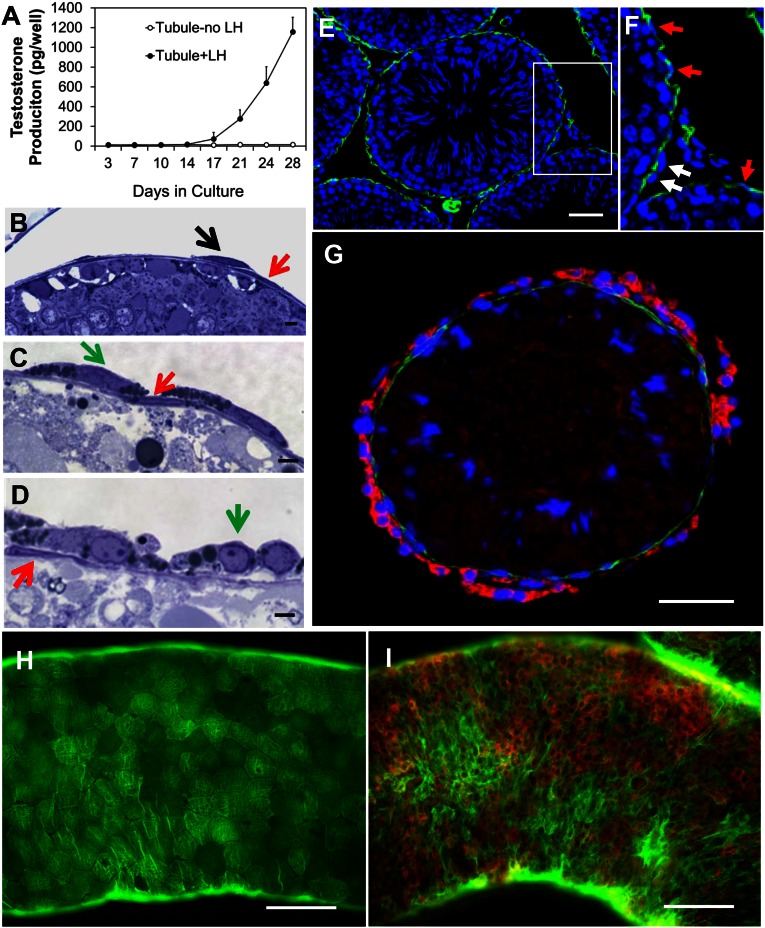
We showed previously that functional Leydig cells can be generated by culturing Leydig cell-free seminiferous tubules with LH (16, 17). With the intent to identify the tubule-associated cells that give rise to T-producing Leydig cells, seminiferous tubule fragments of comparable lengths were cultured in medium containing LH for up to 4 wk. In the presence of LH, little or no T was produced by the tubules for the first week (Fig. 1A). By the end of 2 wk, T began to appear in the medium, and then increased with further culture through 4 wk (Fig. 1A). Examination of the morphology of freshly isolated and cultured tubules revealed a continuous layer of cells surrounding the tubules, on the outer surface of which were scattered, spindle-shaped cells (Fig. 1B). By 2 wk in culture, the continuous layer of cells had not changed in appearance, but lipid droplets had become apparent in the cytoplasm of the cells located on their surface (Fig. 1C). By 4 wk, the outer cells had become round and had far fewer lipid droplets (Fig. 1D).
Fig. 1.
Differentiation of Leydig cells associated with seminiferous tubules in vitro. (A) T production by tubules cultured with LH. (B) Section showing continuous layer of cells surrounding freshly isolated tubules (red arrow), with scattered spindle-shaped cells outside these cells (black arrow). (C and D) Morphological changes in cells on the outside of the tubules (green arrows) after tubule culture with LH for 2 or 4 wk, respectively. (E) α-SMA–positive myoid cells (green) on tubule surfaces 4 d after EDS. (F) α-SMA–positive myoid cells (red arrows) outside of which are α-SMA–negative putative stem Leydig cells (white arrows). (G–I) Tubules costained for α-SMA (green, myoid cells) and CYP11A1 (red, Leydig cells). (G) Cross-section of tubule cultured for 4 wk with LH. (H) Whole-mount tubule before culture. (I) Whole-mount tubule cultured for 4 wk with LH. (Scale bars: white, 100 μm; black, 10 μm.)
The cells in the continuous layer were identified as myoid cells by their location and staining for α-smooth muscle actin (α-SMA), a well-established myoid cell marker (Fig. 1E). The cells on the surface of the myoid cell layer did not stain for α-SMA (Fig. 1F, white arrows) and also did not stain for CYP11A1, a marker for steroidogenic cells. By 4 wk of tubule culture with LH, CYP11A1-positive cells (red) were seen on the surface of the α-SMA–positive (green) myoid cells (Fig. 1G). The relationship of the α-SMA–positive myoid cells and the CYP11A1-positive cells is evident in whole mount tubules before (Fig. 1H) and after (Fig. 1I and Fig. S1) culture for 4 wk with LH. The cells outside the myoid cell layer also became 3βHSD-positive after 4 wk of culture with LH, consistent with their positive staining for CYP11A1 (Fig. S2).
Fig. S1.
(A) Tubules cultured with LH for 4 wk and costained for α-SMA (green) and CYP11A1 (red). Cells stained for α-SMA (B) or CYP11A1 (C), with little overlap (A and D). (Scale bars: 100 μm.)
Fig. S2.
Staining by 3βHSD of freshly isolated tubules (A) and of tubules cultured for 4 wk with LH (B). (Scale bars: 100 μm.)
Putative Niche Factors Involved in the Regulation of Stem Leydig Cell Proliferation and Differentiation.
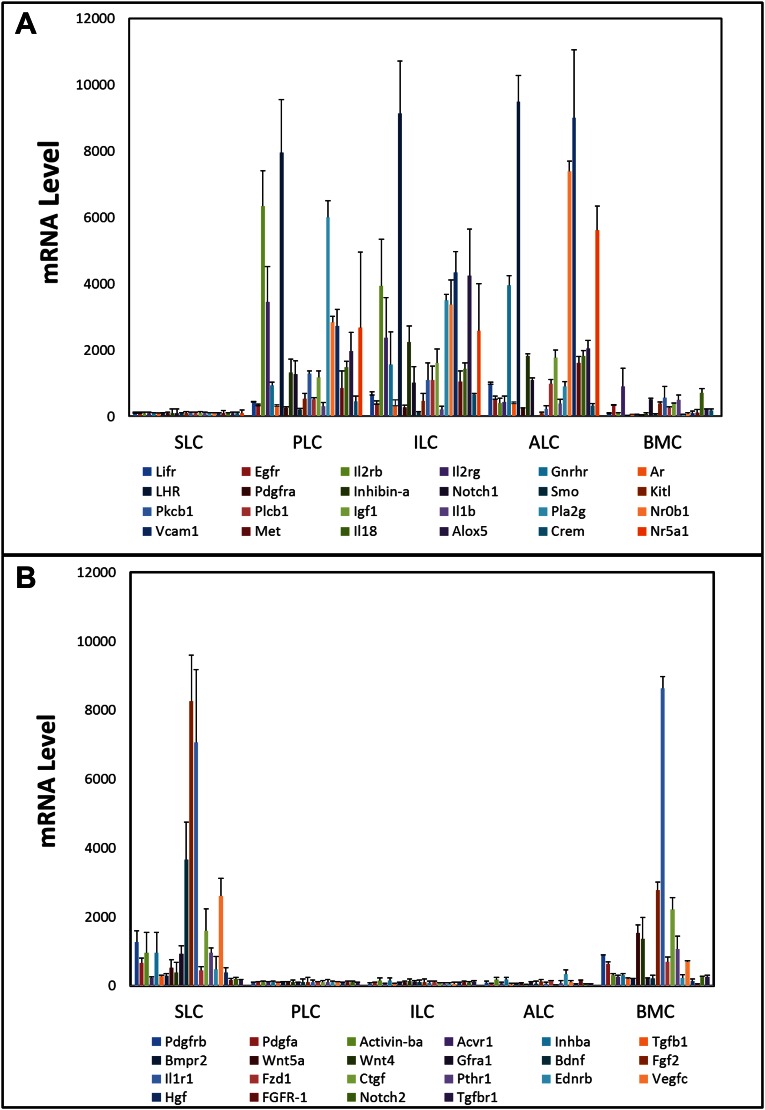
We hypothesized that there are circulatory and local factors that regulate the proliferation and differentiation of the tubule-associated stem Leydig cells. To test this hypothesis, we examined the effects of hormones, growth factors, and cytokines (Table S1) that had been shown by microarray analysis to be up-regulated or down-regulated significantly in the differentiation of stem to progenitor Leydig cells (Fig. S3), or that had been suggested to play roles in Leydig cell development and/or adult stem cell function in other organs. Identified factors were tested by using the cultured seminiferous tubule system that we developed (16, 17). The advantage of this system is that proliferation and differentiation of the stem cells occur sequentially, with most proliferation occurring during the first week of culture and most differentiation occurring subsequently (17). This same sequence occurs in vivo (21, 22).
Table S1.
Factors screened by tubule culture experiments for their roles in the proliferation and differentiation of stem Leydig cells
| Reagent | Signaling pathway | Concentration (range tested) | Manufacturer |
| Ovine LH | 10 ng/mL (0.1–10) | Hormone Program USDA | |
| Rat FSH | 20 ng/mL (0.2–20) | Hormone Program NIDDK | |
| T3 | 10 nM (0.1–10) | Sigma-Aldrich | |
| Estradiol (E2) | 10 ng/mL (0.1–10) | Steraloids | |
| DHT | 10 ng/mL (0.1–10) | Steraloids | |
| R1881 | AR− | 10 nM (0.1–10) | Sigma-Aldrich |
| Dexamethasone | 10 nM (0.1–10) | Sigma-Aldrich | |
| Retinoic acid | 1 μM (0.01–1) | Sigma-Aldrich | |
| Mouse PDGFAA | 10 ng/mL (0.1–10) | Prospec Protein Specialists | |
| Rat PDGFBB | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| PDGFR Inhibitor IV | PDGFBB− | 20 nM (0.2–20) | EMD Bioscience |
| Imatinib Mesylate | c-Kit−/PDGFR− | 0.2 μM (0.02–2) | Selleck Chemicals |
| Human TGFβ1 | 10 ng/mL (0.1–10) | Cell Signaling Technology | |
| Human Activin | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| SB431542 | TGFβ−/Activin− | 0.1 μM (0.01–1) | Selleck Chemicals |
| SB525334 | TGF-β− | 50 nM (0.5–50) | Selleck Chemicals |
| Rat TGFα | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| Mouse EGF | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| Porcine Inhibin | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| Rat IL-1β | 1 ng/mL (0.1–10) | Sigma-Aldrich | |
| Rat IGF1 | 50 ng/mL (0.5–50) | Sigma-Aldrich | |
| Human SCF | 10 ng/mL (0.1–10) | MP Biomedicals | |
| Rat GDNF | 10 ng/mL (0.1–10) | EMD Chemicals | |
| Human FGF2 | 10 ng/mL (0.1–10) | Novus Biologiclas | |
| Mouse LIF | 10 ng/mL (0.1–10) | Sigma-Aldrich | |
| DAPT | Notch− | 2 μM (0.2–20) | EMD Bioscience |
| Lithium | ? | 5 mM (0.5–50) | Sigma-Aldrich |
| Wnt Activator I | Wnt+ | 1 μM (0.01–1) | EMD Bioscience |
| XAV939 | Wnt− | 0.1 μM (0.01–1) | Selleck Chemicals |
| Wortmannin | PI3Ks− | 0.1 μM (0.01–1) | Cayman Chemical |
| SAG | DHH+ | 0.5 μM (0.05–5) | EMD Bioscience |
| Vismodegib | DHH− | 0.1 μM (0.01–1) | Selleck Chemicals |
| Cyclopamine | DHH− | 0.2 μM (0.02–2) | Enzo Life Science Inc |
| Rapamycin | mTOR− | 5 nM (0.05–5) | Invitrogen |
| DFU | Cox2− | 0.2 μM (0.02–2) | Merck Canada Ltd |
| dbcAMP | PKA+ | 0.1 mM (0.01–1) | Sigma-Aldrich |
Fig. S3.
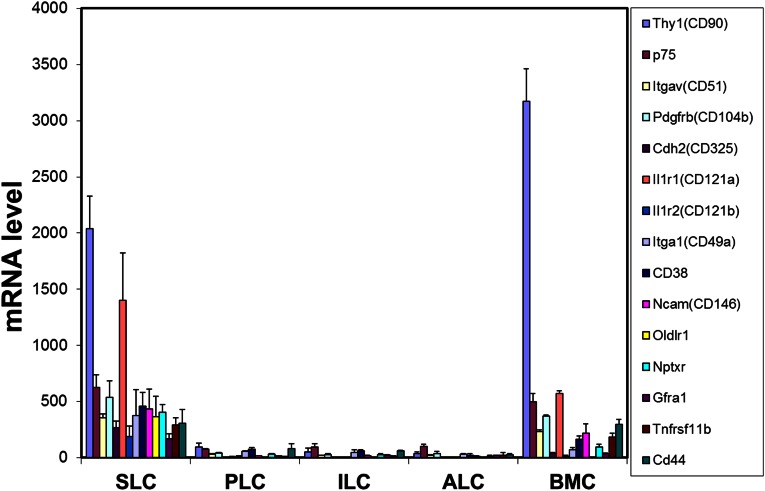
Microarray analysis of gene expression of receptors and/or signaling molecules up-regulated (A) or down-regulated (B) in the transition from stem Leydig cells (SLC) to progenitor Leydig cell (PLC), immature Leydig cells (ILC) and adult Leydig cells (ALC). BMC, bone marrow cells. Data are from analysis of our previously published microarray study (13).
To assess the effects of particular factors on stem cell proliferation, equivalent lengths of tubules were incubated with LH plus a given factor during week 1, the period during which active proliferation of the stem cells occurs, and then switched to LH-only medium for weeks 2 and 3. To assess the effects of given factors on stem cell differentiation, equivalent lengths of tubules were incubated in medium containing LH alone during week 1, and then with LH plus the factor to be tested during weeks 2 and 3, the period during which differentiation of the cells occurs. After week 3, T was assayed in the media, and the results were compared with tubules that had been cultured with LH alone.
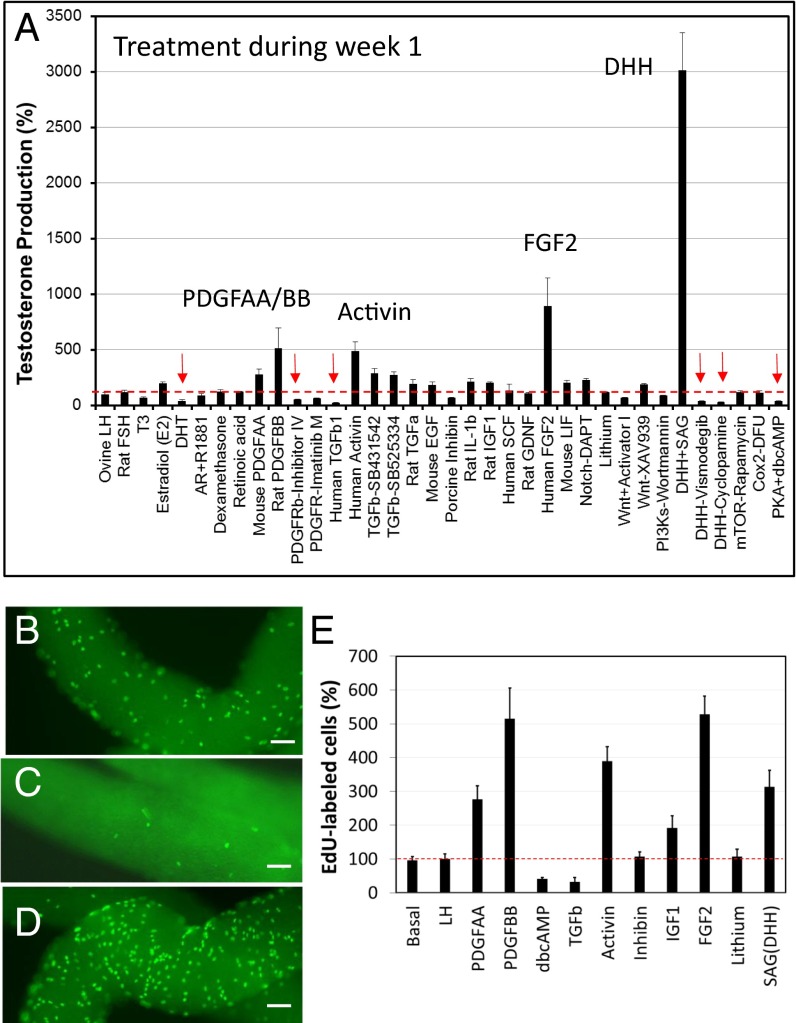
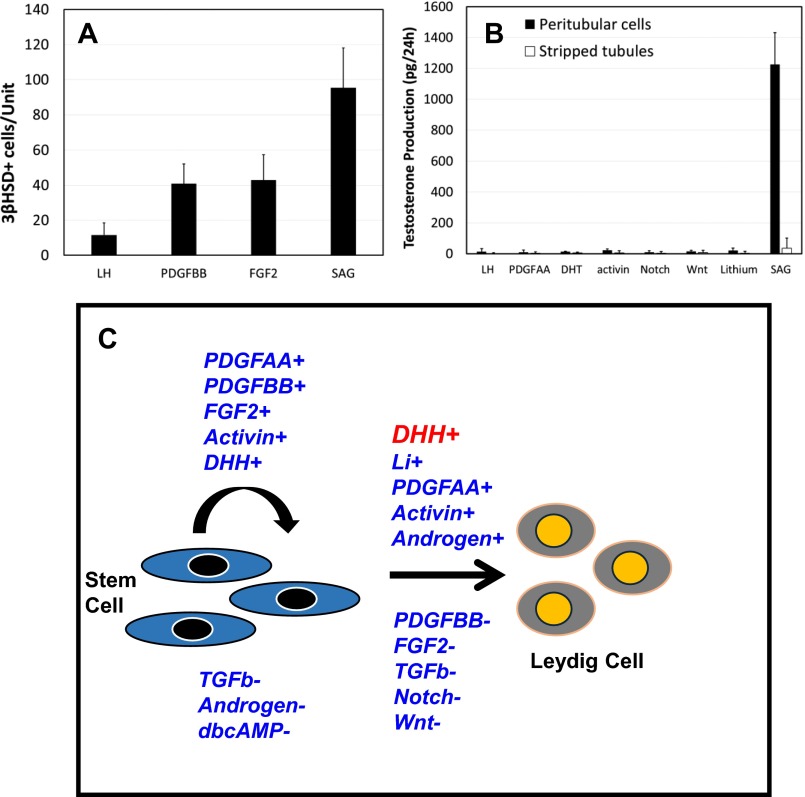
When added to cultures during week 1, SAG (an agonist of DHH), FGF2, activin, PDGFBB, and PDGFAA had stimulatory effects on T production, whereas TGF-β, DHT, dbcAMP, and inhibitors of PDGFR and DHH were among the factors that had inhibitory effects (Fig. 2A). To determine whether the stimulatory or inhibitory effects of these factors on T production were the result of their effects on stem cell proliferation during week 1 and, thus, on the number of cells capable of differentiating to form T-producing cells during weeks 2 and 3, dividing cells were labeled with EdU after tubules had been cultured for 5 d. Treatment with TGF-β inhibited cell proliferation in comparison with incubation with LH alone (compare Fig. 2B vs. Fig. 2C), whereas treatment with FGF2 stimulated cell proliferation (Fig. 2D). The effects of various factors on cell proliferation were quantified (Fig. 2E). PDGFAA, PDGFBB, activin, FGF2, and SAG, the five most active factors found to stimulate T production, resulted in two- to fivefold increases in EdU-positive cells in comparison with LH alone, whereas treatment with TGF-β and dbcAMP, which reduced T production significantly, decreased EdU-positive cells (Fig. 2E). Treatment with inhibin and lithium, factors that did not affect T production when applied during week 1, also did not affect cell proliferation (Fig. 2E). Increased numbers of 3βHSD-stained Leydig cells (Fig. S4A) were seen at 3 wk after treatment of tubules during week 1 with PDGFBB, FGF2, or SAG, consistent with the cell proliferation data seen at 1 wk.
Fig. 2.
(A) Effects on T production of potential regulatory factors added to tubules during week 1 of culture with LH. The tubules were then switched to LH-only medium for weeks 2 and 3. T was assayed in the media after 3 wk and expressed as the percentage of T production by tubules cultured with LH alone in week 1. (B–E) Dividing cells were labeled with EdU after 5 d of cultures with LH-only medium (B), LH plus TGF-β (C) or LH plus FGF2 (D), and cell numbers were quantified (E). Data are expressed as mean ± SEM of 4–7 separate experiments. Red arrows indicate significant inhibition. (Scale bars: 100 μm.)
Fig. S4.
(A) Effect of different treatments during week 1 on the number of 3βHSD-positive cells formed by the end of week 3. Data are expressed as mean ± SEM of three experiments. (B) Effects on testosterone production by peritubular cells isolated after collagenase treatment of the tubules and by stripped tubules after their culture with LH or LH plus PDGFAA, DHT, activin, Notch, Wnt antagonists, lithium, or SAG (DHH). Data are expressed as mean ± SEM of three experiments. (C) Proposed regulatory model of stem Leydig cell proliferation and differentiation. The positive and negative factors are followed by ‘+’ and ‘-’, respectively.
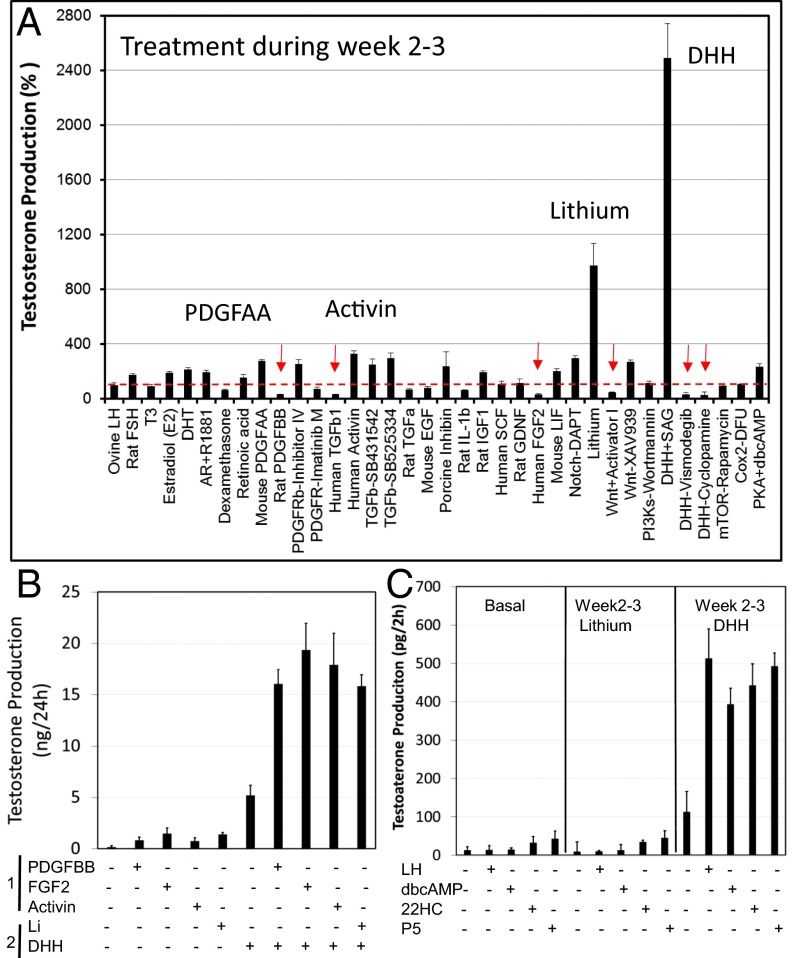
When added to cultured tubules during weeks 2 and 3, the period during which cell differentiation occurs, SAG and lithium resulted in at least 10-fold stimulation of T production (Fig. 3A). Activin, PDGFAA, DHT, and antagonists for TGF-β (SB431542; SB525334), Notch (DAPT), and Wnt (XAV939) also had mild stimulatory effects. In contrast, TGF-β, Wnt activator I, and DHH inhibitors had negative effects on T production. Interestingly, FGF2 and PDGFBB, which had stimulatory effects on cell proliferation during week 1, were found to have inhibitory effects on T production when administered during weeks 2 and 3.
Fig. 3.
Effects on T production of potential regulatory factors. (A) Factors were added during weeks 2 and 3 after culture of the tubules with LH alone during week 1. T was assayed in the media after 3 wk and expressed as the percentage of T production by tubules cultured with LH alone in weeks 2 and 3. Red arrows indicate significant inhibition. (B) T production by tubules stimulated with proliferating factors during week 1 and then with or without SAG and/or lithium during weeks 2 and 3. (C) Effects on T production of treating tubules with SAG or lithium for weeks 2 and 3, in the absence of LH, after which the tubules were incubated (2 h) with LH, dbcAMP, 22HC, or P5. Data are expressed as mean ± SEM of 4–7 separate experiments.
To examine interactions among factors that regulate stem cell proliferation and differentiation, tubules were treated during week 1 with LH plus factors that affect proliferation, and then were switched during weeks 2 and 3 to medium containing LH plus SAG, the most potent differentiation stimulator (Fig. 3B). Incubation of the tubules during week 1 with PDGFAA, FGF2, or activin and then with LH alone for weeks 2 and 3 resulted in relatively modest increases in T production by the end of week 3. However, when tubules that had been incubated with any of the three factors during week 1 were incubated with SAG during weeks 2 and 3, the tubules increased their T production dramatically compared with tubules incubated with SAG alone (Fig. 3B). Adding lithium to SAG-treated tubules during weeks 2 and 3 increased T production compared with SAG alone.
Although LH is essential for the development of adult Leydig cells, stem Leydig cells do not express the LH receptor (9, 23, 24). Therefore, we reasoned that there must be a Leydig cell lineage commitment factor, as yet unidentified, that triggers the transition from stem Leydig cells to LH-responsive progenitor cells. Having found that SAG and lithium have profound stimulatory effects on differentiation during weeks 2 and 3 of culture (Fig. 3A), we examined the possibility that one or both of these factors might induce the transition from stem to progenitor cells in the absence of LH. To that end, the tubules were incubated in culture without LH for 1 wk, and then with SAG or lithium from weeks 2 to 3. At the completion of week 3, the ability of the tubules to produce T in response to a brief (2-h) treatment with LH, dbcAMP, 22-hydroxycholesterol (22HC), or progesterone (P5) was determined. As seen in Fig. 3C, tubules cultured in medium alone for 3 wk did not produce any T in response to brief exposure to LH, dbcAMP, 22HC, or P5. Similarly, treatment with lithium during weeks 2 and 3 failed to increase T production. When tubules were cultured with SAG from weeks 2 to 3, however, there was significant elevation of T production in response to each of LH, dbcAMP, 22HC, and P5, indicating that DHH signaling, even in the absence of LH, can stimulate stem cells to enter the Leydig cell lineage and ultimately become able to produce T.
Sertoli cells have been reported to produce paracrine factors, including DHH, that are involved in the regulation of Leydig cell development (25, 26). We asked whether DHH signaling alone can commit stem Leydig cells to differentiate, or whether commitment also requires other factors produced by the seminiferous tubules. To address this question, the peritubular stem cells were separated from the tubules by collagenase treatment of the tubules. Then, the isolated cells and stripped tubules were cultured separately (Fig. S4B). Neither the tubules nor the isolated peritubular cells produced T in response to 3-wk culture with LH alone, or with LH for 1 wk and then with LH plus PDGFAA, DHT, activin, antagonists of Notch or Wnt, or lithium during weeks 2 and 3. In contrast, SAG (DHH agonist), when added during weeks 2 and 3, induced the isolated cells, but not the stripped tubules, to produce T. These results support the conclusion that DHH may be the long-sought, tubule-derived niche factor required for the commitment of stem cells to Leydig cells. The factors that affect stem Leydig cell proliferation and differentiation are summarized in Fig. S4C.
CD90 as a Specific Surface Marker for the Isolation of Stem Leydig Cells from Adult Testis.
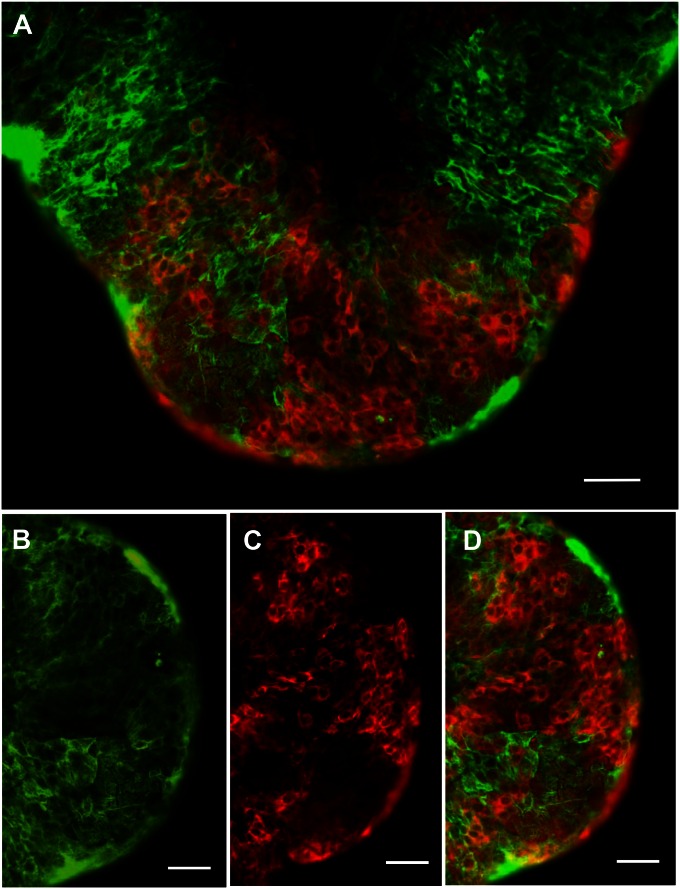
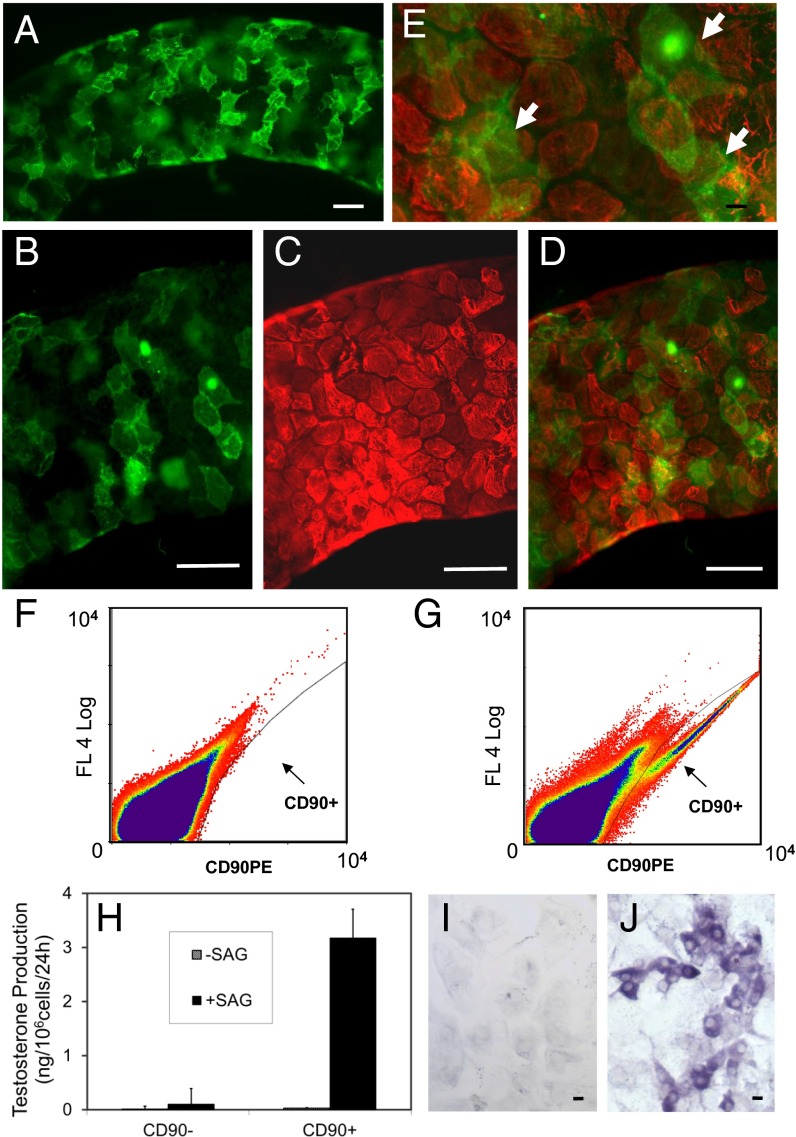
In the studies described above, stem cells obtained by collagenase treatment of seminiferous tubules were shown to differentiate in response to SAG (DHH). We asked whether there might be other cells in these preparations (e.g., germ, Sertoli, myoid) that might contribute factors involved in the differentiation of the SAG-treated stem cells, or whether DHH signaling alone was sufficient. To address this question required the identification of a Leydig stem cell-specific surface marker with which to purify the cells. Analysis of our previously published microarray data (13) identified 15 cell surface proteins whose message levels were highly expressed in stem cells and then turned off at the progenitor stage (Fig. S5). Among these proteins, we tested four (CD90, p75NTR, CD51, and PDGFRα) for their specificity. We found CD90 to be of greatest use for purifying stem Leydig cells. In particular, CD90 localized to cells on the surface of freshly isolated seminiferous tubules (Fig. 4A), which is where stem cells reside. In tubules costained for CD90 (Fig. 4B) and for the myoid cell protein desmin (Fig. 4C), cells on the surfaces of the tubules were stained for one or the other protein, with little overlap (Fig. 4 D and E). This result indicated that the myoid cells do not express CD90.
Fig. S5.
mRNA levels of cell surface proteins whose expressions are high in SLC but turned off in the PLC stage and thereafter (ILC and ALC). BMC, bone marrow cells. Data are from analysis of our previously published microarray study (13).
Fig. 4.
Identification and isolation of CD90-positive cells. (A) Peritubular cells in freshly isolated seminiferous tubules stained for CD90. (B and C) Tubules costained for CD90 (B, green) and the myoid cell marker desmin (C, red). (D and E) Few cells were positive for both CD90 (green) and desmin (red). White arrows indicate the green CD90+ cells on top of red myoid cells. (F) Unstained cell population sorted by flow cytometry. (G) CD90+ cell fraction sorted by flow cytometry after staining with CD90 antibody. (H) T production by CD90+ and CD90− cells cultured with LH plus or minus SAG (DHH agonist) for weeks 2 and 3. (I and J) Staining by 3βHSD of CD90− (I) and CD90+ (J) cells treated with LH for week 1 and then SAG for weeks 2 and 3. Data are expressed as mean ± SEM of three separate experiments. (Scale bars: white, 100 μm; black, 10 μm.)
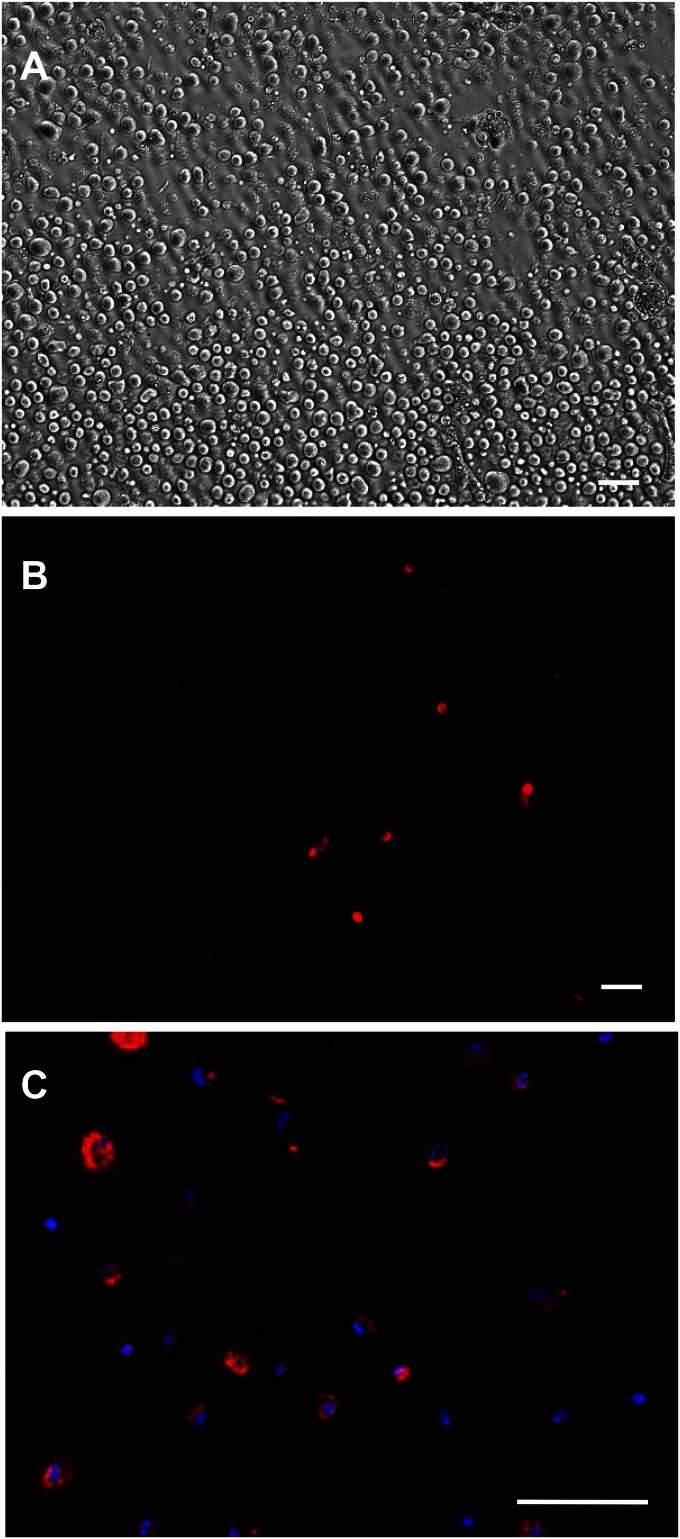
Peritubular cells obtained from collagenase-treated freshly isolated tubules (Fig. S6A) were stained for CD90 (Fig. S6B) and sorted by flow cytometry (Fig. S6C). As shown in Fig. 4G, the CD90+ cells separated into a population not seen in unstained cell preparations (Fig. 4F). The CD90+ cells represented about 0.4% of the total cells. The CD90+ and CD90− cells were cultured with LH for 3 wk plus or minus SAG during weeks 2 and 3, and their ability to differentiate into steroidogenic cells was assessed (Fig. 4H). CD90+ but not CD90− cells were able to form T-producing cells in the presence of LH plus SAG. Staining by 3βHSD of cultured cells was consistent with T production; the CD90+ (Fig. 4J) but not CD90− cells (Fig. 4I) stained positively after the 3-wk period of LH/SAG. These results indicate that in the presence of LH, DHH signaling alone is sufficient to induce the Leydig stem cell to differentiate.
Fig. S6.
(A) Peritubular cells before purification. (B) Peritubular cells stained by CD90 antibody (same field as A). (C) CD90+ cells (red) purified by flow cytometric sorting. Blue, DAPI-stained nuclei. (Scale bars: 100 μm.)
Discussion
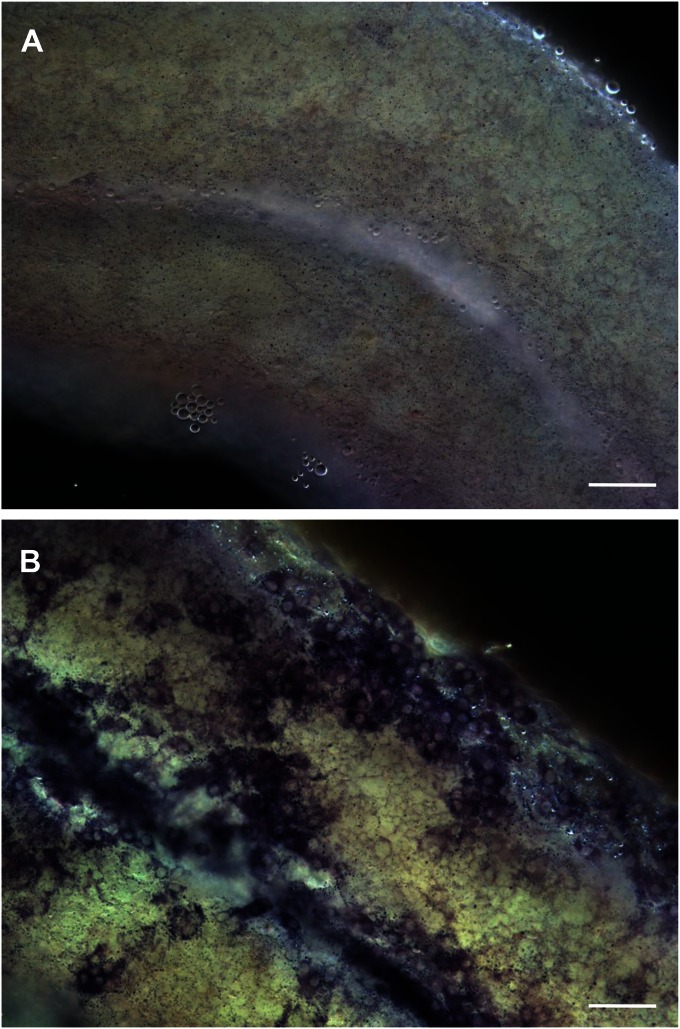
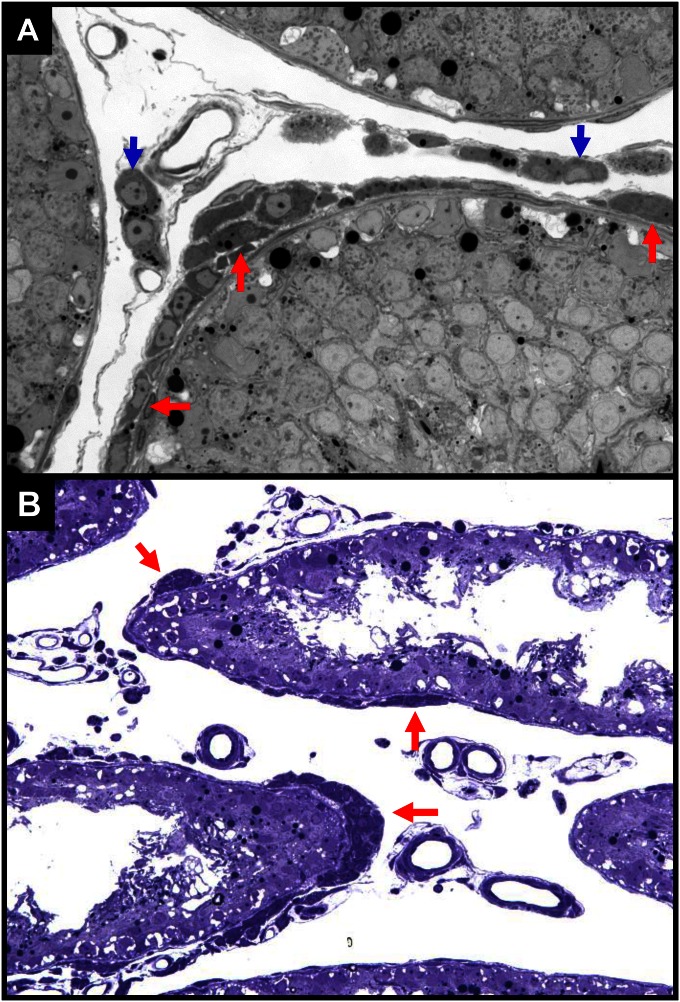
In the normal rat testis, the growth and differentiation of the adult Leydig cell population is largely completed by approximately day 70 postpartum. The number of Leydig cells present in the adult testis (approximately 25 million) does not change with aging. However, there is evidence that although the Leydig cell number is relatively stable, there is some turnover. For example, 3H-thymidine has been shown to be incorporated into cells in the interstitial compartment (27), and injections of rats with high doses of hCG to result in the formation of new Leydig cells (28). These early studies, based on 3H-thymidine labeling, in fact may have underestimated the turnover rate of adult Leydig cells. For example, cells around the tubules were shown to divide actively, but were classified as myoid cells (27). We now know that stem Leydig cells are located both on the tubule surfaces (9, 16, 19, 20) and associated with blood vessels (18, 24). In the event of loss of the adult Leydig cells (14, 15), stem cells in both locations are capable of regenerating new Leydig cells (16, 20–22; Fig. S7A). The peritubular stem cells, however, play a particularly significant role in reestablishing the Leydig cell population when testes are regressed (20; Fig. S7B).
Fig. S7.
Leydig cell development in normal (A) versus atrophic (B) testes 4 wk after EDS treatment. Leydig cells were formed in both peritubular (red arrows) and interstitial (blue arrows) locations in testes with normal spermatogenesis (A), but only in peritubular locations (red arrows) in testes without germ cells (B).
In the present study, we identified a group of cells on the surface of the seminiferous tubules of the adult testis that are distinguishable from myoid cells and are capable of giving rise to functional Leydig cells. In contrast to the continuous layer of α-SMA–staining myoid cells surrounding the tubules, the cells do not express α-SMA and are located outside the layer of cells that do so. These cells became CYP11A1-positive and 3βHSD-positive in association with T production that is induced by culturing tubules with LH for 3–4 wk, indicating that the peritubular cells had become steroidogenic. To begin to assess whether the seminiferous tubules provided niche factors needed for the peritubular cells to differentiate, mild collagenase treatment of the tubules was used to isolate cells from the surface of the tubules. In contrast to LH-cultured tubules, the isolated cells failed to differentiate into steroidogenic cells when cultured with LH, suggesting that the tubules might indeed provide factors needed for the differentiation, and perhaps the proliferation, of the peritubular putative stem cells.
In an earlier study, we had conducted microarray analysis of gene expression during the differentiation of neonatal stem Leydig cells to cells that entered the Leydig cell lineage as progenitor Leydig cells (13). The significantly up-regulated or down-regulated genes for signaling molecules and receptors were among those in which we took particular interest. Others were proteins identified in previous studies that were suggested to play roles in Leydig cell development or in stem cell function in organs outside the testis. We used the cultured seminiferous tubule system that we developed to screen for the effects of these factors. We found that, as is the case in vivo (21, 22), the proliferation of the stem cells on the surfaces of the tubules occurred during the first week of culture, which was followed by their differentiation during weeks 2 and 3 (17). With this system, therefore, factors associated with the tubules that might be involved in cell proliferation, cell differentiation, or both can be tested.
Five factors (PDGFAA, PDGFBB, FGF2, activin, DHH) were identified which, when added to tubules during week 1, resulted in stimulated T production by the end of week 3, and three (TGF-β, androgen, dbcAMP) in suppressed T production. These results suggest that these factors had differential effects on cell proliferation during week 1, resulting in different numbers of cells able to differentiate during weeks 2 and 3 into steroidogenic cells. The effects of these factors on proliferation were assessed in three ways. First, proliferation was tested directly by labeling the dividing cells with EdU during the first week of treatment. Second, the tubules treated with the stimulatory factors during week 1 were further treated during weeks 2 and 3 with the DHH agonist SAG, a potent stem Leydig cell differentiation inducer, and T was measured at the end of week 3. This latter approach was based on the assumption that if there were more stem cells resulting from treatment with proliferation factors during week 1, there would be more T-producing cells formed, and more T produced, in the presence of differentiation inducer. Third, 3βHSD-positive cells were directly quantified after 3 wk of treatment. The results obtained by the three approaches were largely in agreement, except for SAG. In that case, the substantial increase in T production and in 3βHSD-positive cells seen after 3 wk cannot be explained entirely by increase in EdU-labeled cells during week 1, suggesting that in contrast to other factors tested, SAG treatment during week 1 has effects in addition to increasing cell proliferation. With respect to differentiation, DHH, lithium, activin, PDGFAA and androgen were found to stimulate the differentiation of stem cells during weeks 2 and 3, and TGF-β, Wnt, and Notch to be inhibitory. Interestingly, PDGFBB and FGF2, the two most potent stem Leydig cell proliferation stimulators, inhibited stem Leydig cell differentiation, suggesting that these factors may play roles in controlling the balance between stem and differentiated cells. The factors that affect stem Leydig cell proliferation and differentiation are summarized in Fig. S5C.
The critical role of LH in adult Leydig cell steroidogenic function is well established. However, it is unlikely that LH alone could commit stem Leydig cells to the differentiation pathway because the stem cells do not express the LH receptor (9, 23, 24). We found SAG, which stimulates DHH, to have strong effect on differentiation. Consequently, we hypothesized that DHH signaling might play an important role in the early commitment of stem cells into the Leydig cell lineage. When tubules were cultured without LH for 3 wk, no Leydig cells were formed. However, when tubules were incubated with SAG in the absence of LH from weeks 2 to 3, differentiation of the stem cells into LH-responsive, T-producing cells occurred. These results suggest that DHH may be the critical commitment factor that triggers the transition of stem Leydig cells into the Leydig cell lineage. The expression of LH receptors by the progenitor cells makes these cells responsive to LH signaling, which is essential for the further maturation of the cells.
Previous studies indicated that DHH and PDGF are involved in the normal development of the fetal and adult Leydig cell populations (25, 26, 29–32). The specific roles of these factors in the functions of stem Leydig cells in adult testes were not elucidated. Using the tubule culture system that we developed, DHH and PDGF were found to increase both stem cell proliferation and differentiation. Although both PDGFAA and PDGFBB stimulated stem cell proliferation, they played opposite roles in stem cell differentiation, with PDGFAA stimulatory and PDGFBB inhibitory. We also found that there is a robust stimulatory effect of lithium on stem cell differentiation. This metal is of particular interest because of its reported effects on the activation of Wnt signaling by inhibiting GSK3β (33). As yet, the molecular mechanism by which this factor acts remains uncertain.
As discussed above, we had obtained results suggesting a critical role for DHH in differentiation of stem Leydig cells. The isolation of pure stem cells was required to address the question of whether DHH, by itself, is sufficient to induce stem Leydig cell differentiation in the absence of other testicular (niche) cells. The isolation of pure stem cells requires specific cell surface markers. Several proteins had been reported in previous studies to be expressed by stem Leydig cells in neonatal or adult testes, including nestin (11, 18), COUP-TFII (24, 34), Arx (35), CD51 (11), p75NTR (11), and PDGFRα (9, 10, 16). Nestin, COUP-TFII, and Arx are intracellular proteins and, therefore, could not readily be used as markers to purify the cells. PDGFRα, a cell surface protein, had been used to concentrate stem Leydig cells from neonatal (9, 10) and adult (16) rats. CD51 and p75NTR also are cell surface proteins whose expressions were found to overlap with that of nestin in the neonatal mouse testis (11). By reanalysis of an earlier array study (13), we also identified a number of cell surface proteins whose mRNAs were highly expressed by stem cells but turned off with the transit of these cells to progenitor Leydig cells.
Among the proteins that we examined, CD90 was found to be most promising for the specific isolation of stem Leydig cells. Staining freshly isolated seminiferous tubules with CD90 antibody resulted in its specific localization to cells on the outer surface of tubules and not by other seminiferous tubule-associated cells, including myoid cells. Additionally, when the CD90+ and CD90− cells were separated by flow cytometric sorting and then cultured with LH/SAG, the CD90+ cells differentiated into 3βHSD-staining cells and produced T. These observations establish CD90 as a specific cell surface marker for the isolation of the seminiferous tubular-associated stem Leydig cells in the adult rat testis. Using these cells, we found that in the absence of SAG (DHH), the CD90+ cells failed to differentiate, but did so when exposed to SAG. These results suggest that DHH is a critical steroidogenic lineage trigger factor in the commitment of stem to Leydig progenitors and able to induce the stem cells to differentiate even in the absence of other niche cells.
In summary, we have shown that the stem cells associated with the surface of seminiferous tubules are capable of giving rise to new adult Leydig cells. Both the proliferation and differentiation of the stem Leydig cells occur under the apparent influences of the seminiferous tubular cells. Multiple factors have been found that affect the proliferation and differentiation of these stem cells. Among the factors that affect differentiation, DHH signaling apparently is necessary and sufficient. The stem Leydig cells of the rat testis were found to express the surface protein CD90 specifically, and that allows the cells to be isolated. The current study, focused as it is on the stem cells associated with tubules, does not rule out the possibility that there also are stem cells present in the interstitial compartment of the testis, the regulation (and functions) of which may or may not be the same as the stem cells associated with the tubules.
Materials and Methods
Culture of Seminiferous Tubules.
All animal procedures were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals, according to protocols approved by the Johns Hopkins Animal Care and Use Committee. Equal lengths (5 cm) of isolated tubules were cultured in 24-well plates with or without LH (10 ng/mL) in M199 medium supplemented with 0.1% BSA, 15 mM Hepes, 2.2 mg/mL sodium bicarbonate, penicillin/streptomycin (100 U/mL and 100 μg/mL) and insulin/transferrin/selenium for up to 4 wk at 34 °C and 5% (vol/vol) CO2. For screening experiments (Figs. 2A and 3A), each of the 36 different factors (Table S1) was added to the media during either week 1 or weeks 2–3. LH was included in the media all of the times. At the end of 3 wk, the abilities of the cultures to produce T were assayed by incubation of the tubules with LH (10 ng/mL) for 24 h. Each factor was tested at three different concentrations (ranges used are reported in Table S1). The concentrations reported herein (Figs. 2A and 3A) were either the lowest that elicited maximal response, or the highest that had no effect. These were the concentrations also used in subsequent experiments (Figs. 2 B–E, 3 B and C, and 4H and Fig. S4 A and B). For experiments to examine interactions among proliferation and differentiation factors (Fig. 3B), tubules were treated during week 1 with PDGFBB, FGF2, or activin and then were switched to lithium or SAG (DHH agonist) during weeks 2 and 3. T production was assayed by incubation of the tubules with LH for 24 h. For experiments to examine the effects of lithium and SAG on the differentiation of stem Leydig cells in the absence of LH (Fig. 3C), the tubules were cultured with or without lithium or SAG alone for weeks 2 and 3. At the end of the 3 wk, the ability of the cells to produce T was assessed after 2-h incubation with LH (10 ng/mL), dbcAMP (1 mM), 22-hydroxycholesterol (22HC, 25 µM) or pregnenolone (P5, 25 µM). For all experiments, media were frozen for T assay, and tubules were used for either morphology or stained for 3βHSD, CYP11A1, desmin, or α-SMA. The antibodies used in these studies are listed in Table S2.
Table S2.
Antibodies used and their sources
| Antigen | Antibody | Dilutions | Manufacturer |
| α-SMA | Mouse monoclonal | 1:1000 | Sigma-Aldrich |
| Desmin | Rabbit IgG | 1:1000 | Thermo Fisher Scientific |
| CYP11A1 | Rabbit IgG | 1:200 | Chemicon International |
| CD90PE | Mouse monoclonal | 1:100 | Stemcell Technologies |
| CD90-FITC | Mouse monoclonal | 1:100 | Stemcell Technologies |
| CD51PE | Rabbit IgG | 1:100 | Bioss Antibodies |
| CD51PE | Rat IgG | 1:100 | eBioscience |
| p75NTR | Rabbit mAb | 1:100 | Cell Signaling Technology |
| PDGFRα | Rabbit IgG | 1:100 | Santa Cruz |
| Anti-rabbit IgG | Alexa Fluor 488 Goat IgG | 1:1000 | Thermo Fisher Scientific |
| Anti-mouse IgG | Alexa Fluor 555 Goat IgG | 1:1000 | Thermo Fisher Scientific |
| Anti-rabbit IgG | Alexa Fluor 555 Donkey IgG | 1:1000 | Thermo Fisher Scientific |
| Anti-mouse IgG | Alexa Fluor 488 Donkey IgG | 1:1000 | Thermo Fisher Scientific |
Isolation and Culture of Peritubular Cells.
To isolate peritubular cells, the tubules were digested with 1 mg/mL collagenase-D in DMEM/F12 medium at 34 °C for 30 min with slow shaking (90 cycles per min) and allowed to settle. The dispersed cells were filtered through a 100-µm pore nylon mesh and were plated in 24-well plates in DEME/F12 medium containing 5% (vol/vol) FBS for 2 h, washed, and expanded by DEME/F12 medium containing 2.5% (vol/vol) FBS, 10 ng/mL FGF2, and 10 ng/mL PDGFBB. When the cells reached 80% confluence, they were switched into M199 medium containing LH (10 ng/mL) for a week. Then the cells were treated with LH plus PDGFAA (10 ng/mL), activin (10 ng/mL), DHT (10 ng/mL), DAPT (2 µM), XAV939 (0.1 µM), lithium (5 mM), or SAG (0.5 µM) from weeks 2 to 3 to assess the ability of these factors to induce differentiation. At the end of 3 wk, differentiation was determined by assessing the ability of the cells to produce testosterone in response to LH (24 h).
Statistical Analyses.
Data are expressed as the mean ± SEM. Group means were evaluated by one-way ANOVA. If group differences were revealed by ANOVA (P < 0.05), differences between individual groups were determined with the Student–Neuman–Kuels test, using SigmaStat software (Systat Software). Values were considered significant at P < 0.05.
See SI Materials and Methods for additional procedures.
SI Materials and Methods
Chemicals.
The manufactures for hormones, growth factors, and/or their agonists and antagonist are listed in Table S1. The antibodies used in these studies are listed in Table S2. The culture media (M-199, DMEM/F12) and Click-iT EdU (5-ethynyl-2′-deoxyuridine) imaging kit were purchased from Invitrogen. Testosterone and other steroids were obtained from Steraloids. EDS was synthesized according to the method described by Jackson and Jackson (36). All other reagents were obtained from Sigma-Aldrich.
Animals and Treatment.
Adult male brown Norway rats, 3–5 mo of age, were supplied by Harlan Sprague Dawley, through the NIA animal resource program. The rats were housed in the animal facilities at the Johns Hopkins Bloomberg School of Public Health under controlled light (14 h light:10 h dark) and with free access to water and rat chow. All animal procedures were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals, according to protocols approved by the Johns Hopkins Animal Care and Use Committee. To eliminate Leydig cells from the testes, rats were injected with a dose of EDS (i.p., 80 mg/kg body weight) dissolved in a mixture of DMSO:PBS (1:3). Testes were collected 4 d after EDS treatment, by which time all adult Leydig cells had been eliminated (14, 15). Seminiferous tubules were mechanically separated from the interstitium with fine forceps under a transillumination dissection microscope (37).
Purification and Culture of Stem Cells by Flow Cytometry.
Peritubular cells obtained from collagenase-treated freshly isolated tubules were stained for CD90, and then sorted by flow cytometry. CD90 antibodies were conjugated with the fluorochromes PE or FITC. Cells were incubated with CD90 antibody (1:100) in Ca++/Mg2+-free HBSS (0.5% BSA, 5 mM EDTA) for 30 min on ice. After washing three times, the cells were suspended in 1 mL of HBSS (0.5% BSA and 5 mM EDTA) for flow cytometric sorting (MoFlo Sorter; Beckman-Coulter). To compare their ability to form Leydig cells, CD90+ and CD90− cells were expended in 2.5% (vol/vol) FBS in DEME/12 medium containing 10 ng/mL FGF2 and 10 ng/mL PDGFBB. When the cells reached 80% confluent, they were switched into M199 medium containing LH (10 ng/mL) for a week. Then the cells were treated with LH with or without SAG (0.5 µM) for 2 wk. By the end of 3 wk, differentiation was determined by assessing the ability of the cells to produce testosterone in response to LH (24 h) or stained for 3βHSD.
Immunofluorescence and 3βHSD Activity Staining.
Seminiferous tubules, tubule sections, or cell suspensions were washed with Ca++ and Mg2+ free HBSS (0.5% BSA) and then incubated with conjugated primary antibody for 30 min, or with primary antibody for 60 min followed by incubation with conjugated second antibody for 30 min. For some studies, tubules were fixed with Bouin’s or formalin, and incubated with antibody for CYP11A1, α-SMA, or desmin for 1 h. After washing three times, tissues were then treated with fluorescent secondary antibodies (Alexa-conjugated anti-rabbit or anti-mouse IgG, 1:1000) for 1 h. After three washes, the tissues were examined by Nikon Eclipse 800 microscope and photos were taken with a Princeton Instruments 5-Mhz cooled CCD camera, custom CRI color filter, and IP-Lab digital image analysis software (Scanalytics). Cytochemical staining of 3βHSD was carried out according to a previously published protocol (16). In some experiments (Fig. S4A), positive cells were counted along the surface of the tubules and expressed as the number per unit. The unit was defined as a square area with the four sides of the square equal to the diameter of a given tubule. For each treatment, at least 80 square areas were counted from three different experiments.
Labeling Cell Proliferation with Click-iT EdU.
Cell divisions on the surface of the tubules were monitored with the Click-iT EdU imaging kit from Invitrogen and quantified as previously (17). The seminiferous tubules were labeled with EdU (10 µM, 24 h) after their treatment with various factors for 5 d. The labeled nuclei (green) were visualized under a Nikon Eclipse 800 microscope (excitation/emission at 495/519 nm) and quantified. Positive cells were counted along the surface of the tubules and expressed as the number per unit (Fig. 2D). The unit was defined as a square area with the four sides of the square equal to the diameter of a given tubule. For each treatment, at least 100 square areas were counted from at least three different experiments.
Acknowledgments
We thank Dr. William Wright for his valuable suggestions on the experimental design. This work was supported by NIH Grant R37 AG21092 (to B.R.Z.); National Natural Science Foundation of China Grants NSFC31271252 (to H.C.), NSFC81471411 (to H.C.), NSFC30871434 (to R.G.), and NSFC31171425 (to R.G.); and an opening grant from Zhejiang Provincial Top Discipline of Clinical Medicine (to H.C. and Q.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519395113/-/DCSupplemental.
References
- 1.Nef S, Parada LF. Hormones in male sexual development. Genes Dev. 2000;14(24):3075–3086. doi: 10.1101/gad.843800. [DOI] [PubMed] [Google Scholar]
- 2.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHenry Martin C. Testosterone deficiency in older men: A problem worth treating. Consult Pharm. 2012;27(3):152–163. doi: 10.4140/TCP.n.2012.152. [DOI] [PubMed] [Google Scholar]
- 4.Huhtaniemi I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16(2):192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malkin CJ, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 6.Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: A nested cross-sectional study. PLoS One. 2013;8(4):e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder PJ. Might testosterone actually reduce mortality? J Clin Endocrinol Metab. 2008;93(1):32–33. doi: 10.1210/jc.2007-2506. [DOI] [PubMed] [Google Scholar]
- 8.Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum Reprod Update. 2015;21(3):310–328. doi: 10.1093/humupd/dmv008. [DOI] [PubMed] [Google Scholar]
- 9.Ge RS, et al. In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc Natl Acad Sci USA. 2006;103(8):2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372(1-2):96–104. doi: 10.1016/j.mce.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Jiang MH, et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24(12):1466–1485. doi: 10.1038/cr.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: From fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90(4):272–283. doi: 10.1002/bdrc.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley EL, et al. Stem Leydig cell differentiation: Gene expression during development of the adult rat population of Leydig cells. Biol Reprod. 2011;85(6):1161–1166. doi: 10.1095/biolreprod.111.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molenaar R, de Rooij DG, Rommerts FF, Reuvers PJ, van der Molen HJ. Specific destruction of Leydig cells in mature rats after in vivo administration of ethane dimethyl sulfonate. Biol Reprod. 1985;33(5):1213–1222. doi: 10.1095/biolreprod33.5.1213. [DOI] [PubMed] [Google Scholar]
- 15.Kerr JB, Donachie K, Rommerts FF. Selective destruction and regeneration of rat Leydig cells in vivo. A new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res. 1985;242(1):145–156. doi: 10.1007/BF00225571. [DOI] [PubMed] [Google Scholar]
- 16.Stanley E, et al. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153(10):5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odeh HM, Kleinguetl C, Ge R, Zirkin BR, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90(6):123. doi: 10.1095/biolreprod.114.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidoff MS, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167(5):935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landreh L, et al. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99(7):E1227–E1235. doi: 10.1210/jc.2013-4199. [DOI] [PubMed] [Google Scholar]
- 20.O’Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135(6):851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]
- 21.Teerds KJ, De Rooij DG, Rommerts FF, van den Hurk R, Wensing CJ. Stimulation of the proliferation and differentiation of Leydig cell precursors after the destruction of existing Leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of LH. J Androl. 1989;10(6):472–477. doi: 10.1002/j.1939-4640.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 22.Teerds KJ, de Rooij DG, Rommerts FF, van den Hurk R, Wensing CJ. Proliferation and differentiation of possible Leydig cell precursors after destruction of the existing Leydig cells with ethane dimethyl sulphonate: The role of LH/human chorionic gonadotrophin. J Endocrinol. 1989;122(3):689–696. doi: 10.1677/joe.0.1220689. [DOI] [PubMed] [Google Scholar]
- 23.Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65(3):660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- 24.Kilcoyne KR, et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA. 2014;111(18):E1924–E1932. doi: 10.1073/pnas.1320735111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao HHC, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16(11):1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology. 2007;148(8):3704–3710. doi: 10.1210/en.2006-1731. [DOI] [PubMed] [Google Scholar]
- 27.Teerds KJ, De Rooij DG, Rommerts FFG, van der Tweel I, Wensing CJG. Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl. 1989;23(2):105–111. doi: 10.3109/01485018908986831. [DOI] [PubMed] [Google Scholar]
- 28.Teerds KJ, De Rooij DG, Rommerts FF, Wensing CJ. The regulation of the proliferation and differentiation of rat Leydig cell precursor cells after EDS administration or daily HCG treatment. J Androl. 1988;9(5):343–351. doi: 10.1002/j.1939-4640.1988.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawai Y, et al. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction. 2011;141(2):217–225. doi: 10.1530/REP-10-0006. [DOI] [PubMed] [Google Scholar]
- 30.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63(6):1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- 31.Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65(5):1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- 32.Basciani S, Mariani S, Spera G, Gnessi L. Role of platelet-derived growth factors in the testis. Endocr Rev. 2010;31(6):916–939. doi: 10.1210/er.2010-0004. [DOI] [PubMed] [Google Scholar]
- 33.Meffre D, et al. Wnt and lithium: A common destiny in the therapy of nervous system pathologies? Cell Mol Life Sci. 2014;71(7):1123–1148. doi: 10.1007/s00018-013-1378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3(9):e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyabayashi K, et al. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One. 2013;8(6):e68050. doi: 10.1371/journal.pone.0068050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson CM, Jackson H. Comparative protective actions of gonadotrophins and testosterone against the antispermatogenic action of ethane dimethanesulphonate. J Reprod Fertil. 1984;71(2):393–401. doi: 10.1530/jrf.0.0710393. [DOI] [PubMed] [Google Scholar]
- 37.Vihko KK, Suominen JJ, Parvinen M. Cellular regulation of plasminogen activator secretion during spermatogenesis. Biol Reprod. 1984;31(2):383–389. doi: 10.1095/biolreprod31.2.383. [DOI] [PubMed] [Google Scholar]