Abstract
Mice immunized with respiratory syncytial virus (RSV) G glycoprotein or with formalin-inactivated RSV (FI-RSV) exhibit severe disease following RSV challenge. This results in type 2 cytokine production and pulmonary eosinophilia, both hallmarks of vaccine-enhanced disease. RSV G-induced T-cell responses were shown to be restricted to CD4+ T cells expressing Vβ14 in the T-cell receptor (TCR), and the deletion of these T cells resulted in less severe disease. We therefore examined the role of Vβ14+ T cells in FI-RSV-induced disease. BALB/c mice were immunized with vaccinia virus expressing secreted RSV G (vvGs) or with FI-RSV. At the time of challenge with live RSV, mice were injected with antibody to the Vβ14 component of the TCR. vvGs-immunized mice treated with anti-Vβ14 had reduced cytokine levels in the lung. Eosinophil recruitment to the lung was also significantly reduced. In contrast, depletion of Vβ14+ T cells in FI-RSV-immunized mice had little impact on cytokine production or pulmonary eosinophilia. An analysis of TCR Vβ chain usage confirmed a bias toward Vβ14 expression on CD4+ T cells from vvGs-immunized mice, whereas the CD4+ T cells in FI-RSV-immunized mice expressed a diverse array of Vβ chains. These data show that although FI-RSV and vvGs induce responses resulting in similar immunopathology, the T-cell repertoire mediating the response is different for each immunogen and suggest that the immune responses elicited by RSV G are not the basis for FI-RSV vaccine-enhanced disease.
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family of viruses. The negative-sense single-stranded RNA genome contains 10 genes that encode 11 proteins (16). The G glycoprotein, the putative attachment protein of RSV, is expressed on both the surface of the virus and on virally infected cells. The G glycoprotein is naturally produced as both membrane-anchored and secreted forms due to the presence of a second methionine codon in the transmembrane region of the protein (18, 42). The secreted form of RSV G can be detected in culture supernatants soon after infection (at 6 h postinfection), while the detection of all membrane-anchored proteins occurs later (18 to 20 h postinfection). Thus, secreted RSV G is available to modulate early innate immune responses to RSV. Two antigenic strains of virus, A and B, exist and cocirculate each year, and much of the antigenic diversity between and within RSV strains is due to variations in the RSV G glycoprotein, with as little as 35% homology between G glycoproteins of strain A and strain B isolates (20, 21, 33). Therefore, as the attachment protein, the first viral protein expressed, and the source of most of the antigenic diversity, the G glycoprotein is a potentially important target of protective antiviral immune responses and should be considered for inclusion in any vaccine product. RSV G also has features that suggest it should not be included as a vaccine antigen. It is heavily glycosylated (30) and does not induce antibody well in young infants (34). Additionally, it is not stable as a purified protein even when stored at −70°C (E. Walsh, personal communication). Also, G-specific cytolytic CD8+-T-cell responses are very rare (2, 3, 7, 10, 45). Finally, RSV G-specific CD4+-T-cell responses have been associated with severe disease (1, 38, 54).
RSV is the major cause of respiratory disease in infants and young children, resulting in more than 130,000 hospitalizations in the United States each year (43). While RSV infection normally results in mild upper respiratory tract symptoms, a subset of infants progress to a more severe lower respiratory tract disease. Children who experience these severe acute lower respiratory tract symptoms caused by RSV infection have an increased incidence of childhood asthma (reviewed in reference 39). Therefore, development of an RSV vaccine is of high priority.
In the early 1960s, a trial of formalin-inactivated alum-precipitated RSV (FI-RSV) was conducted. However, rather than protecting vaccinees against infection, children immunized with the FI-RSV preparation experienced more severe disease following subsequent natural exposure to the virus, resulting in the hospitalization of 80% of FI-RSV-immunized infants and two deaths, compared to 5% hospitalization and no deaths in children immunized with a similar preparation of parainfluenza virus (26, 28). Subsequent analyses of blood from these children demonstrated significant titers of nonneutralizing serum antibody (28) and heightened lymphoproliferative responses (29). Histopathologic examination of lung tissue from one of the infants that died revealed a prominent eosinophilic infiltrate (15, 28). Animal models of RSV pathogenesis have similarly demonstrated enhanced disease in FI-RSV-immunized animals following challenge with live RSV (9, 13, 35, 41). This vaccine-enhanced disease is typified by pulmonary eosinophilia and the production of type 2 cytokines, especially interleukin-4 (IL-4), IL-5, and pulmonary eosinophilia. Interference with the function of these cytokines decreases disease severity (9, 23, 25, 48-50), underscoring the importance of cytokines in FI-RSV vaccine-enhanced immunopathogenesis.
Recombinant vaccinia viruses expressing RSV G induce CD4+ T cells that secrete IL-4, IL-5, and IL-13 and result in pulmonary eosinophilia upon RSV challenge of vaccinated mice (4, 17, 24, 38, 45, 46). Mice immunized with vaccinia virus expressing only the secreted form of RSV G (vvGs) exhibit more severe disease after challenge than do mice immunized with vaccinia expressing wild-type (vvGwt) or only membrane-anchored (vvGr) G glycoprotein (4, 24). These similar disease patterns of type 2 cytokine production and pulmonary eosinophilia following RSV challenge of FI-RSV- or G-immunized mice have led to the proposal that it is the presence of the G glycoprotein in FI-RSV that predisposes for the vaccine-enhanced illness observed. However, we have demonstrated that while similar endpoints of pulmonary eosinophilia and severe illness occur in FI-RSV- and G-immunized mice, the cytokine requirements of these two immunogens are significantly different. Vaccine-enhanced disease in FI-RSV-immunized mice, in which IL-4 expression predominates, may be modulated by the inhibition of IL-4 or IL-13 alone (23, 25, 48) or by the addition of exogenous IL-12 during priming (49). In contrast, IL-13 expression is greater in vvGs-immunized mice, and disease is reduced only when both IL-4 and IL-13 function is blocked (23, 25). Furthermore, the presence of gamma interferon (IFN-γ) (22) or IL-12 (unpublished data) during vvGs priming has minimal impact on vaccinia virus replication or disease following live RSV challenge.
In vitro analyses have shown that CD4+ T cells from BALB/c mice immunized with RSV G respond to a single peptide spanning amino acids (aa) 183 to 198 (47, 51) or aa 193 to 203 (44) of the G glycoprotein and produce both type 1 and type 2 cytokines following peptide stimulation. Immunization of BALB/c mice with only this immunodominant epitope of RSV G is sufficient to induce those immune responses that result in enhanced disease following RSV challenge (51). Subsequent work demonstrated the presence of a single immunodominant CD4-T-cell-restricted epitope spanning aa 183 to 195 that elicited both Th1 and Th2 CD4+ T cells (55). Further examination of the CD4+ memory T-cell response to the immunodominant epitope spanning aa 183 to 195 demonstrated that this population is highly skewed with Vβ14-T-cell-receptor (TCR)-expressing cells that exhibit striking conservation within the complement determining region 3 (54). When these Vβ14+ T cells were depleted in G-immunized mice prior to RSV challenge, eosinophilia and type 2 cytokine production were reduced. We therefore examined the contribution of Vβ14+ T cells to vaccine-enhanced disease in FI-RSV-immunized mice.
MATERIALS AND METHODS
Viruses and vaccine formulations.
RSV A2 was grown in HEp-2 cells (American Type Culture Collection) in Eagle's modified essential medium supplemented with 10% fetal calf serum, glutamine, and antibiotics (10% EMEM). FI-RSV was prepared as previously described (13). An RSV challenge stock was grown in HEp-2 cells as previously described (14). vvGs and vvGwt were gifts from Gail Wertz (University of Alabama at Birmingham). The viruses were grown in BSC40 cells in 10% EMEM and purified on discontinuous potassium tartrate gradients as described previously (24). PCR showed all virus stocks to be free of mycoplasma and adenovirus contamination.
Immunization, antibody treatment, and RSV challenge of mice.
Six-week-old pathogen-free BALB/c mice were purchased from Charles River Laboratories. Mice were immunized intramuscularly with 0.1 ml of FI-RSV diluted 1:10 in 1× phosphate-buffered saline (PBS) or with vvGs or vvGwt (5 × 105 PFU in 0.05 ml) intradermally at the base of the tail. Six weeks later, all mice were challenged intranasally with 107 PFU of live RSV in 0.1 ml.
To deplete Vβ14+ T cells, subsets of FI-RSV- and vvGs-immunized mice were injected intraperitoneally with 0.1 mg of anti-Vβ14 in 0.2 ml of PBS. Additional immunized mice were injected with 0.1 mg of immunoglobulin M (IgM) isotype-matched control antibody. The mice were injected on days −1, 2, and 5 around RSV challenge. The anti-Vβ14 antibody and the IgM control were purchased from BD Pharmingen.
RSV titers.
Subsets of mice were euthanized 4 and 7 days postchallenge for determination of virus titers. The lungs were removed and transferred to 10% EMEM and quick-frozen. The lungs were stored at −80°C until assayed. RSV titers were measured by plaque assay on subconfluent HEp-2 monolayers as previously described (24). The data are expressed as the log10 PFU per gram of lung tissue.
Cytokine protein levels in the lung.
IL-4, IL-5, IL-13, IFN-γ, eotaxin, MIP-1α, and MIP-1β protein levels were measured by cytokine-specific sandwich enzyme-linked immunosorbent assays (ELISAs; R&D Systems, Minneapolis, Minn.) using the lung supernatants after viral plaque assays were completed. The data are expressed as picograms per milliliter.
BAL.
Seven days after challenge, a subset of mice were euthanized. A tracheotomy was performed, and the large airways were washed with 0.5 ml PBS-1% bovine serum albumin. The bronchoalveolar lavage (BAL) wash was centrifuged, and the supernatant was removed. The BAL pellet was resuspended, total cell counts of the BAL cell pellet were made by trypan blue exclusion, and cytospins were made with the remaining sample. The cytospins were differentially stained with HemaStain, and differential cell counts were determined by counting at least 300 total cells. The data are expressed as the percentage of eosinophils and as the total number of eosinophils present.
Lung histopathology.
Seven days after challenge, mice were euthanized. The left lungs were inflated with 10% formalin and removed and transferred to formalin. The tissue was paraffin embedded, and thin sections were cut. Sections were stained with hematoxylin and eosin and with Giemsa stain.
Analysis of TCR expression.
Mice were immunized with vvGs or FI-RSV and challenged with live RSV. Five days after challenge, lung lymphocytes were isolated from immunized and challenged mice as described previously (55). The cells (2 × 106) were stained with phycoerythrin (PE)-conjugated anti-CD4 and fluorescein isothiocyanate-labeled anti-TCR monoclonal antibodies in staining buffer (PBS containing 2% fetal bovine serum and 0.02% NaN3). All antibodies were purchased from BD Pharmingen, with the exception of anti-TCR Vβ8.2/3, which was purchased from CalTag. Stained cells were fixed and erythrocytes were lysed with fluorescence-activated cell sorter lysing solution (BD Pharmingen), washed, resuspended in staining buffer, and analyzed on a FACSCalibur flow cytometer.
Peptide stimulation and intracellular cytokine production.
Mice were immunized with vac-lac (recombinant vaccinia virus encoding β-galactosidase), vvGs, or FI-RSV and were challenged with live RSV. Lung mononuclear cells were isolated as described previously (55) 5 days after RSV challenge. Lung mononuclear cells (2 × 106) were then stimulated for 5 h in the presence or absence of 1 μM RSV peptides (Table 1) and in the presence of 1 μg of brefeldin A (Sigma)/ml. The cells were then washed in staining buffer and fixed with fluorescence-activated cell sorter lysing solution. After fixation, the cells were washed twice in permeabilization buffer (staining buffer containing 0.5% saponin) and blocked with purified anti-FcγRII/III (clone 2.4G2; BD Pharmingen). The cells were then stained with PerCp/Cy5.5-conjugated anti-CD4 and a combination of either PE-labeled anti-IL-4 (clone 11B11) and allophycocyanin (APC)-labeled anti-IL-5 (clone TRFK5) or PE-labeled anti-IL-6 (clone MP5-20F3) and APC-labeled anti-IFN-γ (clone XMG1.2). PE- and APC-conjugated rat IgG1 (clone R3-34) were used as isotype-matched control antibodies. All antibodies were purchased from BD Pharmingen. Stained cells were washed, resuspended in staining buffer, and analyzed on a FACSCalibur flow cytometer.
TABLE 1.
I-Ed-restricted RSV peptides
| Peptide designation | Peptide sequence | Reference |
|---|---|---|
| G183-195 | WAICKRIPNKKPG | 55 |
| M161-175 | PTYLRSISVRNKDLN | 52 |
| F-1146-160 | VSVLTSKVLDLKNYI | 52 |
| F-2184-199 | SAIASGIAVSKVLH | 52 |
| SH-133-46 | IISIMIAILNKLCE | 52 |
| NSP2-162-75 | LVNYEMKLLHKVGS | 52 |
| NSP2-267-80 | MKLLHKVGSTKYKK | 52 |
| L-1392-406 | VWLYNQIALQLKNHA | 52 |
| L-2790-804 | ISLLDLISLKGKFSI | 52 |
| L-3931-945 | TMPVYNRQVLYKKQR | 52 |
| L-41234-1248 | LINIDKIYIKNKHKF | 52 |
| L-51364-1378 | SIEYILKDLIIKDPN | 52 |
| L-61650-1664 | NEVFSNKLINHKHMN | 52 |
| L-71832-1847 | INGRWIILLSKFLK | 52 |
| L-82090-2105 | STIASGIIIEKYNV | 52 |
| HEL108-119 (hen egg lysozyme) | WVAWRNRCKGTD | 27 |
Statistical analysis.
Data were maintained in a Paradox database. Comparisons between immunization groups were made with a Kruskal-Wallis test by using SAS software. P < 0.05 was defined as a significant difference. For statistical analysis of eosinophil and cytokine data, a Student's t test was performed by using the SigmaStat software package (Jandel Scientific, San Rafael, Calif.) to compare two groups, or one-way analysis of variance was used to compare more than two groups. If the data did not distribute normally, a rank sum test was used to compare two groups.
RESULTS
Depletion of Vβ14+ T cells at the time of RSV challenge reduces pulmonary eosinophilia and type 2 cytokine production in both vvGwt- and vvGs-immunized mice.
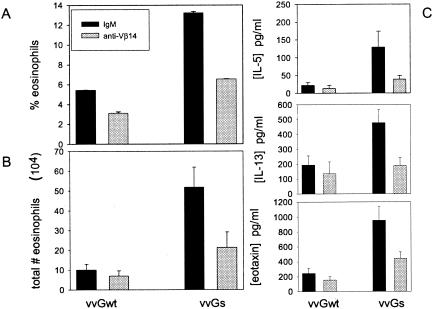
It was previously demonstrated that while immunization with vaccinia virus expressing either vvGwt or only vvGs results in pulmonary eosinophilia and type 2 cytokine production following RSV challenge, the magnitude of the response in vvGs-immunized mice is more similar to that of FI-RSV-immunized mice (24). Prior to Vβ14 depletion studies in parallel with FI-RSV-immunized mice, we compared the relative effects of Vβ14 antibody treatment during immunization with either vvGwt or vvGs. Mice were immunized with vvGwt or vvGs, and 6 weeks later, the mice were challenged with live RSV. At days −1, 2, and 5 of challenge, anti-Vβ14 antibody was administered to deplete mice of Vβ14+ CD4+ T cells. Seven days after RSV challenge, mice were euthanized and BAL was performed. When evaluated as the percentage of cells present in the BAL, depletion of Vβ14+ T cells at the time of RSV challenge reduced eosinophil recruitment to the large airways by 42.7 and 50.6% in mice immunized with vvGwt and vvGs, respectively (Fig. 1A). Similarly, the reduction in total number of eosinophils recruited to the BAL compartment was 30.0 and 58.7% in vvGwt- and vvGs-immunized mice, respectively (Fig. 1B). Thus, as previously reported (54), anti-Vβ14 treatment of vvGwt-primed mice reduced pulmonary eosinophilia to nearly background levels of <5% eosinophils during primary RSV infection, as reported by numerous groups (4, 24, 38, 45, 53). However, with the greater inflammatory response induced in vvGs-immunized mice, administration of Vβ14-specific antibody significantly reduces pulmonary eosinophilia but does not completely abolish the eosinophil-recruiting immune responses. In a similar manner, depletion of Vβ14+ T cells in vvGwt- and vvGs-immunized mice reduced the production of type 2 cytokines and chemokines following RSV challenge (Fig. 1C) but did not completely eliminate production of these cytokines, in agreement with findings of previous work (54). These data demonstrate that RSV-specific immune responses are mediated by Vβ14+ T cells in both vvGwt- and vvGs-immunized mice. Thus, since an impact on disease severity was observed in anti-Vβ14-treated vvGs-immunized mice, comparative studies with FI-RSV immunization utilized vvGs-primed mice, where the magnitude of responses are more similar to those of FI-RSV-immunized mice (23, 24).
FIG. 1.
Depletion of Vβ14+ T cells reduces eosinophil recruitment and cytokine production in both vvGwt- and vvGs-immunized mice. Mice were immunized with vvGwt or vvGs and challenged with RSV 6 weeks later. Anti-Vβ14 antibody or IgM isotype control was administered on days −1, 2, and 5 around RSV challenge. BAL was performed 7 days after challenge, and eosinophils were counted, and the percentage and the total number of eosinophils in the BAL compartment are shown (A and B, respectively). (C) Cytokines were measured in lung supernatants at day 4 postchallenge. The data represent the means ± standard deviations for five mice per group.
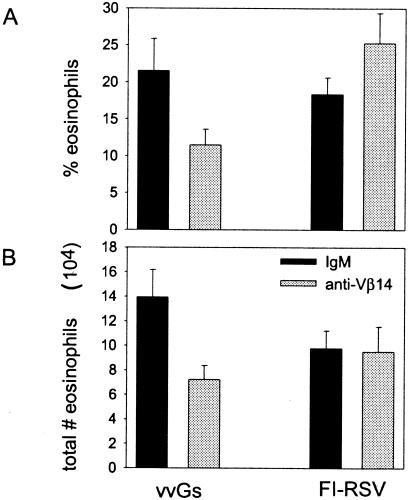
Depletion of Vβ14+ T cells does not reduce pulmonary eosinophilia following RSV challenge in FI-RSV-immunized mice but does so in vvGs-immunized mice.
Immunization with FI-RSV (13, 35), with vaccinia virus expressing the entire RSV G (17, 38, 45, 46), or with vaccinia virus expressing only secreted RSV G (4, 23-25) results in profound pulmonary eosinophilia following challenge with live RSV. We sought to determine whether Vβ14+ T cells contribute to eosinophil infiltration in FI-RSV-immunized mice as has been shown for G-immunized mice (54) in mice with similar magnitudes of disease severity upon RSV challenge. In our model of the pathogenesis of RSV vaccine-enhanced disease, the magnitude of disease severity is most similar in FI-RSV- and vvGs-immunized mice, while vvGwt-primed mice typically exhibit less severe disease following RSV challenge (23, 24). Therefore, to fairly evaluate the contribution of Vβ14+ T cells to disease, we chose to compare FI-RSV-immunized mice with vvGs-immunized mice rather than with vvGwt-primed animals. Mice were immunized with FI-RSV or with vvGs, and 6 weeks later, the mice were challenged with live RSV. At days −1, 2, and 5 of challenge, anti-Vβ14 antibody was administered to deplete mice of Vβ14+ CD4+ T cells. Seven days after challenge of mice immunized with the FI-RSV or vvGs and depleted of Vβ14+ T cells, mice were euthanized and BAL was performed. Differential staining of the BAL cells demonstrated profound eosinophilia in mice primed with FI-RSV or with vvGs and treated with IgM (Fig. 2). Depletion of Vβ14+ T cells significantly reduced the degree of eosinophils in the BAL of vvGs-immunized mice as evaluated by either the percentage (Fig. 2A) or the number of eosinophils present (Fig. 2B) in vvGs-immunized mice (P < 0.01, comparing mice treated with anti-Vβ14 to those treated with IgM isotype control antibody). In contrast, depletion of Vβ14+ T cells in FI-RSV-immunized mice resulted in an increase in the percentage of eosinophils in the BAL compartment (Fig. 2A), although the difference was not statistically significant (P = 0.18). However, the total number of eosinophils (Fig. 2B) was similar between the two treatment groups of FI-RSV-immunized mice. Similar patterns were observed upon histopathologic examination of the lung tissue. Specifically, in vvGs-immunized mice, but not in FI-RSV-primed mice, depletion of Vβ14+ T cells decreased the number of eosinophils recruited to the lung after RSV challenge (data not shown). These data demonstrate that Vβ14+ T cells are involved in the recruitment of eosinophils to the lungs of RSV-challenged vvGs-immunized mice while this subset of T cells plays no significant role in pulmonary eosinophilia of FI-RSV-immunized mice.
FIG. 2.
Depletion of Vβ14+ T cells reduces pulmonary eosinophilia in vvGs-immunized mice but not in FI-RSV-immunized mice. Mice were immunized with vvGs or FI-RSV and challenged with live RSV 6 weeks later. At days −1, 2, and 5 around RSV challenge, anti-Vβ14 antibody or IgM isotype control was administered. Seven days after challenge, BAL was performed, cells were stained, and differential cell counts were performed. The data represent the means ± standard deviations of the percentage of eosinophils (A) and the total number of eosinophils present in the BAL compartment (B). n = 5 mice per group from one of two experiments. Statistical analyses resulted in the following P values when IgM- and anti-Vβ14-treated mice from each priming group were compared. For vvGs-primed mice, P = 0.008 and 0.04 (percentage of eosinophils and total number of eosinophils, respectively). For FI-RSV-immunized mice, P = 0.176 and 0.91 (percentage of eosinophils and total number of eosinophils, respectively).
Significant levels of type 2 cytokines and chemokines are produced in FI-RSV-immunized mice following RSV challenge.
Protein levels of cytokines and chemokines in the lungs were measured by ELISA 4 and 7 days after challenge of immunized mice. IL-4, IL-5, IL-13, IFN-γ, eotaxin, MIP-1α, and MIP-1β production were examined in lung supernatants (Tables 2 and 3, respectively). At day 4 postchallenge (Table 2), all cytokines and chemokines but IL-4 and IL-5 were significantly reduced in vvGs-immunized mice by the depletion of Vβ14+ T cells, whereas only MIP-1β levels were reduced in FI-RSV-immunized mice by anti-Vβ14 treatment. In contrast, at day 7 postchallenge, the levels of nearly all cytokines in anti-Vβ14-treated and IgM-treated control mice were similar in both vvGs- and FI-RSV-immunized groups (Table 3). Only MIP-1β levels in vvGs-immunized mice were significantly altered by the depletion of Vβ14+ T cells. Thus, Vβ14+ T cells contribute to the production of type 2 cytokines and chemokines in vvGs-immunized mice but appear to play no significant role in cytokine production following RSV challenge in FI-RSV-immunized mice.
TABLE 2.
Day 4-cytokine and chemokine levelsa
| Cytokine or chemokine | Cytokine or chemokine level (pg/ml) in:
|
|||
|---|---|---|---|---|
| vvGs-immunized mice treated with:
|
FI-RSV-immunized mice treated with:
|
|||
| IgM | anti-Vβ14 | IgM | anti-Vβ14 | |
| Cytokine | ||||
| IL-4 | 196.7 ± 79.4 | 47.4 ± 18.0 | 84.2 ± 16.2 | 144.1 ± 49.1 |
| IL-5 | 112.7 ± 15.2 | 113.2 ± 22.5 | 190.0 ± 17.4c | 192.7 ± 54.3 |
| IL-13 | 390.6 ± 75.7 | 135.4 ± 36.1b | 377.2 ± 49.6 | 453.7 ± 101.7c |
| IFN-γ | 2417.0 ± 141.5 | 968.2 ± 326.0b | 2,224.8 ± 282.4 | 1,905.3 ± 593.5 |
| Chemokine | ||||
| Eotaxin | 585.6 ± 67.8 | 363.5 ± 57.4b | 1,015.6 ± 85.7c | 935.8 ± 204.7c |
| MIP-1α | 1,036.9 ± 132.5 | 415.1 ± 156.0b | 413.5 ± 53.5c | 416.5 ± 91.6 |
| MIP-1β | 1,122.44 ± 44.8 | 521.0 ± 139.1b | 759.0 ± 39.1c | 517.0 ± 88.9b |
Sandwich ELISAs were used to detect cytokine and chemokine levels in lung supernatants at day 7 after challenge. Data are represented as means ± standard deviation of cytokine or chemokine levels in picograms/milliliter. The limit of detection is 20 pg/ml for all kits but IFN-γ, where the limit of detection is 50 pg/ml.
Statistically different from IgM-treated controls of same priming group with the following P values: for vvGs-primed mice, P = 0.016 for IL-13, P = 0.004 for IFN-γ, P = 0.037 for eotaxin, P = 0.016 for MIP-1α, and P = 0.003 for MIP-1β; for FI-RSV-primed mice, P < 0.001 for MIP-1β.
Statistically different from vvGs-immunized mice with the following P values: for IgM-treated groups, P = 0.010 for IL-5, P = 0.004 for cotaxin, P = 0.002 for MIP-1α, and P < 0.001 for MIP-1β; for anti-Vβ14-treated groups, P = 0.018 for IL-13 and P = 0.027 for eotaxin.
TABLE 3.
Day 7 cytokine and chemokine levelsa
| Cytokine or chemokine | Cytokine or chemokine level (pg/ml) in:
|
|||
|---|---|---|---|---|
| vvGs-immunized mice treated with:
|
FI-RSV-immunized mice treated with:
|
|||
| IgM | anti-Vβ14 | IgM | anti-Vβ14 | |
| Cytokine | ||||
| IL-4 | 54.1 ± 34.1 | 20.0 ± 0.0 | 20.0 ± 0.0 | 23.2 ± 3.2 |
| IL-5 | 61.2 ± 15.0 | 36.1 ± 3.4 | 43.7 ± 11.0 | 29.7 ± 4.5 |
| IL-13 | 39.5 ± 7.2 | 21.9 ± 5.8 | 48.0 ± 8.8 | 67.7 ± 20.1 |
| IFN-γ | 1,895.1 ± 331.5 | 1,513.8 ± 388.5 | 337.3 ± 76.9 | 161.8 ± 20.7 |
| Chemokine | ||||
| Eotaxin | 621.6 ± 62.2 | 454.2 ± 60.7 | 751.7 ± 48.1 | 1,051.9 ± 204.7 |
| MIP-1α | 480.3 ± 35.4 | 356.3 ± 103.6 | 338.7 ± 36.8 | 105.9 ± 19.8 |
| MIP-1β | 702.6 ± 33.3 | 505.1 ± 73.9b | 338.7 ± 33.8c | 280.9 ± 28.7 |
Sandwich ELISAs were used to detect cytokine and chemokine levels in lung supernatants at day 7 after challenge. Data are represented as means ± standard deviation of cytokine or chemokine levels in picograms/milliliter. The limit of detection is 20 pg/ml for all kits but IFN-γ, where the limit of detection is 50 pg/ml.
Statistically different from IgM-treated controls of same priming group with the following P value: for vvGs-primed mice, P = 0.041 for MIP-1β.
Statistically different from vvGs-immunized mice with the following P value: for IgM-treated groups, P = 0.040 for MIP-1β.
Deletion of Vβ14+ T cells significantly decreases viral titers following RSV challenge in vvGs-primed mice.
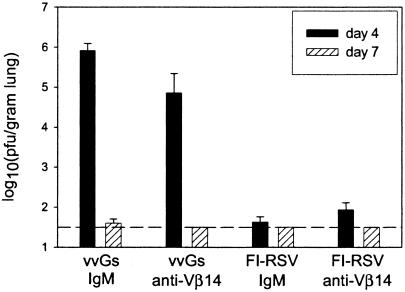
Peak viral replication occurs 4 days after primary RSV infection, and virus clearance occurs between days 7 and 9 in the BALB/c mouse model of RSV (14). To assess the contribution of Vβ14+ T cells to viral clearance, RSV titers in the lung were measured at days 4 and 7 postchallenge. Anti-Vβ14-treated vvGs-immunized mice had lower RSV titers than did IgM-treated mice at both days 4 and 7 after challenge (Fig. 3). While the difference at day 4 postchallenge was statistically significant, high titers of RSV were still present in the lung. In contrast, FI-RSV-immunized mice depleted of Vβ14+ T cells had higher RSV titers at day 4 postchallenge than did IgM-treated controls, although the difference was not significant. All FI-RSV-immunized mice cleared the virus by day 7 postchallenge. These data demonstrate a minimal role for Vβ14+ T cells in RSV clearance in either vvGs- or FI-RSV-immunized mice.
FIG. 3.
RSV titers following challenge are minimally altered by depletion of Vβ14+ T cells. Mice were immunized, treated with anti-Vβ14 antibody, and challenged as described in the legend of Fig. 2. At days 4 and 7 postchallenge, viral titers were measured in subsets of mice from each group as described in Materials and Methods. Data are represented as the means ± standard deviations of the log10 PFU/gram of lung tissue. The limit of detection is 1.8 log10 PFU/g of lung tissue. n = 5 mice per group from one of two experiments.
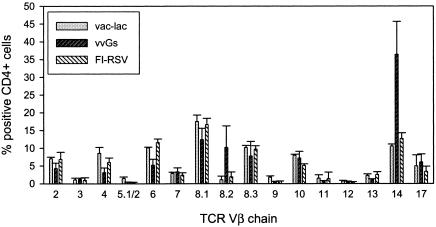
FI-RSV-immunized mice exhibit diverse TCR Vβ receptor usage and do not respond to any single RSV peptide, in contrast to the restricted Vβ14 TCR expression and peptide specificity in vvGs-immunized mice.
Immunization of BALB/c mice with vvGs induces immune responses mediated by an expanded oligoclonal CD4+-T-cell subset that predominantly express the Vβ14 TCR (54). Since immunization with FI-RSV or with vvGs results in very similar patterns of disease, we examined the Vβ TCR diversity of CD4+ T cells from FI-RSV- and vvGs-immunized mice. While a predominance of CD4+ T cells in vvGs-immunized mice were positive for the Vβ14 chain of the TCR, the cells in FI-RSV-immunized mice expressed a balanced array of Vβ chains (Fig. 4).
FIG. 4.
Vβ chain usage is restricted in vvGs-immunized mice but diverse in FI-RSV-immunized mice. Mice were immunized with vvGs or FI-RSV and challenged with live RSV. Five days after challenge, lung lymphocytes were isolated and stained for Vβ chain expression. The stained cells were analyzed by flow cytometry.
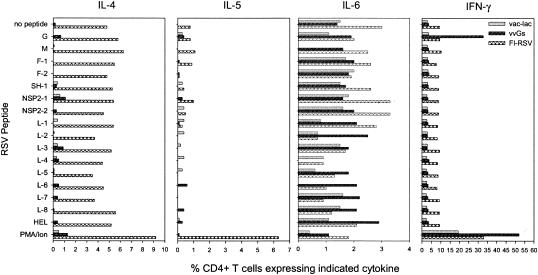
An I-Ed-restricted epitope has been identified in RSV G, and this peptide stimulates both type 1 and type 2 cytokine production (47, 55). Therefore, we examined the ability of a panel of I-Ed-restricted RSV-derived peptides (Table 1) to stimulate cytokine production in FI-RSV- and vvGs-immunized mice. While the majority of the CD4+ T cells in vvGs-immunized mice responded only to the G peptide at aa 183 to 195, CD4+ T cells from FI-RSV-immunized mice responded with a different pattern (Fig. 5). No single peptide used in these studies elicited cytokine production that was greater than that observed during primary RSV infection of vac-lac-immunized and RSV-challenged mice (Fig. 5). Similar patterns of production were observed for IL-4, IL-5, and IL-6. Together, these data demonstrate that vvGs immunization elicits a restricted subset of T cells specific for aa 183 to 195 of RSV G that mediate disease, whereas FI-RSV disease is the result of a diverse set of T cells with unidentified and potentially diverse peptide specificities.
FIG. 5.
CD4+ T cells from vvGs-immunized mice respond primarily to a single peptide, whereas CD4+ T cells from FI-RSV-immunized mice respond to a diverse array of I-Ed-restricted peptides. Mice were immunized with vvGs or FI-RSV and challenged with RSV. Lung lymphocytes were isolated 5 days after challenge and stimulated with a panel of I-Ed-restricted peptides, and intracellular cytokine levels were measured after staining with fluorescently labeled anticytokine antibody. PMA/Iono, positive control cells treated with phorbol myristate acetate and ionomycin.
DISCUSSION
With an increasing incidence of severe disease and increased rates of hospitalization in both infants and the elderly, the development of a safe and effective vaccine against RSV is of tremendous importance. However, an incomplete understanding of RSV pathogenesis and the failed vaccine trials of the 1960s hinder the accomplishment of this goal. With its role as the viral attachment protein (31) and as the source of much of the sequence variation between virus strains (20, 21), it is desirable to include the G glycoprotein as a component of any RSV immunogen. However, the similar patterns of severe disease induced by immunization with FI-RSV or with RSV G complicate the question of the safety of a vaccine construct containing RSV G. While disease in FI-RSV- and G-immunized animals may appear to be analogous, there are several indications that they have distinct pathogenic mechanisms that lead to a common final pathway resulting in enhanced disease.
It has been clearly demonstrated that both immunization with FI-RSV (9, 13, 35, 41, 50) and immunization with RSV G (4, 17, 24, 38, 45, 46) predispose for severe disease typified by severe illness, pulmonary eosinophilia, and type 2 cytokine production. However, although the endpoints may be similar, it is apparent that the cytokine requirements for these two immunogens are distinct. While illness and type 2 cytokine production are reduced in RSV-challenged FI-RSV-immunized mice with IL-4 depletion (23, 48), the enhanced disease is unaltered in G-primed mice when IL-4 function is inhibited either by antibody depletion or in IL-4-deficient mice (23, 25). Furthermore, inhibition of IL-13 alone modulates disease only in FI-RSV-primed mice (25). Both IL-4 and IL-13 function must be blocked to modulate disease in mice immunized with RSV G (25). Additionally, when RSV glycoprotein F is administered as a purified protein in the context of alum, immune responses that result in eosinophilia and IL-4 and IL-5 production following RSV challenge are induced (8, 17, 35). However, in contrast to G-specific responses, F-specific immune responses may be modified by Th1-modulating adjuvants such as monophosphoryl lipid A or QS-21 (17, 35). Thus, the ability to induce disease-enhancing immune responses is not restricted to RSV G but appears to be a consequence of parenteral administration of RSV antigen and presentation to the immune system as a soluble protein that cannot be processed via the major histocompatibility complex class I pathway.
Vaccine-enhanced disease was observed in approximately 80% of FI-RSV vaccinees in the 1960s trial (26, 28) and occurs in many animal models (5, 9, 24, 25, 40, 41, 50). In contrast, RSV G-induced immune responses associated with vaccine-enhanced illness exhibit some degree of genetic restriction, with pulmonary eosinophilia being absent (19, 47) or dramatically reduced (23) in vvGs-immunized mouse strains other than BALB/c. The peptide at aa 183 to 198 of the RSV G glycoprotein is sufficient to elicit pulmonary eosinophilia and both type 1 and type 2 cytokine production in BALB/c mice (44, 47, 51) and is largely restricted to a subset of CD4+ T cells expressing the Vβ14 TCR (54). The existence of this immunodominant epitope that alone is sufficient to induce those immune responses associated with vaccine-enhanced disease underscores the phenomenon of genetically restricted RSV G immunogenicity, which is not consistent with the nearly universal induction of vaccine-enhanced illness by the FI-RSV vaccine in children less than 6 months of age (28).
These observations, together with the distinct cytokine requirements in FI-RSV- and vvGs-immunized mice, led us to hypothesize that while Vβ14+ CD4+ T cells mediate much of the eosinophil recruitment and cytokine production in vvGs-primed RSV-challenged mice, such an oligoclonal T-cell subset would not be expanded and would not significantly contribute to vaccine-enhanced disease in FI-RSV-immunized mice. As reported previously (54), these data confirm that the depletion of Vβ14+ T cells at the time of challenge of vvGs-immunized mice decreases the degree of pulmonary eosinophilia and type 2 cytokine production. In marked contrast, however, anti-Vβ14 treatment of FI-RSV-immunized mice has little impact on any aspect of disease examined in these studies. Furthermore, analyses of TCR expression and T-cell specificity underscore the distinct differences in T-cell subsets. Whereas vvGs-immunized mice show a prevalence of Vβ14+ CD4+ T cells that are specific for a single immunodominant epitope encompassing aa 183 to 195 of RSV G, RSV challenge of FI-RSV-immunized mice amplifies CD4+ T cells expressing a diverse Vβ TCR repertoire with no predominance for an individual peptide apparent. This finding suggests that T cells induced by FI-RSV immunization are specific for an as-yet-unidentified RSV peptide or that they are specific for a diverse array of peptides.
It is not necessarily unexpected to see a selected expansion of a particular Vβ-restricted T-cell subset upon exposure to RSV G. Pathogenic roles for specific T-cell subsets in several models of infection and in various autoimmune diseases, including a disease-causing population of Vβ14+ CD8+ T cells in a mouse model of ulcerative colitis (36), have been described (6, 11, 12, 32, 36, 37). Several of those studies describe a protective effect of selected depletion of the particular T-cell subset associated with disease without any toxicity or observable effect in normal populations (6, 11). In fact, the deletion of the disease-causing T-cell subsets is an effective treatment for at least two autoimmune syndromes in humans (6, 37). Thus, while studies have not described the effects of Vβ14+-T-cell depletion in normal uninfected mice, these studies and the data reported here (for RSV-challenged mice primed with vac-lac or FI-RSV) would suggest that the elimination of this T-cell subset does not adversely affect the general immune status of mice.
These data demonstrate that distinct immune responses mediated by discrete T-cell subsets result in pulmonary eosinophilia and type 2 cytokine production in vvGs- and FI-RSV-immunized mice. Thus, the induction of a single oligoclonal G-specific CD4+-T-cell subset is not the basis for vaccine-enhanced disease induced by FI-RSV immunization. Therefore, these data suggest that RSV G may be safely included in a vaccine product if the potential vaccine is properly formulated to target RSV antigens to endogenous antigen-processing and presentation pathways. Furthermore, these data confirm that a restricted T-cell subset mediates RSV G-specific immune responses in BALB/c mice. In contrast, within the parameters utilized in this study, these data do not support the hypothesis that FI-RSV immunization induces oligoclonal T-cell subsets with restricted peptide specificities and suggest that either the peptide specificity in FI-RSV-immunized mice has yet to be identified or that FI-RSV-induced responses have diverse specificities. These observations may suggest that FI-RSV vaccine-enhanced disease will be produced in nearly all populations with diverse genetic backgrounds. In contrast, the severe disease of RSV G-immunized RSV-challenged mice, which is clearly genetically restricted, may be comparable to the severe disease of children during primary infection. Information gained from an investigation into the pathogenic mechanisms of RSV G-induced disease may provide insights into the immunologic basis for severe primary RSV disease in humans, while further research is necessary to understand the underlying mechanisms of FI-RSV vaccine-enhanced disease and to aid in the proper formulation of a safe vaccine product.
Acknowledgments
This work was funded in part by National Institutes of Health grants AI-37293 (TJB) and HL-33391 (TJB) and by a grant from the Parker B. Francis Foundation (SMV).
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. M. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangham, C. R. M., M. J. Cannon, D. T. Karzon, and B. A. Askonas. 1985. Cytotoxic T-cell response to respiratory syncytial virus in mice. J. Virol. 56:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangham, C. R. M., P. J. M. Openshaw, A. Ball, A. M. Q. King, G. W. Wertz, and B. A. Askonas. 1986. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotien (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J. Immunol. 137:3973-3977. [PubMed] [Google Scholar]
- 4.Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473-2480. [PubMed] [Google Scholar]
- 5.Cannon, M. J., P. J. M. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrion, F., M. Fernandez, M. Iruretagoyena, L. E. Coelho Andrade, M. Odete-Hilario, and F. Figueroa. 2003. Selective depletion of Vβ2+CD8+ T cells in peripheral blood from rheumatic heart disease patients. J. Autoimmun. 20:183-190. [DOI] [PubMed] [Google Scholar]
- 7.Cherrie, A. H., K. Anderson, G. W. Wertz, and P. J. M. Openshaw. 1992. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J. Virol. 66:2102-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors, M., P. L. Collins, C.-Y. Firestone, A. V. Sotnikov, A. Waitze, A. R. Davis, P. P. Hung, R. M. Chanock, and B. R. Murphy. 1992. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine 10:475-484. [DOI] [PubMed] [Google Scholar]
- 9.Connors, M., N. A. Giese, A. B. Kulkarni, C.-Y. Firestone, I. Morse, and B. R. Murphy. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 68:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaddum, R. M., R. S. Cook, J. M. Furze, S. A. Ellis, and G. Taylor. 2003. Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes. Immunology 108:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez, F. J., J. A. Cain, R. S. Gibbons, R. Allendoerfer, and G. S. Deepe, Jr. 1998. Vbeta4(+) T cells promote clearance of infection in murine pulmonary histoplasmosis. J. Clin. Investig. 102:984-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez, F. J., E. O. Woodward, R. Pilcher-Roberts, R. S. Gibbons, and G. S. Deepe, Jr. 2001. Vβ6+ and Vβ4+ T cells exert cooperative activity in clearance of secondary infection with Histoplasma capsulatum. J. Immunol. 166:2855-2862. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., G. S. Henderson, Y.-W. Tang, X. Lu, K. M. Neuzil, and D. G. Colley. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 151:2032-2040. [PubMed] [Google Scholar]
- 14.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 15.Graham, B. S., J. A. Rutigliano, and T. R. Johnson. 2002. Respiratory syncytial virus immunobiology and pathogenesis. Virology 297:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks, D. A., K. Baradaran, K. McIntosh, and J. L. Patterson. 1987. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J. Gen. Virol. 68:1705-1714. [DOI] [PubMed] [Google Scholar]
- 19.Hussell, T., A. Georgiou, T. E. Sparer, S. Matthews, P. Pala, and P. J. M. Openshaw. 1998. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J. Immunol. 161:6215-6222. [PubMed] [Google Scholar]
- 20.Johnson, P. R., R. A. Olmsted, G. A. Prince, B. R. Murphy, D. W. Alling, E. E. Walsh, and P. L. Collins. 1987. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 61:3163-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 84:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, T. R., J. E. Fischer, and B. S. Graham. 2001. Construction and characterization of recombinant vaccinia viruses co-expressing a respiratory syncytial virus protein and a cytokine. J. Gen. Virol. 82:2107-2116. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, T. R., R. A. Parker, J. E. Johnson, and B. S. Graham. 2003. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 170:2037-2045. [DOI] [PubMed] [Google Scholar]
- 26.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shvedoff, and C. E. Stewart. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405-421. [DOI] [PubMed] [Google Scholar]
- 27.Katz, M. E., R. M. Maizels, L. Wicker, A. Miller, and E. E. Sercarz. 1982. Immunological focusing by the mouse major histocompatibility complex: mouse strains confronted with distantly related lysozymes confine their attention to very few epitopes. Eur. J. Immunol. 12:535-540. [DOI] [PubMed] [Google Scholar]
- 28.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 29.Kim, H. W., S. L. Leikin, J. Aerobic, C. D. Brandt, R. M. Chanock, and R. H. Parrott. 1976. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr. Res. 10:75-78. [DOI] [PubMed] [Google Scholar]
- 30.Lambert, D. M. 1988. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology 16:458-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine, S., R. Franco-Klaiber, and P. R. Paradiso. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521-2524. [DOI] [PubMed] [Google Scholar]
- 32.Mendes-da-Cruz, D. A., J. de Meis, V. Cotta-de-Almeida, and W. Savino. 2003. Experimental Trypanosoma cruzi infection alters the shaping of the central and peripheral T-cell repertoire. Microb. Infect. 5:825-832. [DOI] [PubMed] [Google Scholar]
- 33.Morgan, L. A., E. G. Routledge, M. M. Willcocks, A. C. R. Samson, R. Scott, and G. L. Toms. 1987. Strain variation of respiratory syncytial virus. J. Gen. Virol. 68:2781-2788. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, B. R., D. W. Alling, M. H. Snyder, E. E. Walsh, G. A. Prince, R. M. Chanock, V. G. Hemming, W. J. Rodriguez, H. W. Kim, B. S. Graham, and P. F. Wright. 1986. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J. Clin. Microbiol. 24:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuzil, K. M., J. E. Johnson, Y.-W. Tang, J.-P. Prieels, M. Slaoui, N. Gar, and B. S. Graham. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525-532. [DOI] [PubMed] [Google Scholar]
- 36.Nitta, T., H. Iwata, Y. Mori, H. Takagi, K. Kanetake, Y. Iida, K. Sakamoto, T. Yamada, M. Saio, and H. Hirose. 2003. Specific CTL activity of CD8+ TCR Vbeta14+ T cell in mouse 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Dig. Dis. Sci. 48:2095-2103. [DOI] [PubMed] [Google Scholar]
- 37.Olsson, T., C. Edenius, M. Ferm, P. Samuelson, A. Torrang, E. Wallstrom, M. Khademi, M. Andersson, and L. Arfors. 2002. Depletion of Vbeta5.2/5.3 T cells with a humanized antibody in patients with multiple sclerosis. Eur. J. Neurol. 9:153-164. [DOI] [PubMed] [Google Scholar]
- 38.Openshaw, P. J. M., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4:493-500. [DOI] [PubMed] [Google Scholar]
- 39.Peebles, R. S. J., K. Hashimoto, and B. S. Graham. 2003. The complex relationship between respiratory syncytial virus and allergy in lung disease. Viral Immunol. 16:25-34. [DOI] [PubMed] [Google Scholar]
- 40.Piedra, P. A., H. S. Faden, G. Cammussi, D. T. Wong, and P. L. Ogra. 1989. Mechanism of lung injury in cotton rats immunized with formalin-inactivated respiratory syncytial virus. Vaccine 124:34-38. [DOI] [PubMed] [Google Scholar]
- 41.Prince, G. A., A. B. Jenson, V. G. Hemming, B. R. Murphy, E. E. Walsh, R. L. Horswood, and R. M. Chanock. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 57:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, S. R., D. L. Lichtenstein, L. A. Ball, and G. W. Wertz. 1994. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J. Virol. 68:4538-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 44.Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A. Melero, and P. J. M. Openshaw. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 187:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J. Virol. 71:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikiatkhachorn, A., W. Chang, and T. J. Braciale. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang, Y.-W., and B. S. Graham. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J. Clin. Investig. 94:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, Y.-W., and B. S. Graham. 1997. T cell source of Type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J. Clin. Investig. 99:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, Y.-W., K. M. Neuzil, J. E. Fischer, F. W. Robinson, R. A. Parker, and B. S. Graham. 1997. Determinants and kinetics of cytokine expression patterns in lungs of vaccinated mice challenged with respiratory syncytial virus. Vaccine 15:597-602. [DOI] [PubMed] [Google Scholar]
- 51.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripp, R. A., S. Hou, N. Etchart, A. Prinz, D. Moore, J. Winter, and L. J. Anderson. 2001. CD4+ T cell frequencies and Th1/Th2 cytokine patterns expressed in the acute and memory response to respiratory syncytial virus I-Ed-restricted peptides. Cell. Immunol. 207:59-71. [DOI] [PubMed] [Google Scholar]
- 53.Tripp, R. A., D. Moore, L. Jones, W. M. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 15:637-646. [DOI] [PubMed] [Google Scholar]
- 55.Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487-6495. [DOI] [PubMed] [Google Scholar]