Significance
In the current study, we show that brown adipose tissue (BAT) activity is dramatically reduced in a dehydroepiandrosterone (DHEA)-induced polycystic ovary syndrome (PCOS) rat when compared with a normal control rat. Importantly, the key features of PCOS (such as insulin resistance and irregular estrous cycle) are alleviated after BAT transplantation. Mechanistically, transplanted BAT enhances endogenous BAT activity and thereby increases the circulating adiponectin level, which was lower in both the PCOS patient and PCOS rat model. Furthermore, exogenous adiponectin protein administration recapitulates beneficial effects from BAT transplantation in a PCOS rat. Taken together, these data highlight the important role of BAT in the development of PCOS and that BAT-induced adiponectin might open up a new way in the treatment of PCOS.
Keywords: brown adipose tissue, transplantation, ameliorates, PCOS, adiponectin
Abstract
Polycystic ovary syndrome (PCOS), which is characterized by anovulation, hyperandrogenism, and polycystic ovaries, is a complex endocrinopathy. Because the cause of PCOS at the molecular level is largely unknown, there is no cure or specific treatment for PCOS. Here, we show that transplantation of brown adipose tissue (BAT) reversed anovulation, hyperandrogenism, and polycystic ovaries in a dehydroepiandrosterone (DHEA)-induced PCOS rat. BAT transplantation into a PCOS rat significantly stabilized menstrual irregularity and improved systemic insulin sensitivity up to a normal level, which was not shown in a sham-operated or muscle-transplanted PCOS rat. Moreover, BAT transplantation, not sham operation or muscle transplantation, surprisingly improved fertility in PCOS rats. Interestingly, BAT transplantation activated endogenous BAT and thereby increased the circulating level of adiponectin, which plays a prominent role in whole-body energy metabolism and ovarian physiology. Consistent with BAT transplantation, administration of adiponectin protein dramatically rescued DHEA-induced PCOS phenotypes. These results highlight that endogenous BAT activity is closely related to the development of PCOS phenotypes and that BAT activation might be a promising therapeutic option for the treatment of PCOS.
Polycystic ovary syndrome (PCOS) is now recognized as one of the most common endocrine diseases in women of reproductive age. The prevalence of PCOS ranges from 9% to 18%, depending on the criteria used for its definition and ethnicity (1, 2). The core feature of PCOS includes polycystic ovaries, hyperandrogenism, and chronic anovulation. Furthermore, PCOS is a complex and heterogeneous syndrome because it is associated with a high risk for the development of insulin resistance, type 2 diabetes (T2D), obesity, dyslipidemia, and cardiovascular disease (3–5). There are three different criteria used for the diagnosis of PCOS: androgen excess, irregular menstruation, and polycystic ovary appearance on ultrasound after excluding other causes of hyperandrogenism and anovulation (6). Because a single etiologic factor is not able to fully account for all of the clinical features in PCOS, the pathogenesis of PCOS is largely unknown. Several genetic and environmental factors may contribute to the development of PCOS; however, the underlying cellular mechanism of the induction and progression of PCOS remains to be elucidated.
Insulin resistance, which is common among PCOS patients, seems to be a key etiological characteristic, and about 85% of women with PCOS suffer from insulin resistance (7). Compensatory hyperinsulinemia can directly stimulate ovarian and adrenal secretion of androgen and decrease hepatic sex hormone binding globulin (SHBG) synthesis, resulting in an increased bioavailability of free testosterone levels (8, 9). Thus, insulin resistance and hyperandrogenism contribute to the key clinical presentation of PCOS. Because the clinical features are complex and vary among PCOS patients, it is hard to provide the first-line treatment of PCOS. Most treatment guidelines recommend that patients change lifestyles, including exercise and dietary modification. Patients can take oral contraceptive pills (OCPs) to control symptoms of hyperandrogenism or take insulin-sensitizing medicines such as metformin or pioglitazone when they have impaired glucose tolerance or features of a metabolic syndrome (10). However, there is a lack of effective treatment for PCOS at present.
It has been reported that the functional abnormality of adipose tissue in PCOS patients is primarily linked to insulin resistance, even in the absence of obesity (11, 12). In humans and other mammals, there are mainly two types of adipose tissue with opposing functions: white adipose tissue (WAT) and brown adipose tissue (BAT). The main function of WAT is to store excess energy in WAT as a form of triglycerides whereas BAT contains large numbers of mitochondria that uncouple large amounts of fuel for heat generation and the maintenance of body temperature (13). Recent studies using positron emission tomography (PET) have demonstrated that human adults also possess metabolically active BAT (14, 15) and that BAT activation inversely correlates with age and body mass index (BMI) (16). Therefore, increasing BAT mass and/or function is a promising strategy to treat obesity and metabolic diseases. Indeed, studies by our group and others have shown that BAT transplantation reverses metabolic disorders in various obese mouse models (17–19).
Given the several common features between PCOS and a metabolic syndrome, we aimed to investigate whether BAT possibly plays an important role in the development of PCOS phenotypes and the treatment of PCOS. In the current study, we show that BAT activity was dramatically reduced in a dehydroepiandrosterone (DHEA) (a precursor of androgen)-induced PCOS rat compared with a normal control rat. Notably, the key features of PCOS, such as insulin resistance, irregular estrous cycle, and low birth rate, were significantly improved after BAT transplantation in PCOS rats. Interestingly, transplanted BAT in PCOS rats enhanced endogenous BAT activity and thereby increased the circulating adiponectin level, which was lower in both PCOS patients and PCOS rats. In parallel, exogenous adiponectin protein administration in a PCOS rat recapitulated the effects that were seen in a BAT-transplanted PCOS rat. Taken together, these data suggest that BAT is one of the important organs regulating the features of PCOS and that the increase of BAT mass or its activity might provide a new therapeutic strategy for the treatment of PCOS.
Materials and Methods
All animal studies were conducted with the approval of the Institutional Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences. Tissue (0.5 g of BAT or muscle) transplantation experiments were performed in a DHEA-induced PCOS rat. Recombinant adiponectin [10 μg/kg body weight (BW)] was daily injected into a PCOS rat. Written informed consent was obtained from all participants and this study was approved by the Institutional Review Board of Reproductive Medicine of Shandong University. Please refer to SI Materials and Methods for detailed information.
SI Materials and Methods
Human Subjects.
All participants in our study were recruited from the Center for Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong University, during September 2014 to March 2015. Anthropometric variables, such as age, body mass index (BMI), menstrual cycle, and select endocrine and biochemical parameters, were recorded and are shown in Table S3. The diagnosis of PCOS was based on the following revised Rotterdam diagnostic criteria for PCOS (37), which require the presence of at least two of the following: (i) oligo-ovulation and/or anovulation; (ii) clinical and/or biochemical signs of hyperandrogenism; and (iii) polycystic ovaries. Diagnoses of PCOS were made after exclusion of other etiologies for hyperandrogenemia and ovulatory dysfunction (e.g., 21-hydroxylase deficiency, congenital adrenal hyperplasia, Cushing syndrome, androgen-secreting tumors, thyroid disease, and hyperprolactinemia). All subjects in the control group had regular menstrual cycles and normal ovarian morphology, and total testosterone was evaluated to exclude hyperandrogenism. Peripheral blood samples were collected from all subjects during days 2–4 of spontaneous cycles after an overnight fast. FSH, LH, and testosterone were tested by chemiluminescence immunization (Beckman Access Health Company). The hematological biochemical variables were measured at fasting. A 75-g oral glucose tolerance test (OGTT) was carried out, and the plasma glucose and serum insulin at fasting and 2-h postload were measured. The serum was centrifugally separated from procoagulant peripheral blood. Written informed consent was obtained from all participants and this study was approved by the Institutional Review Board of Reproductive Medicine of Shandong University.
Table S3.
Fertility assessment in tissue transplantation experiment
| Group | Total no. | Pregnancy and parturition |
| Control | 9 | 9 |
| DHEA+sham | 8 | 2 |
| DHEA+Mus | 7 | 2 |
| DHEA+BAT | 7 | 6 |
Animals.
Female and male Sprague–Dawley rats (3 wk old) were purchased from Vital River Laboratory Animal Technology Co Ltd. Five rats per cage were housed under constant environmental conditions in the Office of Laboratory Animal Welfare-certified animal facility with a 12-h light–dark cycle. Food and water were provided ad libitum. All animal studies were conducted with the approval of the Institutional Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Establishment of the PCOS Model and Assessment of the Estrous Cycle.
The DHEA used for establishing the PCOS model was purchased from Yangzhou Pharmaceutical Co., Ltd (cat. no. H10940064). From 4 wk of age, female rats were injected daily (s.c.) with DHEA (6 mg/100 g body weight) dissolved in 0.2 mL of PBS for 20 consecutive days. Control rats were injected with 0.2 mL of PBS for an equivalent length of time. The successful PCOS rat model was selected according to the previous criteria (38), which was assessment of estrous cycle by vaginal cytology for eight consecutive days, together with a glucose tolerance test (GTT) for the following experiment.
Tissue Transplantation.
The experiment was conducted according to the methods described previously (17, 18). The operation was carried out in sterile conditions. Age- and sex-matched donor and recipient rats were used for the tissue transplantation experiment. BAT and part of the quadriceps were harvested from the donor rats, which were anesthetized with avertin (400 mg/kg body weight i.p.), and were placed in sterile saline. The recipient rats were anesthetized with avertin (400 mg/kg body weight i.p.), and the donor tissues (0.5 g for each recipient rat) were transplanted into the s.c. space of the dorsal region adjacent to the endogenous BAT as quickly as possible. The sham-operated rats underwent the same procedure, except receiving donor tissues.
Adiponectin Protein Treatment.
Recombinant adiponectin protein (50636-M08H-100) was purchased from Sino Biological Inc. PCOS rats were treated with exogenous recombinant adiponectin protein (10 μg/kg body weight/day i.p.) once a day for 20 consecutive days. The control group was treated with sterile PBS.
Glucose Tolerance Test and Insulin Tolerance Test.
For glucose tolerance tests (GTTs), female rats were fasted for 16 h (1700 to 0900), with free access to drinking water, and injected with d-glucose (2.0 g/kg body weight) intraperitoneally. Blood glucose level was measured before and 15, 30, 60, 90, and 120 min after i.p. glucose injection by using an Accu-Chek glucose monitor (Roche Diagnostics Corp.). For the insulin tolerance test (ITT), female rats were fasted for 4 h (0900 to 1300), with free access to drinking water, and injected with insulin (1 U/kg body weight) (Humulin; Eli Lilly) intraperitoneally. Blood glucose levels were measured before and 15, 30, and 60 min after insulin injection.
Resting Metabolic Rate.
The female rats were housed with one rat per cage, with free access to food and water. Metabolic rate was determined by oxygen consumption measurement performed for two consecutive days using a TSE laboratory master system as previously described (39).
Infrared Thermography and Core Temperature.
Rats were exposed to a cold chamber (4 °C) with one rat per cage for up to 4 h, with free access to food and water. Images were taken using an infrared digital thermographic camera (E60: Compact Infrared Thermal Imaging Camera; FLIR) and were analyzed using FLIR Quick Report software (FLIR ResearchIR Max 3.4; FLIR). Core body temperature was measured using a rectal probe connected to a digital thermometer (Yellow Spring Instruments).
Micro PET/CT.
PET/CT imaging was achieved with the Siemens Inveon Dedicated PET (dPET) System and Inveon Multimodality (MM) System (CT/SPECT) (Siemens Preclinical Solutions) at the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences. Mice were allowed to fast overnight and were lightly anesthetized with isoflurane, followed by a tail vein injection of 18F-FDG (500 mCi). Mice were subjected to PET/CT analysis at 60 min after radiotracer injection. Inveon Acquisition Workplace (IAW) software was used for the scanning process. A 10-min CT X-ray for attenuation correction was scanned with a power of 80 kV and 500 μA and an exposure time of 1,100 ms before PET scan. Ten-minute static PET scans were acquired, and images were reconstructed by an OSEM3D algorithm followed by Maximization/Maximum a Posteriori (MAP) or FastMAP provided by IAW. The 3D regions of interest (ROIs) were drawn over the guided CT images, and the tracer uptake was measured using the software of Inveon Research Workplace (IRW) (Siemens). Individual quantification of the 18F-FDG uptake in each of the ROIs was calculated. The data for the accumulation of 18F-FDG on micro PET images were expressed as the standard uptake values (SUVs), which were determined by dividing the relevant ROI concentration by the ratio of the injected activity to the body weight. The data are presented as the mean ± SEM.
Fertility Assessment.
To examine fertility, female rats mated with proven stud males. Next day, successful mating was judged by observation of a vaginal plug. After 10 d, the few female rats were killed and were examined at implantation sites to confirm pregnancy. The rest of the animals were allowed to undergo natural delivery to produce pups.
Blood Analysis.
The blood samples were collected by cardiac exsanguination under Avertin anesthesia, and the plasma samples were frozen and stored at −80 °C until further analysis. Rat plasma levels of LH, FSH, and insulin were analyzed using ELISA kits (NanJing Jian Cheng Bioengineering Institute). The plasma level of adiponectin in both rat and human samples was analyzed using ELISA kits (R&D Systems).
Gene Expression Analysis.
Total RNA was isolated using the RNeasy Mini Kit. The cDNA was synthesized using random hexamers (Invitrogen) for subsequent real-time quantitative PCR analysis (ABI Prism VIIA7; Applied Biosystems). PCR products were detected using SYBR Green and normalized by cyclophilin expression. Primers were designed using Primer Quest (Integrated DNA Technologies).
Western Blot Analysis.
Tissues were dissolved in RIPA buffer (150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, protease and phosphatase inhibitor mixture (Roche Diagnostics). Protein concentrations were determined using a BCA assay kit (Pierce Diagnostics). Protein was separated by 10% (wt/vol) SDS/PAGE, transferred to a PVDF membrane (Millipore), blocked in 5% (wt/vol) skim milk in TBST (0.02 M Tris base, 0.14 M NaCl, 0.1% Tween 20, pH 7.4), and incubated with primary antibodies overnight at 4 °C and then incubated with secondary antibodies conjugated with HRP. The following primary antibodies were used: anti-UCP1 (1:1,000; Abcam), anti-OXPHOS (1:250; Abcam), and anti-GAPDH (1:1,000; Cell Signaling Technology). Signals were detected with super signal west pico chemiluminescent substrate (Pierce).
Histology and Immunohistochemistry Analysis.
Tissues were fixed in 4% paraformaldehyde overnight at room temperature and then embedded in paraffin. Sections of 5 μm thickness were stained with hematoxylin and eosin (H&E), and then images were taken by microscope (DS-RI1; Nikon). The number of antral follicles (600–1,000 µm in diameter) and corpora lutea were counted based on morphology and diameter. The thickness of theca cell layers was measured using ImageJ software (version 1.48; NIH) (n = 6 per group, serial sections of each ovary were used for measurement). The standard streptavidin-biotin-peroxide immunostaining procedure was used for the detection of tyrosine hydroxylase (TH). Tissue specimens were blocked with 10% normal goat serum for 60 min and then incubated with anti-UCP1 (1:400 dilution; Santa Cruz Biotechnologies) or anti-TH (1:400 dilution; Pel FreeZ) antibody overnight at 4 °C, followed by a 1-h incubation with HRP-conjugated goat anti-rabbit IgG at room temperature.
Statistical Analysis.
Comparisons between groups were made by one-way ANOVA with Tukey’s post hoc test or Student’s t tests. A difference between groups of P < 0.05 was considered significant.
Results
BAT Transplantation Reverses Reduced BAT Activity and Metabolic Abnormality in the PCOS Rat.
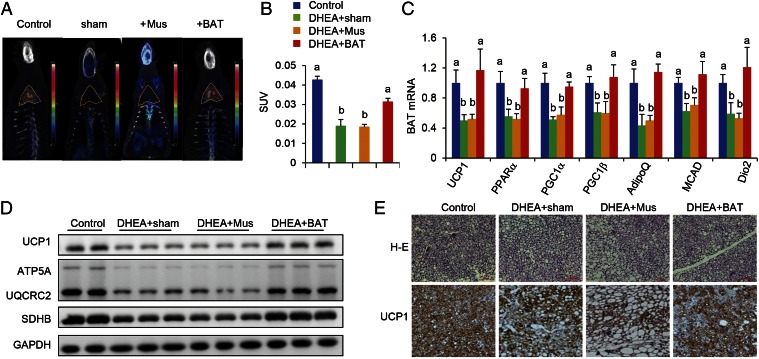
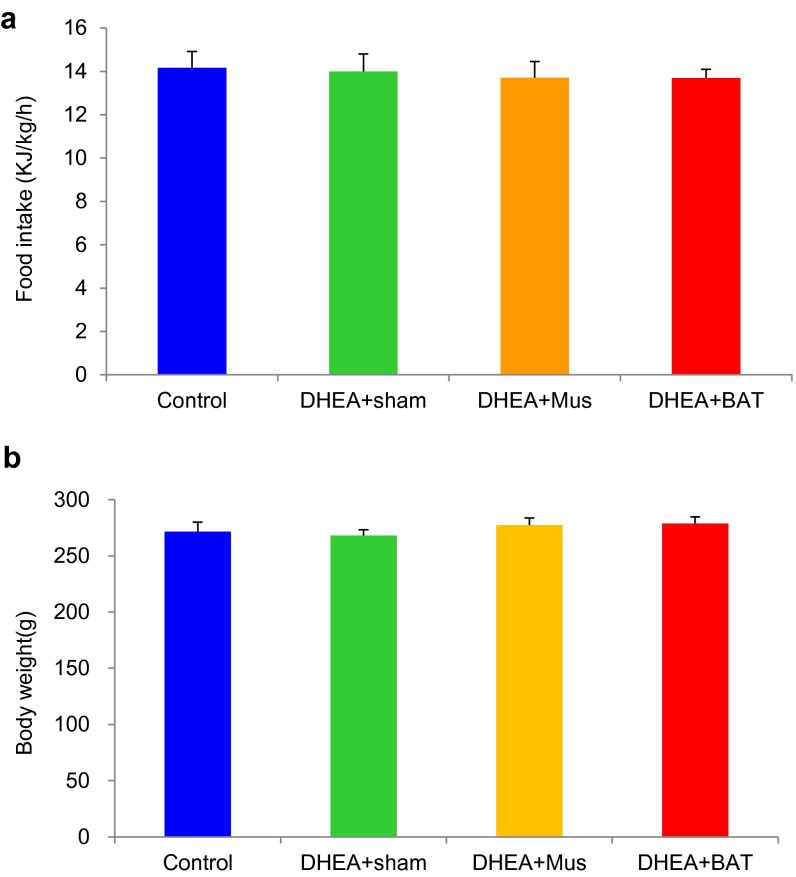
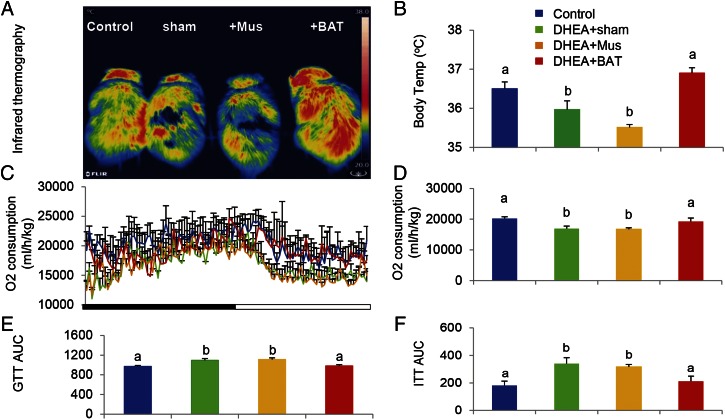
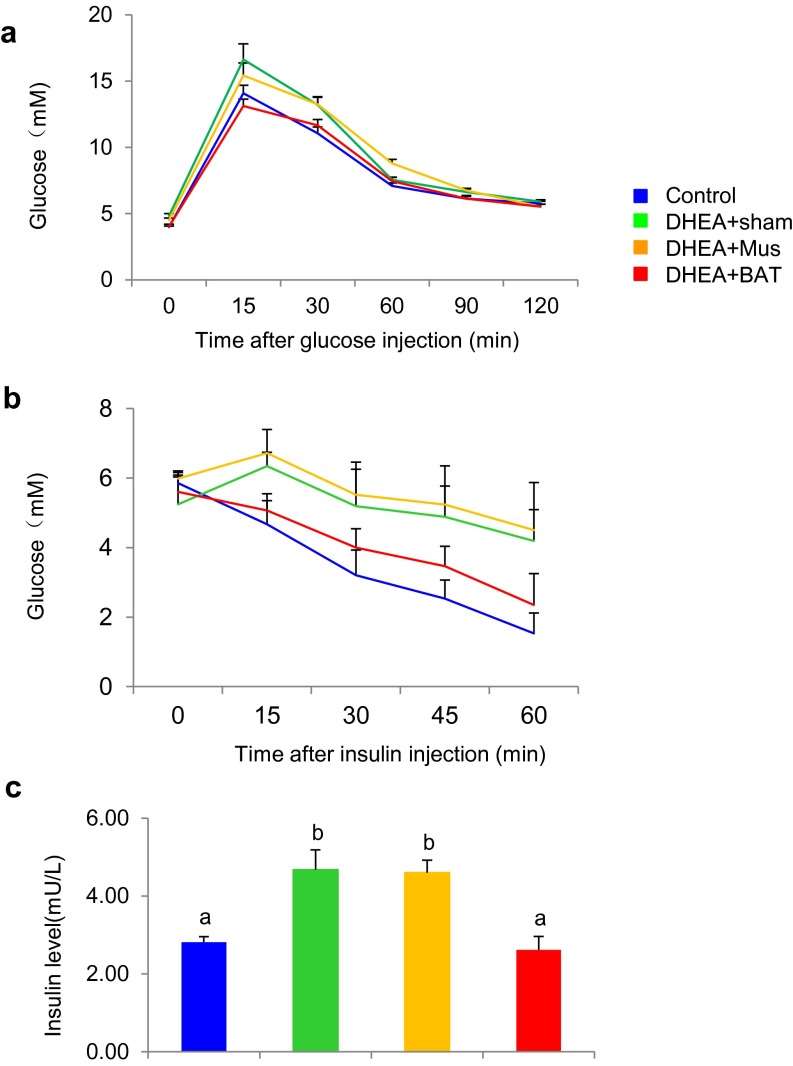
Accumulating evidence indicates that insulin resistance is one of the most common clinical features in PCOS (20) and that insulin resistance is often accompanied with reduced BAT activity (21). Therefore, we hypothesized that BAT mass and/or its activity might be associated with PCOS phenotypes, including polycystic ovaries, hyperandrogenism, and chronic anovulation. To prove our hypothesis, a rat was daily injected with DHEA for 20 d and then the irregular estrous cycle was analyzed by vaginal smear check to confirm the development of PCOS. Next, we transplanted 0.5 g of BAT from an age- and sex-matched donor rat into a PCOS rat (DHEA+BAT), and three other groups—a PBS-treated (control) group, a sham-operated (DHEA+sham) group, or a skeletal muscle-transplanted (DHEA+Mus) group—served as control groups. At 3 wk after tissue transplantation, BAT activity was assessed with positron emission tomography–computed tomography (PET-CT). Results from PET-CT showed that BAT activity was significantly reduced in DHEA+sham and DHEA+Mus groups than in the control group; however, BAT transplantation into a DHEA-induced PCOS rat dramatically increased endogenous BAT activity up to the level of the control group (Fig. 1 A and B). Although obesity is a key feature of PCOS, there was no significant difference of body weight as well as food consumption among groups in the current study (Fig. S1). Uncoupling protein 1 (UCP1) is a BAT-specific protein that dissipates the proton electrochemical gradient in mitochondria to generate heat (22). Peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC1α) and peroxisome proliferator activated receptor gamma coactivator 1 beta (PGC1β) induce the expression of UCP1 and mitochondria thermogenesis-related genes (22). Peroxisome proliferator activated receptor alpha (PPARα) is a major regulator of lipid metabolism (23). Type II iodothyronine deiodinase (Dio2) is a marker gene of BAT activation (24). Therefore, we analyzed the gene expression levels of these genes to assess BAT thermogenic activity. In parallel to BAT activity results, BAT-specific gene expressions were significantly decreased in DHEA+sham and DHEA+Mus groups compared with control and DHEA+BAT groups (Fig. 1C). Moreover, UCP1 and OXPHOS protein expressions were also increased in the DHEA+BAT group compared with DHEA+sham or DHEA+Mus groups (Fig. 1 D and E). It has been reported that postprandial thermogenesis is decreased in PCOS patients (25). In our PCOS rat model, body temperature after cold exposure was significantly decreased in DHEA+sham and DHEA+Mus groups whereas BAT transplantation significantly reversed DHEA-mediated body temperature reduction (Fig. 2 A and B). In addition, BAT transplantation, not sham operation or skeletal muscle transplantation, significantly improved energy expenditure in a DHEA-induced PCOS rat (Fig. 2 C and D). Consequently, glucose homeostasis and insulin sensitivity were dramatically improved in the DHEA+BAT group compared with DHEA+sham or DHEA+Mus groups (Fig. 2 E and F and Fig. S2). These results suggest that BAT transplantation reverses endogenous BAT activity and insulin resistance in the DHEA-induced PCOS rat.
Fig. 1.
BAT transplantation reverses PCOS BAT activity. BAT activity was assessed at the end of the experiment (3 wk after tissue transplantation) by using PET-CT. BAT transplantation could significantly increase endogenous BAT activity in the DHEA+BAT group compared with the DHEA+sham or DHEA+Mus groups (A). Yellow triangle indicates the anatomical site of the interscapular BAT. The activity of brown adipose tissue, expressed as the standard uptake values (SUVs), dramatically decreased in the DHEA+sham and DHEA+Mus groups compared with the control and BAT transplantation groups (B). Furthermore, BAT transplantation could significantly increase BAT-specific marker gene expression (C) and OXPHOS protein expression (D), as well as UCP1 expression (E), compared with the DHEA+sham or DHEA+Mus groups. Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 8–10 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Fig. S1.
Effect of BAT transplantation on body weight and food intake. (A) Food intake and (B) body weight were not changed among groups. n = 8–10 per group. Error bars represent means ± SEM.
Fig. 2.
BAT transplantation reverses PCOS metabolic abnormality. An infrared thermal image demonstrates that cold exposure significantly reduced body temperature of the DHEA+sham and DHEA+Mus groups whereas BAT transplantation significantly reversed DHEA-induced body temperature reduction (A and B). In addition, BAT transplantation significantly increased whole-body energy expenditure compared with the DHEA+sham or DHEA+Mus groups (C and D). Moreover, results from a glucose tolerance test (E) and insulin tolerance test (F) showed that BAT transplantation significantly reversed DHEA-induced glucose intolerance. Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 8–10 per group. (A and B) P < 0.05. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Fig. S2.
BAT transplantation reverses PCOS metabolic abnormality. BAT transplantation could significantly reverse DHEA-induced glucose intolerance as evidenced by (A) glucose tolerance test (GTT) and (B) insulin tolerance test (ITT), as well as insulin levels during GTT (C). Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 8–10 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
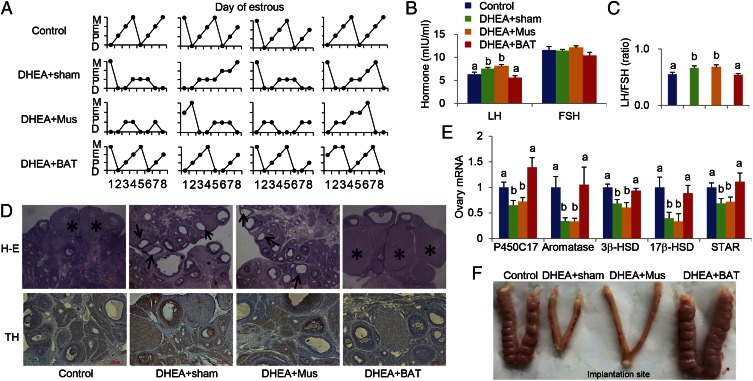
BAT Transplantation Reverses PCOS Acyclicity.
As mentioned above, irregular menstruation is one of the key criteria for the diagnosis of PCOS. We therefore investigated whether BAT transplantation could regulate the estrous cycle in a PCOS rat. After DHEA treatment, acyclicity detected by vaginal cytology was found in the DHEA+sham group and not in the control group, indicating that a rat PCOS model had been successfully developed (Fig. 3A and Table S1). Surprisingly, BAT transplantation normalized menstrual cyclicity in 7 out of 10 DHEA-induced PCOS rats, which was not found in the DHEA+Mus group (Fig. 3A and Table S1). These results highlighted that BAT transplantation could reverse abnormal estrous cycles in the PCOS rat. Abnormal estrous is accompanied with altered plasma gonadotropin concentration. Although plasma follicle-stimulating hormone (FSH) concentration was not altered among groups, plasma-luteinizing hormone (LH), as well as the LH/FSH ratio, which is one of the parameters for the diagnosis of PCOS in clinics, was significantly increased in DHEA+sham and DHEA+Mus groups compared with the control group. Notably, BAT transplantation reversed the plasma LH level and LH/FSH ratio to a normal level (Fig. 3 B and C). Additionally, the plasma testosterone (T) level was significantly attenuated after BAT transplantation in a DHEA-induced PCOS rat. Taken together, these results indicated that BAT transplantation reversed irregular estrous cyclicity in the PCOS rat.
Fig. 3.
BAT transplantation reverses PCOS acyclicity, ovarian phenotypes, and infertility. BAT transplantation could reverse abnormal estrous cycles in the PCOS rodent compared with abnormal estrous cycles in the DHEA+sham and/or DHEA+Mus groups (A). BAT transplantation further significantly reversed the concentrations of luteinizing hormone (LH) levels, as well as the LH/FSH ratio, to normal control levels compared with the DHEA+sham and DHEA+Mus groups (B and C). (D) Ovarian histology revealed that cystic follicles (arrow) appeared in the DHEA+sham and DHEA+Mus groups but not in the DHEA+BAT group. In addition, few corpora lutea (CL, asterisk) and low levels of TH expression were observed in the BAT transplantation group but not in the muscle transplantation group (D). Consistent with histology results, the expression of ovarian steroidogenic enzymes was dramatically reversed after BAT transplantation (E), and the DHEA+BAT group rats, but not the DHEA+sham and DHEA+Mus group rats, were also able to mate with proven stud males and produce a little (F). Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 8–10 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05). D, diestrus; E, estrus; M, metestrus; P, proestrus.
Table S1.
The effect of BAT transplantation on estrous cycle
| Group | Total no. | Normal estrous cycle | Abnormal estrous cycle |
| Control | 8 | 8 | 0 |
| DHEA+sham | 9 | 1 | 8 |
| DHEA+Mus | 9 | 2 | 7 |
| DHEA+BAT | 10 | 7 | 3 |
PCOS Ovarian Phenotypes and Infertility Were Reversed by BAT Transplantation.
Histologically, the number of corpora lutea (CL) was decreased and the thickness of the theca cell layer was increased in DHEA+sham and DHEA+Mus groups compared with the control group (Table S2). However, a normal layer of theca cells, mature follicles, and corpus luteum (CL) were observed in the ovary from the DHEA+BAT group (Fig. 3D and Table S2). Previous studies demonstrated that ovarian sympathetic tone was increased in women with PCOS (26, 27). Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the biosynthesis of norepinephrine (NE), and expression of TH in the ovary is highly restricted to sympathetic nerves. Thus, ovarian tissue sections from four groups were immunostained with an anti-TH antibody to detect sympathetic innervation. A large number of TH-positive sympathetic nerve fibers were found in ovaries from the DHEA+sham and DHEA+Mus groups whereas BAT transplantation significantly reduced the number of TH-expressing sympathetic nerve fibers in the ovaries (Fig. 3D). Consistent with immunostaining results, the expressions of ovarian steroidogenic enzymes, such as P450C17, aromatase, 3β-HSD, 17β-HSD, and STAR, were significantly decreased in the DHEA+sham and DHEA+Mus groups compared with the control group, and BAT transplantation dramatically reversed their expressions up to normal levels (Fig. 3E). In particular, rats in the DHEA+sham and DHEA+Mus groups were infertile and unable to give birth to a litter; however, BAT transplantation enabled the PCOS rat to deliver a litter (Fig. 3F and Table S3). Collectively, these results indicate that BAT transplantation could significantly reverse infertility in the PCOS rat.
Table S2.
The effect of BAT transplantation on ovarian phenotype
| Group | Total no. | No. of corpora lutea | Thickness of theca cell layer, μm |
| Control | 6 | 16 ± 0.58a | 19.34 ± 1.02a |
| DHEA+sham | 6 | 6.67 ± 0.88b | 36.04 ± 0.87b |
| DHEA+Mus | 6 | 5.67 ± 0.33b | 37.26 ± 2.11b |
| DHEA+BAT | 6 | 14.33 ± 1.2a | 19.99 ± 1.03a |
Data were analyzed by one-way ANOVA with Tukey's post hoc test. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Administration of Adiponectin Recapitulates the Beneficial Effects of BAT Transplantation in the PCOS Rat.
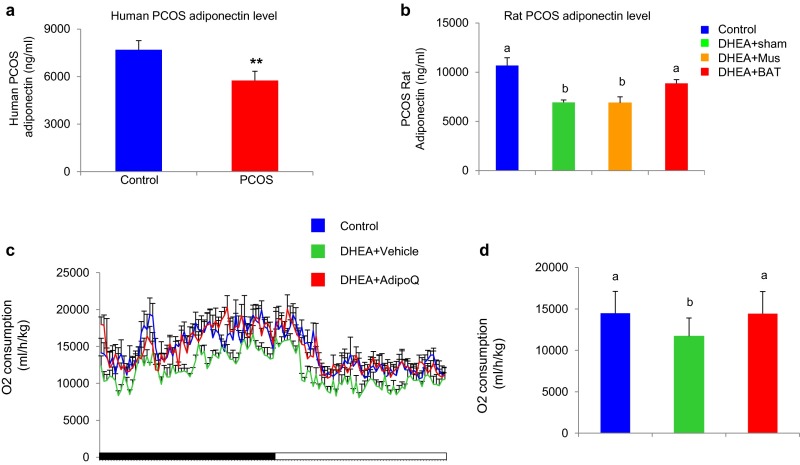
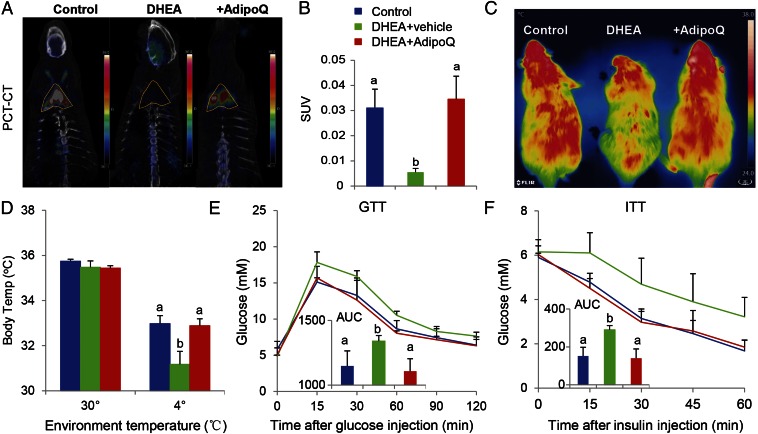
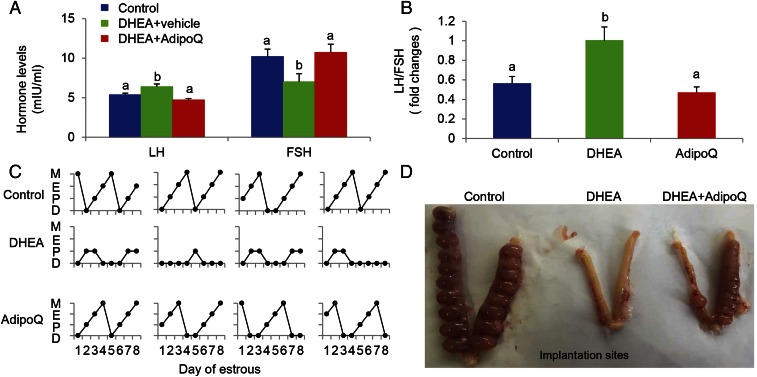
In our previous study, we showed that transplanted BAT activated endogenous BAT and increased the circulating adiponectin level in an obese mouse (17). Thus, we determined whether the adiponectin level is altered in the PCOS human and rat. Consistent with a previous report (28), the circulating adiponectin level was significantly decreased in both the PCOS patient and rat (Fig. S3 A and B and Table S4). Therefore, we reasoned that adiponectin might account, at least in part, for the beneficial effects of BAT transplantation in the PCOS rat. To address this question, a PCOS rat was daily injected with recombinant adiponectin protein (10 μg/kg BW) for 20 d. Results from PET-CT (Fig. 4 A and B), as well as cold-induced thermogenesis (Fig. 4 C and D), showed that administration of adiponectin in a PCOS rat significantly increased endogenous BAT activity up to the level of the control group. Similar to BAT transplantation, adiponectin treatment also increased energy expenditure and glucose homeostasis in the PCOS rat (Fig. 4 E and F). In addition, adiponectin treatment markedly attenuated the plasma LH/FSH ratio that was increased in the DHEA+sham group (Fig. 5 A and B). Interestingly, adiponectin treatment significantly reversed DHEA-induced acyclicity (Fig. 5C and Table S5), ovarian phenotypes (Table S5), and infertility in the PCOS rat (Fig. 5D and Table S5). These results highlight that the beneficial effects of BAT transplantation are partly mediated by an elevated circulating adiponectin level.
Fig. S3.
Adiponectin treatment increases energy expenditure. Circulating adiponectin levels were significantly decreased in both the PCOS patient and rat model compared with their respective controls (A and B). Data were analyzed by unpaired t test in A. n = 40 per group. **P < 0.01 versus control or analyzed by one-way ANOVA with Tukey’s post hoc test in B. n = 8–10 per group. Different characters indicate P < 0.05. Adiponectin treatment increases oxygen consumption (C and D). Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 6 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Table S4.
Clinical characteristics of PCOS patient compared with control subject
| Variable | PCOS | Control | P |
| No. | 40 | 40 | — |
| Age, y | 29.35 ± 4.40 | 29.38 ± 4.30 | 0.980 |
| BMI, kg/m2 | 27.36 ± 5.19 | 28.34 ± 4.63 | 0.379 |
| FSH, mIU/mL | 5.61 ± 1.514 | 6.51 ± 1.42 | <0.001 |
| LH, mIU/mL | 10.09 ± 5.94 | 4.37 ± 1.90 | 0.008 |
| T, ng/dL | 45.44 ± 16.04 | 28.90 ± 13.54 | <0.001 |
| E2, pg/mL | 44.47 ± 21.07 | 29.82 ± 15.08 | 0.001 |
| 0 min Glu, mmol/L | 5.58 ± 0.65 | 5.83 ± 0.97 | 0.210 |
| 120 min Glu, mmol/L | 6.95 ± 2.06 | 6.17 ± 2.05 | 0.101 |
| 0 min INS, mU/L | 20.65 ± 19.67 | 16.43 ± 6.16 | 0.205 |
| 120 min INS, mIU/L | 106.48 ± 103.03 | 61.04 ± 63.81 | 0.025 |
Data represent the mean ± SD. BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone; Glu, glucose; INS, insulin a; LH, luteinizing hormone; T, testosterone. P < 0.05 compared with the control group; P values were determined by Student's t test.
Fig. 4.
Adiponectin recapitulates the beneficial effects of BAT transplantation. Adiponectin treatment could significantly increase endogenous BAT activity compared with DHEA groups as evidenced by PET-CT (A and B). Moreover, Infrared thermal images demonstrated that adiponectin treatment significantly reversed DHEA-mediated body temperature reduction (C and D). A glucose tolerance test (E) and insulin tolerance test (F) showed that adiponectin treatment significantly improved DHEA -induced insulin resistance (inner graph indicating area under the curve of GTT and ITT, respectively). Data were analyzed by one-way ANOVA with Tukey’s post hoc test; n = 6 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Fig. 5.
Adiponectin reverses PCOS acyclicity, ovarian phenotypes, and infertility. The concentrations of luteinizing hormone (LH) and the LH/FSH ratio were significantly increased in the DHEA group compared with the control group, and it was reversed to a normal level after adiponectin treatment (A and B). In addition, adiponectin treatment could significantly reverse DHEA-induced acyclicity (C) and pregnant capacity in the PCOS rodent (D). Data were analyzed by one-way ANOVA with Tukey’s post hoc test. n = 6 per group. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Table S5.
The effect of adiponectin treatment on estrous cycle and ovarian phenotype
| Group | Total no. | Normal estrous cycle | Abnormal estrous cycle | No. of corpora lutea | Thickness of theca cell layer, μm | Pregnancy and parturition |
| Control | 6 | 6 | 0 | 14.33 ± 0.67a | 19.25 ± 1.01a | 6 |
| DHEA+Vehicle | 6 | 1 | 5 | 6.67 ± 1.2b | 36.54 ± 1.23b | 1 |
| DHEA+adipoQ | 6 | 4 | 2 | 11.67 ± 1.2a | 19.06 ± 0.95a | 4 |
Data were analyzed by one-way ANOVA with Tukey's post hoc test. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05).
Discussion
In the current study, we showed that BAT activity was dramatically decreased in the PCOS rat and that BAT transplantation effectively ameliorated most of the symptoms found in the PCOS rat. In addition, we revealed that the beneficial effects of BAT transplantation in the PCOS rat were mediated by the increased circulating adiponectin level. To the best of our knowledge, this study is the first study showing that the activity of BAT is associated with clinical phenotypes of PCOS in an animal model. We believe that the current study points out BAT as a previously unidentified target organ for the treatment of PCOS.
Mice neonatally androgenized with testosterone that induces PCOS showed a significant decrease in energy expenditure (29). It has been speculated that this phenomenon could be due to the BAT hypofunction (30). In agreement with previous findings, BAT-specific thermogenic gene expression, UCP1, and mitochondrial OXPHOS protein expression and cold-induced thermogenic capacity, which are key factors accounting for the reduction of energy metabolism, were reduced in our PCOS rat (Fig. 1), indicating that the DHEA-induced PCOS rat had a significant defect in energy metabolism and BAT activity.
In parallel, it was also reported that women with PCOS show increased sympathetic tone (31). Consistently, we observed that sympathetic innervation, as evidenced by TH staining, was increased in the ovaries of the DHEA-treated PCOS rat (Fig. 2C). Sustained high sympathetic tone causes insensitivity of BAT and later influences disrupted whole-body energy metabolism in PCOS. Taken together, these results suggest that the attenuation of BAT activity might play a significant pathogenic role in PCOS.
It has been widely appreciated that women with PCOS show insulin resistance and glucose intolerance (32). On the other hand, BAT activity is often negatively associated with diabetes status but positively correlated with glucose uptake activity in humans (33). Recently, we demonstrated that BAT transplantation has a beneficial effect on the prevention and treatment of obesity in the HFD-induced obese mouse, as well as in the genetic obese Ob/Ob mouse (17, 18). In addition, we showed that BAT transplantation significantly improved glucose homeostasis in both diet-induced obesity and genetic obesity mice models (17, 18). In agreement with previous results, we observed that DHEA-induced glucose intolerance was significantly reversed by transplantation of BAT, but not muscle (Fig. 2 E and F). These results again emphasize the important role of BAT in glucose homeostasis.
The remaining question we had was how the transplanted BAT displayed beneficial effects on PCOS. We speculated that the beneficial effects of BAT transplantation might be from activated endogenous BAT that might secret systemic brown adipose tissue-derived adipokine (batokine). In our previous report, we demonstrated that BAT transplantation in obese mice significantly increased the circulating adiponectin level (17), which is known to be attenuated in women with PCOS (34). Consistently, we also confirmed that there was a significant reduction of the circulating adiponectin level in both PCOS women and the DHEA-treated rats. Interestingly, we found that the adiponectin level was significantly reversed to normal level after BAT transplantation (Fig. S3 A and B). These results led us to investigate whether adiponectin administration recapitulates the beneficial effects of BAT transplantation in the PCOS rat. After adiponectin treatment, decreased BAT activity, metabolic abnormalities, acyclicity, and abnormal hormonal levels were surprisingly normalized up to normal levels in the PCOS rat. Based on recent publications, BAT also secretes a considerable number of adipokines, such as adiponectin, FGF21, NGF, NRG4, VEGF, and BMPs (16, 35). We have observed that there was no significant difference of FGF21 or NGF levels between groups (Table S6). Gunawardana et al. (36) reported that BAT transplantation can reverse type 1 diabetes in streptozotocin-treated mice without exogenous insulin treatment. Furthermore, we and other group have shown that BAT transplantation reversed metabolic disorders in various obese mouse models (17–19). These results further suggest that BAT secretes systemic mediators that could regulate in whole-body glucose homeostasis. It should be noted that we do not exclude other factors mentioned above that may be involved in the beneficial effects of BAT transplantation in the PCOS rat model. However, in our hands, we observed that adiponectin alone was enough to recapitulate the beneficial effects of BAT transplantation in the PCOS rat. Other mechanisms behind the adiponectin effect for the treatment of PCOS would be necessary to be revealed in the near future. Taken together, our findings highlight that systemic adiponectin treatment significantly improves PCOS phenotypes in an animal model.
Table S6.
Hormonal profiles in tissue transplantation experiment
| Variable | Control (n = 8) | DHEA+Sham (n = 9) | DHEA+Mus (n = 9) | DHEA+BAT (n = 10) |
| T3, pmol/L | 4.48 ± 0.208 | 4.40 ± 0.331 | 4.24 ± 0.151 | 4.45 ± 0.251 |
| T4, pmol/L | 32.74 ± 1.454 | 29.84 ± 1.568 | 28.30 ± 1.916 | 29.35 ± 2.542 |
| Estradiol, pg/mL | 31.13 ± 4.689 | 25.78 ± 1.597 | 28.14 ± 5.616 | 24.10 ± 5.177 |
| Progesterone, ng/mL | 40.00 ± 1.200 | 37.36 ± 1.621 | 38.61 ± 1.311 | 36.95 ± 1.601 |
| Testosterone, ng/mL | 2.41 ± 0.068a | 2.79 ± 0.136b | 2.79 ± 0.077b | 2.35 ± 0.133a |
| CHO, mmol/L | 1.34 ± 0.220 | 1.51 ± 0.116 | 1.33 ± 0.107 | 1.06 ± 0.114 |
| TG, mmol/L | 0.28 ± 0.032 | 0.40 ± 0.044 | 0.38 ± 0.043 | 0.28 ± 0.044 |
| HDL, mmol/L | 1.55 ± 0.174a | 1.34 ± 0.022b | 1.11 ± 0.079b | 1.15 ± 0.072a |
| LDL, mmol/L | 0.12 ± 0.016 | 0.14 ± 0.014 | 0.13 ± 0.017 | 0.12 ± 0.012 |
| NGF, pg/mL | 47.11 ± 5.658 | 48.42 ± 5.860 | 49.33 ± 8.420 | 55.35 ± 7.548 |
| FGF21, pg/mL | 45.50 ± 7.910 | 47.12 ± 8.100 | 50.17 ± 11.078 | 56.79 ± 10.726 |
Data were analyzed by one-way ANOVA with Tukey's post hoc test. Different lowercase letters indicate significant differences among groups (One-way ANOVA, with Tukey’s post hoc test, P < 0.05). CHO, cholesterol; FGF21, fibroblast growth factor 21; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NGF, nerve growth factor; T3, triiodothyronine; T4, thyroxine; TG, triglyceride.
In conclusion, we demonstrate here that BAT transplantation could significantly improve PCOS phenotypes, including disrupted energy metabolism, acyclicity, and infertility. In addition, these beneficial effects of BAT transplantation were at least in part mediated by systemic adiponectin. Because BAT transplantation is not easily applied to human beings, administration of batokines or drugs that enhance BAT activity will be alternative strategies for the treatment of PCOS.
Acknowledgments
This work was supported by Ministry of Science and Technology of China Grant 2012CB944701 (to W.J.), Strategic Priority Research Program Grant XDB13030000 (to W.J.), Key Research Program Grant KJZD-EW-L01-3 (to W.J.), the One Hundred Talents Program of the Chinese Academy of Sciences (W.J.), and National Natural Science Foundation\ of China Grants 31171131 and 81370951 (to W.J.), as well as Ministry of Science and Technology of China Grant 2012CB944700 (to Z.-J.C.) and National Natural Science Foundation of China Grants 81430029, 81490743, 31371453, and 31571548 (to Z.-J.C. and H. Zhao).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523236113/-/DCSupplemental.
References
- 1.March WA, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Muscogiuri G, Colao A, Orio F. Insulin-mediated diseases: Adrenal mass and polycystic ovary syndrome. Trends Endocrinol Metab. 2015;26(10):512–514. doi: 10.1016/j.tem.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Moran LJ, Strauss BJ, Teede HJ. Diabetes risk score in the diagnostic categories of polycystic ovary syndrome. Fertil Steril. 2011;95(5):1742–1748. doi: 10.1016/j.fertnstert.2011.01.133. [DOI] [PubMed] [Google Scholar]
- 5.Lindholm A, Andersson L, Eliasson M, Bixo M, Sundström-Poromaa I. Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;102(1):39–43. doi: 10.1016/j.ijgo.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Spritzer PM. Polycystic ovary syndrome: Reviewing diagnosis and management of metabolic disturbances. Arq Bras Endocrinol Metabol. 2014;58(2):182–187. doi: 10.1590/0004-2730000003051. [DOI] [PubMed] [Google Scholar]
- 7.Stepto NK, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 8.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, et al. Endocrine Society Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozegowska KE, Pawelczyk LA. The role of insulin and selected adipocytokines in patients with polycystic ovary syndrome (PCOS): A literature review. Ginekol Pol. 2015;86(4):300–304. [PubMed] [Google Scholar]
- 12.Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep. 2011;11(3):179–184. doi: 10.1007/s11892-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 13.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 15.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GX, Zhao XY, Lin JD. The brown fat secretome: Metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015;26(5):231–237. doi: 10.1016/j.tem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology. 2015;156(7):2461–2469. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23(6):851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanford KI, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 21.Betz MJ, Enerbäck S. Human brown adipose tissue: What we have learned so far. Diabetes. 2015;64(7):2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, et al. Recent advances in brown adipose tissue biology. Chin Sci Bull. 2014;59(31):4030–4040. [Google Scholar]
- 23.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 24.de Jesus LA, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108(9):1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson S, et al. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 1992;36(6):537–543. doi: 10.1111/j.1365-2265.1992.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 26.Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: A novel therapeutic target? Clin Endocrinol (Oxf) 2012;77(6):791–801. doi: 10.1111/cen.12003. [DOI] [PubMed] [Google Scholar]
- 27.Dissen GA, et al. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology. 2009;150(6):2906–2914. doi: 10.1210/en.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannerås-Holm L, et al. Adipose tissue has aberrant morphology and function in PCOS: Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 29.Nohara K, et al. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152(4):1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nohara K, et al. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013;304(12):E1321–E1330. doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sverrisdóttir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab. 2008;294(3):E576–E581. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 32.Legro RS. Polycystic ovary syndrome and cardiovascular disease: A premature association? Endocr Rev. 2003;24(3):302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 33.Ouellet V, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 34.Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18(7):266–272. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Keipert S, et al. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol Metab. 2015;4(7):537–542. doi: 10.1016/j.molmet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61(3):674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell AS, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 39.Chi QS, Wang DH. Thermal physiology and energetics in male desert hamsters (Phodopus roborovskii) during cold acclimation. J Comp Physiol B. 2011;181(1):91–103. doi: 10.1007/s00360-010-0506-6. [DOI] [PubMed] [Google Scholar]