Abstract
The cytoplasmic domain of pseudorabies virus (PRV) glycoprotein B (gB) contains three putative internalization motifs. Previously, we demonstrated that the tyrosine-based YQRL motif at positions 902 to 905, but not the YMSI motif at positions 864 to 867 or the LL doublet at positions 887 and 888, is required for correct functioning of gB during antibody-mediated internalization of PRV cell surface-bound glycoproteins. In the present study, we demonstrate that the YQRL motif is also crucial to allow spontaneous internalization of PRV gB, and thus, that spontaneous and antibody-mediated internalizations of PRV gB occur through closely related mechanisms. Furthermore, we found that PRV gB colocalizes with the cellular clathrin-associated AP-2 adaptor complex and that this colocalization depends on the YQRL motif. In addition, by coimmunoprecipitation assays, we found that during both spontaneous and antibody-dependent internalization, PRV gB physically interacts with AP-2, and that efficient interaction between gB and AP-2 required an intact YQRL motif. Collectively, these findings demonstrate for the first time that during internalization of an alphaherpesvirus envelope protein, i.e., PRV gB, a specific amino acid sequence in the cytoplasmic tail of the protein interacts with AP-2 and may constitute a common AP-2-mediated mechanism of internalization of alphaherpesvirus envelope proteins.
Pseudorabies virus (PRV), a swine alphaherpesvirus closely related to the human pathogens herpes simplex virus (HSV) and varicella-zoster virus (VZV), is the causative agent of Aujeszky's disease (4, 24). Its genome encodes at least 11 glycoproteins, which have homologs in other herpesviruses (24). In PRV-infected cells, newly synthesized glycoproteins travel from the endoplasmic reticulum via the Golgi to the plasma membrane (25). These glycoproteins play important roles in the viral life cycle, as well as in the pathogenesis of PRV infections (9, 29).
Interestingly, several alphaherpesvirus-encoded cell surface-associated envelope glycoproteins have been reported to be internalized, either spontaneously or upon binding of antigen-specific antibodies (12, 13, 17, 32, 35, 36, 44). The biological function of spontaneous internalization in the virus life cycle is not yet fully understood, although some hypothetical roles have been proposed (reviewed in reference 9), such as the possible involvement of internalization in delivering the viral cell surface proteins to a specific compartment, where viral envelopment takes place; in redirecting viral proteins to specific membrane surfaces (such as the apical, lateral, or basal surfaces of polarized cells); or in immune evasion. Antibody-dependent internalization of viral cell surface proteins may also be implicated in immune evasion, since it has been shown to decrease the efficiency of antibody-dependent lysis of PRV-infected cells (49).
Recently, several groups reported on the amino acid sequence motifs involved in the internalization of different alphaherpesvirus envelope glycoproteins. Two types of motifs, located in the cytoplasmic tails of these viral proteins, have been shown to be of predominant importance: tyrosine-based YXXφ-type motifs (where Y stands for tyrosine, X stands for any amino acid, and φ stands for any bulky hydrophobic amino acid) and LL (dileucine) motifs. Spontaneous internalization of PRV glycoprotein E (gE) requires an intact YTSL motif (where T stands for threonine and S stands for serine) in its cytoplasmic tail (45), and internalization of PRV gB requires the C-terminal 29 amino acids of the gB cytoplasmic domain, which contain an LL motif and a YQRL motif (where Q stands for glutamine and R stands for arginine) (32). In a previous study, it was shown that the gB membrane-distal YQRL motif at positions 902 to 905, but not the membrane-proximal YMSI motif at positions 864 to 867 (where M stands for methionine and I stands for isoleucine) or the LL doublet at positions 887 and 888, is critical for efficient antibody-mediated internalization of PRV cell surface proteins (13). In agreement with these findings, mutation of the HSV gB membrane-distal YSPL motif (where P stands for proline), but not mutation of the membrane-proximal YMAL motif (where A stands for alanine) or the LL motif, was found to abrogate internalization of HSV gB (11). The internalization of some VZV-encoded glycoproteins has also been shown to depend on either a YXXφ motif or an LL motif. Indeed, internalization of VZV gB, gE, and gH requires, respectively, a YSRV (where V stands for valine), a YAGL (where G stands for glycine), or a YNKI (where N stands for asparagine and K stands for lysine) motif located in the respective cytoplasmic domains, while internalization of VZV gI is dependent on an LL motif (3, 17, 35, 36, 37). Thus, the internalization of many alphaherpesvirus envelope proteins is mediated by related tyrosine-based YXXφ-type motifs or LL motifs within their cytoplasmic tails. Similar YXXφ and LL signals, together with acidic amino acid clusters, are known to be mediators of clathrin-mediated endocytosis of cellular receptors (7, 22, 46, 47, 50). Clathrin-mediated endocytosis of cellular receptors is initiated by invagination of certain regions of the plasma membrane, so-called clathrin-coated pits, which are subsequently pinched off from the plasma membrane to form clathrin-coated vesicles (16, 20, 40). Crucial roles in these initial steps of the endocytosis process are attributed to clathrin triskelions and the clathrin-associated AP-2 adaptor complex, which is composed of two 100- to 110-kDa large chains (α and β2), a 50-kDa medium chain (μ2), and a 17-kDa small chain (σ2) (2, 18, 38, 41). Several studies have shown that the AP-2 adaptor complex recognizes and binds (via its medium μ subunit) tyrosine-based or dileucine-type internalization sequences in the cellular receptor on the one hand and to clathrin triskelions on the other hand, forming a physical link between the cellular receptor and clathrin triskelions as a first step in the formation of receptor-containing clathrin-coated endocytosis vesicles (8, 10, 22, 23, 40, 43).
It has been demonstrated that during the early steps of some internalization processes, alphaherpesvirus glycoproteins colocalize with clathrin triskelions (17, 35, 48). Because of their dependence on clathrin- and tyrosine-based motifs or dileucine motifs, alphaherpesviruses seem to initiate the internalization of envelope proteins via a mechanism similar to that of clathrin-mediated endocytosis of cellular receptors. However, no information is available on whether, as in clathrin-mediated endocytosis of cellular receptors, internalization of alphaherpesvirus envelope proteins relies on an interaction with the cellular clathrin-associated AP-2 adaptor complex.
Therefore, in the present study, we wanted to investigate (i) whether PRV gB colocalizes and interacts with the clathrin-associated AP-2 adaptor complex during spontaneous and antibody-mediated internalization processes and, if so, (ii) whether the YQRL motif is required for interaction of PRV gB with AP-2.
MATERIALS AND METHODS
Virus strains.
The wild-type PRV Becker strain and isogenic PRV Becker gB mutants, carrying tyrosine (Y)-to-alanine (A) substitutions in the cytoplasmic tyrosine-based motifs and leucine (L)-to-arginine (R) substitutions in the LL motif in gB (PRV Be, PRV Y864A, PRV Y902A, PRV LL887RR, PRV Y864A/Y902A, and PRV Y864A/Y902A/LL887RR), were described earlier (13).
Cell culture and infection.
Porcine kidney PK15 cells were cultivated in Eagle's minimal essential medium (MEM) supplemented with 5% fetal calf serum, glutamine (0.3 mg/ml), and antibiotics (100 U of penicillin/ml, 0.1 mg of streptomycin/ml, and 0.1 mg of kanamycin/ml). For infection experiments, monolayers of PK15 cells grown on glass coverslips were inoculated with the appropriate PRV strains at a multiplicity of infection (MOI) of 10.
Construction of mammalian expression vectors and transfection assay.
Plasmids pHF3, pHF16, and pHF17, encoding wild-type Be gB and point-mutated Y864A and Y902A gB, respectively, were previously described (13). To construct plasmid pHF50, encoding a gB mutant that lacks a cytoplasmic domain, a 750-bp SalI-SphI fragment of pHF3 was cloned in pALTER-1 (Promega, Madison, Wis.), creating pHF39. Subsequently, oligonucleotide mutagenesis was performed on pHF39 to replace serine 882 (S882) by a stop codon. The oligonucleotide used (5′ CCTACCGGCACATCTAGCGCTTGCGCCGCAACCCC 3′) introduced an additional Eco47III site, which facilitated screening of the resulting plasmid, pHF47. The mutated 750-bp DNA fragment of SalI-SphI-digested pHF47 was then subcloned in SalI-SphI-digested pHF3, creating pHF50. To construct mammalian expression vectors, BamHI-EcoRV restriction fragments of pHF3, pHF16, pHF17, and pHF50 were subcloned into the cytomegalovirus promoter-based eucaryotic expression vector pcDNA3.1D/V5-His-TOPO (Invitrogen, Groningen, The Netherlands). The resulting expression vectors, pGVM3, pGVM16, pGVM17, and pGVM50, encode wild-type gB, Y864A gB, Y902A gB, and gB truncated, respectively. For transfection experiments, 80 to 90% confluent PK15 cells grown on glass coverslips were transiently transfected with pGVM3, pGVM16, pGVM17, or pGVM 50 by use of Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
Antibodies and reagents.
Fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal immunoglobulin G2a (IgG2a) antibodies directed against PRV gB (1C11) were produced at our laboratory, as previously described (28). Monoclonal anti-α-adaptin antibodies (recognizing the α subunit of AP-2), clone AP6 (IgG1), and clone AC1-M11 (IgG2a) were purchased from 10P's (Zandhoven, Belgium). Biotinylated rabbit anti-mouse IgG1-specific antibodies were from Zymed Laboratories Inc. (San Francisco, Calif.). Streptavidin-Texas Red was obtained from Molecular Probes (Eugene, Oreg.). Biotinylated sheep anti-mouse IgG antibodies and streptavidin-biotin-horseradish peroxidase complex and polyvinylidene difluoride membranes were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Goat anti-mouse agarose beads were from Sigma (St. Louis, Mo.).
Antibody-independent internalization assay.
The amounts of PRV gB on the surfaces of PRV-infected PK15 cells, determined by immunofluorescent staining, were used as a measure for spontaneous internalization of gB: low levels correspond to efficient internalization, and high levels correspond to inefficient internalization, essentially as previously described (32). Briefly, at the appropriate time points, paraformaldehyde-fixed, nonpermeabilized cells were stained for cell surface-localized (noninternalized) gB by use of the FITC-labeled primary antibody 1C11. The stained cells were mounted on microscope slides using glycerin-DABCO and were analyzed by confocal microscopy (TCS SP2 laser scanning spectral confocal system; Leica Microsystems GmbH, Heidelberg, Germany).
Antibody-dependent internalization assay.
The antibody-dependent internalization assay was essentially done as described previously with some modifications (13, 48). At the appropriate time points postinoculation (p.i.) with the appropriate PRV strains or 24 h posttransfection with the appropriate gB expression vectors, PK15 cells were cooled on ice for 15 min, rinsed two times with ice-cold phosphate-buffered saline (PBS), and incubated on ice for 1 h with the FITC-labeled anti-gB monoclonal antibody 1C11. After two washings with ice-cold PBS, the cells were shifted for 0 or 90 min (infected cells) or 60 min (transfected cells), respectively, to 37°C by the addition of prewarmed MEM and placed in a 37°C incubator. Afterwards, the cells were paraformaldehyde fixed. The stained cells were mounted, and subcellular localization of gB was analyzed by confocal microscopy as described above.
Double immunofluorescence stainings of PRV gB and α-adaptin.
After an antibody-dependent internalization assay was performed as described above, infected or transfected PK15 cells were paraformaldehyde fixed, Triton X-100 permeabilized, and costained for α-adaptin. To stain α-adaptin, cells were incubated (1 h at 37°C) with anti-α-adaptin IgG1 antibodies (clone AP-6) diluted 1/200 in PBS plus 0.3% gelatin. Afterwards, the cells were washed twice in Tris-buffered saline (20 mM Tris-HCl and 150 mM NaCl)-4.5% (wt/vol) sucrose-2% heat-inactivated goat serum (TBS-SG). The cells were then incubated with biotinylated rabbit anti-mouse IgG1-specific antibodies diluted 1/100 in PBS-0.3% gelatin (1 h at 37°C) and washed twice in TBS-SG. Finally, the cells were incubated (1 h at 37°C) with streptavidin-Texas Red diluted 1/50 in PBS-0.3% gelatin, washed twice in TBS-SG, mounted, and analyzed by confocal microscopy.
Coimmunoprecipitation experiments.
At 6 h (spontaneous internalization) and 10 h (antibody-dependent internalization) p.i., monolayers of PRV-infected PK15 cells were washed twice in MEM (37°C), incubated for 15 min at 37°C with monoclonal anti-gB antibodies diluted in MEM (only for antibody-dependent internalization), rinsed twice in ice-cold PBS, scraped, and lysed for 1 h at 4°C in 750 μl of lysis buffer (1% Triton X-100, 50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1 mM Na3VO4, 10 mM NaF, and a mixture of protease inhibitors). The lysates were precleared by centrifugation at 13,000 × g in a cooled centrifuge and incubated for 90 min at 4°C with monoclonal anti-gB antibodies or with monoclonal anti-α-adaptin antibodies. Subsequently, gB or α-adaptin was precipitated overnight at 4°C using goat anti-mouse agarose beads, washed three times in PBS, and resuspended in 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. Equal amounts of all samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. The blotting membranes were then incubated for 1 h with either monoclonal anti-α-adaptin antibodies (clone AC1-M11) or anti-gB monoclonal antibodies (1C11), washed three times in PBS-0.1% Tween 20, incubated for 1 h with biotinylated sheep anti-mouse IgG, washed again three times in PBS-0.1% Tween 20, and finally incubated with a streptavidin-biotin-horseradish peroxidase complex. Immunoreactive bands, corresponding to gB or α-adaptin, were revealed with an ECL Western blot detection kit from Amersham Pharmacia Biotech according to the manufacturer's instructions.
RESULTS
Efficient spontaneous and antibody-mediated internalizations of PRV gB both depend on an intact YQRL motif in the cytoplasmic tail of gB.
PRV gB has been reported to undergo spontaneous antibody-independent internalization during the early phases of a PRV infection (<6 h p.i.) in PK 15 cells and in rabbit kidney RK13 cells transfected with PRV gB (32, 44), which is mediated by a region of the gB cytoplasmic tail that contains two potential internalization motifs: an LL doublet and a YQRL motif (32). In addition, PRV gB has been shown to be implicated in antibody-mediated internalization of cell surface-bound glycoproteins in PRV-infected monocytes (12), requiring the YQRL motif (13).
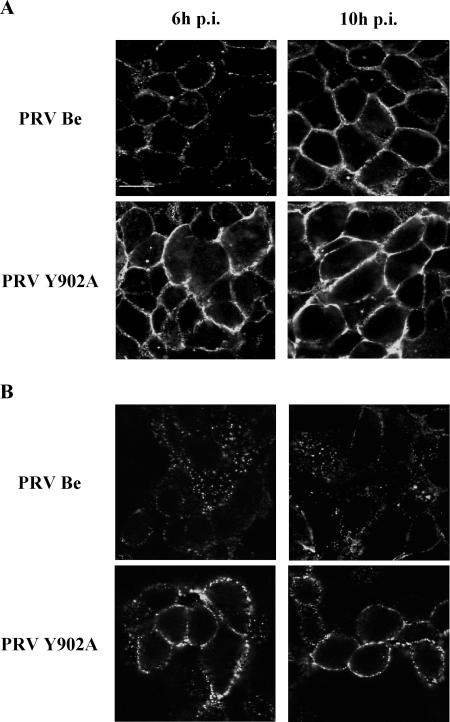
First, we examined whether the mechanisms of spontaneous and antibody-mediated internalization of PRV gB are related by investigating whether they both occur efficiently in PRV-infected PK15 cells and, if so, whether they both rely on the YQRL motif. To this end, PK15 cells were infected at an MOI of 10 either with wild-type PRV (PRV Be) or with an isogenic PRV strain in which the tyrosine (Y) residue of the YQRL motif was replaced by an alanine (A) residue (PRV Y902A). The efficiency of spontaneous internalization of PRV gB was investigated, essentially as described before (32), by determining the amount of gB at the cell surface. Low levels of gB at the cell surface correlate with efficient retrieval of gB from the cell surface. The upper images in Fig. 1A show that in PRV Be-infected PK15 cells, very few gB molecules were detected at the cell surface at 6 h p.i., whereas at 10 h p.i., large amounts of gB were present at the cell surface. In PRV Y902A-infected PK15 cells, high levels of gB Y902A were present at the cell surface at both time points examined. These observations indicate (i) that wild-type Be gB is efficiently internalized in an antibody-independent manner during the early stages of a PRV infection but not at late stages of infection, in agreement with earlier findings (44), and (ii) that replacement of tyrosine 902 by an alanine residue abrogates spontaneous internalization of PRV gB.
FIG. 1.
Spontaneous (A) and antibody-dependent (B) internalization of PRV gB are both dependent on an intact YQRL motif. Confluent monolayers of PK15 cells were infected at an MOI of 10 with either PRV Be or PRV Y902A. At 6 (left column) and 10 (right column) h p.i., the efficiency of spontaneous internalization was studied by determining the levels of gB remaining at the cell surface (A), whereas antibody-dependent internalization was studied using an internalization assay based on FITC-labeled anti-gB antibodies, as described in Materials and Methods (B). The cells were analyzed by confocal microscopy. Bar, 10 μm.
To determine the efficiency of antibody-mediated internalization of PRV gB in PK15 cells at 6- and 10 h p.i. with PRV Be or PRV Y902A, cells were incubated on ice with the FITC-labeled gB-specific monoclonal antibody 1C11, washed, shifted for 90 min to 37°C to allow internalization, and paraformaldehyde fixed. Analysis of the stained cells by confocal microscopy allowed discrimination between cell surface-residing and internalized gB. In Fig. 1B, it can be seen that in PRV Be-infected PK15 cells, gB undergoes highly efficient antibody-mediated internalization at 6 h p.i. and moderately efficient internalization at 10 h p.i. On the other hand, in PRV Y902A-infected cells, no or very little antibody-mediated internalization of Y902A gB could be observed at either time point. Thus, the tyrosine residue in the YQRL motif is critical for antibody-mediated internalization of PRV gB.
Hence, although spontaneous internalization seems to occur only during the early phases of PRV infection, the two PRV gB internalization processes are closely related, since (i) they both occur in PRV-infected PK15 cells and (ii) they both depend on the YQRL motif in the cytoplasmic tail of gB.
Subcellular localization of α-adaptin during internalization of PRV gB in infected PK15 cells.
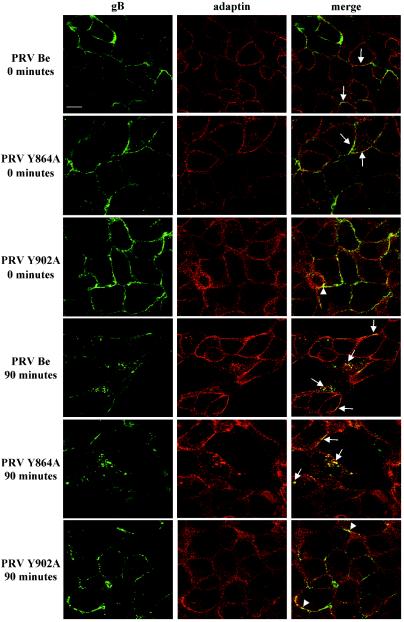
The YQRL motif, which is involved in both spontaneous and antibody-dependent internalization of PRV gB, is highly similar to the tyrosine-based internalization motifs involved in clathrin-mediated endocytosis of cellular receptors. To investigate whether the gB cytoplasmic YQRL motif, like its cellular counterparts, initiates internalization by interacting with the clathrin-associated AP-2 adaptor complex, we determined the subcellular localizations of PRV gB and the α subunit of the AP-2 adaptor complex (α-adaptin) before and after antibody-mediated induction of gB internalization in PRV Be- and PRV Y902A-infected PK15 cells. As an extra control, we determined the subcellular localization of gB in cells infected with a PRV strain (PRV Y864A) that carries a mutation in the tyrosine residue in the YMSI motif in the cytoplasmic domain of PRV gB that has been shown not to be involved in internalization (13). Figure 2 shows that after the induction of internalization in PRV-infected cells, many of the wild-type and Y864A gB molecules are internalized and that the majority of wild-type and Y864A gB molecules, both in internalized vesicles and on the plasma membrane, colocalize with α-adaptin (Fig. 2). Furthermore, we found that Y902A gB is not internalized but that a minority of the internalization-deficient Y902A gB molecules show colocalization with membrane-associated α-adaptin (Fig. 2). Thus, in PRV-infected cells, the AP-2 adaptor complex seems to mediate the internalization of wild-type and Y864A gB but is unable to drive the internalization of Y902A gB, although somewhat surprisingly, a minority of the internalization-deficient Y902A gB molecules colocalize with α-adaptin at the cell surface.
FIG. 2.
Colocalization between gB and α-adaptin in PRV-infected cells. PK15 cells were infected at an MOI of 10 with either PRV Be, PRV Y864A, or PRV Y902A. All infected cells were processed for an antibody-dependent internalization assay and stained for gB (left column) as described in Materials and Methods. Internalization was allowed to proceed for 0 or 90 min at 37°C. Thereafter, the cells were costained for α-adaptin (middle column). To this end, the cells were incubated with monoclonal anti-α-adaptin IgG1 antibodies (clone AP-6), biotinylated rabbit anti-mouse IgG1-specific antibodies, and streptavidin-Texas Red. Merged images (right column), obtained by superposition of the green (gB) and red (α-adaptin) stainings, indicate areas of colocalization (yellow) between PRV gB and α-adaptin. The arrows indicate colocalization between wild-type or Y864A gB and α-adaptin in cytoplasmic internalization vesicles or at the cell surface, whereas the arrowheads indicate colocalization between Y902 gB and α-adaptin at the cell surface. Bar, 10 μm.
Subcellular localization of α-adaptin during internalization of gB in transfected cells.
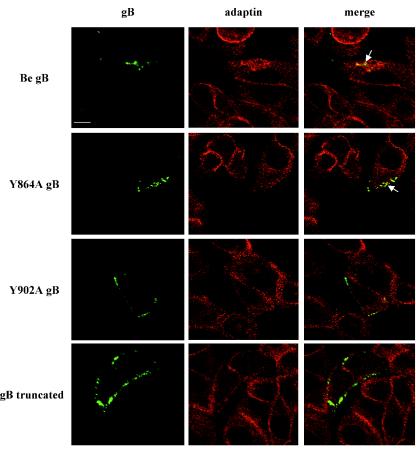
A potential explanation for the somewhat puzzling observation of colocalization of some of the cell surface-localized, internalization-deficient gB molecules and α-adaptin in PRV Y902A-infected PK15 cells could perhaps be found in the presence of internalization motifs in the cytoplasmic tails of some other cell surface-localized PRV glycoproteins, such as gD, gE, or gH, which may be present in close proximity to the Y902A gB molecules. Alternatively, it could be that a gB cytoplasmic sequence, different from the tyrosine 902-based YQRL motif, is also involved in the recruitment of gB Y902A molecules to AP-2-rich regions in the plasma membrane. To investigate these assumptions, and also to test whether AP-2 is associated with the internalization of wild-type and Y864A gB in the absence of other PRV proteins, PK15 cells were transiently transfected with mammalian expression vectors encoding either wild-type gB, Y864A gB, Y902A gB, or a truncated gB that lacks the cytoplasmic domain (gB truncated), and 24 h posttransfection, the cells were subjected to an antibody-mediated internalization assay as described above. Figure 3 shows that, as in infected PK15 cells, wild-type and Y864A gB are efficiently internalized in transfected cells and the cytoplasmic gB-containing internalization vesicles are coated with α-adaptin. On the other hand, Y902A gB and gB truncated molecules are not internalized in transfected cells (Fig. 3) and, importantly, show no or only minimal colocalization with α-adaptin at the cell surface.
FIG. 3.
Colocalization between gB and α-adaptin in gB-transfected cells. PK15 cells were transiently transfected with either Be gB (pGVM3), Y864A gB (pGVM16), Y902A gB (pGVM17), or gB truncated (pGVM50). At 24 h posttransfection, the cells were incubated with FITC-labeled monoclonal anti-gB antibodies at 4°C for 1 h. Subsequently, the cells were shifted for 60 min to 37°C to allow internalization of gB (right column). Thereafter, the cells were fixed, permeabilized, and costained for α-adaptin (middle column) by the use of monoclonal anti-α-adaptin IgG1 antibodies, biotinylated IgG1-specific antibodies, and streptavidin-Texas Red. Merged images (right column), obtained by superposition of the green (gB) and red (α-adaptin) stainings, indicate areas of colocalization (yellow) between gB and α-adaptin. The arrows indicate colocalization between wild-type or Y864A gB and α-adaptin in cytoplasmic internalization vesicles. Bar, 10 μm.
Thus, the subcellular localization of gB and α-adaptin in gB-transfected PK15 cells indicates that, in the absence of other viral proteins, (i) the YQRL motif in the cytoplasmic domain of gB drives AP-2-associated internalization of gB and (ii) very little colocalization can be observed between Y902A gB and AP-2.
The tyrosine-based YQRL motif in the cytoplasmic tail of gB mediates a physical interaction between the AP-2 adaptor complex and PRV gB.
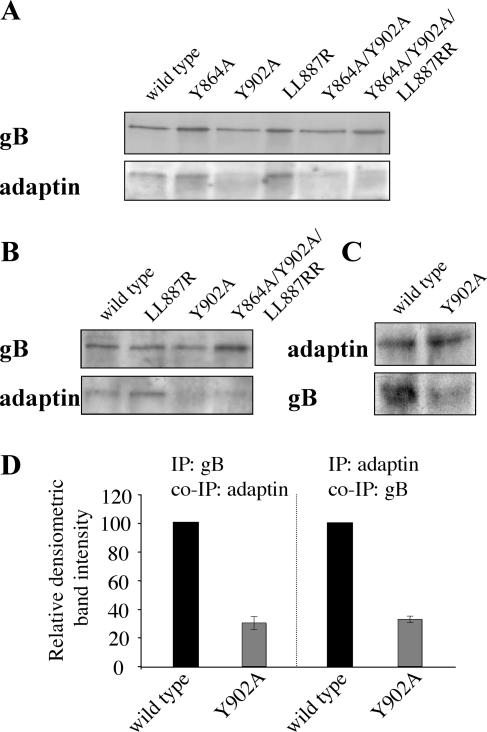
To test whether during internalization PRV gB physically interacts with AP-2 and, if so, whether this occurs via the YQRL motif, we performed coimmunoprecipitation experiments. Figure 4A and B show that immunoprecipitation of gB, during both spontaneous and antibody-mediated internalization of gB in infected PK15 cells, results in coimmunoprecipitation of the α subunit of AP-2, indicating that PRV gB and AP-2 are physically associated during these processes. In addition, the figure shows that mutation of the Y902 residue, but not of two other potential AP-2-interacting motifs (YMSI at position 864 and LL at position 887), severely impairs this interaction between gB and AP-2. The LL887RR mutation resulted in a slightly, but consistently, elevated interaction between α-adaptin and gB. The reverse-immunoprecipitation experiment in cells infected with wild-type or Y902A PRV during spontaneous internalization further confirmed these results: immunoprecipitates of AP-2 contained high levels of wild-type gB and only very low levels of Y902A gB (Fig. 4C and D).
FIG. 4.
PRV gB interacts with α-adaptin, and mutation of the Y902 residue strongly reduces this interaction. (A) Coimmunoprecipitation of α-adaptin with PRV gB during spontaneous gB internalization. At 6 h p.i., PK15 cells infected with either PRV Be, PRV Y864A, PRV Y902A, PRV LL887RR, PRV Y864A/Y902A, or PRV Y864A/Y902A/LL887RR were lysed and immunoprecipitated with monoclonal anti-gB antibodies, and the immunoprecipitates were analyzed for the presence of gB (upper row) and α-adaptin (lower row). (B) Coimmunoprecipitation of α-adaptin with PRV gB during antibody-mediated gB internalization. At 10 h p.i., PK15 cells infected with PRV Be, PRV LL887RR, PRV Y902A, or PRV Y864A/Y902A/LL887RR were incubated for 15 min at 37°C with monoclonal anti-gB antibodies to induce internalization. Subsequently, gB was immunoprecipitated as described for panel A, and the immunoprecipitates were analyzed for gB (upper row) and α-adaptin (lower row). (C) Coimmunoprecipitation of PRV gB with α-adaptin during spontaneous internalization of gB. At 6 h p.i., PK15 cells infected with either PRV Be or PRV Y902A were lysed and immunoprecipitated with monoclonal anti-α-adaptin antibodies, and the immunoprecipitates were analyzed for the presence of α-adaptin (upper row) and gB (lower row). (D) Densitometric quantification of coimmunoprecipitation (IP) of α-adaptin with wild-type or Y902A gB (left) and of wild-type or Y902A gB with α-adaptin (right) (both relative to wild-type gB band intensity).
Taken together, these results demonstrate that (i) during both spontaneous and antibody-mediated internalization, wild-type PRV gB interacts with AP-2, and (ii) efficient interaction between PRV gB and AP-2 is critically dependent on the integrity of the YQRL motif.
DISCUSSION
In the present study, we found that PRV gB and the cellular clathrin-associated AP-2 adaptor complex colocalize and associate with each other and that mutation of the tyrosine residue in the YQRL motif in the cytoplasmic domain of PRV gB abrogates this interaction. The importance of the YQRL motif in establishing interaction between gB and AP-2 most likely explains how this motif drives spontaneous and antibody-mediated internalization of gB, as we observed in the present study, and how it drives efficient internalization of antibody-antigen complexes in PRV-infected monocytes (13), a process that protects these cells from efficient antibody-dependent lysis (49).
Although we show here that spontaneous and antibody-mediated internalizations of gB are closely related processes, spontaneous endocytosis has been shown to occur solely during the early phases of a PRV infection (<6 h p.i.), whereas antibody-mediated internalization can be triggered up to later time points in infection (references 12 and 44 and the present study). Although the viral and/or cellular factors that downregulate spontaneous internalization are still unknown, it is remarkable that inhibition of spontaneous internalization correlates with the time at which most glycoproteins are abundantly expressed. This may imply that the high expression level of viral glycoproteins like gB overwhelm the endocytosis machinery and that antibody-mediated cross-linking of gB is needed to allow efficient internalization at later time points in infection. In line with this, it has been suggested that ligand-induced multimerization of endocytosis motif-bearing cellular receptors results in a stronger internalization signal by increasing the affinity of these receptors for AP-2 (51). Inhibition of spontaneous internalization also correlates with the time at which the virion host shutoff protein (vhs) of PRV, like that of other alphaherpesviruses, shows efficient downregulation of cellular and viral mRNA levels (5, 44). Perhaps, as has been suggested before (44), this vhs protein may negatively regulate the amounts of the different endocytosis components (clathrin, AP-2, dynamin, etc.), requiring stronger internalization signals to allow efficient internalization of PRV gB. Furthermore, recent data indicate that some PRV cell surface glycoproteins, including gB, may be linked and segregate in lipid raft-like microdomains during later stages of infection (14). Although recent studies indicate that at least some raft-associated proteins may be internalized via clathrin-dependent pathways, lipid rafts most likely do not constitute very efficient platforms for clathrin-mediated internalization (1, 27, 30, 31, 42), perhaps also requiring stronger internalization signals for gB internalization at later time points in infection.
We found that, in PRV-infected PK15 cells, mutation of tyrosine 902 to an alanine residue (Y902A) abrogated the internalization of gB. However, somewhat surprisingly, this mutation did not prevent colocalization of a minority of the cell surface-localized gB molecules and AP-2 in these infected cells. At least two hypotheses can be put forward that may explain this. First, recent data indicate that several of the major PRV cell surface glycoproteins (gB, gC, gD, and gE) are linked and therefore most likely are present in close proximity to each other (14). The exact nature of this link is still putative but may rely on the numerous protein-protein interactions that are believed to exist between viral glycoproteins and tegument proteins (26). Many PRV cell surface glycoproteins other than gB (e.g., gD, gE, and gH) contain potential or functional internalization motifs that may also interact with AP-2, and therefore, the colocalization between a minority of the cell surface-localized gB molecules and AP-2 that we observed in cells infected with the PRV Y902A strain may be attributed to the juxtaposition of gB and other AP-2-interacting viral glycoproteins. An alternative explanation for this weak, but significant, colocalization may be that the mutated YQRL motif or a cytoplasmic domain different from the YQRL motif may display a weak affinity for AP-2. We tend to favor the first hypothesis, since we found that, in contrast to infected cells, in Y902A gB-transfected cells, gB did not show significant colocalization with AP-2.
The data from our coimmunoprecipitation and colocalization assays show that the YQRL motif, but not the YMSI or LL motif, in the cytoplasmic domain of PRV gB is required for interaction between gB and AP-2, further confirming an earlier observation that the LL and YMSI motifs are not involved in endocytosis processes (13). Different hypotheses can be put forward to explain why the YMSI motif and the LL doublet do not interact significantly with AP-2. Perhaps these motifs have very low intrinsic affinity for AP-2. In this context, it is important to note that some LL motifs require an acidic amino acid at position −4 and/or −5 (relative to the first leucine of the doublet), which is absent in PRV gB, in order to be functional (39). Furthermore, it is known that some amino acids at the X position in a YXXφ motif seem to be preferred over others to allow interaction with the μ chain of the AP-2 complex (34). Ohno and colleagues have shown that a YQRL motif, identical to the AP-2-interacting motif in PRV gB, is the most favorable YXXφ motif for this interaction (34). On the other hand, the internalization of HSV gB and VZV gB also depend on the membrane-distal YXXφ motif that corresponds to the PRV gB YQRL motif, although these motifs (YSPL and YSRV) contain less-preferred amino acids at the X position (11, 17). Therefore, although the amino acids at the X position in the YXXφ-type motifs in the gB tail may certainly be significant for its capacity to interact with AP-2, other factors, such as the conformational context of the motif, may be of equal importance. The tyrosine-based motifs in the PRV and HSV gB tails form parts of α-helical domains, and at least for PRV gB, it is believed that these domains adopt a tight-turn conformation (32, 15), which, according to the literature, is favorable for interaction with the μ chain of the AP-2 complex (6, 47).
Interestingly, our present finding that the YQRL motif in the cytoplasmic domain of gB allows gB to interact with a member of the clathrin-associated adaptor protein complexes, i.e., AP-2, may be significant in explaining the important roles of this motif in two other processes: basolateral targeting of gB in polarized cells and direct cell-to-cell spread of PRV (13). Indeed, besides AP-2, there are other members of the adaptor protein complex family that interact with YXXφ motifs, such as the YQRL motif in gB. One of these, AP-1B, drives basolateral sorting in polarized cells. It is known that certain YXXφ motifs, e.g., in the human immunodeficiency virus env protein, interact with both AP-1B and AP-2, thereby driving both basolateral sorting and endocytosis of a protein (33). In this context, it is interesting that, of the different YXXφ motifs, the YQRL motif (like that in PRV gB) has been predicted to be among the most preferred to establish an interaction with both types of adaptor protein complexes (34). Hence, the YQRL motif in gB may allow gB to interact not only with AP-2 but perhaps also with AP-1B, thereby explaining how this motif directs gB to basolateral surfaces in polarized cells. Since YXXφ-mediated deposition of specific viral proteins or virus particles at basolateral surfaces enhances cell-to-cell spread of alphaherpesviruses and other viruses, such as human immunodeficiency virus (19, 21), the possibility that the YQRL motif in PRV gB interacts not only with AP-2 but also with other members of the adaptor protein complex family may provide a clue to how this motif facilitates direct cell-to-cell spread of PRV.
Taken together, our findings suggest that both spontaneous and antibody-mediated internalizations of PRV gB occur via an interaction of the membrane-distal tyrosine-based motif in its cytoplasmic tail with the cellular clathrin-associated AP-2 complex. Therefore, internalization of PRV gB can be considered to be a viral mimicry of cellular receptor endocytosis and may perhaps constitute a common mechanism used by several alphaherpesviruses to achieve internalization of envelope proteins from the surfaces of infected cells.
Acknowledgments
We thank Lynn Enquist, Christoph Hengartner, and Greg Smith for invaluable help with the construction of the mutants; Maurice Pensaert, Peter Delputte, and Kristin Geenen for fruitful discussions; and Carine Boone for excellent technical assistance.
This research was supported by a cooperative research action fund of the Research Council of Ghent University.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. Van der Groot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahle, S., A. Mann, U. Eichelsbacher, and E. Ungewickell. 1988. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 7:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phopsphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Porat, T., J. M. Demarchi, B. Lomnicizi, and A. S. Kaplan. 1986. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology 154:325-334. [DOI] [PubMed] [Google Scholar]
- 5.Berthomme, H., B. Jacquemont, and A. Epstein. 1993. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 193:1028-1032. [DOI] [PubMed] [Google Scholar]
- 6.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. C. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-5795. [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremnes, T., V. Lauvrak, B. Lidqvist, and O. Bakke. 1998. A region from the medium chain adaptor subunit (μ) recognizes leucine- and tyrosine-based sorting signals. J. Biol. Chem. 273:8638-8645. [DOI] [PubMed] [Google Scholar]
- 9.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 10.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 Nef with the μ subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 11.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favoreel, H. W., H. J. Nauwynck, H. M. Halewyck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J. Gen. Virol. 80:1283-1291. [DOI] [PubMed] [Google Scholar]
- 13.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the intracytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favoreel, H. W., T. C. Mettenleiter, and H. J. Nauwynck. 2004. Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of pseudorabies virus-infected cells. J. Virol. 78:5279-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster, T. P., J. M. Melancon, and K. G. Kousoulas. 2001. An alpha-helical domain within the carboxyl terminus of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is associated with cell fusion and resistance to heparin inhibition of cell fusion. Virology 287:18-29. [DOI] [PubMed] [Google Scholar]
- 16.Goodman, O. B., and J. H. Keen. 1995. The α-chain of the AP-2 adaptor is a clathrin binding subunit. J. Biol. Chem. 270:23768-23773. [DOI] [PubMed] [Google Scholar]
- 17.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXφ motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 18.Hirst, J., and M. S. Robinson. 1998. Clathrin and adaptors. Biochim. Biophys. Acta 1404:173-193. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keen, J. H. 1990. Clathrin and associated assembly and disassembly proteins. Annu. Rev. Biochem. 59:415-438. [DOI] [PubMed] [Google Scholar]
- 21.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks, M. S., H. Ohno, T. Kirchhausen, and J. S. Bonifacino. 1997. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell. Biol. 7:124-128. [DOI] [PubMed] [Google Scholar]
- 23.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell. Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 24.Mettenleiter, T. C. 1991. Molecular biology of pseudorabies (Aujeszky's disease) virus. Comp. Immunol. Microbiol. Infect. Dis. 14:151-163. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 26.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabi, I. R., and P. U. Le. 2003. Caveolae/raft-dependent endocytosis. J. Cell Biol. 161:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauwynck, H. J., and M. B. Pensaert. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137-1146. [DOI] [PubMed] [Google Scholar]
- 29.Nauwynck, H. J. 1997. Functional aspects of Aujeszky's disease (pseudorabies) viral proteins with relation to invasion, virulence and immunogenicity. Vet. Microbiol. 55:3-11. [DOI] [PubMed] [Google Scholar]
- 30.Nichols, B. J. 2001. Endocytosis without clathrin coats. Trends Cell. Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 31.Nichols, B. J. 2003. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr. Biol. 13:686-690. [DOI] [PubMed] [Google Scholar]
- 32.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 34.Ohno, H., R. C. Aguilar, D. Yeh, D. Taura, T. Saito, and J. S. Bonifacino. 1998. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273:25915-25921. [DOI] [PubMed] [Google Scholar]
- 35.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein E: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson, J. K., and C. Grose. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 72:1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearse, B. M., and M. S. Robinson. 1984. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 3:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pond, L., L. A. Kuhn, L. Teyton, M. P. Schutze, J. A. Tainer, M. R. Jackson, and P. A. Peterson. 1995. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 270:19989-19997. [DOI] [PubMed] [Google Scholar]
- 40.Rapoport, I., M. Miyazaki, W. Boll, B. Duckworth, L. C. Cantley, S. Shoelson, and T. Kirchhausen. 1997. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 16:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson, M. S. 1987. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J. Cell Biol. 104:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoddart, A., M. L. Dykstra, B. K. Brown, W. Song, S. K. Pierce, and F. M. Brodsky. 2002. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17:451-462. [DOI] [PubMed] [Google Scholar]
- 43.Storch, S., and T. Braulke. 2001. Multiple C-terminal motifs of the 46-kDa mannose 6-phosphate receptor tail contribute to efficient binding of medium chains of AP-2 and AP-3. J. Biol. Chem. 276:4298-4303. [DOI] [PubMed] [Google Scholar]
- 44.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trowbridge, I. S. 1991. Endocytosis and signals for internalization. Curr. Opin. Cell Biol. 3:634-641. [DOI] [PubMed] [Google Scholar]
- 47.Trowbridge, I. S., J. F. Collawn, and C. R. Hopkins. 1993. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9:129-161. [DOI] [PubMed] [Google Scholar]
- 48.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2001. Involvement of cellular cytoskeleton proteins in antibody-induced internalization of viral glycoproteins in pseudorabies virus-infected monocytes. Virology 288:129-138. [DOI] [PubMed] [Google Scholar]
- 49.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, and M. B. Pensaert. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protect pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84:939-948. [DOI] [PubMed] [Google Scholar]
- 50.Voorhees, P., E. Deignan, E. Van Donselaar, J. Humphrey, M. S. Marks, P. J. Peters, and J. S. Bonifacino. 1995. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 14:4961-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.York, S. J., L. S. Arneson, W. T. Gregory, N. M. Dahms, and S. Kornfeld. 1999. The rate of internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J. Biol. Chem. 274:1164-1171. [DOI] [PubMed] [Google Scholar]