Significance
Strong associations between cocaine and the environmental contexts where cocaine is administered are thought to drive relapse. The nucleus accumbens (NAc) encodes these cue–reward associations, and here we determined how cocaine alters the ability of cells in NAc to respond to drug-associated environmental stimuli to drive drug seeking. Using fiber photometry calcium imaging we define the specific population of cells, dopamine D1 receptor-expressing neurons, that encodes information about drug associations and show that these cells can be manipulated to attenuate the strength of drug associations and prevent relapse. Together, these data define a basic circuit mechanism underlying drug–context associations and suggest that pharmacotherapeutic agents aimed at D1-type neurons may help to promote sustained abstinence in cocaine abusers.
Keywords: cocaine, reward, medium spiny neuron, calcium imaging, associative learning
Abstract
The reinforcing and rewarding properties of cocaine are attributed to its ability to increase dopaminergic transmission in nucleus accumbens (NAc). This action reinforces drug taking and seeking and leads to potent and long-lasting associations between the rewarding effects of the drug and the cues associated with its availability. The inability to extinguish these associations is a key factor contributing to relapse. Dopamine produces these effects by controlling the activity of two subpopulations of NAc medium spiny neurons (MSNs) that are defined by their predominant expression of either dopamine D1 or D2 receptors. Previous work has demonstrated that optogenetically stimulating D1 MSNs promotes reward, whereas stimulating D2 MSNs produces aversion. However, we still lack a clear understanding of how the endogenous activity of these cell types is affected by cocaine and encodes information that drives drug-associated behaviors. Using fiber photometry calcium imaging we define D1 MSNs as the specific population of cells in NAc that encodes information about drug associations and elucidate the temporal profile with which D1 activity is increased to drive drug seeking in response to contextual cues. Chronic cocaine exposure dysregulates these D1 signals to both prevent extinction and facilitate reinstatement of drug seeking to drive relapse. Directly manipulating these D1 signals using designer receptors exclusively activated by designer drugs prevents contextual associations. Together, these data elucidate the responses of D1- and D2-type MSNs in NAc to acute cocaine and during the formation of context–reward associations and define how prior cocaine exposure selectively dysregulates D1 signaling to drive relapse.
Drug seeking associated with addiction is mediated in part by strong associations between the rewarding effects of drugs and the environment within which they are administered (1). The nucleus accumbens (NAc) is a key neural substrate of context–reward associations because it mediates multiple aspects of this process, including learning, selecting, and executing goal-oriented behaviors (2–5). Over time, contextual cues in the absence of drug can elicit neural responses that resemble that of the drug itself and trigger drug craving and seeking, resulting in relapse in drug-addicted individuals (1). Furthermore, an inability to extinguish previously formed associations is thought to contribute to pathological drug seeking that persists despite periods of abstinence (6–9). Thus, identifying the specific neuronal populations that drive these behaviors, and determining therapeutic strategies to manipulate the cell populations to promote extinction of these associations, would be advantageous to improving treatment outcomes in drug-addicted individuals.
Increased activity of the mesolimbic dopamine system is a central mechanism underlying the reinforcing and rewarding actions of drugs of abuse, including cocaine, as well as the compulsive drug seeking that develops over time and characterizes an addicted state (10–12). Dopamine action in NAc is mediated predominantly via activation of D1 or D2 dopamine receptors that are expressed by largely nonoverlapping populations of medium spiny neurons (MSNs) (13). These two subtypes of MSNs exert opposite effects on behavior, with optogenetic activation of D1-type neurons promoting positive reinforcement and increasing the formation of cocaine reward–context associations and activation of D2-type neurons being aversive and decreasing cocaine reward (14, 15); related differences in behavioral responses are seen in response to D1 vs. D2 receptor agonists or antagonists (16). Changes in NAc MSN activity in vivo have been documented in response to acute and chronic cocaine (17–19); however, it has not been possible to obtain this information in a cell-type-specific manner.
The aim of the current study is to overcome this major gap in knowledge and define changes in D1- and D2-type MSN activity in vivo driven by acute and chronic exposures to cocaine and to determine how these cell types respond during context–reward associations. Thus, whereas the optogenetic studies cited above define the consequences of D1 or D2 MSN stimulation on reward-related behaviors, how the cell types respond under physiological conditions remains unknown. Furthermore, because optogenetic stimulations are experimenter-defined they eliminate the temporally specific nature of the endogenous signaling that encodes information. Advances in Ca2+ imaging have made it possible to record from genetically distinct subpopulations of neurons in awake behaving animals (20, 21). Here, we combine Ca2+ imaging and fiber photometry with conditioned place preference (CPP) to establish the patterns of activity of D1- and D2-type MSNs in NAc during formation of reward–context associations and to determine how these patterns are dysregulated by prior chronic exposure to cocaine. We then causally establish the role of altered firing of a given cell type to key aspects of context–reward associations by use of chemogenetic approaches.
Results
Biphasic Ca2+ Responses in NAc to Cocaine-Associated Contextual Cues.
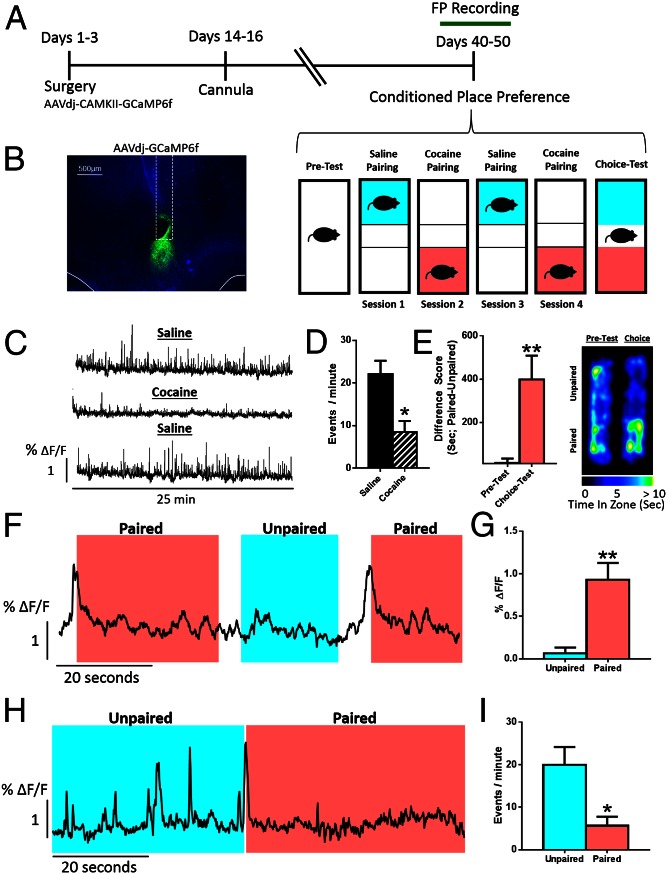
To examine real-time activity of NAc neurons in vivo during associative learning for drug rewards, we injected wild-type C57BL/6J mice with AAV-GCaMP6f—which infects all types of neurons but not nonneuronal cells—into NAc core and recorded Ca2+ transients through an optic fiber (20) while mice were trained for CPP (Fig. 1 A and B). During pairing, cocaine reduced the frequency of Ca2+ transients in NAc (Fig. 1 C and D). In a choice test 24 h after the final pairing, animals spent more time on the side that was previously paired with drug, indicating that cocaine had induced a place preference via an association between the context and the rewarding effects of cocaine (Fig. 1E). Robust spikes in Ca2+ activity were recorded immediately preceding entry into the drug-paired, but not saline-paired, chamber (Fig. 1 F and G). Further, firing was suppressed while the mouse remained in the drug-paired chamber (Fig. 1 H and I).
Fig. 1.
Biphasic responses of NAc neurons to cocaine-associated cues. (A) Timeline of the experimental design. (B) Viral expression of AAV-GCaMP6f and placement of the fiber-optic probe in NAc core. (C) Representative Ca2+ traces from a single animal over pairing sessions. Data are represented as the percent change in fluorescence over the mean fluorescence (ΔF/F). (D) Peak analysis of Ca2+ imaging traces. Cocaine reduces the number of events [Student’s t test; t(5) = 3.48, P < 0.05, n = 6]. (E) (Left) Animals formed a preference for the chamber associated with cocaine [Student’s t test; t(5) = 4.04, P < 0.01, n = 6]. (Right) Heat maps showing time spent in each area of the chamber. (F) Representative traces demonstrating the temporal profile of NAc activity around paired and unpaired chamber entry. (G) Quantification of the peak amplitude of the Ca2+ signal in the five seconds preceding paired and unpaired chamber entry [Student’s t test; t(5) = 4.38, P < 0.01, n = 6]. (H) Representative trace showing the change in event frequency when the animal is in the paired or unpaired chamber. (I) Quantification of event frequency [Student’s t test; t(5) = 2.68, P < 0.05, n = 6]. *P < 0.05, **P < 0.01.
Specific Contribution of D1 and D2 MSNs to Learning Cocaine-Associated Cues.
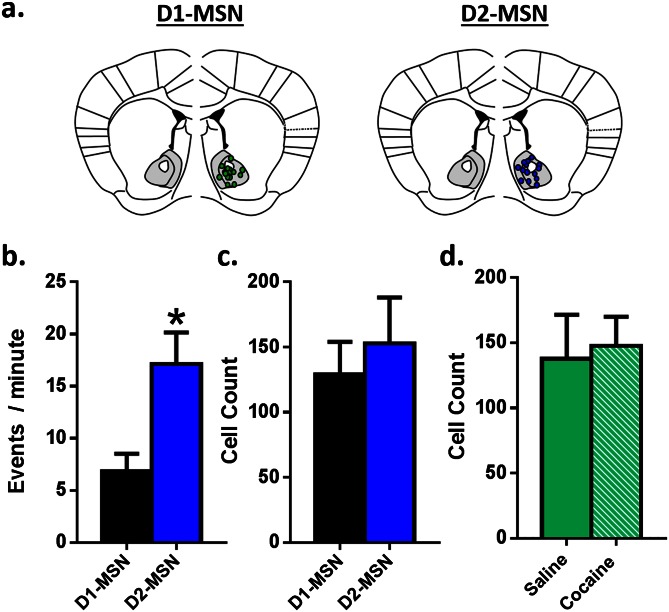
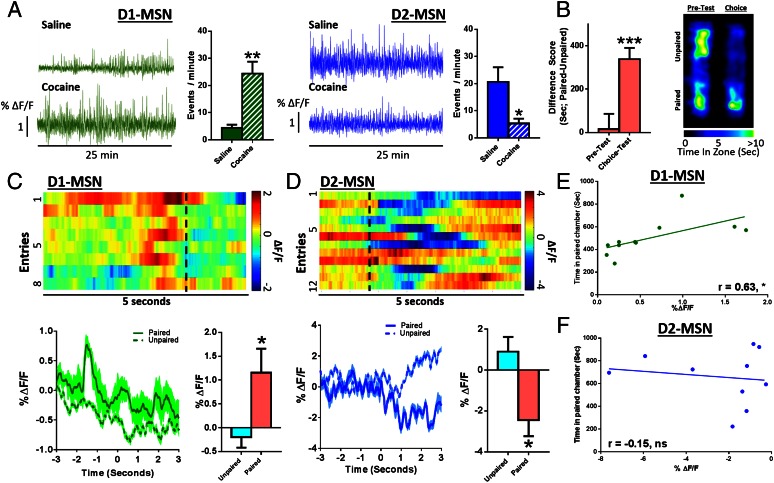
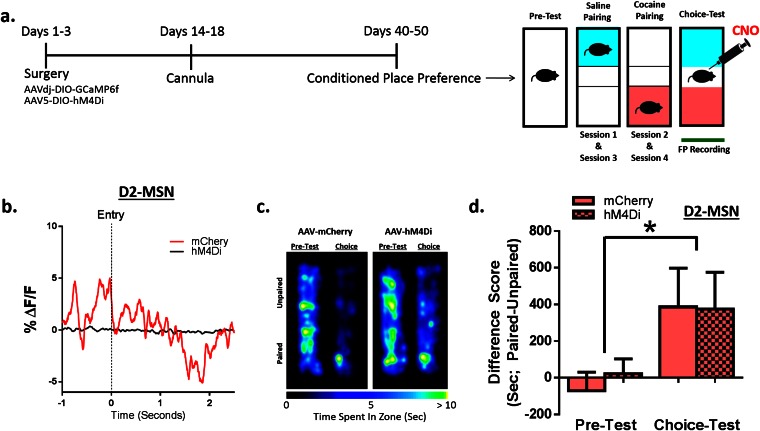
These changes in NAc neuronal activity presumably reflect altered firing of MSNs, which comprise >95% of all NAc neurons. To determine the MSN subtypes that mediate the two distinct phases of NAc activity during context–reward associations, we injected AAV-DIO-GCaMP6f into NAc of mice expressing Cre-recombinase in either D1 or D2 MSNs to induce GCaMP6f expression specifically in each cell type (Fig. S1). At baseline, D2 MSNs displayed greater than fivefold higher Ca2+ transient frequency than D1 MSNs (Fig. 2 C–E). Expression levels of GCaMP6f were not different between the neuron subtypes, indicating that the effect is physiological and not an artifact of the mouse line or viral expression (Fig. S1 A and B). Because MSNs make up the vast majority of NAc neurons, the most parsimonious explanation of these findings is that D2 MSNs are more active at baseline, although one important caveat is that D2 receptors are also expressed by certain NAc interneurons. Single cocaine injections, during training, increased D1 MSN Ca2+ transient frequency, while reducing D2 frequency (Fig. 2A). During the subsequent choice test (Fig. 2B), entry into the drug-paired context similarly increased D1 and decreased D2 firing frequency (Fig. 2 C and D), eliciting the same physiological response as cocaine had previously, suggesting that these contextual cues acquire rewarding properties (22). D1 and D2 MSNs displayed distinct temporal profiles of firing; increased D1 MSN activity immediately preceded entry into the drug-paired context (Fig. 2C and Movie S1), whereas D2 activity was suppressed only after entry (Fig. 2D and Movie S2). It is possible that D1 activity drives the motivation to enter a paired compartment, with the reduced activity of D2 cells after entry driving the motivation to remain in that compartment. Further, these data suggest that the biphasic neural signature of global NAc neuronal activity (Fig. 1 H and I) is mediated by the two distinct populations of MSNs. Additionally, during choice testing, D1 MSN peak amplitude in the 5 s preceding entry into the drug-paired context correlated with time spent in the drug-paired side (Fig. 2E), suggesting that D1, but not D2 (Fig. 2F), signaling specifically drives expression of place preference.
Fig. S1.
Viral targeting and infection rate of the calcium indicator GCaMP6f across mouse strains. (A) Representative image denoting recording sites in D1-Cre (Left) and D2-Cre (Right) mouse lines. (B) D2 MSNs displayed a greater number of events per minute compared with D1 MSNs under baseline conditions. (C) Cell counting was carried out to determine the number of cells within each mouse line that expressed the calcium indicator. There were no differences between D1- or D2-Cre mice. (D) There were also no differences in the number of cells expressing the calcium indicator between saline- and cocaine-pretreated animals. *P < 0.05 vs. control. All data are presented as mean ± SEM.
Fig. 2.
Specific contribution of D1 and D2 NAc MSNs to learning cocaine-associated cues. (A) Representative Ca2+ traces and peak analysis from a D1-Cre (Left, green) and D2-Cre (Right, blue) mouse showing higher activity of D2-MSNs under baseline conditions. Cocaine increases D1 events [Student’s t test; t(3) = 5.84, P < 0.05, n = 4] and decreases D2 events [Student’s t test; t(4) = 2.92, P < 0.05, n = 4] in NAc. (B) (Left) Animals formed a conditioned preference [Student’s t test; t(21) = 3.96, P < 0.001, n = 22]. (Right) Heat maps showing time spent in each area of the CPP chamber. (C) (Top) Representative heat map of D1-MSN-mediated Ca2+ signaling during successive entries into the drug-paired chamber. (Bottom) Averaged D1 traces (also see Movie S1). Quantification of peak amplitude of Ca2+ events 5 s around entry [Student’s t test; t(10) = 2.61, P < 0.05, n = 11]. (D) (Top) Heat map of D2-MSN-mediated Ca2+ signaling during successive entries into the drug-paired chamber. (Bottom) Averaged D2 traces (also see Movie S2). Quantification of peak amplitude of Ca2+ events [Student’s t test; t(9) = 2.70, P < 0.05, n = 10]. (E) Correlation analysis showing the relationship between CPP and the amplitude of D1 events preceding drug-paired chamber entry (r = 0.63, P < 0.05, n = 11). (F) Correlation analysis showing no relationship with D2 events (r = −0.15, P = not significant, n = 10). *P < 0.05, **P < 0.01, ***P < 0.001.
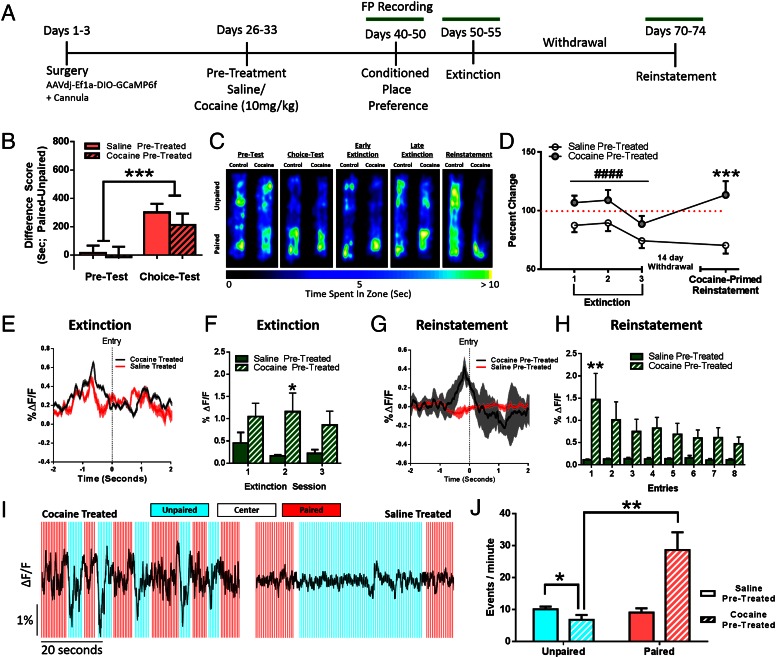
Cocaine Pretreatment Alters D1 MSN Signaling in Association with Reduced Extinction.
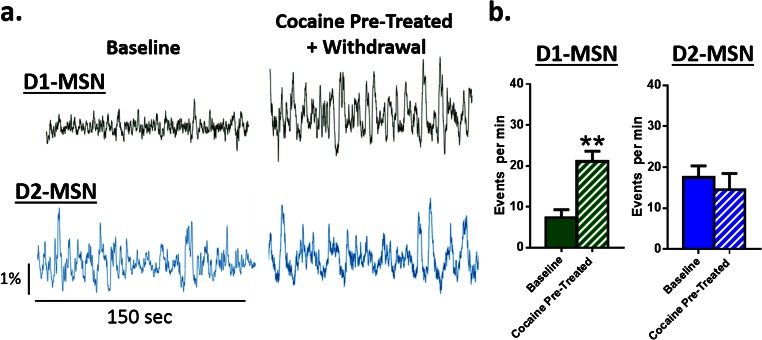
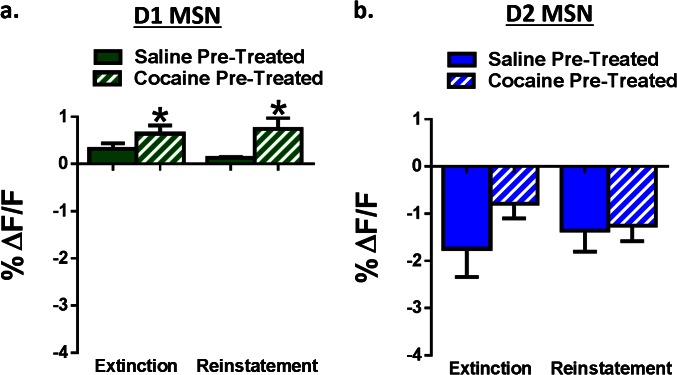
Animals were injected for 7 d with 10 mg/kg i.p. cocaine in their home cage followed by 7 d of withdrawal (Fig. S2) and then baseline MSN activity was recorded followed by CPP (Fig. 3A). Prior cocaine administration selectively increased baseline D1 MSN activity without affecting D2 activity (Fig. S3). Further, although cocaine administration did not alter initial CPP strength (Fig. 3 B and C) it impaired later extinction of the place preference, indicating a latent potentiation of the cocaine–context association (Fig. 3 C and D). This effect associated with heightened D1 activity (Fig. 3E) preceding drug-paired chamber entry that uniquely persisted throughout extinction in cocaine-preexposed animals (Fig. 3F). Cocaine pretreatment did not alter the magnitude of D2 MSN responses to the drug-associated contexts during choice testing, extinction, or reinstatement (Fig. S4). These findings suggest that cocaine experience alters associative learning processes by selectively enhancing D1 MSN activity, to potentiate the association between drugs and cues and promote relapse during periods of abstinence.
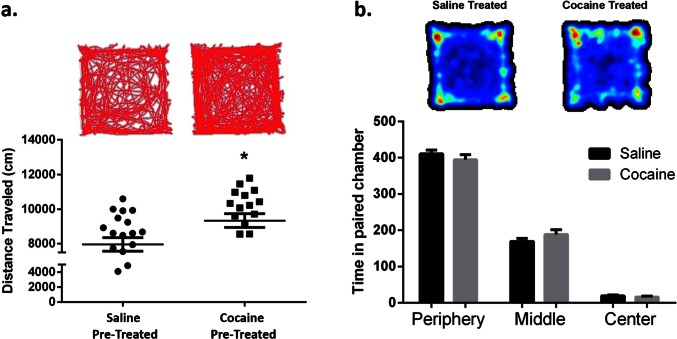
Fig. S2.
Cocaine preexposure increases cocaine-induced locomotor activity. (A) Open-field analysis plotting distance traveled in centimeters over a 10-min session. Animals were placed into the chamber 15 min following an i.p. injection of 10 mg/kg cocaine. Cocaine-pretreated animals exhibited increased cocaine-induced locomotor activity compared with saline treated controls [Student’s t test; t(38) = t = 2.50, P < 0.05, n = 22, 19]. Interestingly, this sensitized locomotor response did not correlate with reduced extinction on an individual animal basis, suggesting the involvement of at least partly different underlying mechanisms. (B) Open-field analysis showing time spent in each area of the open-field chamber. There were no differences between groups. *P < 0.05 vs. control. All data are presented as mean ± SEM.
Fig. 3.
Chronic cocaine administration alters D1 MSN signaling in association with reduced extinction and facilitated reinstatement of CPP. (A) Experimental timeline. (B) Animals form a preference for the drug-paired chamber [two-way ANOVA; F (1, 88) = 14.17, P < 0.001, n = 14]; cocaine pretreatment does not change CPP. (C) Heat maps showing time spent in each chamber. (D) Chronic cocaine administration impairs extinction and facilitates cocaine-primed reinstatement [two-way ANOVA; F (1, 147) = 20.08, P < 0.0001; n = 20]. Data plotted as percent change from the original choice test. During extinction test 1, cocaine pretreated animals increased their preference (one-sample t test; t22 = 2.56, P < 0.05). During reinstatement, cocaine-pretreated animals reinstated above their original choice test values (one-sample t test; t22 = 2.09, P < 0.05). (E) Representative traces averaged over entries showing increased amplitude of D1 MSN Ca2+ activity at paired chamber entry during extinction. (F) Peak amplitude analysis. Saline-treated animals extinguish D1 MSN responses; cocaine pretreated animals do not [two-way ANOVA; F (1, 17) = 4.492, P < 0.05, n = 10]. (G) Representative traces averaged over entries showing increased amplitude of D1 MSN Ca2+ activity at paired chamber entry during cocaine-primed reinstatement. (H) Peak amplitude analysis over trials. Cocaine-treated animals reinstate D1 MSN responses, whereas saline-pretreated animals do not [two-way ANOVA; F (1, 12) = 5.262, P < 0.05, n = 6, 9]. (I) Representative D1 MSN traces from a cocaine-treated (Left) and saline-treated (Right) animal during reinstatement. Acute effects of the cocaine challenge are augmented in cocaine pretreated animals, but only when the animal is in the paired chamber. (J) Peak analysis: cocaine effects are enhanced in cocaine-pretreated animals in the previously drug-paired context [two-way ANOVA; F (1, 4) = 22.16, P < 0.01, n = 3]. *P < 0.05, **P < 0.01, ***P < 0.001, #### P < 0.0001.
Fig. S3.
Cocaine pretreatment alters baseline D1, but not D2, MSN activity. (A) Representative calcium traces from D1 (Top, green) and D2 (Bottom, blue) MSNs at baseline and following 7 d of cocaine injections and withdrawal. (B) Peak analysis of D1 MSNs (Left) showing that cocaine and withdrawal increases the number of events in the NAc relative to baseline [Student’s t test; t(5) = 4.23, P < 0.01, n = 6]. In contrast, D2 MSN activity (Right) was unchanged [Student’s t test; t(6) = 1.24, P = not significant, n = 7]. **P < 0.01 vs. control. All data are presented as mean ± SEM.
Fig. S4.
Cocaine pretreatment alters temporally specific D1, but not D2, MSN activity. (A) Group data showing that peak D1 amplitude is increased in cocaine pretreated animals during both extinction and reinstatement compared with saline-pretreated controls [two-way ANOVA; F (1, 26) = 6.44, P < 0.05, n = 6, 9]. (B) Group data showing that D2 signaling is unchanged between cocaine and saline pretreated animals during both extinction and reinstatement. *P < 0.05. All data are presented as mean ± SEM.
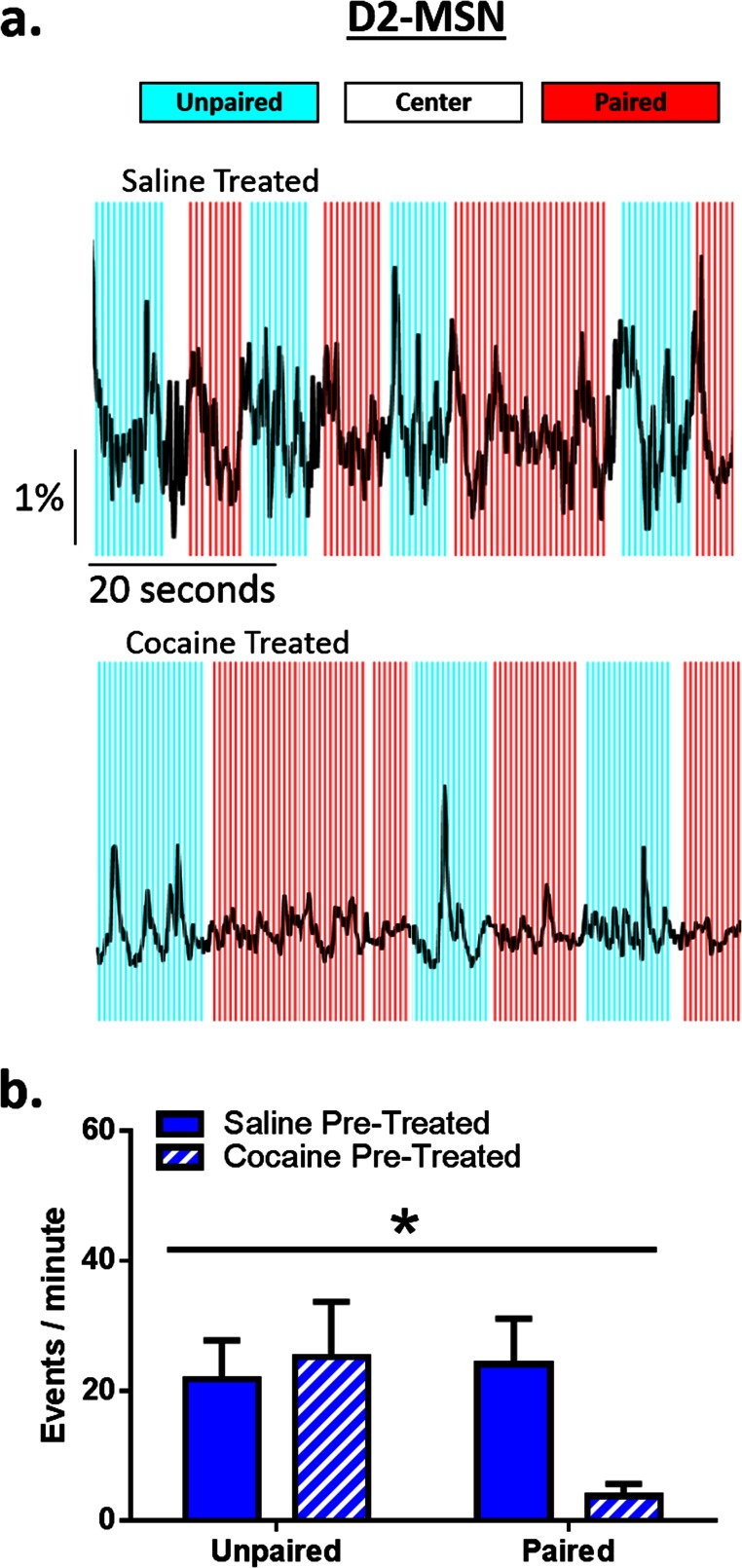
Cocaine Pretreatment Facilitates Reinstatement of Conditioned Place Preferences.
Previous work has shown that prior cocaine exposure induces time-dependent increases in cocaine seeking (6, 23). We found that a subthreshold challenge dose of cocaine [5 mg/kg i.p.; i.e., a dose insufficient to induce preference in cocaine-naïve animals (24)] induced reinstatement of CPP only in animals with a history of prior chronic cocaine exposure and that the reinstated preference exceeded the initial preference for the drug-paired chamber (Fig. 3D). This increased preference was accompanied by an augmented spike in D1, but no change in D2 (Fig. S4), MSN activity preceding drug-paired chamber entry and this persisted over many entries (Fig. 3 G and H). Further, this potentiated reinstatement was associated with selective enhancement of the acute effects of cocaine—opposite in the two MSN subtypes—on both D1 MSNs (Fig. 3 I and J) and D2 MSNs (Fig. S5), only when the animal was in the drug-paired context. Unpaired chamber entry associated with consistent suppression of D1 activity, an effect not seen in animals without prior cocaine experience, and this was accompanied by reduced time spent in the unpaired chamber (Fig. 3 I and J). Thus, chronic cocaine produces a state in which cocaine-associated contexts activate the same neuronal mechanisms as acute cocaine administration, which may be a primary mechanism by which repeated cocaine administration strengthens cue–reward associations that drive relapse, even following periods of extended abstinence (6).
Fig. S5.
The acute effects of cocaine are enhanced by drug-associated contextual cues in cocaine-, but not saline-, pretreated animals. (A) Representative traces showing D2 signaling across 60 s of a conditioned place preference session in saline-treated (Top) and cocaine-treated (Bottom) animals following a challenge dose of 5 mg/kg cocaine. (B) Frequency analysis during time in the paired and unpaired chamber in D2 MSNs during a cocaine-primed reinstatement session. Cocaine reduced D2 firing, only when the animal was present in the previously drug-paired context [two-way ANOVA; interaction; F (1, 4) = 10.12, P < 0.05, n = 3]. *P < 0.05 ANOVA. All data are presented as mean ± SEM.
D1 MSN Activity Is Required for the Acquisition and Expression of Cocaine Conditioned Place Preferences.
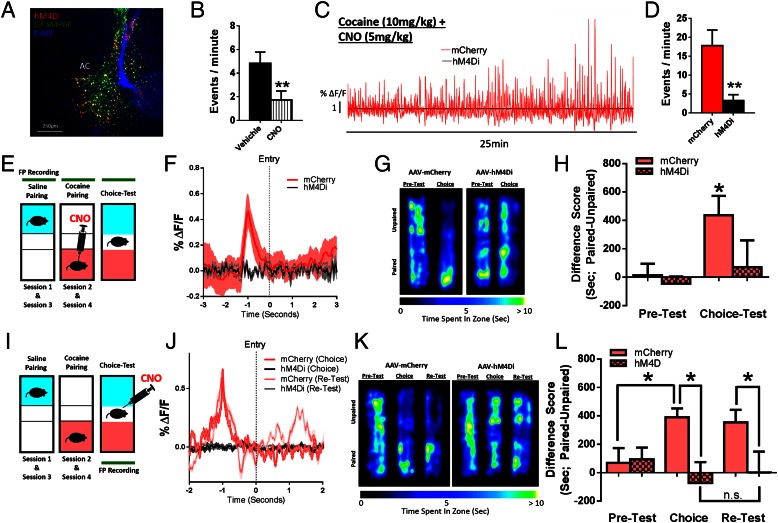
To confirm this causal role of D1 MSNs in associative learning, we expressed the inhibitory designer receptors exclusively activated by designer drugs (DREADD), hM4Di, selectively in D1 MSNs and administered clozapine N-oxide (CNO; 5 mg/kg i.p.) before cocaine pairing to inhibit D1 activity (Fig. 4). CNO administration at this dose inhibited D1-specific Ca2+ activity (Fig. 4B). When CNO was administered 1 h before each cocaine pairing it blocked the cocaine-induced increases in D1 activity (Fig. 4 C and D). Further, it blocked both the preference for the drug-paired chamber as well as the spike in D1 MSN activity that preceded entry into the drug-paired chamber (Fig. 4 E–G), showing that this signal is indeed required for associative learning for drug rewards.
Fig. 4.
D1 MSN activity is required for the expression of cocaine CPP. (A) Confocal image showing hM4Di and GCaMP6f coexpression in NAc. (B) CNO reduces D1-mediated Ca2+ activity. (C) Representative D1 Ca2+ traces from mCherry (red) and hM4D1 (black) animals following cocaine (10 mg/kg i.p.). (D) Peak analysis of D1 MSNs. CNO reduces the frequency of events [Student’s t test; t(5) = 4.87, P < 0.01, n = 6]. (E) Animals were injected with CNO (5 mg/kg i.p.) before pairing sessions. (F) Representative traces averaged over entries. (G) Time spent in each area of the CPP chamber. (H) Animals form a preference for the drug-paired chamber, with CNO reducing the time spent in the drug-paired chamber [two-way ANOVA; F (1, 28) = 4.55, P < 0.05, n = 8]. (I) Animals were injected with CNO before choice test . (J) Representative traces averaged over entries showing increased amplitude of D1 MSN Ca2+ activity at paired chamber entry. (K) Time spent in each area of the CPP chamber. (L) Animals form a preference for the drug-paired chamber, with CNO reducing the time spent in the drug-paired chamber. The reduced preference in hM4Di animals remained 2 wk after the initial choice test [two-way ANOVA; F (1, 37) = 5.32, P < 0.05, n = 5, 10]. *P < 0.05, **P < 0.01.
To assess whether the D1 signal is also causal for the decision to enter the drug-paired chamber, we administered CNO on choice test day (Fig. 4H). CNO administration completely abolished the temporally specific increase in D1 MSN activity that is associated with entry into the drug-paired chamber as well as the expression of a place preference (Fig. 4 I–K). Further, when animals were examined in a choice test 2 wk later, long after CNO had cleared, preference scores remained down (Fig. 4K), suggesting that inhibiting D1 firing in the presence of the associated context is sufficient to extinguish the association indefinitely. This could reflect enhanced extinction learning or blockade of reconsolidation. Regardless, the effect was specific for D1 MSNs: Inhibiting D2 MSN activity during the choice test had no effect on cocaine place preference (Fig. S6).
Fig. S6.
Blocking D2 MSN activity during posttesting does not alter place preference for cocaine. (A) Animals were injected with AAV-DIO-hM4D to inhibit the activity of D2 MSNs. Animals were injected with CNO (5 mg/kg) before posttest sessions to determine the causal relationship between MSN activity and the expression of previously learned associations. (B) Representative traces showing that D2 MSN activity at paired chamber entry was blocked for hM4D (black) but not mCherry (red) animals. (C) Heat maps showing time spent in each area of the CPP chamber for D2-Cre animals that expressed AAV-DIO-mCherry (Left) or AAV-DIO-hM4D (Right). (D) Group data showing that animals form a preference for the drug-paired chamber [two-way ANOVA; F (1, 7) = 6.27, P < 0.05, n = 5] that is not blocked by CNO administered 1 h before preference testing. *P < 0.05. All data are presented as mean ± SEM.
Discussion
Results of the present study demonstrate distinct patterns of D1 and D2 MSN signaling in NAc during cocaine reward learning, extinction, and reinstatement and provide fundamentally new insight into the circuit basis of drug–cue associations and drug seeking. We show that D2 MSNs exhibit manyfold higher activity than D1 MSNs in NAc at baseline, that acute cocaine administration enhances D1 and suppresses D2 MSN activity, and that cocaine-induced facilitation of D1 MSN activity is required for formation of cocaine–context associations. Further, temporally precise, cell-type-specific signaling encodes contextual information about cocaine experiences such that increased D1 activity precedes entry into a drug-paired context, with decreased D2 activity occurring only after entry. Further, prior chronic exposure to cocaine impairs extinction of contextual associations by preventing the concomitant extinction of D1 MSN signaling that precedes drug-paired context entry. Inhibiting this D1 signal by DREADD-induced D1 MSN inhibition blocked the expression of conditioned preference, confirming that D1 signaling is the critical mediator of drug-context learning. More importantly, reducing the activity of D1 MSNs is sufficient to block (i.e., extinguish) the expression of preference, an effect that persists for weeks. Together, these data elucidate cell-type-specific engrams that contribute to the encoding of cocaine reward, and a D1 MSN-mediated mechanism by which cocaine exposure retards extinction of drug–cue associations to promote relapse.
The precise temporal profile of the encoding of cue–reward associations in MSNs provided here now enables elucidation of the circuit-wide signaling that controls these responses to drive reward- and reinforcement-related behaviors. The majority of work outlining the neural networks underlying cue–reward associations for both natural and drug rewards has shown that the ventral tegmental area (VTA) and NAc exhibit temporally specific signaling in response to unexpected rewards (25). However, once animals have learned the association between cues and reward availability the neural response transfers to the reward-predictive cue, thus guiding behavior toward environments that will result in obtaining that reward (26). Studies using in vivo pharmacological approaches have demonstrated differential roles of NAc D1 and D2 receptors in drug conditioning by use of selective receptor agonists or antagonists, further supporting a role for both dopamine and D1 and D2 MSN subtypes in associative learning (27). Whereas this work has focused on the VTA-to-NAc dopamine circuit, tracking postsynaptic responses in NAc MSNs is particularly important because they integrate information not only from VTA dopamine neurons but also from numerous glutamatergic projections (28, 29). From a network perspective, D1 and D2 MSNs receive inputs from several regions known to encode and store information about context or context–drug associations such as the prefrontal cortex, basolateral amygdala, and hippocampus (30). Although dopamine plays an integral role in controlling motivated behaviors, its effects are largely modulatory, and it is the glutamatergic inputs that provide the excitatory drive for these circuits. Thus, interactions between dopamine and glutamate are critical in the case of drugs of abuse where dopaminergic involvement changes over the course of drug administration. Initially, dopaminergic responses to cues predicting drug availability drive drug seeking; however, over time, dopaminergic responses to cocaine-associated cues are blunted, whereas sensitization occurs to the motivational aspects of drug seeking and taking (28). For example, despite blunted dopaminergic responses, multiunit recordings have shown that enhanced cue-induced responses in NAc remain intact (17, 19). Further, recent findings have shown that repeated cocaine injections strengthen glutamatergic inputs onto D1 MSNs specifically (31), which could underlie some of the changes in cue responsivity that are seen in the current study. Together, these data suggest that, although dopamine is likely highly involved, it is the activity of the NAc, regardless of originating influences, that mediates these behaviors.
Although suppressing D1 MSN activity during CPP testing reduced the choice to enter the drug-paired chamber, we were surprised to find that this manipulation also eliminated the association indefinitely. Subsequent testing showed that blocking D1 MSNs while the animal was exposed to the cocaine-paired context facilitated the extinction of these associations. Thus, it is likely that the D1 signal not only guides animals to seek the reward, driving them to enter the drug-paired context, but the lack of signal also functions to update new information as associations change. In other words, eliminating the D1 signal in the drug-paired context indicates that the association no longer exists. The ability to attenuate these associations is important because we show here that prior chronic cocaine exposure inhibits the ability of this signal to be extinguished. These data suggest that pharmacological intervention could help to eliminate or attenuate these associations in drug-addicted individuals in a way that would greatly improve treatment outcomes.
Prior chronic cocaine exposure not only inhibits extinction of place conditioning but also facilitates reinstatement of place conditioning. Similar dysregulated associative learning concerning environmental stimuli and rewards occurs in human drug addicts, and an inability to extinguish previously formed associations is thought to contribute to pathological drug seeking as well as relapse (6, 7). Our findings provide a neural mechanism for the deficits in extinction and enhanced reinstatement, whereby cocaine treatment (i) dysregulates the ability of D1 MSNs to respond to context information and update information when the association no longer occurs and (ii) enhances the effects of challenge doses of cocaine on D1 MSN signaling only when animals are in the drug-paired context. These data show that, in addition to the contextual cues eliciting a temporally specific response, the context in which drug is administered can greatly enhance the pharmacological effects of the drug, as has been demonstrated in many other settings (32, 33). Enhancement of the pharmacological effects of drugs is likely one mechanism by which the presentation of drug-paired cues can increase the motivation to administer cocaine.
Our data highlight the important role played by D1 MSNs in NAc core in establishing context–reward associations and in controlling the strength of these associations after cocaine exposure. This prominent influence of D1 MSNs in cocaine action is consistent with previous studies that have shown that chronic cocaine exposure alters D1 MSNs selectively via both changes in dendritic morphology and function as well as changes in gene transcription and epigenetic mechanisms that last long into the abstinence period (34–36). Further work is needed to determine whether similar patterns of D1 MSN activity in NAc core as shown here are also observed in NAc shell and to establish the function of cocaine-induced changes in D2 MSNs that have also been demonstrated.
Here we show that regulation of associative learning, and its dysregulation by cocaine, is driven primarily through alterations in D1 MSNs in NAc core, which both impair the extinction of previously learned associations and enhance reinstatement following abstinence. The magnitude of D1 firing to reward-associated cues may play a critical role in determining the perceived value of a reward and bias decision making toward outcomes most likely to result in obtaining that reward. Further, we show that inhibiting D1 MSNs in NAc is sufficient to prevent preference for contexts previously paired with a drug reward, an effect that lasts indefinitely. These findings suggest that therapies targeted selectively to D1 MSNs may help to normalize D1 activity following a history of cocaine use and prevent relapse. Additionally, the causal role of temporally specific cue-elicited D1 signaling in the expression of learned associations demonstrates the importance of this cell type in the development of fundamental associative learning processes. Disruption of associative learning is a hallmark of diverse psychopathologies that span developmental stages. Demonstrating the causal role of NAc D1 MSN signaling in mediating these processes provides fundamental insight to guide the development of novel targeted therapeutic interventions not only for addiction but also for a wide range of psychiatric disorders.
Methods
See SI Methods for more detailed explanations.
Experimental Subjects.
D1-Cre and D2-Cre BAC transgenic mice on a C57BL/6J background were obtained from N. Heintz, P. Greengard, C. Gerfen, and National Institute of Neurological Disorders and Stroke/Gene Expression Nervous System Atlas (NINDS/GENSAT) (www.gensat.org/index.html) from Rockefeller University/National Institute of Mental Health. C57BL/6J wild-type mice were obtained from The Jackson Laboratory (SN: 000664). All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
Stereotaxic Virus Injection and Cannula Implantation.
AAVdj-CaMKIIα-GCaMP6f-WPRE (for pan-neuronal recordings), AAVdj-EF1α-DIO-GCaMP6f-WPRE (for cell-type-specific recording), or AAV5/hSyn-DIO-hm4Di-mcherry [University of North Carolina at Chapel Hill (37), for DREADD inhibition studies] was infused into NAc core. Chronically implantable optic fibers (Doric Lenses) were positioned above the injection site.
Fiber Photometry Ca2+ Imaging.
Fiber photometry uses the same fiber to both excite and record from GCaMP in real time. The system used two light-emitting diodes at 490 and 405 (Thor Labs), reflected off dichroic mirrors (Semrock, FF495), and coupled into a 400-μm 0.48 N.A. optical fiber (BFH48-600; Thorlabs). Analysis of the resulting signal was performed with custom-written MATLAB software.
Behavioral Testing.
CPP was performed in a rectangular apparatus consisting of two side chambers with different contextual cues. Mice were paired with cocaine in one of the chambers and saline in the other chamber. After pairing, during a choice test, mice were allowed to freely explore the entire apparatus and preference for each chamber was determined. During extinction animals were placed into the chamber with free access. For reinstatement, animals were injected with 5 mg/kg cocaine i.p. immediately before being placed into the chamber with free access. For DREADD experiments, CNO (5 mg/kg) was administered 1 h before the session.
Statistics.
For groups of three or more, one-way ANOVA was run. For groups of two a two-tailed Student’s t test was run. Significance was set at P < 0.05 for all tests. All data are expressed as mean ± SEM.
SI Methods
Experimental Subjects.
Male 6- to 8-wk-old C57BL/6J mice were maintained on a 12-h light–dark cycle at 22–25 °C with ad libitum access to food and water. Mice were housed five per cage except after fiber implantation surgery, at which point mice were singly housed. The experimenter was blinded to experimental group and order of testing was counterbalanced during behavioral experiments.
Transgenic Mice.
D1-Cre and D2-Cre BAC transgenic mice on a C57BL/6J background were obtained from N. Heintz, P. Greengard, C. Gerfen, and NINDS/GENSAT (www.gensat.org/index.html) from Rockefeller University/NIH/National Institute of Mental Health. Mice were backcrossed at least 10 generations with C57BL/6J wild-type mice before use. C57BL/6J wild-type mice were obtained from JAX (SN: 000664).
Viral Constructs.
For general GCaMP expression, GCaMP6f was cloned into the adeno-associated virus (AAV) vector, AAVdj-CaMKIIα-GCaMP6f-WPRE. To achieve cell-type-specific expression in Cre driver lines, we cloned the GCaMP6f into the double-floxed inverted ORF plasmid AAVdj-EF1α-DIO-GCaMP6f-WPRE. DREADD constructs were purchased from the vector core at the University of North Carolina at Chapel Hill. rAAV5/hSyn-DIO-hm4Di-mcherry was used to selectively inactivate D1 or D2 MSNs with CNO, and rAAV5/hSyn-DIO-mCherry was used as the control.
Stereotaxic Virus Injection and Cannula Implantation.
Under ketamine (100 mg/kg)/xylazine (10 mg/kg) anesthesia, mice were positioned in a stereotaxic frame (Kopf Instruments) and the NAc was targeted (bregma coordinates: anterior/posterior, 1.4 mm; medial/lateral, 1.5 mm; dorsal/ventral, −4.4 mm; 10° angle). Ophthalmic ointment was applied to the eyes to prevent drying. A midline incision was made down the scalp and a craniotomy was made using a dental drill. A 10-mL Nanofil Hamilton syringe (WPI) with a 34-gauge beveled metal needle was used to infuse virus. Virus was infused at a rate of 100 nL/min. Following infusion, the needle was kept at the injection site for 10 min and then slowly withdrawn. AAV vectors expressing GCaMP6f under the control of the CaMKIIa or EF1a promoter (AAVdj-CaMKIIa-GCaMP6f or AAVdj-EF1a-DIO-GCaMP6f) were used for recording from cells in the NAc. Chronically implantable optic fibers constructed with 400-μm core 0.48 N.A. optic fiber (Doric Lenses) threaded through ceramic zirconia ferrules were implanted in NAc. Fiber-optic cannulae were positioned above the viral injection site (bregma coordinates: anterior/posterior, 1.4 mm; medial/lateral, 1.5 mm; dorsal/ventral, −4.3 mm; 10° angle) and were cemented to the skull using dental acrylic (Grip Cement Dentsply). For robust viral expression, viral infusions occurred a minimum of 4–6 wk before stimulation. When fiber-optic cannulae were not implanted the incision was closed using Vetbond tissue adhesive and staples.
Fiber Photometry Calcium Imaging.
Fiber photometry uses the same fiber to both excite and record from the genetically encoded calcium indicator GCaMP in real time and thus can allow for temporally precise measurements of neuronal activity that can then be time-locked to a specific behavioral output. The utilization of this technique in transgenic Cre lines allows for cell-type-specific recording of population activity in freely moving animals during behavioral tasks. The fiber photometry system used two light-emitting diodes at 490 and 405 (Thorlabs), reflected off dichroic mirrors (FF495; Semrock), and coupled into a 400-μm 0.48 N.A. optical fiber (Thorlabs BFH48-600, important for minimal autofluorescence and signal recovery) using a 40 × 0.48 N.A. microscope objective (Olympus) and fiber launch (Thorlabs), with the patch cord linked to an implanted 400-μm optical fiber with zirconia sheath. The light-emitting diode intensity at the interface between the fiber tip and the animal ranged from 30 to 75 μW (but was constant across trials and over days). The power output of the system was tested with a power meter at the start of each experimental session. A real-time signal processor (RX8; Tucker-Davis Technologies), running software that was custom-designed using OpenEx, sinusoidally modulated each laser’s output. The two output signals were projected onto a photodetector (model 2151 femtowatt photoreceiver; Newport), after which they were separated for analysis. Signals were collected at a sampling frequency of 381 Hz.
Fiber Photometry Analysis.
Analysis of the signal was done with custom-written MATLAB software. The bulk fluorescent signals from each channel were normalized to compare across animals and experimental sessions. The 405 channel was used as the control channel. GCaMP signals that are recorded at this wavelength are not calcium-dependent; thus, changes in signal can be attributed to autofluorescence, bleaching, and fiber bending. Accordingly, any fluctuations that occurred in the 405 control channels were removed from the 490 channel before analysis. To do this, the 405 signal was filtered to remove events that exceeded 40 Hz. Change in fluorescence (ΔF) was calculated as (488 nm signal − fitted 405 nm signal), adjusted so that a ΔF/F was calculated by dividing each point in ΔF by the 405-nm curve at that time.
Behavioral variables, such as paired and unpaired chamber entries, were marked in the signaling traces via the real-time processor as TTL signals from Noldus (Ethovision) software. This allowed for precise determinations of the temporal profile of signals in relation to paired and unpaired chamber entries. For each mouse, the time at which the animal entered the drug-paired chamber was determined, and a 5-s window around that point was analyzed. We subsequently determined the maximum or minimum fluorescent signal that occurred within that window over both consecutive entries and across behavioral tasks/sessions.
Peak analysis to determine frequency and amplitude of the signals was done by determining the median average deviation (MAD) of the corrected/normalized data set and peaks were identified as events that exceeded the MAD by 2.91 deviations.
Cocaine Administration.
Animals were i.p. injected in their home cage with 10 mg/kg cocaine or saline for seven consecutive days (at the same time each day) followed by a 7-d abstinence period.
Baseline Recordings.
All baseline recordings were done in the animal’s home cage. All tests were performed during the dark phase, and animals were acclimated to the behavior room for at least 1 h before testing. A 3-m-long fiber-optic patch cord (Doric Lenses) was connected to the chronically implanted optical fiber with a zirconia sheath and was suspended above the behavioral testing environment to allow animals to move freely during stimulation. Calcium transients were recorded for 2.5 min. Baseline recordings were taken the day following the final IP cocaine injection (after seven injections) and again following a 7-d withdrawal period.
Behavioral Testing.
A patch cord was attached to the fiber-optic cannula and suspended above the animal so that the animal could move around freely within the conditioned place preference (CPP) apparatus. CPP was performed in a rectangular apparatus consisting of two side chambers measuring 28 × 24 cm each, and a center chamber measuring 11.5 × 24 cm. One side had black and white stripes on the walls with a metal grid floor, and the other had gray walls with a punched metal floor. An unbiased CPP paradigm was used in the current study. On day 1 mice were allowed to explore the entire apparatus for 20 min. On days 2 and 3 mice were confined to one of the side chambers and injected with cocaine or saline. Side pairings were counterbalanced across animals. On day 4, during the choice test, mice were again allowed to freely explore the entire apparatus and preference for the stimulated chamber was compared with day 1. During extinction testing animals were placed back into the CPP chamber to freely explore for 20 min. This was repeated for three consecutive days until animals showed a reduced preference for the drug-paired chamber. Animals were then given a 2-wk abstinence period after which they underwent drug-induced reinstatement. To test drug-induced reinstatement, animals were injected with 5 mg/kg cocaine i.p. immediately before being placed in the CPP chamber with free access to the entire chamber.
Conditioned Place Preference with DREADD-Induced Inhibition of D1 or D2 MSNs.
To determine the contribution of D1 or D2 MSNs to associative learning AAV-DIO-hM4Di (or AAV-DIO-mCherry) was injected into the NAc of D1 or D2-cre mice. The virus was allowed to express for 5 wk and then the animals went through CPP as described above. CNO (5 mg/kg) was administered 1 h before either the cocaine pairing or the choice-test session. Time spent in each area of the conditioning chamber was recorded as before and animals were considered to have developed a preference when the difference in time spent between the drug paired and chamber paired with saline was significantly greater than before the pairings. Control animals also received CNO (5 mg/kg); however, they were injected with AAV-DIO-mCherry, and thus the drug had no measureable pharmacological effect.
Open-Field Testing.
Ambulatory activity was measured using the open-field test. Mice were allowed to habituate to the behavioral testing room for 1 h before testing. Mice were injected with cocaine (10 mg/kg) and immediately placed in the center of a square open field (50 × 50 × 50 cm) with white walls and were observed for 10 min. Movements and trajectories of mice were videotaped and analyzed by the Noldus Ethovision System. Using the behavioral testing software, the chamber was divided into three areas: periphery, middle, and center. Data were analyzed for motion tracking trajectory, total ambulatory time, and percent ratio of the time spent in all three arenas were recorded, respectively, and were calculated by the same Noldus software. Total distance traveled as well as time spent in each area of the chamber was recorded to determine both cocaine-induced locomotor activity as well as cocaine-induced anxiety (reflected as reduced time in center of the open field chamber), respectively.
Immunohistochemistry.
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and transcardially perfused with 0.1M PBS followed by 4% (wt/vol) paraformaldehyde in PBS. Brains were postfixed overnight in 4% (wt/vol) PFA and then placed for at least 24 h at 4C in PBS. Brains were sectioned into 100-µm-thick slices using a vibrating tissue slicer and stored in 4 °C in the dark until antibody staining. Free-floating sections were washed 10 min in PBS and then blocked for 1 h in 0.3% Triton X and 3% (wt/vol) normal donkey serum. Sections were then incubated in primary antibodies overnight at 4 °C (chicken anti-GFP, AB13970, 1:500; Abcam) in PBST. Sections were then washed three times in PBS and incubated with secondary antibodies (donkey anti-chicken Alexa Fluor 488; 1:500; Jackson ImmunoResearch) for 2 h at room temperature. Sections were washed three times in PBS. Slices were washed 10 min in PBS, 10 min in 1:50,000 DAPI, and 10 min in PBS and mounted on microscope slides with PVA-DABCO.
Statistics.
Graph Pad Prism was used to analyze datasets. For groups of three or more, one-way ANOVA was run. In datasets with two groups a two-tailed Student’s t test was run. Variance was similar between groups being statistically compared and data were normally distributed supporting the use of parametric statistics. Some animals or data points were lost in the course of the experiment, due to loss of fiber implant or equipment failure during behavioral testing. In one-way designs all pairwise comparisons were tested and in two-way repeated measures designs the effect of the between subjects variable was compared at each level of the within subjects variable. Significance was set at P < 0.05 for all tests and only comparisons with P < 0.05 are explicitly reported. Detailed statistics are provided in figure legends. All data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (R01DA014133 and P01DA008227 to E.J.N.) and Brain & Behavior Research Foundation Grant NARSAD 22713 (to R.C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 2560.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521238113/-/DCSupplemental.
References
- 1.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg EE, et al. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16(7):966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 5.du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34(43):14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 8.Reid AG, Lingford-Hughes AR, Cancela LM, Kalivas PW. Substance abuse disorders. Handb Clin Neurol. 2012;106:419–431. doi: 10.1016/B978-0-444-52002-9.00024-3. [DOI] [PubMed] [Google Scholar]
- 9.Xue YX, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336(6078):241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154(2):327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, et al. Cocaine-induced potentiation of synaptic strength in dopamine neurons: Behavioral correlates in GluRA(-/-) mice. Proc Natl Acad Sci USA. 2004;101(39):14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271(5255):1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 17.Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626(1-2):14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27(13):3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui G, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 24.Vialou V, et al. Serum response factor and cAMP response element binding protein are both required for cocaine induction of ΔFosB. J Neurosci. 2012;32(22):7577–7584. doi: 10.1523/JNEUROSCI.1381-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90(4):385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 27.Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 2007;191(3):719–730. doi: 10.1007/s00213-006-0473-5. [DOI] [PubMed] [Google Scholar]
- 28.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo SJ, et al. The addicted synapse: Mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33(6):267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 31.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17(9):1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23(3):335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26(6):703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa M, et al. Dopamine triggers heterosynaptic plasticity. J Neurosci. 2013;33(16):6759–6765. doi: 10.1523/JNEUROSCI.4694-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110(5):1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.