Significance
The cathelicidin antimicrobial peptide (CAMP) is an innate immune element that promotes antimicrobial defense, but excessive CAMP can stimulate inflammation and tumorigenesis. We recently discovered that external perturbations that induce subtoxic levels of endoplasmic reticulum (ER) stress increase sphingosine-1-phosphate (S1P) production, in turn activating NF-κB–mediated CAMP synthesis. We report here that S1P interacts with the heat shock proteins (HSP90α and GRP94) through a previously unidentified S1P receptor-independent intracellular mechanism, followed by the activation of NF-κB leading to stimulation of CAMP production. These studies illuminate the critical role of both ER stress and S1P in orchestrating stress-specific signals that enhance innate immunity.
Keywords: ER stress, sphingosine-1-phosphate, heat shock protein 90, cathelicidin, innate immunity
Abstract
We recently identified a previously unidentified sphingosine-1-phosphate (S1P) signaling mechanism that stimulates production of a key innate immune element, cathelicidin antimicrobial peptide (CAMP), in mammalian cells exposed to external perturbations, such as UVB irradiation and other oxidative stressors that provoke subapoptotic levels of endoplasmic reticulum (ER) stress, independent of the well-known vitamin D receptor-dependent mechanism. ER stress increases cellular ceramide and one of its distal metabolites, S1P, which activates NF-κB followed by C/EBPα activation, leading to CAMP production, but in a S1P receptor-independent fashion. We now show that S1P activates NF-κB through formation of a previously unidentified signaling complex, consisting of S1P, TRAF2, and RIP1 that further associates with three stress-responsive proteins; i.e., heat shock proteins (GRP94 and HSP90α) and IRE1α. S1P specifically interacts with the N-terminal domain of heat shock proteins. Because this ER stress-initiated mechanism is operative in both epithelial cells and macrophages, it appears to be a universal, highly conserved response, broadly protective against diverse external perturbations that lead to increased ER stress. Finally, these studies further illuminate how ER stress and S1P orchestrate critical stress-specific signals that regulate production of one protective response by stimulating production of the key innate immune element, CAMP.
Mammalian epithelial tissues face hostile external environments, where they are repeatedly bombarded by external perturbants, such as UV irradiation, oxidative stress and microbial pathogens that potentially threaten the integrity of epithelial and nonepithelial tissues/cells. Antimicrobial peptides (AMPs) represent highly conserved, innate immune elements that protect the host from microbial pathogens, while also signaling a variety of downstream responses that further fortify innate and adaptive immunity (1, 2). We have shown that AMP production increases not only in response to microbial challenges, but also in response to a wide variety of other external perturbations that converge on the endoplasmic reticulum (ER), where AMP orchestrate a host of ER-initiated stress responses (3). Although high doses of external perturbants can cause excessive ER stress, leading to increased production of the proapoptotic lipid ceramide, threatening cells with apoptosis (4, 5), subtoxic levels of the same perturbants instead provoke lower levels of ER stress, with incrementally reduced ceramide production that rescues cells from apoptosis in part through metabolic conversion of ceramide to sphingosine-1-phosphate (S1P) (6). S1P in turn stimulates production of the key AMP, cathelicidin AMP (CAMP), and its downstream proteolytic product, LL-37, through transactivation of NF-κB–C/EBPα (7). This mechanism operates independently of the well-known vitamin D receptor-dependent mechanism, which instead regulates CAMP synthesis under basal conditions (3, 7). However, CAMP production is not only always beneficial: Although transient increases defend against microbial pathogens, sustained production of this AMP can stimulate downstream inflammatory responses, and even tumorigenesis (1).
S1P modulates a variety of cellular functions (e.g., cell proliferation, differentiation and motility) through well-known G protein coupled, S1P receptor-dependent mechanisms (8). However, we showed recently that ER stress-stimulated CAMP production is likely receptor-independent (7). Although prior studies showed that ER stress activates NF-κB via plasma membrane-localized S1P1, S1P2, and S1P3 receptor activation (9, 10), using specific activators/agonists and inhibitors/antagonists of all five identified S1P receptors (S1P1–S1P5), we showed that these pharmacological interventions did not modify the ER stress-induced increase in CAMP expression (7). Although prior study shows that TNFα receptor activation can initiate S1P binding to TRAF2 on plasma membrane, forming a signaling complex (S1P–TRAF2–TRADD–RIP1) that activates NF-κB (11), this mechanism does not appear to regulate CAMP synthesis. Instead, we identify and delineate a previously unidentified TNFα receptor- and a S1P receptor-independent mechanism that regulate CAMP production through intracellular assembly of a S1P–TRAF2-stress-responsive protein signaling complex that forms in response to ER stress. Elucidation of this previously unidentified regulatory mechanism could point to potentially previously undeveloped therapeutic approaches to either enhance innate immunity or to suppress excessive CAMP production causing inflammation and tumorigenesis.
Results
S1P Generated by SPHK1 Stimulates CAMP Production Through S1P Receptor-Independent Activation of NF-κB in Response to ER Stress.
Because mammalian epidermis is continuously threatened by the external environment, we used cultured human epidermal keratinocytes (KC) as our primarily model in these studies.
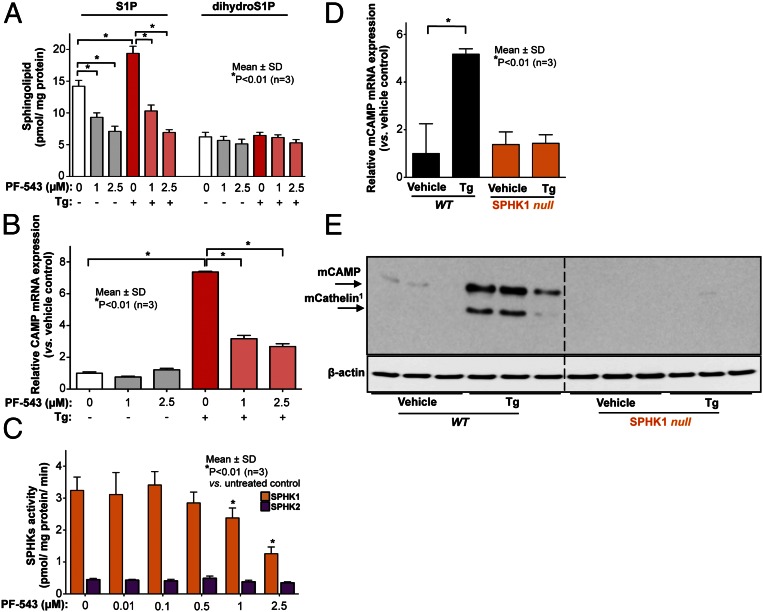
Our prior study characterized that S1P generated by sphingosine kinase (SPHK) 1 activates NF-κB, leading to stimulation of CAMP production in KC in response to ER stress (7). Consistent with this prior study (7), increased S1P and CAMP production in response to ER stress [induced by an ER stressor, thapsigargin (Tg) that releases Ca2+ from ER by inhibition of sarco-ER Ca2+-ATPases; ref. 12] were suppressed by a specific inhibitor of SPHK1, PF-543, which significantly inhibited an isoform of SPHK, SPHK1, but not SPHK2, at concentrations ≥ 1 µM in KC (Fig. 1 A–C) without decreasing cell viability and proliferation. We further confirmed that S1P generated by SPHK1 stimulated CAMP production using SPHK1-null mice skin and SPHK1-silenced cells. Expected ER stress-induced stimulation of CAMP mRNA and protein production did not occur in SPHK1-null mice skin (Fig. 1 D and E) and SPHK1-silenced KC (SI Appendix, Figs. S1A and S2C). In addition, increased phosphorylation of IκBα and nuclear translocation of NF-κB were abrogated in SPHK1-silenced cells (SI Appendix, Fig. S1 B and C). As shown in our prior study (7), exogenous S1P increased CAMP production (SI Appendix, Fig. S2 A and C). Whereas CAMP up-regulation did not occur in SPHK1-silenced KC in response to ER stress, exogenous S1P stimulated CAMP production in both SPHK1 siRNA and control (scrambled) siRNA transfected KC (SI Appendix, Fig. S2C), further supporting the idea that S1P generated by SPHK1 is responsible for stimulation of CAMP production. Moreover, Lys63 (K63)-linked polyubiquitination of RIP1, which is required for NF-κB activation, was suppressed by a blockade of SPHK1 using PF-543 or SPHK1 siRNA (Fig. 2 A and D). These results ascertained our prior findings that S1P signaling generated by SPHK1 is essential for ER stress-induced CAMP expression through NF-κB activation.
Fig. 1.
ER stress-mediated S1P production by SPHK 1 induces CAMP production. KC pretreated with PF-543 (at indicated concentrations) was incubated with Tg for 24 h. (A) S1P and dihydro-S1P contents were assessed by LC-ESI-MS/MS. (B) CAMP mRNA levels were assessed by qRT-PCR. (C) Specificity of PF-543 was confirmed by SPHKs activity assay (see details in Materials and Methods). SPHK1 null mouse skin was obtained 24 h following topical application (20 µL/cm2) of Tg (100 nM) or vehicle (DMSO) on dorsal skin. (C and D) Murine CAMP (mCAMP) mRNA (C) and protein (MW 18kDa) (D) levels were assessed by qRT-PCR or Western blot analysis, respectively. 1mCathelin, an N-terminal domain of mCAMP.
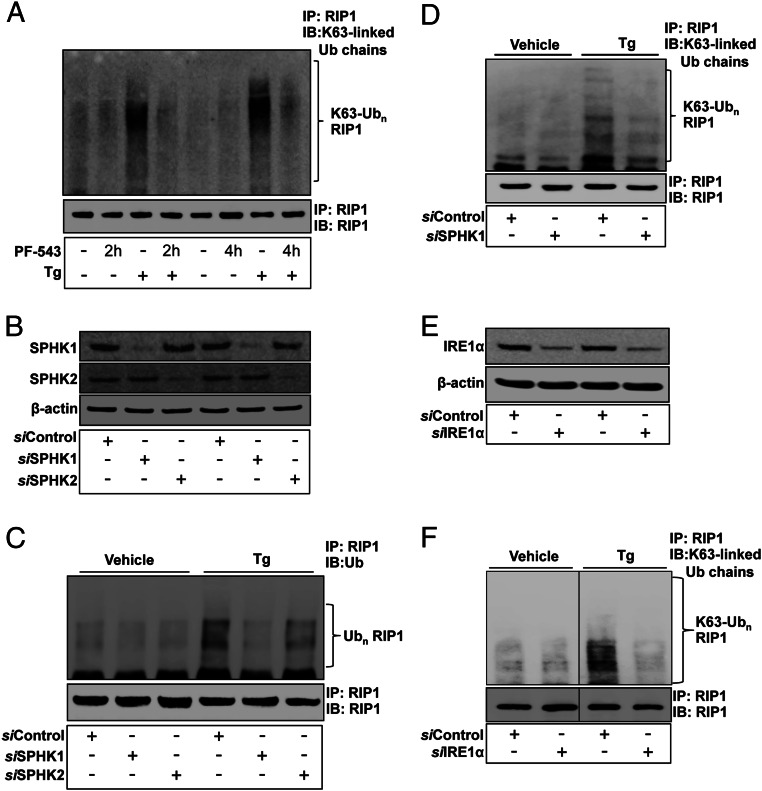
Fig. 2.
IRE1α and S1P generated by SPHK1 are required for ER stress-induced polyubiquitination of RIP1. KC preincubated with PF-543 (1 µM, 2–4 h) (A) or transfected SPHK1 siRNA (B–D), SPHK2 siRNA (B–D), IRE1α siRNA (E and F), or scrambled control siRNA were incubated with Tg (20 nM) for 10 min. Lysates from cells were immunoprecipitated with anti-RIP1 antibody (A, C, D, and F) and immunoblotted with K63-specific polyubiquitin antibody (A, D, and F) or ubiquitin antibody (C). SPHK1 (B), SPHK2 (B), or IRE1α (E) protein levels were assessed by Western blot analysis.
Although previous findings showed that ER stress activates NF-κB via S1P1, S1P2, and S1P3 receptor activation (9, 10), our prior studies revealed that pharmacological interventions using specific activators/agonists and inhibitors/antagonists of all five identified S1P receptors (S1P1–S1P5) did not modify the ER stress-induced increase in CAMP expression (7). We first confirmed whether S1P-mediated stimulation of CAMP expression in vivo is S1P receptor-independent. Topical applications of Tg produced comparable increases in mRNA and protein production of the murine homolog of CAMP (13) in the epidermis of S1P1-, S1P2-, and S1P3-null and wild-type mice (SI Appendix, Fig. S3 A, B, D, and E). Pertinently, topical Tg also comparably increased murine CAMP (mCAMP) expression in S1P1 and S1P3 double knockout mice (SI Appendix, Fig. S3 C and F). Notably, topically applied Tg did not appear to be toxic to the skin of S1P1-3 single, double knockout, or wild-type mice under these conditions; i.e., neither epidermal hyperplasia nor dermal inflammation became evident in hematoxylin and eosin-stained sections from Tg-treated mice (SI Appendix, Fig. S4). Finally, although S1P4 and S1P5 have not been shown to be activated by ER stress (9, 10), we further excluded possibilities of S1P4 and S1P5 by a gene silencing approach. Increases in CAMP production were not affected in cells in which S1P4 or S1P5 was knocked down by siRNA (SI Appendix, Fig. S3 G and H). These studies further confirmed that the ER stress-induced increase in CAMP production is operative not only in vitro, but also in vivo, where it regulates CAMP production in an S1P receptor-independent fashion.
S1P Receptor-Independent CAMP Induction Requires the Stress-Responsive ER Localizing Protein, IRE1α.
Because ER stress can stimulate TNFα production (14), we next investigated whether S1P-mediated TNFα receptor (TNFR)-dependent formation of the signaling complex, S1P–TRAF2–TRADD–RIP1 (11), is responsible for the S1P-induced activation of NF-κB that leads to increased CAMP production in response to ER stress. KC were pretreated with either a specific TNFR inhibitor or anti-TNFα neutralizing antibody, followed by exposure to the ER stressors, Tg or tunicamycin (Tm) (the latter triggers ER stress by inhibiting N-glycosylation). We first confirmed that TNFR activation, assessed as changes in the expression of three known TNFR regulating genes (i.e., MMP-9, ICAM1, and IL-8), increases following exogenous TNFα (SI Appendix, Table S1). However, neither treatment with a TNFR inhibitor nor treatment with the anti-TNFα neutralizing antibody altered ER stress-induced CAMP production (SI Appendix, Table S1). These results indicate that the ER stress-induced increase in CAMP production occurs independently of TNFR-mediated activation.
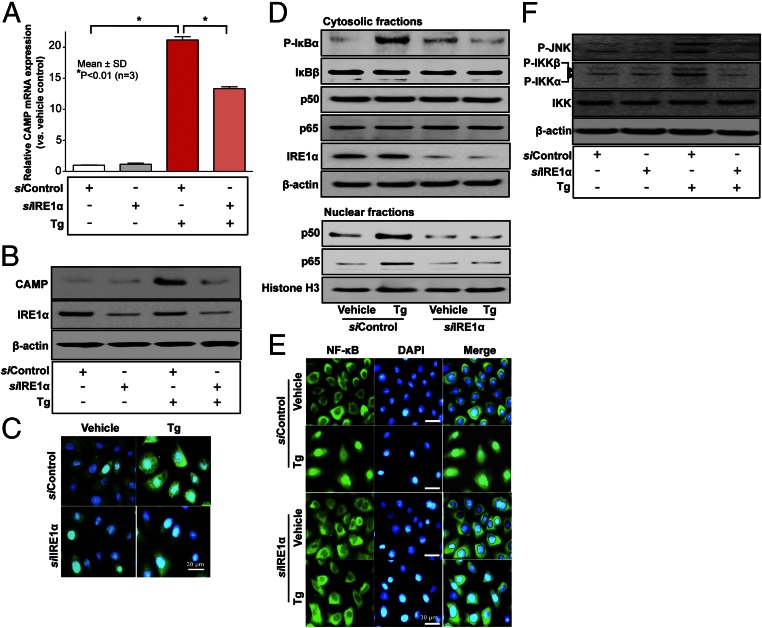
Alternatively, TRAF2 forms a signaling complex with an ER transmembrane molecule, IRE1α, which is activated by endoribonuclease, leading to NF-κB activation following imposition of ER stress (15, 16). Because IRE1α serves as a part of a molecular complex that activates NF-κB, we next assessed whether IRE1α is required for ER stress-induced CAMP production. siRNA directed against IRE1α, but not scrambled siRNA, significantly attenuated CAMP production following ER stress [by either Tg (Fig. 3 A–C) or Tm (SI Appendix, Fig. S5A)]. Furthermore, both Western blot and immunofluorescence analyses revealed that silencing of IRE1α diminished ER stress-induced NF-κB activation, including phosphorylation of IκB, as well as nuclear translocation of both the p65 and p50, isoforms of NF-κB (Fig. 3 D and E and SI Appendix, Fig. S5 B and C). Thus, ER stress-induced CAMP production requires IRE1α-mediated activation of NF-κB.
Fig. 3.
IRE1 is required for ER stress-induced CAMP production through NF-κB activation. KC transfected with IRE1α or scrambled control siRNA were exposed to Tg (20 nM). (A–C) mRNA and protein levels of CAMP or IRE1α were assessed by qRT-PCR (A), Western blot (B), or immunofluorescence staining (C). (D) Alterations of phosphorylated IκBα, IκBβ, isoforms of NF-κB, p50 and p65, and IRE1α levels were assessed by Western blot analysis. (E) Nuclear translocations of NF-κB were also assessed by immunofluorescence staining. (F) Phosphorylations of IKKα/β and JNK, and IKK protein levels were assessed by Western blot analysis. Green and blue staining corresponds to CAMP (C) or NF-κB (E), and nucleus, respectively.
ER Stress-Induced NF-κB Activation Stimulates Formation of a S1P-Mediated TRAF2 Signaling Complex.
Although TNFα receptor activation does not mediate the ER stress→↑NF-κB→↑CAMP mechanism, IRE1α, which we showed above is required for this signaling pathway, can form a complex with TRAF2 (15, 16). Moreover, TRAF2-mediated NF-κB activation requires RIP1 polyubiquitination by an E3 ligase contained in TRAF2, releasing IκB (17). To further characterize TRAF2-mediated NF-κB activation, we assessed IKK activation, which occurs following RIP1 polyubiquitination. As expected, RIP1 polyubiquitination became evident in KC following ER stress (Fig. 2). In addition, a known RIP1 polyubiquitination consequence, JNK phosphorylation, also occurred in KC exposed to ER stress (Fig. 3F). Hence, we next assessed whether silencing TRAF2 alters ER stress-induced increases in CAMP expression. Because siRNA against TRAF2 significantly suppresses CAMP production, TRAF2 likely is also involved in the ER stress-induced stimulation of CAMP production (SI Appendix, Fig. S6).
We next investigated whether IRE1α activation of RIP1, which is polyubiquitinated at the Lys63 in the IRE1α-TRAF2-containing signaling complex, is required for ER stress-initiated NF-κB activation. Lys63-linked polyubiquitination of RIP1 in response to ER stress was markedly decreased in IRE1α-silenced KC, indicating a requirement of IRE1α for the polyubiquitination of RIP1 in response to ER stress (Fig. 2 E and F).
We first confirmed that silencing of IRE1α does not suppress an ER stress-mediated increase in S1P production (SI Appendix, Fig. S7). We then investigated the role of S1P in RIP1 polyubiquitination by blocking SPHK1 and SPHK2. Although siRNA against SPHK1 as well as a specific inhibitor of SPHK1, PF-543, markedly inhibited both polyubiquitination of RIP1 and Lys63-linked polyubiquitination of RIP1 following ER stress (Fig. 2 A, C, and D), knockdown of SPHK2 only slightly reduced polyubiquitination of RIP1 (Fig. 2C). Moreover, silencing of SPHK1 attenuated the ER stress-induced phosphorylation of IκB, as well as the nuclear translocation of NF-κB (SI Appendix, Fig. S1 B and C).
We next assessed the relationship between TRAF2 and S1P-dependent RIP1 polyubiquitination. SPHK1 siRNA significantly diminished TRAF2 binding to RIP1 in cells exposed to ER stress (SI Appendix, Fig. S8 C and D), indicating that S1P is required for TRAF2 binding to RIP1. Together, these results indicate that S1P generation in response to ER stress is responsible for polyubiquitination of RIP1, and furthermore, that IRE1α is required for the formation of the TRAF2–Lys63 polyubiquitinated RIP1 signal complex that results in canonical NF-κB activation.
Identification of Heat Shock Proteins in the S1P-Mediated Signaling Complex.
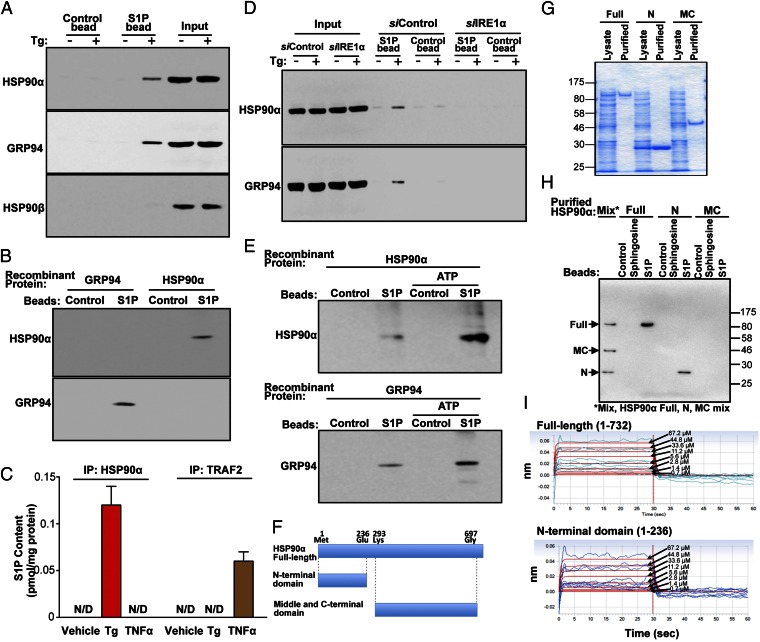
Because the results described above show that an S1P-mediated signaling complex with TRAF2 activates NF-κB, we next investigated whether S1P-conjugated beads (S1P beads) bind to TRAF2 following ER stress. Although Western blot analysis did not show detectable binding of TRAF2 to S1P beads, we detected other unidentified bands in SDS/PAGE gels of such S1P bead-exposed protein fractions. Using LC-MALDI TOF-MS, we subsequently identified two heat shock proteins (HSP) 90s; i.e., HSP90α and the ER resident HSP90, GRP94, within this complex. In addition, β-actin, desmoplakin, myosin-9, and tubulin bound to the S1P beads, but neither TRAF2 nor IRE1α were detected by MS analyses. Although recent studies show that IL-1 induces IRF1 (IFN-regulatory factor 1) following S1P binding to cIAP2 (18), cIAP2 was not detected in the S1P bead complexes by MS analyses.
Both HSP90α and GRP94 function as molecular chaperones that maintain cellular functions in cells exposed to ER stress (19). Hence, HSP90s could play a role in S1P receptor-dependent NF-κB activation. Because HSP90s contain loop structures that often bind electrostatically (nonspecifically) to contiguous molecules, accordingly, we next assessed whether binding of HSP90α or GRP94 to S1P beads is specific or nonspecific. Both HSP90α and GRP94 were recovered from S1P beads incubated with ER-stressed KC lysates, but not from control beads (Fig. 4A). However, HSP90β did not bind to either S1P beads or control beads (Fig. 4A). In addition, recombinant HSP90α and GRP94 bind to S1P beads (Fig. 4B). These results indicate that S1P exhibits a specific binding affinity toward HSP90α and GRP94. To further confirm S1P binding to HSP90, as well as quantitatively measuring S1P content in S1P-HSP90α in cells following ER stress, we assessed S1P content in immunoprecipitates of HSP90α or TRAF2 isolated from TRAF2 overexpressed KC lysates following either Tg or TNFα treatments. LC-ESI-MS/MS analysis demonstrated that consistent with prior studies, S1P binds to TRAF2 in cells in response to TNFα receptor activation (Fig. 4C and SI Appendix, Fig. S9F). Different from TNFα receptor activation (11), S1P did not bind to TRAF2 in ER stressed cells (Fig. 4C and SI Appendix, Fig. S9E). Instead, S1P binds to HSP90α, but not TRAF2, in KC following ER stress (Fig. 4C and SI Appendix, Fig. S9B). In contrast to S1P, dihydro-S1P did not bind to TRAF2 or HSP90α (SI Appendix, Fig. S9 H and L).
Fig. 4.
S1P interacts with HSP90α and GRP94. (A and B) Control or S1P beads were incubated with lysates of Tg (20 nM)-treated KC (A), purified GRP94, and HSP90α (B). (D) Lysates of KC transfected with IRE1α siRNA or scrambled siRNA following exposure to Tg (20 nM). (E) Purified HSP90α and GRP94 preincubated with ATP (1 mM) (E). (A, B, D, and E) The bound proteins were released from beads by boiling in SDS-sample buffer and were assessed by Western blot analysis with indicated antibodies. (C) Lysates from pTRAF2-GFP–overexpressed KC incubated with Tg (20 nM) or TNFα (10 ng/mL) were immunoprecipitated with anti-HSP90α or anti-TRAF2 antibodies, and bound S1P content was determined by LC-ESI-MS/MS. (F) Recombinant HSP90α. Schematic diagram of deletion constructs of HSP90α. Full length (Full), N-terminal (N), or middle/C-terminal (MC) domains of HSP90α were successfully overexpressed in Escherichia coli and purified. (G) The quality of recombinant HSP90α. The purity of recombinant HSP90α was assessed with SDS/PAGE stained with coomassie brilliant blue. (H) S1P-, sphingosine-, or control-bead binding of the purified full length/deletion constructs of HSP90α was also assessed by Western blot analysis with HRP-conjugated anti-His6 antibody (proteins were eluted from beads by buffer containing 0.1 mg/mL S1P). (I) Specific binding affinity of the purified full length and N-terminal domain of HSP90α to S1P was assessed by Bio-Layer Interferometry (Octet).
Because prior studies showed that HSP90α forms a complex with RIP1 that results in NF-κB activation (20), we next assessed whether S1P beads bind to one or both of these HSP90 family proteins, followed by formation of the signal complex that activates NF-κB, along with RIP1. Because IRE1α is a client protein of HSP90s (21), we next examined whether IRE1α is required for interaction with S1P to either HSP90α or GRP94. siRNA silencing of IRE1α abolished binding of both HSP90α and GRP94 to S1P beads in cells exposed to ER stress (Fig. 4D), indicating that IRE1α is required for S1P interaction with HSP90α and GRP94. Together, these studies show that S1P recruits a family of stress responsive proteins, including IRE1α, HSP90α, and GRP94, which activate NF-κB.
HSP90s Are Required for ER Stress-Induced Stimulation of CAMP Production.
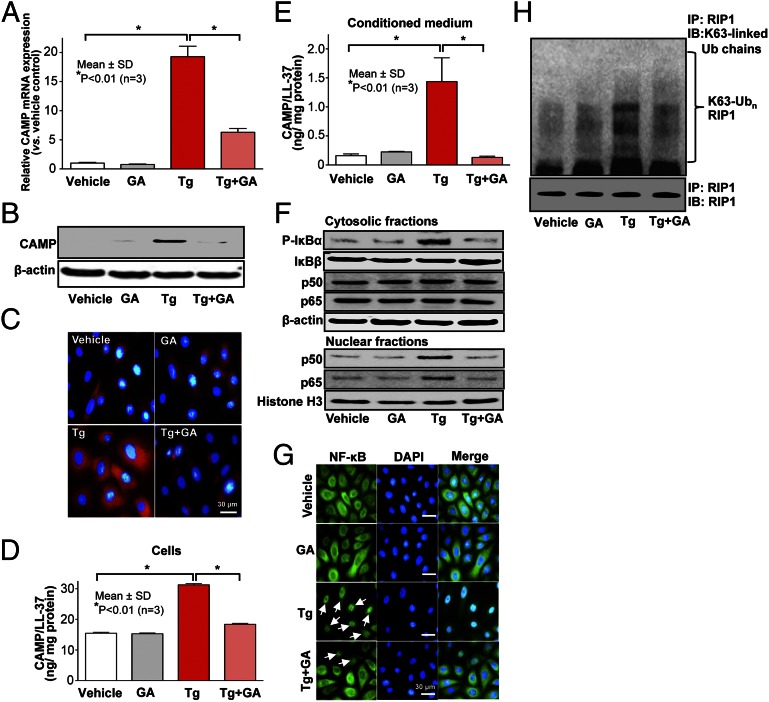
We next addressed whether HSP90α and/or GRP94 are required for ER stress-induced NF-κB activation, leading in turn to increased CAMP production. We first noted that silencing of HSP90 does not suppress ER stress-mediated increases in S1P (SI Appendix, Fig. S7). Pretreatment of ER-stressed KC with geldanamycin (GA), a specific inhibitor of HSP90, significantly attenuated the expected increase in CAMP production (Fig. 5 A–E), diminishing ER stress-induced phosphorylation of IκB (Fig. 5F), as well as the subsequent translocation of both the p50 and p65 NF-κB isoforms from the cytosol to the nucleus (Fig. 5G) and K63-polyubiquitination of RIP1 (Fig. 5H). Moreover, siRNA-induced silencing of either HSP90α or GRP94 diminished the ER stress-induced increase in CAMP production, and double knockdown of both HSP90s further attenuated the expected ER stress-induced stimulation of CAMP (SI Appendix, Fig. S10 A–C). Finally, decreased CAMP production in these knockdown experiments was paralleled by reduced translocation of NF-κB from the cytosol to the nucleus in these cells (SI Appendix, Fig. S10D). Therefore, both cytosolic HSP90α and ER resident GRP94 are required for S1P-dependent NF-κB activation, leading to increased CAMP production.
Fig. 5.
A family of HSP90 proteins is required for increased CAMP production initiated by ER stress. KC-pretreated HSP90 inhibitor, GA (0.5 µM), was exposed to Tg for 24 h. CAMP mRNA (A) and peptide/protein (B–E) were assessed by qRT-PCR (A), Western blot (B), immunofluorescence (C), or ELISA analysis (D and E). (F) Alterations of phosphorylated IκBα, IκBβ, and NF-κB isoforms (p50 and p65) in cytosolic (Upper) and nuclear (Lower) fractions were measured by Western blot analysis. Nuclear translocations of NF-κB were assessed by immunofluorescence. (H) Lysates from cells treated with Tg and/or GA were immunoprecipitated with anti-RIP1 antibody and immunoblotted with K63-specific polyubiquitin antibody.
Further Characterization of the S1P-Mediated Signaling Complex.
Because HSP90α associates with a cochaperone, E3 ubiquitin ligase STIP1 homology, and U-Box containing protein 1 (STUB1) (22), we next assessed whether STUB1 is required for the NF-κB–induced enhancement of CAMP production following ER stress. CAMP expression declined significantly in KC transfected with siRNA against STUB1, indicating an involvement of STUB1 in RIP1 polyubiquitination (SI Appendix, Fig. S6). However, TRAF2 also contains the TRAF2 RING domain, E3 ubiquitin ligase (17). Hence, it remains possible that both STUB1 and the TRAF2 RING domain, E3 ubiquitin ligases, could contribute to RIP1 ubiquitination under ER stressed conditions.
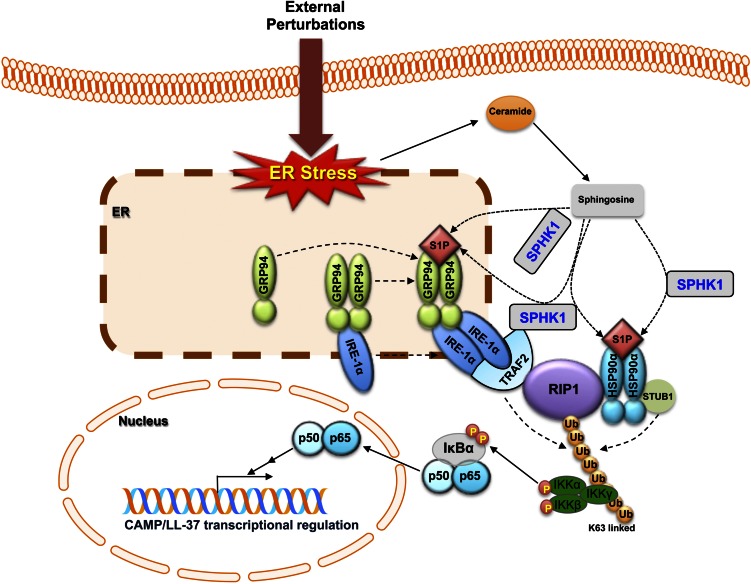
We next assessed the binding partners of the intracellular signaling complex. Immunoprecipitation studies show that the ER residential heat shock protein, GRP94, is already bound to IRE1α under basal conditions, and that blockade of S1P production by SPHK1 siRNA did not affect binding of IRE1α to GRP94 under either basal or ER-stressed conditions (SI Appendix, Fig. S8E). Moreover, neither R1P1 nor TRAF2 was recovered from immunoprecipitates preexposed to the GRP94 antibody (SI Appendix, Fig. S8E). Likewise, HSP90α immunoprecipitation studies demonstrated only low background levels of IRE1α bound to HSP90α under both basal and ER stressed conditions (SI Appendix, Fig. S8F). In contrast, HSP90α binding to RIP1 was evident in immunoprecipitated fractions following ER stressed condition, whereas this association was significantly declined in SPHK1-silenced KC (SI Appendix, Fig. S8F). Similar to GRP94, TRAF2 bands were not observed in immunoprecipitated fractions (SI Appendix, Fig. S8F). Finally, consistent with prior studies (11, 23), SPHK1 bound to TRAF2 (SI Appendix, Fig. S8A). However, it still remains unknown whether a preformed pool of GRP94, bound to IRE1α under basal conditions, is selectively recruited and contributes to formation of the signaling complex, or whether free IRE1α also is used. Nonetheless, taken together with prior studies that show IRE1α–TRAF2 binding (15, 16), we can now predict the likely interaction/binding partners and member architecture of the S1P intracellular signaling complex (Fig. 6).
Fig. 6.
Proposed S1P-mediated signaling complex activates NF-κB, leading to stimulation of CAMP production. Increased cellular S1P in response to ER stress, which can be induced by external perturbations, specifically interacts with HSP90s (HSP90α and GRP94) to form a signaling complex with an ER residential stress responsive protein, IRE1α, TRAF2, and RIP1, leading to NF-κB activation that stimulates CAMP production. Both TRAF2-bound and unbound SPHK1 could be responsible for a signaling complex formation.
HSP90 families contain three conserved functional domains: an N-terminal ATP-binding domain required for dimerization following ATP binding; a middle domain containing a client protein binding sequence; and a C-terminal spontaneous dimerization domain with an alternative ATP-binding site (24). Preincubation of recombinant HSP90α and GRP94 with ATP enhanced the binding of both HSP90α and GRP94 to S1P beads (Fig. 4E). Thus, ATP-dependent dimerization of HSP90α and GRP94 allows more efficient interaction of S1P with these HSP90s.
The S1P beads binding assay using recombinant HSP90α demonstrated that the full-length and the N-terminal portion of HSP90 bound to only S1P beads but neither to sphingosine beads nor control beads. However, the middle/C-terminal domain of HSP90α did not bind at all to the beads examined (Fig. 4 F–H). Finally, we assessed the S1P interaction domain of HSP90α using a sensitive, quantitative method; i.e., interferometry. Consistent with the S1P beads binding assay, S1P bound to full-length and N-terminal domain of HSP90α [Kd of 120 and 75 µM, respectively; because these Kd are determined by Bio-Layer Interferometry (Octet) in vitro, these values should not be the same as S1P–HSP90α binding in physiological conditions in cells] (Fig. 4I). However, S1P did not bind to the middle/C-terminal domain of HSP90α (Fig. 4I). Moreover, neither of two structurally related lipids, sphingosine nor lysophosphatidic acid, bound to HSP90α (SI Appendix, Fig. S11). Together, these studies demonstrate that S1P specifically interacts with the N-terminal domain of HSP90α.
The S1P-Mediated Signaling Complex Regulates Innate Immunity in Multiple Cell and Tissue Types.
As we reported previously, ER stress induces CAMP expression both in primary normal human KC, as well as in other epithelial cell types; i.e., HeLa and HaCaT cells (3). We next investigated the role of HSP90s in the S1P-dependent increase in CAMP production, following ER stress administrated to other epithelial and nonepithelial cell types, including the human alveolar epithelial cell line, A549. Activation of XBP1 (ER stress marker) became evident in A549 cells, suggesting that ER stress occurs under comparable conditions in these cells (SI Appendix, Fig. S12A). CAMP mRNA levels also increased significantly in these cells under these conditions, whereas pretreatment with the HSP90 inhibitor, GA, significantly attenuated the expected ER stress-induced increase in CAMP expression in A594 cells (SI Appendix, Fig. S12B). Because CAMP also is produced by macrophages, we next assessed whether ER stress-initiated increases in CAMP production also occurs in murine macrophages (RAW264.7) cell line through the S1P–HSP90–NF-κB mechanism. CAMP mRNA levels increased in RAW264.7 cells following ER stress, whereas GA again suppressed the expected increase in CAMP expression in these cells (SI Appendix, Fig. S12B). Finally, inhibition of SPHK1 suppressed CAMP production in both A549 and RAW264.7 cells without suppressing ER stress (SI Appendix, Fig. S12 C and D). Together, these results indicate that the S1P-initiated signal complex operates universally in both epithelial and nonepithelial cell types when subjected to ER stressed conditions.
Discussion
A variety of external insults, including UV irradiation and oxidative stress, induce ER stress. We have shown an enhancement of innate immunity through S1P-dependent transcriptional up-regulation of CAMP (3, 7). We now show that S1P initiates such S1P receptor-independent signaling through a previously unidentified intracellular signaling complex, S1P–GRP94–IRE1α–TRAF2–RIP1–HSP90α–S1P, which in turn activates NF-κB, leading to increased CAMP production (Fig. 6). Consistent with prior studies (11, 23), SPHK1 binds to TRAF2 in KC, and this interaction significantly increases in cells in response to ER stress. However, in contrast to another NF-κB activation mechanism that requires S1P association with TRAF2 in response to TNFα receptor activation (11), S1P generated in response to ER stress interacts with HSP90s, independent of TNFα receptor activation. Likely, the presence of IRE1α and HSP90s in this signaling complex prevents S1P from interacting with TRAF2, and/or the binding affinity of S1P to HSP90s could exceed the affinity of S1P for TRAF2 binding in such conditions.
Although we focused here on S1P signaling of the innate immune element, CAMP, the participation of multiple S1P interacting/binding partners, including TRAF2, cIAP2, HSP90α, GRP94, and S1P receptors, suggests that S1P could regulate diverse cellular functions through varying combinations of these binding partners in response to different stimuli, consistent with the diverse roles of S1P in cells, including both pro- and antiapoptotic effects (25). We demonstrated that S1P specifically interacts with (binds to) N-terminal domain of HSP90α. A specific binding site(s) of S1P to S1P receptors have been largely characterized (26). However, likewise TRAF2 and cIAP2, a specific interaction (binding) site(s) of S1P to HSP90s remains unknown.
S1P displays a specific interaction with an N-terminal domain in HSP90α, and addition of ATP increases the binding affinity of S1P to both HSP90α and GRP94. ATP binding to HSP90s leads to conformational changes at N-terminal toroidal structures that increase binding affinities with client proteins (24), likely also facilitating interaction of S1P to these HSP90s. Because both HSP90α and GRP94 are highly conserved members of the HSP90 family (27), S1P likely has the same interacting preference for GRP94 as for HSP90α; i.e., a comparable N-terminal domain structure. Further studies are still needed to identify the specific S1P-binding sequence in the N-terminal domain of HSP90α, as well as other cofactors that regulate S1P binding to HSP90s and/or increases binding affinity to S1P–HSP90s interaction. Similar to previous findings that have been reported the Kd values in micromolar (µM) to millimolar (mM) range for HSP90 binding to certain small molecules assessed by Octet (28, 29), our Octet assay indicates the low-affinity binding S1P to HSP90s (Kd: 75–120 µM). However, in cells, the presence of ATP (Fig. 4) and/or unidentified possible cofactors could increase S1P–HSP90s binding affinity.
Importantly, S1P interaction with HSP90s was only detected following ER stress in the presence of IRE1α, suggesting that IRE1α forms a platform on the ER membrane that facilitates S1P association with GRP94, thereby initiating formation of the intracellular signaling complex. In contrast to cells/cell lysate systems, which contain numerous components that can interact with HSP90s and/or S1P, nonphysiologic (high) concentrations of S1P or purified recombinant HSP90s could induce this interaction in the absence of IRE1α as well as of another proteins. In addition, IRE1α–GRP94 signaling platform in vivo (stable foundation) could allow S1P to interact with HSP90s; we speculate that, in in vitro studies using S1P beads and Octet system, S1P is conjugated to agarose beads or to HSP90s that are immobilized onto the Ni-NTA sensor, both conditions that may provide a platform (or foundation). Similar to our findings in response to subtoxic levels of external perturbations that induce ER stress, preconditioning with hypoxia reportedly enhances cardioprotection through an S1P signal (30). Together, these studies suggest that subtoxic levels of ER stress exert broad benefits in cells, protecting cellular functions through S1P signaling.
Our prior (3, 7) and present studies further illuminate how ER stress-mediated S1P production constitutes a metabolic rescue mechanism that protects against ceramide-induced apoptosis in response to external insults, in turn enhancing innate immunity through increased CAMP expression. These studies further suggest that pharmacological stimulation of S1P signaling mechanisms could represent a previously unidentified therapeutic approach to enhance antimicrobial defense through increased CAMP production. However, excessive and/or sustained elevations of cellular S1P and CAMP can increase inflammation and cancer development (31). Hence, interventions that interdict S1P signaling could be exploited as either anti-inflammatory and/or antineoplastic therapeutic strategy.
Materials and Methods
Reagents, antibodies, expression vectors, recombinant proteins, detailed methods for cell culture and transfection, mice and ex vivo studies, expression and/or purification of TRAF2 and HSP90 proteins, RNA isolation and quantitative RT-PCR (qRT-PCR), Western blot analysis, ELISA for CAMP quantification, histological and immunofluorescence, immunoprecipitation, sphingolipids-coated bead binding assay, protein identification by mass spectrometry, bio-layer interferometry assay, S1P quantification, enzyme activity assays for sphingosine kinases, and statistical analysis used in this study can be found in SI Appendix, SI Materials and Methods. This study was approved by the University of California, San Francisco, Institutional Review Board approval protocol and the San Francisco VA Medical Center Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank Dr. Timothy Hla (Weill Cornell Medical College), Dr. Julie Saba (Children's Hospital Oakland Research Institute), and Dr. Richard Proia for providing transgenic mice and useful suggestions; Dr. Julie Saba for critical review of the manuscript; Ms. Sally Pennypacker and Ms. Lillian Adame for technical support in cell culture; and Ms. Joan Wakefield for superb editorial assistance. We acknowledge the support of the Medical Research Services of the Veterans Affairs Medical Center, San Francisco. This study was supported by a Grant-in-Aid for Scientific Research (C) 25440036 from the Japan Society for the Promotion of Science (JSPS) and the Japan Foundation for Applied Enzymology (to H.I.), the National Rosacea Society, the San Francisco Foundation, and National Institutes of Health Grants AR051077 and AR062025 (the National Institute of Arthritis and Musculoskeletal and Skin Diseases) (to Y.U.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504555113/-/DCSupplemental.
References
- 1.Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: Key components of the innate immune system. Crit Rev Biotechnol. 2012;32(2):143–171. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- 2.Nakatsuji T, Gallo RL. Antimicrobial peptides: Old molecules with new ideas. J Invest Dermatol. 2012;132(3 Pt 2):887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park K, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286(39):34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2 beta)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283(50):34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2009;24(1):296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida Y, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130(10):2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- 7.Park K, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33(4):752–762. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55(8):1596–1608. doi: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom T, et al. An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival. BMC Cell Biol. 2010;11:45. doi: 10.1186/1471-2121-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ki SH, Choi MJ, Lee CH, Kim SG. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J Biol Chem. 2007;282(3):1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inesi G, Wade R, Rogers T. The sarcoplasmic reticulum Ca2+ pump: Inhibition by thapsigargin and enhancement by adenovirus-mediated gene transfer. Ann N Y Acad Sci. 1998;853:195–206. doi: 10.1111/j.1749-6632.1998.tb08267.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallo RL, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272(20):13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 14.Menon MB, et al. Endoplasmic reticulum-associated ubiquitin-conjugating enzyme Ube2j1 is a novel substrate of MK2 (MAPKAP kinase-2) involved in MK2-mediated TNFα production. Biochem J. 2013;456(2):163–172. doi: 10.1042/BJ20130755. [DOI] [PubMed] [Google Scholar]
- 15.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26(7):931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416(6878):345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 18.Harikumar KB, et al. K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nat Immunol. 2014;15(3):231–238. doi: 10.1038/ni.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275(14):10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 21.Marcu MG, et al. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22(24):8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galigniana MD, et al. Retrograde transport of the glucocorticoid receptor in neurites requires dynamic assembly of complexes with the protein chaperone hsp90 and is linked to the CHIP component of the machinery for proteasomal degradation. Brain Res Mol Brain Res. 2004;123(1-2):27–36. doi: 10.1016/j.molbrainres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Xia P, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277(10):7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 24.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93(3):211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling. Development. 2014;141(1):5–9. doi: 10.1242/dev.094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen H, Germana Sanna M, Gonzalez-Cabrera PJ, Roberts E. The organization of the sphingosine 1-phosphate signaling system. Curr Top Microbiol Immunol. 2014;378:1–21. doi: 10.1007/978-3-319-05879-5_1. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics. 2005;86(6):627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Song X, et al. Identification of epipolythiodioxopiperazines HDN-1 and chaetocin as novel inhibitor of heat shock protein 90. Oncotarget. 2015;6(7):5263–5274. doi: 10.18632/oncotarget.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews SB, et al. Characterization of a novel novobiocin analogue as a putative C-terminal inhibitor of heat shock protein 90 in prostate cancer cells. Prostate. 2010;70(1):27–36. doi: 10.1002/pros.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: A decade of progress. Biochim Biophys Acta. 2013;1831(1):203–212. doi: 10.1016/j.bbalip.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280(1):22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.