Significance
In a study investigating mechanisms whereby period 2 (PER2) stability can set the pace of biological rhythms, we have looked at molecular, cellular, and structural features of the mouse mutant, early doors (Edo). Early doors is a novel N-ethyl-N-nitrosourea (ENU)-induced point mutation that shortens the circadian period of mice by 1.5 h. The mutation results in an amino acid substitution in the interdomain linker between the tandem PAS domains of PER2. Biophysical analyses confirm that increased flexibility of this interdomain linker reduces stability of the PAS domain core. This flexibility can accelerate circadian rhythms by destabilizing the PER2Edo protein through faster kinase-mediated degradation. Furthermore, we find that Per2Edo mice carrying the casein kinase 1ε (Csnk1e) Tau mutation have extremely fast but very stable circadian clocks.
Keywords: mouse mutant, behavior, protein stability, circadian period, genetic interaction
Abstract
The suprachiasmatic nucleus (SCN) defines 24 h of time via a transcriptional/posttranslational feedback loop in which transactivation of Per (period) and Cry (cryptochrome) genes by BMAL1–CLOCK complexes is suppressed by PER–CRY complexes. The molecular/structural basis of how circadian protein complexes function is poorly understood. We describe a novel N-ethyl-N-nitrosourea (ENU)-induced mutation, early doors (Edo), in the PER-ARNT-SIM (PAS) domain dimerization region of period 2 (PER2) (I324N) that accelerates the circadian clock of Per2Edo/Edo mice by 1.5 h. Structural and biophysical analyses revealed that Edo alters the packing of the highly conserved interdomain linker of the PER2 PAS core such that, although PER2Edo complexes with clock proteins, its vulnerability to degradation mediated by casein kinase 1ε (CSNK1E) is increased. The functional relevance of this mutation is revealed by the ultrashort (<19 h) but robust circadian rhythms in Per2Edo/Edo; Csnk1eTau/Tau mice and the SCN. These periods are unprecedented in mice. Thus, Per2Edo reveals a direct causal link between the molecular structure of the PER2 PAS core and the pace of SCN circadian timekeeping.
Circadian rhythms, as exemplified by the sleep/wake cycle, are the outward manifestation of a 24-h timing mechanism that coordinates many physiological processes (1). The principal circadian clock in mammals is the suprachiasmatic nucleus (SCN). At a molecular level, the SCN clockwork consists of interacting positive and negative transcriptional/translational feedback loops (TTFLs) that drive rhythms in the RNA and protein levels of key clock components. Heterodimers of the PER-ARNT-SIM (PAS) domain-containing transcriptional activators CLOCK and BMAL1 drive rhythmic transcription of Per (period) and Cry (cryptochrome) genes by binding to E-box elements. PER proteins associate with CRY, translocating to the nucleus to inhibit their own transcription by interacting with the CLOCK–BMAL1 complex (2, 3). An additional feedback loop involves the nuclear orphan receptors REV-ERBα (4) and RAR-related orphan receptor A (RORA) (5) that modify the transcription of Bmal1, thereby stabilizing and amplifying the CLOCK–BMAL complex-dependent oscillation. Once suppressed, CLOCK–BMAL complex-mediated transactivation can only reoccur after PER and CRY have been degraded.
The circadian TTFL is therefore sustained by timely synthesis and degradation of PER and CRY proteins. Changes in PER stability in flies (6, 7) and changes in PER or CRY stability in mammals lead to a faster or slower clock (8). Phosphorylation-dependent licensing of PER proteins for ubiquitination and subsequent proteasomal degradation is a sensitive checkpoint in setting clock speed (9–11). Moreover, two familial advanced sleep phase syndromes (FASPSs) are associated with site-specific phosphorylation of human period 2 (PER2) (12, 13). Finally, the Tau mutation of casein kinase 1ε (Csnk1e) (14) is a hypermorph that accelerates the clock of rodents (15). Thus, phosphorylation, PER protein stability, and circadian period are intimately linked (16).
Current knowledge of the structural basis of circadian transcription and its repression is limited, but it is becoming clear that modular PAS domains form protein interactions that are important for progression of the circadian TTFL. The crystal structures of mouse CLOCK and BMAL1 have revealed how tandem PAS domains contribute to the formation of the dimeric transcription factor (17). PAS domains are also found in circadian negative regulators: tandem PAS domains mediate formation of PER dimers observed in vivo (18). Unlike the asymmetrical, extended conformation seen in CLOCK-BMAL1, the PER PAS dimer core forms a more symmetrical and compact fold (19, 20). In PER2, the PAS domain core is followed immediately by a CK1ε-dependent phosphodegron that recruits the E3 ubiquitin ligase β-TrCP to control PER2 stability (10, 16, 21). Thus, control of PER2 repressive function and/or PER2 stability may be controlled by interactions of its PAS domain core.
To gain new insight into the mechanisms that determine the pace of the mammalian circadian clock, we used N-ethyl-N-nitrosourea (ENU) mutagenesis (22). We identified a founder mouse with a significantly shortened circadian period. The mutation, early doors (Edo), mapped to the Per2 gene within the linker between the PAS-A and PAS-B domains. PER2Edo reveals the determinative relationship between protein stability and circadian pacemaking, and, more importantly, it reveals a structural framework within which to understand this defining property of the circadian system.
Results
Per2Edo Shortens Circadian Wheel-Running Period.
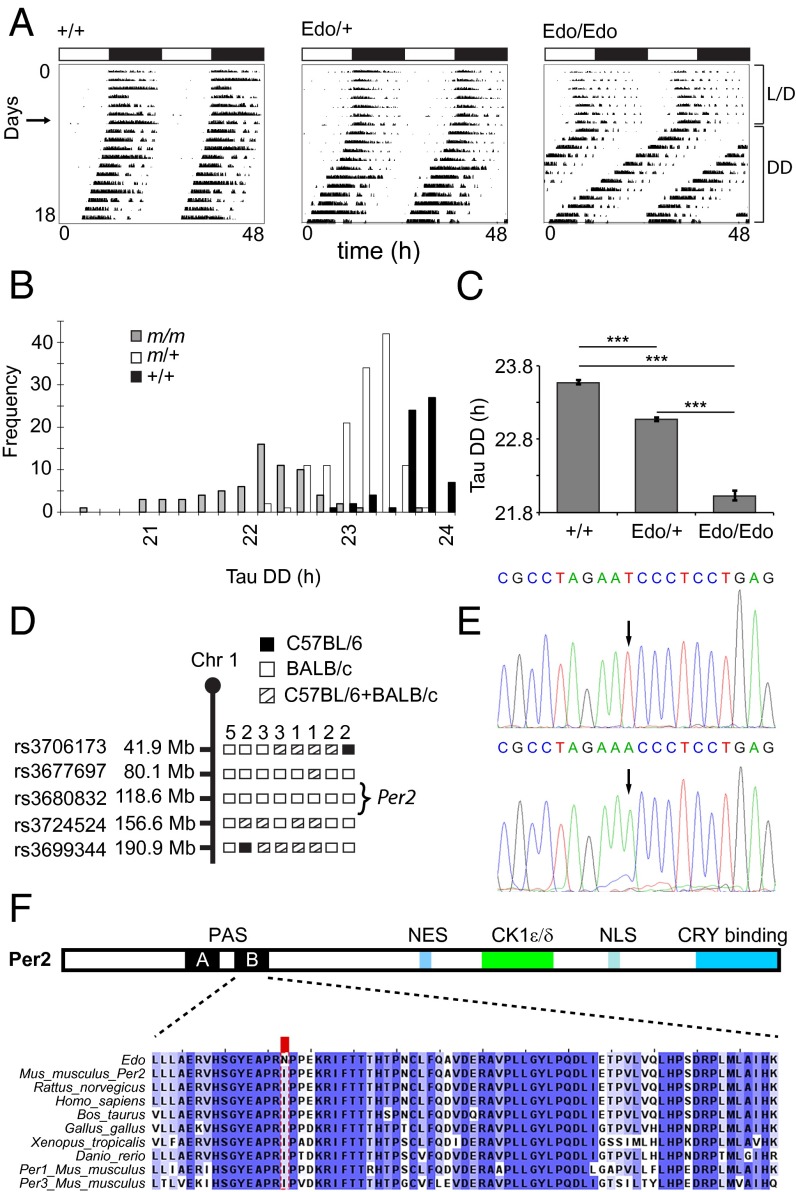
We screened for genetic factors affecting overt behavioral circadian rhythms by monitoring daily wheel-running activity in progeny of ENU mutagenized mice. Within G1/dominant inheritance screens, we identified a mouse with a significantly shortened circadian period (τDD) (Fig. 1A). Test mating confirmed semidominant heritability of the phenotype, with the following mean circadian periods recorded in outcross/intercross offspring: WT τDD of ∼23.6 h, heterozygote τDD of ∼23 h, and homozygote τDD of ∼22 h (Fig. 1 B and C). Homozygotes also displayed a clear advanced phase of entrainment, with activity onsets preceding the onset of the dark phase by several hours. This mutant line was named early doors (Edo; Mouse Genome Informatics accession ID MGI:3606316).
Fig. 1.
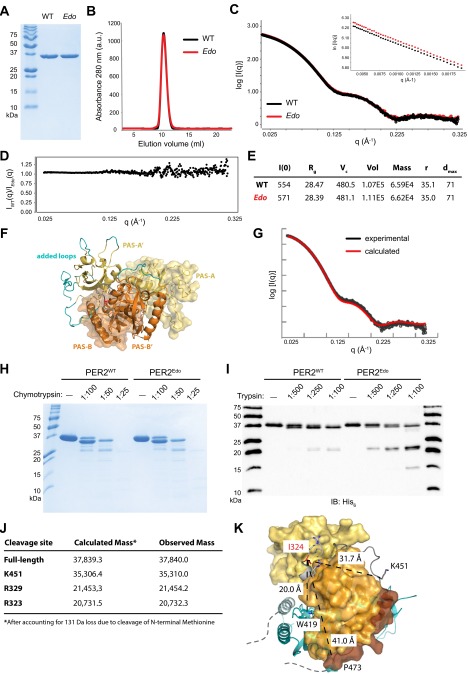
Phenotype and cloning of the Per2Edo mutation. (A) Representative double-plotted actograms of wheel-running activity of +/+, Edo/+, and Edo/Edo mice, recorded under light/dark (L/D) conditions (days 1–7) followed by constant darkness (DD). Horizontal bars at the top indicate L/D conditions over the first 7 d. (B) Frequency distribution of the endogenous period [Tau DD (τDD)] of WT (+/+, black), Edo/+ (m/+, white), and Edo/Edo (m/m, gray) littermates. (C) Free-running period of mutants and littermate controls in constant darkness (mean ± SEM; n = 66, 134, 70; one-way ANOVA, P < 0.001; ***Bonferroni pairwise comparisons, P < 0.001). (D) Graphical representation of chromosome 1 (Chr 1) showing positions of SNP markers used to genotype the affected animals. Informative haplotypes for phenotypically homozygous animals are indicated. (E) Chromatograms from resequencing the Per2 gene in +/+ (Top) and Edo/Edo (Bottom) DNA. (F) Functional domains of PER2 (Top) and sequence conservation in PER2 (Bottom) proteins. CK1ε/δ, CK1ε/δ binding domain; NES, nuclear export signal; NLS, nuclear localization signal. The protein sequence alignment of the interdomain linker and PAS-B domain showing the I324N substitution (red box) in Per2Edo is illustrated. High sequence conservation of the interdomain linker and PAS-B domain is evident in vertebrate proteins and in paralogous mouse proteins (PER1, PER3).
The mutation was mapped by genetic linkage analysis to a 76.5-Mb region of mouse chromosome 1 (Fig. 1D). Within this region lies the period homolog 2 (Per2). With an established role in the circadian oscillator, this gene was selected as a candidate, and the coding regions of Per2 were resequenced in a mutant animal. Sequencing revealed a T-to-A transversion in exon 9 of the gene (Fig. 1E). This nonsynonymous missense mutation results in an Ile-to-Asn substitution at residue 324 (I324N) of PER2, which lies in the interdomain linker between two tandem PAS domains (Fig. 1F). The residue is highly conserved within the protein sequence of all mouse Per genes and in the orthologous genes of other vertebrates.
Per2Edo Is a Gain-of-Function Allele.
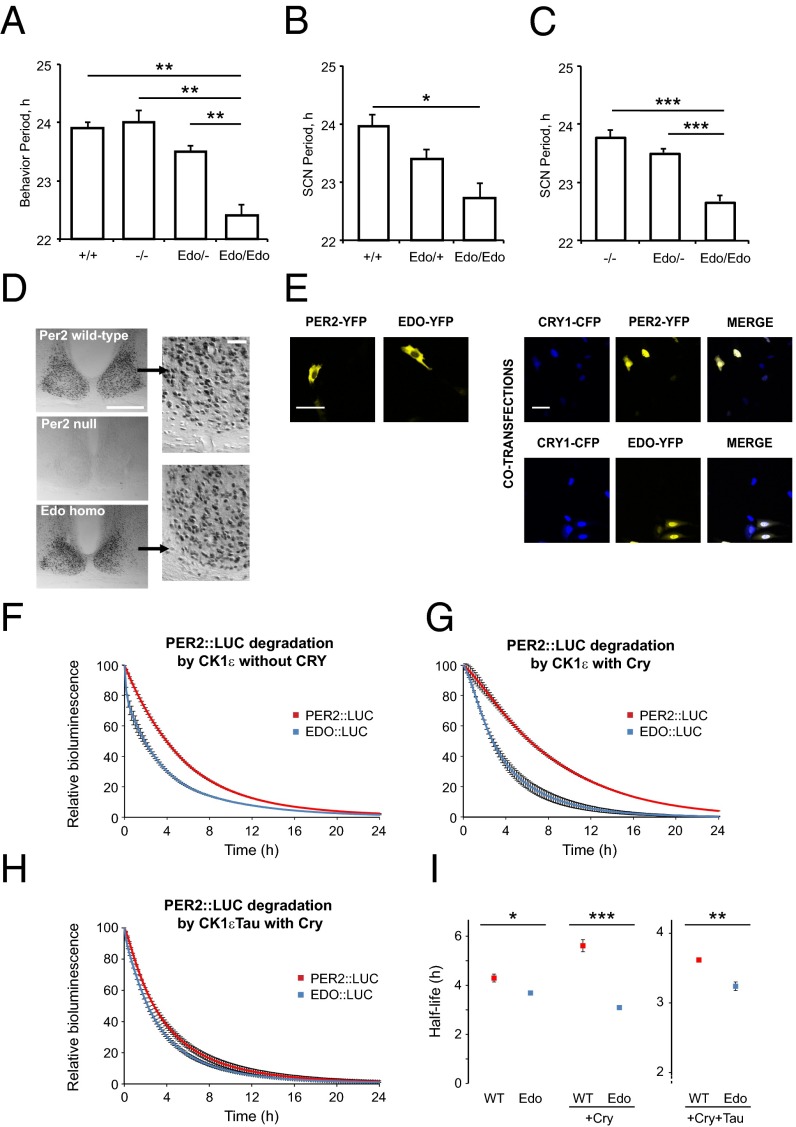
Several null alleles of mPer2 have been described, and have been shown to have either no effect on circadian period (23) or to disrupt rhythms (24, 25). To define the genetic status of the Per2Edo allele, we created compound heterozygous mice alongside single mutants and controls and then monitored wheel-running behavior. The Per2 genotype had an overall significant effect on the period of behavioral rhythms (1× ANOVA F = 22.4; 3,20 df; P < 0.001; Fig. 2A). Whereas Per2Edo/Edo mice exhibited accelerated period compared with WT (period: Per2+/+ = 23.9 ± 0.1 h, Per2Edo/Edo = 22.4 ± 0.2 h; n = 3, 6; P < 0.01), Per2−/− mice had periods (24.0 ± 0.2 h; n = 6) not significantly different from WT and significantly (P < 0.01) longer than Per2Edo/Edo. Moreover, the period of Per2Edo/− compound heterozygotes (23.5 ± 0.1 h; n = 9) was intermediate between Per2Edo/Edo and Per2−/− mutants, although still significantly (P < 0.01) longer than the period of Per2Edo/Edo. At a behavioral level, therefore, Per2Edo is not a loss-of-function mutation. To explore the dose dependence of this effect, SCN slices were prepared from Per1-Luc reporter mice, heterozygous or homozygous for Per2Edo. As with behavior, heterozygous mice showed a period acceleration of 0.56 h, and a second copy of Per2Edo accelerated the clock further, to 1.23 h shorter than WT (1× ANOVA F = 5.0; 2,36 df; P < 0.02; Per2+/+ vs. Per2Edo/Edo: t = 3.1, P < 0.05; Fig. 2B). There was no effect of Per2Edo genotype on the amplitude of oscillation reported by Per1-luc. We then examined SCN function in compound heterozygotes and controls using the Per1-luc reporter (Fig. 2C). As with behavioral rhythms, Per2 genotype had an overall effect on circadian period (1× ANOVA F = 39.4; 2,55 df; P < 0.001). The SCN of Per2−/− mice had a period comparable to WT SCN (period: Per2+/+ = 23.9 ± 0.1 h, Per2−/− = 23.8 ± 0.1 h; n = 9). In contrast, but consistent with behavioral findings, Per2Edo dose-dependently accelerated the SCN clock. The period of SCN from Per2Edo/Edo mice was significantly shorter than the period of SCN from Per2−/− mice, and in compound heterozygous Per2Edo/− mice, the single copy of Per2Edo shortened the period to a value not different from the period seen in Per2Edo/+ SCN but shorter than in Per2−/− SCN (1× ANOVA F = 39.4; 2,55 df; P < 0.001; Per2−/− vs. Per2Edo/−: t = 1.8 ns; Per2−/− vs. Per2Edo/Edo: t = 7.2, P < 0.001; Per2Edo/− vs. Per2Edo/Edo: t = 7.5, P < 0.001). Thus, data from SCN ex vivo studies support the behavioral observations that Per2Edo is a gain-of-function, probably antimorphic, mutation.
Fig. 2.
Reporter oscillations and PER2 stability are affected in Per2Edo. (A) Comparison of wheel-running period in Per2−/−, Per2Edo/Edo, and Per2Edo/− compound heterozygous mutants with littermate controls in constant darkness. (B) Circadian period reported by Per1-luc bioluminescence of SCN slices from WT, Per2Edo/+, and Per2Edo/Edo mice. (C) Circadian period reported by Per1-luc bioluminescence of SCN slices from Per2−/−, Per2Edo/−, and Per2Edo/Edo mice. (D) Immunostaining of PER2 in SCN of WT, Per2−/− (null), and Per2Edo/Edo mice at Zeitgeber time 12. (Scale bar, 500 μm.) (Insets) Nuclear localization of PER2. (Scale bar, 50 μm.) (E) Nuclear/cytoplasmic distribution of YFP-tagged PER2WT and PER2Edo in COS7 cells, transfected alone or with CFP-CRY1. A representative experiment of three independent experiments is shown. (Scale bars, 100 μm.) (F–H) Graphs showing decay of PER2::LUC and EDO::LUC bioluminescence in cycloheximide-treated HEK293 cells. Conditions are as indicated on each graph (n = 3 per condition, mean ± SEM). (I) Comparison of t1/2 of bioluminescent decay for each condition depicted in F–H (*P < 0.05; **P < 0.01; ***P < 0.001 by t test).
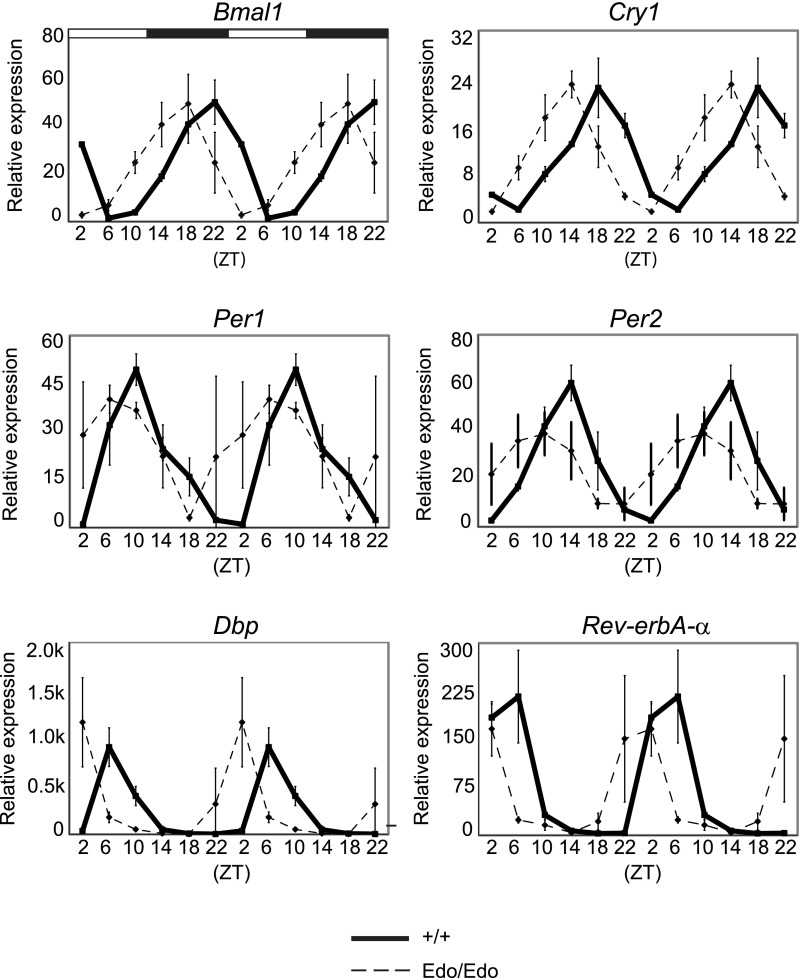
We tested whether the accelerated Per2Edo SCN affected peripheral clocks. The liver of Per2Edo/Edo mice held on a 24-h light/dark (L/D) schedule exhibited very robust daily cycles of gene expression, with amplitudes comparable to the amplitudes of liver from WT mice (Fig. S1). There was a marked advance of ca. 3 h in the phase of the peripheral oscillations for both core clock genes (Bmal1, Per1, Per2, and Cry1) and the canonical circadian output gene Dbp. This phase advance is likely a consequence of the advanced phase of the behavioral rhythm exhibited in the homozygous mutants when entrained to the L/D cycle (Fig. 1A). Thus, the shortened period of the Per2Edo/Edo SCN, and perhaps also an accompanying acceleration of the local liver clock, had a significant effect on peripheral circadian organization.
Fig. S1.
Circadian gene expression in the liver of Per2+/+ and Per2Edo/Edo mice. Relative expression of core oscillator and clock-controlled genes in Per2+/+ (solid lines) or Per2Edo/Edo (dashed lines) in ex vivo liver samples collected at six time points across the 12-h L/D cycle. Each value represents the mean ± SEM (n = 3 independent samples). Graphs are double-plotted for clarity. ZT, Zeitgeber time.
Finally, to confirm that Per2Edo is not a null allele, serial sections of SCN were immunostained with anti-PER2 serum (26). In WT SCN collected at the peak of PER2 expression (Zeitgeber time 12, lights-off), nuclear immunolabeling was extensive across the SCN (Fig. 2D). In homozygous PER2-null SCN, immunolabeling was absent. In contrast, SCN from Per2Edo/Edo mice exhibited widespread nuclear immunoreactivity, comparable to the nuclear immunoreactivity of WT (cell counts: Per2+/+ = 413 ± 26; Per2Edo/Edo = 256 ± 53; Per2−/− = 2 ± 1; n = 3 per group; 1× ANOVA F = 36.3; 2,6 df; P < 0.001; Per2+/+ vs. Per2Edo/Edo: t = 3.2 ns; Per2+/+ vs. Per2−/−: t = 8.4, P < 0.001; Per2Edo/Edo vs. Per2−/−: t = 5.2, P < 0.01). Per2Edo thus encodes a PER2 variant highly expressed and trafficked appropriately to the nucleus of SCN neurons.
PER2Edo Maintains Its Ability to Dimerize but Is Less Stable than PER2WT.
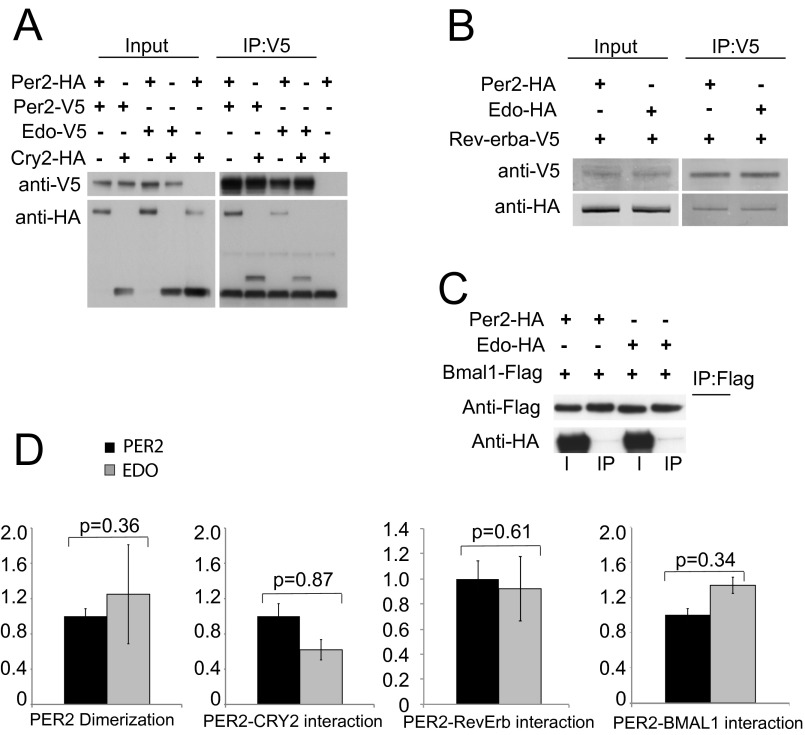
To characterize the biochemical properties of PER2Edo likely to underpin the accelerated clock, we initially focused on protein dimerization, a function determined by the PAS domains where the mutation is located. PER2 PAS domains mediate the formation of homodimers and heterodimers with both PER1 and PER3 (19, 20). Using site-directed mutagenesis, we created the I324N mutation in full-length PER2 and expressed proteins in COS7 cells with various C-terminal tags. Immunoblotting of cell lysates confirmed the appropriate expression of all tagged proteins (Fig. S2 A–C). Coimmunoprecipitation showed that PER2Edo exhibited the expected homodimerization and associations with CRY2, whereas minimal interactions were found with REV-ERBα and BMAL1. Importantly, PER2Edo exhibited comparable associations that, within the limits of the assay, were not significantly different from the associations of PER2WT (Fig. S2D). Given that association with CRY is thought to direct nuclear translocation of PER, we expressed tagged PER2 and CRY1 and monitored their cellular localization. In the absence of CRY1, both PER2 variants were predominantly cytoplasmic (Fig. 2E). Cotransfection with CRY1, however, localized both variants to the nucleus. Thus, the mutation did not compromise functional interaction between PER2 and CRY1, and it did not appear to affect the subcellular localization of either.
Fig. S2.
PER2Edo maintains an ability to dimerize and form complexes with other clock proteins. (A) Representative Western blots of lysates (Input) from COS7 cells transfected with either WT (Per2) or mutant (Edo) PER2-V5 and either PER2-HA or CRY2-HA immunoprecipitated (IP) with an Ab to V5. Lane 5 is a negative control. (B) Representative Western blots of lysates from COS7 cells transfected with either WT (Per2) or mutant (Edo) PER2-HA and REV-ERBα-V5 immunoprecipitated with an Ab to V5. (C) Representative Western blots of lysates from COS7 cells transfected with either WT (Per2) or mutant (Edo) PER2-HA and Flag-tagged BMAL1 immunoprecipitated with an Ab to FLAG. I, input. (D) Relative differences in protein interactions were determined by normalizing the amount of coimmunoprecipitated proteins to the amount of the primary precipitated protein. Values represent the mean ± SEM of four independent experiments. No significant differences were identified between Per2WT and Per2Edo (t test, P values as shown).
Steady-state analyses of the intracellular behavior of PER2Edo failed to reveal significant effects of the mutation. Given that nuclear localization and complex formation can influence the degradation of circadian proteins, we monitored the real-time decay of bioluminescent PER2::LUC fusion proteins in HEK293 cells to test the relative vulnerability of PER2Edo to proteasomal degradation triggered by CK1ε (15). Following treatment with the translational inhibitor cycloheximide, bioluminescent signal for cytoplasmic PER2::LUC declined progressively in the presence of CK1ε. Degradation of EDO::LUC, however, was more rapid (Fig. 2 F and I). The t1/2 of EDO::LUC was slightly but significantly shorter (36 min) than PER2::LUC (P = 0.0223, t test). In the presence of CRY, the vulnerability of nuclear EDO::LUC to CK1ε-mediated degradation was even more pronounced (151 min shorter; P = 0.0005, t test; Fig. 2 G and I). Although the t1/2 of nuclear PER2::LUC was substantially longer in this study, the EDO::LUC t1/2 was shorter. Finally, coexpression with CK1εTau in the presence of CRY resulted in a consistent shorter t1/2 in EDO::LUC (23 min; P = 0.0035, t test; Fig. 2 H and I). Thus, although the Edo mutation did not prevent PER2Edo from forming complexes with other circadian proteins, the vulnerability of PER2Edo to CK1ε-mediated degradation was nevertheless greater.
PER2Edo Increases Flexibility of Interdomain Linker to Destabilize the PAS Domain Core of PER2.
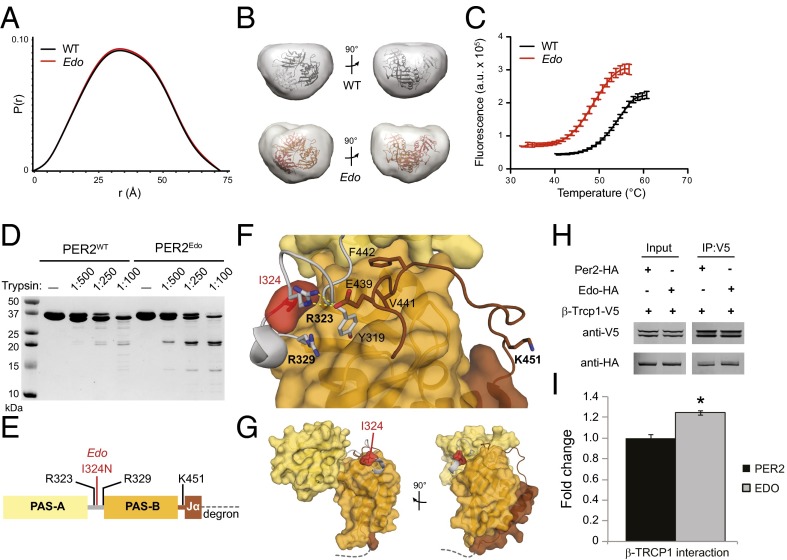
The Edo mutation is located in a conserved linker between the two PAS domains that is distant from the dimer interface observed in the crystal structure (19), consistent with our finding that Edo does not have an impact on dimerization in full-length proteins or in the purified tandem PAS domains [PAS-A and PAS-B (PAS-AB) domains; Fig. S3 A and B]. However, we reasoned that substitution of the more polar Asn at this site might disrupt packing of the linker to influence conformation of the PER2Edo PAS-AB dimer in solution. This possibility was assessed using small-angle X-ray scattering (SAXS), which provides low-resolution envelopes of protein structures in solution (27). Scattering curves for PER2WT and PER2Edo were essentially identical and confirmed dimer formation (Fig. S3 C–E). The pair-distance distribution function (Fig. 3A) indicated that both PAS-AB dimers maintain a compact fold in solution, as demonstrated by oblate spherical envelopes that were well fit by the PAS-AB structure with missing loops modeled in Fig. 3B and Fig. S3 F and G. We then used differential scanning fluorimetry to assess thermodynamic stability of the two proteins. We found that the PER2Edo PAS dimer core was significantly less stable than PER2WT, exhibiting a decrease in melting temperature (Tm) of more than 5 °C (Fig. 3C), which corresponds to a change in the free energy of unfolding, ΔΔG, of 0.66 kcal/mol at the WT Tm of 54.6 °C. Therefore, Edo decreases the intrinsic stability of the PER2 PAS domain core without causing global changes in the overall structure.
Fig. S3.
PAS-AB domains of PER2Edo maintain a similar overall structure as the WT protein. (A) Recombinant PER2WT and PER2Edo PAS-AB domains visualized by Coomassie staining after separation by 18% SDS/PAGE. (B) PER2WT and PER2Edo migrate identically down a Superdex 75 10/300 GL analytical column. a.u., arbitrary units. (C) Merged scattering curves from multiple SAXS exposures taken from 1–5 mg/mL PER2WT (black) and PER2Edo (red). (Inset) Guinier plot of scattering data is linear, indicating the absence of radiation damage. (D) Comparison of SAXS scattering over all q values. A value of 1 for the comparison represents identical data. IEdo, scattering intensity (I) for Edo mutant; IWT, scattering intensity (I) for WT protein. (E) Table of parameters derived from analysis of SAXS data. Dmax, maximal particle size (in angstroms); I(0), extrapolated scattering intensity at zero scattering angle q; r, particle radius (in angstroms); Rg, radius of gyration; Vc, volume of correlation; Vol, volume. (F) Loops missing from the PER2 crystal structure (Protein Data Bank ID code 3GDI) were modeled using AllosMod FoXS (36) to find the top 10 models that best fit the SAXS data. The model shown here represents the best fit to the SAXS data (χ = 2.70). One monomer is shown in surface representation and the other as a cartoon with PAS-A domains (yellow), PAS-B domains (orange), and interdomain linker (gray) with I324 in red. Added loops are depicted on one monomer in teal. (G) Comparison of experimental scattering curve with the curve calculated from the structure shown in F. (H) Limited proteolysis of PER2WT and PER2Edo by chymotrypsin. TEV-cleaved (tagless) proteins were digested with the indicated concentrations of chymotrypsin (wt/wt) relative to PER2. (I) Edo causes differential trypsin proteolysis of the C-terminal Jα helix and interdomain linker. An immunoblot (IB) of the His6 tag on purified proteins digested with trypsin (1:50 dilution of samples visualized by Coomassie staining in Fig. 3D) shows that both proteins retain the N-terminal His6 tag. (J) Table of trypsin cleavage sites in His6-PER2 PAS-AB domain based on liquid chromatography/MS analysis. (K) Proximity of Edo mutation site (I324N) to relevant functional sites on the PER2 PAS-AB dimer. One monomer of the dimer is shown in surface representation (same coloring as in Fig. 3F), and the other is shown in cyan as a cartoon representation. Distances are shown between I324 and (i) the dimer interface at W419 (19); (ii) the trypsin cleavage site at K451 in the linker to the Jα helix; and (iii) P473, the last residue of the structure before the CK1ε-dependent phosphodegron (16). Note that the Jα helix and phosphodegron from the cyan monomer are also located in close proximity to the W419 dimerization interface on the left.
Fig. 3.
PER2Edo mutation increases flexibility of the interdomain linker to destabilize the PAS domain core. (A) SAXS pairwise distribution curves for the PAS-AB dimer of PER2WT (black) or PER2Edo (red). (B) Envelopes calculated from SAXS data for PER2WT and PER2Edo PAS-AB dimers fitted with the crystal structure (Protein Data Bank ID code 3GDI). (C) Thermal denaturation curves of purified proteins in the presence of SYPRO Orange dye (Sigma–Aldrich). Mean fluorescence (n = 6 from one representative experiment) is shown with SD error bars. Tms derived from nonlinear regression: PER2WT, 54.6 ± 0.4 °C; PER2Edo, 49.4 ± 0.3 °C. a.u., arbitrary units. (D) Limited proteolysis of His6-tagged proteins with indicated ratios of trypsin (wt/wt) for 1 h at room temperature. Cleavage products were resolved by 18% SDS/PAGE and visualized by Coomassie staining. (E) Schematic of the PER2 PAS-AB domain region with trypsin cleavage sites determined by liquid chromatography/MS. The dashed line represents the phosphodegron immediately downstream of the Jα helix. (F) Close-up view of the interdomain and Jα helix linkers in the PER2 PAS-AB domain core. Domain coloring is as in E; trypsin cleavage sites detected by liquid chromatography/MS are shown in bold. (G) Relationship of PAS domains, Edo and Jα helix to the PER2 phosphodegron (dashed line). (H) Representative Western blots of lysates (Input) from HEK293 cells transfected with either WT (Per2) or mutant (Edo) PER2-HA and V5-tagged TRCP1 immunoprecipitated (IP) with an Ab to V5. (I) Relative differences in protein interactions were determined by normalizing the amount of coimmunoprecipitated proteins to the amount of the primary precipitated protein. Values represent the mean ± SEM of four independent experiments (*P < 0.05, t test).
To determine how Edo influences stability of the PAS domain core, we probed flexibility of the protein backbone using limited proteolysis. Digestion with chymotrypsin showed no difference between PER2WT and PER2Edo (Fig. S3H), but we observed a pattern of differential cleavage by trypsin (Fig. 3D): first, a short peptide was preferentially cleaved from the C terminus of PER2WT; second, PER2Edo preferentially accumulates ∼21-kDa fragments (Fig. S3 I and J). Specific cleavage sites were identified by liquid chromatography/MS. Cleavage at K451 in the linker to the Jα helix occurred preferentially in PER2WT, whereas cleavage of the interdomain linker at R323 and R329 occurred preferentially in PER2Edo (Fig. 3E). The interdomain and Jα linkers interact with one another and the PAS-B domain through hydrophobic packing and electrostatic interactions, including a salt bridge between R323 and E439 (Fig. 3F). This network of interactions suggests how Edo could influence the flexibility of two linkers that are separated by the PAS-B domain in the primary sequence (Fig. S3K).
The phosphodegron that regulates CK1ε-dependent turnover of PER2 is located immediately downstream of the Jα helix (Fig. 3 E and G). The structure ends at P473 and is followed by the sequence HSGSSGYGS (residues 474–482), where phosphorylation by CK1ε at the underlined Ser residues facilitates binding of the E3 ubiquitin ligase β-TRCP1 (10, 16, 21). Therefore, alterations to the stability and flexibility of the PAS-AB domains may further contribute to destabilization of the mutant in vivo by altering accessibility of this motif. In support of this argument, we found a stronger interaction between β-TRCP1 and PER2Edo when coimmunoprecipitated from HEK293 cell protein complexes (P = 0.022, t test; Fig. 3 H and I).
PER2Edo Is More Sensitive to CK1ε-Mediated Degradation in Vivo, Generating an Extremely Rapid Circadian Clock.
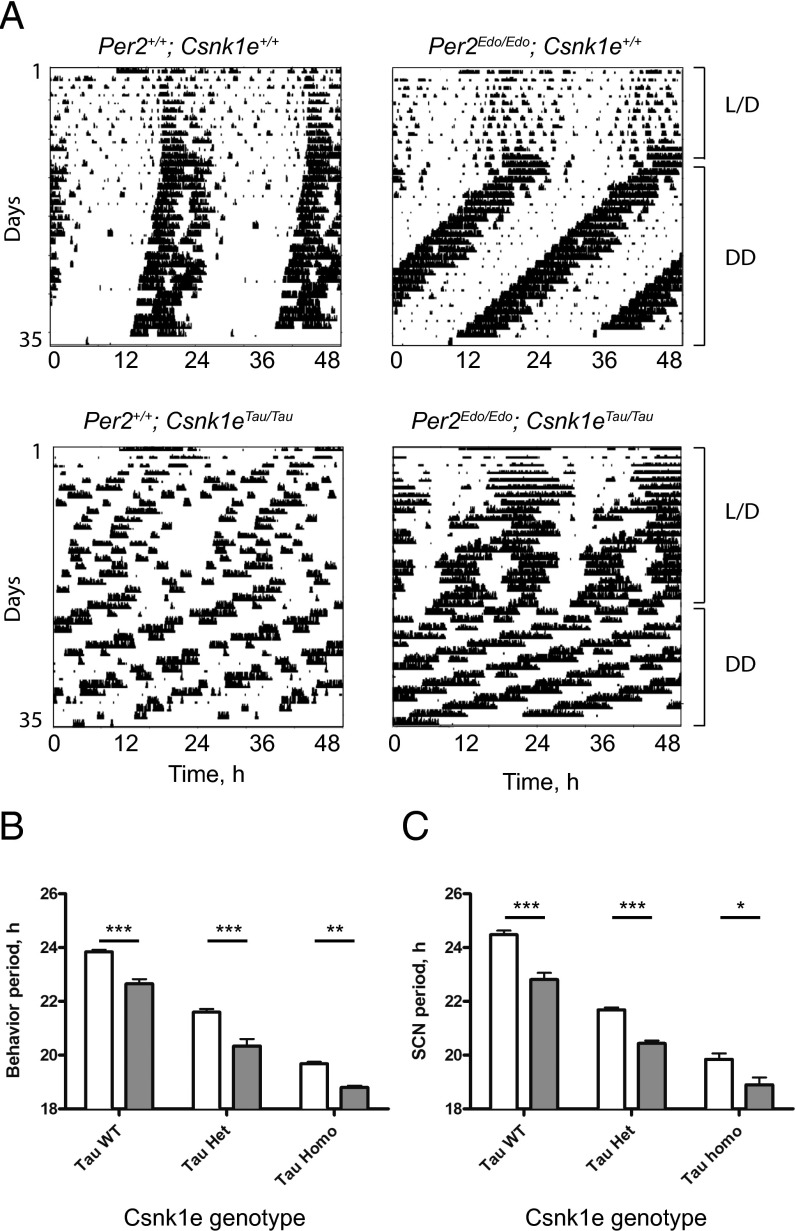
To test predictions based on our biochemical and biophysical analyses in vivo, we crossed the Per2Edo mutation with the Csnk1eTau mutation. Our prediction was that availability of the more vulnerable PER2Edo substrate would allow the mutant CK1ε to accelerate the circadian TTFL even further than in a Per2+/+ background. As anticipated, Csnk1eTau/Tau mice were unable to entrain to the 24-h lighting cycle regardless of whether they carried the additional Per2Edo/Edo allele (Fig. 4 A and B), whereas mice carrying Per2Edo/Edo alone could still entrain. Under continuous dim red light, the free-running periods revealed an additive effect of the two mutations. Both alleles contributed to a shortening of the behavioral circadian period, as revealed by significant main effects of Csnk1eTau and Per2Edo by two-way ANOVA (P < 0.0001 for both). There was not, however, any interaction (P = 0.35). For each of the three Csnk1eTau genotypes tested, the periods of Per2Edo/Edo mice were significantly shorter than the periods of Per2+/+ controls. Notably, the period of the double-homozygous animals was 18.80 ± 0.04 h, which is an unprecedentedly fast behavioral rhythm in mice, and comparable to the ultrashort period of “super-duper” hamsters (28).
Fig. 4.
Per2Edo/Edo; Csnk1eTau/Tau double mutants have an extremely short circadian period in vivo and in vitro. (A) Representative double-plotted actograms of wheel-running for Per2+/+; Csnk1e+/+, Per2Edo/Edo; Csnk1e+/+, Per2+/+; Csnk1eTau/Tau, and Per2Edo/Edo; Csnk1eTau/Tau mice. Animals were entrained to cycles of 12-h L/D and then placed into continuous dim red light as indicated. (B) Circadian period of wheel-running activity rhythms from Per2+/+ (white bars) and Per2Edo/Edo (gray bars) mice in the presence or absence of Csnk1eTau mutant alleles. (C) Circadian period reported by Per1-luc bioluminescence rhythms of SCN slices from Per2+/+ (white bars) and Per2Edo/Edo (gray bars) mice in the presence or absence of Csnk1eTau mutant alleles (mean ± SEM; ***P < 0.001, **P < 0.01, *P < 0.05 by t test).
To confirm that these additive genotypic effects occurred within the SCN, slices were made and the circadian period was determined by recording Per1-luc bioluminescence (Fig. 4C). As with behavior, both alleles contributed to a shortening of the circadian period (main effects two-way ANOVA: P < 0.0001 for both). Again, there was no interaction (P = 0.18), confirming their additive, independent actions. Thus, for all three Csnk1eTau genotypes tested, the periods of Per2Edo/Edo SCN were significantly shorter than the periods of Per2+/+ controls, creating, again, an unprecedentedly short, genetically specified circadian period for the SCN of 18.80 ± 0.06 h.
Discussion
Edo is a novel ENU-induced mutation that shortens the circadian wheel-running period in mice, an effect mediated by accelerated molecular pacemaking in the SCN. The point mutation in Edo lies in the interdomain linker (I324N) between two PAS domains in PER2, and has the effect of increasing linker mobility and reducing stability of the core PAS dimer in PER2Edo in vitro. This mutation is associated with increased vulnerability of PER2Edo to CK1ε-dependent degradation in vivo. Thus, in Per2Edo; Csnk1eTau mice, the combination of increased vulnerability of the substrate with the gain-of-function enzyme leads to extremely short circadian periods of behavior and SCN gene expression.
By uncovering a new allelic variant of Per2, the current study illustrates the continuing value of forward genetic screens to understand circadian mechanisms. The accelerated oscillation in the Edo SCN emphasizes the critical role of protein stability in setting the circadian period. The interaction between PER2 and CK1ε is clearly pace-setting, as revealed by accelerated clocks in mutants of CK1ε and CK1δ in rodents and humans, respectively, and in the human PER2 FASPS mutant (12) now alongside mouse PER2Edo. These observations are consistent with the view that the rhythmic availability of PER2 “defines a critical nodal point for negative feedback” within the TTFL (29). Moreover, the Edo mutation accelerates/phase-advances mRNA rhythms in the liver that are, again, as well defined as the mRNA rhythms seen in WT animals. Thus, a genetic background that accelerates PER2 degradation generates an ultrashort <19-h circadian period in the SCN and in activity/rest cycles. In hamsters, a comparably fast period is seen in compound mutants for Csnk1eTau and “duper,” an unidentified mutation that does not reside in hamster Per2 (28). Critically, the rhythms in hamsters and mice alike are as robust and coherent as in the 24-h WT, highlighting the precision and stability of the SCN oscillator remaining unperturbed in the face of a >20% acceleration of the TTFL.
Importantly, Edo does not phenocopy the Per2 null mutant. First, by immunostaining and circadian recording, it is clear that PER2Edo is expressed and functional. Second, it displays semidominant inheritance, with progressively severe heterozygous and homozygous phenotypes. This phenotype is a notable variation from targeted deletions of Per2, which are phenotypically recessive. Third, this phenotype is expressed in the compound heterozygote Per2Edo/−. It therefore acts as a gain-of-function mutation. We speculate that this gain of function is due to maintenance of normal interactions of PER2Edo with other clock proteins (e.g., CRY), whereas its more rapid turnover leads to a change in the repressive “nodal set point” established by PER2 (29).
Having characterized the phenotypic consequences of the mutation in vivo, we explored its biochemical and structural features to discover that Edo destabilizes the PAS domain dimer core to accelerate PER2 degradation. Notably, Edo does not induce global changes in dimer structure as assessed by size-exclusion chromatography and SAXS. Here, we show that Edo alters the flexibility of linkers in the PAS domain core to elicit its dramatic overall effect on PER2 function. Edo makes the PAS domain core less intrinsically stable and also leads to increased interaction with its E3 ubiquitin ligase β-TRCP1, leading to increased turnover in vivo. Given the modest buried surface area between the interdomain linker and the PAS domains (590 Å2), it is perhaps surprising that substitution of one residue has such profound implications for PER2 structure and function. However, the interdomain and Jα linkers are linked through a series of interactions on the PAS-B domain, potentially connecting the Edo site to the CK1ε-dependent phosphodegron immediately downstream of the Jα helix (16). The biochemical basis of the interaction between PER2 and CK1ε is, however, unclear. A current model posits that the Tau mutation changes the relative efficacy of CK1ε at two competing phosphorylation sites that determine whether PER2 has a fast or slow degradation rate (16). Further work will be necessary to understand the structural basis by which linker flexibility enhances interaction with CK1ε-dependent β-TRCP1 activity to control PER2 stability in vivo. Our data reaffirm the central role of PAS-mediated mechanisms in tuning the period of the mammalian circadian clock, and thereby extend the scope of molecular structure-based analysis of cellular timekeeping.
Methods
Animals.
All animal studies were carried out in accordance with the 1986 Animals (Scientific Procedures) Act, United Kingdom, with Medical Research Council Harwell and Laboratory of Molecular Biology ethical approval and were compliant with the guidance issued by the Medical Research Council (30). Inbred strains were obtained from in-house stock (C57BL/6J, C3H/HeH; hereafter termed C3H) or were purchased from Harlan-Olac (BALB/cOlaHsd, hereafter termed BALB/c). Mutant and reporter lines were obtained from the following: D. Weaver [University of Massachusetts Medical School, Worcester, MA; Per2tm1Drw null mutant (24)], H. Okamura [University of Kyoto, Kyoto; mPer1-luc reporter (31)], J. S. Takahashi [University of Texas Southwestern Medical Center, Dallas; mPer2::Luc reporter, Per2tm1Jt (32)], and A. S. Loudon [University of Manchester, Manchester, United Kingdom; Ck1ε mutant, Csnk1etm1Asil (15)]. All molecular and behavioral studies were carried out in mice bred on a congenic C57BL/6J background.
Mutagenesis, Genetic Mapping, and Mutation Detection.
Mutagenesis, screening, genetic linkage, positional candidate analysis, and sequencing were carried out as described previously (22, 33).
SCN Slices.
SCN organotypic slices for bioluminescence recordings and immunostaining of SCN PER2 expression were as described previously (26).
Cell Culture and Transfection.
COS7 and HEK293 cells were maintained in DMEM supplemented with 10% (vol/vol) FBS and antibiotics (100 μg/mL penicillin and streptomycin). Transient transfection was carried out using Fugene 6 (Roche) according to the manufacturer’s protocol. For degradation studies, HEK293 cells were transfected with either PER2::LUC (a generous gift from Y.-H. Fu and A. Hirano, University of California, San Francisco, San Francisco, CA) or EDO::LUC generated by site-directed mutagenesis (Stratagene). Reporter constructs were cotransfected with combinations of CK1εWT, CK1εTau, and mCry2-HA plasmids. Bioluminescence degradation studies were conducted as in the study by Hirano et al. (34). Luciferase activity of PER-LUC was recorded at 10-min intervals at 37 °C with Lumicycle (Actimetrics). The data were fitted to an exponential curve of the form N(t) = N0 e(−λt) where N(t) is the luminescence (counts per second) at time t, N0 is the initial luminescence (counts per second), λ is the decay rate, and t is the time. The rate of λ was determined using a MATLAB (MathWorks) Curve Fitting Toolbox, and t1/2 was calculated using the t1/2 equation t1/2 = ln (2)/λ.
For coimmunoprecipitation experiments, cells were transfected with mPER2WT-V5-YFP-His, mPER2Edo-V5-YFP-His, or β-TRCP1–V5-His plasmids and mCry2-HA, HA-mPER2, HA-mPER2Edo, mBmal1-FLAG, mRev-erba-V5, respectively, in 10-cm dishes in medium without antibiotics. The total amount of transfected DNA was 6 μg (composed of 3 μg of mPer2 WT or mPer2 mutant and 3 μg of mCry2-HA, HA-mPer2, HA-mEdo, HA-mPer1, mBmal1-FLAG, mClock-HA, mRev-erba-V5). Forty-eight hours after transfection, cells were washed three times with cold PBS, lysed in 700 μL of lysis buffer, and treated as described above.
Coimmunoprecipitation and Western Blotting.
Tagged proteins were immunoprecipitated from COS7 or HEK293 cell lysates with anti-V5 (R960-25; Invitrogen), anti-HA (HA.11; Covance), or anti-FLAG M2 (F3165; Sigma) following 2 h of gentle agitation at 4 °C with 25 μL of protein G agarose. Samples were washed in buffer, boiled at 95 °C for 5 min, separated by SDS/PAGE on a 4–12% gel, and transferred to a PVDF membrane. Immunoblotting was performed using anti-V5 (1:3,000), anti-HA (1:1,000), anti-FLAG (1:800), and anti-GFP (1:5,000) (11814460001; Roche). Secondary Ab was HRP-conjugated anti-mouse (Sigma) at a 1:10,000 dilution. Chemiluminescence was performed using ECL Plus (Amersham).
Recombinant Protein Expression and Purification.
A fragment containing the tandem mouse PER2 PAS-AB domains (residues 170–473) was expressed in Escherichia coli as a fusion with a tobacco etch virus (TEV)-cleavable N-terminal His6 tag. The Edo mutation (I324N) was introduced by site-directed mutagenesis and validated by sequencing. Protein expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside at an OD600 of ∼0.8, and E. coli was grown for an additional 16 h at 18 °C. Soluble proteins were purified by Ni2+-nitrilotriacetic acid affinity chromatography (QIAGEN), followed by cleavage of the His6 tag with TEV protease for either 4 h at room temperature or overnight at 4 °C. Subsequent Ni2+-nitrilotriacetic acid affinity chromatography was performed to remove the protease and cleaved tag. Proteins were further purified by size-exclusion chromatography on a Superdex 75 16/60 prep grade column (GE Healthcare) equilibrated in 25 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM DTT, and then concentrated to 10 mg/mL. Concentration of the purified, cleaved proteins (residues 170–473 with an N-terminal “GAMDP” remaining after TEV cleavage) was determined at A280 using a calculated extinction coefficient of 32,890 M−1⋅cm−1.
SI Methods
ENU Mutagenesis and Wheel-Running Activity Screen.
Briefly, male BALB/c mice were injected i.p. with two weekly injections of 100 mg/kg ENU (Sigma) at ∼10 wk of age (35). After a period of temporary sterility (about 15 wk), they were crossed to C3H females, and the F1 progeny were screened for abnormal circadian behavior from 6 to 12 wk of age. To test inheritance of an abnormal phenotype, F1 founder mice were backcrossed to C3H mice and the backcross progeny were assessed for the inheritance of the abnormal phenotype. Mutant mouse lines were also transferred onto a C55BL/6J background once they could be genotyped. Screens were carried out by transferring the mice to individual cages equipped with running wheels and maintained within light-tight ventilated chambers with timer-controlled lighting. Wheel-running data were collected and analyzed using Clocklab (Actimetric Software).
Genotyping.
For genotyping Edo mice, ear biopsy DNA was subjected to a PCR discrimination assay, with amplification of exon 9 and the flanking region of Per2 by the primers 5′ TTGAGTATTTCCCTAGTTATTGCAG 3′ and GCGACACGGAGGACACAG, followed by HinfI digest. Agarose gel electrophoresis revealed bands of 241 bp (WT), 241 bp, 144 bp, and 97 bp (heterozygote) and of 144 bp and 97 bp (homozygote). Genotyping for other lines was as described (24, 26, 31, 32).
RNA Extraction, RT, and Real-Time PCR.
Homozygous Edo mutants and littermate controls on a 12-h/12-h L/D cycle were killed at 4-h intervals by cervical dislocation, and livers were harvested, dissected, immediately snap-frozen on dry ice, and stored at −80 °C. RNA was extracted using the standard TRIzol extraction protocol (Life Technologies). The total RNA concentration was determined using a nanodrop ND-1000 (Thermo Fisher Scientific). mRNA expression was determined using SYBR Green RT-PCR assays utilizing the following primers:
Bmal1 (forward: CCGTGCTAAGGATGGCTGTT, reverse: TTGGCTTGTAGTTTGCTTCTGTGT)
Cry1 (forward: GTGGATCAGCTGGGAAGAAG, reverse: CACAGGGCAGTAGCAGTGAA)
Dbp (forward: GAGCCTTCTGCAGGGAAACA, reverse: GCCTTGCGCTCCTTTTCC)
Nr1d1 (forward: CGTTCGCATCAATCGCAACC, reverse: GATGTGGAGTAGGTGAGGTC)
Per1 (forward: CCCCTGCCTCCCAGTGA, reverse: CTGAAAGTGCATCCTGATTGGA)
Per2 (forward: AGCTACACCACCCCTTACAAGCT, reverse: GACACGGCAGAAAAAAGATTTCTC)
Housekeeping control gene Rlpl3a (forward: TGCTGCTCTCAAGGTTGTTC, reverse: TGCTTCTTCTTCGATAGTGC).
The RT reactions were conducted using a SuperScript III First-Strand Synthesis for qRT-PCR kit with 1 μg of RNA (protocol for part 11752-050; Life Technologies). The RT-PCR reactions were performed in triplicate using 10 μL of 2× Power SYBR Green PCR Master Mix (Life Technologies), 2 μL of product from the RT reaction (diluted 1:4), 1 μL of 2 μM primer mix (containing both the forward and reverse primers), and 7 μL of nuclease-free water. A sample minus RT buffer was used as a negative control for the RT-PCR reactions. None of the negative controls reached threshold by 45 cycles. RT-PCR reactions were run on a 7500 Fast Real-Time PCR System (Applied Biosystems) in 96-well format. Relative expression was calculated using the standard 2−(Δ(Ct)) cycle threshold relative expression method in Microsoft Excel. The relative expression of the individual samples was normalized to the Rlpl3a.
Plasmids.
Plasmids for Per2-HA, Bmal1-Flag, and CK1ε were a gift from Filippo Tamanini (Erasmus University Medical Center, Rotterdam, The Netherlands). V5-YFP-His epitope–tagged mPer2 and mCry2-HA were generated in-house. To create the mREV-ERBA-V5-His expression vector, Rev-erba cDNA was PCR-amplified using primer pairs 5′-CAACATGACGACCCTGGACTCC-3′ and 5′-CTGGGCGTCCACCGGGAA-3′ and cloned into a pENTR/D-TOPO vector using a pENTR Directional TOPO Cloning Kit (K2400; Invitrogen) to produce an entry clone. The latter, containing an Rev-erba gene, was then introduced into the pcDNA-DEST40 expression vector by following the Gateway Technology with Clonase II (12535-029; Invitrogen). Similarly, the bTRCP1-V5-His expression vector was created by amplifying βTrcp1 cDNA from primer pairs 5′-CACCATGGACCCGGCAGAGGCGGTG-3′ and 5′-TCTGGAGATGTAGGTGTATGT-3′ and subcloned as above. The sequences of pENTR/D-TOPO and pcDNA-DEST40 vectors are available for downloading from the Invitrogen web site (www.thermofisher.com).
Site-Directed Mutagenesis.
Amino acid substitution in the mPER2 PAS-B domain of mPER2 (full)-V5-YFP-His and HA-mPER2 (full) was generated using a QuikChange XL Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s directions. Mutagenesis primers for the Edo mutant were 5′-CTCAGGAGGGTTTCTAGGCGCTTCATAGCC-3′ and its complementary strand. The presence of the expected base pair substitution was confirmed by DNA sequencing.
Size-Exclusion Chromatography.
For estimation of dimerization state by size-exclusion chromatography, purified proteins were injected on a Superdex 75 10/300 GL analytical column at 160 μM (∼6.4 mg/mL) in 25 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM DTT. All size-exclusion columns were calibrated with a low-molecular-weight gel filtration standards kit (GE Healthcare Life Sciences).
SAXS.
SAXS data were collected on the SIBYLS beamline (12.3.1) at the Advanced Light Source (Lawrence Berkeley National Laboratory). Multiple exposures of three concentrations (1, 3, and 5 mg/mL) of freshly purified protein were taken to check for concentration dependence of scattering and radiation damage (neither was detected). After buffer subtraction, experimental scattering curves were compared with a theoretical scattering curve derived from the X-ray crystal structure (Protein Data Bank ID code 3GDI) using FoXS (36), giving a χ fit of 16.0. To improve on the fit, AllosMod FoXS was used to build in the missing loops from the crystal structure, improving the χ fit to 2.70. Scattering data were further analyzed using the ATSAS suite of programs (37). Briefly, data were merged using PRIMUS, and the radius of gyration was determined using the Guinier approximation. The pair-distance distribution function [P(r)] and maximal particle size (Dmax) were generated in GNOM, and the output data were used by GASBOR to calculate 10 independent solution envelopes that were averaged together using DAMAVER. The improved model of the solution structure was used in UCSF Chimera (38) to fit into the averaged solution envelope. Scattering datasets, analysis, and models have been deposited with BioISIS (www.bioisis.net) with the following deposition codes: PLCSWP (PER2WT) and PLCSEP (PER2Edo).
Limited Proteolysis.
Limited proteolysis of purified proteins was performed at 1.5 mg/mL in 25 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM DTT with sequencing-grade proteases (Promega) for 1 h at room temperature with the following mass (wt/wt) ratios: chymotrypsin (1:25, 1:50, and 1:100) or trypsin (1:100, 1:250, and 1:500). To visualize proteins by Coomassie staining, reactions were quenched with addition of an equal volume of 2× SDS Laemmli buffer (Bio-Rad) and samples were boiled at 95 °C for 5 min. Digested fragments were resolved by 18% SDS/PAGE and visualized by Coomassie staining. For immunoblotting, the same gel samples were first diluted 50-fold in 1× SDS Laemmli buffer and then separated by 18% SDS/PAGE and transferred to a nitrocellulose membrane. Immunoblotting was performed using anti-His6 1:2,000 (AM1010a; Abgent). Secondary Ab was HRP-conjugated anti-mouse (A9917; Sigma) at a 1:10,000 dilution, spiked with a 1:10,000 dilution of anti-Streptactin-HRP (161-0380; Bio-Rad) for direct visualization of Western C protein markers (Bio-Rad). Chemiluminescence was detected with Clarity reagent (Bio-Rad) and detected via a CCD camera on a ChemiDoc gel imaging system (Bio-Rad).
Proteolytic samples for MS were quenched by addition of formic acid to a final concentration of 1% (vol/vol). Samples were desalted and separated by HPLC (Surveyor; Thermo Finnegan) on a Proto 300 C4 reverse-phase column with a 100-mm × 2.1-mm inner diameter and 5-μm particle size (Higgins Analytical, Inc.) using a mobile phase consisting of solvent A (0.1% formic acid in HPLC grade water) and solvent B (0.1% formic acid in acetonitrile). The samples were analyzed on an LTQ Orbitrap linear ion trap mass spectrometer system (Thermo Finnegan). Proteins were detected by full-scan MS mode (over an m/z of 300–2,000) in positive mode with the electrospray voltage set to 5 kV. Mass measurements of deconvoluted electrospray ionization mass spectra of the reversed-phase peaks were generated by Magtran software. Observed masses were within 0.01% error of calculated masses after accounting for cleavage of the N-terminal methionine (loss of 131 Da), a common occurrence for proteins expressed recombinantly in E. coli (39).
Differential Scanning Fluorimetry.
Stability of purified proteins was assessed by miniaturized differential scanning fluorimetry using SYPRO Orange dye (Sigma–Aldrich) (40). Proteins were diluted to 10 μM with 25 mM Hepes (pH 7.5), 200 mM NaCl, 5 mM DTT, and SYPRO Orange dye at a final concentration of twofold. Fifty-microliter aliquots of PER2WT and PER2Edo were plated in replicate (n = 6) into a 96-well PCR plate and sealed with optically clear film (Invitrogen). Thermal denaturation was performed in a ViiA7 Real-Time PCR instrument (Applied Biosystems) using changes to the default Melt Curve program as previously described (40). Raw fluorescence data were processed in Microsoft Excel, and Tms were obtained by nonlinear regression of sigmoidal denaturation curves using GraphPad Prism 6 as previously described (40). Data shown are representative of three independent assays. The equilibrium constant for unfolding (KU) was determined after estimating the fraction of protein unfolded (FU) as a function of temperature using the following equation:
where Flobs corresponds to the fluorescence observed at a given temperature, Flnative is the baseline fluorescence for natively folded protein, and Flunfolded corresponds to fluorescence at the upper plateau (determined after subtraction of postpeak fluorescence quenching). KU was determined with the following equation:
and used to calculate the free energy for unfolding of WT and Edo protein at the Tm for WT PER2 PAS-AB domain (∼55 °C).
Acknowledgments
We thank Drs. Ying-Hui Fu and Arisa Hirano (University of California, San Francisco) for providing us with the PER2::LUC construct. We are grateful for excellent technical support from the Biomedical Staff at the Medical Research Council Mary Lyon Centre and the Ares facility. We thank Steve Thomas for work on the figures. SAXS data were collected at the Advanced Light Source (ALS), a national user facility supported by the US Department of Energy Office of Biological and Environmental Research. Additional support comes from the US NIH project MINOS R01 GM105404. We thank Greg Hura and Jane Tanamachi at the ALS SIBYLS beamline for their assistance. This study was supported by Medical Research Council, United Kingdom Grants MC_U142684173 and MC_U105170643; Sixth Framework Project EUCLOCK Grant 018741 (to M.H.H. and P.M.N.); and US NIH Grant R01 GM107029 (to C.L.P.). The University of California, Santa Cruz Mass Spectrometry Facility receives support from the W. M. Keck Foundation (Grant 001768) and the US NIH Center for Research Resources (Grant S10 RR020939).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Scattering datasets, analysis, and models have been deposited with BioISIS (www.bioisis.net) [deposition codes PLCSWP (PER2WT) and PLCSEP (PER2Edo)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517549113/-/DCSupplemental.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111(7):919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 3.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 5.Sato TK, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Shigeyoshi Y, et al. Restoration of circadian behavioural rhythms in a period null Drosophila mutant (per01) by mammalian period homologues mPer1 and mPer2. Genes Cells. 2002;7(2):163–171. doi: 10.1046/j.1356-9597.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29(2):453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 8.Maywood ES, et al. Tuning the period of the mammalian circadian clock: Additive and independent effects of CK1εTau and Fbxl3Afh mutations on mouse circadian behavior and molecular pacemaking. J Neurosci. 2011;31(4):1539–1544. doi: 10.1523/JNEUROSCI.4107-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms E, Kivimäe S, Young MW, Saez L. Posttranscriptional and posttranslational regulation of clock genes. J Biol Rhythms. 2004;19(5):361–373. doi: 10.1177/0748730404268111. [DOI] [PubMed] [Google Scholar]
- 10.Eide EJ, et al. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25(7):2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reischl S, et al. Beta-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J Biol Rhythms. 2007;22(5):375–386. doi: 10.1177/0748730407303926. [DOI] [PubMed] [Google Scholar]
- 12.Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 14.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288(5465):483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng QJ, et al. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58(1):78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M, Kim JK, Eng GW, Forger DB, Virshup DM. A Period2 Phosphoswitch Regulates and Temperature Compensates Circadian Period. Mol Cell. 2015;60(1):77–88. doi: 10.1016/j.molcel.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Huang N, et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337(6091):189–194. doi: 10.1126/science.1222804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107(7):855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 19.Hennig S, et al. Structural and functional analyses of PAS domain interactions of the clock proteins Drosophila PERIOD and mouse PERIOD2. PLoS Biol. 2009;7(4):e94. doi: 10.1371/journal.pbio.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucera N, et al. Unwinding the differences of the mammalian PERIOD clock proteins from crystal structure to cellular function. Proc Natl Acad Sci USA. 2012;109(9):3311–3316. doi: 10.1073/pnas.1113280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohsaki K, et al. The role of beta-TrCP1 and beta-TrCP2 in circadian rhythm generation by mediating degradation of clock protein PER2. J Biochem. 2008;144(5):609–618. doi: 10.1093/jb/mvn112. [DOI] [PubMed] [Google Scholar]
- 22.Bacon Y, et al. Screening for novel ENU-induced rhythm, entrainment and activity mutants. Genes Brain Behav. 2004;3(4):196–205. doi: 10.1111/j.1601-183X.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 23.Maywood ES, Chesham JE, Smyllie NJ, Hastings MH. The Tau mutation of casein kinase 1ε sets the period of the mammalian pacemaker via regulation of Period1 or Period2 clock proteins. J Biol Rhythms. 2014;29(2):110–118. doi: 10.1177/0748730414520663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30(2):525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 25.Oster H, Yasui A, van der Horst GT, Albrecht U. Disruption of mCry2 restores circadian rhythmicity in mPer2 mutant mice. Genes Dev. 2002;16(20):2633–2638. doi: 10.1101/gad.233702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng QJ, et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA. 2010;107(34):15240–15245. doi: 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hura GL, et al. Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS) Nat Methods. 2009;6(8):606–612. doi: 10.1038/nmeth.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monecke S, Brewer JM, Krug S, Bittman EL. Duper: A mutation that shortens hamster circadian period. J Biol Rhythms. 2011;26(4):283–292. doi: 10.1177/0748730411411569. [DOI] [PubMed] [Google Scholar]
- 29.Chen R, et al. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell. 2009;36(3):417–430. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medical Research Council 1993. Responsibility in the Use of Animals for Medical Research (Medical Research Council, London)
- 31.Yamaguchi S, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302(5649):1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 32.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godinho SI, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316(5826):897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 34.Hirano A, et al. FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell. 2013;152(5):1106–1118. doi: 10.1016/j.cell.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 35.Nolan PM, et al. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet. 2000;25(4):440–443. doi: 10.1038/78140. [DOI] [PubMed] [Google Scholar]
- 36.Schneidman-Duhovny D, Hammel M, Tainer JA, Sali A. Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys J. 2013;105(4):962–974. doi: 10.1016/j.bpj.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petoukhov MV, et al. New developments in the ATSAS program package for small-angle scattering data analysis. J Appl Cryst. 2012;45(Pt 2):342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 39.Frottin F, et al. The proteomics of N-terminal methionine cleavage. Mol Cell Proteomics. 2006;5(12):2336–2349. doi: 10.1074/mcp.M600225-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Huynh K, Partch CL. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci. 2015;79:28.9.1–28.9.14. doi: 10.1002/0471140864.ps2809s79. [DOI] [PMC free article] [PubMed] [Google Scholar]