Abstract
The introduction of drinking water disinfection greatly reduced waterborne diseases. However, the reaction between disinfectants and natural organic matter in the source water leads to an unintended consequence, the formation of drinking water disinfection byproducts (DBPs). The haloace-taldehydes (HALs) are the third largest group by weight of identified DBPs in drinking water. The primary objective of this study was to analyze the occurrence and comparative toxicity of the emerging HAL DBPs. A new HAL DBP, iodoacetaldehyde (IAL) was identified. This study provided the first systematic, quantitative comparison of HAL toxicity in Chinese hamster ovary cells. The rank order of HAL cytotoxicity is tribromoacetaldehyde (TBAL) ≈ chloroacetaldehyde (CAL) > dibromoacetaldehyde (DBAL) ≈ bromochloroacetaldehyde (BCAL) ≈ dibromochloroacetaldehyde (DBCAL) > IAL > bromoacetaldehyde (BAL) ≈ bromodichloroacetaldehyde (BDCAL) > dichloroacetaldehyde (DCAL) > trichloroacetaldehyde (TCAL). The HALs were highly cytotoxic compared to other DBP chemical classes. The rank order of HAL genotoxicity is DBAL > CAL ≈ DBCAL > TBAL ≈ BAL > BDCAL > BCAL ≈ DCAL > IAL. TCAL was not genotoxic. Because of their toxicity and abundance, further research is needed to investigate their mode of action to protect the public health and the environment.
Graphical abstract
INTRODUCTION
The disinfection of drinking water was an outstanding contribution for the protection of the public health.1 An unintended consequence of water disinfection is the generation of disinfection byproducts (DBPs). Trihalomethanes (THMs) were discovered as the first chemical class of DBPs in 1974.2 Since then, research has led to the identification of emerging DBPs3–7 and determination of their formation kinetics,8–10 toxicity,7,11,12 exposure, and risk assessment.13–17 To date, more than 600 DBPs have been identified, and many are reported to be cytotoxic, genotoxic, teratogenic, or carcinogenic.7,11,12,18–21 Epidemiologic studies have demonstrated associations between DBPs and increased risk for bladder and colon cancers.22–26 Furthermore, evidence associating DBPs and adverse pregnancy outcomes, including spontaneous abortion, low birth weight, small-for-gestational-age, still birth, and preterm delivery has also been reported.14,27–35
Haloacetaldehydes (HALs) are an important class of emerging (nonregulated) DBPs.36 HALs were the third largest DBP class by weight in a U.S. Nationwide DBP Occurrence Study, with dichloroacetaldehyde (DCAL) as the most abundant individual HAL reported (maximum concentration: 16 µg/L).37 Individual HAL concentrations in finished water are dependent on the source water quality, including natural organic matter and bromide levels, and disinfection treatment type. The contribution of trichloroacetaldehyde (TCAL), another ubiquitous HAL, that is present in water in its hydrated form (chloral hydrate), to total HALs in water was reported to be highly variable (5–60%), thus, it is important to evaluate other HAL species in order not to underestimate the overall HAL amount present in drinking water.38 In the U.S. EPA Information Collection Rule, TCAL was found at median and maximum concentrations of 1.7 µg/L and 46 µg/L, respectively, and concentrations observed in finished water did not significantly vary among the investigated disinfection treatments (including chlorine, chloramine, chlorine/chloramine, chlorine dioxide, and ozone).39 In Canadian drinking water distribution systems, the highest TCAL concentration was 263 µg/L, with the highest HAL concentrations found in waters disinfected with ozone and chlorine.38 Waters from chloraminated systems had lower levels.38 In the U.S. Nationwide Occurrence Study, DCAL levels were maximized with chloramines and ozone, but TCAL formation was reduced with this disinfectant combination.37,40 Brominated HALs, including bromochloroacetaldehyde (BCAL), dibromoacetaldehyde (DBAL), bromodichloroacetaldehyde (BDCAL), dibromochloroacetaldehyde (DBCAL), and tribromoacetaldehyde (TBAL), were formed after chlorination of bromide-containing waters and similarly as for trihalomethanes, bromine incorporation increased with bromide concentration in source waters.38 Six di- and tri-HALs were measured recently in two microfiltration/reverse osmosis (RO) water recycling plants in Perth, Australia, where HALs were formed by chloramination (used to prevent membrane fouling) but were, for the most part, effectively removed by RO.41
The toxicity of a few specific HALs was examined in previous studies.20 TCAL was mutagenic in Salmonella typhimurium42–45 and induced chromosomal aberrations42,46 and aneuploidy47,48 in mammalian cells. TCAL was also reported to induce micronuclei,49–53 mitotic aberrations,53–56 and DNA strand-breaks57–59 in mammalian cells. The toxicity of chloroacetaldehyde (CAL) was studied as a metabolite of the industrial chemical vinyl chloride.60 CAL was cytotoxic in rat hepatocytes61 and induced nephrotoxicity in human renal proximal tubule cells.62 Further, CAL formed DNA adducts, caused mutations,63–69 and generated mitotic chromosome malsegregation70 and interstrand cross-links.71 Similar to TCAL, DCAL induced mitotic aneuploidy.72 Regarding the toxicity of brominated HALs, BAL irreversibly bound to DNA and proteins in rat liver microsomes,73 and TBAL induced single- and double-strand DNA breaks.59 Despite these studies, a systematic investigation of other emerging HAL DBPs has not been conducted, and there is no quantitative, comparative database on the toxicity of the complete set of chloro-bromo HALs or iodo-HALs.
In this context, the objectives of our research were to (i) develop and validate an analytical method to determine 10 chloro-bromo-iodo-HALs in water, (ii) evaluate for the first time the occurrence of iodoacetaldehyde (IAL) in source and drinking waters and to compare its concentrations to those of other target HALs, (iii) analyze the in vitro cytotoxicity and genotoxicity of HALs in mammalian cells, (iv) determine the cytotoxicity and genotoxicity index values of HALs and develop a quantitative, comparative toxicity database, and (v) conduct a mechanism-based structure–activity relationship analysis for the observed HAL-mediated cytotoxicity and genotoxicity.
MATERIALS AND METHODS
Chemicals and Reagents
General reagents were certified ACS reagent grade and were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Itasca, IL). Media and fetal bovine serum (FBS) were purchased from Fisher Scientific (Itasca, IL). HAL standards were purchased from Sigma-Aldrich, CanSyn Chem. Corp. (Toronto, ON), Aldlab Chemicals (Woburn, MA), and TCI America (Waltham, MA) at the highest level of purity available (chemical properties, purity, and CAS numbers of investigated HALs are provided in Supporting Information (SI), Table S1). 4-Fluorobenzaldehyde and 1,2-dibromopropane, used as the surrogate standard (SS) and internal standard (IS), respectively, and O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA), used as the derivatizing agent, were purchased from Sigma-Aldrich. Oasis HLB cartridges (6 cc, 150 mg, 30 µm particle size) for solid-phase extraction (SPE) were purchased from Waters (Milford, MA). All solvents (acetonitrile, n-hexane, methyl tert-butyl ether (MTBE), methanol, and ethyl acetate) were of highest purity and were purchased from Fisher Scientific, EMD Millipore (Billerica, MA) or VWR International (Radnor, PA).
Preparation of HAL Solutions
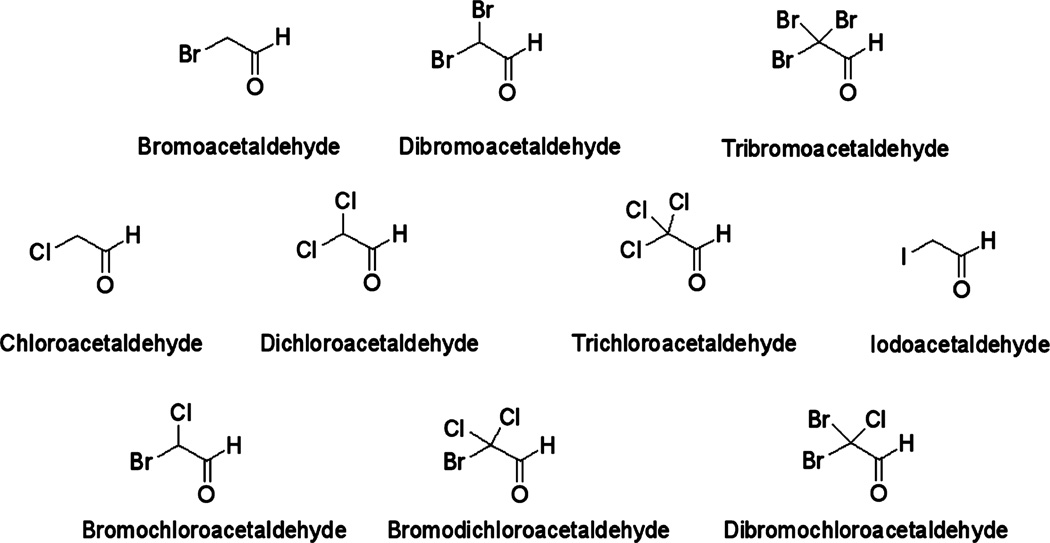
The chemical structures of the investigated HALs are shown in Figure 1. For chemical analyses, individual HAL stock solutions were prepared at a concentration of 100 µg/L by dissolving the appropriate amount of HAL standard in MTBE. Stock solutions were stored in the dark at −20 °C for up to two months. Working solutions were prepared in acetonitrile prior to method validation experiments and sample analyses. Calibration curves were made at concentrations ranging between 0.01 ng/L and 10,000 ng/L by spiking different levels of the calibration standards into purified water and carrying through the complete extraction/derivatization process.
Figure 1.
Chemical structures of the ten HALs analyzed in this study.
Prior to toxicological analyses, individual HAL stock solutions were prepared in dimethyl sulfoxide (DMSO) from HAL commercial standard solutions, and immediately stored in sterile glass vials under dark conditions at −20 °C.
Chemical Analyses
The methods developed for HAL analysis are based partly on methodologies used to evaluate DBPs in the U.S. Nationwide Occurrence Study.37,40 Mono-and di-HALs were derivatized with PFBHA, and subsequently liquid–liquid extracted (LLE) with n-hexanes, whereas tri-HALs were preconcentrated by means of SPE with Oasis HLB cartridges. Analyte detection was performed by gas chromatography-electron ionization-mass spectrometry (GC/EI-MS) with selected ion monitoring. Further details are provided in the SI (Table S2). These methodologies were evaluated in terms of linearity, sensitivity, repeatability, and recovery. Method performance is discussed later in the Results section. Total organic carbon (TOC), UV absorbance, bromide, and iodide content were also measured in source waters (Table S3, SI).
Water Samples
Source and treated drinking water samples were collected at different water treatment plants (WTPs) in the U.S. from 6 cities in 3 states from geographically diverse regions. Five of the seven investigated plants used chloramines, and two used chlorine, for disinfection. Although treated waters were available for all investigated WTPs the source waters could be collected for five of them only (Table S4, SI). Samples were collected in headspace-free 2-L polytetrafluoroethylene (PTFE) bottles. Ascorbic acid (12.5 mg/L) was used to quench the residual disinfectant, and sulfuric acid was used to lower the sample pH to 3.5 for analyte preservation.74 Stability of target analytes during sampling, transport, and storage conditions until sample extraction (within 48 h) was evaluated and is discussed in the SI (Table S5). Source waters were passed through 0.45 µm Durapore hydrophilic filters (EMD Millipore) prior to extraction.
Chinese Hamster Ovary Cells. Chinese hamster ovary (CHO) cell line AS52, clone 11-4-8 was used for the toxicity studies.75–77 The CHO cells were maintained in Ham’s F12 medium containing 5% FBS, 1% antibiotics (100 U/mL sodium penicillin G, 100 µg/mL streptomycin sulfate, 0.25 µg/mL amphotericin B in 0.85% saline), and 1% glutamine at 37 °C in a humidified atmosphere of 5% CO2.
CHO Cell Chronic Cytotoxicity Assay
This 96-well microplate assay measures the reduction in cell density as a function of the HAL concentration over a period of 72 h (~3 cell cycles) 11,20 The detailed procedure has been published elsewhere 11,20 and is presented in the SI. In general, for each HAL concentration, 8 replicates were analyzed, and the experiments were repeated 2–4 times. A concentration–response curve was generated for each HAL, and a regression analysis was conducted for each curve. The LC50 values were calculated, where the LC50 represents the HAL concentration that induced a 50% reduction in cell density as compared to the concurrent negative controls.
Single Cell Gel Electrophoresis Assay
The single cell gel electrophoresis (SCGE) or “comet assay” quantitatively 78–80 measures genomic DNA damage in individual nuclei. The detailed procedure of the microplate methodology used in this study is presented in the SI80. The SCGE metric for genomic DNA damage induced by the HALs was the %Tail DNA value, which is the amount of DNA that migrated from the nucleus into the microgel.81 For each HAL concentration range where the cell viability was >70%, a concentration–response curve was generated. A regression analysis was used to fit the curve, and the concentration inducing a 50% Tail DNA value was calculated.
Statistical Analyses
For the cytotoxicity assay, a one-way analysis of variance (ANOVA) test was conducted to determine if the HAL induced a statistically significant level of cell death. If a significant F value (P ≤ 0.05) was obtained, a Holm-Sidak multiple comparison versus the control group analysis was performed to identify the lowest cytotoxic concentration. The power of the test statistic (1-β) was maintained as ≤0.8 at α = 0.05.
For the SCGE assay, the %Tail DNA values are not normally distributed, which limits the use of parametric statistics.82 The mean %Tail DNA value for each microgel was calculated, and these values were averaged among all of the microgels for each HAL concentration. A one-way ANOVA test was conducted on these averaged % Tail DNA values. If a significant F value of P ≤ 0.05 was obtained, a Holm-Sidak multiple comparison versus the control group analysis was conducted with the power ≥ 0.8 at α = 0.05.
A bootstrap statistical approach was used to generate a series of multiple LC50 values or %Tail DNA values for each HAL.84,85 For each LC50 value, a cytotoxicity index (CTI) value was calculated as (LC50)−1(103). For each %Tail DNA value, a genotoxicity index (GTI) value was calculated as (50%Tail DNA)−1(103). These values (1/M) were then analyzed using an ANOVA test to determine significant differences among the HALs. A Pearson’s Product Moment correlation test was conducted to test for correlations among cytotoxicity and genotoxicity data and HAL chemical characteristics.
RESULTS AND DISCUSSION
Analysis of HALs in Water
Due to the wide range in volatility and polarity, two separate analytical methods were required to analyze the group of 10 HALs. Most HALs are separated by conventional GC/MS, but the mono- and di-HALs are highly volatile and a few coelute with the extraction solvent. In addition, the mono-HALs are highly polar and are not extracted efficiently by SPE. Therefore, derivatization with PFBHA was advantageous to increase their molecular weight and decrease their volatility so that they elute later in the GC/ MS chromatogram, away from the extraction solvent, and so that they are extracted effectively from water. PFBHA derivatization has been used similarly for highly volatile and highly polar nonhalogenated aldehydes.86−88 Although it would be ideal to measure all 10 HALs using this PFBHA-GC/MS method, the tri-HALs were not derivatized effectively by PFBHA. As a result, they were measured without derivatization using SPE and GC/MS. Table 1 summarizes the performance of the analytical methodologies. Total ion chromatograms obtained for the analysis of investigated HALs are shown in the SI (Figures S1 and S2). Analyte quantification was performed with the internal standard method. Mono- and di-HAL response was normalized to the SS area count, whereas the IS peak area was used to normalize tri-HAL signal. Calibration curves were constructed with the extraction and analysis of fortified Milli-Q water solutions. In general, seven data points were fitted by linear least-squares regression. Coefficients of determination (r2) above 0.99 were obtained for all analytes (Table 1, Figure S3 in the SI). Linearity was observed from the analyte limit of quantification (LOQ) up to 8 µg/L for IAL, 10 µg/L for the remaining mono-HALs and di-HALs, or 25 µg/L for tri-HALs.
Table 1.
Linearity, Sensitivity, Repeatability, and Recovery Obtained for the Analysis of the Target Analytes in Waters
| linearity |
sensitivity |
precisiona |
||||||
|---|---|---|---|---|---|---|---|---|
| Target analytes | range (µg/L) | R2 | LODc (µg/L) | LOQd (µg/L) | RSDe (%) low level | RSD (%) high level | recoveryb (%) | |
| Mono-HALs and di-HALs | CAL | 0.25–10 | 0.99 | 0.05 | 0.25 | N.A.f | ||
| BAL | 0.50–10 | 0.99 | 0.25 | 0.50 | 7.1 | 5.4 | N.A.f | |
| IAL | 0.50–8 | 0.99 | 0.25 | 0.50 | 8.3 | 14.5 | N.A.f | |
| DCAL | 0.25–10 | 0.99 | 0.10 | 0.25 | 6.1 | 4.9 | N.A.f | |
| DBAL | 0.25–10 | 0.99 | 0.05 | 0.25 | 3.8 | 5.4 | N.A.f | |
| BCAL | 0.10–10 | 0.99 | 0.05 | 0.10 | 5.6 | 6.9 | N.A.f | |
| tri-HALs | TCAL | 0.25–25 | 0.99 | 0.10 | 0.25 | 11.7 | 4.7 | 30/23 |
| TBAL | 1–25 | 0.99 | 0.50 | 1 | 4.4 | 3.8 | 82/97 | |
| BDCAL | 1–25 | 0.99 | 0.50 | 1 | 6.4 | 3.1 | 68/43 | |
| DBCAL | 1–25 | 0.99 | 0.50 | 1 | 5.8 | 2.8 | 72/58 | |
Mono- and di-HALS: n = 5 analyses at 0.5 µg/L (low level) and n = 5 analyses at 5 µg/L (high level). RSD of the SS peak area was 22% at 0.5 µg/L and 14% at 5 µg/L. Tri-HALs: n = 6 analyses at 2 µg/L (low level) and n = 6 analyses at µg/L (high level). RSD of the IS peak area was <8% at both spiking levels.
Replicate analysis (n = 6) of spiked waters at levels of 2 µg/L and 10 µg/L.
LOD = limit of detection.
LOQ = limit of quantification.
RSD = relative standard deviation.
N.A. = not available.
Method sensitivity was estimated from the concentrations observed in analyzed samples and calibration standard solutions. Limits of detection (LODs), i.e., the analyte concentration that provides a signal-to-noise (S/N) ratio of 3, ranged from 0.05 µg/L (CAL, BCAL, and DBAL) to 0.5 µg/L (TBAL, DBCAL, and BDCAL). LOQs, i.e., the analyte concentration that provides a S/N ratio of 10, varied between 0.1 µg/L (BCAL) and 1 µg/L (TBAL, DBCAL, and BDCAL).
Method precision was evaluated with the replicate analysis of fortified Milli-Q water solutions at two different levels (Table 1). Relative standard deviation (RSD) values of the normalized analyte peak areas were <10%, except for IAL (5 µg/L) and TCAL (2 µg/L), which were <15%.
Recovery could not be calculated for mono- and di-HALs because analytical standards of derivatized compounds are not commercially available. Moreover, the smallest ones e.g., CAL and DCAL, are not amenable to GC-MS without derivatization. Haloacetaldehyde conversion during PFBHA derivatization was consistently observed to be 75%.40 In this respect, any artifact affecting oxime yield during the derivatization step would also affect the SS, and, therefore, it can be corrected. In the case of tri-HALs, SPE recoveries were calculated via IS quantification of the analyte peak areas obtained in the recovery studies using MTBE-based calibration curves. Three different SPE sorbents were tested for tri-HAL extraction, i.e., Oasis HLB, Supelclean LC-18, and StrataC18-E. Best recoveries were achieved with Oasis HLB (SI). The analyte most efficiently extracted with Oasis HLB cartridges was TBAL (>82%), followed by DBCAL (58–72%) and BDCAL (43–68%). In contrast, TCAL had low recovery (23–30%). TCAL is the most polar and soluble compound of the investigated tri-HALs (Table S1, SI), and thus, lower sorption onto the cartridge would be expected. SPE recoveries for IAL and BCAL were also evaluated because these compounds provided good MS signals without derivatization. However, recoveries below 10% were obtained for these polar compounds, and thus, they are determined more reliably with the derivatization approach. Despite this, IAL was kept in the analytical GC/MS analysis of tri-HALs for confirmation purposes.
Occurrence of HALs in Source and Finished Waters
Source waters were similar with regard to TOC concentration (6.4–8.3 mg/L) and specific UV absorbance (SUVA) (2.1–3.1 L/m·mg) (Table S3, SI). The main variation was in bromide content (20–540 µg/L). Iodide levels were below the method LOD (5 µg/L) in all samples.
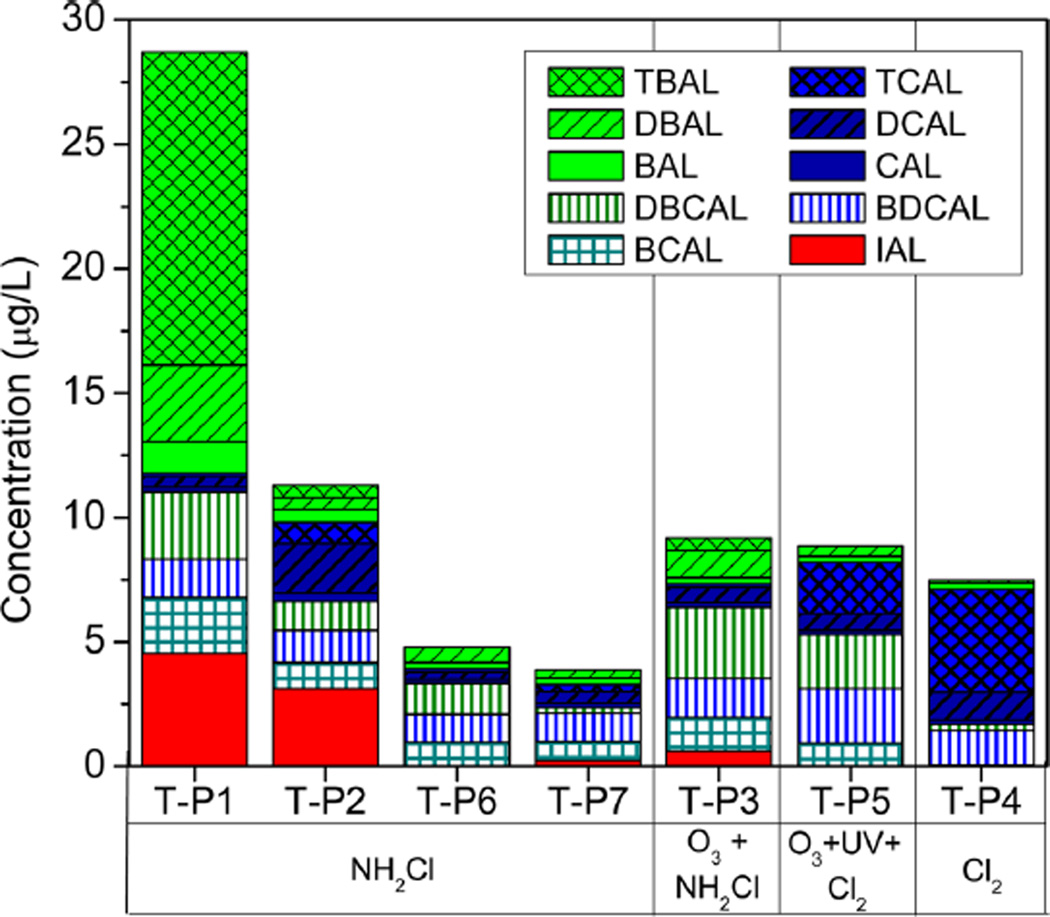
HALs were barely detected in source waters (Table S6). Only trace levels of DBAL, IAL, TBAL, and DBCAL were observed in the source water of Plant 1, and they could originate from the recirculation of disinfected water within the WTP. All target HALs were detected in all finished waters (Table S7, SI), with the exception of TBAL and IAL, which were detected only in 43% and 57% of the samples, respectively. HAL concentrations in treated waters are summarized in Figure 2.
Figure 2.
Levels of HALs in the investigated finished waters. Samples are classified according to the disinfection process applied at the water treatment plant. <LOQ levels were included as half of the analyte LOQ value.
IAL was only detected in chloraminated waters. This is consistent with previous research in which other iodinated DBPs (iodo-THMs, iodo-acids, and an iodo-amide) maximized with chloramination12,89–93 because unlike chlorine, which oxidizes iodide rapidly to iodate, monochloramine preferentially forms iodo-DBPs.90,91 Lower IAL concentrations were observed in the plant where preozonation was also applied. However, more research is needed to understand the effect of preozonation on IAL levels because it could also be related to a low iodide concentration and the type of NOM present in the source water.94 Previous research has shown that the application of ozone before chlorination can significantly increase HAL formation, likely due to initial formation of aldehydes by ozone and subsequent halogenation.38,95
IAL was detected (0.62–4.5 µg/L) in chloraminated drinking water even with iodide below the detection limit (5 µg/L) in the source waters. In this respect, other iodide sources, e.g., X-ray contrast media present in the water, could also contribute to IAL formation.96 IAL together with TBAL were the HALs detected at highest levels in treated waters. Their maximum levels (4.5 µg/L for IAL and 12.6 µg/L for TBAL) were observed in finished water from Plant 1, which originated from a source water with the highest content of bromide (540 µg/L). Moreover, these two DBPs are the main contributors (16% and 44% in the case of IAL and TBAL, respectively) to the total load of HALs in Plant 1 (29 µg/L), which presents the highest HAL load of all the drinking water samples. Chromatograms for Plant 1 are shown in the SI (Figures S4, S5). DBCAL (≤2.85 µg/L) and/or BDCAL (≤2.20 µg/L) were the predominant brominated acetaldehydes in treated waters originating from source waters with low bromide (≤120 µg/L). This is in agreement with data published previously on HAL occurrence in treated waters.38 These results highlight the importance of monitoring bromine-and iodine-containing HALs in drinking water.
High levels of chlorinated HALs, particularly TCAL (4 µg/ L), were found in finished waters from Plant 4, which is the only investigated plant applying chlorine exclusively for water disinfection. However, this level is below the maximum TCAL concentrations found in treated waters in Canada38 and Spain.97 Overall, despite being ubiquitous, CAL was found at the lowest concentrations in the treated water samples (below 1 µg/L). Concentrations of DCAL varied between 0.3 and 1.99 µg/L, and its formation appeared independent of the disinfection treatment applied.
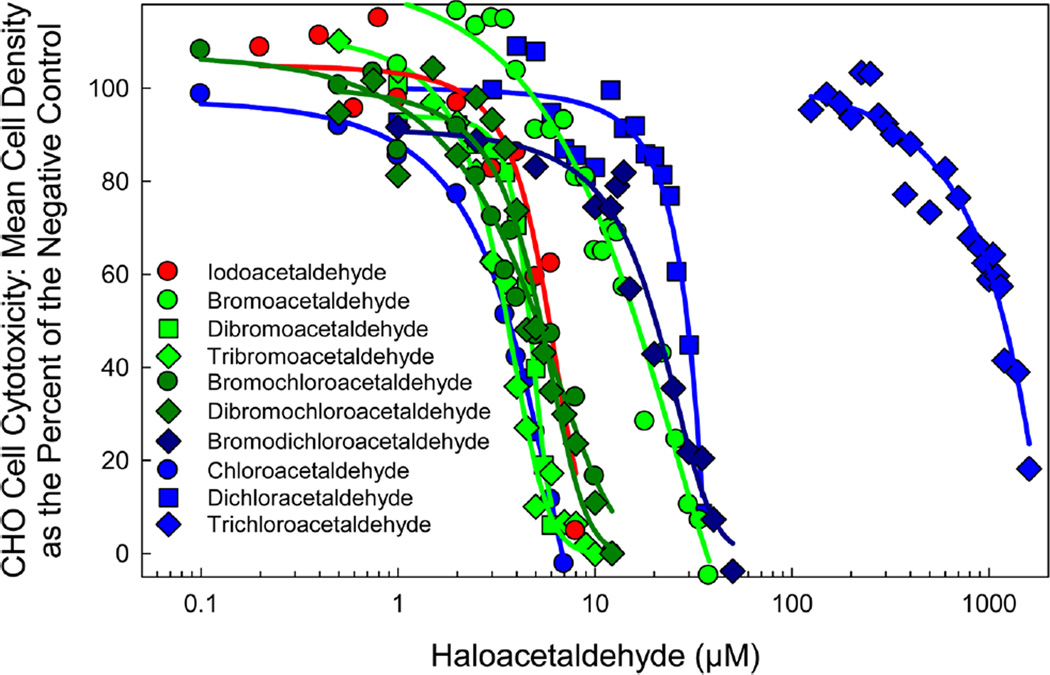
CHO Cell Chronic Cytotoxicity
CHO cell chronic cytotoxicity analyses (72 h exposures) of each HAL are summarized in Table 2. Figure 3 illustrates the concentration–response curves for the HALs. The individual concentration–response curves of each HAL are presented in the SI (Figures S6–S15).
Table 2.
Summary of the CHO Cell Chronic Cytotoxicity of the Target HALs
| concn range (µM) | lowest cytotoxic concn (µM)a | LC50 (µM)b | R2c | ANOVA test statisticd | ||
|---|---|---|---|---|---|---|
| mono-HALs and di-HALs | CAL | 0.1–7 | 0.5 | 3.51 | 0.99 | F11,176 = 241; P ≤ 0.001 |
| BAL | 1–42 | 8 | 17.28 | 0.98 | F23,248 = 76.1; P ≤ 0.001 | |
| IAL | 0.2–10 | 5 | 6.00 | 0.96 | F12,163 = 79.6; P ≤ 0.001 | |
| DCAL | 1–15 | 8 | 29.25 | 0.91 | F20,335 = 37.5; P ≤ 0.001 | |
| DBAL | 1–6 | 2 | 4.7 | 0.99 | F10,177 = 165; P ≤ 0.001 | |
| BCAL | 0.1–10 | 2.5 | 5.34 | 0.97 | F14,169 = 31.5; P ≤ 0.001 | |
| tri-HALs | TCAL | 125–1600 | 375 | 1163 | 0.94 | F24,333 = 34.0; P ≤ 0.001 |
| TBAL | 0.5–10 | 2 | 3.58 | 0.99 | F15,316 = 256; P ≤ 0.001 | |
| BDCAL | 1–50 | 10 | 20.35 | 0.89 | F14,209 = 23.4; P ≤ 0.001 | |
| DBCAL | 0.5–10 | 4 | 5.15 | 0.95 | F16,167 = 36.1; P ≤ 0.001 |
Lowest cytotoxic concentration was the lowest concentration of the HAL in the concentration–response curve that induced a statistically significant reduction in cell density as compared to the concurrent negative controls.
The LC50 value is the concentration of the HAL, determined from a regression analysis of the data, that induced a cell density of 50% as compared to the concurrent negative controls.
R2 is the coefficient of determination for the regression analysis upon which the LC50 value was calculated.
The degrees of freedom for the between-groups and residual associated with the calculated F-test result and the resulting probability value.
Figure 3.
Comparison of the CHO cell chronic cytotoxicity concentration–response curves of the target HALs.
CHO Cell Acute Genotoxicity
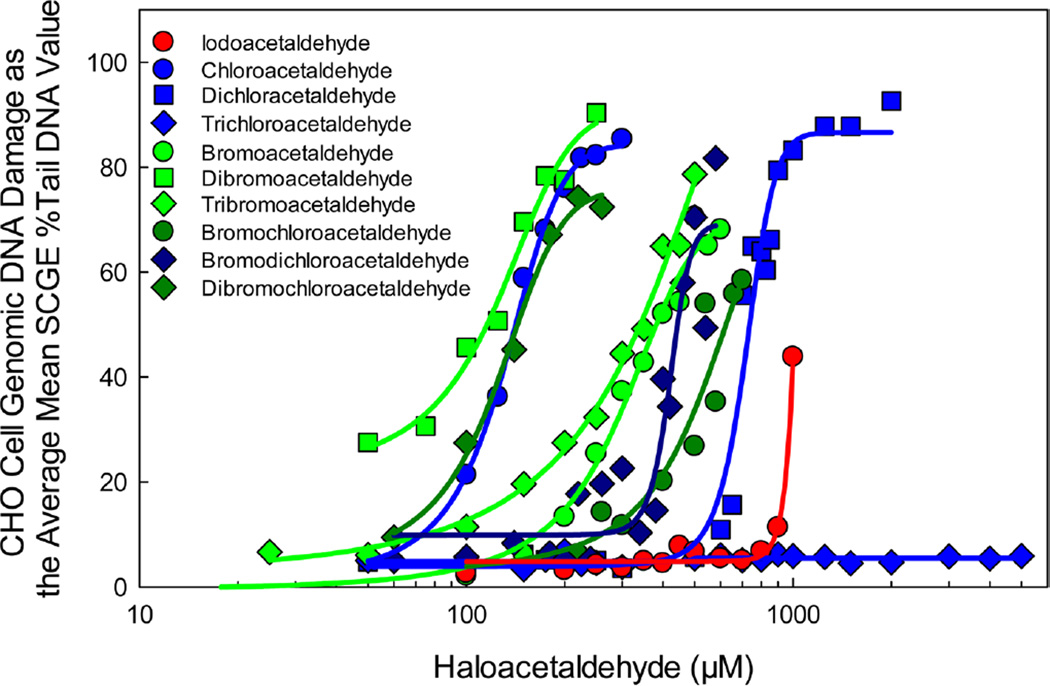
CHO cell acute genotoxicity analyses (4 h exposures) of each HAL are summarized in Table 3. Figure 4 illustrates the concentration–response curves for the HALs. The individual concentration–response curves of each HAL with the cell viability data are presented in the SI (Figures S17–S26).
Table 3.
Summary of the CHO Cell Acute Genotoxicity of the Target HALs
| concn range (µM) | lowest %TDNA genotoxic concn (µM)a | 50% TDNA (µM)b | R2c | ANOVA test statisticd | ||
|---|---|---|---|---|---|---|
| Mono-HALs and di-HALs | CAL | 50–500 | 100 | 142.8 | 0.99 | F10,59 = 62.6; P ≤ 0.001 |
| BAL | 100–550 | 200 | 381.2 | 0.98 | F10,68 = 57.2; P ≤ 0.001 | |
| IAL | 100–1000 | 900 | 1009 | 0.98 | F13,103 = 22.5; P ≤ 0.001 | |
| DCAL | 50–2000 | 800 | 795 | 0.98 | F19,60 = 64.0; P ≤ 0.001 | |
| DBAL | 50–300 | 50 | 111.3 | 0.98 | F9,44 = 41.5; P ≤ 0.001 | |
| BCAL | 100–700 | 500 | 621.4 | 0.92 | F10,51 = 22.0; P ≤ 0.001 | |
| tri-HALs | TCAL | 50–5000 | NSe | NSe | NSe | F20,37 = 0.556; P = 0.918 |
| TBAL | 25–500 | 100 | 340.3 | 0.99 | F11,64 = 168; P ≤ 0.001 | |
| BDCAL | 60–600 | 300 | 470.4 | 0.91 | F17,106 = 16.4; P ≤ 0.001 | |
| DBCAL | 60–220 | 100 | 143.7 | 0.99 | F5,29 = 34.4; P ≤ 0.001 |
The lowest genotoxic concentration was the lowest concentration of the haloacetaldehyde in the concentration–response curve that induced a statistically significant amount of genomic DNA damage as compared to the negative control.
The SCGE 50% Tail DNA value is the haloacetaldehyde concentration determined from a regression analyses of the data that was calculated to induce, on average, 50% of the genomic DNA of the nucleoids to migrate into the gel.
R2 is the coefficient of determination for the regression analysis upon which the SCGE % Tail DNA value was calculated.
The degrees of freedom for the between-groups and residual associated with the calculated F-test result and the resulting probability value.
NS = not significantly different from the negative control.
Figure 4.
Comparison of the CHO cell acute genotoxicity concentration–response curves of the target HALs.
Structure–Activity Relationships of Haloacetaldehyde Toxicity
This study presents the first systematic, quantitative comparison of HAL cytotoxicity and genotoxicity. An all pairwise ANOVA test of the CTI values generated a descending rank order of chronic cytotoxicity as TBAL ≈ CAL > DBAL ≈ BCAL ≈ DBCAL > IAL > BAL ≈ BDCAL > DCAL > TCAL. The mean bootstrap CTI (±SE) values are presented in Table 4 and Figure S16. An all pairwise ANOVA test of the GTI values generated a descending rank order of genotoxicity of the ten HALs as DBAL > CAL ≈ DBCAL > TBAL ≈ BAL > BDCAL > BCAL ≈ DCAL > IAL. TCAL was not genotoxic. The mean bootstrap GTI (±SE) values are presented in Table 4 and Figure S27.
Table 4.
Comparison of Calculated Khydration Values, Cytotoxicity Index (CTI) Values, and Genotoxicity Index (GTI) Values of HALs
| SPARCa Khydration |
CTI (±SE)b | GTI (±SE)c | ||
|---|---|---|---|---|
| Mono-HALs | CAL | 17.8 | 279.0 ± 7.0 | 7.20 ± 0.42 |
| and di-HALs | BAL | 11.0 | 64.6 ± 3.5 | 2.68 ± 0.11 |
| IAL | 4.37 | 170.4 ± 7.3 | 0.96 ± 0.03 | |
| DCAL | 1.95 × 103 | 35.7 ± 0.8 | 1.26 ± 0.03 | |
| DBAL | 1.58 × 103 | 207.5 ± 2.1 | 9.11 ± 0.60 | |
| BCAL | 1.70 × 103 | 207.4 ± 11.0 | 1.61 ± 0.21 | |
| tri-HALs | TCAL | 3.24 × 104 | 0.94 ± 0.03 | NSd |
| TBAL | 1.15 × 104 | 279.8 ± 4.8 | 3.00 ± 0.03 | |
| BDCAL | 4.37 × 104 | 51.1 ± 4.3 | 2.24 ± 0.05 | |
| DBCAL | 2.00 × 104 | 200.2 ± 1.4 | 6.99 ± 0.28 |
SPARC (SPARC Performs Automated Reasoning in Chemistry) models are mechanistic perturbation models developed by the U.S. EPA to calculate chemical reactivity and physical processes for compounds from molecular structure.100,101
The Cytotoxicity index (CTI) value was calculated from the individual LC50 values generated from the bootstrap analyses. The mean CTI was calculated as the (LC50)−1(103).
The Genotoxicity index (GTI) value was calculated from the individual 50%TDNA values generated from the bootstrap analyses. The mean GTI was calculated as the (50%TDNA)−1(103). A Pearson correlation analysis demonstrated that no significant correlation exists among the hydration constants and the CTI or the GTI.
NS = not significantly different from the negative control.
The cytotoxicity and genotoxicity of these ten HALs were not significantly correlated (r = 0.36; P = 0.308). The HALs did not follow the pattern in which the halogen affected toxicity (iodinated > brominated > chlorinated DBPs) in contrast to other DBP classes including the haloacetic acids (HAAs),98 THMs,20 or haloacetamides (HAcAms).93
The toxicity of HALs is complex in that these compounds possess two potential sites to react with nucleophiles in cells. One is the halogen α-carbon bond, which is associated with SN2 type reactions. The halogen substituent, through bond dissociation energy and other factors, determines the relative bimolecular nucleophilic substitution (SN2) reactivity of the compound. With monohaloacetic acids (mono-HAAs) and monohaloacetamides, the rank order of toxicity followed I > Br ≫ Cl, which corresponds to the leaving tendency of the halogens of alkyl halide.93,98,99 We found that the mono-HAAs irreversibly inhibited glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity with a high correlation with the dissociation energy of the halogen α-carbon bond and with the alkylation potential of the HAA.98,99 The rate of GAPDH inhibition and the toxic potency of the mono-HAAs showed the same rank order (I > Br ≫ Cl) in a concentration-dependent manner. With the combined data of tri-HALs and di-HALs from the present study (Table 4), a strong significant correlation was found between the number of Br atoms and the CTI (r = 0.90; P ≤ 0.006), while a good but not significant correlation was found with the GTI values (r = 0.63; P = 0.13). However, the impact of the halogen was not observed in the mono-HALs.
The other reactive site of the HALs is the carbonyl C=O bond of the aldehyde group. Aliphatic aldehydes are able to undergo Schiff base formation (Figure S28, SI). The Schiff base formation is a mechanism used by enzymes to catalyze reactions between an amine group with either an aldehyde or ketone. It proceeds through the carbinolamine intermediate resulting in an imine as a final product. HALs may induce genotoxic effects, such as DNA adducts, DNA–DNA crosslinks, or DNA–protein cross-links by reacting with DNA chains through Schiff base formation.63,68,69 Therefore, the overall toxic potency may differ by individual compound depending on the combinative reactivity of SN2 type reaction and Schiff base formation in a biological system.
In an aqueous phase, HALs exist in equilibrium between an aldehyde and a hydrate form (Figure S29). This hydration equilibrium constant is defined as Khydration = [hydrate]/[aldehyde]. As Khydration increases, the hydrate species is dominant in the aqueous system. Theoretical Khydration values were calculated for each HAL from a predictive modeling system, SPARC (SPARC Performs Automated Reasoning in Chemistry), that was developed by the U.S. EPA100,101 (Table 4). Based on these values, Khydration increases as the number of halogens increases. As the number of halogens increases, the electron withdrawing capacity of the C(X)n group is greater and the carbonyl carbon becomes more partial positive, enabling attack of a water molecule and hydration. The Khydration values for mono-HALs were 2–4 orders of magnitude lower than those for di-HALs and tri-HALs. The halogen-induced toxicity pattern seen with other DBP classes was not expressed in the mono-HALs. Mono-HALs have distinct Khydration values where the distribution of reactive aldehyde species will differ by halogen type. It is interesting that there was no correlation between the Khydration values and cytotoxicity or genotoxicity. Therefore, mono-HALs may induce overall toxicity outcomes through more than one mode of action. For the di- and tri-HALs the halogen-mediated SN2 reaction may perform the predominant role in the induction of toxicity.
Comparison of the Toxicity of Haloacetaldehydes to Other DBP Classes
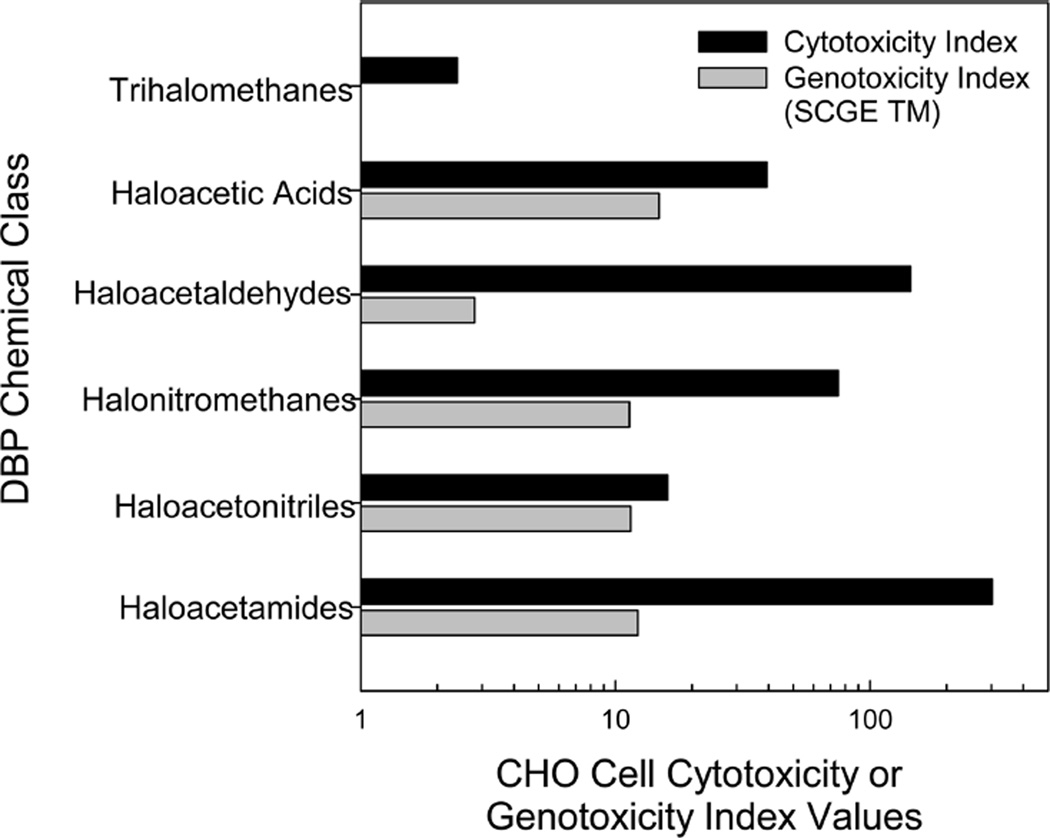
We compared the CHO cell toxic potencies of the HALs to those for other DBP chemical classes using calculated cytotoxicity and genotoxicity indices (Figure 5). The cytotoxicity index was determined by calculating the mean LC50 value of all of the individual compounds of a single class of DBPs. The genotoxicity index was determined by calculating the mean SCGE genotoxic potency value, which is defined by the SCGE tail moment from the individual compounds within a single class of DBPs.20 Six DBP chemical classes were compared, including THMs, HAAs, HALs, halonitromethanes, haloacetonitriles, and HAcAms. HALs constitute the second most cytotoxic DBP class, whereas they rank as the second least genotoxic DBP class.
Figure 5.
Comparison of the CHO cell chronic cytotoxicity index values and acute genotoxicity index values of various DBP chemical classes. Of the THMs analyzed, none were genotoxic in the CHO cell assay.
Research Implications
This study presented a precise analytical chemical method for the most comprehensive HAL identification and quantification to date and reports for the first time the formation of IAL during water disinfection. We conducted systematic quantitative comparative analyses of the cytotoxicity and genotoxicity in mammalian cells of the HALs, performed structure–activity relationships analyses on their toxicity, and compared the HALs with other DBP classes. Considering that HALs constitute the third largest group by weight of identified DBPs, attention should be given to determine their possible health risks and to their control by engineering practices.
Supplementary Material
Acknowledgments
We would like to thank the drinking water treatment plants for generously providing us with samples and Yusupha Sey for excellent technical support. This work was supported by the U.S. EPA STAR Grant R834867. We appreciate support by the Center of Advanced Materials for the Purification of Water with Systems (WaterCAMPWS), a National Science Foundation Science and Technology Center, under Award CTS-0120978. C.J. was supported by a NIEHS Predoctoral Fellowship under Grant No. T32 ES007326. C.P. acknowledges support from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 274379 (Marie Curie IOF). This work has been financially supported by the Generalitat de Catalunya (Consolidated Research Groups “2014 SGR 418 -Water and Soil Quality Unit” and 2014 SGR 291 - ICRA). This work reflects only the authors’ views. The EU is not liable for any use that may be made of the information contained therein. The information in this document was funded in part by the U.S. Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
ASSOCIATED CONTENT
Additional information on experimental methods, including additional figures and tables. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/es506358x.
The authors declare no competing financial interest.
REFERENCES
- 1.Cutler D, Miller G. The role of public health improvements in health advances: the twentieth-century United States. Demography. 2005;42:1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 2.Rook JJ. Formation of haloforms during chlorination of natural waters. Water Treat. Exam. 1974;23:234–243. [Google Scholar]
- 3.Bond T, Huang J, Templeton MR, Graham N. Occurrence and control of nitrogenous disinfection by-products in drinking water-a review. Water Res. 2011;45:4341–4354. doi: 10.1016/j.watres.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Chu W, Gao N, Yin D, Krasner SW. Formation and speciation of nine haloacetamides, an emerging class of nitrogenous DBPs, during chlorination or chloramination. J. Hazard. Mater. 2013;260:806–812. doi: 10.1016/j.jhazmat.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Krasner SW, Mitch WA, McCurry DL, Hanigan D, Westerhoff P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: a review. Water Res. 2013;47:4433–4450. doi: 10.1016/j.watres.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg HS. Modern approaches to the analysis of disinfection by-products in drinking water. Philos. Trans. R Soc, A. 2009;367:4097–4118. doi: 10.1098/rsta.2009.0130. [DOI] [PubMed] [Google Scholar]
- 7.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Kimura SY, Komaki Y, Plewa MJ, Mariñas BJ. Chloroacetonitrile and N,2-dichloroacetamide formation from the reaction of chloroacetaldehyde and monochloramine in water. Environ. Sci. Technol. 2013;47:12382–12390. doi: 10.1021/es4029638. [DOI] [PubMed] [Google Scholar]
- 9.Krasner SW. The formation and control of emerging disinfection by-products of health concern. Philos. Trans. R. Soc., A. 2009;367:4077–4095. doi: 10.1098/rsta.2009.0108. [DOI] [PubMed] [Google Scholar]
- 10.Pereira RO, Postigo C, de Alda ML, Daniel LA, Barcelo D. Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere. 2011;82:789–799. doi: 10.1016/j.chemosphere.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 11.Plewa MJ, Kargalioglu Y, Vankerk D, Minear RA, Wagner ED. Mammalian cell cytotoxicity and genotoxicity analysis of drinking water disinfection by-products. Environ. Mol. Mutagen. 2002;40:134–142. doi: 10.1002/em.10092. [DOI] [PubMed] [Google Scholar]
- 12.Plewa MJ, Wagner ED, Muellner MG, Hsu KM, Richardson SD. Comparative mammalian cell toxicity of N-DBPs and C-DBPs. In: Karanfil T, Krasner SW, Westerhoff P, Xie Y, editors. Occurrence, Formation, Health Effects and Control of Disinfection By-Products in Drinking Water. Vol. 995. Washington, DC: American Chemical Society; 2008. pp. 36–50. [Google Scholar]
- 13.Feder PI, Ma ZJ, Bull RJ, Teuschler LK, Schenck KM, Simmons JE, Rice G. Evaluating sufficient similarity for disinfection by-product (DBP) mixtures: multivariate statistical procedures. J. Toxicol. Environ. Health, Part A. 2009;72:468–481. doi: 10.1080/15287390802608965. [DOI] [PubMed] [Google Scholar]
- 14.Grellier J, Bennett J, Patelarou E, Smith RB, Toledano MB, Rushton L, Briggs DJ, Nieuwenhuijsen MJ. Exposure to disinfection by-products, fetal growth, and prematurity: a systematic review and meta-analysis. Epidemiology. 2010;21:300–313. doi: 10.1097/EDE.0b013e3181d61ffd. [DOI] [PubMed] [Google Scholar]
- 15.Hrudey SE. Chlorination disinfection by-products, public health risk tradeoffs and me. Water Res. 2009;43:2057–2092. doi: 10.1016/j.watres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Legay C, Rodriguez MJ, Serodes JB, Levallois P. Estimation of chlorination by-products presence in drinking water in epidemiological studies on adverse reproductive outcomes: a review. Sci. Total Environ. 2010;408:456–472. doi: 10.1016/j.scitotenv.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Ngwenya N, Ncube EJ, Parsons J. Recent advances in drinking water disinfection: successes challenges. Rev. Environ. Contam. Toxicol. 2013;222:111–170. doi: 10.1007/978-1-4614-4717-7_4. [DOI] [PubMed] [Google Scholar]
- 18.Hunter ES, III, Rogers EH, Schmid JE, Richard A. Comparative effects of haloacetic acids in whole embryo culture. Teratology. 1996;54:57–64. doi: 10.1002/(SICI)1096-9926(199606)54:2<57::AID-TERA1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES, III, Simmons JE. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reprod. Toxicol. 2011;31:59–65. doi: 10.1016/j.reprotox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Plewa MJ, Wagner ED. Mammalian Cell Cytotoxicity and Genotoxicity of Disinfection By-Products. Denver, CO: Water Research Foundation; 2009. p. 134. [Google Scholar]
- 21.Wei X, Wang S, Zheng W, Wang X, Liu X, Jiang S, Pi J, Zheng Y, He G, Qu W. Drinking water disinfection byproduct iodoacetic acid induces tumorigenic transformation of NIH3T3 cells. Environ. Sci. Technol. 2013;47:5913–5920. doi: 10.1021/es304786b. [DOI] [PubMed] [Google Scholar]
- 22.Costet N, Villanueva CM, Jaakkola JJ, Kogevinas M, Cantor KP, King WD, Lynch CF, Nieuwenhuijsen MJ, Cordier S. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case-control studies. Occup. Environ. Med. 2011;68:379–385. doi: 10.1136/oem.2010.062703. [DOI] [PubMed] [Google Scholar]
- 23.Rahman MB, Driscoll T, Cowie C, Armstrong BK. Disinfection by-products in drinking water and colorectal cancer: a meta-analysis. Int. J. Epidemiol. 2010;39:733–745. doi: 10.1093/ije/dyp371. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva CM, Cantor KP, Cordier S, Jaakkola JJ, King WD, Lynch CF, Porru S, Kogevinas M. Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology. 2004;15:357–367. doi: 10.1097/01.ede.0000121380.02594.fc. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castano-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M. Bladder cancer exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am. J. Epidemiol. 2007;165:148–156. doi: 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- 26.Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, Malats N, Chanock S, Yeager M, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castano-Vinyals G, Samanic C, Rothman N, Kogevinas M. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ. Health Perspect. 2010;118:1545–1550. doi: 10.1289/ehp.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costet N, Garlantezec R, Monfort C, Rouget F, Gagniere B, Chevrier C, Cordier S. Environmental and urinary markers of prenatal exposure to drinking water disinfection by-products, fetal growth, and duration of gestation in the PELAGIE birth cohort (Brittany, France, 2002–2006) Am. J. Epidemiol. 2012;175:263–275. doi: 10.1093/aje/kwr419. [DOI] [PubMed] [Google Scholar]
- 28.Grazuleviciene R, Nieuwenhuijsen MJ, Vencloviene J, Kostopoulou-Karadanelli M, Krasner SW, Danileviciute A, Balcius G, Kapustinskiene V. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: a prospective Kaunas cohort study. Environ. Health. 2011;10:32. doi: 10.1186/1476-069X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinckley AF, Bachand AM, Reif JS. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ. Health Perspect. 2005;113:1808–1813. doi: 10.1289/ehp.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong CH, Wagner ED, Siebert VR, Anduri S, Richardson SD, Daiber EJ, McKague AB, Kogevinas M, Villanueva CM, Goslan EH, Luo W, Isabelle LM, Pankow JF, Grazuleviciene R, Cordier S, Edwards SC, Righi E, Nieuwenhuijsen MJ, Plewa MJ. The occurrence and toxicity of disinfection byproducts in European drinking waters in relation with the HIWATE epidemiology study. Environ. Sci. Technol. 2012;46:12120–12128. doi: 10.1021/es3024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanueva CM, Gracia-Lavedan E, Ibarluzea J, Santa Marina L, Ballester F, Llop S, Tardon A, Fernandez MF, Freire C, Goni F, Basagana X, Kogevinas M, Grimalt JO, Sunyer J, Project I. Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ. Health Perspect. 2011;119:1824–1830. doi: 10.1289/ehp.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JM, Schwartz J, Dockery DW. Effect of trihalomethane exposure on fetal development. Occup. Environ. Med. 2003;60:173–180. doi: 10.1136/oem.60.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CY, Xiao ZP, Ho SC, Wu TN, Tsai SS. Association between trihalomethane concentrations in drinking water and adverse pregnancy outcome in Taiwan. Environ. Res. 2007;104:390–395. doi: 10.1016/j.envres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Waller K, Swan SH, DeLorenze G, Hopkins B. Trihalomethanes in drinking water and spontaneous abortion. Epidemiology. 1998;9:134–140. [PubMed] [Google Scholar]
- 35.Savitz DA, Singer PC, Hartmann KE, Herring AH, Weinberg HS, Makarushka C, Hoffman C, Chan R, Maclehose R. Drinking Water Disinfection By-products and Pregnancy Outcome. Denver, CO: AWWA Research Foundation; 2005. [Google Scholar]
- 36.Richardson SD, Postigo C. Emerging Organic Contaminants and Human Health. Berlin, Germany: Springer; 2012. Drinking water disinfection byproducts; pp. 93–137. [Google Scholar]
- 37.Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad GD, Thruston AD., Jr The occurrence of a new generation of disinfection by-products. Environ. Sci. Technol. 2006;40:7175–7185. doi: 10.1021/es060353j. [DOI] [PubMed] [Google Scholar]
- 38.Koudjonou B, LeBel GL, Dabeka L. Formation of halogenated acetaldehydes, and occurrence in Canadian drinking water. Chemosphere. 2008;72:875–881. doi: 10.1016/j.chemosphere.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 39.McGuire MJ, McLain JL, Obolensky A. Information Collection Rule Data Analysis. Denver, CO: American Water Works Association Research Foundation and AWWA; 2002. [Google Scholar]
- 40.Weinberg HS, Krasner SW, Richardson SD, Thruston AD., Jr . The Occurrence of Disinfection By-Products (DBPs) of Health Concern in Drinking Water: Results of a Nationwide DBP Occurrence Study; EPA/600/R02/068. Athens, GA: U.S. Environmental Protection Agency National Exposure Research Laboratory; 2002. pp. 1–460. [Google Scholar]
- 41.Linge KL, Blythe JW, Busetti F, Blair P, Rodriguez C, Heitz A. Formation of halogenated disinfection by-products during microfiltration and reverse osmosis treatment: Implications for water recycling. Sep. Pur. Technol. 2013;104:221–228. [Google Scholar]
- 42.Beland FA. NTP technical report on the toxicity and metabolism studies of chloral hydrate (CAS No 302-17-0). Administered by gavage to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 1999;1–66:A1–E7. [PubMed] [Google Scholar]
- 43.Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. 1983;5(Suppl 1):1–142. [PubMed] [Google Scholar]
- 44.Leuschner J, Leuschner F. Evaluation of the mutagenicity of chloral hydrate in vitro and in vivo. Arzneim. Forsch. 1991;41:1101–1103. [PubMed] [Google Scholar]
- 45.Ni YC, Wong TY, Kadlubar FF, Fu PP. Hepatic metabolism of chloral hydrate to free radical(s) and induction of lipid peroxidation. Biochem. Biophys. Res. Commun. 1994;204:937–943. doi: 10.1006/bbrc.1994.2550. [DOI] [PubMed] [Google Scholar]
- 46.Furnus CC, Ulrich MA, Terreros MC, Dulout FN. The induction of aneuploidy in cultured Chinese hamster cells by propionaldehyde and chloral hydrate. Mutagenesis. 1990;5:323. doi: 10.1093/mutage/5.4.323. [DOI] [PubMed] [Google Scholar]
- 47.Mailhes JB, Preston RJ, Yuan ZP, Payne HS. Analysis of mouse metaphase II oocytes as an assay for chemically induced aneuploidy. Mutat. Res. 1988;198:145–152. doi: 10.1016/0027-5107(88)90049-8. [DOI] [PubMed] [Google Scholar]
- 48.Russo A, Pacchierotti F, Metalli P. Nondisjunction induced in mouse spermatogenesis by chloral hydrate, a metabolite of trichloroethylene. Environ. Mutagen. 1984;6:695–703. doi: 10.1002/em.2860060507. [DOI] [PubMed] [Google Scholar]
- 49.Allen JW, Collins BW, Evansky PA. Spermatid micronucleus analyses of trichloroethylene and chloral hydrate effects in mice. Mutat. Res. 1994;323:81–88. doi: 10.1016/0165-7992(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 50.Migliore L, Nieri M. Evaluation of twelve potential aneuploidogenic chemicals by the in vitro human lymphocyte micronucleus assay. Toxicol. In Vitro. 1991;5:325–336. doi: 10.1016/0887-2333(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 51.Nesslany F, Marzin D. A micromethod for the in vitro micronucleus assay. Mutagenesis. 1999;14:403–410. doi: 10.1093/mutage/14.4.403. [DOI] [PubMed] [Google Scholar]
- 52.Parry JM, Parry EM, Bourner R, Doherty A, Ellard S, O’Donovan J, Hoebee B, de Stoppelaar JM, Mohn GR, Onfelt A, Renglin A, Schultz N, Soderpalm-Berndes C, Jensen KG, Kirsch-Volders M, Elhajouji A, Van Hummelen P, Degrassi F, Antoccia A, Cimini D, Izzo M, Tanzarella C, Adler ID, Kliesch U, Hess P. The detection and evaluation of aneugenic chemicals. Mutat. Res. 1996;353:11–46. doi: 10.1016/0027-5107(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 53.Parry JM, Parry EM, Warr T, Lynch A, James S. The detection of aneugens using yeasts and cultured mammalian cells. Prog. Clin. Biol. Res. 1990;340B:247–266. [PubMed] [Google Scholar]
- 54.Brunner M, Albertini S, Wurgler FE. Effects of 10 known or suspected spindle poisons in the in vitro porcine brain tubulin assembly assay. Mutagenesis. 1991;6:65–70. doi: 10.1093/mutage/6.1.65. [DOI] [PubMed] [Google Scholar]
- 55.Eichenlaub-Ritter U, Baart E, Yin H, Betzendahl I. Mechanisms of spontaneous and chemically-induced aneuploidy in mammalian oogenesis: basis of sex-specific differences in response to aneugens and the necessity for further tests. Mutat. Res. 1996;372:279–294. doi: 10.1016/s0027-5107(96)00147-9. [DOI] [PubMed] [Google Scholar]
- 56.Warr TJ, Parry EM, Parry JM. A comparison of two in vitro mammalian cell cytogenetic assays for the detection of mitotic aneuploidy using 10 known or suspected aneugens. Mutat. Res. 1993;287:29–46. doi: 10.1016/0027-5107(93)90143-4. [DOI] [PubMed] [Google Scholar]
- 57.Moore MM, Harrington-Brock K. Mutagenicity of trichloroethylene and its metabolites: implications for the risk assessment of trichloroethylene. Environ. Health Perspect. 2000;108(Suppl 2):215. doi: 10.1289/ehp.00108s2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson MA, Bull RJ. Induction of strand breaks in DNA by trichloroethylene and metabolites in rat mouse liver in vivo. Toxicol. Appl. Pharmacol. 1988;94:45–54. doi: 10.1016/0041-008x(88)90335-3. [DOI] [PubMed] [Google Scholar]
- 59.Liviac D, Creus A, Marcos R. DNA damage induction by two halogenated acetaldehydes, byproducts of water disinfection. Water Res. 2010;44:2638–2646. doi: 10.1016/j.watres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Guengerich FP, Crawford WM, Jr, Watanabe PG. Activation of vinyl chloride to covalently bound metabolites: roles of 2-chloroethylene oxide and 2-chloroacetaldehyde. Biochemistry. 1979;18:5177–5182. doi: 10.1021/bi00590a023. [DOI] [PubMed] [Google Scholar]
- 61.Sood C, O’Brien PJ. Molecular mechanisms of chloroacetaldehyde-induced cytotoxicity in isolated rat hepatocytes. Biochem. Pharmacol. 1993;46:1621–1626. doi: 10.1016/0006-2952(93)90332-q. [DOI] [PubMed] [Google Scholar]
- 62.Benesic A, Schwerdt G, Mildenberger S, Freudinger R, Gordjani N, Gekle M. Disturbed Ca2+ -signaling by chloroacetaldehyde: a possible cause for chronic ifosfamide nephrotoxicity. Kidney Int. 2005;68:2029–2041. doi: 10.1111/j.1523-1755.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 63.Biernat J, Ciesiolka J, Gornicki P, Adamiak RW, Kryzosiak WJ, Wiewiorowski M. New observations concerning the chloroacetaldehyde reaction with some tRNA constituents. Stable intermediates, kinetics and selectivity of the reaction. Nucleic Acids Res. 1978;5:789–804. doi: 10.1093/nar/5.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiang SY, Swenberg JA, Weisman WH, Skopek TR. Mutagenicity of vinyl chloride and its reactive metabolites, chloro-ethylene oxide and chloroacetaldehyde, in a metabolically competent human B-lymphoblastoid line. Carcinogenesis. 1997;18:31–36. doi: 10.1093/carcin/18.1.31. [DOI] [PubMed] [Google Scholar]
- 65.Dosanjh MK, Chenna A, Kim E, Fraenkel-Conrat H, Samson L, Singer B. All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1024–1028. doi: 10.1073/pnas.91.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen RJ, Nagasubramanian R, Delaney SM, Samson LD, Dolan ME. Role of O6-methylguanine-DNA methyltransferase in protecting from alkylating agent-induced toxicity and mutations in mice. Carcinogenesis. 2007;28:1111–1116. doi: 10.1093/carcin/bgl218. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen JS, Humayun MZ. Mechanisms of mutagenesis by the vinyl chloride metabolite chloroacetaldehyde. Effect of gene-targeted in vitro adduction of M13 DNA on DNA template activity in vivo and in vitro. Biochemistry. 1990;29:496–504. doi: 10.1021/bi00454a025. [DOI] [PubMed] [Google Scholar]
- 68.Palejwala VA, Simha D, Humayun MZ. Mechanisms of mutagenesis by exocyclic DNA adducts. Transfection of M13 viral DNA bearing a site-specific adduct shows that ethenocytosine is a highly efficient RecA-independent mutagenic noninstructional lesion. Biochemistry. 1991;30:8736–8743. doi: 10.1021/bi00100a004. [DOI] [PubMed] [Google Scholar]
- 69.Pandya GA, Moriya M. 1,N6-ethenodeoxyadenosine, a DNA adduct highly mutagenic in mammalian cells. Biochemistry. 1996;35:11487–11492. doi: 10.1021/bi960170h. [DOI] [PubMed] [Google Scholar]
- 70.Crebelli R, Conti G, Conti L, Carere A. Chloroacetaldehyde is a powerful inducer of mitotic aneuploidy in Aspergillus nidulans. Mutagenesis. 1990;5:165–168. doi: 10.1093/mutage/5.2.165. [DOI] [PubMed] [Google Scholar]
- 71.Spengler SJ, Singer B. Formation of interstrand cross-links in chloroacetaldehyde-treated DNA demonstrated by ethidium bromide fluorescence. Cancer Res. 1988;48:4804–4806. [PubMed] [Google Scholar]
- 72.Crebelli R, Conti G, Conti L, Carere A. Induction of somatic segregation by halogenated aliphatic hydrocarbons in Aspergillus nidulans. Mutat. Res. 1984;138:33–38. doi: 10.1016/0165-1218(84)90082-x. [DOI] [PubMed] [Google Scholar]
- 73.Guengerich FP, Mason PS, Stott WT, Fox TR, Watanabe PG. Roles of 2-haloethylene oxides and 2-haloacetaldehydes derived from vinyl bromide and vinyl chloride in irreversible binding to protein and DNA. Cancer Res. 1981;41:4391–4398. [PubMed] [Google Scholar]
- 74.Koudjonou BK, LeBel GL. Halogenated acetaldehydes: analysis, stability and fate in drinking water. Chemosphere. 2006;64:795–802. doi: 10.1016/j.chemosphere.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 75.Hsie AW, Brimer PA, Mitchell TJ, Gosslee DG. The dose-response relationship for ultraviolet-light-induced mutations at the hypoxanthine-guanine phosphoribosyltransferase locus in Chinese hamster ovary cells. Somatic Cell Genet. 1975;1:383–389. doi: 10.1007/BF01538669. [DOI] [PubMed] [Google Scholar]
- 76.Tindall KR, Stankowski LF, Jr, Machanoff R, Hsie AW. Detection of deletion mutations in pSV2gpt-transformed cells. Mol. Cell. Biol. 1984;4:1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner ED, Rayburn AL, Anderson D, Plewa MJ. Analysis of mutagens with single cell gel electrophoresis, flow cytometry, and forward mutation assays in an isolated clone of Chinese hamster ovary cells. Environ. Mol. Mutagen. 1998;32:360–368. [PubMed] [Google Scholar]
- 78.Rundell MS, Wagner ED, Plewa MJ. The comet assay: genotoxic damage or nuclear fragmentation? Environ. Mol. Mutagen. 2003;42:61–67. doi: 10.1002/em.10175. [DOI] [PubMed] [Google Scholar]
- 79.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 80.Wagner ED, Plewa MJ. Microplate-based comet assay. In: Dhawan A, Anderson D, editors. The Comet Assay in Toxicology. London: Royal Society of Chemistry; 2009. pp. 79–97. [Google Scholar]
- 81.Kumaravel TS, Jha AN. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006;605:7–16. doi: 10.1016/j.mrgentox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Box GEP, Hunter WG, Hunter JS. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. New York, NY: Wiley & Sons Inc; 1978. [Google Scholar]
- 83.Lovell DP, Omori T. Statistical issues in the use of the comet assay. Mutagenesis. 2008;23:171–182. doi: 10.1093/mutage/gen015. [DOI] [PubMed] [Google Scholar]
- 84.Efron B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987;82:171–185. [Google Scholar]
- 85.Singh K, Xie M. Bootstrap: A Statistical Method; Rutgers University. 2008:14. [Google Scholar]
- 86.Richardson SD, Thruston AD, Jr, Caughran TV, Chen PH, Collette TW, Floyd TL, Schenck KM, Lykins BW, Jr, Sun GR, Majetich G. Identification of new drinking water disinfection byproducts formed in the presence of bromide. Environ. Sci. Technol. 1999;33:3378–3383. [Google Scholar]
- 87.Glaze W, Weinberg H. Am. Water Works Assoc. Res. Denver, CO: Foundation; 1993. Identification and Occurrence of Ozonation By-products in Drinking Water. [Google Scholar]
- 88.Sclimenti MJ, Krasner SW, Glaze WH, Weinberg HS. Proceedings of the American Water Works Association Water Quality Technology Conference; Denver, CO. Denver, CO: Am. Water Works Assoc; 1990. [Google Scholar]
- 89.Richardson SD, Fasano F, Ellington JJ, Crumley FG, Buettner KM, Evans JJ, Blount BC, Silva LK, Waite TJ, Luther GW, McKague AB, Miltner RJ, Wagner ED, Plewa MJ. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ. Sci. Technol. 2008;42:8330–8338. doi: 10.1021/es801169k. [DOI] [PubMed] [Google Scholar]
- 90.Bichsel Y, von Gunten U. Oxidation of iodide and hypoiodous acid in the disinfection of natural waters. Environ. Sci. Technol. 1999;33:4040–4045. [Google Scholar]
- 91.Bichsel Y, von Gunten U. Formation of iodo-trihalomethanes during disinfection and oxidation of iodide containing waters. Environ. Sci. Technol. 2000;34:2784–2791. [Google Scholar]
- 92.Jones DB, Saglam A, Triger A, Song H, Karanfil T. I-THM formation and speciation: preformed monochloramine versus prechlorination followed by ammonia addition. Environ. Sci. Technol. 2011;45:10429–10437. doi: 10.1021/es202745t. [DOI] [PubMed] [Google Scholar]
- 93.Plewa MJ, Muellner MG, Richardson SD, Fasano F, Buettner KM, Woo YT, McKague AB, Wagner ED. Occurrence, synthesis and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection by-products. Environ. Sci. Technol. 2008;42:955–961. doi: 10.1021/es071754h. [DOI] [PubMed] [Google Scholar]
- 94.Allard S, Nottle CE, Chan A, Joll C, von Gunten U. Ozonation of iodide-containing waters: selective oxidation of iodide to iodate with simultaneous minimization of bromate and I-THMs. Water Res. 2013;47:1953–1960. doi: 10.1016/j.watres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Richardson SD, Thruston AD, Jr, Caughran TV, Chen PH, Collette TW, Floyd TL, Schenck KM, Lykins BW, Jr, Sun GR, Majetich G. Identification of new ozone disinfection byproducts in drinking water. Environ. Sci. Technol. 1999;33:3368–3377. [Google Scholar]
- 96.Duirk SE, Lindell C, Cornelison C, Kormos JL, Ternes TA, Attene-Ramos MS, Osiol J, Wagner ED, Plewa MJ, Richardson SD. Formation of toxic iodinated disinfection by- products from compounds used in medical imaging. Environ. Sci. Technol. 2011;45:6845–6854. doi: 10.1021/es200983f. [DOI] [PubMed] [Google Scholar]
- 97.Serrano M, Silva M, Gallego M. Micro liquid-liquid extraction combined with large-volume injection gas chromatography–mass spectrometry for the determination of haloacetaldehydes in treated water. J. Chromatogr. A. 2011;1218:8295–8302. doi: 10.1016/j.chroma.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 98.Plewa MJ, Simmons JE, Richardson SD, Wagner ED. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ. Mol. Mutagen. 2010;51:871–878. doi: 10.1002/em.20585. [DOI] [PubMed] [Google Scholar]
- 99.Pals J, Ang J, Wagner ED, Plewa MJ. Biological mechanism for the toxicity of haloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol. 2011;45:5791–5797. doi: 10.1021/es2008159. [DOI] [PubMed] [Google Scholar]
- 100.Hilal SH, Bornander LL, Carreira LA. Hydration equilibrium constants of aldehydes, ketones, and quinazolines. QSAR Comb. Sci. 2005;24:631–638. [Google Scholar]
- 101. [accessed May 1, 2015];U. S. Environmental Protection Agency SPARC Performs Automated Reasoning in Chemistry. http://www.epa.gov/extrmurl/research/sparc.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.