Summary
Western blotting is one of the most commonly used laboratory techniques for identifying proteins and semi-quantifying protein amounts, however, several recent findings suggest that western blots may not be as reliable as previously assumed. This is not surprising since many labs are unaware of the limitations of western blotting. In this manuscript we review essential strategies for improving confidence in the accuracy of western blots. These strategies include selecting the best normalization standard, proper sample preparation, determining the linear range for antibodies and protein stains relevant to the sample of interest, confirming the quality of the primary antibody, preventing signal saturation and accurately quantifying the signal intensity of the target protein. Although western blotting is a powerful and indispensable scientific technique that can be used to accurately quantify relative protein levels, it is necessary that proper experimental techniques and strategies are employed.
Keywords: Western blotting, loading control, immunoblot, quantification, Stain-Free gels, total protein normalization, housekeeping proteins, western blotting accuracy, western blotting strategies
The western blot
The western blot, also sometimes referred to as the immunoblot, involves separating native or denatured proteins by gel electrophoresis, transferring these separated proteins to a protein binding membrane and subsequent detection of a target protein by an antibody specific to the target protein (Figure 1). Although western blotting is mainly carried out on complex samples such as tissue or cell extracts, this procedure is also used to detect target proteins in less complex samples such as purified proteasome complexes [1,2]. The name “western blot” was coined by W. Neal Burnette [3], but actually originated in the laboratory of Harry Towbin [4]. Over the last three decades the use of western blotting has continued to increase, currently making it one of the most widely used analytical techniques in scientific laboratories worldwide. This review highlights the key issues with western blotting that cause inconsistent results between similar experiments that are reported in the literature. These issues include sample preparation, sample fractionation, protein loading amounts, antibody specificity, linear dynamic range of antibodies, normalization controls, detection method, blotting reagents, incubation times, and the quantitative analysis method. The strategies described are limited to the main western blotting techniques currently utilized, as strategies for all of the different western blotting variations are not possible in a single review.
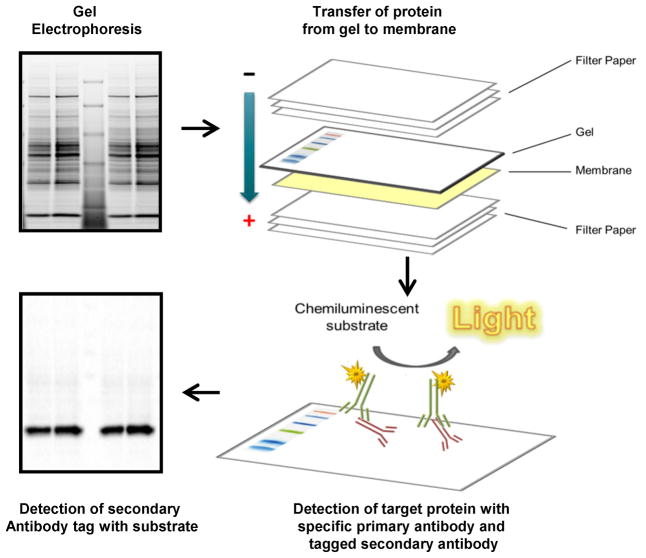
Figure 1. Schematic diagram of a typical western blot.
The typical western blot requires gel electrophoresis to separate proteins based upon molecular mass with subsequent transfer of the proteins from the gel to a protein binding membrane. The membrane is then incubated with the primary antibody to the target protein and the primary antibody is detected by a tagged secondary antibody. The tagged secondary antibody catalyzes an enzymatic reaction with the substrate which can be detected by film or a digital imager.
Why do we need western blotting?
Besides being an essential analytical tool to identify a protein of interest in a complex mixture, western blot data can also be used as a semi-quantitative method to determine and compare the expression of specific proteins in various cells and tissues [5]. Although the western blotting technique can also be used for absolute quantification [6], this requires a linear standard curve of purified target protein. The target protein in the homogenate must be within the range of the standard curve; hence western blotting is very rarely used for absolute quantification. However, semi-quantification of protein levels using western blots is common in most life science laboratories.
The advantages of western blots include the ability to detect picogram levels of protein in a sample [7], allowing the technique to be used for many purposes including as an effective early diagnostic tool [8,9]. The sensitivity and specificity of western blots is due to two main factors: 1) the separation of proteins which are different in size, charge and conformation by gel electrophoresis. For sodium-dodecyl sulfate (SDS)-polyacrylamide gel-electrophoresis (PAGE) the proteins are denatured and given a negative charged by binding to SDS, then separated based on size. The molecular mass of the protein identified by western blot can be determined by using standards of known molecular weights. 2) The specificity of the antibody-antigen interaction. The selective nature of the specific antibody allows the detection of a target protein in complex mixtures containing > 100,000 different proteins. When two-dimensional (2D) electrophoresis is used instead of one dimensional (1D) electrophoresis (2DE westerns), isoforms and post-translationally modified target proteins with similar molecular masses can be identified [10].
Limitations of western blotting
As with all techniques, western blotting has its limitations [4,5,11]. The main limitation of western blotting is that it can only be carried out if a primary antibody against the protein of interest is available. To detect post-translational modifications such as phosphorylation of target proteins, specific antibodies against the phosphorylated residues are needed. While antibodies for many different proteins are available from biotech companies, they are not cheap, and if primary antibodies are not available for a given protein, it will not be possible to perform a western blot to detect that particular protein. Another major limitation is that many antibodies exhibit off-target effects by interacting with other proteins. There are also many commercially available antibodies that do not detect the target protein when tested in the laboratory with particular tissues or cell types, resulting in what can only be described as expensive buffer. Another important limitation of the western blotting technique is the technical demand on the scientist. It is not uncommon for simple mistakes such as using too little or too much primary antibody to result in unusable results. An investigation of quantitative western blotting using erythropoietin showed that the interoperator variability was the main error source accounting for nearly 80% of the total variance [12]. Other western blotting limitations include the need for each antibody to be independently optimized and the cost of modern western blotting equipment such as advanced digital imagers. The basic western blot protocol is often ineffective in detecting a particular protein and modified protocols exist in most laboratories. One problem that may be encountered is variations in transfer efficiency. Small proteins (< 10kDa) may not be retained by the membrane, large proteins (> 140kDa) may not being transferred to the membrane, and varying gel concentrations may affect transfer efficiency [13]. Other problems include the primary antibody not recognizing the immobilized antigen in its denatured state, the detection signal decaying too quickly and high background to name a few. However, many of these limitations can be overcome with proper experimental techniques.
Sample preparation and amount of protein loaded onto polyacrylamide gels
Sample preparation is an often overlooked source of inaccuracy in western blotting. Tissues and cells should be rapidly frozen with liquid nitrogen and lysed as quickly as possible to avoid degradation of proteins by endogenous proteases. Repeated freeze/thaw cycles should be avoided as they can have an adverse effect on the quality of protein. Samples are usually centrifuged to remove “cellular debris” or fractionated to enrich the sample of interest [14]. However, in some cases removal of “cellular debris” or fractionation could lead to a significant loss of the protein of interest, resulting in incorrect results. A misconception is that the “cellular debris” of a homogenate interferes with the detection and quantification of the target proteins. When homogenates are run on SDS-PAGE at high concentrations the “cellular debris” can sometimes affect western blotting, but these homogenates can be run at significantly lower concentrations, resulting in more accurate western blotting. This “cellular debris” was shown to contain almost 50% of the myosin and two-thirds of calsequestrin-2 found in skeletal muscle cells [14]. Many other proteins of interest are likely to be associated with this “cellular debris.” Hence, it is important to determine if the cellular debris contains the target protein when quantifying total sample homogenates. Some cultured cell samples show streaks in the gel due to high levels of DNA; this can be reduced by adding DNase to the sample during homogenization or just before lysis in SDS-PAGE sample buffer. Lysis buffers used to prepare samples for western blotting should facilitate efficient protein extraction and preserve antibody recognition sites on proteins [15].
The amount of protein loaded onto gels for subsequent western blotting is a major source of inconsistency between laboratories. Most laboratories use a range of 20–40μg of total protein for loading homogenates for western blotting. However, some labs use greater than 100μg of total protein to detect lower abundance proteins. Under certain conditions loading large amounts of protein samples may actually decrease the relative amount of low abundance proteins that bind to nitrocellulose and PVDF membranes due to saturation of the membrane by highly expressed proteins. The benefits of loading lower amounts of sample for western blotting are often overlooked. Quantification of poorly expressed proteins has been demonstrated with less than 2μg of total sample [11]. When small amounts of whole skeletal muscle homogenate were utilized, μ-calpain was found to occur at a concentration of approximately 200nM [16]. Two other proteins, calsequestrin 1 (CSQ1) and AMP-activated kinase (AMPK) β1, were found to be linear up to 70 μg (CSQ1) or 150 μg (AMPK β1) of purified protein, while signal saturation was obtained with only approximately 12 μg of protein sample loaded [17]. These latter results suggest that loading 20 μg of total protein for quantification of either of these proteins will result in incorrect results. It is important that proper protein determination assays which are compatible with the homogenization buffers be utilized and that scientists realize different methods can give different protein concentrations for the same sample in buffers compatible for these assays [18]. An alternate approach to using whole tissue sample for western blot is using single cell western blotting, which utilizes individual cells. This method enables the detection of specific proteins in a very small sample and avoids the common problem of tissue heterogeneity which increases the detection of non-specific proteins. Quantitative protein analysis can be performed in single segments of individual human skeletal muscle fiber allowing for the detection of low abundance proteins such as the ryanodine receptor 1 (RyR1) which was present at approximately 200nM levels [19]. The quantification of the bands obtained in this case required a standard curve of the tissue homogenate (non-fractionated) to detect the target protein and to compare the expected band specificities of the antibodies used.
Antibody quality, antibody interaction solution and incubation time
To carry out western blot successfully, the quality of the primary antibody is a critical factor. Therefore validation of the antibody is vital to avoid inaccurate results. Validation includes determining the optimal antibody concentration for the protein of interest. The occurrence of multiple bands does not necessarily mean that the antibody recognizes non-specific bands, as some proteins may be post-translationally modified or alternately spliced resulting in forms which run at different molecular masses but contain the same epitope recognized by the antibody [20,21]. However, many commercial antibodies show non-specific binding to antigens other than the targeted protein. Some of the antibodies that recognize non-specific proteins can still be utilized for western blotting if the non-specific interactions occur at a molecular mass which is sufficiently different from the target protein to allow accurate quantification of the target protein. Non-specific interactions can sometimes be reduced by lowering the concentration of the primary antibody and/or by varying the period of incubation time. Inefficient blocking reagent or insufficient blocking times are common mistakes which result in increased non-specific bands; however, caution is needed as overblocking also results in poor signal intensity of the target protein. We have found that some antibodies give stronger signals when incubated at room temperature for 2–4 hours than when incubated overnight at 4ºC. The amount of Tween 20 used in the buffers is also important for reducing background staining [22].
The specificity of the antibodies can readily be determined using positive and negative controls. The best positive controls are purified proteins or lysates which overexpress the target protein, while the best negative controls are tissues from knock-out animal tissues or cell lysates. Some companies sell peptides to the epitope recognized by the antibodies which can be used to verify that the antibody binds specifically to the target epitope. As a last resort, if purified protein or lysates which overexpress the target protein are unavailable many companies such as Aviva Systems Biology, Cell Signaling Technology and Santa Cruz Biotechnology all have available lysates for many tissues and cells, which can be used as positive controls if the target proteins are known to be highly expressed in these tissues or cells. Antibody specificity can also be comprehensively determined using whole proteome microarrays [23]. The need for verifying antibodies is exemplified by a recent publication which showed a high risk of artifactual signal when performing western blotting with routinely used anti-tau antibodies [24]. This manuscript strongly recommends the use of negative and positive controls in all experiments for tau detection [24]. We recommend the use of negative and positive controls in at least one western blotting experiment for each antibody.
Using the ability of the antibody to detect the correct molecular weight as the only criteria for judging whether the antibody is specific for the target is not satisfactory, since many proteins migrate anomalously on SDS-PAGE. The target protein itself may migrate to a different molecular mass than expected, or a non-specific antibody-interacting protein may migrate at the same expected molecular mass as the target protein. The same protein can also migrate differently relative to other proteins on different types of SDS-PAGE gels. Examples include calmodulin and cardiac troponin C, which migrate differently when bound to calcium compared to when EGTA is present, preventing calcium from binding to the proteins [25,26]. Another example is CSQ1, which has a predicted molecular mass of approximately 45.3 kDa but runs on a Tris-HCl pH 8.3 SDS-PAGE gel above CSQ2 at approximately 63kDa [6], but on a Bis-Tris-HCl pH 7.3 gel migrates faster than CSQ2 [27]. An important principle of SDS-PAGE is that during denaturation of the sample proteins SDS binds to proteins in excess, resulting in a net overall negative charge which supersedes the intrinsic net charge of the proteins [28]. However, proteins which are highly anionic or contain regions with high levels of glutamic and/or aspartic acid residues often migrate at higher molecular masses than predicted, possibly because the high amount of intrinsic negatively charged residues prevent significant binding of SDS to certain regions of the protein. Human CSQ1 has 14% glutamic acid and 12.1% aspartic acid residues (Uniprot accession number P31415), while the average natural occurrence of glutamic and aspartic acid in proteins are 6.3 and 5.2% respectively [29]. Depending on the protein investigated the use of specific enzymes such as protein kinases could also affect the migration pattern of proteins. One example is recombinant troponin I incubated with protein kinase A and subsequent determination of troponin I phosphorylation levels by antibodies to dephosphorylated and phosphorylated troponin I: a lower molecular weight band was detected due to contaminants in the protein kinase A that partially degraded troponin I [30].
After validating that the antibody specifically recognizes the target protein it is important to determine the linear dynamic range for the target protein using the validated antibody against the samples being investigated. Different antibodies show different linear dynamic ranges, especially at high total protein levels (Figure 2). Using western blotting, Bag3 protein expression was found to increase 23 fold when co-expressed with HspB8 in HEK293 cells [31]. When the amount of HEK293 sample was increased 10 fold, Bag3 expression was determined to increase by only 4 fold [31]. The fold changes in protein expression detected decreased with increasing protein loading. Hence it is critical that western blots are carried out within the linear range of the antibody being utilized. This can be easily carried out by doing western blots using different sample dilutions. To improve data accuracy in western blotting these calibration or dilution curves must be carried out for every antibody.
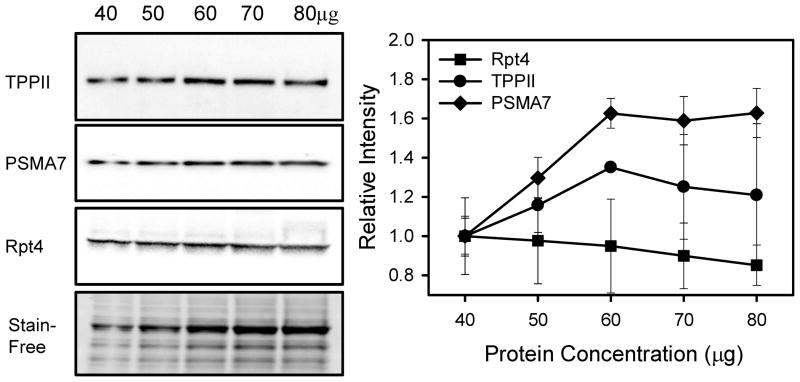
Figure 2. The linear dynamic range for each target protein may be different.
Western blotting of heart homogenates (40 to 80μg) using anti-TPPII (Santa Cruz), anti-PSMA7 (Abcam) and anti-Rpt4 (Enzo). A Stain-Free image of a representative region of the blot is shown. The figure shows the relative intensity of the different target proteins without any normalization. Both anti-TPPII and anti-PSMA7 were linear within the range of linearity of the total protein stain (see Figure 4) but the anti-Rpt4 antibody was not linear within the protein range utilized. Use of the anti-Rpt4 antibody in samples in which 50μg of total protein was loaded would give incorrect results.
Normalization and quantification of western blots
Attaining quantitative data from western blots requires the proper execution of the western blotting procedures including gel loading and transfer of proteins. Inconsistency in any of the above methods could result in variations in signal intensity between the same protein bands in different gel lanes. For this reason, researchers typically opt for lane to lane normalization using housekeeping proteins (HKPs) or total protein to rectify the errors related to the variations in sample loading and transfers.
While HKPs are excellent normalization references when validated for the tissue or cell being investigated, recent findings suggest that these HKPs are substandard loading controls under many conditions [32–34]. Commonly used loading controls include the expression of HKPs such as β-actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and β-tubulin. Reasons for the poor reliability of HKPs as loading controls include the presence of numerous poor quality antibodies to each HKP, the relatively high expression of HKPs in many tissues and cells, and the change in expression of HKPs in some tissues under certain experimental conditions. It has been demonstrated that HKP levels vary under certain conditions, such as hypoxia, serum starvation, exercise, and transplantation [35–38]. The levels of GAPDH and α-tubulin have also been shown to change with the density of cultured cells [39]. A recent mass spectrometry-based approach found that the protein expression levels of housekeeping proteins such as GAPDH vary substantially between tissues [40]. Both the protein of interest and the HKP need to be with the linear detection range for their expression levels to be accurately determined. HKPs are high abundance proteins and therefore are often overloaded, particularly when large amounts of total protein are loaded to detect low abundance target proteins, so that HKP expression levels cannot be quantified accurately [22,33,41]. Another overlooked aspect of HKPs is that they are highly post-translationally modified (Figure 3), which can also potentially affect quantification depending on the epitope of the antibody utilized.
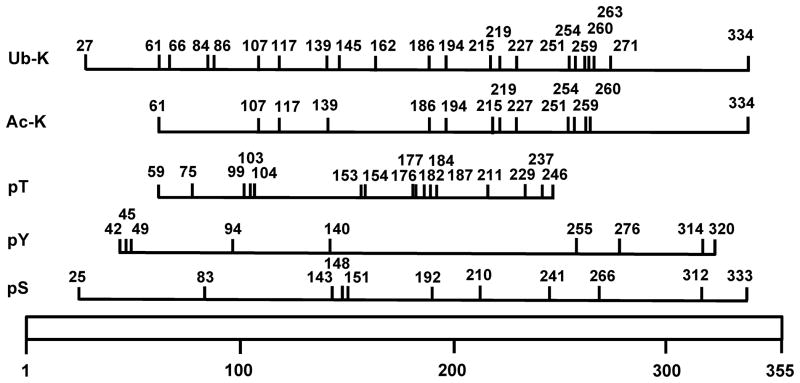
Figure 3. Schematic diagram of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) showing known post-translational modifications identified by different methods including mass spectrometry.
The diagram is drawn to scale to show the location of the post-translational modifications (PTMs) on the 355 residue long GAPDH. The residue number for each PTM is shown. Ub-K, ubiquitinated lysine; Ac-K, acetylated lysine; pT, phosphorylated threonine; pS, phosphorylated serine; pY, phosphorylated tyrosine. PTMs on GAPH were obtained from PhosphoSitePlus (http://www.phosphosite.org/).
Over the last five years, the use of total protein normalization has increased and has been found to be a better normalization control than HKPs for several cells and tissues investigated. Total protein normalization utilizes the intensity of all the proteins in a lane on either a nitrocellulose or PVDF membrane. Total protein quantification by Coomassie blue, Ponceau S, and the Stain-Free method has been shown to have advantages over HKPs for normalization of western blots mainly due to the fact that this normalization does not depend on expression of a single protein [22,42–44]. Other staining methods for total protein detection on blots are also available [45]. Stain-Free gels contain trihalo compounds that modify tryptophans when exposed to UV light, producing a strong fluorescent signal that is proportional to the total amount of protein present. Total protein quantification with the Stain-Free method has been shown to serve as a reliable loading control and conveniently allows for the investigator to ensure that proteins have been separated and transferred before continuing with the procedure [32,41,46,47]. We currently utilize the Stain-Free method for our western blots because of its high sensitivity compared to Ponceau S staining (Figure 4), as well as its cost effectiveness when compared to HKPs as a loading control. Figures 2 and 4 show that the Stain-Free protein normalization method for determining changes in PSMA7 amounts in rat heart homogenates would allow for accurate determination of the amount of protein in each lane.
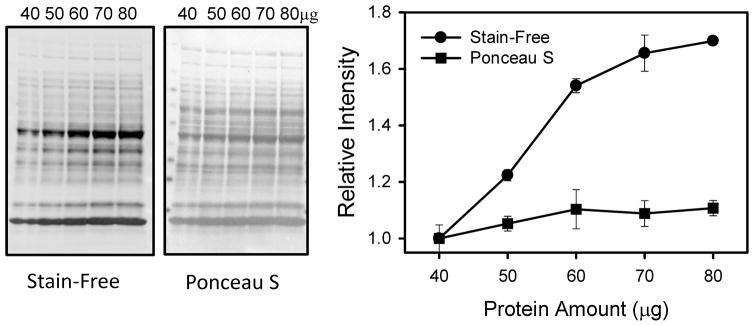
Figure 4. The linear dynamic range for total protein detection varies with the staining reagent utilized.
Both Stain-Free and Ponceau S staining of total proteins (40 to 80μg) in heart homogenates show that both stains were linear between 40 to 60 μg of total protein. Images shown are nitrocellulose membranes post-transfer. Due to the larger relative changes in intensity using the Stain-Free method when compared to the Ponceau S stain, it is likely that smaller differences in target protein changes could be distinguished using the Stain-Free method.
Another major concern of western blot quantification is the difficulty in determining whether the signal is saturated using film for developing western blots. Digital imagers, which are available from many companies (Azure Biosystems, Bio-Rad, BioSpectrum, GE Healthcare, Li-cor, ProteinSimple, SYNGENE, and UVP to name a few) show higher sensitivity, better linearity, and easier detection of saturated bands than film-based processors. Digital imagers have a 3-fold increase in linear dynamic range when compared to film [48]. While digital imagers will continue to improve, resulting in lower background, higher resolution and increased dynamic range, advances in film technology are likely to be minimal at best.
Quantification of the image detected has been described as being based on traditions and guesswork [49]. Software for the quantification and determination of molecular mass of the target proteins has improved considerably in the last two decades. Depending on the digital imager, the optimal image that prevents signal saturation of the most intense band can be determined. Better tools are now available for automatically detecting bands and lanes and for moving and bending individual bands and lanes. For molecular mass determination, chemiluminescent and colorimetric images for detection of pre-stained molecular weight standards can be imaged and merged. Assignment of the molecular masses to the unknown molecular weight standards or automatic assignment to previously assigned molecular weight standards allows for the automatic determination of the molecular masses of the target proteins which removes human error and bias. In many gels some of the lanes do not run as a straight line but often run as curved or wavy lines, which make it more difficult to properly detect lanes. Software has advanced such that using automatic detection of lanes has improved from members of our lab being unsatisfied with any automatic lane detection method to being satisfied about 50% of the time. Helpful tools to discriminate between two closely detected bands such as the 3-D viewer allow the investigator to determine if the detected bands are sufficiently separated from each other for protein semi-quantification of individual bands. Recommendations for scientifically sound densitometry determination have been presented but are not utilized [49–51].
The background subtraction problem
An optimized semi-quantification scheme which utilizes the Chemidoc MP (Bio-Rad) and ImageLab 5 is described below. ImageLab is a recently developed software program which is now supplied with all Bio-Rad imagers and contains an array of analysis tools for quantification and determination of the molecular weights of the target proteins in the samples. One of the most important improvements in semi-quantification software is in determining the levels of intensity of each lane. A major problem with semi-quantification of western blots is the determination of the level of background. All western blots contain non-specific background signals due to many factors including secondary antibodies and auto-chemiluminescence of the membranes utilized. Proper background subtraction is critical for accurate semi-quantification of a western blot. The most common method for background subtraction involves determining the intensity of an area of blot that is the same size as the area of blot that is used for the determination of the protein of interest. The area (usually a rectangle) that is used for background subtraction is usually placed above or below or is an average of the intensity of the area above or below the band of interest. While this method has been shown to be accurate under many conditions, on some blots the background intensity varies considerably such that the background intensity at the target protein is different from either above, below or an average of these two intensities (above and below). In many cases the area above or below a band of interest is unavailable to be used for background subtraction due to signals from other bands. The ImageLab software utilizes an optional rolling disc background subtraction algorithm which can be used to manually or automatically subtract background. In the rolling disc background subtraction method, the desired disc size can be set for each band or lane background subtraction to specify the size of the rolling disc that selects the band or lane along its length and corrects the background levels with the aid of a lane profile tool [50]. The lane profile tool shows the intensity of the target protein or proteins as well as the background associated with the samples. The size of the disc determines the level of background removal. Since the intensity before and after the target proteins are also visualized with the lane profile tool it is convenient to determine the optimal disc size to remove the background. We have found that this method is as good and in many cases better than previously used methods for background subtraction. We suggest using test samples at different concentrations when using this feature for the first time so that the advantages and disadvantages of this feature are understood, as a larger disc size than necessary leads to only partial removal of the background while a smaller disc size than needed will result in the deletion of the actual target protein signal. Hence, an appropriate disc size should be selected and a value of ≤ 10mm is usually considered optimum for removal of most backgrounds. The advantage of the rolling disc background subtraction is evident when total protein intensity determinations are carried out. Blank lanes containing no proteins are not needed for background correction. For total protein detection individual bands can be detected in each selected lane by selecting the sensitivity for which the lowest intensity band can be detected. Using too high a value for sensitivity sometimes results in the detection of background staining as bands. This can easily be prevented by using the lane profile tool together with the band detection sensitivity tool. When the protein signal is developed based on methods like immuno-probing (either chemiluminescence or fluorescence), commassie, silver staining, or Sypro Ruby where the substrate or the dye itself can contribute to uneven background on the blot, a small disc size would help generate more consistent lane profiles among the samples. As previously noted programs containing rolling ball algorithms that are applied to the entire image for immunoblots such as found in ImageJ [101] should be avoided [49]. When the protein signal is developed by methods that do not contribute to the background (signal is generated only when the dye binds or reacts with proteins), such as Stain-Free, a large disc size for a global background subtraction is more appropriate.
Other problems associated with inaccurate quantification of western blots
Protein transfer method
Two main types of protein transfer exist: wet transfer and semi-dry transfer. Traditional wet transfer is still the most common method for protein transfer from gel to membrane and offers high efficiency, but at a cost of time and effort. Life Technologies’ iBlot2® systems are referred to as dry systems but are really still semi-dry blotters since they still utilize small amounts of buffer for transfer. Semi-dry transfer is faster than wet transfer [52]. The iBlot2® and Trans-Blot® Turbo (Bio-Rad) are two semi-dry systems that can efficiently transfer proteins to membranes in 7 minutes. In our hands the quality of the transfer are similar between the new semi-dry blotters and the traditional wet transfer method.
A common problem observed during protein transfer to a membrane is the appearance of air bubbles. This problem typically arises due to improper assembly of the transfer stack. The use of a plastic rod (shaped like a thick pencil) or a miniature roller is very effective at significantly reducing air bubbles. Air bubbles should be rolled out and excess transfer buffer should be used at every step during the transfer assembly before protein transfer to the membrane is started. Although not obvious, several small bubbles may lead to inaccuracies in protein quantification depending on the location of the bubbles and the method of quantification of the target protein. Improper assembly of the transfer stack can also lead to parts of the gel having better transfer than other parts of the gels, such as what happens due to partly worn fiber pads when using wet transfer.
Another issue faced by researchers is low signal detection where the target proteins cannot be detected even after 2–5 minutes of exposure. This often happens due to incomplete transfer or over-transfer. Depending on the transfer method, membrane type, current and voltage used, the appropriate transfer time should be determined using an extra membrane below the membrane touching the gel to determine whether proteins are being over-transferred. The pore size of the membrane, as well as the transfer buffer and the type and percentage of acrylamide gel used all affect protein transfer from a gel to a membrane. The reuse of transfer buffer for protein transfer to membrane can also be problematic and is not recommended [53].
Blocking reagent and blocking time
Appropriate blocking reagents must be selected in order to avoid high background and non-specific binding [54]. Under certain blocking conditions as much as 60% of proteins has been shown to be lost from membranes [55]. 3–5% non-fat dry milk, bovine serum albulmin (BSA), or goat serum is usually recommended to be incubated with the membrane for 1 hour in order to block non-specific sites. However excessive blocking must be avoided, as it may produce low signal. We have found that including 1% non-fat dry milk in the antibody buffer typically yields better results than using 5% non-fat dry milk. In some cases using milk as a blocking buffer results in high background, such as when using phospho-antibodies. The casein in the milk is sometimes recognized by the phospho-specific antibodies leading to non-specific binding. Therefore, before the initiation of any western blot experiment using new antibodies, the researchers should first determine the best blocking reagent for their antibody and the duration of incubation which will help in attaining reproducible results. If manufacturers’ recommendations are suggested then the researchers should start optimizing using these recommendations. For some antibodies the manufacturers recommend using PBS instead of TBS; this is important as some antibodies show significantly better results when incubated in PBS than TBS. However, most antibodies work well in TBS. An easy way to check for nonspecific binding of primary antibody to the membrane blocker is to incubate a small piece of blocked membrane with the primary antibody working solution followed by the secondary antibody working solution. If a background signal is detected on the membrane after addition of detection substrate it is recommended to use either a different blocking solution or less primary antibody.
Enhanced chemiluminescence (ECL) substrate
Detection of the tagged secondary antibody by ECL is the most common technique currently used in western blotting [56]. The secondary antibody is tagged with the enzyme horseradish peroxidase (HRP), which catalyzes the oxidation of luminol (substrate) in the presence of peroxide to 3-aminophthalate, leading to the emission of light at 428nm which is detected by film or digital imagers [57]. Modern enhanced luminol-based chemiluminescent substrates are non-radioactive and show high sensitivity for the detection of conjugated secondary antibodies bound to primary antibodies interacting with low picogram (1–3 pg) amounts of antigen. An advantage of using ECL substrate is that the blots can be successfully stripped of the reagents and re-probed with a different antibody to detect a new protein. Stripping of the blot and reprobing require careful analysis of the stripping method to verify that improper or uneven stripping do not occur. Other concerns with stripping and reprobing exist, such as the loss of protein bound to the membrane [58]. The signal detected in the presence of ECL reagent is transient in nature, in that the signal can be found only when a reaction occurs between the enzyme and the substrate. Addition of too much secondary antibody enzyme conjugate and/or incubation of the secondary enzyme for prolonged periods are major causes of high background, short signal duration, signal variability, and low sensitivity. It is important to obtain signal emission curve that decays slowly because this allows reproducible results, as longer lasting signals reduce variability due to transfer efficiency, different manufacturer lots of substrate and other factors. HRP can become inactive with prolonged exposure to substrate, as free radicals produced during the oxidation reaction can bind to HRP reducing the enzyme’s ability to interact with the substrate [59]. High levels of HRP produce free radicals that not only affect HRP itself but can also damage the antigen, antibodies and membrane.
Although not as common as ECL, fluorescently labelled secondary antibodies has been shown to be powerful reagents in the western blotting scheme [60]. Using fluorescence instead of chemiluminescence for signal detection has its advantages and disadvantages. A major advantage of using fluorescently labelled secondary antibodies is the ability to detect multiple targets by using fluorophores with non-overlapping excitation-emission spectra. One factor that has limited the use of fluorescence detection in western blotting is the relatively poor performance of fluorophores in the visible range [61]. However, fluorophores in the infrared spectrum are as sensitive as chemiluminescence but have the advantage of a wider linear dynamic range than chemiluminescent detection [61].
Automated western blots
A few companies currently sell automated western blotting machines, which have their advantages and disadvantages. However, several of the concerns that plague non-automated western blotting will also affect automated western blotting. The use of capillary columns in some new automated machines [62,63] introduces new technical concerns including how we can relate some of the results from capillary electrophoresis westerns back to traditional western blotting results.
Conclusion
Western blotting is entering an exciting time period in which new equipment and reagents are being introduced annually. No researcher can compare all of the numerous equipment currently available for western blotting. While some equipment reduces the time needed for western blotting, most do not actually improve western blotting. The more essential improvements needed for improving confidence in the accuracy of western blots include better documentation of primary antibodies, better training of researchers to use quantification software, use of antibodies within their linear range and proper choice of a loading control.
The average scientist would like to detect specific proteins and quantify relative changes in these proteins under different conditions in an efficient and robust manner. Many laboratories waste valuable and costly antibodies because of the lack of guidelines for properly using these antibodies. Even antibodies that have been pre-tested and packaged with recommended methods often do not work well for all tissues and cell types. Companies selling antibodies should not only test the antibodies against overexpressed proteins, but in tissue samples which are more complex and typically show more non-specific bands than cells. Relying exclusively on commercially available antibody datasheets is a common mistake made by investigators. Most antibody datasheets do not typically provide complete sample preparation information, tissue specific data for the antibody or adequate negative control data. Hence researchers must do their own antibody validation for their tissues of interest. Ultimately, having the best equipment and reagents will still not prevent inaccurate western blots results if the researchers carrying out the blots are not well-trained.
Expert commentary
Few researchers who do western blotting have a detailed understanding of the theory and/or proper practice needed to obtain accurate results. This is because western blotting is often done by researchers who are shown how to do the blots but are never taught the detailed theory behind the technique. At a recent seminar we attended where a graduate student presented her research and showed normalization to total protein staining a faculty told the student that she should have normalized to a HKP. Misconceptions like these hinder scientists’ ability to fully utilize western blots for quantification of relative protein expression levels. When HKPs are utilized as loading controls they should be validated so that HKP levels are within the linear range at which the protein samples are being utilized. It is important to note that different laboratories utilize different methods to determine protein concentration, so it is advisable that each laboratory do an independent determination that the HKP used is within the linear range for their protein sample. This needs to be carried out each time a new batch of HKP antibody is utilized, since each antibody batch may have a different linear dynamic range. When total protein staining is used for normalization the linear detection range for the sample being investigated should also be carried out.
As a scientific group we need to do a better job of documenting poor quality antibodies to prevent or significantly reduce their use in scientific discovery. Another concern is the current inability of any laboratory to reproduce published western blots since the details published in journals about western blotting are typically minimal. More details about the amount of sample loaded and method used to determine protein concentration, antibody used (catalog, lot number and company), antibody concentrations, type of imager used, basic imaging conditions, type of membrane used, protein transfer method, time of exposure to developer (if used), type of film (if used), ECL reagent (if used), and quantification software all need to be provided either in the main publication or in the supplement.
Five-year view
Western blotting techniques, equipment and software will all considerably advance within the next five years. Hence, the sensitivity and reliability of this powerful technique will continue to improve. The use of total protein normalization will substantially increase as more concerns about housekeeping proteins are uncovered. The amount of protein samples loaded on a gel will slowly be reduced as researchers realize the advantages of loading less protein as well as the improved sensitivity of new chemiluminescent reagents and imagers. The type and numbers of automated western blotting machines will significantly improve and begin to be adopted by pharmaceutical researchers, core facilities and larger research laboratories.
Table 1.
Common problems that affect the accuracy of western blot quantification and solutions to correct them.
| Parameter | Important considerations | Common problems | Solutions |
|---|---|---|---|
| Sample preparation | Buffer, homogenization procedure, fractionation procedure, protease inhibitors, temperature, sample storage | Inefficient homogenization or fractionation of sample, improper handling of tissue/cell samples | Verify homogenization is optimal and that protein of interest is not lost during fractionation |
| Sample quantification | Compatibility of buffer and protein quantification method | Incorrect quantification due to buffer components that are incompatible with the protein determination method | Use only protein determination methods that are compatible with the sample buffer |
| Polyacrylamide gel | Manufacturer, percent polyacrylamide, age, lot | Use of gels which are not satisfactory for the molecular weight of the target protein, expired gels | Determine the optimal percentage gels for separation of target protein and use these gels for blots. |
| Membrane | Age, manufacturer, type, lot | High background staining, poor binding of protein to membrane | Use membranes which are well documented in the literature to work well for the western blotting application that is being used. |
| Sample loading amount | Total protein amount, polypeptide sizes | Protein of interest is not within its linear detection range. | A dilution curve needs to be carried out to determine the linearity of the target protein with the antibody that is being used. |
| Transfer conditions | protein size, transfer buffer, transfer time, current/voltage | High and low molecular weight proteins are not observed on the membrane or observed in small amounts | The optimal transfer of both high and low molecular mass proteins needs to be optimized depending on membrane and transfer method utilized. |
| Blocking solution | type of blocking solution, concentration, cross-reactivity | High levels of non-specific protein signal and back ground. | Verify that the blocking solution is not interacting with the primary antibody. Change blocking reagent or incubation time. |
| Primary Antibody | Specificity, titer, affinity, incubation time, source animal, concentration, source, lot, and temperature. Primary antibody concentrations have a profound effect on signal intensity. | Antibody recognizes multiple bands near the expected molecular mass of the target protein. Assuming different lots of the same antibody are identical. |
Minimal primary antibody is advantageous, as it reduces non-target binding and background. Use positive and negative controls. |
| Secondary Antibody | HRP conjugate enzyme activation level and activity, source animal, concentration, temperature and incubation time, | Detection signal is loss within a few minutes or ghost bands are observed. | Too much secondary antibody. Use less secondary antibody. |
| Appropriate reference for normalization | Suitability of housekeeping proteins and total protein staining as normalization methods | Normalization reference is outside its linear dynamic range resulting in inaccurate results. | The linear dynamic range for the total protein and housekeeping protein needs to be determined and used within this range for western blots. The linear dynamic range of total protein stains is greater than most antibodies making it a better normalization method for most western blots |
| Washing | Buffer, buffer volume, duration, frequency | High background | Increasing volume of buffer, wash duration, and frequency all reduce the background. |
| Detection and imaging | Substrate sensitivity, substrate manufacturer, lot, age, detection method, film age, imaging instrument manufacturer, exposure time | Film suffers from a small dynamic range allowing band signals to quickly become saturated. | Use a digital imager if possible. Otherwise validate that the film based signal is not saturated. |
| Quantification | Software, image quality, signal saturation | Improper quantification | Use test blots of different concentrations of target protein to improve understanding of the quantification software utilized. Follow recommendations from references quoted in text. |
The parameters associated with the causing the most inaccuracies in western blotting are bolded.
Key Issues.
Signal detection. The ease at which x-ray film becomes saturated with signal gives investigators a false sense that film is more sensitive that digital imagers. Laboratories should be encouraged to switch from x-ray films to digital imagers or learn how to validate that signals are not saturated on x-ray films.
The misconception that HKPs are the best normalization method for western blots needs to be addressed.
The need for researchers to load sample amounts in which target detected by the antibody is within the linear range
High volume of poor quality antibodies available.
Need for positive and negative controls to validate antibodies.
Need for determining and stating the molecular weight of the target protein on blots.
Need for an unbiased database allowing researchers to document good and poor quality antibodies.
Need for publications to describe the western blotting technique utilized in more detail. A minimum reporting standard for western blotting data should be established.
Need for recommended procedures for protein quantification.
Acknowledgments
This work was supported by National Institutes of Health Grants HL096819 and HL080101, and UC Davis research funds. AV Gomes presented a talk on ‘Can Western blots be trusted?’ at the Experimental Biology 2014 meeting which was sponsored by Bio-Rad.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
No writing assistance was utilized in the production of this manuscript.
References
Papers of special interest have been highlighted as:
*of interest
**of considerable interest
- 1.Gomes AV, Young GW, Wang Y, et al. Contrasting proteome biology and functional heterogeneity of the 20 S proteasome complexes in mammalian tissues. Molecular & cellular proteomics : MCP. 2009;8(2):302–315. doi: 10.1074/mcp.M800058-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes AV, Zong C, Edmondson RD, et al. Mapping the murine cardiac 26S proteasome complexes. Circulation research. 2006;99(4):362–371. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 3*.Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Analytical biochemistry. 1981;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. This paper was the first to introduce the term “western blotting.”. [DOI] [PubMed] [Google Scholar]
- 4**.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. This is the original publication which introduced the western blotting methodology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurien BT, Scofield RH. Western blotting. Methods. 2006;38(4):283–293. doi: 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. The Journal of physiology. 2009;587(Pt 2):443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coorssen JR, Blank PS, Albertorio F, et al. Quantitative femto- to attomole immunodetection of regulated secretory vesicle proteins critical to exocytosis. Analytical biochemistry. 2002;307(1):54–62. doi: 10.1016/s0003-2697(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni TA, Perenha-Viana MC, Patussi EV, Cardoso RF, Svidzinski TI. Western blotting is an efficient tool for differential diagnosis of paracoccidioidomycosis and pulmonary tuberculosis. Clinical and vaccine immunology : CVI. 2012;19(11):1887–1888. doi: 10.1128/CVI.00252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson AB, Ethridge SF, Wesolowski LG, et al. Costs and outcomes of laboratory diagnostic algorithms for the detection of HIV. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;58(Suppl 1):e2–7. doi: 10.1016/j.jcv.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Canelle L, Bousquet J, Pionneau C, et al. An efficient proteomics-based approach for the screening of autoantibodies. Journal of immunological methods. 2005;299(1–2):77–89. doi: 10.1016/j.jim.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Murphy RM, Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. The Journal of physiology. 2013;591(Pt 23):5823–5831. doi: 10.1113/jphysiol.2013.263251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koller A, Watzig H. Precision and variance components in quantitative gel electrophoresis. Electrophoresis. 2005;26(12):2470–2475. doi: 10.1002/elps.200500024. [DOI] [PubMed] [Google Scholar]
- 13.Smejkal G, Gallagher S. Determination of semidry protein transfer efficiency with transverse gradient gel electrophoresis. BioTechniques. 1994;16(2):196–198. 200–192. [PubMed] [Google Scholar]
- 14.Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Experimental cell research. 2009;315(6):1015–1028. doi: 10.1016/j.yexcr.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 15.MacPhee DJ. Methodological considerations for improving Western blot analysis. Journal of pharmacological and toxicological methods. 2010;61(2):171–177. doi: 10.1016/j.vascn.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of mu-calpain in rat skeletal muscle. The Journal of physiology. 2006;576(Pt 2):595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Mollica JP, Oakhill JS, Lamb GD, Murphy RM. Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Analytical biochemistry. 2009;386(2):270–275. doi: 10.1016/j.ab.2008.12.029. This paper showed how critical the sample loading amounts are for proper western blotting quantification. [DOI] [PubMed] [Google Scholar]
- 18.Cui Z, Gilda JE, Gomes AV. Crude and purified proteasome activity assays are affected by type of microplate. Analytical biochemistry. 2014;446:44–52. doi: 10.1016/j.ab.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-alpha1 and -beta1. J Appl Physiol (1985) 2011;110(3):820–825. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- 20.Jinwal UK, Trotter JH, Abisambra JF, et al. The Hsp90 kinase co-chaperone Cdc37 regulates tau stability and phosphorylation dynamics. The Journal of biological chemistry. 2011;286(19):16976–16983. doi: 10.1074/jbc.M110.182493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bicer S, Reiser PJ. Complex tropomyosin and troponin T isoform expression patterns in orbital and global fibers of adult dog and rat extraocular muscles. Journal of muscle research and cell motility. 2013;34(3–4):211–231. doi: 10.1007/s10974-013-9346-9. [DOI] [PubMed] [Google Scholar]
- 22.Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. Journal of proteome research. 2011;10(3):1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 23.Michaud GA, Salcius M, Zhou F, et al. Analyzing antibody specificity with whole proteome microarrays. Nature biotechnology. 2003;21(12):1509–1512. doi: 10.1038/nbt910. [DOI] [PubMed] [Google Scholar]
- 24.Petry FR, Pelletier J, Bretteville A, et al. Specificity of Anti-Tau Antibodies when Analyzing Mice Models of Alzheimer's Disease: Problems and Solutions. PloS one. 2014;9(5):e94251. doi: 10.1371/journal.pone.0094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess WH. Characterization of calmodulin and calmodulin isotypes from sea urchin gametes. The Journal of biological chemistry. 1982;257(4):1800–1804. [PubMed] [Google Scholar]
- 26.Tanokura M, Imaizumi M, Yamada K, Shiraishi F, Ohtsuki I. Preparation and characterization of troponin C from bullfrog skeletal muscle. Journal of biochemistry. 1992;112(6):800–803. doi: 10.1093/oxfordjournals.jbchem.a123979. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez A, Schiffer TA, Ivarsson N, et al. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. The Journal of physiology. 2012;590(Pt 15):3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CE, Musich PR, Johnson DA. Sodium dodecyl sulfate enhancement of quantitative immunoenzyme dot-blot assays on nitrocellulose. Analytical biochemistry. 1989;177(1):212–219. doi: 10.1016/0003-2697(89)90043-2. [DOI] [PubMed] [Google Scholar]
- 29.Simpson RJ. Proteins and Proteomics: A laboratory Manual. Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 30.Gomes AV, Harada K, Potter JD. A mutation in the N-terminus of troponin I that is associated with hypertrophic cardiomyopathy affects the Ca(2+)-sensitivity, phosphorylation kinetics and proteolytic susceptibility of troponin. Journal of molecular and cellular cardiology. 2005;39(5):754–765. doi: 10.1016/j.yjmcc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 31*.Charette SJ, Lambert H, Nadeau PJ, Landry J. Protein quantification by chemiluminescent Western blotting: elimination of the antibody factor by dilution series and calibration curve. Journal of immunological methods. 2010;353(1–2):148–150. doi: 10.1016/j.jim.2009.12.007. This paper describes the importance of a dilution series and calibration curve when quantifying protein by western blot using chemiluminescent signal. This is applicable to any detection method. [DOI] [PubMed] [Google Scholar]
- 32.Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Analytical biochemistry. 2013;440(2):186–188. doi: 10.1016/j.ab.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27(14):2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- 34*.Eaton SL, Roche SL, Llavero Hurtado M, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PloS one. 2013;8(8):e72457. doi: 10.1371/journal.pone.0072457. This report demonstrated that different regions of the same tissue show differences in expression of housekeeping proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan W, Lai M. Actin, a reliable marker of internal control? Clinica chimica acta; international journal of clinical chemistry. 2007;385(1–2):1–5. doi: 10.1016/j.cca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. Journal of biochemical and biophysical methods. 2000;46(1–2):69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Mulero S, Montanya E. Selection of a suitable internal control gene for expression studies in pancreatic islet grafts. Transplantation. 2005;80(5):650–652. doi: 10.1097/01.tp.0000173790.12227.7b. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5(2):566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- 39.Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L. Housekeeping genes; expression levels may change with density of cultured cells. Journal of immunological methods. 2010;355(1–2):76–79. doi: 10.1016/j.jim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelm M, Schlegl J, Hahne H, et al. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509(7502):582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 41.Posch A, Kohn J, Oh K, Hammond M, Liu N. V3 stain-free workflow for a practical, convenient, and reliable total protein loading control in western blotting. Journal of visualized experiments : JoVE. 2013;(82):50948. doi: 10.3791/50948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng L, Guo J, Xu HB, et al. Direct Blue 71 staining as a destaining-free alternative loading control method for Western blotting. Electrophoresis. 2013;34(15):2234–2239. doi: 10.1002/elps.201300140. [DOI] [PubMed] [Google Scholar]
- 43.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. Journal of neuroscience methods. 2008;172(2):250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero-Calvo I, Ocon B, Martinez-Moya P, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Analytical biochemistry. 2010;401(2):318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Sundaram RK, Balasubramaniyan N, Sundaram P. Protein stains and applications. Methods Mol Biol. 2012;869:451–464. doi: 10.1007/978-1-61779-821-4_39. [DOI] [PubMed] [Google Scholar]
- 46.Gurtler A, Kunz N, Gomolka M, et al. Stain-Free technology as a normalization tool in Western blot analysis. Analytical biochemistry. 2013;433(2):105–111. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Colella AD, Chegenii N, Tea MN, Gibbins IL, Williams KA, Chataway TK. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Analytical biochemistry. 2012;430(2):108–110. doi: 10.1016/j.ab.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Khoury MK, Parker I, Aswad DW. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Analytical biochemistry. 2010;397(1):129–131. doi: 10.1016/j.ab.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Gassmann M, Grenacher B, Rohde B, Vogel J. Quantifying Western blots: pitfalls of densitometry. Electrophoresis. 2009;30(11):1845–1855. doi: 10.1002/elps.200800720. This paper provides strong evidence that the current lack of recommended procedures in densitometry results in inaccurate quantification of many westerns. This group applied different common densitometry procedures to the identical western blot and obtained p-values of the correlations of plasma erythropoietin ranging from 0.000013 to 0.76. [DOI] [PubMed] [Google Scholar]
- 50.Taylor SC, Berkelman T, Yadav G, Hammond M. A defined methodology for reliable quantification of Western blot data. Molecular biotechnology. 2013;55(3):217–226. doi: 10.1007/s12033-013-9672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Taylor SC, Posch A. The Design of a Quantitative Western Blot Experiment. BioMed research international. 2014;2014:361590. doi: 10.1155/2014/361590. This paper focuses on critical issues of sample preparation and data analysis for obtaining quantitative western blots. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wisdom GB. Protein blotting. Methods Mol Biol. 1994;32:207–213. doi: 10.1385/0-89603-268-X:207. [DOI] [PubMed] [Google Scholar]
- 53.Dorri Y, Kurien BT, Scofield RH. Problems with multiple use of transfer buffer in protein electrophoretic transfer. Journal of biomolecular techniques : JBT. 2010;21(1):1–2. [PMC free article] [PubMed] [Google Scholar]
- 54.Spinola SM, Cannon JG. Different blocking agents cause variation in the immunologic detection of proteins transferred to nitrocellulose membranes. Journal of immunological methods. 1985;81(1):161–165. doi: 10.1016/0022-1759(85)90132-2. [DOI] [PubMed] [Google Scholar]
- 55.Pluskal MG, Przekop MB, Kavonian MR, Vecoli C, Hicks DA. Immobilon PVDF Transfer Membrane: A New Membrane Substrate For Western Blotting of Proteins. BioTechniques. 1986;4(3):272–282. [Google Scholar]
- 56.Mattson DL, Bellehumeur TG. Comparison of three chemiluminescent horseradish peroxidase substrates for immunoblotting. Analytical biochemistry. 1996;240(2):306–308. doi: 10.1006/abio.1996.0364. [DOI] [PubMed] [Google Scholar]
- 57.Durrant I. Enhanced chemiluminescent detection of horseradish peroxidase labeled probes. Methods Mol Biol. 1994;31:147–161. doi: 10.1385/0-89603-258-2:147. [DOI] [PubMed] [Google Scholar]
- 58.Alegria-Schaffer A, Lodge A, Vattem K. Performing and optimizing Western blots with an emphasis on chemiluminescent detection. Methods in enzymology. 2009;463:573–599. doi: 10.1016/S0076-6879(09)63033-0. [DOI] [PubMed] [Google Scholar]
- 59.Kapeluich YL, Rubtsova M, Egorov AM. Enhanced chemiluminescence reaction applied to the study of horseradish peroxidase stability in the course of p-iodophenol oxidation. Journal of bioluminescence and chemiluminescence. 1997;12(6):299–308. doi: 10.1002/(SICI)1099-1271(199711/12)12:6<299::AID-BIO459>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 60.Bergendahl V, Glaser BT, Burgess RR. A fast Western blot procedure improved for quantitative analysis by direct fluorescence labeling of primary antibodies. Journal of immunological methods. 2003;277(1–2):117–125. doi: 10.1016/s0022-1759(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 61.Mathews ST, Plaisance EP, Kim T. Imaging systems for westerns: chemiluminescence vs. infrared detection. Methods Mol Biol. 2009;536:499–513. doi: 10.1007/978-1-59745-542-8_51. [DOI] [PubMed] [Google Scholar]
- 62.Rustandi RR, Loughney JW, Hamm M, et al. Qualitative and quantitative evaluation of Simon, a new CE-based automated Western blot system as applied to vaccine development. Electrophoresis. 2012;33(17):2790–2797. doi: 10.1002/elps.201200095. [DOI] [PubMed] [Google Scholar]
- 63.Anderson GJ, CMC, Kennedy RT. Western blotting using capillary electrophoresis. Analytical chemistry. 2011;83(4):1350–1355. doi: 10.1021/ac102671n. [DOI] [PMC free article] [PubMed] [Google Scholar]