Abstract
The right temporoparietal junction (rTPJ) is frequently associated with different capacities that to shift attention to unexpected stimuli (reorienting of attention) and to understand others’ (false) mental state [theory of mind (ToM), typically represented by false belief tasks]. Competing hypotheses either suggest the rTPJ representing a unitary region involved in separate cognitive functions or consisting of subregions subserving distinct processes. We conducted activation likelihood estimation (ALE) meta-analyses to test these hypotheses. A conjunction analysis across ALE meta-analyses delineating regions consistently recruited by reorienting of attention and false belief studies revealed the anterior rTPJ, suggesting an overarching role of this specific region. Moreover, the anatomical difference analysis unravelled the posterior rTPJ as higher converging in false belief compared with reorienting of attention tasks. This supports the concept of an exclusive role of the posterior rTPJ in the social domain. These results were complemented by meta-analytic connectivity mapping (MACM) and resting-state functional connectivity (RSFC) analysis to investigate whole-brain connectivity patterns in task-constrained and task-free brain states. This allowed for detailing the functional separation of the anterior and posterior rTPJ. The combination of MACM and RSFC mapping showed that the posterior rTPJ has connectivity patterns with typical ToM regions, whereas the anterior part of rTPJ co-activates with the attentional network. Taken together, our data suggest that rTPJ contains two functionally fractionated subregions: while posterior rTPJ seems exclusively involved in the social domain, anterior rTPJ is involved in both, attention and ToM, conceivably indicating an attentional shifting role of this region.
Keywords: Attention, Theory of mind, ALE, MACM, RSFC
Introduction
The human temporoparietal junction (TPJ) is a supramodal association area located at the intersection of the posterior end of the superior temporal sulcus, the inferior parietal lobule, and the lateral occipital cortex. It integrates input from the lateral and posterior thalamus, as well as visual, auditory, somaesthetic, and limbic areas (Bzdok et al. 2013a; Mars et al. 2012b; Nieuwenhuys et al. 2007).
Functionally, in particular the right TPJ (rTPJ) has been associated with—at least at first sight—quite distinct cognitive processes (Corbetta et al. 2008; Decety and Lamm 2007; Mars et al. 2012b; Young et al. 2010). On the one hand, its influential role in attentional tasks, e.g. the detection of deviant stimuli in oddball paradigms (Bledowski et al. 2004; Vossel et al. 2008), has been demonstrated in many functional magnetic resonance imaging (fMRI) studies (Arrington et al. 2000; Corbetta et al. 2000; Jakobs et al. 2009; Macaluso et al. 2002). On the other hand, rTPJ has been frequently implicated in social cognition tasks, such as perspective taking or empathy (Hooker et al. 2010; Lombardo et al. 2009). Above all, it has been found to be involved in reorienting of attention and theory of mind (ToM; Corbetta et al. 2008; Decety and Lamm 2007; Mars et al. 2012b; Young et al. 2010).
Reorienting of attention is defined as the capacity to alter the focus of attention to unexpected, external stimuli while actually expecting another task/situation (rTPJ as a ‘circuit breaker’; see Corbetta et al. 2008 for a review). The ability to do so is essential for survival, as human beings have to be capable to respond quickly to unexpected events in their surroundings. Reorienting of attention has been frequently investigated using the Posner task. Cues are shown to indicate whether a target appears on the right or the left side of a screen (Fig. 1; Giessing et al. 2004; Mayer et al. 2006; Posner et al. 1980). In the original version of the Posner task, the actual target is presented on the cued side (valid trials) on 80 % of the trials, and on the remaining 20 %, the target appears on the opposite position (invalid trials). The (fMRI) contrast invalid versus valid trials depicts the reorienting effect, i.e. it displays the costs to shift attention from a cued location to an uncued one and unravels the corresponding neural mechanisms.
Fig. 1.
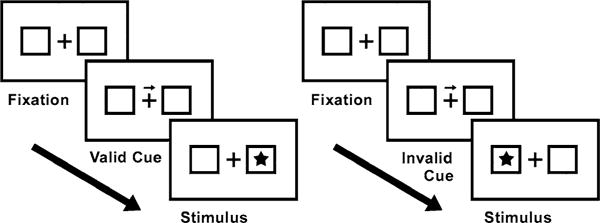
Example of a typical Posner reorienting of attention task in which cues are shown to indicate whether a target (asterisk) appears on the right or the left side of the screen (here indicated by two rectangles). A valid cue (left) is presented in 80 % of the trials, whereas an invalid cue (right) is shown in 20 % of the trials. Valid cues correctly predict the position of the target. Invalid trials include miscued targets where an asterisk suddenly appears on the opposite, uncued position. The contrast invalid versus valid trials finally reveals the reorienting effect and accordingly displays the costs it takes to externally shift attention
ToM, in contrast, is an indispensable function in successful human social relationships as it enables people to understand and predict others’ mental states (Saxe et al. 2004). ToM capacities are typically tested with the aid of false belief paradigms, such as the Sally–Ann task (Fig. 2; Wellman et al. 2001; Wimmer and Perner 1983). It belongs to the change-of-location tasks in which first Sally puts a marble into a basket. While Sally is away, Ann quickly puts the marble into another box. Then, the subjects are asked at which location Sally will search for the marble. Hence, the participants have to perform a shift in mental states and breach with their former expectation (true location) to understand Sally’s false belief of the location of the marble. This involves inhibiting their intuitive response of naming the true location.
Fig. 2.
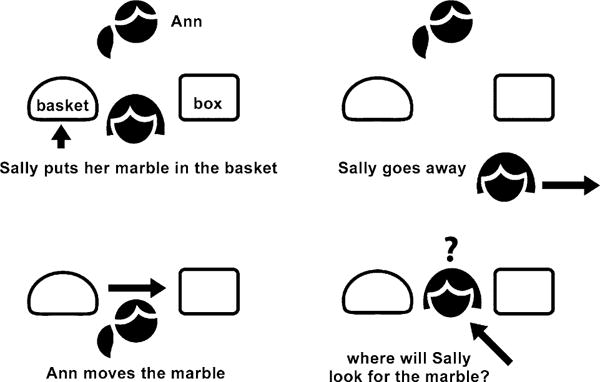
Example of a Sally–Ann task. First Sally puts a marble into a basket. While Sally is away, Ann quickly puts the marble into another box. Hereafter, subjects are asked at which location Sally will search for the marble. Hence, the participants have to perform a shift in mental states to understand Sally’s false belief of the location of the marble
Although at first glance, reorienting of attention and ToM seem to be two rather independent cognitive processes, there is also evidence for some overlap across these different domains from a developmental as well as clinical perspective. During typical development, the abilities to shift attention and to engage in joint attention with others seem to be important prerequisites for ToM development during infancy (Mundy and Newell 2007). With respect to neuropsychiatric disorders, subjects with attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) often show deficits in attentional functions and social cognition (Elsabbagh et al. 2013; Uekermann et al. 2010). Even though the previous literature primarily focused on attentional deficits in ADHD and social disturbances in ASD, more recent literature pointed out social and attentional deficits in both disorders. This suggests an interdependence of the two cognitive domains and may indicate shared neural mechanisms. However, to date, rTPJ’s involvement in attention and social cognition remains to be elucidated. This debate is further complicated by the absence of commonly accepted micro- and macroanatomical landmarks to define the exact location of this functional region, as well as by uncertainties about homologous areas in nonhuman primates (Bzdok et al. 2013a; Mars et al. 2012b, 2013).
Generally, two competing views on the role of the rTPJ have been put forward: (1) the overarching view assumes that rTPJ indeed represents a unitary region that is involved in separate functions (cf. Cabeza et al. 2012). It has been hypothesized that reorienting of attention and false belief activate the rTPJ since both functions rely on the shared phenomenon of breach of expectation (Corbetta et al. 2008) or require a ‘where-to’ function (Van Overwalle 2009). This is in line with the attention hypothesis, which poses that shifting between mental states as required in false belief tasks (or more general ToM tasks) can be interpreted in terms of attentional shifting (reorienting of attention; cf. Young et al. 2010). By contrast, (2) the fractionation view assumes that the rTPJ consists of a number of—yet to be defined—separate subregions involved in distinct cognitive processes (cf. also Cabeza et al. 2012). In line with this view, it has been demonstrated that the rTPJ can be subdivided into at least two subregions (anterior and posterior rTPJ) on the basis of its structural and functional connectivity (Bzdok et al. 2013a; Mars et al. 2012b).
To date, only few experimental studies directly examined the relative contribution of the rTPJ in attention and social cognition in order to test whether they reflect the same or different functions in relation to the attention hypothesis. So far, these fMRI studies provided conflicting results. While Mitchell (2008)—in line with a meta-analysis by Decety and Lamm (2007)—reported spatial overlap between rTPJ activations during attentional selection (reorienting of attention) and attribution of beliefs (ToM), Scholz et al. (2009) obtained contrary findings. Here clear-cut inferior–superior segregation was identified in the rTPJ by a bootstrap analysis.
Thus, to date, the debate on the role of rTPJ in reorienting of attention and false belief remains controversial. The inclusion of findings based upon relatively small sample sizes as well as variations in naming of certain brain regions and, consequently, inexact localization of neural activations may all have contributed to the heterogeneity of previous findings (Bzdok et al. 2013a). One way to overcome these limitations is to identify convergent regions of activation over multiple studies by random effect meta-analysis, using the activation likelihood estimation (ALE) method (Eickhoff et al. 2009; Turkeltaub et al. 2002). This approach allows estimating the probability at each location in the brain that a reorienting of attention or false belief study would report a focus of activity, given the underlying set of analysed neuroimaging studies. It thus identifies brain regions with statistically significant convergence across the input studies.
Recent ALE meta-analyses have already reviewed the role of the rTPJ from different perspectives (Bzdok et al. 2012; Decety and Lamm 2007; Geng and Vossel 2013; Kubit and Jack 2013). However, previous meta-analyses did not directly test the overarching versus fractionation view in association with the attention hypothesis (primarily focussing on the concepts of reorienting of attention and false belief). They included studies on agency and empathy (besides ToM) as well. In addition, in the study by Decety and Lamm (2007), the selection of studies was a priori restricted to studies demonstrating TPJ activity (primarily defined by the authors as posterior superior temporal sulcus and inferior parietal lobule/angular gyrus). This led to a biased statistical ALE analysis.
Contrary to those previous studies, we here focus on two homogeneous concepts, namely, reorienting of attention and false belief. In these experiments, the requirements for a breach of expectation (Corbetta et al. 2008) and mental state shifting (Van Overwalle 2009) in union with the attention hypothesis are maximal. As a consequence, only fMRI and PET studies that used reorienting of attention tasks—similar to the classic Posner version—or false belief experiments—resembling the classic Sally–Ann tasks—were included. If the overarching hypothesis was true, an overlap of the two cognitive tasks in the rTPJ due to their common role in state shifting would be expected. Based on this concept, it is hypothesized that both—reorienting of attention and false belief—rely on some fundamental form of switching attention either from previously expected stimuli (reorienting of attention) or mental states (false belief). In contrast, according to the fractionation hypothesis, distinct neural mechanism of false belief and reorienting of attention in the rTPJ would be predicted. To further evaluate potentially separate functional modules within the rTPJ based on the results of our ALE meta-analysis, we performed task-restricted meta-analytic connectivity mapping (MACM) and task-free resting-state functional connectivity (RSFC) analyses on the ensuing significant findings within the rTPJ.
Methods
Selection of studies
Neuroimaging experiments to be included in this current meta-analysis were obtained through PubMed (http://www.pubmed.org). Further studies were identified by review articles and reference tracing of retrieved studies. The following key words were used in order to identify relevant studies: For reorienting of attention studies: functional fMRI OR positron emission tomography (PET) AND reorienting OR orienting OR valid OR invalid OR validity OR congruent OR incongruent OR congruency OR Posner OR attention OR attention network test (ANT). For false belief studies: fMRI OR PET AND ToM OR false belief OR true belief OR perspective taking OR perspective shifting. Note, we did not use (r)TPJ as a key word in the literature search.
Additionally, only fMRI and PET studies with random effects and whole-brain group analyses were enclosed, while single-subject findings and region-of-interest analyses were excluded. Studies were included only if they reported results either in Talairach (TAL) standard reference space or Montreal Neurological Institute (MNI) coordinate space. The coordinate space was matched by transforming TAL coordinates into MNI coordinates by means of a linear transformation (Lancaster et al. 2007). All analyses were consequently performed in MNI space. Experiments addressing any pharmacological manipulation, brain lesions, mental/neurological disorders, connectivity analyses, transcranial magnetic stimulation, genetic effects, or data on children and elderly were excluded.
Several fMRI/PET studies allowed the extraction of multiple experiments. For instance, the study by Döhnel et al. (2012) comprised a false belief task versus distinct control conditions leading to various suitable experiments to be enclosed (see Tables 1, 2 for an overview of false belief and reorienting of attention experiments). Therefore, based on these studies, data from the same set of subjects entered the final ALE analysis more than once. Overall, the average number of contrasts enclosed in the ALE analysis was approximately 1.15 (54 contrasts from 47 studies). A significant influence of a single-subject population is therefore extremely unlikely. The inclusion of a high number of contrasts (total of 54) further ensured the robustness of the current ALE meta-analysis results.
Table 1.
Overview of reorienting of attention studies
| References | Contrast | Imaging method | No. of Foci | No. of participants | Modality | Type of cue |
|---|---|---|---|---|---|---|
| Arrington et al. (2000) | Invalid > valid | fMRI | 7 | 12 | Visual | Arrow |
| Chen et al. (2012) | Invalid > valid | fMRI | 9 | 20 | Visual | Arrow |
| Corbetta et al. (2000) | Invalid > valid | fMRI | 5 | 12 | Visual | Arrow |
| Corbetta et al. (2002) | Invalid > valid | fMRI | 10 | 12 | Visual | Arrow |
| Coull et al. (2000) | Invalid > valid | fMRI | 6 | 6 | Visual | Brief brightening of inner or outer circle |
| Doricchi et al. (2010) | Invalid > valid | fMRI | 6 | 24 | Visual | Arrow-like |
| Engelmann et al. (2009) | Invalid > valid | fMRI | 35 | 20 | Visual | Arrow-like |
| Giessing et al. (2004) | Invalid > valid | fMRI | 3 | 13 | Visual | Arrow-like |
| Gillebert et al. (2013) | Invalid > valid | fMRI | 6 | 16 | Visual | Arrow-like |
| Indovina and Macaluso (2004) | Invalid > valid | fMRI | 8 | 3 | Visual | Arrow |
| Indovina and Macaluso (2007) | Invalid > valid | fMRI | 12 | 12 | Visual | Arrow-like |
| Konrad et al. (2005) | Invalid > valid | fMRI | 1 | 16 | Visual | Asterisks |
| Macaluso et al. (2002) | Invalid > valid | fMRI | 2 | 8 | Visual | LED light |
| Macaluso et al. (2002) continued | Invalid > valid | fMRI | 2 | 8 | Tactile | Air-puff |
| Macaluso and Patria (2007) | Invalid > valid | fMRI | 6 | 12 | Visual | Arrow-like |
| Mattler et al. (2006) | Invalid > valid | fMRI | 17 | 12 | Visual + auditory | Red light + letters H or S |
| Mayer et al. (2006) | Invalid > valid | fMRI | 10 | 17 | Auditory | Tone pip |
| Mayer et al. (2009) | Invalid > valid | fMRI | 21 | 24 | Auditory | Tone pip |
| Mitchell (2008) | Invalid > valid | fMRI | 1 | 20 | Visual | Arrow |
| Natale et al. (2009) | Invalid > valid | fMRI | 11 | 22 | Visual | Change of colour arrowhead |
| Santangelo et al. (2008) | Invalid > valid | fMRI | 3 | 17 | Visual | Fixation cross—change into red or green |
| Thiel et al. (2004) | Invalid > valid | fMRI | 7 | 13 | Visual | Arrow-like |
| Vossel et al. (2006) | Invalid > valid | fMRI | 2 | 12 | Visual | Arrow-like |
| Vossel et al. (2008) | Invalid > valid | fMRI | 4 | 20 | Visual | Arrow-like |
| Weissman and Prado (2012) | Invalid > valid | fMRI | 9 | 14 | Visual | Arrow-like |
fMRI functional magnetic resonance imaging, TAL Talairach standard reference space, MNI Montreal Neurological Institute coordinate space
Table 2.
Overview of false belief studies
| References | Contrast | Imaging method | No. of foci | No. of participants | Study type |
|---|---|---|---|---|---|
| Aichhorn et al. (2009) | False belief > false photo | fMRI | 7 | 21 | Stories |
| Dodell-Feder et al. (2011) | False belief > false photo/map | fMRI | 16 | 62 | Stories |
| Döhnel et al. (2012) | False belief > true belief | fMRI | 9 | 18 | Cartoons |
| Döhnel et al. (2012) continued | False belief > state of reality | fMRI | 3 | 18 | Cartoons |
| Fletcher et al. (1995) | False belief > unlinked sentences | PET | 5 | 6 | Stories |
| Fletcher et al. (1995) continued | False belief > physical stories | PET | 4 | 6 | Stories |
| Gallagher et al. (2000) | False belief > physical stories | fMRI | 5 | 6 | Stories |
| Gallagher et al. (2000) continued | False belief > neutral cartoon | fMRI | 5 | 6 | Stories |
| Gobbini et al. (2007) | False belief > physical stories | fMRI | 19 | 12 | Stories |
| Hartwright et al. (2012) | False belief > false photo | fMRI | 7 | 19 | Stories |
| Hooker et al. (2008) | False belief > true belief | fMRI | 19 | 20 | Cartoons |
| Jenkins and Mitchell (2010) | False belief > preference | fMRI | 2 | 15 | Stories |
| Jimura et al. (2010) | False belief > factual aspect of false belief | fMRI | 4 | 34 | Stories |
| Kobayashi et al. (2006) | False belief > physical stories | fMRI | 5 | 16 | Stories |
| Mitchell (2008) | False belief > false photo | fMRI | 4 | 20 | Stories |
| Moran et al. (2012) | False belief > false photo | fMRI | 15 | 31 | Stories |
| Perner et al. (2006) | False belief > false photo | fMRI | 6 | 19 | Stories |
| Perner et al. (2006) continued | False belief > temporal change story | fMRI | 7 | 19 | Stories |
| Rothmayr et al. (2011) | False belief > true belief | fMRI | 16 | 12 | Cartoons |
| Samson et al. (2008) | False belief > visual pun cartoon | fMRI | 11 | 17 | Cartoons |
| Samson et al. (2008) continued | False belief > semantic cartoon | fMRI | 7 | 17 | Cartoons |
| Saxe and Kanwisher (2003) | False belief > mechanical inference | fMRI | 5 | 25 | Stories |
| Saxe and Kanwisher (2003) continued | False belief > false photo | fMRI | 5 | 21 | Stories |
| Saxe and Powell (2006) | False belief > false photo | fMRI | 9 | 12 | Stories |
| Saxe et al. (2006) | False belief > false photo | fMRI | 8 | 12 | Cartoons |
| Sommer et al. (2007) | False belief > true belief | fMRI | 8 | 16 | Cartoons |
| Van der Meer et al. (2011) | False belief > state of reality | fMRI | 23 | 19 | Movie clips |
| Young et al. (2007) | False belief > false photo | fMRI | 6 | 10 | Stories |
| Young et al. (2010) | False belief > false photo | fMRI | 6 | 17 | Stories |
fMRI functional magnetic resonance imaging, TAL Talairach standard reference space, MNI Montreal Neurological Institute coordinate space
The attentional reorienting category enclosed in the statistical meta-analysis is best represented by the Posner task contrast ‘invalid–valid’ including the essential invalidity effect. Attentional studies using the attention network task (e.g. Fan et al. 2005), oddball tasks (Bledowski et al. 2004), or studies testing the detection/translation of sensory information (Geng and Mangun 2011) were explicitly excluded as these types of tasks/studies do not involve the targeted ‘reorienting of attention’ effect. The included experiments comprised auditory, visual, haptic, or cross-sensory studies as well as diverse versions of cues (e.g. arrows; see Table 1 for an overview of all reorienting of attention studies). In studies using variable stimulus onset asynchronies (SOAs), only those with short SOAs were enclosed to minimize the danger of an accidental inclusion of an inhibition of return effect. Moreover, to ensure homogeneity, only contrasts with a minimum ratio of 1:3 (invalid vs. valid) were included, as only these contrasts lead to an invalidity (top-down) effect (Alvarez and Freides 2004). Coordinates were also extracted for three studies, which were based on a combination of a 50/50 contrast (including 50 % invalid and 50 % valid trials) and the preferable ratio. This was done as these only led to differences in intensity of the activations, but not in the locations themselves (for the ALE analyses only the location matters).
In the case of false belief studies, solely those experiments were included which contrasted a false belief condition to (variable) control conditions. The stories and cartoons highly resembled typical Sally–Ann tasks, which best encompassed the requested ‘breach of expectation’ (Corbetta et al. 2008) and ‘mental shifting’ (Van Overwalle 2009) effects. The control conditions comprised, for example, true belief attribution, false photograph stories, or unlinked sentences. In general, the inclusion of multiple false belief studies ensured that our results are not an artefact of a specific contrast, but pinpoint the concept of false belief reflected in various task combinations used in previous studies (cf. also Dodell-Feder et al. 2011). Table 2 shows an overview of all false belief and control studies.
Data analyses
A total of 25 experiments for reorienting of attention and 29 studies for false belief met all inclusion criteria for the meta-analysis. Overall, 798 subjects [note that for the reorienting of attention and false belief experiments extracted from Mitchell (2008), the very same set of subjects was measured] and 449 activation foci (3D peak coordinates) were included in the final analyses.
All analyses were accomplished using the revised ALE algorithm for coordinate-based meta-analysis neuroimaging results (Eickhoff et al. 2009, 2012; Laird et al. 2009a, b; Turkeltaub et al. 2002, 2012). The main goal of ALE is the identification of cerebral regions revealing convergences of 3D peak coordinates across contrasts, which have to be higher than expected under a random spatial association in order to be considered significant. Importantly, the peak coordinates reported in the respective papers were modelled not as single points, but as centres for 3D Gaussian probability distributions, thereby acquiring spatial uncertainty correlated with each point. The width of the 3D Gaussian probability distribution was calculated based on the between-subject and between-template variance. This was done by an algorithm weighting the between-subject variance by the number of participants per experiment, building on the assumption that larger sample sizes yield more reliable results. Therefore, these have to be modelled by smaller Gaussian distributions (Eickhoff et al. 2009).
Probability values of all foci in a particular experiment were calculated and combined for each voxel, resulting in one modelled activation (MA) map per experiment (Turkeltaub et al. 2012). The union of these MA maps across experiments was then computed, resulting in voxel-wise ALE scores reflecting the convergence of results at each location. In the next step, ALE scores were compared against a null distribution reflecting the random versus true convergence of regions across the brain. The null distribution was acquired by an analytical solution of a randomization procedure (Eickhoff et al. 2012) consisting of randomly sampling a voxel from each of the MA maps and subsequently taking the union of these values (analogously as in the true analysis).
The p value of a “true” ALE is then given by the proportion of equal or higher values obtained under the null distribution. These nonparametric p values for each meta-analysis were then thresholded at a cluster-level corrected threshold of p < 0.05 (cluster-forming threshold at voxel level p < 0.001) and transformed into Z scores for display. A Monte Carlo simulation was performed of the excursion set above a cluster-forming threshold founding on an analysis of randomly distributed foci under otherwise identical context. This simulation was implemented to gather an extent threshold to control for the cluster-level FWE rate. A final null distribution of above threshold cluster sizes was derived from 10,000 simulations of these random analyses. This distribution was then used to identify the cluster size, which was only exceeded in 5 % of all random realizations (reflecting the critical threshold for cluster-level FWE correction). Finally, a grey matter mask was applied to exclude coordinates located in deep white matter.
Individual ALE analyses reorienting of attention and false belief tasks
For the reorienting of attention contrast, 25 experiments (358 subjects and 203 foci) were chosen as a basis for the identification of regions showing convergence across reorienting of attention studies higher than expected under a random spatial association. For the false belief contrast, 29 experiments (460 subjects and 246 foci) were chosen to determine converging activation clusters. Both analyses were cluster-level family-wise error (FWE) corrected at p < 0.05.
Conjunction ALE analysis reorienting of attention and false belief tasks
Both individual meta-analyses were then combined into a conjunction analysis. The major aim of this analysis was to identify those voxels showing a significant effect in both individual analyses (reorienting of attention and false belief). The conservative minimum statistic (Nichols et al. 2005) was used to perform the conjunction analysis, which is identical to the voxel-wise minimum between two cluster-level FWE corrected results (Caspers et al. 2010). Therefore, only areas that were significant in the individual analyses were included. An additional extent threshold of k > 15 voxels was applied to exclude those regions showing incidental overlap between the ALE maps of the separate analyses.
Difference ALE analyses reorienting of attention versus false belief tasks
Difference analyses allowed identifying those areas of activation which converged significantly more for either reorienting of attention or false belief studies. This was analysed by performing individual ALE analyses for both studies and computing voxel-wise differences between the ensuing ALE maps (Eickhoff et al. 2012). In a subsequent step, all experiments were pooled and randomly divided into two groups. These artificially created groups were identical in size as the two original data sets. Consequently, ALE scores for the randomly assembled groups were calculated, and the voxel-wise difference was documented. This process was repeated 10,000 times generating an expected distribution of ALE score differences based on an exchangeability assumption. The actual “true” difference score was then contrasted against this null distribution. The final probability values were thresholded at p > 0.95 (95 % chance for true difference) and masked by the main effects of reorienting of attention and false belief.
Comparison of conjunction and difference ALE analyses
A conjunction analysis aims at identifying overlapping convergences of brain activity across both reorienting of attention and false belief experiments, whereas the main goal of the difference analyses is the identification of areas more strongly associated with one or the other task, i.e. either false belief or reorienting of attention. Therefore, contrasting reorienting of attention with false belief tasks reveals locations where a significantly stronger convergence exists among the former relative to the latter. Nevertheless, this does not preclude a significant convergence of the latter. Even in areas where significantly stronger convergence of activation is reported in reorienting of attention, the convergence reported for false belief tasks might be significant as well. Consequently, both the contrast as well as the conjunction would be significant at the very same location. Therefore, contrast and conjunction effects are not mutually exclusive, but strictly complementary (cf. Rottschy et al. 2012).
Follow-up analyses: MACM and RSFC: anterior and posterior rTPJ
In order to identify whole-brain connectivity patterns for the anterior (x = 54, y = −44, z = 18; Z = 4.61, 111 voxels) and posterior rTPJ (x = 54, y = −52, z = 26; Z = 6.5, 519 voxels; as identified in the conjunction and difference analyses, respectively, cf. results) and therefore disentangle their functional modules, MACM and RSFC were assessed (Jakobs et al. 2012; Rottschy et al. 2013). The comprehensive assessment of functional connectivity patterns of certain seed regions was given by performing a meta-analysis unravelling significant co-activation in all experiments activating the respective seed area (MACM), as well as the identification of all voxels in the human brain whose time series in a task-free/resting-state depict significant correlation with the reference time course extracted from the coherent seed. The goal of these follow-up analyses was to test the distinctness of the anterior and posterior rTPJ by different whole-brain connectivity patterns. Task-based MACM determined those regions across the entire brain that significantly co-activated with a specific seed region across a large number of functional neuroimaging experiments. For this purpose, an ALE meta-analysis was performed across all BrainMap experiments (Fox and Lancaster 2002; Laird et al. 2011, 2013; http://www.brainmap.org) comprising at least one 3D peak of activation within the clusters. Only fMRI and PET studies were included based on ‘normal mapping’ neuroimaging studies (no interventions and no group comparisons) in healthy subjects and reporting coordinates in either MNI or TAL space. The final implementation of the ALE analysis was identical to the one described in “Data analyses” section. Overall, this approach allowed determining how likely any other voxel throughout the brain was co-activated with the selected seed voxels (cFWE corrected; p < 0.05) in contrast to the respective seed region.
Task-free RSFC analysis was performed on the anterior and posterior rTPJ clusters, by which whole-brain connectivity profiles related to the selected clusters were determined by resting-state correlations. Hereby, it was possible to quantify the connectivity strength of the selected clusters with any other voxel in contrast to the second seed region. This was achieved through the calculation of correlations between spontaneous fluctuations throughout the human brain while mind wandering. The RSFC analysis was performed using the Nathan Kline Institute “Rockland” sample, which is accessible online and belongs to the International Neuroimaging Data-sharing Initiative (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). Overall, 132 healthy volunteers (78 male, 54 female) between 18 and 85 years (mean age: 42.3 ± 18.08 years) for whom 260 RSFC echo-planar imaging (EPI) volumes were collected during rest using blood-oxygen-level-dependent (BOLD) contrast [Siemens TrioTrim 3T scanner; gradient-echo EPI pulse sequence, repetition time (TR) = 2.5 s, echo time (TE) = 30 ms, flip angle = 80°, in-plane resolution = 3.0 × 3.0 mm, 38 axial slices (3.0 mm thickness) covering the entire brain].
After standard preprocessing steps using SPM8 (Well-come Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; see Bzdok et al. 2013a for details), the final time-series data were further processed to account for confounds possibly biasing voxel-wise correlations of BOLD signal time series. This was achieved by iteratively discarding variance which could be explained by nuisance variables (Fox et al. 2009; Weissenbacher et al. 2009). Thereby, BOLD signal fluctuations highly correlated with the six head motion parameters acquired by image realignment and the first derivative of the realignment parameters were removed. Furthermore, the mean grey/white matter and cerebrospinal fluid (CSF) signal per time point derived by averaging across voxels associated with the respective tissue class in the SPM 8 segmentation were discarded. Finally, these nuisance variables entered the model as first-order and second-order terms (Jakobs et al. 2012; cf. Satterthwaite et al. 2013 for an evaluation of this framework). Data were band-pass filtered removing frequencies outside the 0.01–0.08 Hz range (cf. Biswal et al. 1995; Fox and Raichle 2007). The time-course correlations (after temporal preprocessing) between every individual seed voxel with any other voxel throughout the brain were calculated. The final correlation values were then transformed into Fisher’s Z scores and documented in a connectivity matrix. Here, we contrasted whole-brain connectivity estimates computed for the anterior rTPJ and posterior rTPJ (and vice versa), which allowed to determine those regions in the whole brain showing significantly higher connectivities with the one or the other seed region.
Finally, to delineate those areas showing both task-dependent and task-independent functional connectivity with the anterior and posterior rTPJ in contrast to each other, a conjunction analysis on MACM and RSFC results was carried out (Jakobs et al. 2012; Rottschy et al. 2013) by the strict minimum statistic (cf. above). Hence, those regions co-activated with the seed clusters were outlined by the computation of the intersection of the connectivity maps derived by MACM and RSFC. Hereby, networks of cerebral regions correlated with the anterior versus posterior rTPJ were identified that are robustly connected in both task-related (MACM) but also task-free (RSFC) brain states. Furthermore, a conjunction analysis across anterior and posterior rTPJ (across MACM and RSFC) was performed identifying those regions showing a significant effect in both individual connectivity analyses (see “Conjunction ALE analysis reorienting of attention and false belief tasks” section for a description of the conjunction procedure).
Labelling
Clusters were anatomically labelled with respect to their most probable macroanatomical and cytoarchitectonic location using the Anatomy Toolbox (Eickhoff et al. 2005, 2007), an SPM plugin (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Foci of activations were attributed to their most likely histological cerebral region at their corresponding location by recourse to a maximum probability (MA) Map from the Jülich brain atlas (Amunts et al. 1999; Caspers et al. 2006; Geyer 2004; Gitelman et al. 1999). Note, however, that the rTPJ has not yet been mapped cytoarchitectonally, making it difficult to apply neuroanatomically well-defined labels (Bzdok et al. 2013a; Mars et al. 2012b; Mitchell 2008). Final peak maxima are presented in MNI space.
Results
Individual ALE analyses reorienting of attention and false belief tasks
The ALE meta-analysis of 25 reorienting of attention experiments unravelled consistent activations (ordered from biggest to smallest cluster) in the rTPJ, the right inferior frontal junction (cf. Brass et al. 2005 for a labelling of the inferior frontal junction), bilateral inferior parietal lobule, bilateral precuneus, and the right insula lobe.
The ALE meta-analysis of 29 false belief experiments revealed the rTPJ, lTPJ, bilateral superior medial gyrus, left posterior cingulate cortex, and bilateral middle temporal gyrus as converging clusters. Table 3 presents the results for the peak maxima identified by the individual ALE analyses on reorienting of attention and false belief tasks.
Table 3.
Peak activations for individual activation likelihood estimation meta-analyses on reorienting of attention and false belief
| Macroanatomical location | Cluster size | x | y | z |
|---|---|---|---|---|
| Reorienting of attention | ||||
| Right temporoparietal junction | 627 | 62 | −44 | 12 |
| Right inferior frontal junction | 260 | 46 | 16 | 28 |
| Right inferior parietal lobule | 253 | 44 | −40 | 52 |
| Left inferior parietal lobule | 228 | −36 | −46 | 46 |
| Right precuneus | 196 | 6 | −60 | 52 |
| Left precuneus | 191 | −6 | −70 | 42 |
| Right insula lobe | 88 | 44 | 18 | −4 |
| False belief | ||||
| Right temporoparietal junction | 519 | 54 | −52 | 26 |
| Left temporoparietal junction | 479 | −52 | −58 | 24 |
| Bilateral superior medial gyrus | 404 | −2 | 56 | 28 |
| 4 | 60 | 16 | ||
| Left posterior cingulate cortex | 393 | −2 | −56 | 38 |
| Right middle temporal gyrus | 227 | 54 | −18 | −14 |
| Left middle temporal gyrus | 149 | 60 | −26 | −8 |
Montreal Neurological Institute coordinate space; family-wise error corrected p < 0.05; extent threshold = 10 voxels
Conjunction ALE analysis reorienting of attention and false belief tasks
The conjunction analysis showed the anterior rTPJ (x = 54, y = −44, z = 18; Z = 4.61, 111 voxels) as the only brain region consistently activated across reorienting of attention and false belief tasks. Figure 3 presents the result of the conjunction analysis. Note that we did not restrict a priori our ALE analyses to TPJ, but entered all maxima across the whole brain.
Fig. 3.
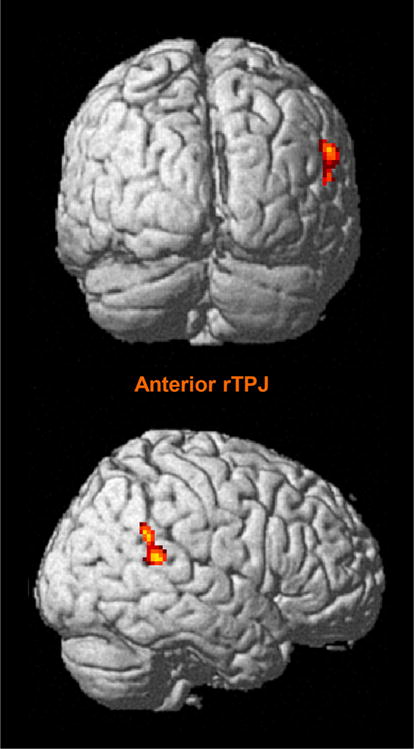
Cerebral region identified in conjunction analysis across reorienting of attention and false belief studies. Family-wise error corrected p < 0.05; extent threshold = 10 voxels; anterior rTPJ anterior right temporoparietal junction
Difference ALE analyses reorienting of attention versus false belief tasks
Reorienting of attention in contrast to false belief tasks revealed stronger convergence for reorienting of attention in the bilateral inferior parietal lobule and right inferior frontal junction. For false belief tasks in contrast to reorienting of attention, the lTPJ, bilateral superior medial gyrus, left precuneus, posterior rTPJ, and bilateral middle temporal gyrus were identified. Table 4 and Fig. 4 show the results of the difference analyses.
Table 4.
Peak activations for difference analyses reorienting of attention versus false belief
| Macroanatomical location | Cluster size | x | y | z |
|---|---|---|---|---|
| Subtraction reorienting of attention—false belief | ||||
| Right inferior parietal lobule | 247 | 42 | −48 | 59 |
| Right inferior frontal junction | 233 | 50 | 8 | 33 |
| Left Inferior Parietal Lobule | 194 | −36 | −52 | 49 |
| Subtraction false belief—reorienting of attention | ||||
| Left temporoparietal junction | 429 | −52 | −62 | 29 |
| Bilateral superior medial gyrus | 391 | 0 | 54 | 19 |
| Left precuneus | 338 | −2 | −58 | 41 |
| Posterior right temporoparietal junction | 292 | 54 | −58 | 27 |
| Left middle temporal gyrus | 147 | −60 | −28 | −3 |
Montreal Neurological Institute coordinate space; uncorrected p < 0.001; extent threshold = 10 voxels
Fig. 4.
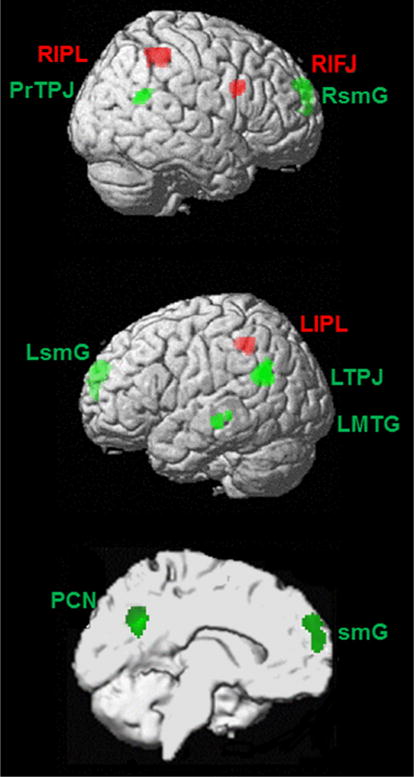
Neural areas identified in difference analyses reorienting of attention (red) and false belief (green). Findings are uncorrected p < 0.001; extent threshold = 10 voxels; LIPL left inferior parietal lobule, RIPL right inferior parietal lobule, RIFJ right inferior frontal junction, LsmG left superior medial gyrus, RsmG right superior medial gyrus, LMTG left middle temporal gyrus, LTPJ left temporoparietal junction, PrTPJ posterior right temporoparietal junction, PCN Precuneus
MACM and RSFC analyses: anterior and posterior rTPJ
Combined MACM and RSFC analyses on the anterior and posterior rTPJ unravelled connectivity patterns for the two regions (see Supplementary Material for individual results of MACM and RSFC difference and conjunction analyses). The anterior rTPJ in contrast to the posterior rTPJ co-activated more significantly (ordered in cluster size) with the anterior lTPJ, right inferior frontal gyrus, bilateral middle cingulate cortex, left insula lobe, right precuneus, and right inferior parietal lobule (Table 5; Fig. 5). On the contrary, the posterior rTPJ compared with the anterior rTPJ showed connectivity to bilateral precuneus, posterior lTPJ, and right middle temporal gyrus (Table 5; Fig. 5). The identification of converging connectivity between the anterior and posterior rTPJ did not reveal common regions besides the rTPJ itself (including the anterior and posterior subregions; x = 54, y = −48, z = 22; Z = 8.41, 1044 voxels).
Table 5.
Peak activations of connectivity regions associated with the anterior rTPJ and posterior rTPJ
| Macroanatomical location | Cluster size | x | y | z |
|---|---|---|---|---|
| Anterior right temporoparietal junction | ||||
| Anterior right temporoparietal junction | 863 | 58 | −44 | 14 |
| Anterior left temporoparietal junction | 346 | −58 | −42 | 32 |
| Right inferior frontal gyrus | 346 | 46 | 24 | −2 |
| 60 | 44 | 34 | 0 | |
| Bilateral middle cingulate cortex | 246 | 4 | 2 | 52 |
| −4 | 8 | 40 | ||
| Left insula lobe | 142 | −38 | 12 | 2 |
| Right precuneus | 84 | 8 | −68 | 56 |
| Right inferior parietal lobule | 33 | 48 | −44 | 56 |
| Posterior right temporoparietal junction | ||||
| Posterior right temporoparietal junction | 925 | 56 | −60 | 15 |
| Bilateral precuneus | 347 | −6 | −58 | 45 |
| 6 | −62 | 33 | ||
| Posterior left temporoparietal junction | 169 | −52 | −68 | 45 |
| Right middle temporal gyrus | 94 | 54 | −20 | −5 |
Montreal Neurological Institute coordinate space; family-wise error corrected p < 0.05; extent threshold = 10 voxels
Fig. 5.
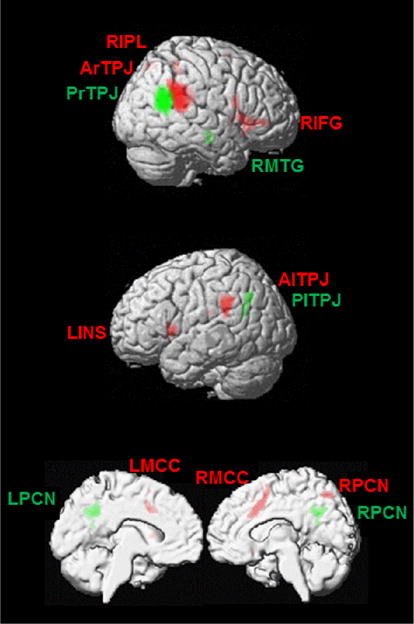
Distinct connectivity patterns for anterior (red) and posterior (green) rTPJ based on a combination of task-related meta-analytic connectivity mapping analysis and task-free resting-state functional connectivity analysis. Family-wise error corrected p < 0.05; extent threshold = 10 voxels; LPCN left precuneus, RPCN right precuneus, RIFG right inferior frontal gyrus, RMTG right middle temporal gyrus, PlTPJ posterior left temporoparietal junction, PrTPJ posterior right temporoparietal junction, AlTPJ anterior left temporoparietal junction, ArTPJ anterior right temporoparietal junction, RIPL right inferior parietal lobule, LINS left insula lobe, LMCC left middle cingulate cortex, RMCC right middle cingulate cortex
Discussion
In the following discussion, we will examine present theories on the overlap of reorienting of attention and false belief in the anterior rTPJ and the specific involvement of the posterior rTPJ in false belief in contrast to reorienting of attention. Additionally, connectivity patterns of the anterior and posterior rTPJ as identified by task-restricted MACM and task-free RSFC analyses will be discussed. These findings gathered by ALE and connectivity analyses will be further interpreted in a more global context considering overarching versus fractionated roles of the rTPJ. Finally, the clinical relevance of this meta-analysis will be highlighted with a special focus on ADHD and ASD.
Shared cerebral region of reorienting of attention and false belief
The conjunction ALE meta-analysis including reorienting of attention and false belief tasks demonstrated an overlap in the anterior rTPJ. This was identified by the voxel-wise conjunction analysis of the two individual ALE meta-analyses. Hereby, only the anterior rTPJ significantly converged for both separate ALE analyses across 54 studies. Therefore, the results support the overarching view for this region. Although it has been questioned that reorienting of attention is processed on the same abstract and verbal level as ToM stimuli and hence draws upon the same neural resources (Young et al. 2010), our data suggest the rTPJ is involved in both attention and social interaction. This is at odds with the results of Young et al. (2010) who did not identify an interaction in the rTPJ in a combined reorienting of attention-ToM task. Presumably, because their inclusion of ‘higher-level’ attention stimuli (unexpected/expected endings in verbal stories reflecting reorienting of attention) did not activate the rTPJ as ‘low-level’ reorienting of attention stimuli (as the classic Posner task) would have done. These common mechanism of ‘low-level’ reorienting of attention and false belief might be explained by Corbetta’s breach of expectation (attention shifting), or alternatively Overwalle’s concept of ‘where-to shifting’ (Corbetta et al. 2008; Van Overwalle 2009). Both focus on the capacity to shift attention between states. For reorienting of attention, an attentional shifting capacity is necessary after an invalid cue. For false belief, the very same capacity is needed during mental state shifting to further understand a false belief. Our ALE meta-analysis data can thus be interpreted in the light of the attention hypothesis indicating that false belief is not feasible without successful attentional processes.
Alternatively, one might interpret these findings in the following way: false belief capabilities are needed for successful attention shifting (cf. Mitchell 2008). A reorienting of attention task would then be based on a participant’s trial-by-trial formation of a new belief about the upcoming target. The cost of an invalid cue would therefore represent the shifting or breach of that former (false) belief. A dispute against this link is that other attentional tasks do activate the rTPJ as well, such as attentional capture tasks using distractors in the same colour as the targets (Serences et al. 2005). In these tasks, one might quite certainly exclude an involvement of a (false) belief formation. Another argument against this interpretation is that lesions in (or near) the rTPJ commonly lead to attentional deficits but not social problems. This is consistent with the assumption of a more general role of attention in the right parietal lobe (Mesulam 1981). Taken together, it seems that the attention hypothesis, where attentional shifting is fundamental for false belief formation, best describes the overlap of reorienting of attention and false belief in anterior rTPJ.
Beyond interpreting these findings in the light of attentional shifting, one might also reconsider the role of the anterior rTPJ in the context of a more domain-global process, such as neural prediction coding (Bzdok et al. 2013a; Koster-Hale and Saxe 2013). Basically, predictive coding can be seen as a ‘neural’ attempt to decrease computational load. This can be achieved by reducing neural responses to predictable input and increasing neural activity to unexpected input (Jakobs et al. 2009). Therefore, incoming information is compared against (learned) expectations rather than being analysed as a new, unknown stimulus. The anterior rTPJ might thus be involved in the working memory formation of predictions about the location of targets (reorienting of attention) and about person’s beliefs (ToM; cf. Koster-Hale and Saxe 2013). Heightened activity in the rTPJ might in consequence be related to the unexpectedness of the input, such as an invalid cue or a question about someone’s false belief. This recognition of an unexpected pattern might further lead to an increase in attention towards these unpredicted stimuli and thus trigger attentional shifting. Therefore, it could be possible that the concept of predictive coding is fundamental to attentional shifting. This renders these two hypotheses not mutually exclusive, but rather complementary.
The further identification of the posterior rTPJ significantly converging for false belief in contrast to reorienting of attention (in the difference analysis) led to various speculations about the actual distinctness of these subregions: (1) the anterior and posterior rTPJ could indeed represent two functionally distinct subregions of the rTPJ or (2) they reflect transition zones between attentional and social processes. Unfortunately, to date, neuroimaging research lacks an agreement regarding which amount of spatial separation between two clusters justifies the conclusion that these reflect distinct neural activations (Decety and Lamm 2007). In fact, the two peak activation convergences in the anterior (conjunction analysis) and posterior rTPJ (difference analysis) were 16.64 mm apart from each other. This basically already hints towards a relatively clear separation. To thus further unravel these divergent findings (conjunction vs difference analyses) and identify the distinctness/transition between these areas, we additionally performed post hoc MACM and RSFC. This allowed testing for separate functional connectivity patterns of the two regions in contrast to each other. Moreover, by the intersection of regionally specific architecture and connectivity patterns, a more precise attribution to a certain functional specialization was feasible (Eickhoff and Grefkes 2011). Regions identified in the conjunction analysis over MACM and RSFC co-activations can be regarded as robustly connected with the respective seed regions because of the incorporation in similar networks and the (potential) engagement by the same cognitive functions. Connectivity pattern results gained by task-restricted MACM and task-free RSFC finally indicated distinct connectivity patterns. This supports the assumption that these two foci indeed reflect activations of separate neural networks and do not represent transition zones. The anterior rTPJ seed region in contrast to the posterior rTPJ seed area featured co-activations to a ventral attention network (Corbetta and Shulman 2002). Thus, MACM and RSFC analyses identified the right inferior frontal gyrus, middle cingulate cortex, left insula lobe, right precuneus, and right inferior parietal lobule to co-activate significantly with the anterior rTPJ compared with the posterior rTPJ. Similar connectivity profiles were found in parcellation studies performed by Bzdok et al. (2013a) or Mars et al. (2012b; see supplementary material for a visualization of peak coordinates assigned to the anterior and posterior rTPJ by Bzdok et al. (2013a), Mars et al. (2012b), and the current meta-analysis). Anterior rTPJ activity seemed to significantly increase in alliance with a midcingulate–motor–insular network and was attributed to attentional functions by network mapping and functional forward/reverse inference analyses (Bzdok et al. 2013a). In particular, pain perception, tactile-attentive tasks, action execution, and motor control had been identified with an anterior rTPJ network by functional profiling. Similarly, Mars et al. (2012b) unravelled the anterior rTPJ to be significantly interacting with ventral prefrontal cortex and anterior insula.
The idea of the ventral attention network conceptualizes the rTPJ as a core region of this mostly right-lateralized frontoparietal network (Corbetta and Shulman 2002). It has also been shown that this ventral network reacts to sudden changes not only in visual stimulation, but also in response to auditory and tactile stimuli (Downar et al. 2000). By the inclusion of multiple modalities in reorienting of attention studies in the current ALE meta-analysis, this cross-modality connection is identified too.
Altogether, it seems that the significant co-activation between the anterior rTPJ and the ventral attention network when compared with the posterior rTPJ represents attentional shifting. Strong connectivities to the ventral attention network/saliency network regions (Corbetta and Shulman 2002; Sridharan et al. 2008) seem to facilitate the allocation of attention to behaviourally relevant stimuli. Thereby, they interrupt automated, predicted routines needed for both reorienting of attention and false belief tasks. On the contrary, the posterior rTPJ compared with the anterior rTPJ showed a significant connectivity pattern with a social cognition network. This implies two separate roles for these subregions identified by task-dependent MACM and task-independent RSFC. Therefore, interpreting the ALE and connectivity results from a more systematic perspective, one might rather clearly advocate for a fractionated role of the rTPJ on the whole. The overlap of reorienting of attention and false belief tasks in the anterior part of the rTPJ does not contradict a fractionated view of the rTPJ as tasks are attributable to a common, unique cognitive function: attentional shifting. The connectivity analyses performed in this study enabled investigating this functional overlap through assessing whether certain neural regions are differentially interacting and thereby adding essential information to standard fMRI activations (Eickhoff and Grefkes 2011). It allowed delineation of brain regions not based on activation in a certain task or their structural properties but on their functional coupling properties across distances with either the anterior or posterior rTPJ. Thereby, it enabled generalization beyond the task set (reorienting of attention and false belief) and tested whether the association is steadily expressed over numerous tasks (MACM) or whether it can be reflected in a task-free state as well. The final results depicting separate connectivity profiles for anterior and posterior parts of the rTPJ thus clearly point to a fractionation in the rTPJ into an anterior and posterior part. It seems that the anterior rTPJ and its connected regions aid attentional shifting, while the posterior rTPJ network enables social interaction capacities. The posterior rTPJ and its connectivities are further discussed below.
Specific neural mechanism of false belief
Difference analysis on false belief versus reorienting of attention revealed the lTPJ, superior medial gyrus, left precuneus, middle temporal gyrus, and the posterior rTPJ as showing significantly higher convergence for the false belief task. This specific neural activity pattern related to false belief in contrast to reorienting of attention has also been repeatedly associated with a more global role of recognizing and processing of others’ mental states (Gallagher and Frith 2003; Van Overwalle 2009). It is in consonance with other ToM meta-analyses (Bzdok et al. 2012; Decety and Lamm 2007). In particular, the involvement of bilateral TPJ is repeatedly shown in ToM neuroimaging studies, in contrast to attentional experiments. In attentional shifting studies primarily the rTPJ has been found, but for ToM the lTPJ seems to be specifically involved in verbal compared with nonverbal ToM tasks (Saxe and Kanwisher 2003). In line with a more influential role of the lTPJ in social interaction compared with attention, lesion studies showed that damage to this region frequently results in a belief-reasoning impairment (Apperly et al. 2004). RTPJ damage, on the contrary, results more often in attentional difficulties (Bultitude et al. 2009).
In the context of our MACM and RSFC analyses delineating the differential connectivity of the anterior and posterior rTPJ, a connectivity network for the posterior rTPJ seed region in contrast to the anterior seed was unravelled. The identified correlations with the precuneus, posterior lTPJ, and right middle temporal gyrus have been repeatedly linked to the social cognition network (Bzdok et al. 2013a, b; Mars et al. 2012b; Schilbach et al. 2012). In a connectivity-based subdivision study, it was shown that posterior rTPJ interacted with posterior cingulate, temporal pole, and anterior medial prefrontal cortex (Mars et al. 2012b). Bzdok et al. (2013a) identified that activity in the posterior rTPJ significantly increased with a parietal network which was further functionally decoded to be associated with explicit (notably episodic) memory retrieval, semantic discrimination, social cognition, and theory of mind tasks. Overall, the identified connectivity profile of the posterior rTPJ appears to resemble results from previous studies, especially considering the activations in the precuneus and middle temporal gyrus.
Basically, it has been suggested that the function of the posterior rTPJ network is particularly related to a more internally oriented network, also relating to the default mode network reflecting stimulus-independent mental processes (Bzdok et al. 2013a; Kubit and Jack 2013; Mars et al. 2012a). In general, this network has been identified not only in ToM tasks, but also in other higher social processes, such as perspective taking (e.g. Ruby and Decety 2004; Vogeley et al. 2004), empathy (e.g. Jackson et al. 2006), or agency (see a review by Sperduti et al. 2011), pointing towards a more (domain-) global role of this region in ToM abilities.
Overall, it thus seems that the posterior rTPJ—as a subregion of the rTPJ—shows stronger convergence for false belief in contrast to reorienting of attention as suggested by our difference analysis. It also is an essential part of the social cognition network linked to multiple aspects of ToM as reflected in our MACM and RSFC findings.
Clinical relevance
Social interaction and attention are commonly affected across different neurodevelopmental disorders, such as in ADHD and ASD (Alvarez and Freides 2004; Korkmaz 2011; Paynter and Peterson 2010). ADHD is typically characterized by difficulties of inattention or hyperactivity or impulsiveness. ASD is marked by social and communication deficits, stereotyped or repetitive behaviours and interests (American Psychiatric Association 2000). Although diagnostic criteria for the two disorders show little overlap, both disorders frequently co-occur. Attention problems, social-cognitive deficits and emotion regulation problems are frequently observed in both ADHD and ASD (see Rommelse et al. 2011 for a review). In addition to behavioural overlap between ADHD and ASD patients, there is increasing evidence for shared genetic (Simonoff et al. 2008), neurocognitive, and brain dysfunction (Brieber et al. 2007). For example, Uekermann et al. (2010) demonstrated in a meta-analysis that ADHD is associated with social cognition impairments, especially face and prosody perception. In addition, a recent meta-analysis on visual orienting in autism (focussing on Posner-type tasks) demonstrated that subjects with ASD indeed showed a small impairment in reorienting of attention compared with controls (effect size across studies: d′ = 0.44; Landry and Parker 2013). In line with this, Elsabbagh et al. (2013) unravelled an association between anomalies in disengagement of attention in a high-risk group of children (having a sibling with ASD) and ASD at the age of 3 years. Polderman et al. (2013) demonstrated that the link between attention problems (attentional shifting difficulties) and autistic traits in adults could be explained by a shared genetic factor, as assessed in a population-based twin-sibling design. On the neural level, recent studies also suggest that children and adolescents with ADHD and ASD share some cortical and subcortical abnormal brain mechanisms. These are reflected in voxel-wise network centrality abnormalities in the precuneus (Di Martino et al. 2013) or task-related (sustained attention task) deviations in bilateral striato-thalamic regions, left dorsolateral PFC, superior parietal cortex, precuneus, and DMN suppression (Christakou et al. 2013). Furthermore, also grey matter reductions in the left medial temporal lobe and higher grey matter volumes in the left inferior parietal cortex were observed in ADHD and ASD adolescents compared with healthy controls (Brieber et al. 2007). Thus, shared neural network dysfunction might be present in both groups of patients. Reconsidering these findings in the context of the results gathered in the present meta-analysis, an interesting question for future studies is the involvement of the anterior rTPJ in attentional and social interaction difficulties in patients with ADHD and ASD. As there is a huge lack in neuroimaging studies focusing on reorienting of attention and false belief tasks in ADHD and ASD, it is currently infeasible to extend our ALE analyses to clinical groups. However, hopefully, our ideas on a potential linkage between attention and social cognitive processes assigned to the rTPJ leads to more neuroimaging studies investigating potential difficulties in these patient groups.
Limitations
There are some limitations of the present meta-analysis that should be considered. Unfortunately, the ALE analysis does not include information on cluster and effect sizes (Decety and Lamm 2007). Additionally, the usage of peak coordinates might lead to mis- or over-interpretation of the results. Nonetheless, up-to-date ALE meta-analyses are as advanced as possible since statistical meta-analyses based on original data sets are not yet available. Only, when an agreement between researchers on data sharing has been achieved, meta-analyses with original data might be possible (Mar 2011).
Furthermore, the MACM and RSFC connectivity analyses imply some limitations regarding functional coupling with specific seed regions. For the MACM, the origin of the ensuing activation maps greatly differs from other connectivity approaches as this method does not rely on time series in the strict sense or any particular neuroimaging experiment (Eickhoff and Grefkes 2011). Rather, functional connectivity is reflected by congruency in co-activation probability across a large quantity of diverse experiments, not across time, which complements existing approaches of functional connectivity analyses. For the RSFC analysis, fMRI time series are obtained while participants are scanned in the absence of an externally structured task set. It has been recently shown that conclusions drawn on functional properties merely based on resting-state connectivity results should be interpreted carefully (Rehme et al. 2013). This is because resting-state and task-based connectivity reflect distinct components of functional integration depending on the functional state of the scanned subjects (reflection of inner mental states or reaction to external tasks, respectively; Rehme et al. 2013). Therefore, independent functional connectivity approaches such as dynamic causal modelling might add important information on the actual functional involvement of the anterior and posterior rTPJ networks in reorienting of attention and false belief tasks.
Conclusion
The current (ALE) meta-analysis resolves important aspects of the debate about the role of the rTPJ in attention and social interaction. The anterior rTPJ was identified as a single converging zone for reorienting of attention and false belief, which supports the overarching hypothesis (Cabeza et al. 2012). Findings are consistent with the concept of an attentional shifting capacity in this region as previously proposed by Corbetta et al. (2008). ToM abilities may therefore be rooted on attentional shifting. This potential link is also reflected in developmental studies showing a strong association between early attentional capacities and later social cognition abilities (Rothbart et al. 2011; Mundy and Newell 2007).
On the other hand, posterior rTPJ was identified by a difference (ALE) analysis as significantly converging for false belief in contrast to reorienting of attention studies. This provides support for the fractionation view postulating that independent regions within the rTPJ support distinct cognitive functions (Cabeza et al. 2012). It seems that besides the importance of an attentional shifting capacity in false belief, linked to the anterior part of the rTPJ, the posterior part is more specifically involved in false belief compared with reorienting of attention (Bzdok et al. 2013a; Mars et al. 2012b).
MACM and RSFC were further performed to identify the differences in functional connectivity between the anterior and posterior rTPJ subregions. The anterior rTPJ in contrast to the posterior rTPJ showed connectivity to regions previously identified as part of a ventral attention network (Corbetta et al. 2008) associated with shifting of attention to behaviourally relevant stimuli. In contrast, the posterior rTPJ compared with the anterior rTPJ co-activated with a social network more globally involved in ToM-like abilities (Jackson et al. 2006; Ruby and Decety 2004). As MACM and RSFC are capable of adding essential information on the underlying connectivity of different regions aiding specific cognitive functions, it was possible to identify these two clearly functionally fractionated regions in the rTPJ: the anterior rTPJ involved in attentional shifting and the posterior rTPJ involved in social interaction capacities. Importantly, our ALE conjunction result identifying an overlap of reorienting of attention and false belief tasks in the anterior rTPJ is not contradicting the fractionated interpretation of our findings, as our connectivity analyses clearly suggest a contribution of this region to a role in attentional shifting.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-014-0803-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
S. C. Krall, Email: s.krall@fz-juelich.de, Institute of Neuroscience and Medicine (INM-3), Jülich Research Center, Jülich, Germany; Department of Child and Adolescent Psychiatry, University Hospital Aachen, Aachen, Germany.
C. Rottschy, Institute of Neuroscience and Medicine (INM-1), Jülich Research Center, Jülich, Germany Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany.
E. Oberwelland, Institute of Neuroscience and Medicine (INM-3), Jülich Research Center, Jülich, Germany Department of Child and Adolescent Psychiatry, University Hospital Aachen, Aachen, Germany.
D. Bzdok, Institute of Neuroscience and Medicine (INM-1), Jülich Research Center, Jülich, Germany Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany.
P. T. Fox, Research Imaging Institute, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA South Texas Veterans Administration Medical Center, San Antonio, Texas, USA.
S. B. Eickhoff, Institute of Neuroscience and Medicine (INM-1), Jülich Research Center, Jülich, Germany Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany.
G. R. Fink, Institute of Neuroscience and Medicine (INM-3), Jülich Research Center, Jülich, Germany Department of Neurology, University Hospital Cologne, Cologne, Germany.
K. Konrad, Institute of Neuroscience and Medicine (INM-3), Jülich Research Center, Jülich, Germany Department of Child and Adolescent Psychiatry, University Hospital Aachen, Aachen, Germany.
References
- Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G. Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J Cogn Neurosci. 2009;21:1179–1192. doi: 10.1162/jocn.2009.21082. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Freides D. Research on attention deficit hyperactivity disorder using the covert orienting paradigm of Posner. Dev Neuropsychol. 2004;26:627–645. doi: 10.1207/s15326942dn2602_6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington: 2000. [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comput Neurosci. 1999;412:319–341. doi: 10.1002/(SICI)1096-9861(19990920)412:2<319:AID-CNE10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Chiavarino C, Humphreys GW. Frontal and temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. J Cogn Neurosci. 2004;16:1773–1784. doi: 10.1162/0898929042947928. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DEJ. Attentional systems in target and distractor processing: a combined ERP and fMRI study. NeuroImage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Fink GR, Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatr. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Bultitude JH, Rafal RD, List A. Prism adaption reverses the local processing bias in patients with right temporo-parietal junction lesions. Brain. 2009;132:1669–1677. doi: 10.1093/brain/awp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann D, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci. 2013a;7:1–17. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation complementary connectivity analyses, and functional decoding. NeuroImage. 2013b;17:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of activation observation and imitation in the human brain. NeuroImage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Weidner R, Vossel S, Weiss PH, Fink GR. Neural mechanisms of attentional reorienting in three-dimensional space. J Neurosci. 2012;32:13352–13362. doi: 10.1523/JNEUROSCI.1772-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, Daly E, Ecker C, Robertson D, MRC AIMS consortium. Murphy DG, Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Mol Psychiatr. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Büchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/S0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neurosci. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, Rodman J, Lord C, Castellanos FX, Milham MP. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. NeuroImage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Döhnel K, Schuwerk T, Meinhardt J, Sodian B, Hajak G, Sommer M. Functional activity of the right temporo-parietal junction and of the medial prefrontal cortex associated with true and false belief reasoning. NeuroImage. 2012;60:1652–1661. doi: 10.1016/j.neuroimage.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Macci E, Silvetti M, Macaluso E. Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the Posner task. Cereb Cortex. 2010;20:1574–1585. doi: 10.1093/cercor/bhp215. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin EEG Neurosci. 2011;42:107–121. doi: 10.1177/155005941104200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbas MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36:1325–1335. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Webb SJ, Dawson J, Charman T, Johnson MH, The British Autism Study of Infant Siblings Team Disengagement of visual attention in infancy is associated with emerging Autism in toddlerhood. Biol Psychiat. 2013;74:189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 2009;3:1–17. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-R. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nat Rev Neurosci. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/S1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Mangun GR. Right temporoparietal junction activation by a salient contextual cue facilitates target discrimination. NeuroImage. 2011;54:594–601. doi: 10.1016/j.neuroimage.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cel. 2004;174:1–89. doi: 10.1007/978-3-642-18910-4_1. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Stephan KE, Rösler F, Fink GR. Visuospatial attention: how to measure effects of infrequent, unattended events in a blocked stimulus design. NeuroImage. 2004;23:1370–1381. doi: 10.1016/j.neuroimage.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Mantini D, Peeters R, Dupont P, Vandenberghe R. Cytoarchitectonic mapping of attentional selection and reorienting in parietal cortex. NeuroImage. 2013;67:257–272. doi: 10.1016/j.neuroimage.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Hartwright CE, Apperly IA, Hansen PC. Multiple roles for executive control in belief-desire reasoning: distinct neural networks are recruited for self perspective inhibition and complexity of reasoning. NeuroImage. 2012;61:921–930. doi: 10.1016/j.neuroimage.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. SCAN. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Macaluso E. Occipital–parietal interactions during shifts of exogenous visuospatial attention: trial-dependent changes of effective connectivity. MRI. 2004;22:1477–1486. doi: 10.1016/j.mri.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Indovina I, Macaluso E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb Cortex. 2007;17:1701–1711. doi: 10.1093/cercor/bhl081. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imaging how I feel versus how you feel pain. Neuropsycholgia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. NeuroImage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. NeuroImage. 2009;47:667–677. doi: 10.1016/j.neuroimage.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Mentalizing under uncertainty: dissociated neural responses to ambiguous and unambiguous mental state inference. Cereb Cortex. 2010;20:404–410. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Asari T, Miyashita Y. Temporal pole activity during understanding other persons’ mental states correlates with neuroticism trait. Brain Res. 2010;1328:104–112. doi: 10.1016/j.brainres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Cultural and linguistic influence on neural bases of ‘theory of mind’: an fMRI study with Japanese bilinguals. Brain Lang. 2006;98:210–220. doi: 10.1016/j.bandl.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR. Development of attentional networks: an fMRI study with children and adults. NeuroImage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Korkmaz B. Theory of mind and neurodevelopmental disorders of childhood. Pediatr Res. 2011;69:101R–108R. doi: 10.1203/PDR.0b013e318212c177. [DOI] [PubMed] [Google Scholar]
- Koster-Hale J, Saxe R. Theory of mind: a neural prediction problem. Neuron. 2013;79:836–848. doi: 10.1016/j.neuron.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubit B, Jack AI. Rethinking the role of the rTPJ in attention and social cognition in light of the opposing domains hypothesis: findings from an ALE-based meta-analysis and resting-state functional connectivity. Front Hum Neurosci. 2013;7:1–18. doi: 10.3389/fnhum.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:1–9. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009a;3:1–11. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. NeuroImage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analysed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry O, Parker A. A meta-analysis of visual orienting in autism. Front Hum Neurosci. 2013;7:1–12. doi: 10.3389/fnhum.2013.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, MRC AIMS Consortium. Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2009;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Supramodal effects of covert spatial orienting triggered by visual or tactile events. J Cogn Neurosci. 2002;14:389–401. doi: 10.1162/089892902317361912. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Patria F. Spatial re-orienting of visual attention along the horizontal or the vertical axis. Exp Brain Res. 2007;180:23–34. doi: 10.1007/s00221-006-0841-8. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012a;6:1–9. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Neubert F-X, Rushworth MFS. Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. Proc Natl Acad Sci USA. 2013;110:10806–10811. doi: 10.1073/pnas.1302956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS. Connectivity-based subdivisions of the right “temporo-parietal junction area”: evidence for different areas participating in different networks. Cereb Cortex. 2012b;22:1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Mattler U, Wüstenberg T, Heinze H-J. Common modules for processing invalidly cued events in the human cortex. Brain Res. 2006;1109:128–141. doi: 10.1016/j.brainres.2006.06.051. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Harrington DL. Neuronal modulation of auditory attention by informative and uninformative spatial cues. Hum Brain Mapp. 2009;30:1652–1666. doi: 10.1002/hbm.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Harrington D, Adair JC, Lee R. The neural networks underlying endogenous auditory covert orienting and reorienting. NeuroImage. 2006;30:938–949. doi: 10.1016/j.neuroimage.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal is not selective for theory of mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. J Neurosci. 2012;32:5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Newell L. Attention, joint attention, and social cognition. Curr Dir Psychol Sci. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale E, Marzi CA, Macaluso E. FMRI correlates of visuo-spatial reorienting investigated with an attention shifting doublecue paradigm. Hum Brain Mapp. 2009;30:2367–2381. doi: 10.1002/hbm.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system: a synopsis and atlas. 4th. Springer; Berlin: 2007. [Google Scholar]
- Paynter J, Peterson C. Language and ToM development in autism versus Asperger syndrome: contrasting influences of syntactic versus lexical/semantic maturity. Res Autism Spectr Dis. 2010;4:377–385. doi: 10.1016/j.rasd.2009.10.005. [DOI] [Google Scholar]
- Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G. Thinking of mental and other representations: The roles of the left and right temporo-parietal junction. Soc Neurosci. 2006;1:245–258. doi: 10.1080/17470910600989896. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Hoekstra RA, Vinkhuyzen AA, Sullivan PF, van der Sluis S, Posthuma D. Attentional switching forms a genetic link between attention problems and autistic traits in adults. Psychol Med. 2013;43:1985–1996. doi: 10.1017/S0033291712002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. doi: 10.1037//0096-3445.109.2.160. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Grefkes C. State-dependent differences between functional and effective connectivity of the human cortical motor system. NeuroImage. 2013;63:237–246. doi: 10.1016/j.neuroimage.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing mechanisms of self-regulation in early life. Emot Rev. 2011;3:207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmayr C, Sodian B, Hajak G, Döhnel K, Meinhardt J, Sommer M. Common and distinct neural networks for false-belief reasoning and inhibitory control. NeuroImage. 2011;56:1705–1713. doi: 10.1016/j.neuroimage.2010.12.052. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Kleiman A, Dogan I, Lagner R, Mirzazade S, Kronen-buerger M, Werner C, Shah NJ, Schulz JB, Eickhoff SB, Reetz K. Diminished activation of motor working-memory networks in Parkinson’s disease. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0061786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emtoions. J Cogn Neurosci. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Samson AC, Zysset S, Huber O. Cognitive humor processing: different logical mechanisms in nonverbal cartoons—an fMRI study. Soc Neurosci. 2008;3:125–140. doi: 10.1080/17470910701745858. [DOI] [PubMed] [Google Scholar]
- Santangelo V, Belardinelli MO, Spence C, Macaluso E. Interactions between voluntary and stimulus-driven spatial attention mechanisms across sensory modalities. J Cogn Neurosci. 2008;21:2384–2397. doi: 10.1162/jocn.2008.21178. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in ‘theory of mind’. NeuroImage. 2003;19:1835–1842. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: dissociating theory of mind and executive control in the brain. Soc Neurosci. 2006;1:284–298. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social and unconstrained cognition. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4:1–7. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sommer M, Döhnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G. Neural correlates of true and false belief reasoning. NeuroImage. 2007;35:1378–1384. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct. 2011;216:151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. NeuroImage. 2004;21:318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analysis. Hum Brrain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann BG, Hebebrand J, Daum I, Wiltfang J, Kis B. Social cognition in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobahav Rev. 2010;34:734–743. doi: 10.1016/j.neubiorev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Van der Meer L, Groenewold NA, Nolen WA, Pijnenborg M, Aleman A. Inhibit yourself and understand the other: neural basis of distinct processes underlying theory of mind. NeuroImage. 2011;56:2364–2374. doi: 10.1016/j.neuroimage.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. NeuroImage. 2006;32:1257–1264. doi: 10.1016/j.neuroimage.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Vossel S, Weidner R, Thiel CM, Fink GR. What is “odd” in Posner’s location-cueing paradigm? Neural responses to unexpected location and feature changes compared. J Cogn Neurosci. 2008;21:30–41. doi: 10.1162/jocn.2009.21003. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Prado J. Heightened activity in a key region of the ventral attention network is linked to reduced activity in a key region of the dorsal attention network during unexpected shifts of covert visual spatial attention. NeuroImage. 2012;61:798–804. doi: 10.1016/j.neuroimage.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001;72:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0022-0965(85)90051-7. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. PNAS. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dodell-Feder D, Saxe R. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia. 2010;48:2658–2664. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


