Abstract
Purpose of review
To review recent clinical and epidemiological studies regarding the prevention, diagnosis, and treatment of trachoma.
Recent Findings
Newer studies propose novel diagnostic tests that appear sensitive for the detection of ocular chlamydial infection. Immunologic studies suggest that chronic inflammation can lead to progressive scarring, even in the absence of chlamydia. Confocal microscopy can obtain accurate grading of scarring progression. Mass oral azithromycin distributions remain a mainstay of treatment; studies have assessed the appropriate frequency and duration of treatment programs. Current studies have also explored ancillary effects of azithromycin distribution on mortality and bacterial infections.
Summary
Trachoma programs have had remarkable success at reducing chlamydial infection and clinical signs of trachoma. Recent work suggests improved methods to monitor infection and scarring, and better ways to distribute treatment. While studies continue to demonstrate reduction in infection in hyperendemic areas, more work will need to be done to achieve elimination of this blinding disease.
Keywords: trachoma, chlamydia, neglected tropical disease, azithromycin, confocal microscopy
Introduction
Trachoma is the leading infectious cause of blindness worldwide, resulting from recurrent infection with ocular strains of Chlamydia trachomatis.[1-3]. While once widespread in Europe and the Americas, its significant decline by the end of the 20th century was attributed to improved socioeconomic conditions and hygiene. The World Health Organization (WHO) estimates that 40 million people continue to be affected by trachoma, and that 1.2 billion people worldwide live in endemic areas. Estimates from India and China could influence these calculations, but information from these countries is limited.[2] The economic burden is estimated to be $3-8 billion is lost in productivity annually, and the disease burden an estimated 1.3 million disability-adjusted life years.[4,5]
Recurrent inflammation of the eyelids leads to a cascade of conjunctival scarring, entropion, and inturned eyelashes (trichiasis) scratch the cornea further putting those affected at risk for secondary ulcers and eventually blindness.[2] Many feel that the prevention of blindness from trachoma may require more than just surgical campaigns and mass distribution of antibiotics, and that if underlying environmental factors that facilitate its transmission are not identified, reinfection and its clinical sequelae may continue.
The WHO Global Alliance for the Global Elimination of Trachoma (GET 2020) promotes implementation of the SAFE strategy: Surgery for trichiasis, Antibiotic distribution, Facial cleanliness, and Environmental improvements.[6,7] Current WHO recommendations include mass treatment with a single dose of azithromycin to those over 6 months of age in areas where follicular trachoma prevalence is 10% or greater in children 1-9 years of age.[7,8]
Recent studies provide new insight into the diagnosis and treatment of trachoma: immunopathologic studies further elucidate etiology and clinical sequelae; new diagnostic tests such as confocal microscopy are supplementing current diagnostic criteria; mass treatment strategies are proposing effective methods for antibiotic distribution; and studies exploring the external benefits of trachoma control programs show promising effects in regards to the reduction of childhood mortality and systemic bacterial infections.
Etiology and Immunopathology of Clinical Signs
A challenge with control is that chlamydia has evolved mechanisms to evade host immune responses through intracellular invasion. Recent studies have suggested that pathways leading to clinical sequelae are related to inflammation rather than infection by C. trachomatis itself. An early stage involves formation of lymphoid follicles--the clinical hallmark of active trachomatous inflammation (Figure 1).[3,7,9] These follicles represent subepithelial collections of lymphoid cells with parafollicular infiltrates dominated by polymorphonuclear leucocytes, macrophages, dendritic cells, plasma cells, and B and T cells. [10] If severe enough, papillary hypertrophy obscures the deep tarsal vessels and vascular infiltrates appear in the upper cornea (pannus). Pathognomonic limbal follicles can leave shallow depressions after resolution (Herbert’s pits).
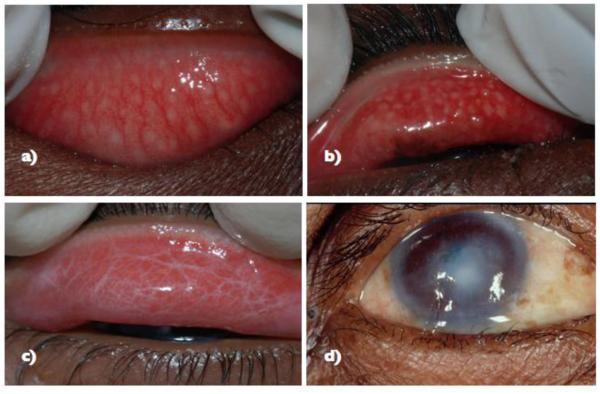
Figure 1.
Clinical features of Trachoma Based on the WHO clinical grading scheme9
a) Follicular Trachoma (FT) with follicles > 0.5mm
b) Active trachoma characterized by TF and intense trachoma
c) Tarsal congunctival scarring (trachomatous scarring, TS)
d) Corneal opacification (CO) over the pupil with trachomatous trichiasis
Blindness from trachoma occurs from conjunctival scarring which is attributed to recurrent infection. Subtarsal conjunctival scarring leave fibrotic bands in which scar contraction leads to entropion, trichiasis, and eventually corneal opacification.[11] Scar-tissue formation can result in loss of meibomian glands and degeneration of the tarsal plate. Dry eye can further compromise host immune responses at the ocular surface.[11,12]
New studies have demonstrated that host immunity plays a significant role in disease severity. Clinical signs are only poorly associated with documented infection.[13-15] New studies explore the relationship between adaptive immune responses and scarring, TH1 pro-inflammatory responses, TH2 role in fibrosis and induction of anti-inflammatory responses through IL-10, and MMP associations with degradation of collagen and consequent chemotactic properties). [16,17]. The presumption is that increased chemokine responses lead to neutrophil activation, chemotaxis, and fibrosis. Thus, molecules such as MMPs and chemokines provide a link between inflammation and fibrosis in immunpathological consequences of trachoma. Previously, scarring complications were often ascribed to adaptive immune responses, since adults have consistently lower bacterial loads than those of children and experience short duration of infection, presumably as a result of acquired immune responses.[7,18,19]
Until recently, there has been limited information regarding fibrogenic pathways in the cicatricial stage preceding tissue damage. It has been more difficult to assess the relationship between repeated infection and progressive scaring. Burton et al. [16] studied conjunctival gene expression profiles among Ethiopians and found that trichiasis cases demonstrated ongoing inflammation and tissue remodeling relative to controls. In a study in The Gambia, active trachoma was associated with increased expression of profibrotic cytokines and IL17A, which is proinflammatory in both innate and adaptive immune responses.[20].
Other nonchlamydial pathogens have been are associated with clinical signs of trachoma. [17] In Tanzania, there was no association between clinically active follicular trachoma (TF) and infection, but there was a more than a four-fold relationship between TF and bacterial pathogens, such as Streptococcus pneumoniae and Haemophilus influenzae.[21] Innate proinflammatory responses (psoriasin, interleukin-1β, tumor necrosis factor alpha, defensin-β4A, chemokine ligand 5, and serum amyloid A1) were associated with nonchlamydial infection.[22] Thus proinflammatory states, possibly unrelated to chlamydial infection, can lead to the immunofibrogenic pathways and conjunctival scarring.
Conclusion
Early vaccine trials in the 1960s were inconclusive, short-lived, and possibly associated with more severe disease.[23-25] Recently, Kari et al. [26] reported that live-attenuated vaccination in cynomolgus macaques with a plasma-deficient strain of a C. trachomatis produced an anti-chlamydial immune response without production of inflammatory ocular pathology. Thus, the plasmid may play a key role in virulence, and vaccination could generate a protective immune response without induction of pro-inflammatory processes. However, upon virulent challenge, the group found no difference in ocular pathology between vaccinated and control groups, indicating that the introduction of a plasmid strain and low levels of bacterial growth are enough to sustain inflammation. Further studies of human models and the role of the plasmid in the induction of inflammation need to be performed.
Diagnosis & Treatment
Since clinical signs of trachoma are poorly correlated with actual evidence of infection, diagnostic criteria that are both sensitive and specific for infection can be further developed. The weak correlation between infection and clinical signs may be in part due to a latent phase (infection without clinical signs) and long recovery phase (with persistant clinical signs) [7,15,27]. Munoz et al. [28] found that high-risk clinical signs were not predictive of infection in communities after multiple rounds of mass drug administration (MDA). Stare et al. [29] found that in an area of The Gambia with a 6% prevalence of follicular trachoma in children, the actual rate of infection detected by nucleic acid amplification tests (NAATs) was <1%. Recent studies continue to find that actual rates of clinical signs in children are higher than actual infection rates. [30, 31].
Accurate estimates of infection are not only important in the determination of reliable estimates for prevalence, but are also necessary to ensure that treatment control programs are cost-effective. Infection has been studied using several nucleic acid tests, most commonly with DNA-based NAATs. However, rRNA-based NAATs appear more sensitive, with comparable specificities. [27,32-34]. Latent class analysis suggests that clinical signs may be relatively sensitive, but that NAATs are far more specific. [33] Point-of-care (POC) assays of chlamydial lipopolysacchrides have yet to be found reliable in hot, humid environments [30,35].
A novel diagnostic approach to visualizing pathological changes in trachoma involve the use of in vivo confocal microscopy (IVCM). Hu et al. [36*] have applied IVCM for grading inflammatory and scarring changes. The group characterized scarring by inflammatory infiltrate density, tissue edema, and presence of dendritic cells, with good inter-observer agreement. [37] The grading system represents a novel approach to characterizing pathologic changes seen in trachoma.
In regards to treatment strategies, WHO recommends MDA in communities where follicular trachoma is >10% in children. The reemergence of infection after 1-2 rounds in hyperendemic communities has been demonstrated.[38,39] Important questions in MDA programs include how long it is necessary to treat communities and how high coverage needs to be in order to achieve elimination.
West et al., [40*] evaluated 71 previously hyper-endemic communities in Tanzania after 3-7 years of annual treatment, where MDA coverage was >50%. They found: i) a linear relationship between prevalence of clinical trachoma and years of treatment; ii) greater than 7 years of annual treatment would be needed to reach a prevalence of <5%; and iii) no communities after 3 years of treatment had achieved the WHO target of <5% prevalence of follicular trachoma. This suggest that in hyperendemic regions the effect of consecutive, long-term programs are still required.
It seems likely that the frequency of treatment may also influence the rate of elimination. Gebre et al.[41*] compared the effect of annual versus biannual mass distribution of azithromycin in 24 Ethiopian subdistricts where coverage was >80% and prevalence of infection was near 40% at baseline. The prevalence of infection was similar at 42-month follow up. However, elimination was more rapid in biannually treated groups with prevalence of infection lower at 12, 24, & 36 months in the biannually treated groups. In comparison to West et al. [40], this study population had higher antibiotic coverage (>80% vs >50%). Given that biannual treatment may expedite elimination efforts, current and future studies can explore the frequency, duration of treatment, and coverage estimates needed to achieve elimination as they relate to prevalence in endemic areas.
Determination of effective community-based strategies is integral to ensure efficient elimination efforts. Recent studies have demonstrated that after multiple rounds of treatment the following are not associated with more infection: living with an untreated infant (azithromycin is not approved for children<6 months)[42], non-participation[43], household density, and lack of antibiotic use in the past 3 months.[44] Ayele et al. [44] found that infection was associated with missing prior antibiotic treatments, having a sibling with ocular chlamydia, and living in a larger community. Studies such as these provide insight into why targeted strategies are reasonable in endemic communities.
Azithromycin has been the drug of choice for trachoma due to its safety profile in children, efficacy as a single oral dose (20 mg/1kg), long half-life in tissues, and efficacy against intra-cellular bacteria such as C. trachomatis. Although tetracyclines can be used to treat infection, oral use is contraindicated in children, and poor compliance has been demonstrated with topical tetracycline ointment.[45] Erythromycin, requires a longer course, and sulfonamides are associated with more severe side effects such as Stevens-Johnson syndrome. [1]
The majority of azithromycin distribution studies report no serious adverse events [41]. In a cluster-randomized clinical trial in Ethiopia, two surveillance rounds reported adverse events in 4.9-7.0% of children, with complaints primarily of abdominal pain and vomiting.[46] These rates are consistent to western populations taking single high dose azithromycin.[47]
Surgery to avert complications from trichiasis and environmental improvements to prevent transmission have been extensively studied. Bilamellar tarsal rotation has demonstrated mixed outcomes.[48] Surgery can clearly correct trichiasis and prevent blinding complications.[6,48,49] Yet, coverage in programs has been low, attrition of surgeons has been consistently high,[50] acceptance of surgery can be poor, and recurrence can be frequent.[51]
The WHO currently warrants tarsal rotation surgery for individuals with a single trichiatic lash. However, new studies suggest that not all trichiasis is associated with clinically significant entropion. Rajak et al [52] studied 2,256 individuals with trachomatous trichiasis (TT) in Ethiopia and found that over half presented with little evidence of entropion. Rather, trichiasis was attributed often to metaplastic or misdirected eyelashes. The same group found that epilation was associated with less central corneal opacity, suggesting that this may be a viable option for mild TT cases and individuals with barriers to acceptance of surgery.[53] In a large randomized controlled trial, Rajak et al. found no evidence that use of absorbable sutures was associated with a lower prevalence of trichiasis recurrence than silk sutures. [54*]
The challenges in surgical interventions can be ascribed to the need for sufficient and sustainable health systems in workforce training and in management of infrastructure. Habtamu et al. [50] found that high attrition of surgeons and low surgical productivity at outreach campaigns were associated with lack of support, shortage of consumables, and equipment. Fifty-nine percent of surgeons who received extensive training had switched jobs during study. Thus, health systems infrastructure and management of supply chains could be further examined in future studies.
Beyond Trachoma: Positive Externalities and Integrated Studies
While antibiotic resistance has been demonstrated in previous studies, mathematical models using longitudinal data from trachoma trials have described that such resistance tends to drop after distribution is stopped, even with an initial rise in resistance. Maher et al.[55] specifically examined the fitness cost of antibiotic resistance in S. pneumoniae, and found that antibiotic resistance has a selective disadvantage in the absence of antimicrobial drugs of approximately 12-14%.
Despite the risk of adverse events and concerns regarding antibiotic resistance associated with antibiotic research, new studies have explored additive benefits trachoma control programs. Previously, azithromycin has been shown to be effective against upper respiratory infections, diarrhea, and malaria.[56,57] Recent studies have suggested associations with mortality prevention, diarrhea, and acute lower respiratory infection.[57-59] In a cohort of 35,502 individuals > 1 and 5,507 children ages 1-5 in 24 communities in Ethiopia, Keenan et al. [58*] found that all-cause mortality (OR=0.35, 95% CI=0.17-0.74) and infectious childhood mortality (OR=0.20, 95% CI=0.07-0.58) were associated with oral azithromycin treatment, even when compared to members of the same household who had not been treated. Coles et al. [59] studied 1,036 children in 8 rural communities in Tanzania and found that a single dose of azithromycin reduced the risk of acute lower respiratory infection (ALRI) by 38%. Notably, S. pneumoniae, H. Influenzae b, and S. aureus are common causes of ALRI in sub-saharan Africa, where greater than half of ALRI mortalities occur. [60,61] In the same cohort, the group found that treatment was also associated with 39% and 24% percent lower risk of diarrhea at 0-1 and 1-3 months follow-up, respectively.[62] Overall, ancillary effects of oral azithromycin may outweigh the risks associated with adverse events and antibiotic resistance. Future studies may focus on integrated monitoring and treatment strategies in order to understand how mass antibiotic administration can be optimized in communities.
Environmental and Social Studies
The WHO’s goal is to control infection to a low enough level that resulting blindness is not a public health concern. Recent evidence from the Gambia has shown that infection in once hyper-endemic areas has dropped to well below 1%. [29] Infection rates have dramatically fallen over the past decade in hyperendemic areas. For example, a survey of 14 villages in Northern Sudan estimated a drop in prevalence of clinical signs from 47% to to 5-9% in 11 districts.[63] Similarly, a recent national survey described that the Sichuan province in China has also seen dramatic reduction in prevalence.[64] However, elimination efforts have been slower in remote Aboriginal communities.[65]
Mathematical models suggest that complete elimination of infection is possible, perhaps even more possible than previously thought. A mathematical model [66] demonstrated that large-scale elimination may be an achievable goal in hyper-endemic regions, even with incomplete coverage. Return of infection is sufficiently slow that surveillance could be effective in stopping re-emergence.[67,68]
In a cross-sectional study of 8358 children aged 1-9 years across Ethiopia, Roba et al. [69] found that all indicators for the SAFE strategy, except for surgery, showed a statistically significant effect. Other recent studies have shown risk factors such as water, flies, lack of latrines, face washing less than once per day, and lack of access to safe water sources (within 30 minutes of walking distance) to be associated with active clinical trachoma. [7,69-72]. Studies have associated some of the following risk factors clinically active trachoma: living in indigenous, mobile communities[73], isolated households whose distance is > 1400 meters from social gathering sites[74], and hot temperatures[75].Contrary to previous findings, new studies in Ethiopia were unable to demonstrate an association between latrine promotion and infection or mortality. [76,77].
Conclusion
As trachoma elimination nears, research will require more accurate diagnosis of infection and monitoring of clinical sequelae (Table 1). NAATs and confocal microscopy can assist in accurate diagnosis of infection and scarring, respectively. Recent research suggests ancillary benefits from mass antibiotic distributions, including possible reductions in infectious diseases and even childhood mortality. Integrating trachoma programs with other neglected tropical diseases may prove cost-effective, since close to 95% of drug distribution costs have been attributed to recurring costs (personnel, travel, etc.)[78]. However, overlap between diseases would need to be considered to ensure effective integration.[75,79] Perhaps the most important finding in the past few years is that trachoma elimination is possible, even in areas that had previously been severely affected.
Table 1.
Selection of recent trachoma studies
| Studies | Findings | Implications | |
|---|---|---|---|
| Etiology of clinical sequelae | |||
| Immunopathology | Burton et al.16
Burton et al.20 |
|
|
| Non-chlamydial pathogens | Hu et al.17
Burton et al.21 |
|
|
| Diagnosis & Monitoring | |||
| Clinical signs | Munoz et al.28
Stare et al.29 Michel et al.30 |
|
|
| Confocal microscopy | Hu et al.36, 37 |
|
|
| RNA-based nucleic acid amplification tests (NAATs) |
Keenan et al.32 |
|
|
| Point-of-care tests | Michel et al.
30
Hardin-Esch et al.35 |
|
|
| Treatment Strategies | |||
| Frequency and length of antibiotic distribution |
West et al.40
Gebre et al.41 |
|
|
| Surgery | Rajak et al.52-54
Habtamu et al.50 |
|
|
| Ancillary benefits of antibiotic programs |
Keenan et al.58
Coles et al.59 Coles et al.62 |
|
|
Key Points.
Nonchlamydial bacterial pathogens have been closely associated with clinical signs of trachoma, suggesting that fibrogenic pathways may be a factor of both adaptive and innate immune responses.
Clinical signs may be present despite no identification of C. trachomatis, suggesting that complications may be a consequence of chronic inflammation rather than direct infection.
In vivo confocal microscopy has been used to propose a grading system for trachoma based on inflammatory and scarring changes.
Mass single-dose oral azithromycin treatment remains the WHO-recommended treatment in districts with greater than 10% prevalence of active clinical signs in children ages 1-9 years; at least 3 years of annual treatment is needed in moderately endemic areas. Biannual treatment may expedite elimination efforts.
Trachoma programs have been associated with reduction in mortality, diarrhea, and upper respiratory infections.
Acknowledgments
Acknowledgements: None
Footnotes
Conflicts of interest: The authors have no personal or financial conflicts to report for this review.
References
- 1.West S. Trachoma and antibiotic use: the “A” in SAFE. Expert Rev Anti Infectv Ther. 2012;10:75–83. doi: 10.1586/eri.11.150. [DOI] [PubMed] [Google Scholar]
- 2.Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–568. doi: 10.1136/bjo.2008.148494. [DOI] [PubMed] [Google Scholar]
- 3.Wright HR, Turner A, Taylor HR. Trachoma. Lancet. 2008;371:1945–1954. doi: 10.1016/S0140-6736(08)60836-3. [DOI] [PubMed] [Google Scholar]
- 4.Kumaresan J, Mecaskey J. The global elimination of blinding trachoma: Progress and promise. American Journal of Tropical Medicine and Hygiene. 2003:24–28. doi: 10.4269/ajtmh.2003.69.24. [DOI] [PubMed] [Google Scholar]
- 5.Frick KD, Hanson CL, Jacobson GA. Global burden of trachoma and economics of the disease. Am. J. Trop. Med. Hyg. 2003;69:1–10. doi: 10.4269/ajtmh.2003.69.5_suppl_1.0690001. [DOI] [PubMed] [Google Scholar]
- 6.Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3:372–381. doi: 10.1016/s1473-3099(03)00659-5. [DOI] [PubMed] [Google Scholar]
- 7.Hu VH, Harding-Esch EM, Burton MJ, Bailey RL, Kadimpeul J, Mabey DCW. Epidemiology and control of trachoma: systematic review. Trop. Med. Int. Health. 2010;15:673–691. doi: 10.1111/j.1365-3156.2010.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack S, Brooker S, Kuper H, Mariotti S, Mabey D, Foster A. Mapping the global distribution of trachoma. Bull. World Health Organ. 2005;83:913–919. [PMC free article] [PubMed] [Google Scholar]
- 9.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bulletin of the World Health Organization. 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 10.Abu el-Asrar AM, Geboes K, Missotten L. Immunology of trachomatous conjunctivitis. Bull Soc Belge Ophtalmol. 2001 [no volume] [PubMed] [Google Scholar]
- 11.Guzey M, Ozardali I, Basar E, Aslan G, Satici A, Karadede S. A survey of trachoma: the histopathology and the mechanism of progressive cicatrization of eyelid tissues. Ophthalmologica. 2000;214:277–284. doi: 10.1159/000027504. [DOI] [PubMed] [Google Scholar]
- 12.Guzey M, Satici A, Karaman SK, Ordulu F, Sezer S. The effect of topical cyclosporine A treatment on corneal thickness in patients with trachomatous dry eye. Clin Exp Optom. 2009;92:349–355. doi: 10.1111/j.1444-0938.2009.00369.x. [DOI] [PubMed] [Google Scholar]
- 13.Burton M, Kinteh F, Jallow O, Sillah A, Bah M, Faye M, Aryee E, Ikumapayi U, Alexander N, Adegbola R, et al. A randomised controlled trial of azithromycin following surgery for trachomatous trichiasis in the Gambia. Br J Ophthalmol. 2005;89:1282–1288. doi: 10.1136/bjo.2004.062489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lietman TM, Dawson CR, Osaki SY, Zegans ME. Clinically active trachoma versus actual Chlamydial infection. Med. J. Aust. 2000;172:93–94. doi: 10.5694/j.1326-5377.2000.tb139214.x. [DOI] [PubMed] [Google Scholar]
- 15.Bird M, Dawson CR, Schachter JS, Miao Y, Shama A, Osman A, Bassem A, Lietman TM. Does the diagnosis of trachoma adequately identify ocular chlamydial infection in trachoma-endemic areas? J. Infect. Dis. 2003;187:1669–1673. doi: 10.1086/374743. [DOI] [PubMed] [Google Scholar]
- 16.Burton MJ, Rajak SN, Bauer J, Weiss HA, Tolbert SB, Shoo A, Habtamu E, Manjurano A, Emerson PM, Mabey DCW, et al. Conjunctival transcriptome in scarring trachoma. Infect. Immun. 2011;79:499–511. doi: 10.1128/IAI.00888-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu VH, Massae P, Weiss HA, Chevallier C, Onyango JJ, Afwamba IA, Mabey DCW, Bailey RL, Burton MJ. Bacterial infection in scarring trachoma. Invest. Ophthalmol. Vis. Sci. 2011;52:2181–2186. doi: 10.1167/iovs.10-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassly NC, Ward ME, Ferris S, Mabey DC, Bailey RL. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Negl Trop Dis. 2008;2:e341. doi: 10.1371/journal.pntd.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey R, Duong T, Carpenter R, Whittle H, Mabey D. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiology and Infection. 1999:479–486. doi: 10.1017/s0950268899003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton MJ, Ramadhani A, Weiss HA, Hu V, Massae P, Burr SE, Shangali W, Holland MJ, Mabey DCW, Bailey RL. Active trachoma is associated with increased conjunctival expression of IL17A and profibrotic cytokines. Infect. Immun. 2011;79:4977–4983. doi: 10.1128/IAI.05718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton MJ, Hu VH, Massae P, Burr SE, Chevallier C, Afwamba IA, Courtright P, Weiss HA, Mabey DCW, Bailey RL. What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Invest. Ophthalmol. Vis. Sci. 2011;52:6012–6017. doi: 10.1167/iovs.11-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu VH, Weiss HA, Ramadhani AM, Tolbert SB, Massae P, Mabey DCW, Holland MJ, Bailey RL, Burton MJ. Innate immune responses and modified extracellular matrix regulation characterize bacterial infection and cellular/connective tissue changes in scarring trachoma. Infect. Immun. 2012;80:121–130. doi: 10.1128/IAI.05965-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayston JT, Wang SP, Woolridge RL, Alexander ER. Prevention of trachoma with vaccine. Arch. Environ. Health. 1964;8:518–526. doi: 10.1080/00039896.1964.10663711. [DOI] [PubMed] [Google Scholar]
- 24.Wang SP, Grayston JT. Pannus with experimental trachoma and inclusion conjunctivitis agent infection of Taiwan monkeys. Am. J. Ophthalmol. 1967;63(Suppl):1133–45. doi: 10.1016/0002-9394(67)94095-0. [DOI] [PubMed] [Google Scholar]
- 25.Wand SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am. J. Ophthalmol. 1967;63(Suppl):1615–30. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 26.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J. Exp. Med. 2011;208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan JD, Lakew T, Alemayehu W, Melese M, House JI, Acharya NR, Porco TC, Gaynor BD, Lietman TM. Slow Resolution of Clinically Active Trachoma Following Successful Mass Antibiotic Treatments. Arch Ophthalmol-Chic. 2011;129:512–513. doi: 10.1001/archophthalmol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz B, Stare D, Mkocha H, Gaydos C, Quinn T, West SK. Can clinical signs of trachoma be used after multiple rounds of mass antibiotic treatment to indicate infection? Invest. Ophthalmol. Vis. Sci. 2011;52:8806–8810. doi: 10.1167/iovs.11-8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stare D, Harding-Esch E, Munoz B, Bailey R, Mabey D, Holland M, Gaydos C, West S, Group OBOPS Design and Baseline Data of a Randomized Trial to Evaluate Coverage and Frequency of Mass Treatment with Azithromycin: The Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiol. 18:20–19. doi: 10.3109/09286586.2010.545500. [DOI] [PubMed] [Google Scholar]
- 30.Michel C-EC, Roper KG, Divena MA, Lee HH, Taylor HR. Correlation of clinical trachoma and infection in Aboriginal communities. PLoS Negl Trop Dis. 2011;5:e986. doi: 10.1371/journal.pntd.0000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon AW, Foster A, Mabey DCW. Clinical examination versus Chlamydia trachomatis assays to guide antibiotic use in trachoma control programmes. Lancet Infect Dis. 2006;6:5–6. doi: 10.1016/S1473-3099(05)70304-2. author reply 7–8. [DOI] [PubMed] [Google Scholar]
- 32.Keenan JD, Ayele B, Gebre T, Moncada J, Stoller NE, Zhou Z, Porco TC, McCulloch CE, Gaynor BD, Emerson PM, et al. Ribosomal RNA Evidence of Ocular Chlamydia trachomatis Infection Following 3 Annual Mass Azithromycin Distributions in Communities With Highly Prevalent Trachoma. Clin. Infect. Dis. 2012;54:253–256. doi: 10.1093/cid/cir791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.See CW, Alemayehu W, Melese M, Zhou Z, Porco TC, Shiboski S, Gaynor BD, Eng J, Keenan JD, Lietman TM. How reliable are tests for trachoma?--a latent class approach. Invest. Ophthalmol. Vis. Sci. 2011;52:6133–6137. doi: 10.1167/iovs.11-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JL, Schachter J, Moncada J, Habte D, Zerihun M, House JI, Zhou Z, Hong KC, Maxey K, Gaynor BD, et al. Comparison of an rRNA-based and DNA-based nucleic acid amplification test for the detection of Chlamydia trachomatis in trachoma. Br J Ophthalmol. 2007;91:293–295. doi: 10.1136/bjo.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding-Esch EM, Holland MJ, Schémann J-F, Molina S, Sarr I, Andreasen AA, Roberts CH, Sillah A, Sarr B, Harding EF, et al. Diagnostic accuracy of a prototype point-of-care test for ocular Chlamydia trachomatis under field conditions in The Gambia and Senegal. PLoS Negl Trop Dis. 2011;5:e1234. doi: 10.1371/journal.pntd.0001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu VH, Massae P, Weiss HA, Cree IA, Courtright P, Mabey DCW, Bailey RL, Burton MJ. In vivo confocal microscopy of trachoma in relation to normal tarsal conjunctiva. Ophthalmology. 2011;118:747–754. doi: 10.1016/j.ophtha.2010.08.029. * This study proposes a grading system to characterize conjunctival scarring and inflammation, representing the first time confocal microscopy has been applied to trachoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu VH, Weiss HA, Massae P, Courtright P, Makupa W, Mabey DCW, Bailey RL, Burton MJ. In vivo confocal microscopy in scarring trachoma. Ophthalmology. 2011;118:2138–2146. doi: 10.1016/j.ophtha.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West S, Munoz B, Mkocha H, Holland M, Aguirre A, Solomon A, Foster A, Bailey R, Mabey D. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet. 2005;366:1296–1300. doi: 10.1016/S0140-6736(05)67529-0. [DOI] [PubMed] [Google Scholar]
- 39.Burton M, Holland M, Makalo P, Aryee E, Alexander N, Sillah A, Faal H, West S, Foster A, Johnson G, et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet. 2005;365:1321–1328. doi: 10.1016/S0140-6736(05)61029-X. [DOI] [PubMed] [Google Scholar]
- 40.West SK, Munoz B, Mkocha H, Gaydos CA, Quinn TC. Number of Years of Annual Mass Treatment With Azithromycin Needed to Control Trachoma in Hyper-endemic Communities in Tanzania. J. Infect. Dis. 2011;204:268–273. doi: 10.1093/infdis/jir257. * This study examined communities after multiple years of MDA in a previously hyperendemic areas (average prevalence 51% in children under 5). A regression model predicts that >7 years of annual treatment is needed to achieve control of hyperendemic communities (infection <5%). Further examination of antibiotic coverage and its relation to the reduction of infection can provide insight into effective treatment strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebre T, Ayele B, Zerihun M, Genet A, Stoller NE, Zhou Z, House JI, Yu SN, Ray KJ, Emerson PM, et al. Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)61515-8. doi:10.1016/S0140-6736(11)61515-8. * This community-randomized trial in 24 subdistricts in northern Ethiopia demonstrated rapid elimination of ocular infection in biannual treatment groups. However, after 42 months, prevalence was similar to annual treatment groups. [DOI] [PubMed] [Google Scholar]
- 42.West SK, Stare D, Mkocha H, Munoz B, Gaydos C, Quinn TC. Do infants increase the risk of re-emergent infection in households after mass drug administration for trachoma? Invest. Ophthalmol. Vis. Sci. 2011;52:6040–6042. doi: 10.1167/iovs.11-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keenan JD, Moncada J, Gebre T, Ayele B, Chen MC, Yu SN, Emerson PM, Stoller NE, McCulloch CE, Gaynor BD, et al. Chlamydial infection during trachoma monitoring: are the most difficult-to-reach children more likely to be infected? Trop. Med. Int. Health. 2011 doi: 10.1111/j.1365-3156.2011.02919.x. doi:10.1111/j.1365-3156.2011.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayele B, Gebre T, Moncada J, House JI, Stoller NE, Zhou Z, Porco TC, Gaynor BD, Emerson PM, Schachter J, et al. Risk factors for ocular Chlamydia after three mass azithromycin distributions. PLoS Negl Trop Dis. 2011;5:e1441. doi: 10.1371/journal.pntd.0001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowman RJ, Sillah A, Van Dehn C, Goode VM, Muqit MM, Muquit M, Johnson GJ, Milligan P, Rowley J, Faal H, et al. Operational comparison of single-dose azithromycin and topical tetracycline for trachoma. Invest. Ophthalmol. Vis. Sci. 2000;41:4074–4079. [PubMed] [Google Scholar]
- 46.Ayele B, Gebre T, House JI, Zhou Z, McCulloch CE, Porco TC, Gaynor BD, Emerson PM, Lietman TM, Keenan JD. Adverse events after mass azithromycin treatments for trachoma in Ethiopia. Am. J. Trop. Med. Hyg. 2011;85:291–294. doi: 10.4269/ajtmh.2011.11-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cochereau I, Goldschmidt P, Goepogui A, Afghani T, Delval L, Pouliquen P, Bourcier T, Robert P-Y. Efficacy and safety of short duration azithromycin eye drops versus azithromycin single oral dose for the treatment of trachoma in children: a randomised, controlled, double-masked clinical trial. Br J Ophthalmol. 2007;91:667–672. doi: 10.1136/bjo.2006.099275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yagci A, Palamar M. Long-Term Results of Tarsal Margin Rotation and Extended Posterior Lamellae Advancement for End Stage Trachoma. Ophthal Plast Reconstr Surg. 2011 doi: 10.1097/IOP.0b013e318229b50e. doi:10.1097/IOP.0b013e318229b50e. [DOI] [PubMed] [Google Scholar]
- 49.Reacher M, Huber M, Canagaratnam R, Alghassany A. A Trial of Surgery for Trichiasis of the Upper Lid From Trachoma. Br J Ophthalmol. 1990;74:109–113. doi: 10.1136/bjo.74.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habtamu E, Rajak SN, Gebre T, Zerihun M, Genet A, Emerson PM, Burton MJ. Clearing the backlog: trichiasis surgeon retention and productivity in northern Ethiopia. PLoS Negl Trop Dis. 2011;5:e1014. doi: 10.1371/journal.pntd.0001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reacher MH, Munoz B, Alghassany A, Daar AS, Elbualy M, Taylor HR. A controlled trial of surgery for trachomatous trichiasis of the upper lid. Arch Ophthalmol-Chic. 1992;110:667–674. doi: 10.1001/archopht.1992.01080170089030. [DOI] [PubMed] [Google Scholar]
- 52.Rajak SN, Habtamu E, Weiss HA, Bedri A, Gebre T, Bailey RL, Mabey DCW, Khaw PT, Gilbert CE, Emerson PM, et al. The clinical phenotype of trachomatous trichiasis in Ethiopia: not all trichiasis is due to entropion. Invest. Ophthalmol. Vis. Sci. 2011;52:7974–7980. doi: 10.1167/iovs.11-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajak SN, Habtamu E, Weiss HA, Bedri A, Gebre T, Genet A, Khaw PT, Bailey RL, Mabey DCW, Gilbert CE, et al. Epilation for trachomatous trichiasis and the risk of corneal opacification. Ophthalmology. 2012;119:84–89. doi: 10.1016/j.ophtha.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajak SN, Habtamu E, Weiss HA, Kello AB, Gebre T, Genet A, Bailey RL, Mabey DCW, Khaw PT, Gilbert CE, et al. Absorbable versus silk sutures for surgical treatment of trachomatous trichiasis in Ethiopia: a randomised controlled trial. PLoS Med. 2011;8:e1001137. doi: 10.1371/journal.pmed.1001137. * This large surgical randomized clinical trial (RCT) found no difference in the risk of recurrent trichiasis after 1 year follow-up between absorable and silk suture use. This is the only RCT in 2011 that examines surgical interventions for trachoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maher MC, Alemayehu W, Lakew T, Gaynor BD, Haug S, Cevallos V, Keenan JD, Lietman TM, Porco TC. The Fitness Cost of Antibiotic Resistance in Streptococcus pneumoniae: Insight from the Field. PLoS ONE. 2012;7:e29407. doi: 10.1371/journal.pone.0029407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Patel K, Mohapatra MK, Lakhani J, Devi CU, Adak T, et al. A double-blind, randomized study of azithromycin compared to chloroquine for the treatment of Plasmodium vivax malaria in India. Am. J. Trop. Med. Hyg. 2005;73:1108–1111. [PubMed] [Google Scholar]
- 57.Fry AM, Jha HC, Lietman TM, Chaudhary JSP, Bhatta RC, Elliott J, Hyde T, Schuchat A, Gaynor B, Dowell SF. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin. Infect. Dis. 2002;35:395–402. doi: 10.1086/341414. [DOI] [PubMed] [Google Scholar]
- 58.Keenan JD, Ayele B, Gebre T, Zerihun M, Zhou Z, House JI, Gaynor BD, Porco TC, Emerson PM, Lietman TM. Childhood Mortality in a Cohort Treated With Mass Azithromycin for Trachoma. Clin. Infect. Dis. 2011;52:883–888. doi: 10.1093/cid/cir069. * In a cohort of 35,052 individuals >1 year of age and 5507 children 1–5 years of age, there was a reduction in all-cause mortality in children who received azithromycin (odds ratio [OR] =0.35 [95% confidence interval {CI}, 0.17–0.74]). This suggests that MDAs have secondary implications that extend beyond trachoma control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coles CL, Levens J, Seidman JC, Mkocha H, Munoz B, West S. Mass Distribution of Azithromycin for Trachoma Control is Associated with Short-Term Reduction in Risk of Acute Lower Respiratory Infection in Young Children. Pediatr Infect Dis J. 2011 doi: 10.1097/INF.0b013e31824155c9. doi:10.1097/INF.0b013e31824155c9. [DOI] [PubMed] [Google Scholar]
- 60.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 61.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coles CL, Seidman JC, Levens J, Mkocha H, Munoz B, West S. Association of mass treatment with azithromycin in trachoma-endemic communities with short-term reduced risk of diarrhea in young children. Am. J. Trop. Med. Hyg. 2011;85:691–696. doi: 10.4269/ajtmh.2011.11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan A, Ngondi JM, King JD, Elshafie BE, Ginaid Al G, Elsanousi M, Abdalla Z, Aziz N, Sankara D, Simms V, et al. The prevalence of blinding trachoma in northern states of Sudan. PLoS Negl Trop Dis. 2011;5:e1027. doi: 10.1371/journal.pntd.0001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, Wu X, Wei M, Eichner JE, Fan Y, Zhang Z, Lei C, Stone DU, Yang J. Changes in the Prevalence of Visual Impairment Due to Blinding Trachoma in Sichuan Province, China: A Comparative Study Between 1987 and 2006. Ophthalmic Epidemiol. 2011 doi: 10.3109/09286586.2011.615451. doi:10.3109/09286586.2011.615451. [DOI] [PubMed] [Google Scholar]
- 65.Taylor Ac HR, English DR, Field MBA, Spicer PE, Graham DM. The Prevalence of Trachoma in a Single Community, 1975 - 2007. Clin. Experiment. Ophthalmol. 2011 doi: 10.1111/j.1442-9071.2011.02672.x. doi:10.1111/j.1442-9071.2011.02672.x. [DOI] [PubMed] [Google Scholar]
- 66.Lietman TM, Gebre T, Ayele B, Ray KJ, Maher MC, See CW, Emerson PM, Porco TC, TANA Study Group The epidemiological dynamics of infectious trachoma may facilitate elimination. Epidemics. 2011;3:119–124. doi: 10.1016/j.epidem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaynor BD, Miao Y, Cevallos V, Jha H, Chaudary JSP, Bhatta R, Osaki-Holm S, Yi E, Schachter J, Whitcher JP, et al. Eliminating trachoma in areas with limited disease. Emerging Infect. Dis. 2003;9:596–598. doi: 10.3201/eid0905.020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solomon A, Holland M, Alexander N, Massae P, Aguirre A, Natividad-Sancho A, Molina S, Safari S, Shao J, Courtright P, et al. Mass treatment with single-dose azithromycin for trachoma. New England Journal of Medicine. 2004:1962–1971. doi: 10.1056/NEJMoa040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roba AA, Wondimu A, Patel D, Zondervan M. Effects of intervention with the SAFE strategy on trachoma across Ethiopia. J Epidemiol Community Health. 2011;65:626–631. doi: 10.1136/jech.2009.094763. [DOI] [PubMed] [Google Scholar]
- 70.Koroma JB, Heck E, Vandy M, Sonnie M, Hodges M, MacArthur C, Sankara DP. The epidemiology of trachoma in the five northern districts of Sierra Leone. Ophthalmic Epidemiol. 2011;18:150–157. doi: 10.3109/09286586.2011.594204. [DOI] [PubMed] [Google Scholar]
- 71.Gower EW. Solving the trachoma elimination puzzle, one piece at a time. Lancet. 2011 doi: 10.1016/S0140-6736(11)61707-8. doi:10.1016/S0140-6736(11)61707-8. [DOI] [PubMed] [Google Scholar]
- 72.Frick KD, Colchero MA, Dean D. Modeling the economic net benefit of a potential vaccination program against ocular infection with Chlamydia trachomatis. Vaccine. 2004;22:689–696. doi: 10.1016/j.vaccine.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 73.Medina NH, Lopes M de F, Durkin SR, Cardoso MRA, Luna EA, Koizumi IK, Brock KR, Freitas HS de A, Maurício MA. Survey of Trachoma within school students in the state of Roraima, Brazil. Ophthalmology. 2011;118:1938–1943. doi: 10.1016/j.ophtha.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 74.Montgomery MA, Desai MM, Groce NE, Elimelech M. Relationship between distance to social gathering facilities and risk of trachoma for households in rural Tanzanian communities. Soc Sci Med. 2011;73:1–5. doi: 10.1016/j.socscimed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Koukounari A, Touré S, Donnelly CA, Ouedraogo A, Yoda B, Ky C, Kaboré M, Bosqué-Oliva E, Basáñez M-G, Fenwick A, et al. Integrated monitoring and evaluation and environmental risk factors for urogenital schistosomiasis and active trachoma in Burkina Faso before preventative chemotherapy using sentinel sites. BMC Infect. Dis. 2011;11:191. doi: 10.1186/1471-2334-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, Zhou Z, Porco TC, Keenan JD, House JI, et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. Int Health. 2011;3:75–84. doi: 10.1016/j.inhe.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gebre T, Ayele B, Zerihun M, House JI, Stoller NE, Zhou Z, Ray KJ, Gaynor BD, Porco TC, Emerson PM, et al. Latrine promotion for trachoma: assessment of mortality from a cluster-randomized trial in Ethiopia. Am. J. Trop. Med. Hyg. 2011;85:518–523. doi: 10.4269/ajtmh.2011.10-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolaczinski JH, Robinson E, Finn TP. The cost of antibiotic mass drug administration for trachoma control in a remote area of South Sudan. PLoS Negl Trop Dis. 2011;5:e1362. doi: 10.1371/journal.pntd.0001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH. How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis. 2010;4:e755. doi: 10.1371/journal.pntd.0000755. [DOI] [PMC free article] [PubMed] [Google Scholar]